Published online Jul 15, 2019. doi: 10.4251/wjgo.v11.i7.538
Peer-review started: January 28, 2019
First decision: April 15, 2019
Revised: May 1, 2019
Accepted: May 28, 2019
Article in press: May 29, 2019
Published online: July 15, 2019
Processing time: 170 Days and 21 Hours
Intraoperative intraperitoneal chemotherapy is an emerging treatment modality for locally advanced rectal neoplasms. However, its impacts on postoperative complications remain unknown. Anastomotic leakage (AL) is one of the most common and serious complications associated with the anterior resection of rectal tumors. Therefore, we designed this study to determine the effects of intraoperative intraperitoneal chemotherapy on AL.
To investigate whether intraoperative intraperitoneal chemotherapy increases the incidence of AL after the anterior resection of rectal neoplasms.
This retrospective cohort study collected information from 477 consecutive patients who underwent an anterior resection of rectal carcinoma using the double stapling technique at our institution from September 2016 to September 2017. Based on the administration of intraoperative intraperitoneal chemotherapy or not, the patients were divided into a chemotherapy group (171 cases with intraperitoneal implantation of chemotherapy agents during the operation) or a control group (306 cases without intraoperative intraperitoneal chemotherapy). Clinicopathologic features, intraoperative treatment, and postoperative complications were recorded and analyzed to determine the effects of intraoperative intraperitoneal chemotherapy on the incidence of AL. The clinical outcomes of the two groups were also compared through survival analysis.
The univariate analysis showed a significantly higher incidence of AL in the patients who received intraoperative intraperitoneal chemotherapy, with 13 (7.6%) cases in the chemotherapy group and 5 (1.6%) cases in the control group (P = 0.001). As for the severity of AL, the AL patients who underwent intraoperative intraperitoneal chemotherapy tended to be more severe cases, and 12 (92.3%) out of 13 AL patients in the chemotherapy group and 2 (40.0%) out of 5 AL patients in the control group required a secondary operation (P = 0.044). A multivariate analysis was subsequently performed to adjust for the confounding factors and also showed that intraoperative intraperitoneal chemotherapy increased the incidence of AL (odds ratio = 5.386; 95%CI: 1.808-16.042; P = 0.002). However, the survival analysis demonstrated that intraoperative intraperitoneal chemotherapy could also improve the disease-free survival rates for patients with locally advanced rectal cancer.
Intraoperative intraperitoneal chemotherapy can improve the prognosis of patients with locally advanced rectal carcinoma, but it also increases the risk of AL following the anterior resection of rectal neoplasms.
Core tip: Intraoperative intraperitoneal chemotherapy is gradually administered during operations for locally advanced rectal cancer patients. It is believed that this treatment can improve the oncological outcomes and survival rates. However, surgical complications related to intraoperative chemotherapy have also been reported. We conducted this study to determine the relationship between intraperitoneal chemotherapy and anastomotic leakage to help surgeons weigh the benefits and risks of intraoperative chemotherapy.
- Citation: Wang ZJ, Tao JH, Chen JN, Mei SW, Shen HY, Zhao FQ, Liu Q. Intraoperative intraperitoneal chemotherapy increases the incidence of anastomotic leakage after anterior resection of rectal tumors. World J Gastrointest Oncol 2019; 11(7): 538-550
- URL: https://www.wjgnet.com/1948-5204/full/v11/i7/538.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i7.538
Tumor recurrence can lead to an unfavorable prognosis for patients with locally advanced rectal carcinoma. The peritoneum is a common recurrence site for patients who undergo a radical resection of rectal carcinoma. In a retrospective study, it was reported that of the 1354 patients with rectal cancer who were included in the report, 5.4% went on to develop peritoneal recurrence after radical surgery[1]. Furthermore, T stage and N stage are independent risk factors that affect the incidence of peritoneal carcinomatosis[2]. Therefore, it is recommended that patients with locally advanced rectal cancer of stage T3/T4 or N1/N2 receive preoperative chemoradiotherapy to decrease the risk of recurrence. Previous randomized trials have shown that patients who undergo preoperative chemoradiotherapy have significantly reduced local recurrence rates and improved rates of disease-free survival (DFS)[3]. However, for a subset of patients who have already suffered from peritoneal metastases, cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) have been widely used to improve their oncological prognosis[4]. Few reports have focused on the use of intraoperative intraperitoneal chemotherapy without hyperthermia to prevent peritoneal carcinomatosis in rectal cancer patients. Intraoperative intraperitoneal chemotherapy is an emerging modality that can improve the prognosis of rectal cancer patients with a high risk of cancer recurrence, and represents a distinct approach from both preoperative chemoradiotherapy and HIPEC that can be easily administrated in the clinic[5,6].
Tumor recurrence results from residual tumor cells that are present after the removal of a primary tumor. Previous studies have shown that residual tumor cells can exhibit a transient period of increased growth rate and become more vulnerable to chemotherapeutic agents during the first 3 d following the resection of a primary tumor, which provides the rationale for the administration of intraoperative intraperitoneal chemotherapy to decrease the incidence of peritoneal recurrence[7,8]. Over the past few years, intraoperative intraperitoneal chemotherapy has been gradually incorporated into the treatment for rectal carcinoma patients in Eastern countries[5,6]. Locally advanced rectal carcinoma has a higher risk of peritoneal recurrence, and as a result, clinical stages T3/T4 and N1/N2 of the disease are often regarded as indications for the use of intraoperative intraperitoneal chemotherapy. In this procedure, chemotherapy agents are placed into the pelvic cavity after neoplasm resection and digestive reconstruction to inhibit the proliferation and dissemination of the remaining tumor cells. However, there are no uniform guidelines for the type and dose of chemotherapeutic agent and most decisions are determined by the surgeon’s recommendations and the patient’s economic conditions. Common drug options include fluorouracil implants, lobaplatin, and raltitrexed. Reports have shown that intraoperative intraperitoneal chemotherapy can reduce locoregional recurrence rates and increase long-term survival rates[5]. Nevertheless, the effects of intraoperative intraperitoneal chemotherapy on postoperative complications have rarely been explored, which raises concerns about the safety and feasibility of this new treatment modality. Given that anastomotic leakage (AL) is the most common and serious operation-associated complication after rectal surgery, we aimed to evaluate the role of intraoperative intraperitoneal chemotherapy in the occurrence of AL.
AL is a common and severe postoperative complication that can develop after an anterior resection of rectal neoplasms and has a high incidence that ranges from 6.1% to 11.9%[9-11]. AL prolongs hospitalization times and increases short-term morbidity and mortality. Moreover, several studies have shown that AL contributes to the risk of local recurrence and decreased overall survival[12-14]. Previous reports have indicated that male sex, history of smoking and ischemic heart disease, tumor location and size, malnutrition, and intersections of staple lines are possible factors that can lead to AL in rectal tumor patients[10,15-18]. Prophylactic ileostomy, transanal tube placement, and intracorporeal reinforcing sutures may decrease the incidence of AL[19,20]. Additionally, some assay indexes and prediction models have been established to evaluate the possibility of AL[21-23]. Exploring the association between intraoperative intraperitoneal chemotherapy and AL can improve our understanding of the indications and contradictions of this new treatment modality.
Our investigation received approval from the ethics committee of our center and was performed in accordance with the Helsinki Declaration of World Medical Association. Every patient signed an informed consent form before participation in the study. We extracted data from 477 consecutive patients who underwent anterior resection of rectal cancer at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College from September 2016 to September 2017. Information regarding the intraoperative use of chemotherapy agents was carefully collected from medical records. Follow-up data were acquired by outpatient reexamination and telephones.
The inclusion criteria were defined as follows: (1) All patients were definitively diagnosed with rectal cancer through abdominal and pelvic enhanced computed tomography (CT) scans, rectal magnetic resonance imaging (MRI), colonoscopy, tissue biopsy, and pathological examination; (2) All patients were confirmed to have TNM stage II-III rectal cancer through rectal MRI at the time of diagnosis; (3) The distal border of the tumor from the anal verge was less than 15 cm; and (4) All patients underwent an anterior resection surgery using the double stapling technique.
The exclusion criteria were defined as follows: (1) Patients who were considered to have TNM stage I or IV rectal cancer at the time of diagnosis; (2) Patients who received hand suture anastomosis, Hartmann’s surgery, intersphincteric resection, or abdominal perineal resection; and (3) Patients whose information was not clearly and accurately presented in the medical records.
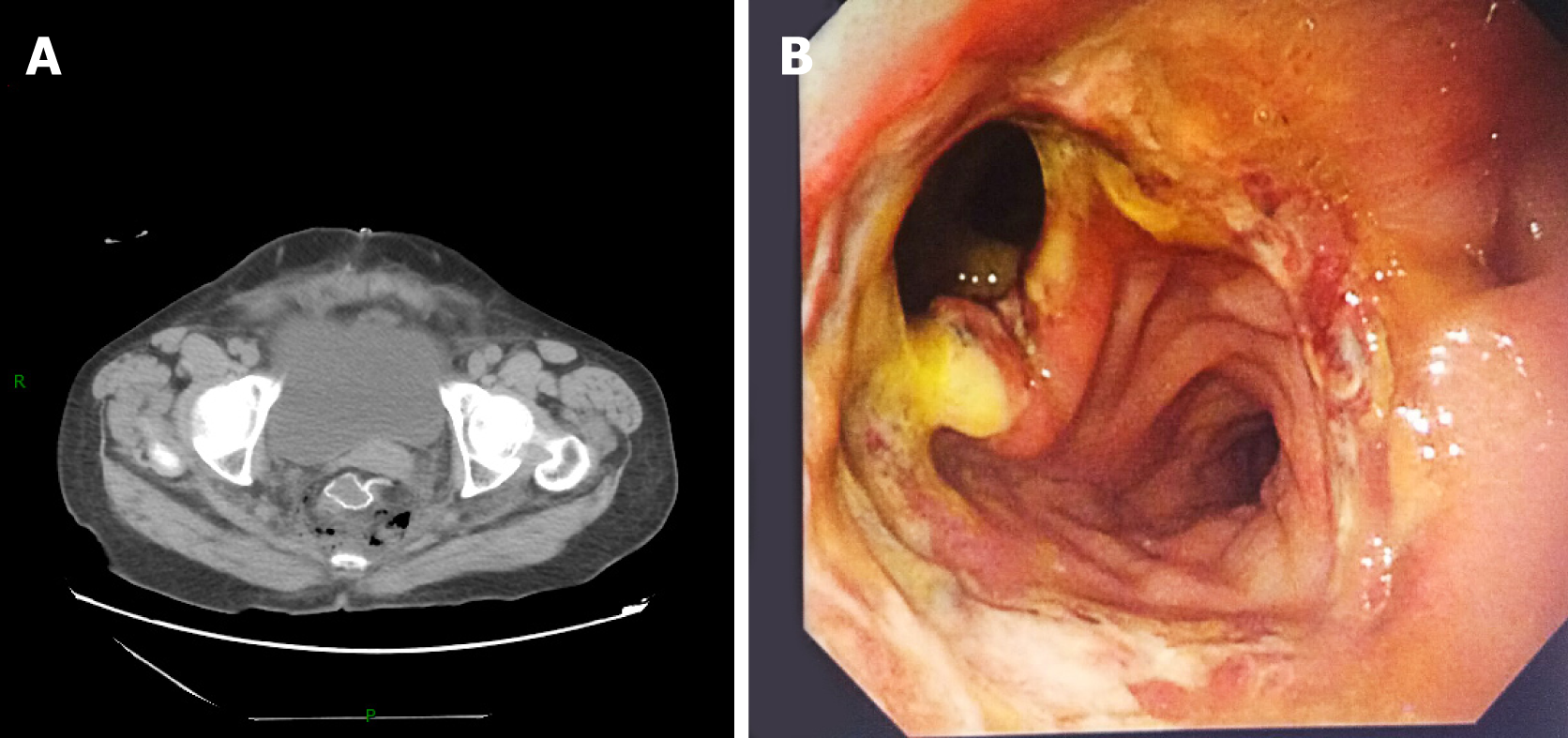
AL was diagnosed through clinical symptoms and signs of fever, abdominal pain, peritonitis, and discharge of intestinal contents from pelvic drainage. Pelvic CT scans and rectoscopy can be used to provide additional confirmation of AL. Furthermore, hydrops and pneumatosis in the pelvic cavity in CT images or anastomotic defects in endoscopy images can also imply the existence of AL (Figure 1). We classified all AL patients as grade A, B, or C according to the proposal from the International Study Group of Rectal Cancer in 2010. Grade A AL patients require no active medical intervention whereas grade B AL patients require only a conservative treatment. In contrast, grade C AL patients require a secondary operation[24]. Given the limited number of grade A AL individuals due to its low rate of clinical manifestation and the lack of a need for active medical intervention, only grade B and C patients were analyzed in our study.
All patients underwent bowel preparation by taking oral sulfate-free polyethylene glycol electrolyte powder the day before surgery. A standardized anterior resection of rectal neoplasms was then performed for each patient by surgeons specialized in colorectal tumors. Both laparotomy and laparoscopic surgeries were performed at our institution. A double stapling technique was used to form an end-to-end anastomotic stoma when the surgeons reconstructed the intestinal tract. Peritoneal lavage was routinely performed after the intestinal anastomosis. One to two pelvic drainage tubes were then placed around the anastomotic stoma. Based on the patient’s condition, a transanal tube was selectively placed. The resected specimens were delivered to professional pathologists to determine the tumor stage.
Lobaplatin or fluorouracil implants were utilized for the patients who received intraoperative intraperitoneal chemotherapy. The dosage of lobaplatin was 60 mg, and it was dissolved in 500 mL of glucose solution at a concentration of 0.05 g/mL. The solution was then poured into the pelvic cavity through the drainage tube after the abdominal incision was closed. The tubes were occluded for 4 to 6 h to prevent drainage of the agents. Fluorouracil implants were placed directly into the pelvic cavity before the incision was closed and remained permanently inside the body. The common dosage ranged from 500 to 1000 mg.
To evaluate the comparability between the chemotherapy group and the control group, and to decrease any confounding bias, a total of 32 variables were included in our investigation. All factors can be roughly divided into demographic characteristics, comorbidities, preoperative oncological therapies, operative treatments, and tumor staging. All of these data were described in detail in the medical records. We carefully examined the accuracy of our data to reduce bias from data collection.
Our study was statistically reviewed by a biomedical statistician from our institution. All data were analyzed using the Statistical Package for the Social Sciences (SPSS version 24.0; IBM Corp., Armonk, NY). Since we excluded patients whose information was not clearly and accurately presented in their medical records, there were no missing data in this study. Quantitative data that were normally distributed are presented as the mean ± SD and were further analyzed using a t-test. Quantitative data that were not normally distributed are presented as the median (range) and were compared using Mann-Whitney U tests. Qualitative data are expressed as the number of cases and percentage and were further compared using Pearson’s χ2 test or Fisher’s exact test. Ordinal data are also presented as cases and percentage but were further examined using Mann-Whitney U tests. To control confounding biases, factors that were regarded to be clinically associated with AL and imbalanced factors between the two groups with a P-value < 0.05 were included in the multivariate logistic regression analysis and stratification analysis to determine the independent risk factors for AL. Overall survival rates and DFS rates were calculated by the Kaplan-Meier method and further compared by a log-rank test. All tests were two-sided, and a P-value < 0.05 was regarded as statistically significant.
Our investigation included 477 patients with an average age of 58.7 ± 10.9 years. Of these patients, 301 (63.1%) were male and 176 (36.9%) were female. A total of 171 patients received intraoperative intraperitoneal chemotherapy, including 8 treated with lobaplatin alone, 157 treated with fluorouracil implants alone, and 6 treated with both. The remaining 306 patients did not receive intraoperative intraperitoneal chemotherapy. Patient-related factors are presented in Table 1. Patient demographics, habits, comorbidities, preoperative therapy, nutritional status, and American Society of Anesthesiologists grade were comparable between the chemotherapy group and the control group. Surgery-related factors are presented in Table 2. Most patients received laparoscopic surgery in our study, including 167 (97.7%) in the chemo-therapy group and 300 (98.0%) in the control group (P = 0.751). Natural orifice specimen extraction (NOSE) surgery is a surgical method that emerged in the last decade in which resected specimens are obtained from the vagina or anus instead of an additional abdominal incision. More patients in the control group underwent the NOSE procedure (1.8% in the chemotherapy group vs 5.9% in the control group, P = 0.035). The placement of a transanal tube was more common in patients who did not receive intraoperative intraperitoneal chemotherapy (43.9% in the chemotherapy group vs 55.6% in the control group, P = 0.014). Additionally, more patients from the control group received more than 2 stapler firings during the digestive reconstruction (14.6% in the chemotherapy group vs 25.2% in the control group, P = 0.007). No obvious differences were observed between the two groups for operation time, reinforcing suture, defunctioning stoma, blood loss, perioperative transfusion, or preservation of the left colic artery. Tumor-related variables are detailed in Table 3. All patients enrolled in our study presented with TNM stage II or III disease at the date of diagnosis prior to treatment. However, of the 97 patients who received neoadjuvant therapy, a total of 11 achieved complete pathological remission and 19 regressed to TNM stage I through the postoperative pathological examination. Tumor location, pathological stage, and degree of differentiation were comparable between the two groups.
| Variable | Intraoperative chemotherapy (+) (n = 171) | Intraoperative chemotherapy(-) (n = 306) | P-value |
| Age (yr, mean ± SD) | 58.1 ± 10.1 | 59.1 ± 11.3 | 0.627 |
| Sex, n (%) | 0.829 | ||
| Male | 109 (63.7) | 192 (62.7) | |
| Female | 62 (36.3) | 114 (37.3) | |
| BMI (kg/m2, mean ± SD) | 24.4 ± 3.1 | 23.9 ± 3.4 | 0.123 |
| Smoking, n (%) | 59 (34.5) | 87 (28.4) | 0.168 |
| Alcohol, n (%) | 46 (26.9) | 67 (21.9) | 0.218 |
| Hypertension, n (%) | 46 (26.9) | 75 (24.5) | 0.565 |
| Ischemic heart disease, n (%) | 6 (3.5) | 7 (2.3) | 0.622 |
| Diabetes, n (%) | 22 (12.9) | 39 (12.7) | 0.970 |
| Hepatitis, n (%) | 14 (8.2) | 16 (5.2) | 0.202 |
| History of malignancy, n (%) | 9 (5.3) | 12 (3.9) | 0.493 |
| Incomplete intestinal obstruction, n (%) | 20 (11.7) | 25 (8.2) | 0.206 |
| Preoperative chemotherapy, n (%) | 32 (18.7) | 65 (21.2) | 0.511 |
| Preoperative radiotherapy, n (%) | 21 (12.3) | 44 (14.4) | 0.522 |
| Preoperative hemoglobin, (g/L, mean ± SD) | 136.8 ± 18.1 | 136.2 ± 18.0 | 0.813 |
| Preoperative albumin (g/L, mean ± SD) | 43.6 ± 3.5 | 44.2 ± 3.7 | 0.075 |
| ASA grade, n (%) | 0.987 | ||
| 1 | 6 (3.5) | 14 (4.6) | |
| 2 | 154 (90.1) | 269 (87.9) | |
| 3 | 11 (6.4) | 23 (7.5) |
| Variable | Intraoperative chemotherapy (+) (n = 171) | Intraoperative chemotherapy(-) (n = 306) | P-value |
| Approach, n (%) | 0.751 | ||
| Open | 4 (2.3) | 6 (2.0) | |
| Laparoscopic | 167 (97.7) | 300 (98.0) | |
| NOSE surgery, n (%) | 3 (1.8) | 18 (5.9) | 0.035a |
| Operation time, (min, mean ± SD) | 164.0 ± 54.7 | 172.0 ± 62.9 | 0.187 |
| Consolidation suture, n (%) | 27 (15.8) | 37 (12.1) | 0.256 |
| Defunctioning stoma, n (%) | 46 (26.9) | 64 (20.9) | 0.137 |
| Estimated blood loss (mL, mean ± SD) | 59.7 ± 54.4 | 67.1 ± 100.1 | 0.764 |
| Transfusion, n (%) | 4 (2.3) | 14 (4.6) | 0.219 |
| Left colic artery preservation, n (%) | 11 (6.4) | 20 (6.5) | 0.965 |
| Transanal tube, n (%) | 75 (43.9) | 170 (55.6) | 0.014a |
| Number of stapler firing, n (%) | 0.007a | ||
| 1 and 2 | 146 (85.4) | 229 (74.8) | |
| More than 2 | 25 (14.6) | 77 (25.2) |
| Variable | Intraoperative chemotherapy (+) (n = 171) | Intraoperative chemotherapy(-) (n = 306) | P-value |
| Distance of tumor from anal verge (cm, mean ± SD) | 7.9 ± 3.0 | 8.3 ± 2.9 | 0.092 |
| Tumor location, n (%) | 0.621 | ||
| Above peritoneal reflection | 121 (70.8) | 223 (72.9) | |
| Below peritoneal reflection | 50 (29.2) | 83 (27.1) | |
| Pathological T stage, n (%) | 0.865 | ||
| T1, T2, and no tumor residual after preoperative therapy | 25 (14.6) | 43 (14.1) | |
| T3 and T4 | 146 (85.4) | 263 (85.9) | |
| Pathological N stage, n (%) | 0.337 | ||
| N0 and no tumor residual after preoperative therapy | 81 (47.4) | 131 (42.8) | |
| N1 and N2 | 90 (52.6) | 175 (57.2) | |
| TNM stage, n (%) | 0.374 | ||
| No tumor residual after preoperative therapy | 3 (1.8) | 8 (2.6) | |
| I | 8 (4.7) | 11 (3.6) | |
| II | 70 (40.9) | 112 (36.6) | |
| III | 90 (52.6) | 175 (57.2) | |
| Degree of differentiation, n (%) | 0.628 | ||
| Low and low-middle grades | 58 (30.2) | 94 (30.8) | |
| Middle, high-middle, and high grades and no tumor residual after therapy | 134 (69.8) | 211 (69.2) |
The details for the occurrence of AL are presented in Table 4. In total, 18 (3.8%) individuals developed AL in our study. A significantly higher incidence of AL was observed in the group that received intraoperative intraperitoneal chemotherapy than in the control group (7.6% vs 1.6%, P = 0.001). Among the AL patients, none with grade A were enrolled in our study. A total of 4 of the 18 AL patients were classified as grade B and received a conservative treatment, while 14 of the 18 patients were classified as grade C and received a secondary surgery according to the standards from the International Study Group of Rectal Cancer released in 2010. The AL patients who underwent intraoperative intraperitoneal chemotherapy tended to be more severe cases and were more likely to receive a secondary operation (P = 0.044). Additionally, the majority of the cases developed AL within a week after surgery and 1 case occurred two months after the procedure. No deaths were observed during the perioperative period.
Descriptive analysis identified significant imbalances between the chemotherapy group and the control group for NOSE surgery (P = 0.035), placement of transanal tubes (P = 0.014), and the number of stapler firings (P = 0.007). These variables and other factors that have a confirmed association with AL from previous reports were included in the subsequent multivariate analyses (Table 5). After adjusting for confounding factors, intraoperative intraperitoneal chemotherapy was confirmed to significantly increase the incidence of AL [odds ratio (OR) = 5.386; 95% confidence interval (CI): 1.808-16.042; P = 0.002].
| Variable | P-value | OR | 95%CI |
| Intraoperative intraperitoneal chemotherapy | 0.002 | 5.386 | 1.808-16.042 |
| Gender | 0.077 | 3.235 | 0.882-11.859 |
| Diabetes | 0.985 | 1.015 | 0.213-4.843 |
| Incomplete intestinal obstruction | 0.752 | 1.261 | 0.301-5.287 |
| Distance of tumor from anal verge | 0.869 | 0.985 | 0.824-1.178 |
| NOSE surgery | 0.388 | 2.696 | 0.283-25.675 |
| Consolidation suture | 0.319 | 0.340 | 0.041-2.835 |
| Defunctioning stoma | 0.157 | 0.312 | 0.062-1.565 |
| Transanal tube | 0.518 | 0.708 | 0.248-2.019 |
| Number of stapler firing | 0.733 | 0.811 | 0.244-2.698 |
Stratification analysis was also performed to control for confounding biases. The influence of intraoperative intraperitoneal chemotherapy on AL was further analyzed in subgroups defined by sex, diabetes, incomplete intestinal obstruction, tumor location, NOSE surgery, consolidation suture, defunctioning stoma, transanal tube, and the number of stapler firings. Intraoperative intraperitoneal chemotherapy significantly promotes the occurrence of AL in individuals who fell into the subgroups of male, nondiabetic, without incomplete intestinal obstruction, with tumors located above the peritoneal reflection, without NOSE surgery, without consolidation sutures, without defunctioning stoma, without transanal tubes, and both who underwent 1 or 2 stapler firings and who underwent more than 2 stapler firings. Although the OR values were different in different subgroups, the OR homogeneity test through the Woolf method demonstrated that the data between the two subgroups were homogenous (P > 0.05). Therefore, we calculated the overall OR values through the Mantel-Haenszel method. The overall P-values and OR values showed that intraoperative intraperitoneal chemotherapy remained significantly associated with AL even though the discussed variables caused weak confounding effects (Table 6).
| Variable | P-value | OR | 95%CI | Woolf homogeneity test |
| Gender | P = 0.805 | |||
| Male | 0.002 | 5.276 | 1.637-17.000 | |
| Female | 0.589 | 3.767 | 0.335-42.393 | |
| Overall | 0.003 | 4.962 | 1.733-14.206 | |
| Diabetes | P = 0.441 | |||
| Yes | 1.000 | 1.810 | 0.108-30.436 | |
| No | 0.001 | 5.759 | 1.823-18.193 | |
| Overall | 0.002 | 4.941 | 1.732-14.093 | |
| Incomplete intestinal obstruction | P = 0.305 | |||
| Yes | 0.161 | 2.471 | 1.712-3.565 | |
| No | 0.009 | 3.915 | 1.313-11.673 | |
| Overall | 0.003 | 4.936 | 1.714-14.217 | |
| Tumor location | P = 0.964 | |||
| Above peritoneal reflection | 0.009 | 4.932 | 1.513-16.081 | |
| Below peritoneal reflection | 0.296 | 5.234 | 0.529-51.758 | |
| Overall | 0.002 | 4.997 | 1.748-14.286 | |
| NOSE surgery | P = 0.209 | |||
| Yes | 0.143 | 10.000 | 2.685-37.239 | |
| No | 0.003 | 4.354 | 1.506-12.585 | |
| Overall | 0.002 | 4.855 | 1.727-13.649 | |
| Consolidation suture | P = 0.588 | |||
| Yes | 0.422 | 2.423 | 1.805-3.253 | |
| No | 0.002 | 4.800 | 1.656-13.910 | |
| Overall | 0.002 | 5.162 | 1.797-14.829 | |
| Defunctioning stoma | P = 0.302 | |||
| Yes | 1.000 | 1.400 | 0.085-22.978 | |
| No | <0.001 | 6.319 | 1.994-20.026 | |
| Overall | 0.002 | 5.092 | 1.793-14.461 | |
| Transanal tube | P = 0.468 | |||
| Yes | 0.259 | 3.136 | 0.684-14.375 | |
| No | 0.013 | 6.931 | 1.463-32.844 | |
| Overall | 0.004 | 4.894 | 1.682-14.241 | |
| Number of stapler firings | P = 0.480 | |||
| 1 and 2 | 0.011 | 4.136 | 1.272-13.446 | |
| More than 2 | 0.045 | 10.364 | 1.026-104.656 | |
| Overall | 0.002 | 4.942 | 1.733-14.096 |
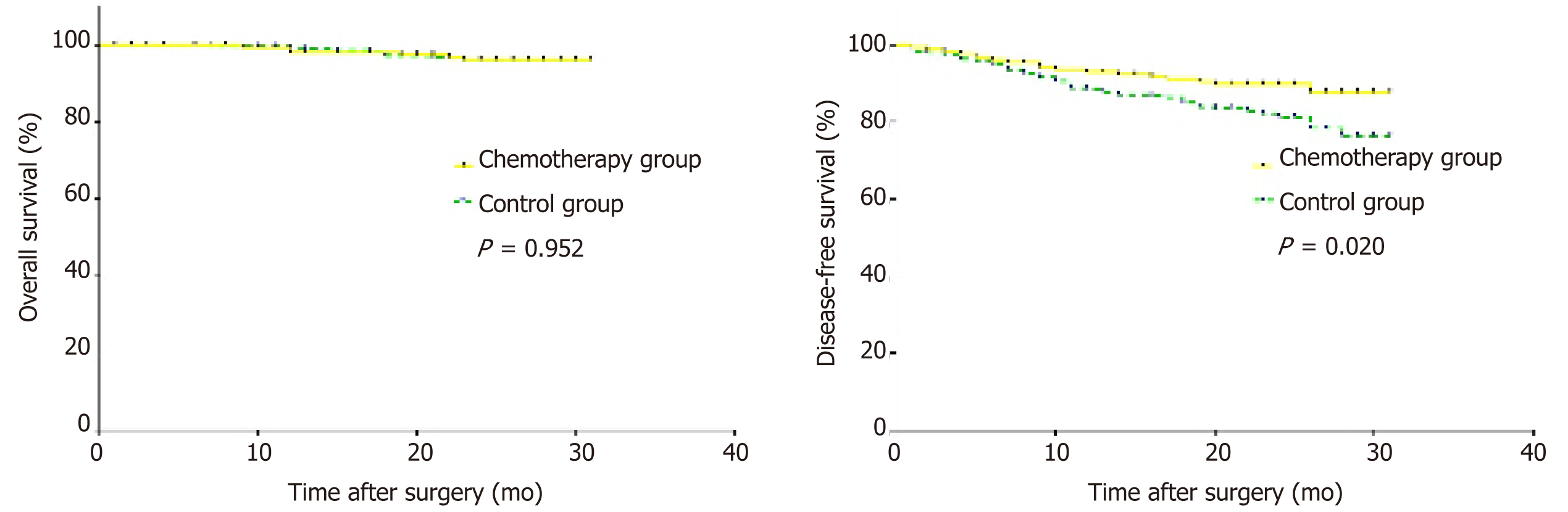
The patients were followed for a median period of 24 mo (range: 1-31 mo). The 1-year survival rate and 2-year survival rate were 98.2% and 96.2% in the chemotherapy group and 99.3% and 96.1% in the control group, respectively. There were no significant differences in the survival rates between the two groups (P = 0.952). However, an increased DFS rate was confirmed in patients who received intraoperative intraperitoneal chemotherapy (P = 0.020). Both the 1-year DFS rate (92.8% in the chemotherapy group vs 88.4% in the control group) and the 2-year DFS rate (89.7% in the chemotherapy group vs 81.3% in the control group) were higher in the chemotherapy group (Figure 2).
Locally advanced (T3/T4 or N1/N2) rectal cancer patients are at an increased risk of local recurrence and distant metastasis. Neoadjuvant therapy has been accepted as a standard treatment for locally advanced rectal carcinoma and can significantly improve the prognosis of these patients[25]. However, we observed that only 13.6% of patients had received preoperative radiotherapy and 20.3% of patients had received preoperative chemotherapy. This might be because many patients were reluctant to receive neoadjuvant treatment due to their poor economic conditions and fear of side effects. However, based on the postoperative pathological stage, most of the patients in our study who did not receive neoadjuvant therapy were recommended to receive postoperative radiotherapy or chemotherapy. Intraoperative intraperitoneal chemotherapy is a newly developed independent modality to treat locally advanced rectal tumor that is distinct from neoadjuvant therapy. Furthermore, patients who have already received neoadjuvant treatment can still receive intraoperative intraperitoneal chemotherapy.
Previous reports have demonstrated that recurrent rectal carcinoma derives from residual intraperitoneal tumor cells after radical resection of the primary tumor site. These residual tumor cells tend to be more sensitive to chemotherapeutic agents since they show a transiently increased growth rate after surgery. However, systematic chemotherapy is not viable due to the poor physical condition of postoperative patients. Intraperitoneal chemotherapy can reach a higher concentration inside the abdominal cavity while maintaining a lower concentration in blood, which can improve the efficacy of killing residual tumor while decreasing systematic side effects. Based on these mechanisms and the high recurrence rate in locally advance rectal cancer patients, intraoperative intraperitoneal chemotherapy has been increasingly used for these patients over the last decade[5,26]. This treatment is administered at the end of the surgery by placing the chemotherapeutic agents into the pelvic cavity and aims to kill the exfoliated tumor cells. A prospective randomized clinical trial confirmed that patients treated by intraoperative implantation of fluorouracil implants experienced improved oncological and survival outcomes[5]. In our study, there were no overall improvements in the survival rate in the chemotherapy group relative to the control group. This might be due to our much shorter follow-up period when compared to previous reports. The longest follow-up time was only 31 months, and only 6 of 171 patients in the chemotherapy group and 10 of the 306 patients in the control group died from the tumor recurrence. However, there was a significant increase in DFS rates for the chemotherapy group, which is consistent with the findings of previous studies.
Although it has been confirmed that intraoperative intraperitoneal chemotherapy improves clinical outcomes in locally advanced rectal cancer patients, its impacts on postoperative complications remain controversial. Considering that AL is one of the most common complications associated with the anterior resection of rectal carcinomas, we evaluated the safety of intraoperative intraperitoneal chemotherapy through the incidence of AL. AL is an extremely severe complication for rectal cancer patients, and most symptomatic AL patients require a secondary operation. Previous studies have identified risk factors for AL after an anterior resection of rectal carcinoma, but the connection between intraoperative intraperitoneal chemotherapy and AL has not been thoroughly examined.
We found that AL occurred more frequently in patients who underwent intraperitoneal chemotherapy, and this trend was verified both in the univariate and multivariate analyses. Lobaplatin and fluorouracil implants were used in our procedures. Lobaplatin was dissolved in solution and poured into the pelvic cavity, which led to the anastomotic stoma being immersed in the lobaplatin solution. In contrast, the fluorouracil implants are solid microcapsules that are placed primarily around the anastomotic stoma in the pelvic wall. Therefore, the chemotherapy drugs can have a direct impact on anastomotic stoma. Both agents are cytotoxic drugs that can inhibit cell proliferation and induce apoptosis, particularly for cells with rapid proliferation[27,28]. Given that the healing process of the rectal anastomotic stoma requires the rapid proliferation of regenerative cells, we hypothesize that intraoperative intraperitoneal chemotherapy increases the incidence of AL by inhibiting cell proliferation.
Previous reports have demonstrated that systematic chemotherapy could delay and impair the healing process of wound. Moreover, treatment for this problem is really difficult as the proliferation of cells in the wound is inhibited[29-31]. Similarly, the side effects of chemotherapy on intestinal anastomosis healing were also observed in experimental studies. AL tended to develop more often in rats receiving intra-operative administration of antineoplastics[32,33]. The anastomotic strength is generally evaluated through bursting pressure, which increases slowly in the first four postoperative days but quickly thereafter[34]. However, the bursting pressure was significantly lower in rats receiving intraoperative intraperitoneal chemotherapy compared to those not. Decreased fibroblast activity and collagen deposition were found in further histological examinations, which contributed to the mechanical strength of the anastomoses[35]. In addition, a series of animal experiments have also indicated that intraoperative intraperitoneal chemotherapy could bring detrimental effects on the intestinal anastomosis by increasing the inflammatory reaction, promoting oxidative stress, and reducing neoangiogenesis at the anastomotic site. These effects were observed in almost all the antitumor agents commonly used in intrapertoneal chemotherapy, including mitomycin C, cisplatin, oxaliplatin, 5-fluorouracil, iritotecan, and doxorubicin[32,36,37]. Moreover, the combination use of different chemotherapeutic agents showed enhanced negative effects on the healing process of intestinal anastomoses compared to those receiving only one type of agent[38]. However, previous clinical studies on the relationship between intraoperative intraperitoneal chemotherapy and AL are very limited. In our study, a significantly higher incidence of AL was observed in the chemotherapy group, which is in line with previous experimental studies. In addition, intraperitoneal usage of antitumor agents appeared to be associated with the severity of AL. AL patients in the chemotherapy group were at higher risks of undergoing a secondary operation. Finally, we recognize that the sample size of the cases treated with lobaplatin was far less than the number of cases treated with fluorouracil implants, which might introduce bias. Further investigations with larger and more sufficient sample sizes are needed to determine the association between the respective types of chemo-therapeutic agents and AL.
Our study has several limitations. First, since this is a retrospective cohort study and the patients were divided into chemotherapy and control groups, there is the risk of selection bias, information bias, and confounding bias, although we tried to collect as many variables as possible and incorporated them into the multivariate analysis and stratification analysis. Additional large multicenter cohort studies or randomized controlled trials are still required to assess the safety of intraoperative intraperitoneal chemotherapy. Second, the incidence of AL at our center is relatively low when compared with most other reports. There were only 18 AL patients observed in this study, which made it difficult to perform dose-response relationship analyses to control for bias.
In conclusion, this study determined that intraoperative intraperitoneal chemotherapy increased the incidence of postoperative AL after the anterior resection of rectal carcinoma, but it also improved the DFS rates in patients with locally advanced rectal carcinoma. Surgeons should carefully weigh the short-term risks of postoperative AL with the long-term benefits of improved oncological outcomes before choosing to utilize intraoperative intraperitoneal chemotherapy.
Tumor recurrence is common for patients with locally advanced rectal carcinoma after radical resection surgery. Over the past few years, intraoperative intraperitoneal chemotherapy has been gradually incorporated into the treatment for rectal carcinoma patients to decrease the recurrence rate and showed improved clinical outcomes. Nevertheless, the effects of intraoperative intraperitoneal chemotherapy on postoperative complications have rarely been explored. We conducted this research to determine the effects of intraoperative intraperitoneal chemotherapy on the incidence of anastomotic leakage (AL), which would be meaningful to promote our knowledge about the safety and feasibility of this emerging therapy modality.
Our study explored the safety of intraoperative intraperitoneal chemotherapy for patients receiving the anterior resection of rectal carcinoma. This is significant for surgeons to weigh the benefits and risks of this treatment technique.
Our research aimed to evaluate the role of intraoperative intraperitoneal chemotherapy in the occurrence of AL. Meanwhile, the prognosis of patients receiving this therapy was also analyzed.
We performed a retrospective cohort study and patients were divided into a chemotherapy group and a control group. Important demographic variables and confounding factors were collected and analyzed through univariate analysis, stratification analysis, and multivariate analysis to control confounding bias. The oncological outcomes of the two groups were compared through the Kaplan-Meier method and log rank test.
We found that intraoperative intrapertitoneal chemotherapy increased the incidence of AL in patients receiving the anterior resection of rectal carcinoma, but this treatment also contributed to improved disease-free survival rate. This finding can help surgeons to weigh the benefits and risks of this emerging treatment method. Moreover, the mechanisms of intraoperative intraperitoneal chemotherapy leading to AL need to be further investigated in more basic studies. The effects of different types of chemotherapeautic agents on AL can also be explored.
Intraoperative intraperitoneal chemotherapy can improve the prognosis of patients with locally advanced rectal cancer, but it also increases the risks of AL in patients receiving anterior resection of rectal carcinoma. Patients who have other risks of postoperative AL may not be suitable to receive this therapy.
Surgeons need to think deeply about the indications and contraindications of intraoperative intraperitoneal chemotherapy so that better clinical outcomes can be achieved in patients with rectal carcinoma. Moreover, our research is a retrospective study, and biases from data collection and analysis may exist. More prospective randomized controlled trials need to be conducted to explore the safety and feasibility of intraoperative intraperitoneal chemotherapy.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdel-Rahman WM, Kuo SH, Senchukova M S-Editor: Ji FF L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 598] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 349] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | Rödel C, Hofheinz R, Fokas E. Rectal cancer: Neoadjuvant chemoradiotherapy. Best Pract Res Clin Gastroenterol. 2016;30:629-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Maciver AH, Lee N, Skitzki JJ, Boland PM, Francescutti V. Cytoreduction and hyperthermic intraperitoneal chemotherapy (CS/HIPEC) in colorectal cancer: Evidence-based review of patient selection and treatment algorithms. Eur J Surg Oncol. 2017;43:1028-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Yuan H, Zheng B, Tu S. Clinical research of intraperitoneal implantation of sustained-release 5-fluorouracil in advanced colorectal cancer. World J Surg Oncol. 2015;13:320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Zhang RX, Lin JZ, Lei J, Chen G, Li LR, Lu ZH, Ding PR, Huang JQ, Kong LH, Wang FL, Li C, Jiang W, Ke CF, Zhou WH, Fan WH, Liu Q, Wan DS, Wu XJ, Pan ZZ. Safety of intraoperative chemotherapy with 5-FU for colorectal cancer patients receiving curative resection: a randomized, multicenter, prospective, phase III IOCCRC trial (IOCCRC). J Cancer Res Clin Oncol. 2017;143:2581-2593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Kim MS, Choi C, Yoo S, Cho C, Seo Y, Ji Y, Lee D, Hwang D, Moon S, Kim MS, Kang H. Stereotactic body radiation therapy in patients with pelvic recurrence from rectal carcinoma. Jpn J Clin Oncol. 2008;38:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Fisher B, Gunduz N, Saffer EA. Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res. 1983;43:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | García-Granero E, Navarro F, Cerdán Santacruz C, Frasson M, García-Granero A, Marinello F, Flor-Lorente B, Espí A. Individual surgeon is an independent risk factor for leak after double-stapled colorectal anastomosis: An institutional analysis of 800 patients. Surgery. 2017;162:1006-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Shinji S, Ueda Y, Yamada T, Koizumi M, Yokoyama Y, Takahashi G, Hotta M, Iwai T, Hara K, Takeda K, Okusa M, Kan H, Uchida E, Yoshida H. Male sex and history of ischemic heart disease are major risk factors for anastomotic leakage after laparoscopic anterior resection in patients with rectal cancer. BMC Gastroenterol. 2018;18:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Zhang W, Lou Z, Liu Q, Meng R, Gong H, Hao L, Liu P, Sun G, Ma J, Zhang W. Multicenter analysis of risk factors for anastomotic leakage after middle and low rectal cancer resection without diverting stoma: a retrospective study of 319 consecutive patients. Int J Colorectal Dis. 2017;32:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | den Dulk M, Marijnen CA, Collette L, Putter H, Påhlman L, Folkesson J, Bosset JF, Rödel C, Bujko K, van de Velde CJ. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg. 2009;96:1066-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | Hain E, Maggiori L, Manceau G, Mongin C, Prost À la Denise J, Panis Y. Oncological impact of anastomotic leakage after laparoscopic mesorectal excision. Br J Surg. 2017;104:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Noh GT, Ann YS, Cheong C, Han J, Cho MS, Hur H, Min BS, Lee KY, Kim NK. Impact of anastomotic leakage on long-term oncologic outcome and its related factors in rectal cancer. Medicine (Baltimore). 2016;95:e4367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Tanaka K, Okuda J, Yamamoto S, Ito M, Sakamoto K, Kokuba Y, Yoshimura K, Watanabe M. Risk factors for anastomotic leakage after laparoscopic surgery with the double stapling technique for stage 0/I rectal carcinoma: a subgroup analysis of a multicenter, single-arm phase II trial. Surg Today. 2017;47:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Lee S, Ahn B, Lee S. The Relationship Between the Number of Intersections of Staple Lines and Anastomotic Leakage After the Use of a Double Stapling Technique in Laparoscopic Colorectal Surgery. Surg Laparosc Endosc Percutan Tech. 2017;27:273-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Yasui M, Takemasa I, Miyake Y, Hata T, Ikeda M, Miyake Y, Hasegawa J, Ota H, Matsuda C, Mizushima T, Doki Y, Mori M; Clinical Study Group of Osaka University (CSGO), Colorectal Group. Tumor Size as an Independent Risk Factor for Postoperative Complications in Laparoscopic Low Anterior Resection for Advanced Rectal Cancer: A Multicenter Japanese Study. Surg Laparosc Endosc Percutan Tech. 2017;27:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Kawada K, Sakai Y. Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J Gastroenterol. 2016;22:5718-5727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Goto S, Hida K, Kawada K, Okamura R, Hasegawa S, Kyogoku T, Ota S, Adachi Y, Sakai Y. Multicenter analysis of transanal tube placement for prevention of anastomotic leak after low anterior resection. J Surg Oncol. 2017;116:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Maeda K, Nagahara H, Shibutani M, Ohtani H, Sakurai K, Toyokawa T, Muguruma K, Tanaka H, Amano R, Kimura K, Sugano K, Ikeya T, Iseki Y, Hirakawa K. Efficacy of intracorporeal reinforcing sutures for anastomotic leakage after laparoscopic surgery for rectal cancer. Surg Endosc. 2015;29:3535-3542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Watanabe T, Miyata H, Konno H, Kawai K, Ishihara S, Sunami E, Hirahara N, Wakabayashi G, Gotoh M, Mori M. Prediction model for complications after low anterior resection based on data from 33,411 Japanese patients included in the National Clinical Database. Surgery. 2017;161:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Liu XH, Wu XR, Zhou C, Zheng XB, Ke J, Liu HS, Hu T, Chen YF, He XW, He XS, Chen YL, Zou YF, Wang JP, Wu XJ, Lan P. Conversion is a risk factor for postoperative anastomotic leak in rectal cancer patients - A retrospective cohort study. Int J Surg. 2018;53:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Reynolds IS, Boland MR, Reilly F, Deasy A, Majeed MH, Deasy J, Burke JP, McNamara DA. C-reactive protein as a predictor of anastomotic leak in the first week after anterior resection for rectal cancer. Colorectal Dis. 2017;19:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 1029] [Article Influence: 68.6] [Reference Citation Analysis (4)] |
| 25. | Ahmed S, Eng C. Neoadjuvant Strategies: Locally Advanced Rectal Cancer. Clin Colon Rectal Surg. 2017;30:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Huang XE, Wei GL, Huo JG, Wang XN, Lu YY, Wu XY, Liu J, Xiang J, Feng JF. Intrapleural or intraperitoneal lobaplatin for treatment of patients with malignant pleural effusion or ascites. Asian Pac J Cancer Prev. 2013;14:2611-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | McKeage MJ. Lobaplatin: a new antitumour platinum drug. Expert Opin Investig Drugs. 2001;10:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Saris CP, van de Vaart PJ, Rietbroek RC, Blommaert FA. In vitro formation of DNA adducts by cisplatin, lobaplatin and oxaliplatin in calf thymus DNA in solution and in cultured human cells. Carcinogenesis. 1996;17:2763-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Hu G, Liu P, Feng J, Jin Y. Transplantation with bone marrow stromal cells promotes wound healing under chemotherapy through altering phenotypes. Int J Biol Sci. 2011;7:912-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Erinjeri JP, Fong AJ, Kemeny NE, Brown KT, Getrajdman GI, Solomon SB. Timing of administration of bevacizumab chemotherapy affects wound healing after chest wall port placement. Cancer. 2011;117:1296-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Cunningham D, Stenning SP, Smyth EC, Okines AF, Allum WH, Rowley S, Stevenson L, Grabsch HI, Alderson D, Crosby T, Griffin SM, Mansoor W, Coxon FY, Falk SJ, Darby S, Sumpter KA, Blazeby JM, Langley RE. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol. 2017;18:357-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 32. | Makrin V, Lev-Chelouche D, Even Sapir E, Paran H, Rabau M, Gutman M. Intraperitoneal heated chemotherapy affects healing of experimental colonic anastomosis: an animal study. J Surg Oncol. 2005;89:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Akyuz C, Yasar NF, Uzun O, Peker KD, Sunamak O, Duman M, Sehirli AO, Yol S. Effects of melatonin on colonic anastomosis healing following chemotherapy in rats. Singapore Med J. 2018;59:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Blouhos K, Pramateftakis MG, Tsachalis T, Kanellos D, Zaraboukas T, Koliakos G, Betsis D. The integrity of colonic anastomoses following the intraperitoneal administration of oxaliplatin. Int J Colorectal Dis. 2010;25:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Kanellos D, Pramateftakis MG, Mantzoros I, Zacharakis E, Raptis D, Despoudi K, Zaraboukas T, Koliakos G, Lazaridis H. The effects of the intraperitoneal administration of oxaliplatin and 5-FU on the healing of colonic anastomoses: an experimental study. Tech Coloproctol. 2011;15 Suppl 1:S111-S115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Aghayeva A, Benlice C, Bilgin IA, Atukeren P, Dogusoy G, Demir F, Atasoy D, Baca B. The effects of hyperthermic intraperitoneal chemoperfusion on colonic anastomosis: an experimental study in a rat model. Tumori. 2017;103:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Arapoglou S, Kambaroudis A, Grivas I, Delis GA, Karkos C, Ballas K, Zacharioudakis G, Petras P, Aftzoglou M, Gouziotis I, Koliakos G, Karakwta M, Hahalis G. The Effect of Iloprost in the Healing of Colonic Anastomosis in Rats under Chemotherapy with Irinotecan. Chirurgia (Bucur). 2017;112:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Pramateftakis MG, Kanellos D, Mantzoros I, Despoudi K, Raptis D, Angelopoulos S, Koliakos G, Zaraboukas T, Lazaridis C. Intraperitoneally administered irinotecan with 5-fluorouracil impair wound healing of colonic anastomoses in a rat model: an experimental study. Tech Coloproctol. 2011;15 Suppl 1:S121-S125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |