Published online Oct 15, 2020. doi: 10.4251/wjgo.v12.i10.1146
Peer-review started: June 4, 2020
First decision: July 21, 2020
Revised: August 4, 2020
Accepted: September 2, 2020
Article in press: September 2, 2020
Published online: October 15, 2020
Processing time: 132 Days and 8.1 Hours
Gastric cancer (GC) is one of the most common malignant tumors in the world. Although in recent years tremendous progress has been made in its early detection, the postoperative overall survival (OS) of GC patients remains extremely low. A number of studies have shown that age, to varying degrees, affects the prognosis of patients with GC. Therefore, this study retrospectively analyzed the clinical and pathologic data of patients with GC to explore the differences in the clinical characteristics and prognostic factors in different age groups.
To explore the difference in clinicopathological characteristics and prognostic factors in GC patients in different age groups.
In this retrospective study, we analyzed 1037 GC patients admitted to Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine from May 2010 to January 2013. The patients were divided into two groups based on age: Younger group (less than 70 years old) and older group (no less than 70 years old). In the younger group, we subdivided the patients in two subgroups by a cut-off value of 45 years. The clinical features and prognostic factors were analyzed in both groups. Subsequently, we retrieved studies that evaluated the predictive role of neutrophil-lymphocyte ratio (NLR) by searching two medical databases, PubMed and EMBASE, to conduct a meta-analysis. Random-effects model was used to pool the data.
In the retrospective study, the mean OS time of the younger group (64.7 mo) was significantly longer than that of the older group (48.1 mo) (P < 0.001). Among patients under 70 years of age, hospitalization time, tumor–node–metastasis (TNM) stage, vascular invasion, and preoperative low pre-albumin were independently associated with OS (P < 0.005). In patients aged 70 years and above, TNM stage, esophageal invasion, histological type, and preoperative NLR were independent factors for OS (P < 0.05). The OS of these older patients was also significantly shorter (P < 0.05). In the meta-analysis, 19 retrieved studies included a total of 8312 patients, among whom 3558 had elevated NLR values. The results showed that high NLR value was a risk factor for the prognosis of GC (P < 0.01).
The OS of elderly patients is significantly worse than that of younger patients. There are significant differences in clinicopathological characteristics and prognostic factors between younger and older patients. NLR is a convenient, inexpensive, and reproducible marker that can be used as an important predictor of the prognosis of GC.
Core Tip: This is a retrospective study that explored the prognostic factors in different age groups and the prognostic significance of neutrophil-lymphocyte ratio in gastric cancer patients. We compared the clinicopathological features of patients in two age groups (< 70 years and ≥ 70 years), analyzed the prognostic factors, and performed a thorough meta-analysis. The findings indicated that the improvement of preoperative nutritional status may be beneficial to the prognosis in younger patients, while the alleviation of inflammatory status should be emphasized for older patients before surgery. The conclusion can provide reliable reference for clinicians to identify and rectify the independent prognostic influencing factors in gastric cancer patients.
- Citation: Li Q, Huang LY, Xue HP. Comparison of prognostic factors in different age groups and prognostic significance of neutrophil-lymphocyte ratio in patients with gastric cancer. World J Gastrointest Oncol 2020; 12(10): 1146-1166
- URL: https://www.wjgnet.com/1948-5204/full/v12/i10/1146.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i10.1146
Gastric cancer (GC) is one of the most common malignant tumors in the world, with approximately 1 million new cases each year[1,2]. It is the second most lethal malignancy worldwide[3], with the 5-year overall survival (OS) rate being only 29.7%[2]. Although in recent years tremendous progress has been made in its early detection, surgical techniques, and multidisciplinary treatment, including neoadjuvant chemotherapy and radiotherapy, the postoperative OS of GC patients remains extremely low[4]. Therefore, it is essential to identify the most significant independent prognostic factors for GC patients.
Due to the decline of physiological function and poor nutritional status in elderly patients (the cut-off value was 70 years old in this study), trauma due to radical gastrectomy may readily lead to a higher incidence of postoperative complications and, accordingly, increased postoperative mortality rate[5,6]. It is urgent and necessary to accurately identify the pivotal prognostic factors for GC patients in different age groups. In the present study, we performed a retrospective analysis to compare different clinical and pathologic factors and explore their prognostic roles in patients with GC in different age groups.
In recent years, the relationship between inflammation and cancer has been widely explored[7-9], in which neutrophil-lymphocyte ratio (NLR) is one of the inflammatory markers, and its prognostic role has been demonstrated in pancreatic[10], colorectal[11], and lung cancer[12], as well as lymphoma[13]. NLR may affect the prognosis of cancer patients via activation of natural killer cells. But the prognostic role of NLR in GC remains controversial[14] though Hirashima et al[15] first found its role in GC. Therefore, we also conducted a systemic review and meta-analysis to explore the prognostic role of NLR in GC in this study.
This study was reviewed and unanimously approved by the Ethics Committee of Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. In the retrospective analysis portion, we investigated the clinical and pathologic data of GC patients admitted to the hospital from May 2010 to January 2013. A total of 1037 patients were included in our study. The inclusion criteria were as follows: (1)Postoperative, pathologically confirmed diagnosis of stages I to IV gastric adenocarcinoma; (2) Complete clinicopathological information; and (3) Complete follow-up data.
In the meta-analysis portion, we searched all articles through PubMed and EMBASE without limitation of publication time. The following terms were used: (“neutrophil-lymphocyte ratio” or “neutrophil-to-lymphocyte ratio” or NLR) and (“gastric cancer” or “GC” or “gastric adenocarcinoma” or “stomach neoplasms” or “gastric carcinoma”) and (“prognosis” or ”prognostic value” or ”overall survival” or ”OS”). We repeatedly executed the search strategy to ensure that no other related article was missed. By checking the authors' names and the affiliations for each study, we excluded any article with overlapping or duplicated results with other included articles.
Studies were included if they met the following criteria: (1) The diagnosis of GC was confirmed pathologically; (2) The relationship between preoperative NLR and OS was evaluated; and (3) The sufficient data (hazard ratio [HR] and 95% confidence interval [CI] for OS) were provided. The exclusion criteria were as follows: (1) Reviews, case reports, letters, or conference abstracts; (2) Studies on cancer cells and animal models; and (3) Studies that failed to provide the cut-off value of NLR elevation.
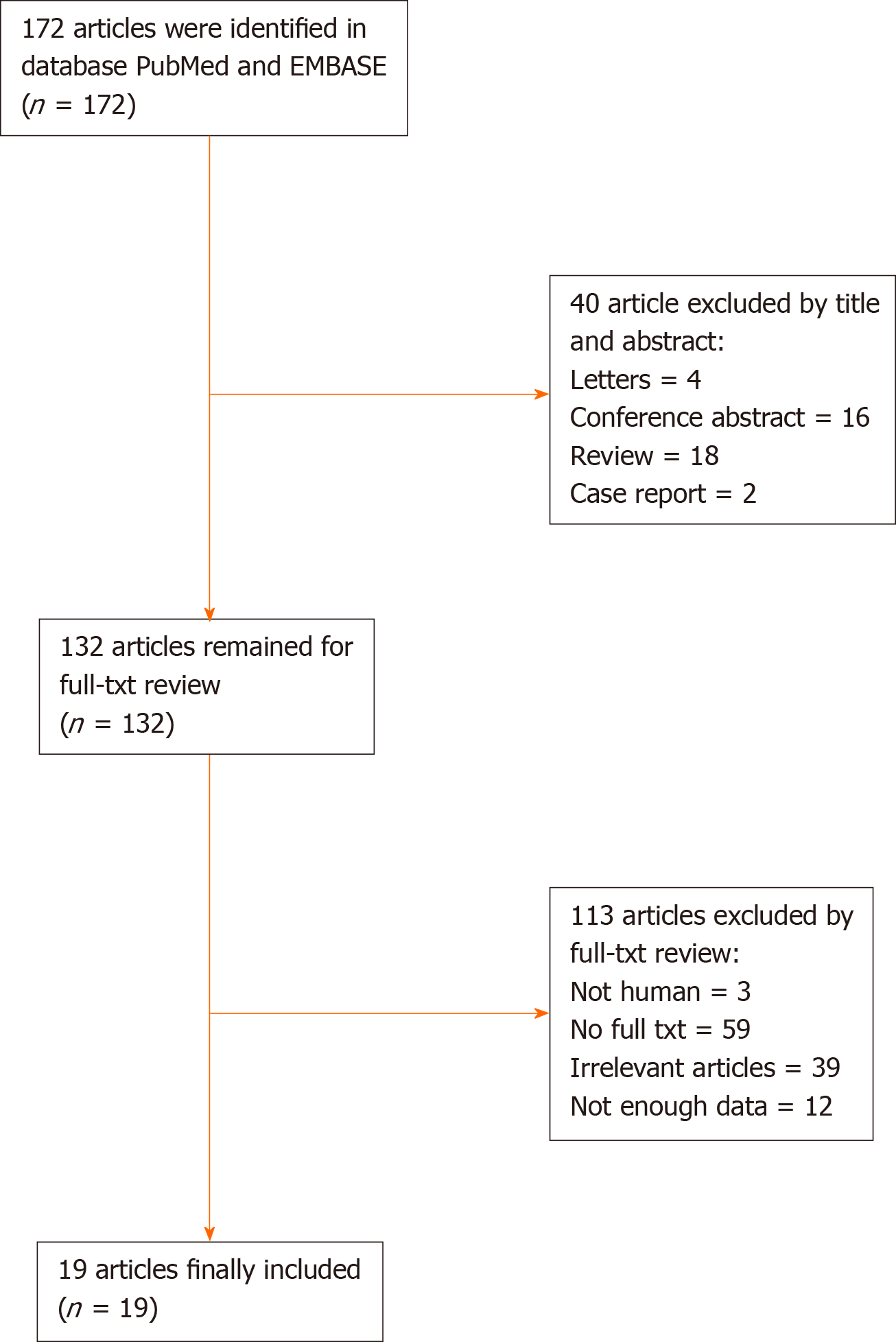
All searches were carried out independently by two authors. The flow chart of selection of the article is shown in Figure 1.
In the retrospective analysis portion, all the data were obtained from the inpatient and outpatient records, including demographic information (age and gender), tumor-specific data (tumor size, neural invasion, vascular invasion, esophageal invasion, histopathologic type, and tumor location), therapeutic modalities (surgical procedures, chemotherapy, and radiotherapy), and survival data. Peripheral blood detection data were collected preoperatively, including routine blood examination, serum albumin, serum pre-albumin, etc.
All the patients underwent routine assessments after surgery, including physical examination, laboratory examination, CT scan, and upper gastrointestinal endoscopy. The latest follow-up date was January 20, 2018, with a median follow-up duration of 61.3 mo (range, 0-92 mo). All patients were followed for at least 5 years except for those who died during the follow-up period. The OS was calculated from the date of surgery until death or final available follow-up.
In the meta-analysis portion, the extracted data were as follows: (1) Publication details including the first author’s name, publication year, and origin of population studied; (2) Demographic characteristics including sample size, gender distribution, age, and disease stage; (3) HR of NLR for OS and its 95%CI; (4) Follow-up time; and (5) Cut-off value for elevated NLR. If several estimates were reported in the same article, we chose the most powerful one.
In the retrospective analysis portion, according to Onodera, the prognostic nutritional index (PNI) was calculated as follows: 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (per cubic millimeter)[16]. Its cut-off value was defined as 47 according to the Youden’s index of the receiver operating characteristic (ROC) curve. The optimal cut-off values for hospitalization time and low preoperative pre-albumin were 18 d and 212 mg/L, respectively, based on the Youden’s index of the ROC curves. Similarly, the optimal cut-off values for preoperative NLR and platelet lymphocyte ratio (PLR) were 2.6 and 133, respectively. Those for preoperative anemia and hypoalbuminemia were defined as 120 g/L and 35 g/L, respectively, according to the normal ranges used in our hospital.
Tumor stage was determined in accordance with the tumor–node–metastasis (TNM) staging system (the seventh edition) proposed by the American Joint Committee on Cancer[17]. Histopathologically, papillary and tubular adenocarcinomas were classified as intestinal-type adenocarcinomas, while poorly differentiated signet ring cell and mucinous adenocarcinomas were classified as diffuse-type adenocarcinomas[18]. The size of the tumor was bounded by 4 cm according to the Youden index of the ROC curve.
We used the Youden’s index of the ROC curve to divide patients into two different groups based on the age of 70 years. We compared the features of patients in the two groups in terms of general characteristics, pathologic findings, inflammatory markers, nutritional immune status, and OS. To obtain more information about the younger group (age < 70 years), we further divided the patients in this group into two subgroups, according to the middle-age definition of the World Health Organization (WHO).
Informed consent was approved by the Ethics Committee of Renji Hospital. This study design and protocol were thoroughly reviewed and unanimously approved by the Ethics Committee of Renji Hospital (Microsoft1).
In the meta-analysis portion, the quality of studies was assessed by the Newcastle-Ottawa Quality Assessment Scale[19]. The scale includes three aspects of assessment: Selection, comparability, and results of case group and control group. Studies scored over 6 were considered high quality ones.
In the retrospective analysis portion, the patients’ quantitative characteristics were defined using descriptive statistics, with their differences analyzed by chi-square test. Univariate and multivariate analyses were performed using the Cox proportional hazard model to explore the major prognostic factors. The ROC curves were plotted, and the optimal cut-off values of hospitalization time, tumor size, pre-albumin, PNI, NLR, and PLR were determined by the Youden’s index [maximum (sensitivity + specificity)-1]. We used Kaplan -Meier methodology to estimate the cumulative survival, and its statistical difference was assessed by the log rank test. All statistical analyses were carried out using the SPSS statistical software package version 24.0 (IBM Corporation, Armonk, NY, United States).
In the meta-analysis portion, all statistical analyses were performed via Stata 12.0. HRs and their associated 95%CI from each study were pooled. Cochran’s Q test and I-squared statistics were used to evaluate heterogeneity. If I2 values < 50% or P ≥ 0.05, which indicates that there was no significant heterogeneity, a fixed-effects model was used; otherwise, we applied a random-effects model. Subsequently, to find potential sources of heterogeneity, we performed meta-regression and subgroup analyses. We also used sensitivity analysis to evaluate the stability of the results. Finally, publication bias of the literature was assessed through Begg’s funnel plot and Egger’s linear regression test.
The alpha was set at 0.05, and all tests were two-sided.
In the retrospective analysis portion, a total of 1037 patients with GC were included in our study (Table 1); 728 (70.2%) were male and 309 (29.8%) were female, with a male-to-female ratio of 2.36: 1. The patients ranged in age from 19 to 90 years, with an average age of 62.9 years. All the patients were followed for 5 years; 452 patients (43.6%) died and 585 (56.4%) survived. Up to the end of follow-up, 482 (46.5%) patients died, and 555 (53.5%) remained alive. In this study, we divided patients into two groups in accordance with their age: 716 cases (69%) in the younger group (< 70 years) with an age range of 19-69 years and an average of 57 years; and 321 cases (31%) in the older group (≥ 70 years) with an age range of 70-90 years and an average of 76 years.
| Characteristic | Patients n (%) | Overall survival months (95%CI) | P value |
| Sex | 0.864 | ||
| Male | 728 (70.2%) | 59.6 (56.9-62.2) | |
| Female | 309 (29.8%) | 59.7 (55.7-63.8) | |
| Age (yr) | < 0.001 | ||
| < 70 | 716 (69%) | 64.7 (62.2-67.3) | |
| ≥ 70 | 321 (31%) | 48.1 (44.1-52.2) | |
| Hospitalization (d) | < 0.001 | ||
| < 18 | 494 (47.6%) | 68.7 (65.7-71.6) | |
| ≥ 18 | 543 (52.4%) | 51.5 (48.4-54.6) | |
| TNM stage | < 0.001 | ||
| I | 251 (24.2%) | 87.1 (84.9-89.2) | |
| II | 197 (19.0%) | 75.5 (71.4-79.5) | |
| III | 509 (49.1%) | 46.2 (43.1-49.2) | |
| IV | 80 (7.7%) | 19.1 (14.5-23.8) | |
| Tumor size (cm) | < 0.001 | ||
| < 4 | 405 (39.1%) | 75.2 (72.3-78.1) | |
| ≥ 4 | 632 (60.9%) | 49.7 (46.8-52.6) | |
| Lymph node metastasis (N) | < 0.001 | ||
| 0 | 393 (37.9%) | 80.6 (78.1-83.2) | |
| 1-3 | 644 (62.1%) | 46.8 (44.0-49.6) | |
| Distant metastasis (M) | < 0.001 | ||
| 0 | 957 (92.3%) | 62.9 (60.7-65.2) | |
| 1 | 80 (7.7%) | 19.1 (14.5-23.8) | |
| Tumor location | < 0.001 | ||
| Cardia | 200 (19.3%) | 50.9 (45.9-56.0) | |
| Non-cardia | 837 (80.7%) | 61.7 (59.2-64.1) | |
| Hematological type | < 0.001 | ||
| Intestinal type | 555 (53.5%) | 64.5 (61.6-67.4) | |
| Diffuse type | 482 (46.5%) | 53.9 (50.6-57.2) | |
| Depth of invasion | < 0.001 | ||
| 1-2 | 313 (30.2%) | 84.4 (82.0-86.8) | |
| 3-4 | 724 (69.8%) | 48.9 (46.3-51.6) | |
| Neural invasion | < 0.001 | ||
| No | 822 (74.3%) | 63.1 (60.7-65.6) | |
| Yes | 215 (25.7%) | 45.9 (41.1-50.7) | |
| Vessel invasion | < 0.001 | ||
| No | 771 (74.3%) | 65.7 (63.2-68.1) | |
| Yes | 266 (25.7%) | 42.0 (37.7-46.3) | |
| Esophageal invasion | < 0.001 | ||
| No | 949 (91.5%) | 61.6 (59.3-63.9) | |
| Yes | 88 (8.5%) | 38.7 (31.5-45.8) | |
| NLR | < 0.001 | ||
| < 2.6 | 634 (61.1%) | 64.5 (61.8-67.3) | |
| ≥ 2.6 | 382 (38.9%) | 51.8 (48.2-55.5) | |
| HB (g/L) | < 0.001 | ||
| < 120 | 407 (39.2%) | 53.2 (49.6-56.8) | |
| ≥ 120 | 630 (60.8%) | 63.8 (61.1-66.6) | |
| PLR | < 0.001 | ||
| < 133 | 486 (46.9%) | 64.9 (61.8-68.0) | |
| ≥ 133 | 551 (53.1%) | 54.9 (51.8-58.0) | |
| Albumin | < 0.001 | ||
| < 35 | 165 (15.9%) | 46.4 (40.8-52.0) | |
| ≥ 35 | 872 (84.1%) | 62.1 (59.8-64.5) | |
| Pre-albumin | < 0.001 | ||
| < 212 | 354 (34.1%) | 45.7 (41.8-49.5) | |
| ≥ 212 | 683 (65.9%) | 66.9 (64.3-69.4) | |
| PNI | < 0.001 | ||
| < 47 | 387 (37.3%) | 48.3 (44.7-52.0) | |
| ≥ 47 | 65 (62.7%) | 66.4 (63.7-69.0) |
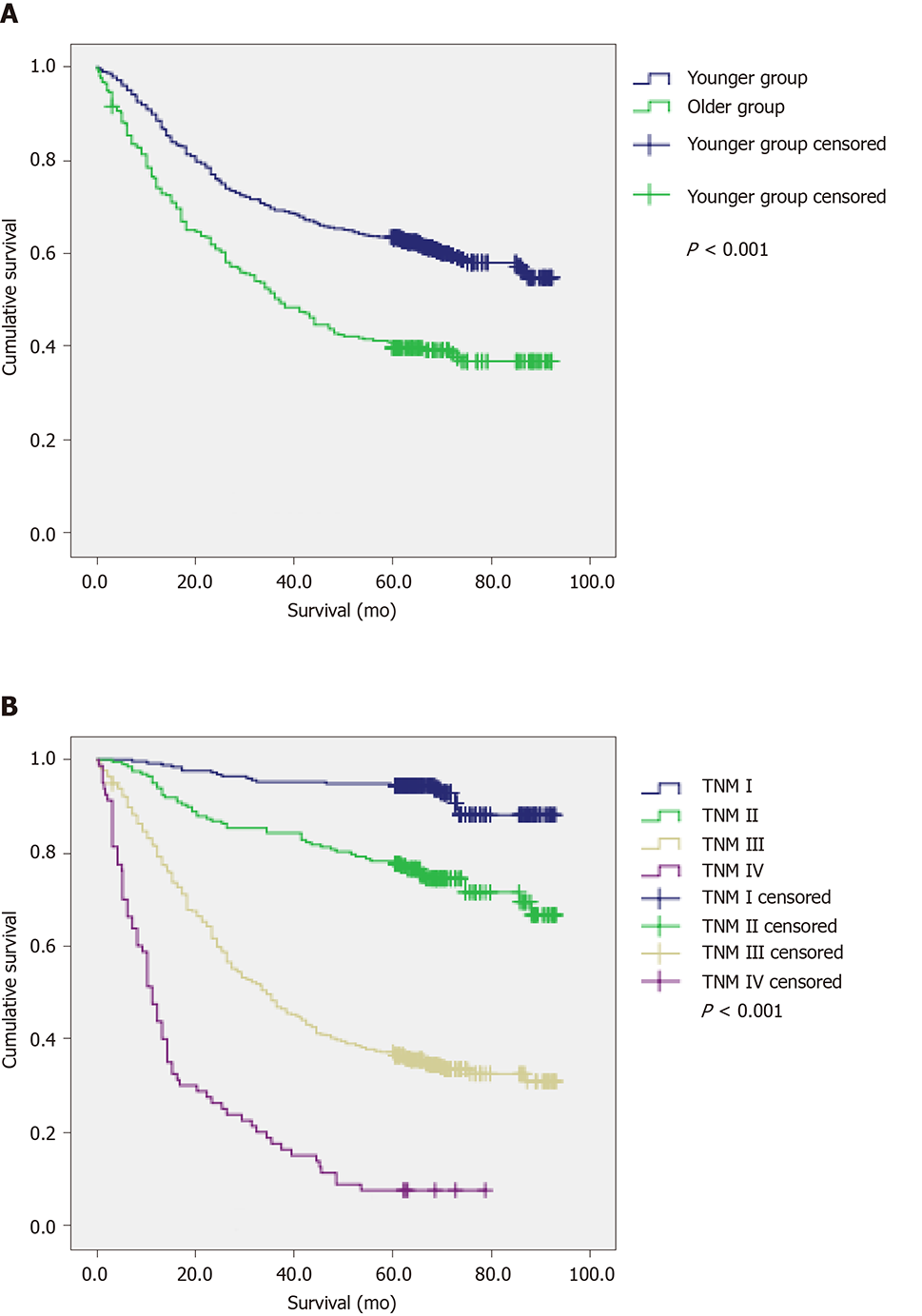
The 5-year survival rate was 63.4% for the younger and 40.8% for the elderly, respectively (Figure 2A). The mean OS (64.7 mo) in the younger group was significantly longer than that in the older group (48.1 mo) (P < 0.001). In addition, the 5-year survival rates for patients with TNM stages I-IV disease were 94.4%, 78.2%, 36.9%, and 7.5%, respectively (Figure 2B), and their OS values were 87.1, 75.5, 46.2, and 19.1 mo, respectively (P < 0.001) (Table 1).
In the meta-analysis portion, the flow chart of our literature search is shown in Figure 1. A total of 172 articles were obtained based on the search strategy; finally, we retrieved 19 articles that met our inclusion criteria[20-38].
Supplementary Table 1 summarizes the characteristics of the included studies. We collected data from 19 studies with a total of 8312 patients. Four studies were prospective and 15 were retrospective. Twelve enrolled studies had less than 400 patients and seven had more than 400 patients. The cut-off value of NLR in ten studies was less than 3, and that of the remaining nine was more than 3. HR and 95%CI were reported directly in all included studies.
The clinicopathological features of the two groups are summarized in Table 2. There was no significant difference in gender distribution, distant metastasis, neural or vascular invasion, esophageal invasion, or tumor location between the two groups. Compared with younger patients, older patients had longer hospitalization, deeper tumor invasion, more advanced TNM stage, larger tumor size, and more lymph node metastasis (P < 0.005). And they had higher preoperative NLR and PLR values, and lower preoperative PNI values, being more likely to have anemia, hypopreal-buminemia, and hypoalbuminemia (P < 0.005). With regard to pathologic type, more patients in the older group had intestinal-type adenocarcinomas, while more younger patients had diffuse-type adenocarcinomas (P < 0.005).
| Characteristic | Younger group n (%) | Older group n (%) | P value |
| Sex | 0.407 | ||
| Male | 497 (69.4%) | 231 (72.0%) | |
| Female | 219 (30.6%) | 90 (28.0%) | |
| Hospitalization (d) | < 0.001 | ||
| < 18 | 369 (51.5%) | 125 (38.9%) | |
| ≥ 18 | 347 (48.5%) | 196 (61.6%) | |
| TNM stage | 0.003 | ||
| I-II | 331 (46.2%) | 117 (36.4%) | |
| III-IV | 385 (53.8%) | 204 (63.6%) | |
| T stage | < 0.001 | ||
| 1-2 | 240 (33.5%) | 73 (22.7%) | |
| 3-4 | 476 (66.5%) | 248 (77.3%) | |
| N stage | 0.021 | ||
| 0 | 288 (40.2%) | 105 (32.7%) | |
| 1-3 | 428 (59.8%) | 216 (67.3%) | |
| M stage | 0.343 | ||
| 0 | 657 (91.8%) | 300 (93.5%) | |
| 1 | 59 (8.2%) | 21 (6.5%) | |
| Tumor size (cm) | < 0.001 | ||
| < 4 | 318 (44.4%) | 87 (27.1%) | |
| ≥ 4 | 398 (55.6%) | 234 (72.9%) | |
| Tumor location | 0.059 | ||
| Cardia | 127 (17.7%) | 73 (22.7%) | |
| Non-cardia | 589 (82.3%) | 248 (77.3%) | |
| Histological type | 0.001 | ||
| Intestinal type | 359 (50.1%) | 196 (61.1%) | |
| Diffuse type | 357 (49.9%) | 125 (38.9%) | |
| Neural invasion | 0.358 | ||
| No | 562 (78.5%) | 260 (81%) | |
| Yes | 154 (21.5%) | 61 (19%) | |
| Vessel invasion | 0.719 | ||
| No | 530 (74%) | 241 (75.1%) | |
| Yes | 186 (26%) | 80 (24.9%) | |
| Esophageal invasion | 0.855 | ||
| No | 656 (91.6%) | 293 (91.3%) | |
| Yes | 60 (8.4%) | 28 (8.7%) | |
| NLR | 0.003 | ||
| < 2.6 | 459 (64.1%) | 175 (54.5%) | |
| ≥ 2.6 | 257 (35.9%) | 146 (45.5%) | |
| Anemia | < 0.001 | ||
| No | 499 (69.7%) | 131 (40.8%) | |
| Yes | 217 (30.3%) | 190 (59.2%) | |
| PLR | 0.003 | ||
| < 133 | 358 (50%) | 128 (39.9%) | |
| ≥ 133 | 358 (50%) | 193 (60.1%) | |
| Low albumin | < 0.001 | ||
| No | 650 (90.8%) | 222 (69.2%) | |
| Yes | 66 (9.2%) | 99 (30.8%) | |
| Low pre-albumin | < 0.001 | ||
| No | 534 (74.6%) | 149 (46.4%) | |
| Yes | 182 (25.4%) | 172 (53.6%) | |
| PNI | < 0.001 | ||
| < 47 | 191 (26.7%) | 196 (61.1%) | |
| ≥ 47 | 525 (73.3%) | 125 (38.9%) |
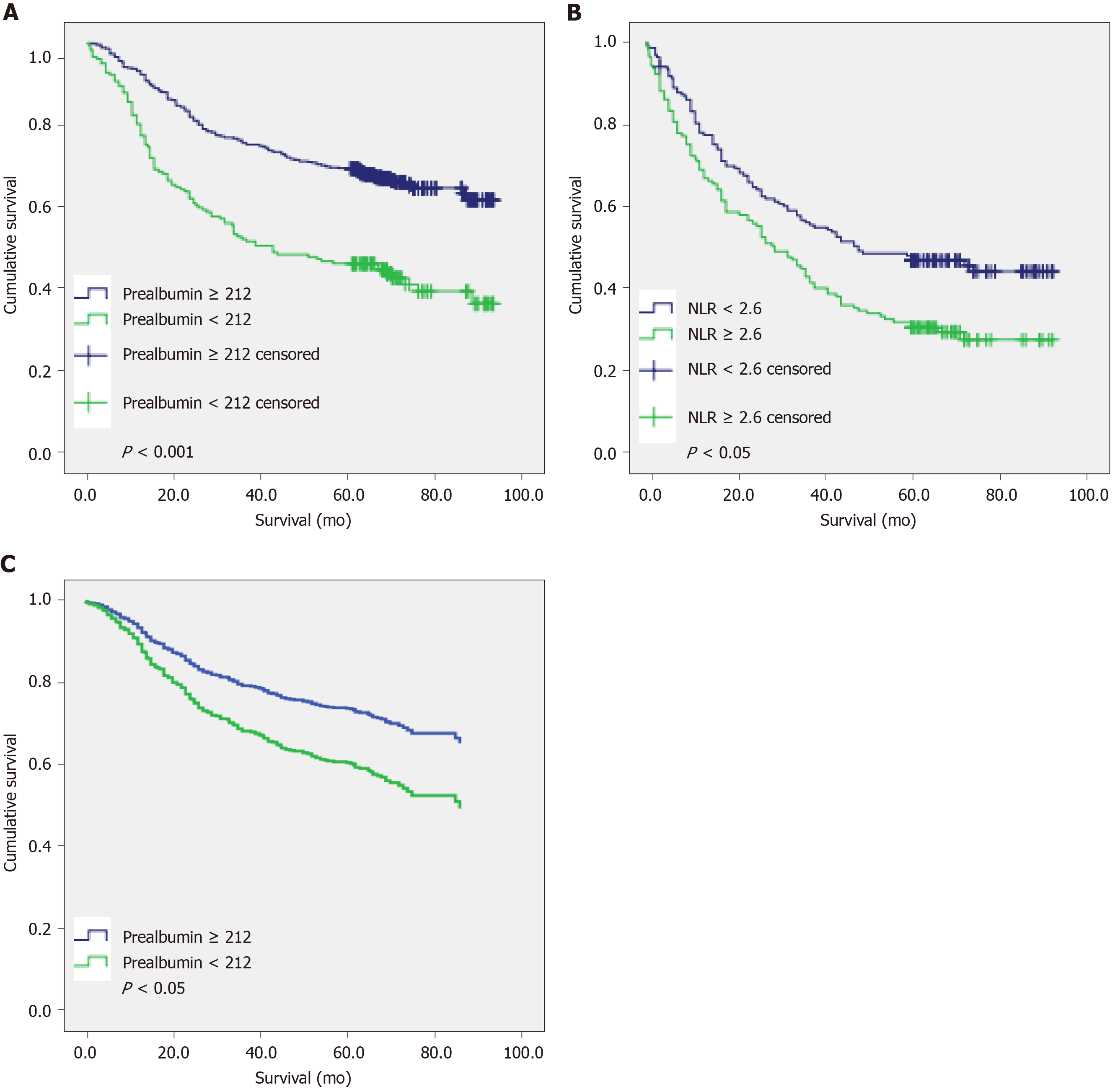
The results of univariate Cox analysis (Table 3) showed that in the younger group (less than 70 years old), hospitalization time (P < 0.001), TNM stage (P < 0.001), tumor location (P = 0.001), histological type (P < 0.001), neural invasion (P < 0.001), vascular invasion (P < 0.001), esophageal invasion (P < 0.001), preoperative NLR (P < 0.001), preoperative PLR (P < 0.001), preoperative anemia (P = 0.046), preoperative low albumin (P = 0.010), preoperative low pre-albumin (P < 0.001), and preoperative PNI (P < 0.001) were significantly correlated with OS. The results of multivariate Cox regression analysis (Table 3) indicated that hospitalization time (P < 0.001), TNM stage (P < 0.001), vascular invasion (P = 0.002), and preoperative low pre-albumin (P < 0.001) were independent prognostic factors for OS, respectively. The 5-year survival rates of patients with low and normal pre-albumin were 46.2% and 69.3%, respectively; the OS of the former (50.7 mo) was significantly shorter than that of the latter (69.6 mo) (P < 0.001) (Figure 3A). Therefore, preoperative nutritional status may affect the survival prognosis of young patients with gastric cancer.
| Characteristic | Univariate | Analysis | Multivariate | Analysis |
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (Male/Female) | 0.983 (0.765-1.263) | 0.893 | ||
| Hospitalization (≥ 18/< 18 mo) | 2.439 (1.909-3.115) | < 0.001 | 1.801 (1.394-2.327) | < 0.001 |
| T stage (3 + 4/1 + 2) | 9.948 (6.242-15.855) | < 0.001 | ||
| N stage (1-3/0) | 6.841 (4.797-9.756) | < 0.001 | ||
| M stage (1/0) | 5.799 (4.278-7.860) | < 0.001 | ||
| TNM (III-IV/I-II) | 7.791 (5.586-10.867) | < 0.001 | 5.418 (3.731-7.869) | < 0.001 |
| Location (Cardia/non-cardia) | 1.567 (1.190-2.064) | < 0.001 | 1.042 (0.753-1.441) | 0.804 |
| Tumor size (≥ 4/< 4 cm) | 2.962 (2.267-3.871) | < 0.001 | 1.123 (0.831-1.517) | 0.452 |
| Histological type (Diffuse/Intestinal) | 1.661 (1.311-2.105) | < 0.001 | 1.249 (0.973-1.604) | 0.081 |
| Neural invasion (Y/N) | 2.024 (1.573-2.604) | < 0.001 | 0.987 (0.758-1.286) | 0.925 |
| Vessel invasion (Y/N) | 2.865 (2.260-3.632) | < 0.001 | 1.501 (1.163-1.936) | 0.002 |
| Esophageal invasion(Y/N) | 2.197 (1.566-3.082) | < 0.001 | 1.214 (0.814-1.810) | 0.341 |
| NLR (≥ 2.6/< 2.6) | 1.602 (1.267-2.024) | < 0.001 | 1.151 (0.881-1.505) | 0.303 |
| Anemia (Y/N) | 1.282 (1.004-1.637) | 0.046 | 0.746 (0.559-0.995) | 0.046 |
| PLR (≥ 133/< 133) | 1.540 (1.217-1.948) | < 0.001 | 1.102 (0.835-1.453) | 0.493 |
| Low albumin (Y/N) | 1.585 (1.117-2.249) | 0.010 | 0.781 (0.515-1.183) | 0.243 |
| Low prealbumin (Y/N) | 2.149 (1.688-2.735) | < 0.001 | 1.637 (1.254-2.138) | < 0.001 |
| PNI (< 47/≥ 47) | 1.839 (1.444-2.343) | < 0.001 | 1.226 (0.896-1.679) | 0.203 |
In the older group, however, univariate analysis (Table 4) showed that TNM stage (P < 0.001), histological type (P < 0.001), neural invasion (P < 0.001), vascular invasion (P < 0.001), esophageal invasion (P < 0.001), preoperative NLR (P = 0.002), preoperative low albumin (P = 0.019), preoperative low pre-albumin (P < 0.001), and preoperative PNI (P = 0.002) were significantly correlated with OS. In the same way, these factors were tested by a multivariate Cox regression analysis (Table 4): TNM stage (P < 0.001), pathohistological type (P = 0.014), esophageal invasion (P < 0.001), and preoperative NLR (P = 0.028) were identified as the independent prognostic factors for OS. The 5-year survival rates of patients with preoperative NLR ≥ 2.6 and 2.6 were 32.2% and 48%, respectively. The OS values were 41.3 mo and 53.8 mo, respectively, and the difference was statistically significant (Figure 3B).
| Characteristic | Univariate | Analysis | Multivariate | Analysis |
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (Male/Female) | 1.016 (0.742-1.392) | 0.920 | ||
| Hospitalization (≥ 18/< 18 mo) | 1.328 (0.991-1.780) | 0.058 | ||
| T stage (3 + 4/1 + 2) | 4.356 (2.709-7.003) | < 0.001 | ||
| N stage (1-3/0) | 3.690 (2.544-5.352) | < 0.001 | ||
| M stage (1/0) | 5.142 (3.210-8.237) | < 0.001 | ||
| TNM (III-IV/I-II) | 5.462 (3.723-8.001) | < 0.001 | 3.873 (2.571-5.836) | < 0.001 |
| Location (Cardia/non-cardia) | 1.314 (0.955-1.807) | 0.094 | ||
| Tumor size (≥ 4/< 4 cm) | 2.711 (1.854-3.963) | < 0.001 | 1.468 (0.965-2.232) | 0.073 |
| Histological type (Diffuse/Intestinal) | 1.675 (1.264-2.220) | < 0.001 | 1.441 (1.078-1.928) | 0.014 |
| Neural invasion (Y/N) | 1.928 (1.392-2.671) | < 0.001 | 1.191 (0.828-1.713) | 0.347 |
| Vessel invasion (Y/N) | 1.983 (1.464-2.684) | < 0.001 | 1.299 (0.927-1.822) | 0.129 |
| Esophageal invasion (Y/N) | 2.602 (1.716-3.944) | < 0.001 | 2.330 (1.505-3.607) | <0.001 |
| NLR (≥ 2.6/< 2.6) | 1.554 (1.174-2.056) | 0.002 | 1.396 (1.038-1.880) | 0.028 |
| Anemia (Y/N) | 1.336 (0.999-1.785) | 0.051 | ||
| PLR (≥ 133/< 133) | 1.221 (0.913-1.633) | 0.179 | ||
| Low albumin (Y/N) | 1.420 (1.060-1.904) | 0.019 | 1.121 (0.792-1.587) | 0.519 |
| Low prealbumin (Y/N) | 1.781 (1.336-2.374) | < 0.001 | 1.190 (0.857-1.653) | 0.299 |
| PNI (< 47/≥ 47) | 1.589 (1.179-2.142) | 0.002 | 0.962 (0.659-1.404) | 0.840 |
We further made univariate and multivariate analyses in the younger group (less than 70 years old) by age sub-stratification. In the subgroup A (45 ≤ age < 70), univariate analysis (Table 5) demonstrated that hospitalization (P = 0.01), T stage (P < 0.001), N stage (P < 0.001), M stage (P < 0.001), TNM stage (P < 0.001), histological type (P < 0.001), tumor location (P = 0.007), tumor size (P < 0.001), neural invasion (P < 0.001), vascular invasion (P < 0.001), esophageal invasion (P < 0.001), preoperative NLR (P = 0.001), PLR (P = 0.002), preoperative low albumin (P = 0.002), preoperative low pre-albumin (P < 0.001), and preoperative PNI (P < 0.001) were significantly correlated with OS. In the multivariate analysis (Table 5), TNM stage (P < 0.001), vascular invasion (P < 0.001), and preoperative low pre-albumin (P < 0.001) were indicated to be independent factors. The survival curve of preoperative low pre-albumin is shown in Figure 3C (P < 0.001). In the subgroup B (age < 45), univariate analysis demonstrated that hospitalization (P = 0.022), T stage (P < 0.015), N stage (P = 0.01), M stage (P < 0.001), TNM stage (P < 0.001), tumor location (P = 0.011), tumor size (P = 0.004), vascular invasion (P < 0.001), preoperative NLR (P = 0.012), PLR (P = 0.025), and preoperative PNI (P = 0.019) were significantly correlated with OS. TNM stage (P < 0.001) and vascular invasion (P = 0.013) were indicated to be the only significant factors in the multivariate analysis (Table 6).
| Characteristic | Univariate | Analysis | Multivariate | Analysis |
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (Male/Female) | 0.555 | |||
| Hospitalization (≥ 18/< 18 mo) | 2.698 (1.153-6.312) | 0.022 | 0.072 | |
| T stage (3 + 4/1 + 2) | 60.674 (2.194-1677.654) | 0.015 | ||
| N stage (1-3/0) | 8.062 (2.361-27.534) | 0.01 | ||
| M stage (1/0) | 6.437 (2.599-15.946) | < 0.001 | ||
| TNM (III-IV/I-II) | 10.933 (3.217-37.162) | < 0.001 | 9.253 (2.644-32.377) | < 0.001 |
| Location (Cardia/non-cardia) | 3.684 (1.344-10.092) | 0.011 | 0.301 | |
| Tumor size (≥ 4/< 4 cm) | 4.293 (1.599-11.527) | 0.004 | 0.271 | |
| Histological type (Diffuse/Intestinal) | 0.161 | |||
| Neural invasion (Y/N) | 0.63 | |||
| Vessel invasion (Y/N) | 4.518 (1.988-10.266) | < 0.001 | 2.898 (1.249-6.723) | 0.013 |
| Esophageal invasion (Y/N) | 0.31 | |||
| NLR (≥ 2.6/< 2.6) | 2.878 (1.257-6.588) | 0.012 | 0.445 | |
| Anemia (Y/N) | 0.094 | |||
| PLR (≥ 133/<133) | 3.083 (1.148-8.276) | 0.025 | 0.958 | |
| Low albumin (Y/N) | 0.295 | |||
| Low prealbumin (Y/N) | 0.051 | |||
| PNI (< 47/≥ 47) | 2.760 (1.178-6.466) | 0.019 | 0.244 |
| Characteristic | Univariate | Analysis | Multivariate | Analysis |
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (Male/Female) | 0.953 | |||
| Hospitalization (≥ 18/< 18 mo) | 1.546 (1.210-1.976) | 0.01 | 0.127 | |
| T stage (3 + 4/1 + 2) | 8.995 (5.635-14.358) | < 0.001 | ||
| N stage (1-3/0) | 6.752 (4.659-9.786) | < 0.001 | ||
| M stage (1/0) | 5.792 (4.186-8.013) | < 0.001 | ||
| TNM (III-IV/I-II) | 7.558 (5.348-10.682 | < 0.001 | 5.994 (4.183-8.590) | < 0.001 |
| Histological type (Diffuse/Intestinal) | 0.598 (0.468-0.764) | < 0.001 | 0.104 | |
| Location (Cardia/non-cardia) | 1.481 (1.111-1.973) | 0.007 | 0.947 | |
| Tumor size (≥ 4/< 4 cm) | 2.859 (2.165-3.776) | < 0.001 | 0.302 | |
| Neural invasion (Y/N) | 2.075 (1.596-2.696) | < 0.001 | 0.723 | |
| Vessel invasion (Y/N) | 2.749 (2.146-3.523) | < 0.001 | 1.603 (1.242-2.069) | < 0.001 |
| Esophageal invasion (Y/N) | 2.209 (1.559-3.130) | < 0.001 | 1.431 (1.285-2.130) | 0.046 |
| NLR (≥ 2.6/< 2.6) | 1.541 (1.206-1.970) | 0.001 | 0.335 | |
| Anemia (Y/N) | 0.157 | |||
| PLR (≥ 133/< 133) | 1.486 (1.163-1.898) | 0.002 | 0.436 | |
| Low albumin (Y/N) | 1.721 (1.216-2.438) | 0.002 | 0.484 | |
| Low prealbumin (Y/N) | 2.128 (1.656-2.735) | < 0.001 | 1.654 (1.285-2.130) | < 0.001 |
| PNI (< 47/≥ 47) | 1.806 (1.404-2.323) | < 0.001 | 0.265 |
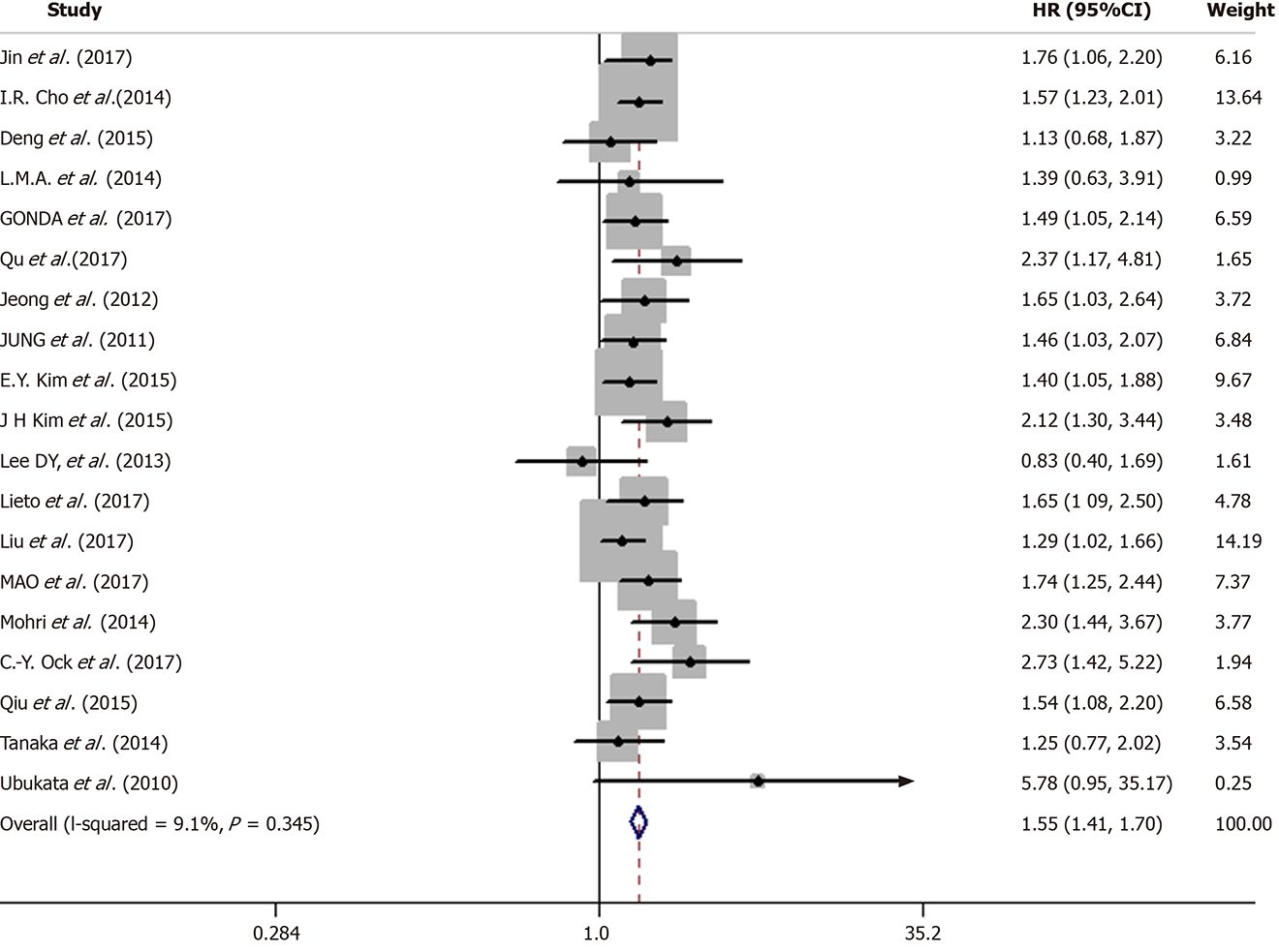
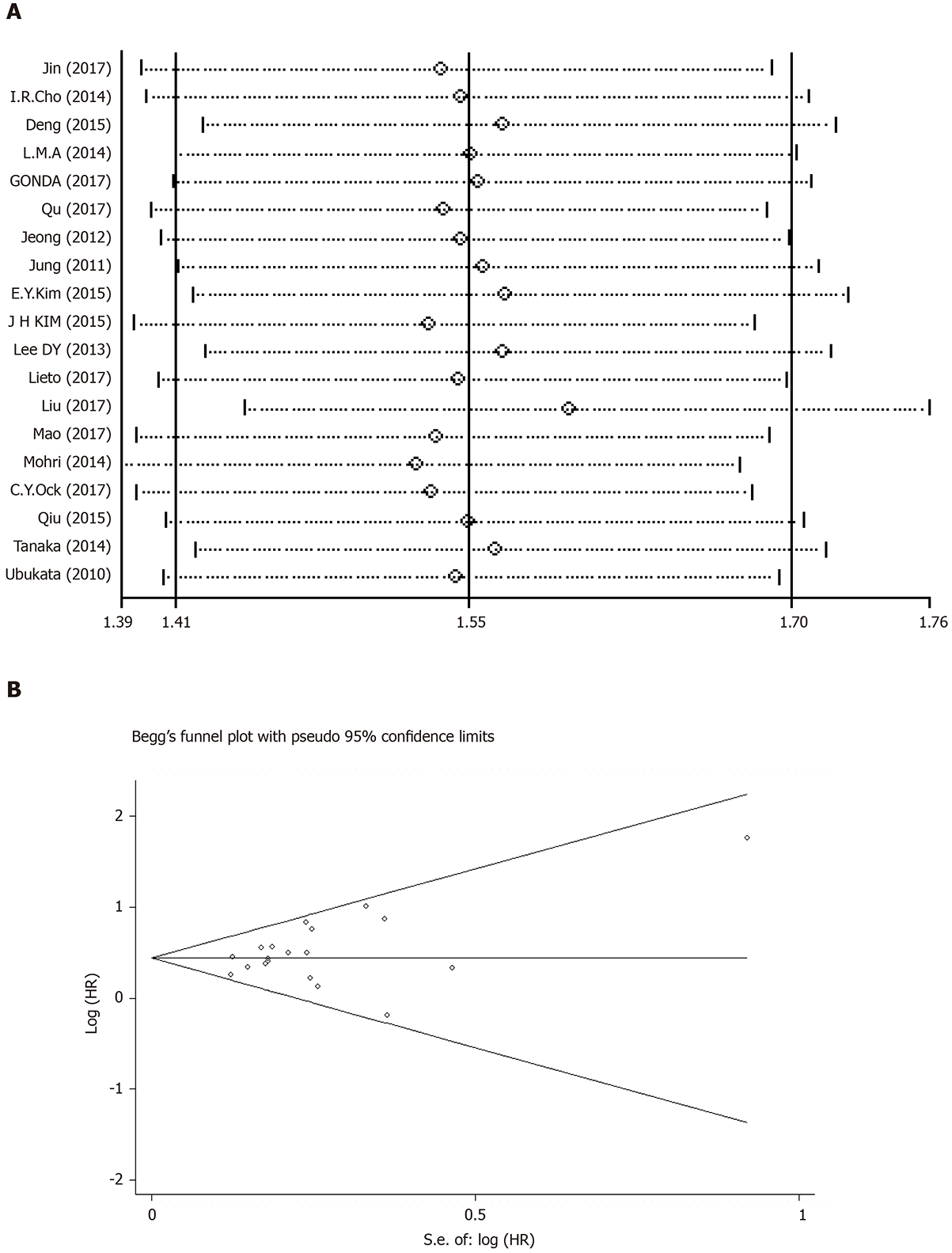
In the 19 studies included, there was no significant heterogeneity (I-squared = 9.1%; P = 0.345). Thus, we applied a fixed-effects model for analysis. The results showed that the pooled HR was 1.55 [95%CI: (1.41-1.70)], indicating that patients with an elevated NLR had a shorter OS (Figure 4).
Subsequently, meta-regression analyses (Table 7) showed that treatment method, study design, NLR threshold, sample size, and proportion of males were not the sources of heterogeneity (P > 0.01). Subgroup analyses showed that, regardless of surgery, chemotherapy, or comprehensive therapy, there was a significant correlation between elevated NLR and poor prognosis (surgery 1.49, 95%CI: 1.34-1.67; chemotherapy 1.55, 95%CI: 1.29-1.86; and multiple therapy 1.95, 95%CI: 1.46-2.60). When performing subgroup analyses stratified by study design, we found that a high NLR value was associated with a poor prognosis in both prospective [HR = 1.61, 95%CI: (1.31–1.98)] and retrospective studies [HR = 1.54, 95%CI: (1.39-1.70)]. When performing subgroup analyses stratified by the cut-off value < 3 and ≥ 3, we found that elevated NLR value was still an indicator for poor OS in both prospective [HR = 1.46, 95%CI: (1.29–1.66)] and retrospective studies [HR = 1.65, 95%CI: (1.45-1.88)] (Table 7).
| Subgroup | 1P value | n | HR | 95%CI | I2 | 2P value |
| Design | 0.715 | |||||
| Retrospective | 15 | 1.54 | 1.39-1.70 | 14.8 | 0.287 | |
| Prospective | 4 | 1.61 | 1.31-1.98 | 9.1 | 0.345 | |
| Sample size | 0.879 | |||||
| < 400 | 12 | 1.56 | 1.38-1.76 | 2 | 0.424 | |
| ≥ 400 | 7 | 1.54 | 1.37-1.77 | 29.8 | 0.201 | |
| Cut-off value | 0.223 | |||||
| < 3 | 10 | 1.46 | 1.29-1.66 | 33.2 | 0.142 | |
| ≥ 3 | 9 | 1.65 | 1.45-1.88 | 0 | 0.798 | |
| Male/all | 0.225 | |||||
| < 0.7 | 14 | 1.50 | 1.36-2.66 | 0 | 0.469 | |
| ≥ 0.7 | 5 | 1.75 | 1.43-2.13 | 25.6 | 0.251 | |
| Treatment | 0.162 | |||||
| Surgery | 13 | 1.49 | 1.34-1.67 | 25.1 | 0.190 | |
| Chemotherapy | 4 | 1.55 | 1.29-1.86 | 0 | 0.982 | |
| Multiple | 2 | 1.95 | 1.46-2.60 | 0 | 0.375 |
The results of the sensitivity analysis, as shown in Figure 5A, indicated that no single literature can significantly affect the entire result, confirming that the results of this meta-analysis were stable.
We applied Begg’s funnel plot and Egger’s test to assess publication bias of the literature. As shown in Figure 5B, no obvious asymmetry was found in the funnel plot shape. Thus, the publication bias in this meta-analysis was not evident. The P values for Begg’s test and Egger’s test were 0.248 and 0.134, respectively.
In general, the 5-year survival rate of GC is still low worldwide, especially in Asian countries such as China, South Korea, and Japan[39], probably due to late diagnosis and inadequate management. Early diagnosis of GC can be achieved through popular science education and endoscopic screening, while appropriate management is a complex task involving many considerations. The quantitative evaluation of postoperative survival in patients with GC relies on a complex mathematical function, determined by the interactions of various known and unknown factors[40].
The WHO defines “elderly” as older than 65 years old[41]. In previously published studies in older patients with GC, age thresholds ranged from 65 to 85 years old, so 65 years old may not be best suitable for “elderly” patients with GC[42-45]. In our study, we used a survival ROC curve in terms of OS to determine the borderline age in patients with GC, and found that the optimal cut-off age was 70 years old. Therefore, the patients were divided into either a younger group (69 years and younger) or an older group (70 years and older) in the present study based on the cutoff value of 70 years. In the younger group, 716 patients were included and their age distribution was from 19 to 69 years old. Given the large amount of data available, this group can be considered for further subgroup analysis. Accordingly, we stratified the younger group into two subgroups (cut off value was 45 years old) according to the middle age definition of the WHO.
Our retrospective analysis showed that the OS of elderly patients was significantly worse than that of younger patients. The reason may be that elderly patients are more likely to exhibit some organ dysfunction, making them more difficult to overcome operative stress. We also found that advanced age was closely associated with longer hospitalization time, advanced tumor stage, poor nutrition, and severe inflammatory state.
As for the analyses of prognostic factors, in the younger group, hospitalization time, TNM stage, vascular invasion, and preoperative low pre-albumin level were independently associated with OS. In the older group, TNM stage was also identified to be the independent risk factor for OS; however, the length of stay, vascular invasion, and preoperative low pre-albumin level were not related to OS, but esophageal invasion, pathohistological type, and preoperative NLR could independently predict OS for the older group.
It is easy to understand that TNM stage can serve as a prognostic factor[46], but its prognostic value is limited because it can only be used after surgery[17]. Similarly, vascular invasion, esophageal invasion, and histological types are also determined by the patient’s postoperative pathologic features, so their prognostic values for GC are also limited. Therefore, we mainly focused on some indicators that are easy to measure as well as inexpensive and convenient to perform, and objective to evaluate preoperatively. From the above results, preoperative pre-albumin in the younger group and preoperative NLR in the older group should be stressed.
There is increasing evidence that cancer-related malnutrition is a common but often unrecognized problem[47], and the prognosis of cancer is closely linked to nutritional status[48-50]. Several potential mechanisms have been hypothesized for their relationship. First, malnutrition weakens human immune defense system, including cellular and humoral immunity and phagocytic functions, resulting in increased risks of postoperative infection and metastasis[51]. Second, malnutrition, as a subacute or chronic state, accompanied by varying degrees of nutritional deficiencies and increased inflammatory responses, contributes to body compositional changes and functional decline[52,53], and thus diverse postoperative complications and reduced therapeutic efficacy of drugs[54]. Finally, malnutrition can also promote tumor development by inhibiting immunity[55]. At present, pre-albumin, as a marker of nutritional status, has become the research focus owing to the fact that its half-life (about 1.9 d) is shorter than albumin and it is a negative acute phase protein synthesized by the liver. Therefore, the pre-albumin level is highly sensitive in identifying the body’s metabolic status and immune function. Han et al[56] also showed that pre-albumin was an independent predictor of postoperative survival outcome[56]. The finding is consistent with that in our study. Therefore, we propose that younger GC patients’ nutritional status should be ameliorated considerably before surgery, especially for those with low pre-albumin.
It is often taken for granted that age is an important factor when clinicians choose the optimal therapeutic modality for GC patients. It is self-evident that the operation risk of senile GC patients is much higher than that of young counterparts. We also found significant differences in clinicopathological characteristics and prognostic factors between different age groups of GC patients. However, there is increasing evidence that the senile GC patients with radical gastrectomy have a relatively longer OS than those without[57,58]. Therefore, according to the findings in our study, the preoperative alleviation of inflammatory status should be emphasized for elderly GC patients after their surgical benefits have been evaluated to prevail over their surgical risks. Certainly, if elder patients cannot bear the risk of surgery, palliative chemotherapy can be an alternative therapeutic method.
It is well known that chronic inflammation induces carcinogenesis and promotes the development of cancers[59]. NLR reflects the patient's inflammatory status and is known to have prognostic value in patients with cancer. Some possible mechanisms may account for the relationship between NLR and poor prognosis in GC patients. First, an increase in the number of neutrophils around the tumor may inhibit the anti-tumor responses of natural killer cells and activated T cells[60]. Furthermore, neutrophils promote tumor progression by producing cytokines such as tumor necrosis factor, IL-1, IL-6, angiogenic factors, and vascular endothelial growth factor[61]. Finally, the decrease of lymphocyte counts attenuates lymphocyte-mediated anti-tumor cellular immune responses. Consequently, preoperative NLR should be given a high priority in elderly patients. For those with high NLR, it is essential to find out whether there is acute or chronic inflammation, and effective anti-inflammatory treatment should be recommended to cut down NLR to a suitable level before surgery, which, to a large extent, may improve the prognosis of GC.
To further verify our results in retrospective analysis portion, we attempted to conduct a comprehensive meta-analysis based on previous studies. Nevertheless, there were few studies for the prognostic significance of pre-albumin. Therefore, we conducted a meta-analysis on the prognostic value of NLR for GC. The results showed that elevated NLR was significantly associated with a shorter OS.
Certainly, some potential limitations may exist in our study. The retrospective analysis was a single-institution study. Moreover, we lacked the data on progression-free survival, although OS has widely been considered to be the gold end-point standard for prognostic studies on cancer. In the meta-analysis portion, due to the lack of sufficient data, the correlation between NLR and disease-free survival cannot be explored. For the whole study, in terms of the prognostic significance of NLR for GC, our retrospective analysis was aimed at the elderly patients, and the meta-analysis was aimed at the whole GC patients, which cannot be divided into age groups. Considering the limitations of this study, if possible, we will perform a larger scale, multi-center, and prospective study to validate our findings.
The OS of elderly patients is significantly worse than that of younger patients. There are significant differences in clinicopathological characteristics and prognostic factors between younger and older patients. NLR is a convenient, inexpensive, and reproducible marker that can be used as an important predictor of the prognosis of GC. In particular, elderly patients should be focused more on the improvement of inflammatory status preoperatively if NLR values are high, whereas preoperative nutritional status improvement may be especially beneficial for the prognosis of younger patients.
Gastric cancer (GC) is one of the most common malignant tumors in the world. The postoperative overall survival (OS) of GC patients remains extremely low. Therefore, we attempted to analyze the clinical and pathologic data of GC patients in different age groups to explore the differences in the clinical characteristics and prognostic factors.
We attempted to provide reliable reference for clinicians to identify and rectify the independent prognostic influencing factors for GC patients in different age groups.
We designed this retrospective study to analyze the clinical and pathologic data of patients with GC, and explore the differences in the clinical characteristics and prognostic factors between different age groups.
We analyzed 1037 GC patients admitted to Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine from May 2010 to January 2013. The patients were divided into two groups based on age: Younger group (less than 70 years old) and older group (no less than 70 years old). The clinical features and prognostic factors were analyzed in both groups. Further, a meta-analysis was conducted to explore the prognostic significance of neutrophil-lymphocyte ratio (NLR) in GC patients.
In the retrospective study, the mean OS of the younger group (64.7 mo) was significantly longer than that of the older group (48.1 mo; P < 0.001). Among patients under 70 years of age, hospitalization time, TNM stage, vascular invasion, and preoperative low pre-albumin were independently associated with OS (P < 0.005), whereas in patients older than 70 years, TNM stage, esophageal invasion, histological type, and preoperative NLR were independent factors for OS (P < 0.005). The OS of these older patients was significantly shorter (P < 0.005). In meta-analysis, the results showed that high NLR value was a risk factor for the prognosis of GC.
There are significant differences in clinicopathological characteristics and prognostic factors between the two age groups of GC patients. NLR is a convenient, inexpensive, and reproducible marker that can be used as an important predictor of the prognosis of GC. The OS of elderly patients is significantly worse than that of younger patients. Elderly patients should focus more on the improvement of inflammatory status, whereas preoperative nutritional status improvement may be particularly beneficial for the prognosis of younger patients. There is still little research to assess the significance of pre-albumin in the prognosis of GC, which needs to be stressed in the future.
Clinicians should attach great importance to the impact of objective indicators on the prognosis of GC, identifying high-risk patients as many as possible and improving their overall prognosis. NLR is a widely used, inexpensive, and reproducible marker that can be used as an important predictor of the prognosis of GC. In particular, elderly patients should be focused more on the improvement of inflammatory status preoperatively if NLR values are high, whereas preoperative nutritional status improvement may be especially beneficial for the prognosis of younger patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Funel N, Karakoyun R S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 308] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20512] [Article Influence: 2051.2] [Reference Citation Analysis (20)] |
| 3. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 4. | Park JY, von Karsa L, Herrero R. Prevention strategies for gastric cancer: a global perspective. Clin Endosc. 2014;47:478-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Wu GH, Liu ZH, Wu ZH, Wu ZG. Perioperative artificial nutrition in malnourished gastrointestinal cancer patients. World J Gastroenterol. 2006;12:2441-2444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Yamamoto K, Nagatsuma Y, Fukuda Y, Hirao M, Nishikawa K, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M, Fujitani K, Tsujinaka T. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer. 2017;20:913-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 7. | Graziosi L, Marino E, De Angelis V, Rebonato A, Cavazzoni E, Donini A. Prognostic value of preoperative neutrophils to lymphocytes ratio in patients resected for gastric cancer. Am J Surg. 2015;209:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Li QQ, Lu ZH, Yang L, Lu M, Zhang XT, Li J, Zhou J, Wang XC, Gong JF, Gao J, Li J, Li Y, Shen L. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, Kim HJ. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, Stojakovic T, Samonigg H, Hoefler G, Pichler M. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 404] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 11. | Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134:2403-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 338] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 12. | Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, Nakano K, Tsuboi M, Shibata K, Furuse K, Fukushima M. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. 2009;45:1950-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 13. | Porrata LF, Ristow K, Habermann T, Inwards DJ, Micallef IN, Markovic SN. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/Lymphocyte ratio. Am J Hematol. 2010;85:896-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Wang DS, Ren C, Qiu MZ, Luo HY, Wang ZQ, Zhang DS, Wang FH, Li YH, Xu RH. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour Biol. 2012;33:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Hirashima M, Higuchi S, Sakamoto K, Nishiyama T, Okada H. The ratio of neutrophils to lymphocytes and the phenotypes of neutrophils in patients with early gastric cancer. J Cancer Res Clin Oncol. 1998;124:329-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001-1005. [PubMed] |
| 17. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 18. | Ueno D, Matsumoto H, Kubota H, Higashida M, Akiyama T, Shiotani A, Hirai T. Prognostic factors for gastrectomy in elderly patients with gastric cancer. World J Surg Oncol. 2017;15:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. “The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses”. Available from: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 20. | Jin H, Sun J, Zhu K, Liu X, Zhang Q, Shen Q, Gao Y, Yu J. The prognostic value of neutrophil-lymphocyte ratio is superior to derived neutrophil-lymphocyte ratio in advanced gastric cancer treated with preoperative chemotherapy and sequential R0 resection: a 5-year follow-up. Onco Targets Ther. 2017;10:2655-2664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Cho IR, Park JC, Park CH, Jo JH, Lee HJ, Kim S, Shim CN, Lee H, Shin SK, Lee SK, Lee YC. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014;17:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, Sun H, Chen J, Wang F, Gao T, Zhang L, Wang S. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 23. | el Aziz LM. Blood neutrophil-lymphocyte ratio predicts survival in locally advanced cancer stomach treated with neoadjuvant chemotherapy FOLFOX 4. Med Oncol. 2014;31:311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Gonda K, Shibata M, Sato Y, Washio M, Takeshita H, Shigeta H, Ogura M, Oka S, Sakuramoto S. Elevated neutrophil-to-lymphocyte ratio is associated with nutritional impairment, immune suppression, resistance to S-1 plus cisplatin, and poor prognosis in patients with stage IV gastric cancer. Mol Clin Oncol. 2017;7:1073-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Qu J, Qu X, Li Z, Zhang J, Teng Y, Jin B, Zhao M, Yu P, Wang Z, Liu Y. Role of patient-, tumor- and systemic inflammatory response-related factors in predicting survival of patients with node-negative gastric cancer. Tumour Biol. 2017;39:1010428317698374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Jeong JH, Lim SM, Yun JY, Rhee GW, Lim JY, Cho JY, Kim YR. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology. 2012;83:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Jung MR, Park YK, Jeong O, Seon JW, Ryu SY, Kim DY, Kim YJ. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The Platelet-to-Lymphocyte Ratio Versus Neutrophil-to-Lymphocyte Ratio: Which is Better as a Prognostic Factor in Gastric Cancer? Ann Surg Oncol. 2015;22:4363-4370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 29. | Kim JH, Han DS, Bang HY, Kim PS, Lee KY. Preoperative neutrophil-to-lymphocyte ratio is a prognostic factor for overall survival in patients with gastric cancer. Ann Surg Treat Res. 2015;89:81-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Lee DY, Hong SW, Chang YG, Lee WY, Lee B. Clinical significance of preoperative inflammatory parameters in gastric cancer patients. J Gastric Cancer. 2013;13:111-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Lieto E, Galizia G, Auricchio A, Cardella F, Mabilia A, Basile N, Del Sorbo G, Castellano P, Romano C, Orditura M, Napolitano V. Preoperative Neutrophil to Lymphocyte Ratio and Lymphocyte to Monocyte Ratio are Prognostic Factors in Gastric Cancers Undergoing Surgery. J Gastrointest Surg. 2017;21:1764-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Liu X, Chen S, Liu J, Xu D, Li W, Zhan Y, Li Y, Chen Y, Zhou Z, Sun X. Impact of systemic inflammation on gastric cancer outcomes. PLoS One. 2017;12:e0174085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Mao M, Wei X, Sheng H, Chi P, Liu Y, Huang X, Xiang Y, Zhu Q, Xing S, Liu W. C-reactive protein/albumin and neutrophil/Lymphocyte ratios and their combination predict overall survival in patients with gastric cancer. Oncol Lett. 2017;14:7417-7424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Mohri Y, Tanaka K, Ohi M, Saigusa S, Yasuda H, Toiyama Y, Araki T, Inoue Y, Kusunoki M. Identification of prognostic factors and surgical indications for metastatic gastric cancer. BMC Cancer. 2014;14:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Ock CY, Nam AR, Lee J, Bang JH, Lee KH, Han SW, Kim TY, Im SA, Kim TY, Bang YJ, Oh DY. Prognostic implication of antitumor immunity measured by the neutrophil-lymphocyte ratio and serum cytokines and angiogenic factors in gastric cancer. Gastric Cancer. 2017;20:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Qiu M, Zhou Y, Jin Y, Wei XL, Wang DS, Ren C, Bai L, Yang DJ, Xu RH. Prognostic effect of high pretreatment neutrophil to lymphocyte ratio on survival of patients with gastric adenocarcinoma in China. Int J Biol Markers. 2015;30:e96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Tanaka H, Muguruma K, Toyokawa T, Kubo N, Ohira M, Hirakawa K. Differential impact of the neutrophil-lymphocyte ratio on the survival of patients with stage IV gastric cancer. Dig Surg. 2014;31:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/Lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010;102:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, Azevedo e Silva G, Chen WQ, Ogunbiyi OJ, Rachet B, Soeberg MJ, You H, Matsuda T, Bielska-Lasota M, Storm H, Tucker TC, Coleman MP; CONCORD Working Group. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385:977-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 1735] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 40. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 41. | Kowal P, Dowd Edward J. Proposed working definition of an older person in Africa for the MDS Project. 2001. [DOI] [Full Text] |
| 42. | Wu CW, Lo SS, Shen KH, Hsieh MC, Lui WY, P'eng FK. Surgical mortality, survival, and quality of life after resection for gastric cancer in the elderly. World J Surg. 2000;24:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Kim DY, Joo JK, Ryu SY, Park YK, Kim YJ, Kim SK. Clinicopathologic characteristics of gastric carcinoma in elderly patients: a comparison with young patients. World J Gastroenterol. 2005;11:22-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Takeshita H, Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E. Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg. 2013;37:2891-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 45. | Yamada H, Shinohara T, Takeshita M, Umesaki T, Fujimori Y, Yamagishi K. Postoperative complications in the oldest old gastric cancer patients. Int J Surg. 2013;11:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Toiyama Y, Yasuda H, Ohi M, Yoshiyama S, Araki T, Tanaka K, Inoue Y, Mohri Y, Kusunoki M. Clinical impact of preoperative albumin to globulin ratio in gastric cancer patients with curative intent. Am J Surg. 2017;213:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Liu X, Qiu H, Liu J, Chen S, Xu D, Li W, Zhan Y, Li Y, Chen Y, Zhou Z, Sun X. A Novel Prognostic Score, Based on Preoperative Nutritional Status, Predicts Outcomes of Patients after Curative Resection for Gastric Cancer. J Cancer. 2016;7:2148-2156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Capozzi LC, McNeely ML, Lau HY, Reimer RA, Giese-Davis J, Fung TS, Culos-Reed SN. Patient-reported outcomes, body composition, and nutrition status in patients with head and neck cancer: Results from an exploratory randomized controlled exercise trial. Cancer. 2016;122:1185-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 49. | Arrieta O, De la Torre-Vallejo M, López-Macías D, Orta D, Turcott J, Macedo-Pérez EO, Sánchez-Lara K, Ramírez-Tirado LA, Baracos VE. Nutritional Status, Body Surface, and Low Lean Body Mass/Body Mass Index Are Related to Dose Reduction and Severe Gastrointestinal Toxicity Induced by Afatinib in Patients With Non-Small Cell Lung Cancer. Oncologist. 2015;20:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Ma L, Luo GY, Ren YF, Qiu B, Yang H, Xie CX, Liu SR, Liu SL, Chen ZL, Li Q, Fu JH, Liu MZ, Hu YH, Ye WF, Liu H. Concurrent chemoradiotherapy combined with enteral nutrition support: a radical treatment strategy for esophageal squamous cell carcinoma patients with malignant fistulae. Chin J Cancer. 2017;36:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Xu J, Zhong Y, Jing D, Wu Z. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. 2006;30:1284-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Soeters PB, Reijven PL, van Bokhorst-de van der Schueren MA, Schols JM, Halfens RJ, Meijers JM, van Gemert WG. A rational approach to nutritional assessment. Clin Nutr. 2008;27:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 53. | Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9 Suppl 2:S51-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 396] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 54. | Sun KY, Xu JB, Chen SL, Yuan YJ, Wu H, Peng JJ, Chen CQ, Guo P, Hao YT, He YL. Novel immunological and nutritional-based prognostic index for gastric cancer. World J Gastroenterol. 2015;21:5961-5971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 55. | Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest. 2003;32:201-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 56. | Han WX, Chen ZM, Wei ZJ, Xu AM. Preoperative pre-albumin predicts prognosis of patients after gastrectomy for adenocarcinoma of esophagogastric junction. World J Surg Oncol. 2016;14:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 57. | Saif MW, Makrilia N, Zalonis A, Merikas M, Syrigos K. Gastric cancer in the elderly: an overview. Eur J Surg Oncol. 2010;36:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 58. | Joharatnam-Hogan N, Shiu KK, Khan K. Challenges in the treatment of gastric cancer in the older patient. Cancer Treat Rev. 2020;85:101980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 59. | Xiao LJ, Zhao S, Zhao EH, Zheng X, Gou WF, Takano Y, Zheng HC. Clinicopathological and prognostic significance of Ki-67, caspase-3 and p53 expression in gastric carcinomas. Oncol Lett. 2013;6:1277-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Shau HY, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol. 1988;141:4395-4402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, He YJ, Xu RH, Jiang WQ. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |