Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1755
Peer-review started: June 30, 2021
First decision: July 18, 2021
Revised: July 30, 2021
Accepted: September 29, 2021
Article in press: September 29, 2021
Published online: November 15, 2021
Processing time: 135 Days and 1.4 Hours
Dietary zinc deficiency has been shown to be associated with the development of esophageal cancer in humans, but the exact mechanism of action is not known
To observe the effects of dietary zinc deficiency on esophageal squamous cell proliferation.
Thirty C57BL/6 mice were randomly divided into three groups: A zinc-sufficient (ZS) group, zinc-deficient (ZD) group, and zinc-replenished (ZR) group. For weeks 1–10, zinc levels in the mice diets were 30.66–30.89 mg/kg in the ZS group and 0.66–0.89 mg/kg in the ZD and ZR groups. During weeks 10–12, the ZR group was switched to the ZS diet; the other two groups had no changes in their diets. Changes in body weight, serum, and esophageal tissue zinc concentrations were assessed as well as differences in the expression of proliferating cell nuclear antigen (PCNA), mitogen-activated protein kinase p38 (p38MAPK), nuclear factor kappa B (NF-κB) p105, NF-κB p65, and cyclooxygenase (COX)-2 proteins in the esophageal mucosa.
The body weight and zinc concentration in the serum and esophageal mucosa were significantly lower in the ZD and ZR groups than in the ZS group (P < 0.05). In ZD mice, there was a marked proliferation of basal cells in the esophageal mucosa, resulting in a disturbance in the arrangement of basal cells in layers 2–4, a thickening of the squamous layer, and a significant increase in the expression of the above-mentioned five proteins involved in proliferation and inflammation in the esophageal mucosa. Two weeks after switching to the ZS diet, the serum zinc concentration in the ZR group increased, and the expression of PCNA, NF-κB p105, and COX-2 decreased, but the concentration of zinc in the esophageal mucosa and the structure of the esophageal mucosa did not display any significant changes
The ZD diet decreased the growth rate and promoted the proliferation of esophageal squamous cells in mice. The mechanism of proliferation was related to the induced overexpression of COX-2, P38, PCNA, and NF-κB (p105 and p65), and the ZR diet reduced the expression of PCNA, NF-κB p105, and COX-2, thereby reversing this process.
Core Tip: Dietary zinc deficiency has been shown to be associated with the development of esophageal cancer in humans, but the exact mechanism of action is not known. The aim of this study was to observe the effects of dietary zinc deficiency on esophageal squamous cell proliferation. In addition, we investigated the pathway of zinc deficiency-induced esophageal squamous cell proliferation by detecting the expression of five predictive biomarkers. The results of the study showed that zinc-deficient diet decreased the growth rate and promoted the proliferation of esophageal epithelial squamous cells in mice. The mechanism was related to the induced overexpression of cyclooxygenase-2, P38, proliferating cell nuclear antigen, and nuclear factor kappa B (p105 and p65), and zinc replenishment reduced the expression of proliferating cell nuclear antigen, nuclear factor kappa B p105, and cyclooxygenase-2, thereby reversing this process.
- Citation: Chen Y, Liu FX, Liu H. Effects of dietary zinc deficiency on esophageal squamous cell proliferation and the mechanisms involved. World J Gastrointest Oncol 2021; 13(11): 1755-1765
- URL: https://www.wjgnet.com/1948-5204/full/v13/i11/1755.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i11.1755
Esophageal squamous cell carcinoma (ESCC), the predominant type of esophageal cancer, is a deadly disease with a 5-year survival rate of only 10%[1]. Because of the absence of early symptoms, patients with ESCC are usually diagnosed at a late stage and, therefore, have a poor prognosis. To improve the prevention and treatment of this deadly cancer, understanding its causes and discovering new early biomarkers are essential for chemoprevention and therapeutic options.
Risk factors for ESCC include alcohol and tobacco use, nutritional deficiencies, and exposure to environmental carcinogens such as N-nitrosomethylbenzylamine[2]. Epidemiological studies have shown an association between dietary zinc deficiency and the etiology of ESCC[3,4]. Studies from Linxian, China, an area of high ESCC prevalence, showed that zinc concentration in biopsy specimens was inversely associated with the risk of cancer development, providing the strongest evidence for an association between dietary zinc deficiency and the occurrence of esophageal cancer in humans[3]. It has been found that zinc deficiency can lead to the overexpression of various genes associated with immune response, apoptosis, cell proliferation, and transcriptional regulation. These overexpressed genes, such as pro-inflammatory cytokines interleukin-1β, interleukin-6, tumor necrosis factor alpha, and cyclic nucleotide phosphodiesterases, may affect esophageal cancer development[5]. However, this is just one of many possibilities. Because of the multiple functions of this element, it can be assumed that the role of zinc in antitumor initiation and promotion is multi-pathway, although its mechanism of action is not fully understood.
The expression of five predictive biomarkers, proliferating cell nuclear antigen (PCNA), mitogen-activated protein kinase p38 (p38MAPK), nuclear factor kappa B (NF-κB) p105, NF-κB p65, and cyclooxygenase (COX)-2 proteins, was shown to be important in the development of esophageal cancer. PCNA is an indicator of cell proliferation[6]. NF-κB p105 and NF-κB p65 are members of the NF-κB family and play important roles in the transcriptional regulation of genes related to inflammation, cell proliferation, differentiation, apoptosis, immune response, and tumorigenesis[7]. Zinc can negatively regulate the NF-κB signaling pathway through numerous mechanisms[8]. COX-2 is an inducible enzyme that converts arachidonic acid to prostaglandins and is involved in inflammatory diseases and tumor development[9]. High COX-2 expression increases the risk of esophageal cancer in healthy people, and inhibition of COX-2 may be useful in the prevention and treatment of this cancer[10-12]. p38MAPK signaling has been linked to the development and progression of cancer, and altered p38MAPK expression has been associated with poor outcomes in patients with esophageal cancer[13]. Hence, the aim of this study was to investigate the effects of dietary zinc deficiency on the growth, development, and proliferation of esophageal squamous cells in mice. In addition, we examined the pathway of zinc deficiency-induced esophageal squamous cell proliferation by detecting the expression of the above-mentioned five predictive biomarkers.
Immunohistochemical detection kits purchased from Abcam Inc. (Cambridge, United Kingdom) were used, and immunostaining was performed according to the manufacturer’s instructions. The D19410B egg white-based AIN-76A diet and D19401 egg white-based AIN-76A diet were purchased from Research Diets Inc. (New Brunswick, NJ, United States). These diets were assayed and found to contain 30.66–30.89 and 0.66–0.89 mg/kg zinc, respectively, and were accordingly used as zinc-sufficient (ZS) and zinc-deficient (ZD) diets (Table 1). This study was approved by the Institutional Research Ethics Committee of Beijing Shijitan Hospital. The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press).
Thirty C57BL/6 mice (age, 3 wk; weaned) of specific-pathogen-free grade were procured from Sparford Laboratory Animal Technology Co. [Beijing, China; certificate number: SCXK (Beijing) 2011-0004]. The animals were housed in groups in stainless steel cages in a temperature-and humidity-controlled room with a 12 h light/dark cycle. The mice were randomized into three groups: ZS, ZD, and zinc-replenished (ZR) groups, with 10 mice in each group. For weeks 1–10, mice in the ZD and ZR groups were fed the ZD diet, and mice in the ZS group were fed the ZS diet. During weeks 10–12, the ZR group was switched to the ZS diet, and the other two groups were fed the same diets without change. All mice had free access to deionized water. The body weight and weight of the ingested feed for all mice were weighed weekly. All animals were euthanized after 12 wk of feeding. The changes in zinc concentrations in the serum and esophageal tissues, as well as differences in the expression levels of PCNA, p38MAPK, NF-κB p105, NF-κB p65, and COX-2 proteins in the esophageal mucosa, were assessed.
The animals were anesthetized with pentobarbital, which was provided by the animal room at our research facility, before euthanization. Blood was collected from the tail of each animal, and the serum was obtained and prepared for zinc analysis. Subsequently, whole esophagi were excised, opened longitudinally, and rinsed with normal saline. The esophageal mucosa was divided into three sections: One part for the detection of zinc concentration and the other two parts fixed in buffered formalin and embedded in paraffin. Cross-sections of the esophagus were cut to a thickness of 4 mm. Half of the sections were stained with hematoxylin and eosin and examined for histopathology under an optical microscope (Olympus, Tokyo, Japan) at 200 × magnification. The remaining tissue sections were dewaxed, hydrated, and analyzed with diluted primary antibodies (Abcam Inc.): PCNA (1:50; ab92552), P38 (1:50; ab31828), NF-κB p105 (1:50; ab32360), NF-κB p65 (1:50; ab16502), and COX-2 (1:50; ab15191).
Serum and esophageal mucosal zinc content was measured by inductively coupled plasma mass spectrometry (inductively coupled plasma mass spectrometry; model ELAN DRC II, Perkin Elmer Inc., Waltham, MA, United States). Prior to sample loading, serum samples were diluted 20 times, and esophageal mucosal samples were weighed, pretreated for microwave digestion, and diluted 20 times. The inductively coupled plasma mass spectrometry was set up with the following parameters: Nebulization gas flow rate, 0.98 L/min; auxiliary gas flow rate, 1.20 L/min; plasma gas flow rate, 15.0 L/min; retention time, 100 ms; sample absorption rate, 1 mL/min; scanning mode, single-point peak-jumping; resolution, 0.7–0.9 aum; 45Sc; 166Er; detection limit, Sc 0.03 ng/mL, Er 0.0003 ng/mL.
Statistical analyses were performed using SPSS software (version 22.0; IBM Inc., Armonk, NY, Unites States). Measurements conforming to a normal distribution were expressed as the mean ± SD. Differences between the groups were evaluated by one-way analysis of variance or the Kruskal–Wallis test for continuous variables. Statistical significance was set at P < 0.05.
As shown in Table 1, the body weight of mice at 3 wk of age did not differ significantly among the three groups. At 10 wk, the body weight of mice was significantly lower in the ZD and ZR groups than in the ZS group (P < 0.05). At 12 wk, the body weight of mice in the ZD group was significantly lower than that of mice in the ZS and ZR groups (P < 0.05). The body weight of mice in the ZR group at 12 wk was higher than that at 10 wk, but there was no significant difference in the body weight of mice in the ZS group, suggesting that dietary zinc replenishment remedied the decrease in body weight caused by zinc deficiency in mice.
As shown in Table 2, the food intake of mice between the ages of 3 and 10 wk did not differ significantly among the three groups. At 12 wk, the food intake of mice in the ZD group was significantly lower than that of mice in the ZS group (P < 0.01), while there was no significant difference in food intake between the ZR and ZS groups.
As shown in Table 3, serum zinc levels of mice in the ZD group were significantly lower than those of mice in the ZS group (P < 0.05), while there was no significant difference between the serum zinc levels in the ZR and ZS groups. Esophageal mucosal zinc levels were significantly lower in the ZD group than in the ZS group (P < 0.01). Although esophageal mucosal zinc levels of mice in the ZR group increased, they were still significantly lower than those of mice in the ZS group (P < 0.01), suggesting that the improvement in the esophageal mucosal zinc level was significantly lower than that in the serum zinc level.
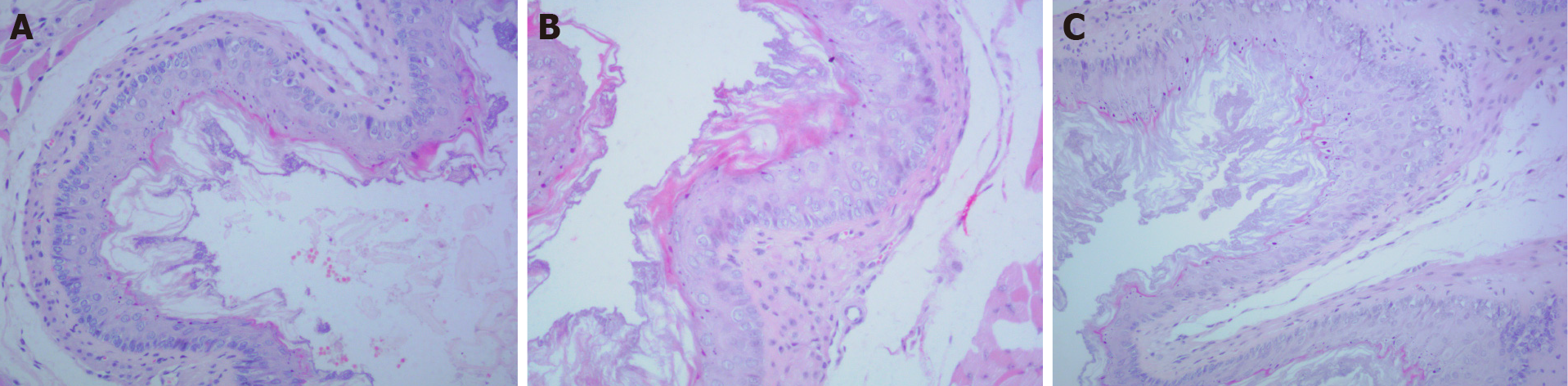
Hematoxylin and eosin staining showed that the esophageal mucosa of mice in the ZS group consisted of a layer of basal cells that were arranged in an orderly manner. In ZD mice, there was apparent basal cell hyperplasia in the esophageal mucosa, which resulted in two to four layers of basal cells arranged in a disordered manner, along with a visible squamous layer thickening. However, columnar metaplasia, inflammation, or ulcers was not observed. Two weeks after zinc replenishment, the esophageal mucosa showed no obvious improvement (Figure 1).
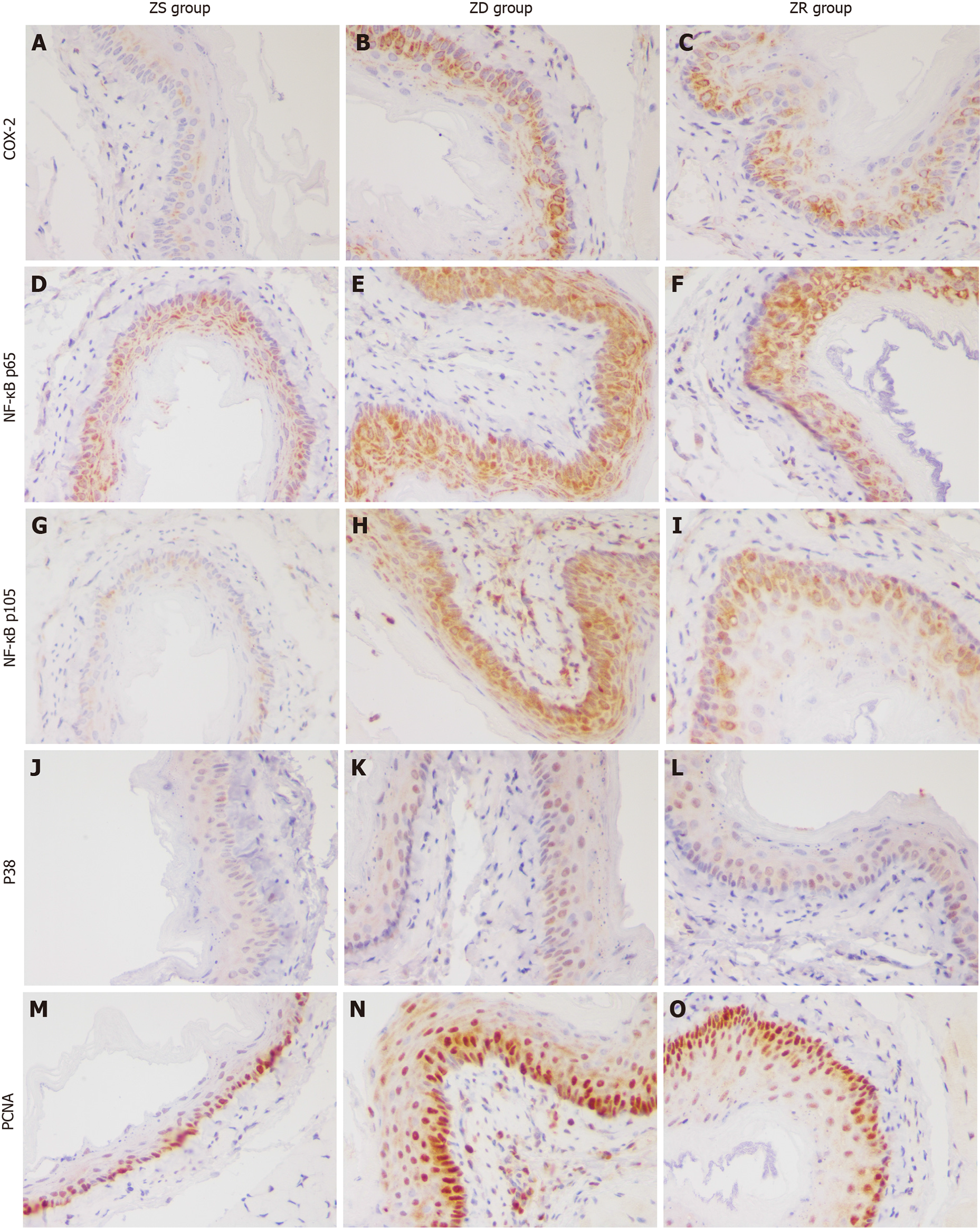
Immunohistochemical staining showed that compared with mice in the ZS group, those in the ZD group showed increased expression levels of PCNA, P38, NF-κB p105, NF-κB p65, and COX-2 in the esophageal mucosa. Two weeks after zinc replenishment, the expression levels of PCNA, NF-κB p105, and COX-2 in the esophageal mucosa of ZR group decreased, while those of NF-κB, p65, and P38 showed no significant change (Figure 2).
Over the past several decades, zinc has been known to be an essential trace element that is widely distributed in vivo and plays a regulatory role in the immune system through its availability, which is tightly regulated by several transporters and regulators. As a functional component or activator of a variety of enzymes, zinc can promote human growth and development, augment nucleic acid and protein synthesis, and increase cell-mediated immune functions[14-16]. Zinc deficiency due to an improper diet is very common, as the human body cannot store zinc reserves[5]. Consequently, zinc deficiency is a global health problem, affecting approximately one-third of the world’s population, predominantly in developing countries[17,18]. Dietary zinc intake is less than half the recommended dose in more than 10% of the population[5]. Acute zinc deficiency causes a decrease in innate and adaptive immunity, whereas chronic deficiency increases inflammation and the risk of cancer[19-22]. Many epidemiological studies have shown that zinc deficiency in humans is associated with an increased risk of developing ESCC, although the mechanism through which this occurs is not fully understood[3,23,24]. By using the ZD rat model, we designed this study to elucidate the effects of dietary zinc deficiency on esophageal squamous cells and the possible mechanisms. In addition, the effect of zinc deficiency on the esophageal mucosa of ZD rats was observed.
Our results showed that after 12 wk of the ZD diet, food intake, body weight, and zinc content in the serum and esophageal mucosal tissues of ZD mice decreased significantly, which differed considerably from that in the ZS group. After 2 wk of zinc replenishment, the body weight and serum zinc content of the ZR mice increased, with no difference compared to the ZS group, while the esophageal mucosal zinc content was very low, indicating that the zinc content in the esophageal mucosal tissues recovered more slowly than the serum zinc content after zinc replenishment. In both animal and human studies, zinc has been shown to participate in the regulation of cell proliferation in several ways; therefore, zinc deficiency can restrict growth[25-26]. It is essential for enzyme systems to influence cell division and proliferation. In humans, an early symptom of zinc deficiency is diarrhea, followed by listlessness and depression[27]. Severe zinc deficiency can lead to hepatic encephalopathy, growth retardation, cell-mediated immune dysfunction, and cognitive impairment[25-28]. Zinc supplementation may be a crucial intervention for improving these clinical problems[29]. Furthermore, epidemiological studies have revealed an association between high circulating zinc concentrations and reduced risk of cancer[30-33]. The mean serum zinc levels in high-risk regions were significantly low. The elemental concentrations of zinc showed a significant difference (P < 0.001) in tumor and non-tumor tissues from the same individual[34]. Zinc deficiency is closely related to the risk of esophageal cancer in multiple populations[32,33]. Zinc is presumed to play a pivotal role in defending against the initiation and promotion of several malignancies, although the mechanism of this role is not fully known[21].
Since the genetic characteristics of mice are clearer than those of rats, mice are the best animal model for studying the development and progression of diseases. The pathological results of this study showed that esophageal mucosal basal cells of mice in the ZD group demonstrated obvious hyperplasia, disordered cell arrangement, and a thickened squamous layer. Previous studies have shown that dietary zinc deficiency may also lead to hyperplasia and hyperkeratosis of esophageal squamous cells in rats and that cell proliferation is directly related to the development of esophageal cancer[35]. Rats fed a low-zinc diet for 5 wk developed esophageal preneoplasia with unique genetic characteristics[36]. Feeding a low-zinc diet to rats for 23 wk resulted in increased expression of cancer-related inflammatory factors that could lead to the development of esophageal cancer when combined with a non-carcinogenic dose of the environmental carcinogen N-nitrosomethylbenzylamine[37-39], while replenishing zinc through a ZS diet reduced these effects in ZD rats. Studies from areas with a high incidence of esophageal cancer have shown that a higher total number of proliferating cells in the esophageal epithelium found in hyperplasia and dysplasia was associated with an increased risk of cancer[40]. Zinc is important for maintaining healthy esophageal epithelium, and zinc deficiency results in abnormal esophageal cell proliferation, promoting tumor development[41].
The expression of five predictive biomarkers, COX-2, NF-κB p65, NF-κB p105, PCNA, and P38, was examined via immunohistochemistry in this study. The results showed that dietary zinc deficiency could induce the overexpression of COX-2, P38, PCNA, NF-κB (p65 and p105), and other inflammatory factors, which may be related to the occurrence of ESCC. After 2 wk of ZR, the expression level of PCNA, NF-κB p105, and COX-2 in the esophageal mucosa decreased. This finding is consistent with the results of Fong et al[42], who found that zinc deficiency also affected cancer development by regulating the expression of NF-κB p65, COX-2, and leukotriene A4 hydrolase. Zinc deficiency significantly increased the incidence of esophageal cancer by inducing the overexpression of inflammatory factors, while zinc supplementation reversed this process[37]. Zinc deficiency-induced inflammation is a critical factor in ESCC development. Furthermore, Taccioli et al[36] found that short-term zinc deficiency could induce the overexpression of proinflammatory genes S100A8 and S100A9 in the esophageal mucosa. Chronic inflammation has been implicated in the pathogenesis of ESCC[37]. Zinc deficiency upregulates oncogenic miR-21, miR-31, and miR-223 and downregulates the tumor suppressor gene miR-375, all of which are accompanied by the dysregulation of their target genes in esophageal cancer[42-44].
In conclusion, dietary zinc deficiency can inhibit growth and promote the proliferation of esophageal epithelial squamous cells in mice. The mechanism may be related to the induced overexpression of COX-2, P38, PCNA, NF-κB (p65 and p105), and other tumor-related factors. Zinc replenishment reversed this process by reducing the expression of PCNA, NF-κB p105, and COX-2. Because China has a high incidence of esophageal cancer, it may be beneficial to prevent the occurrence of esophageal cancer by promoting an increase in the intake of foods rich in zinc, such as fish, seafood, meat, fresh vegetables, and fruits.
Zinc is an element with multiple functions. Zinc deficiency can lead to overexpression of several genes related to immune response, apoptosis, cell proliferation, and transcriptional regulation. Dietary zinc deficiency has been shown to be associated with the development of esophageal cancer in humans, but the exact mechanism of action is not known.
Esophageal squamous cell carcinoma is a deadly disease with a 5-year survival rate of only 10%. Because of the absence of early symptoms, patients with esophageal squamous cell carcinoma ESCC are usually diagnosed at a late stage and, therefore, have a poor prognosis. To improve the prevention and treatment of this deadly cancer, understanding its causes and discovering new early biomarkers are essential for chemoprevention and therapeutic options.
The aim of this study was to investigate the effects of dietary zinc deficiency on the growth, development, and proliferation of esophageal squamous cells in mice. In addition, we investigated the pathway of zinc deficiency-induced proliferation of esophageal squamous cells by detecting the expression of five predictive biomarkers, namely proliferating cell nuclear antigen (PCNA), mitogen-activated protein kinase p38 (p38MAPK), nuclear factor kappa B (NF-κB) p105, NF-κB p65, and cyclooxygenase (COX)-2 protein.
Thirty C57BL/6 mice were randomly divided into three groups: a zinc-sufficient (ZS) group, zinc-deficient (ZD) group, and zinc-replenished (ZR) group. For weeks 1–10, zinc levels in the mice diets were 30.66–30.89 mg/kg in the ZS group and 0.66–0.89 mg/kg in the ZD and ZR groups. During weeks 10–12, the ZR group was switched to the ZS diet; the other two groups had no changes in their diets. Changes in body weight, serum, and esophageal tissue zinc concentrations were assessed as well as differences in the expression of PCNA, p38MAPK, NF-κB p105, NF-κB p65, and COX-2 proteins in the esophageal mucosa.
The body weight and zinc concentration in the serum and esophageal mucosa were significantly lower in the ZD and ZR groups than in the ZS group. In ZD mice, there was a marked proliferation of basal cells in the esophageal mucosa, resulting in a disturbance in the arrangement of basal cells in layers 2–4, a thickening of the squamous layer, and a significant increase in the expression of the above-mentioned five proteins involved in proliferation and inflammation in the esophageal mucosa.
The results indicated that the ZD diet decreased the growth rate and promoted the proliferation of esophageal squamous cells in mice. The mechanism of proliferation was related to the induced overexpression of COX-2, P38, PCNA, and NF-κB (p105 and p65), and the ZR diet reduced the expression of PCNA, NF-κB p105, and COX-2, thereby reversing this process.
In this study, all five proteins were detected by immunohistochemistry staining, which is a semi-quantitative method. In future studies, we will try to increase the sample size and use a quantitative approach to make the results more meaningful.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koma YI, Ozawa S, Tsai NM S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11836] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 2. | Magee PN. The experimental basis for the role of nitroso compounds in human cancer. Cancer Surv. 1989;8:207-239. [PubMed] |
| 3. | Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM, Taylor PR, Dong ZW, Mark SD, Dawsey SM. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst. 2005;97:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Dar NA, Mir MM, Salam I, Malik MA, Gulzar GM, Yatoo GN, Ahmad A, Shah A. Association between copper excess, zinc deficiency, and TP53 mutations in esophageal squamous cell carcinoma from Kashmir Valley, India--a high risk area. Nutr Cancer. 2008;60:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Skrajnowska D, Bobrowska-Korczak B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 6. | Boehm EM, Gildenberg MS, Washington MT. The Many Roles of PCNA in Eukaryotic DNA Replication. Enzymes. 2016;39:231-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 7. | Van der Heiden K, Cuhlmann S, Luong le A, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond). 2010;118:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4:676-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 9. | Jin J, Guo T, Guo Y, Liu J, Qu F, He Y. Methylationassociated silencing of miR128 promotes the development of esophageal cancer by targeting COX2 in areas with a high incidence of esophageal cancer. Int J Oncol. 2019;54:644-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Peng Q, Yang S, Lao X, Tang W, Chen Z, Lai H, Wang J, Sui J, Qin X, Li S. Meta-analysis of the association between COX-2 polymorphisms and risk of colorectal cancer based on case-control studies. PLoS One. 2014;9:e94790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Jiang JG, Tang JB, Chen CL, Liu BX, Fu XN, Zhu ZH, Qu W, Cianflone K, Waalkes MP, Wang DW. Expression of cyclooxygenase-2 in human esophageal squamous cell carcinomas. World J Gastroenterol. 2004;10:2168-2173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Cheng Y, Qiao Z, Dang C, Zhou B, Li S, Zhang W, Jiang J, Song Y, Zhang J, Diao D. p38 predicts depression and poor outcome in esophageal cancer. Oncol Lett. 2017;14:7241-7249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Fong LY, Taccioli C, Jing R, Smalley KJ, Alder H, Jiang Y, Fadda P, Farber JL, Croce CM. MicroRNA dysregulation and esophageal cancer development depend on the extent of zinc dietary deficiency. Oncotarget. 2016;7:10723-10738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Wellinghausen N. Immunobiology of gestational zinc deficiency. Br J Nutr. 2001;85 Suppl 2:S81-S86. [PubMed] |
| 15. | Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009;6:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015;29:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Caro CR, Del C Coronell M, Arrollo J, Martinez G, Majana LS, Sarmiento-Rubiano LA. Zinc deficiency: A global problem that affect the health and cognitive development. Arch Latinoam Nutr. 2016;66:165-175. [PubMed] |
| 18. | Wessells KR, Singh GM, Brown KH. Estimating the global prevalence of inadequate zinc intake from national food balance sheets: effects of methodological assumptions. PLoS One. 2012;7:e50565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Mocchegiani E, Costarelli L, Giacconi R, Piacenza F, Basso A, Malavolta M. Zinc, metallothioneins and immunosenescence: effect of zinc supply as nutrigenomic approach. Biogerontology. 2011;12:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Bonaventura P, Benedetti G, Albarède F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 482] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 21. | Dhawan DK, Chadha VD. Zinc: a promising agent in dietary chemoprevention of cancer. Indian J Med Res. 2010;132:676-682. [PubMed] |
| 22. | Zowczak M, Iskra M, Torliński L, Cofta S. Analysis of serum copper and zinc concentrations in cancer patients. Biol Trace Elem Res. 2001;82:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Fong LY, Magee PN. Dietary zinc deficiency enhances esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in C57BL/6 mouse. Cancer Lett. 1999;143:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Fong LY, Nguyen VT, Farber JL. Esophageal cancer prevention in zinc-deficient rats: rapid induction of apoptosis by replenishing zinc. J Natl Cancer Inst. 2001;93:1525-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013;4:176-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 520] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 26. | MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000;130:1500S-1508S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 543] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 27. | Jeejeebhoy KN. Human zinc deficiency. Nutr Clin Pract. 2007;22:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Muhamed PK, Vadstrup S. [Zinc is the most important trace element]. Ugeskr Laeger. 2014;176. [PubMed] |
| 29. | Barnett JB, Dao MC, Hamer DH, Kandel R, Brandeis G, Wu D, Dallal GE, Jacques PF, Schreiber R, Kong E, Meydani SN. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2016;103:942-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Wu T, Sempos CT, Freudenheim JL, Muti P, Smit E. Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann Epidemiol. 2004;14:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Leone N, Courbon D, Ducimetiere P, Zureik M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 32. | Zhang ZF, Kurtz RC, Yu GP, Sun M, Gargon N, Karpeh M Jr, Fein JS, Harlap S. Adenocarcinomas of the esophagus and gastric cardia: the role of diet. Nutr Cancer. 1997;27:298-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Hashemian M, Poustchi H, Abnet CC, Boffetta P, Dawsey SM, Brennan PJ, Pharoah P, Etemadi A, Kamangar F, Sharafkhah M, Hekmatdoost A, Malekzadeh R. Dietary intake of minerals and risk of esophageal squamous cell carcinoma: results from the Golestan Cohort Study. Am J Clin Nutr. 2015;102:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Xie B, Lin J, Sui K, Huang Z, Chen Z, Hang W. Differential diagnosis of multielements in cancerous and non-cancerous esophageal tissues. Talanta. 2019;196:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Fong LY, Farber JL, Magee PN. Zinc replenishment reduces esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in zinc-deficient rats. Carcinogenesis. 1998;19:1591-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Taccioli C, Wan SG, Liu CG, Alder H, Volinia S, Farber JL, Croce CM, Fong LY. Zinc replenishment reverses overexpression of the proinflammatory mediator S100A8 and esophageal preneoplasia in the rat. Gastroenterology. 2009;136:953-966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Taccioli C, Chen H, Jiang Y, Liu XP, Huang K, Smalley KJ, Farber JL, Croce CM, Fong LY. Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature. Oncogene. 2012;31:4550-4558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Fong LY, Zhang L, Jiang Y, Farber JL. Dietary zinc modulation of COX-2 expression and lingual and esophageal carcinogenesis in rats. J Natl Cancer Inst. 2005;97:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Wan SG, Taccioli C, Jiang Y, Chen H, Smalley KJ, Huang K, Liu XP, Farber JL, Croce CM, Fong LY. Zinc deficiency activates S100A8 inflammation in the absence of COX-2 and promotes murine oral-esophageal tumor progression. Int J Cancer. 2011;129:331-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Wang LD, Lipkin M, Qui SL, Yang GR, Yang CS, Newmark HL. Labeling index and labeling distribution of cells in esophageal epithelium of individuals at increased risk for esophageal cancer in Huixian, China. Cancer Res. 1990;50:2651-2653. [PubMed] |
| 41. | Choi S, Cui C, Luo Y, Kim SH, Ko JK, Huo X, Ma J, Fu LW, Souza RF, Korichneva I, Pan Z. Selective inhibitory effects of zinc on cell proliferation in esophageal squamous cell carcinoma through Orai1. FASEB J. 2018;32:404-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Fong LY, Jiang Y, Riley M, Liu X, Smalley KJ, Guttridge DC, Farber JL. Prevention of upper aerodigestive tract cancer in zinc-deficient rodents: inefficacy of genetic or pharmacological disruption of COX-2. Int J Cancer. 2008;122:978-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Mei LL, Qiu YT, Zhang B, Shi ZZ. MicroRNAs in esophageal squamous cell carcinoma: Potential biomarkers and therapeutic targets. Cancer Biomark. 2017;19:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | Liu CM, Liang D, Jin J, Li DJ, Zhang YC, Gao ZY, He YT. Research progress on the relationship between zinc deficiency, related microRNAs, and esophageal carcinoma. Thorac Cancer. 2017;8:549-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |