Published online Dec 15, 2019. doi: 10.4251/wjgo.v11.i12.1115
Peer-review started: May 21, 2019
First decision: July 31, 2019
Revised: August 5, 2019
Accepted: October 1, 2019
Article in press: October 1, 2019
Published online: December 15, 2019
Processing time: 208 Days and 17.2 Hours
Chronic atrophic gastritis (CAG) is a common disease of the digestive system with pathological characteristics of a decreasing number, or disappearance, of inherent glands of the gastric mucosa. CAG has been defined as a precancerous condition of gastric cancer. Intestinal metaplasia or intraepithelial neoplasia accompanying atrophied glands of the stomach is regarded as one of the most important precancerous lesions of gastric cancer. As a common malignant tumour, gastric cancer remains without a satisfactory therapy and its pathogenesis remains unclear, seriously threatening human life. Therefore, some scholars have proposed to prevent the incidence of gastric cancer by avoiding precancerous lesions. If CAG can be reversed, the incidence of gastric cancer can be substantially reduced. To reverse and prevent CAG and study its pathogenesis and therapy, it is necessary to develop an ideal, safe, stable, animal model.
To study a rapid, stable, and safe method of establishing a mouse model of human CAG.
Six-week-old Kunming mice were divided into a phosphate buffered solution control group, a Helicobacter pylori (H. pylori) group, an N-methyl-N'-nitroguanidine (MNNG) group, an ammonia water group, and a group combining H. pylori, MNNG, and ammonia water (hereinafter referred to as the combined group). The mice were administrated with drinking water containing ammonia or infected with H. pylori through gavage. At the 30th, 60th, 90th, and 120th day after the last H. pylori infection, mice were selected randomly to collect their gastric mucosa for hematoxylin eosin staining, terminal nick-end labelling staining detection, and immunohistochemical staining for Bax and Bcl-2. In addition, H. pylori was isolated, cultured, and identified, and its extent of colonisation calculated. Blood was collected to detect inflammatory factors interleukin (IL)-1β, IL-8, and tumor necrosis factor (TNF)-α and immune function markers CD4 and CD8 to confirm successful establishment of the CAG model.
The combined group showed slight CAG at the 90th day and moderate CAG at the 120th day, while other groups did not show CAG at that time.
The combination of H. pylori, MNNG, and ammonia is an effective method of developing a mouse model of human CAG.
Core tip: A chronic atrophic gastritis (CAG) model was successfully established at the 90th day after the last Helicobacter pylori (H. pylori) infection of six-week-old mice, with a success rate reaching 90%. The method overcomes the difficulty in colonising H. pylori in mice and the fact that the colonisation time is short. In addition, rats are replaced with mice, and the method is thus simpler and also cheaper. The established models are stable and safe, therefore, the method is a relatively ideal method for establishing a mouse model of CAG. It provides a significant help when exploring the mechanisms of occurrence and prevention CAG.
- Citation: Wei X, Feng XP, Wang LY, Huang YQ, Liang LL, Mo XQ, Wei HY. Improved method for inducing chronic atrophic gastritis in mice. World J Gastrointest Oncol 2019; 11(12): 1115-1125
- URL: https://www.wjgnet.com/1948-5204/full/v11/i12/1115.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i12.1115
Chronic atrophic gastritis (CAG) is a common disease of the digestive system with pathological characteristics of a decreasing number, or disappearance, of inherent glands of gastric mucosa[1,2]. As early as 1978, the World Health Organization had announced a close relationship between CAG and the incidence of gastric cancer[3]. CAG has been defined as a precancerous condition of gastric cancer. Intestinal metaplasia or intraepithelial neoplasia accompanying atrophied glands of the stomach is regarded as one of the most important precancerous lesions of gastric cancer. As a common malignant tumour, gastric cancer remains without a satisfactory therapy and its pathogenesis remains unclear, seriously threatening human life[4,5]. Therefore, some scholars have proposed to prevent the incidence of gastric cancer by avoiding precancerous lesions. If CAG can be reversed, the incidence of gastric cancer can be substantially reduced. To reverse and prevent CAG and study its pathogenesis and therapy, it is necessary to develop an ideal, safe, stable, animal model. An ideal animal model of CAG needs to meet the following conditions: (1) The model develops disease to resemble the same condition in humans; (2) Animals are abundant and easily raised; and (3) The model is simple and provides a high success rate, and readily guarantees survival of the animals. In recent years, scholars mainly established animal models by imitating human CAG etiology. Commonly used animals include rats and Mongolian gerbils. Methods based on live Helicobacter pylori (H. pylori), N-methyl-N'-nitroguanidine (MNNG), ammonia, sodium deoxycholate, sodium salicylate, and immunology are commonly used to establish animal models[6-8]. In addition to this, the animal model can also be developed through combination of diseases and syndromes according to traditional Chinese medicine (TCM)[9]. Regardless of what method is used, the results differ to some extent from the ideal animal model and certain deficiencies therein require to be overcome. It is a challenge to build a CAG model with mice. For example, even if the methods of chemical mutagens and stimulation of the gastric mucosa with a combination of factors are used for establishing animal models with a high success rate, they retain certain shortcomings. These include various problems, such as the selection of experimental animals, drug combination, drug dosages, and lack of a unified standard for the duration of the modelling. Besides, spontaneous animal models are seldom studied. TCM diagnoses and treats CAG mainly based on an overall analysis of patient condition, rather than the method applied by Western medicine that treats CAG in a general way according to the classification of diseases. Therefore, it is a preferable research approach to establish experimental animals combining diseases and syndromes using TCM to carry out TCM basic and clinical research. Even so, there are also some drawbacks. For instance, there is a lack of a recognised method of developing models and objective evaluation indices thereof. Therefore, it is necessary to improve the animal model of CAG, and explore an ideal method of developing mouse models of CAG. H. pylori is the primary pathogenic factor of CAG; the MNNG method is an acknowledged method of making mouse models of CAG; and ammonia simulates the injury of alkaline environment to the gastric mucosa, so it is also a clinically common method for establishing animal models based on etiology. Considering this, this study proposed an improved comprehensive method for establishing an animal model by combining H. pylori, MNNG, and ammonia. To our knowledge, the combined modelling method has not yet been reported.
Female six-week-old Kunming mice, weighing between 180 and 200 g, were purchased from the Experimental Animal Center, Youjiang Medical University for Nationalities. The animals were housed at controlled humidity (50% ± 5%) and temperature (25 ± 2 °C) with a light-dark cycle of 12 h. Animal treatment was in accordance with National Institute of Health guidelines and experimental protocols were approved by the Institutional Animal Care and Use Committee of Youjiang Medical University for Nationalities.
The study involved H. pylori strains (Affiliated Hospital of Youjiang Medical University for Nationalities), MNNG (BIDE PHARMATECH Co., ltd), ammonia water (Sinopharm Chemical Reagent Co., Ltd.), IL-1β, IL-8, TNF-α, CD4, CD8, CagA, Bax, a Bcl-2 kit (Beijing Solarbio Science and Technology Co., ltd.), and a DNA kit (TIANGEN, BIOTECH Co., ltd.). All reagents were of analytical grade.
A total of 220 six-week-old Kunming mice were randomly divided into a phosphate buffered solution control group, an H. pylori group, an MNNG group, an ammonia water group, and a group combining H. pylori, MNNG, and ammonia water (hereinafter referred to as the combined group). Each group contained equal numbers of male and female mice.
Mice in the MNNG and combined groups were administrated with MNNG (5 mL/kg body mass) at a concentration of 120 μg/mL through daily gavage for seven successive days.
Mice were fed drinking water containing 0.02 wt% ammonia throughout. Mice in the control group were fed regularly without intervention.
Clinically isolated H. pylori strains were used, and they were subjected to Gram staining, urease testing, oxidase testing, and catalase testing, and identified as H. pylori through 16S ribosomal RNA sequencing. Strains were CagA and VagA positive and were proliferated and cultured for three days. The concentration of biosynthetic human insulin (BHI) medium was adjusted to 1 × 109 CFU/mL. H. pylori was applied to the mice through gavage after intragastric administration of MNNG. The mice were fasted for 12 h before gavage. The mice in the H. pylori and combined groups were intragastrically administrated with 5 × 108 CFU of H. pylori. Thereafter, the mice were fasted for solids and liquids for 2 h. The administration was conducted every other day, five times in succession. In comparison, 0.5 mL of BHI medium was applied to each mouse in the control group through gavage (also five times).
Five male and five female mice were chosen from each group on the 30th, 60th, 90th, and 120th days. Then, gastric mucosal samples were collected for pathological examination by hematoxylin eosin (HE) staining, terminal nick-end labelling (TUNEL) staining, and immunohistochemical staining for Bax and Bcl-2. In addition, H. pylori was isolated, cultured, and identified, and the extent of its colonisation calculated. Blood was collected to detect the inflammatory factors IL-1β, IL-8, and TNF-α, and immune function markers CD4 and CD8. Besides, the liver, kidney, and spleen samples of the modelling groups were detected by HE staining to confirm whether or not CAG has been successfully induced.
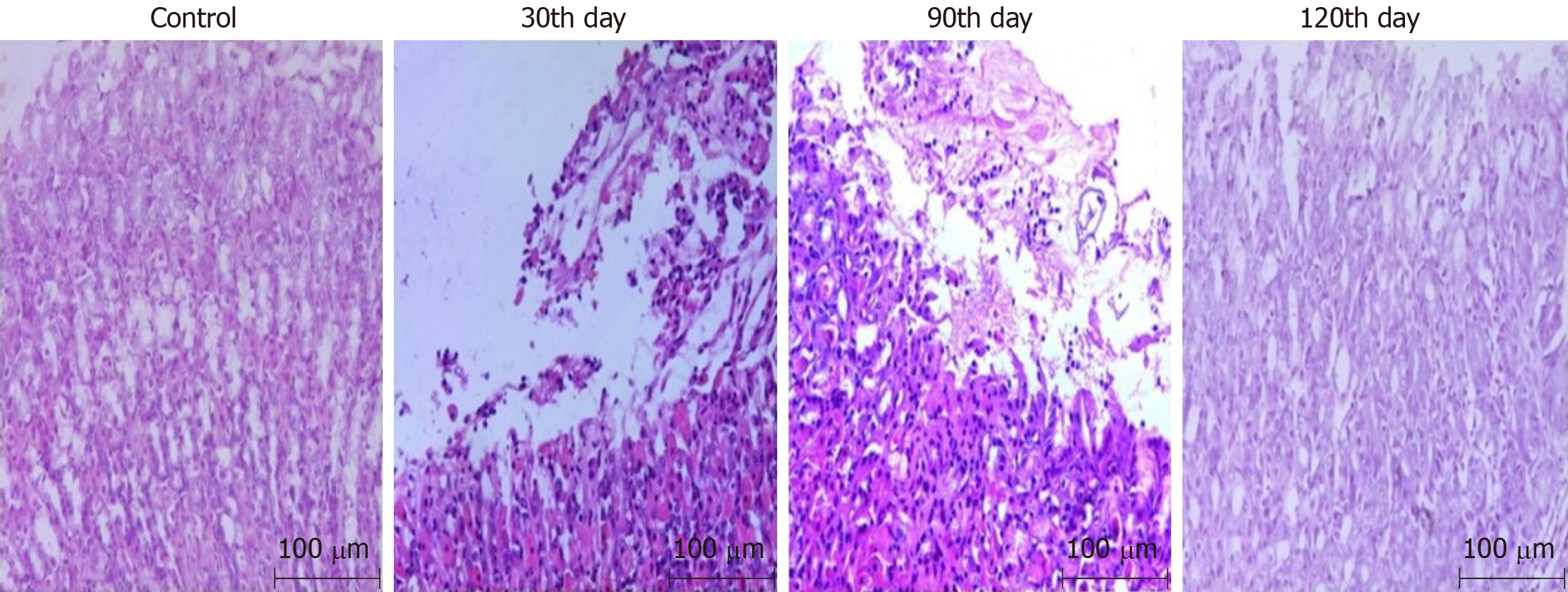
On the 30th day after infection, all of the mice in the combined group were found to have developed acute gastritis, while there were no CAG mice. No significant difference was shown on the 60th day compared with that on the 30th day. On the 90th day, 90% (nine out of ten) of mice had developed slight CAG which developed to moderate CAG on the 120th day, as shown in Figure 1.
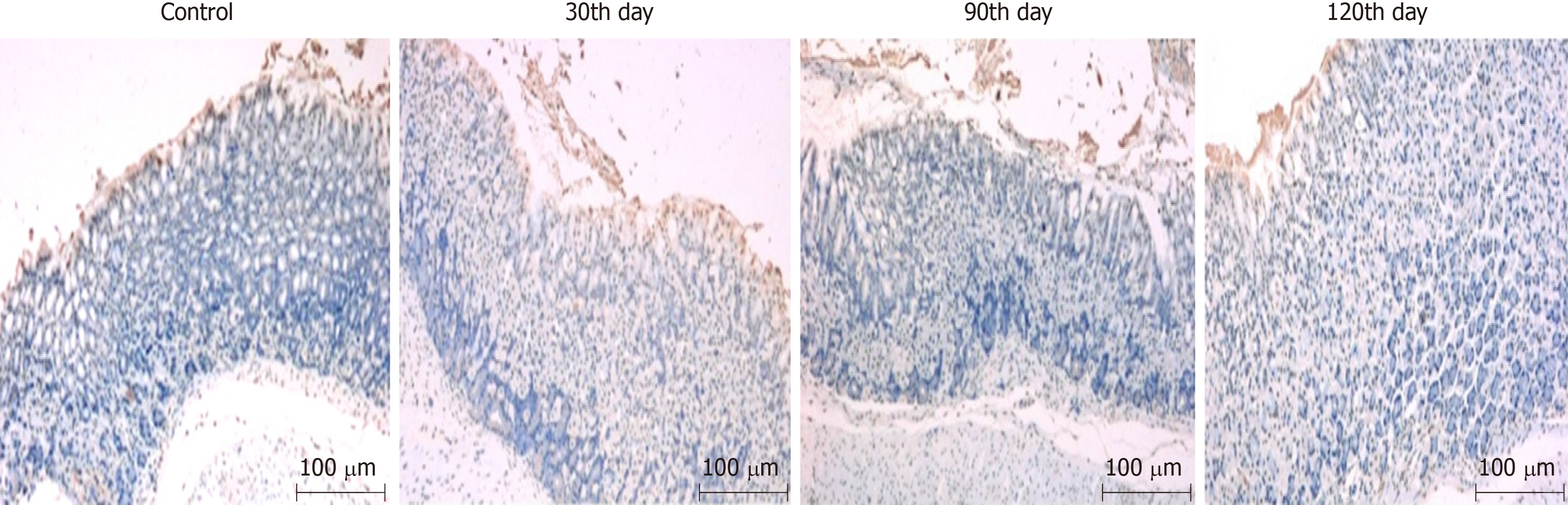
The detection of TUNEL-stained gastric mucosa of mice in the combined group on the 30th, 60th, 90th, and 120th days after infection revealed that epithelial cells of the gastric mucosa all presented significant apoptosis. There was greater cell apoptosis by the 30th day, while at the 90th and 120th days, similar, less severe, apoptosis than that at the 30th day was found, as shown in Figure 2.
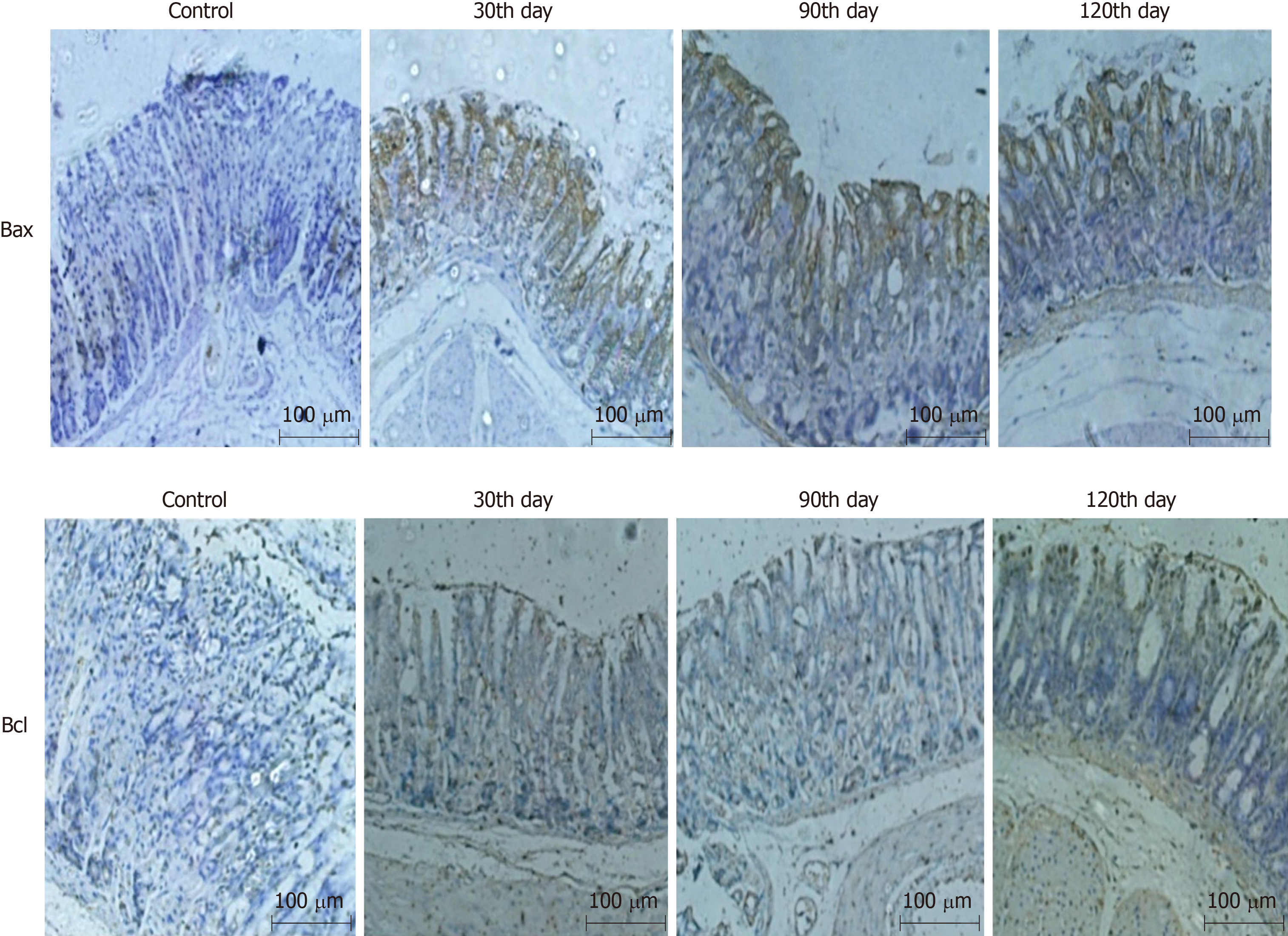
Bax, directly activated by p53, can improve apoptotic sensitivity and promote p53-mediated cell apoptosis. Bcl-2 protein can form a heterodimer with other pro-apoptotic proteins to inhibit cell apoptosis. It also plays an anti-apoptosis effect by interacting with apoptotic protease activating factor or inhibiting the release of cytochrome c, an activator of mitochondrial caspases. Therefore, cell apoptosis can be examined by detecting the expression of Bcl-xL and Bax. The expression of Bax in the combined group was up-regulated at the 90th and 120th day, while that of Bcl-2 was down-regulated, which indicated that the mice in the combined group had undergone significant cell apoptosis, conforming to the characteristics of CAG, as shown in Figure 3.
According to the postulates of Robert Koch, infecting H. pylori has to be isolated and cultured from the modelling groups, so the gastric mucosa was collected for isolation and culture of H. pylori, and the cultures were identified. The specific results are described below.
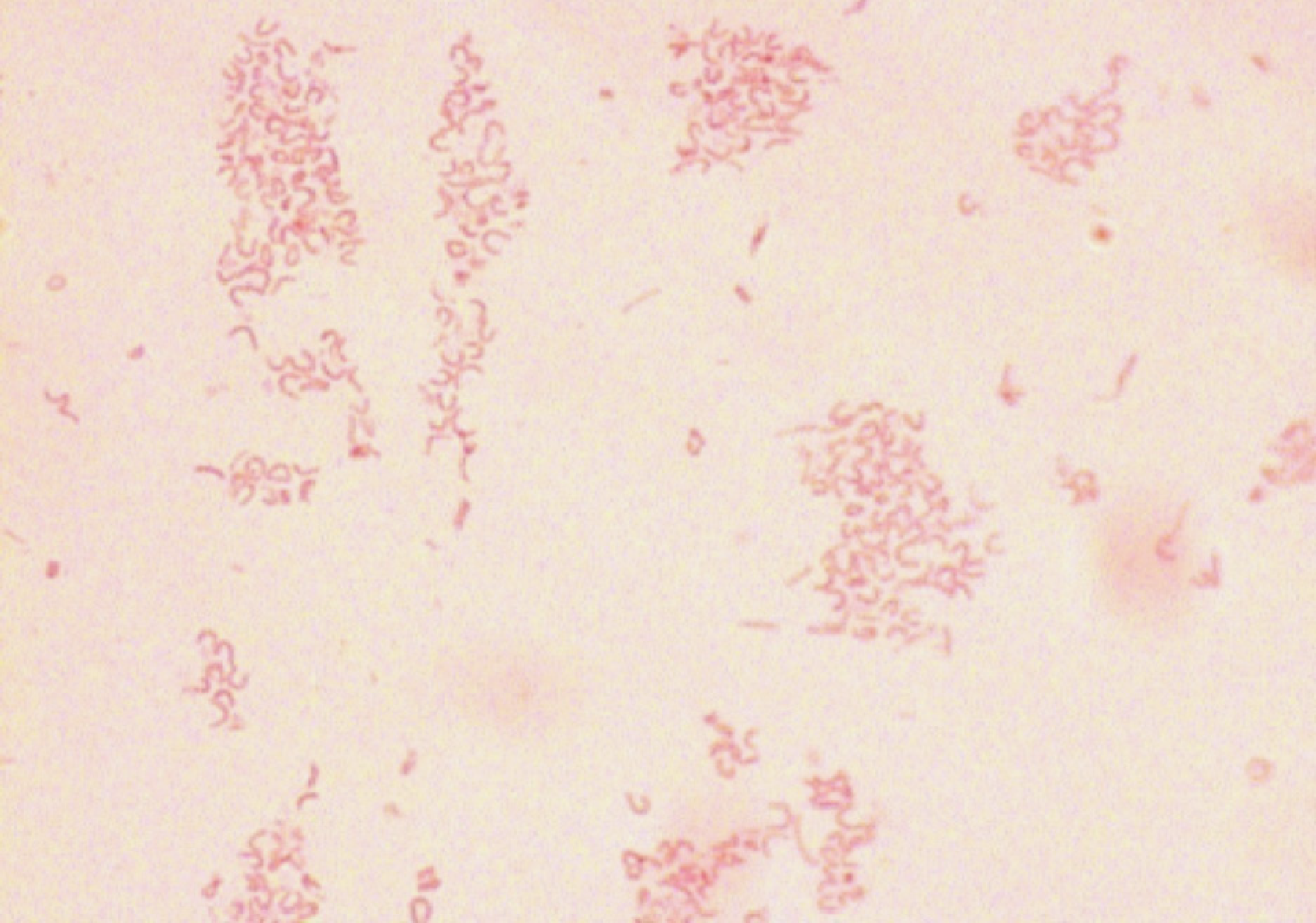
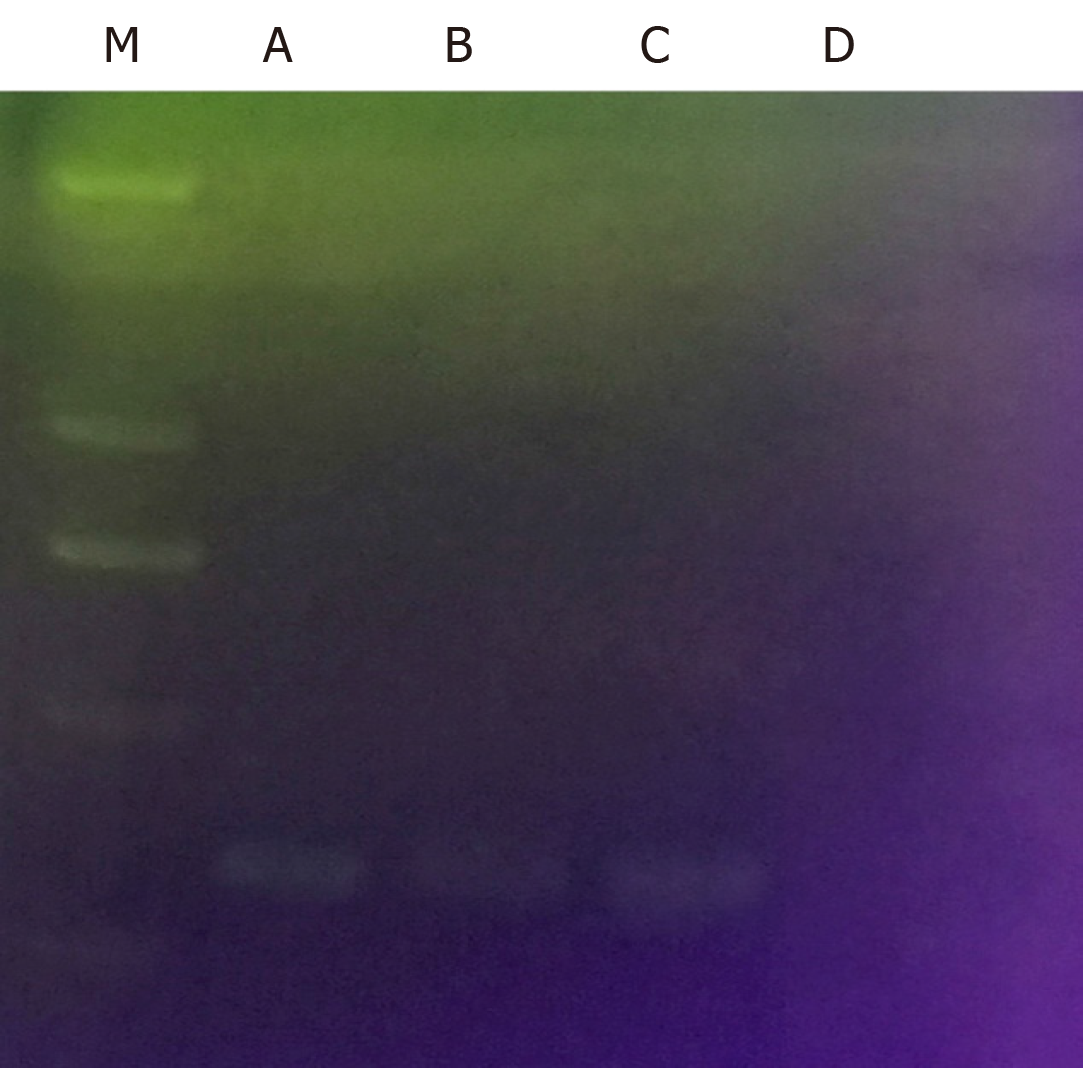
Gram staining: Isolated cultures were selected for Gram staining. The bacteria were Gram negative under a microscope and curving, which is consistent with the morphological characteristics of stained H. pylori, as shown in Figure 4.
CagA detection: CagA is a typical virulence factor of H. pylori. It had been verified that the infecting H. pylori strains contain the CagA gene before H. pylori infection through gavage. Therefore, if the isolated bacterium was H. pylori, the genome was bound to contain the CagA gene. It was verified that the isolated cultures did contain the CagA gene, as shown in Figure 5.
Detection of oxidase, peroxidase, and urease:H. pylori shows the biochemical characteristic of positive oxidase, peroxidase, and urease, so the three factors are commonly used as biochemical indices for identifying H. pylori. Detection of the isolated cultures showed that the three were also positive, which accorded with the biochemical characteristics of H. pylori, as summarised in Table 1.
| Group | Oxidase | Peroxidase | Urease |
| Positive control | + | + | + |
| Negative control | - | - | - |
| Isolated culture | + | + | + |
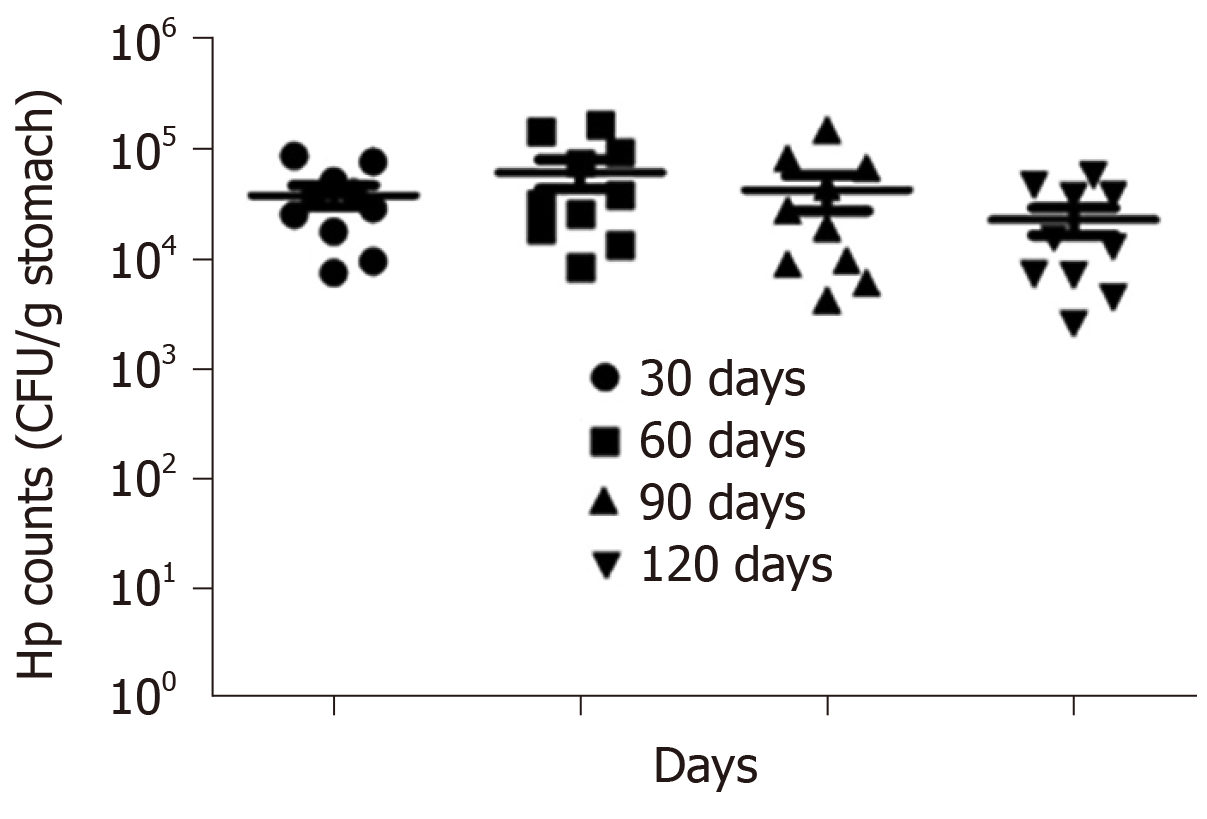
Extent of H. pylori colonisation: The above identifications revealed that the isolated and cultured bacterium was H. pylori, whose population was calculated then to estimate the extent of its colonisation. At the 30th, 60th, 90th, and 120th days after infection, every gram of gastric mucosa of mice in the combined group was found to contain 1 × 104 to 1 × 105 CFU of H. pylori, and there was no significant difference across different time periods. This indicated that the colonisation of H. pylori did not decrease within 120 d after H. pylori infection, so H. pylori probably played a significant role in the pathogenic process of CAG, as shown in Figure 6.
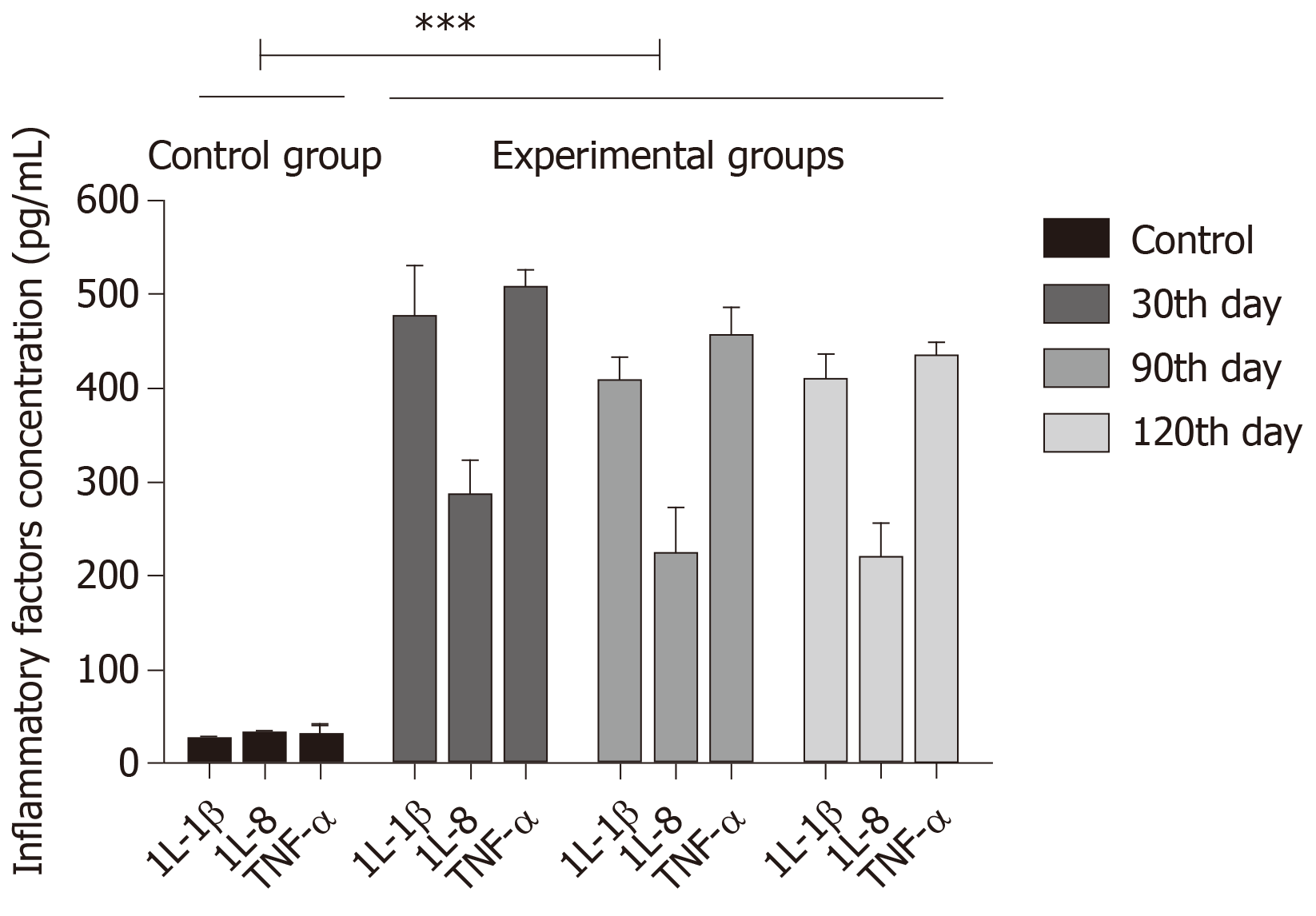
The incidence and development of CAG can trigger the release of various inflammatory factors such as IL-1β, IL-8, and TNF-α. Inflammatory factors IL-1α, IL-8, and TNF-α were found to have increased expression on the 30th, 90th, and 120th days after mice in the combined group were infected with H. pylori. The increase was greater the first month, while the inflammatory factors had equivalent expression at the 90th and 120th days, both being lower than that on the 30th day. The results are consistent with the results of TUNEL staining, also showing the up-regulation of CAG inflammatory factors, as shown in Figure 7.
The expression of CD4+ and CD8+ immune cells of mice in the combined group were both decreased by the 30th, 90th, and 120th days after infection. The expression was down-regulated significantly by the 30th day, while the two immune cells were expressed in a similar manner on the 90th and 120th days, both having been down-regulated to a lesser extent than that on the 30th day. This was mainly because, by the 30th day, the mice were still affected by the immunosuppressor MNNG, and their immunological functions were impaired. After eliminating the influences of the immunosuppressor, the immunological functions improved while were unable to recover to normal levels. This is also probably because CAG itself is characterised by the impairment of immunological function, as seen from Table 2.
| Group | CD4+ | CD8+ | ||||
| 30th d | 90th d | 120th d | 30th d | 90th d | 120th d | |
| PBS control | 52.56 ± 1.82 | 51.27 ± 2.05 | 52.84 ± 1.49 | 28.13 ± 1.92 | 29.41 ± 2.39 | 30.07 ± 1.24 |
| Combined | 40.17 ± 1.36 | 44.38 ± 2.11 | 43.97 ± 2.08 | 22.08 ± 1.42 | 25.53 ± 1.87 | 25.19 ± 1.53 |
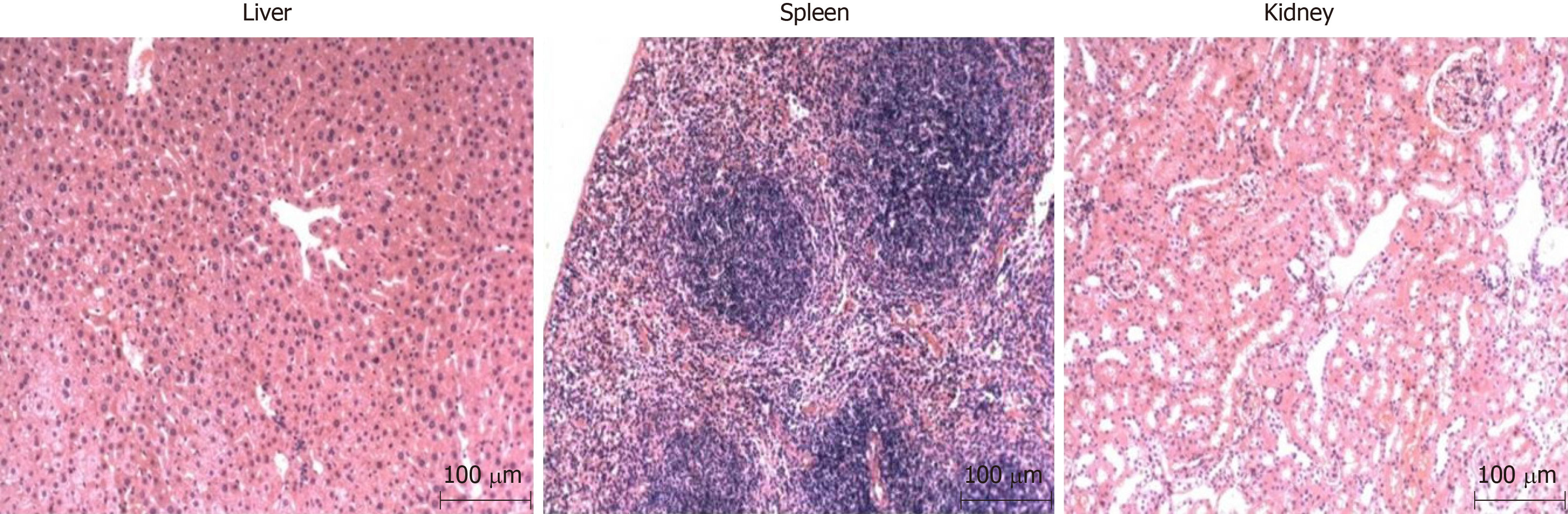
As chemical and biological methods were combined in the study to establish the model, chemical reagents were likely to lead to impairment of organ functions of the mice, and even cancers therein. Therefore, it is necessary to detect the impairment of organs of the mice. By collecting and staining the liver, kidney, and spleen tissues with HE, it revealed that there was no impairment. This indicated that the modelling method did not induce complications of the liver, kidney, and spleen except for the development of CAG, so the method could be deemed safe, as shown in Figure 8.
The aforementioned identifications proved that the established animal models were CAG models. Statistical analysis revealed that 90% of mice in the combined group developed CAG at the 90th and 120th day after infection and H. pylori colonisation was found in all such mice. In contrast, the H. pylori group showed H. pylori colonisation but no CAG. Other groups did not exhibit CAG or H. pylori colonisation, as can be seen from the data in Table 3.
| Group | The number of mice developing CAG | The number of mice with Hp colonisation | Success rate | ||
| 90th d after infection | 120th d after infection | 90th d after infection | 120th d after infection | ||
| PBS control | 0 | 0 | 0 | 0 | 0 |
| Hp | 0 | 0 | 90% (9/10) | 100% (10/10) | 0 |
| MNNG | 0 | 0 | 0 | 0 | 0 |
| Ammonia water | 0 | 0 | 0 | 0 | 0 |
| Combined | 90% (9/10) | 90 (9/10) | 100% (10/10) | 100% (10/10) | 90% |
CAG is one of the most common gastrointestinal diseases and an early stage of gastric cancer. CAG is actually one event in a chronic degenerative process beginning with gastritis, proceeding to CAG, then to intestinal metaplasia, dysplasia and, finally, to carcinoma[10]. CAG is hard to be cured with recurrent attacks, severely affecting the health of human. The research and treatment of CAG are very popular. An animal model of CAG is an important basis for research into chronic gastritis. Although there have been numerous reports on the animal models of CAG, it is still not clear which model is best suited to investigating the mechanism of occurrence of human chronic gastritis and there are no unified standards for the type and age of animals to be selected[11-13]. As the modelling of chronic gastritis takes a long time, there is an urgent need to find a more rapid, stable, safe model. At present, biological modelling, chemical injury, and active immunisation are commonly used methods of establishing animal models, and rats are generally used. MNNG, sodium deoxycholate, and sodium salicylate are commonly model materials with chemical methods. Rats and Mongolian gerbils are commonly used animals to develop CAG models, while mice are unusually used. The reasons may be that mice do not control the dose of the intervention and are prone to other complications or toxic side effects.
H. pylori is a human-specific pathogen, which leads to gastric pathologies including gastritis and gastric ulcers. H. pylori is a common risk factor for CAG and can be used to create CAG model. The animal model is due to H. pylori infection, more like a human infection model, and beneficial to studying the pathogenesis of CAG. However, H. pylori is relatively easy to implant in rats and Mongolian gerbils, and the incidence rate is relatively high, but it is difficult to plant in the mouse. Clinically isolated H. pylori strains must be domesticated for adapted planting, and the time of retaining in the stomach is relatively short. After the first month of infection, the maximum number of plants is reached, and it will decrease in the second and third months. The mice will not be able to maintain a relatively high infection rate. Although the C57BL/6 mice and BALB/c mice with H. pylori Sydney strain (SS1) can maintain a high amount of plants in the stomach for a long time, the strain was CagA negative and CAG did not appear in 24 wk after the last infection[14]. “H. pylori + N-methyl-N-nitrourea can induce CAG in 23.1% of animals after infection for 36 wk, which required a long time, and the success rate is low[15]. As it is difficult to colonise H. pylori in the stomach in the long term, and particularly difficult to infect mice for a long time, biological modelling commonly uses rats. However, H. pylori does not readily infect rats without injury to the gastric mucosa, therefore, the gastric mucosa of rats has to be impaired before being infected with H. pylori in many experiments. So, it is very difficult to establish CAG with H. pylori infected mice in a short period of time.
In reference to the methods of Bergin et al[16], Nagahara et al[17], and Jin et al[18], this study established a CAG model with Kunming mice by combining H. pylori, MNNG, and ammonia water. The method was proposed on the basis of the fact that infection with H. pylori can induce gastritis[19,20], the functions of MNNG as a chemical mutagen and carcinogen[21,22], and the role of ammonia water in neutralising gastric acid and impairing gastric mucosa[23,24]. A CAG model was successfully established by the 90th day after the last H. pylori infection of six-week-old mice, with a success rate reaching 90%. The method overcomes the difficulty in colonising H. pylori in mice and the fact that the colonisation time is short. In addition, rats are replaced with mice, and the method is thus simpler and also cheaper. The established models are stable and safe, therefore, the method is a relatively ideal method for establishing a mouse model of CAG. It is demonstrated to be possible to build an animal model that is analogous to human CAG, which provides a significant help when exploring the mechanisms of occurrence and prevention of CAG.
Chronic atrophic gastritis (CAG) is a precancerous lesion of gastric cancer. If it is effectively treated, GC will be prevented. So the prevention and treatment of CAG are very important. It needs a good animal model, which can provide etiological research and screening of therapeutic drugs. It is an important tool for studying CAG.
Current animal models of chronic atrophic gastritis are not ideal, long cycle, high cost, difficult to operate, and unstable, so better animal models are needed.
To construct a more rapid, safe, stable and efficient chronic atrophic gastritis model with mice.
The mice selected for this method were six-week-old Kunming mice, and Helicobacter pylori, N-methyl-N'-nitroguanidine, and ammonia water were combined to develop a CAG model.
The method showed slight CAG at the 90th day and moderate CAG at the 120th day.
The method presented here is more rapid, safe, stable, and efficient.
Starting from the etiology of chronic atrophic gastritis, the course of the disease is simulated to explore a simple, fast, convenient, and stable method of modelling.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kay TM, Lin JK, Wittkopf N S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Sonnenberg A, Lash RH, Genta RM. A national study of Helicobactor pylori infection in gastric biopsy specimens. Gastroenterology. 2010;139:1894-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev. 2015;20:25-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 3. | Corso G, Seruca R, Roviello F. Gastric cancer carcinogenesis and tumor progression. Ann Ital Chir. 2012;83:172-176. [PubMed] |
| 4. | Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 734] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14:302-308. |
| 6. | Oda T, Murakami K, Nishizono A, Kodama M, Nasu M, Fujioka T. Long-term Helicobacter pylori infection in Japanese monkeys induces atrophic gastritis and accumulation of mutations in the p53 tumor suppressor gene. Helicobacter. 2002;7:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Peraino C, Staffeldt EF, Carnes BA, Ludeman VA, Blomquist JA, Vesselinovitch SD. Characterization of histochemically detectable altered hepatocyte foci and their relationship to hepatic tumorigenesis in rats treated once with diethylnitrosamine or benzo(a)pyrene within one day after birth. Cancer Res. 1984;44:3340-3347. [PubMed] |
| 8. | Mitaka T, Tsukada H. Sexual difference in the histochemical characteristics of "altered cell foci" in the liver of aged Fischer 344 rats. Jpn J Cancer Res. 1987;78:785-790. [PubMed] |
| 9. | Lu Weimin, Shan Zhaowei, Wu Jing, Shen Hong, Zhang Jianning, Zhu Yunhua, and Zhu Changle. Rat model of qi-deficiency-blood-stasis syndrome of precancerous lesion developed from chronic atrophic gastritis. Journal of Nanjing University of Traditional Chinese Medicine (Natural Science). 2000;16:156-158. [DOI] [Full Text] |
| 10. | Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H, Shimizu Y, Takeshita T, Mohara O, Ichinose M. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 373] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Si J, Zhou W, Wu J, Cao Q, Xiang Z, Jiang L, Lü W, Huang H. Establishment of an animal model of chronic atrophic gastritis and a study on the factors inducing atrophy. Chin Med J (Engl). 2001;114:1323-1325. [PubMed] |
| 12. | Liu Fuhua, Qin Xiaoguang, Long Junhua, Li Mei. Chinese Medicine Modern Distance Education of China. 2018;16:1-2 Research progress of animal model of chronic atrophic gastritis. [DOI] [Full Text] |
| 13. | Lahner E, Bernardini G, Possenti S, Renzone G, Scaloni A, Santucci A, Annibale B. Immunoproteomics of Helicobacter pylori infection in patients with atrophic body gastritis, a predisposing condition for gastric cancer. Int J Med Microbiol. 2011;301:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Zeng zhirong, Hu pinjin, Chen minhu, Yu feng-an, Chen wei, Peng xiao-zhong. Development of Mouse Model Long-term Helicobacter pylori infection. Zhongguo Shengwu Zhipin Xue Zazhi. 1998;11:233-236. [DOI] [Full Text] |
| 15. | Wang Jian, Wang Ji-yao, Shen Xizhong, Qi Wei-dong, Gao Hong, Wang Yi-qing. Establishment of Helicobacter py lori infection model and influence of its infection on N-methyl-Nnitrosourea-induced gastric carcinogenesis in Balb/c mice. Clin J Dig. 2005;25:146-149. [DOI] [Full Text] |
| 16. | Bergin IL, Sheppard BJ, Fox JG. Helicobacter pylori infection and high dietary salt independently induce atrophic gastritis and intestinal metaplasia in commercially available outbred Mongolian gerbils. Dig Dis Sci. 2003;48:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Nagahara A, Watanabe S, Miwa H, Endo K, Hirose M, Sato N. Reduction of gap junction protein connexin 32 in rat atrophic gastric mucosa as an early event in carcinogenesis. J Gastroenterol. 1996;31:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Jin Z, Hu FL, Wei H, Tang XY, Dong Y. [Establishment of Mongolian gerbil model of long-term Helicobacter pylori infection]. Zhonghua Yi Xue Za Zhi. 2008;88:1518-1522. [PubMed] |
| 19. | Valenzuela MA, Canales J, Corvalán AH, Quest AF. Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis. World J Gastroenterol. 2015;21:12742-12756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Zhang H, Ding C, Suo Z, Kang Y. Effect of Helicobacter pylori on cyclooxygenase-2 and inducible nitric oxide synthase in patients with gastric precancerous lesions and its clinical significance. Exp Ther Med. 2015;9:2364-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Miwa H, Endo K, Wada R, Hirai S, Hirose M, Misawa H, Nagahara A, Ohta K, Watanabe S, Sato N. Cellular proliferation and differentiation in rat atrophic gastric mucosa induced by N'-methyl-N'-nitro-N-nitrosoguanidine. J Clin Gastroenterol. 1997;25 Suppl 1:S116-S121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Yuan Xiaobing. Research progress of rat models of precancerous lesions. Journal of Anhui Traditional Chinese Medical College. 2004;23:62-64. [DOI] [Full Text] |
| 23. | Nagahashi S, Suzuki H, Miyazawa M, Nagata H, Suzuki M, Miura S, Ishii H. Ammonia aggravates stress-induced gastric mucosal oxidative injury through the cancellation of cytoprotective heat shock protein 70. Free Radic Biol Med. 2002;33:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 24. | Liu WL, Chen SJ, Chen Y, Sun LM, Zhang W, Zeng YM, Zhou TH, Si JM. Protective effects of heat shock protein70 induced by geranylgeranylacetone in atrophic gastritis in rats. Acta Pharmacol Sin. 2007;28:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |