Published online Aug 25, 2015. doi: 10.4253/wjge.v7.i11.1003
Peer-review started: April 29, 2015
First decision: June 4, 2015
Revised: June 25, 2015
Accepted: July 29, 2015
Article in press: August 3, 2015
Published online: August 25, 2015
Processing time: 122 Days and 11.1 Hours
Endoscopic management of biliary obstruction has evolved tremendously since the introduction of flexible fiberoptic endoscopes over 50 years ago. For the last several decades, endoscopic retrograde cholangiopancreatography (ERCP) has become established as the mainstay for definitively diagnosing and relieving biliary obstruction. In addition, and more recently, endoscopic ultrasonography (EUS) has gained increasing favor as an auxiliary diagnostic and therapeutic modality in facilitating decompression of the biliary tree. Here, we provide a review of the current and continually evolving role of gastrointestinal endoscopy, including both ERCP and EUS, in the management of biliary obstruction with a focus on benign biliary strictures.
Core tip: Benign biliary strictures (BBSs) are commonly encountered by advanced endoscopists. As our understanding of longstanding techniques involving biliary dilation and plastic stent placement evolves, newer therapeutic options such as self-expandable metal stents and endoscopic ultrasound have become available. Here we review the literature pertaining to the most common etiologies of BBSs with current considerations for their respective endoscopic management.
- Citation: Visrodia KH, Tabibian JH, Baron TH. Endoscopic management of benign biliary strictures. World J Gastrointest Endosc 2015; 7(11): 1003-1013
- URL: https://www.wjgnet.com/1948-5190/full/v7/i11/1003.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i11.1003
Benign biliary strictures (BBSs) originate from a variety of etiologies (Table 1), most commonly post-operative injury (e.g., post-cholecystectomy), chronic pancreatitis, and chronic cholangiopathies (e.g., primary sclerosing cholangitis). The clinical presentation of BBSs depends greatly on the context, including the onset, degree, and sterility of obstruction, and ranges from subclinical (i.e., incidentally detected biochemical abnormalities) to severe and life-threatening[1,2]. The diagnostic evaluation to determine the etiology of a BBS and exclude the possibility of underlying malignancy generally entails cholangiography via magnetic resonance (MRCP) and/or endoscopic retrograde cholangiopancreatography (ERCP) (with biliary brushings for cytology and/or intraductal biopsies for histology) in addition to serologic testing with serum liver tests and tumor marker carbohydrate antigen 19-9 (CA 19-9). Therapeutic interventions are aimed at providing durable biliary decompression, with options including ERCP, percutaneous, and surgical techniques.
| Postsurgical |
| Cholecystectomy (open or laparoscopic) |
| Liver transplantation (i.e., anastomotic biliary stricture) |
| Bilio-enteric anastomosis |
| Sphincterotomy |
| Inflammatory |
| Chronic pancreatitis |
| Primary sclerosing cholangitis |
| Immunoglobulin G4-related cholangiopathy |
| Acquired immune deficiency syndrome cholangiopathy |
| Vasculitis |
| Other |
| Ischemia (e.g., post-liver transplantation) |
| Trauma |
| Portal biliopathy |
| Infection (e.g., Clonorchiasis) |
| Radiation injury |
| Idiopathic |
Given its efficacy, safety, and less disruptive nature, ERCP has become the first-line therapeutic option for management of most cases of biliary obstruction, including but not limited to BBSs[3]. Since the introduction of ERCP in the 1970s, this technique has progressively evolved and enhanced the management of a variety of disorders of the biliary tract[4]. Currently, a wide array of catheters, guidewires, papillotomes, stents, and other accessories are available to facilitate diagnostic and therapeutic maneuvers in the management of BBSs.
In this review, we discuss the current role of, evidence for, and approach to endoscopic management in patients with BBSs.
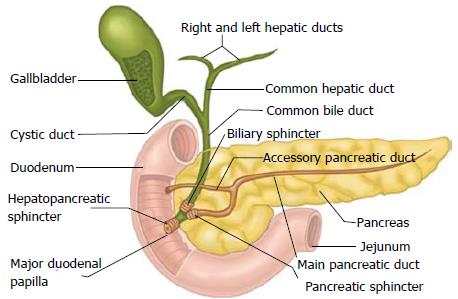
Owing to advancements in non-invasive imaging, ERCP has largely been supplanted by cross-sectional imaging for purposes of initial diagnosis. MRCP, facilitated by the high T2-signal intensity of bile as well as improvements in MR imaging methods and post-processing tools, has essentially become the preferred modality for diagnostic cholangiography, with relatively few indications remaining for diagnostic ERCP[5]. Not all patients require cross-sectional imaging with MRCP or computed tomography prior to ERCP; however, having such data available can provide a useful roadmap and clarify the pre-procedural plan by shedding light on the patient’s pancreatobiliary anatomy, which often does not follow the conventional teaching (Figure 1), and underlying disease. Patients who proceed to ERCP should, as with other endoscopic procedures, be fasting for a sufficient amount of time to allow gastric emptying (e.g., 4-6 h), and careful review and management of antithrombotic medications (if applicable) should be undertaken[6]. Pre-procedural antibiotics should be administered in selected patients in whom adequate drainage is not anticipated such as those with complex hilar strictures and PSC.
Once bile duct cannulation has been achieved, attempts at guidewire passage beyond the BBS may prove challenging depending on the severity and anatomic location of obstruction. BBSs can be more difficult to traverse than neoplastic strictures due to greater asymmetry, angulation, and density of fibrous tissue[7]; nevertheless, forceful maneuvers should be avoided, as these may result in the creation of a false tract or perforation. If necessary, guidewire passage can be facilitated by: (1) positioning an inflated stone extraction balloon just below the stricture and withdrawing it, which allows for traction and better alignment between the guidewire and stricture axes; or by (2) selection of an alternative guidewire tailored to the particular stricture anatomy.
Multiple types of guidewires are commercially available and vary in their properties, including diameter, construction material (nitinol, stainless steel), type of coating (hydrophilic vs nonhydrophilic), and tip morphology (straight, angled) (Table 2). Comparative studies between guidewires are lacking, but standard 0.035-inch hydrophilic guidewires can be used for most BBSs, whereas tighter strictures may require guidewires with a smaller diameter and/or angled tip. Once a stricture has been traversed, the guidewire can be exchanged, if needed, for a stiffer or nonhydrophilic guidewire to facilitate dilation and stenting. Biliary sphincterotomy (i.e., papillotomy) is also frequently necessary if large (cumulative) caliber stenting is anticipated.
| Diameter (inch) | Length (cm) | Core material | Sheath material | Tip material/properties | Tip shape | Comments | Cost ($) | |
| Monofilament | ||||||||
| Amplatz (Boston Scientific) | 0.038 | 260 | Stainless steel | Uncoated | Platinum | Straight | Extremely stiff | 1491 |
| Coiled | ||||||||
| Standard (Cook Medical) | 0.035 | 480 | Stainless steel | Uncoated | Stainless steel coil | Straight | Must remove prior to sphincterotomy | 90 |
| Coated | ||||||||
| Tracer metro direct (Cook Medical) | 0.021, 0.025, 0.035 | 260, 480 | Nitinol | Teflon | Platinum; hydrophilic (5 cm) | Straight, angled | Kink resistant, graduated endoscopic markings | 196 |
| Delta (Cook Medical) | 0.025, 0.035 | 260 | Nitinol | Polyurethane | Hydrophilic (fully) | Straight | Kink resistant, fully hydrophilic, must remove prior to sphincterotomy | 151 |
| Roadrunner (Cook Medical) | 0.018 | 260, 480 | Nitinol | Teflon | Platinum | Straight, angled | Kink resistant, must remove prior to sphincterotomy | 184 |
| Jagwire (Boston Scientific) | 0.025, 0.035 0.038 (260) | 260, 480 | Nitinol | Endo-Glide™ | Tungsten, hydrophilic (5 cm) | Straight, angled; trim, round | Kink resistant, guidewire extension (0.035, 200) available | 357/box of 2 |
| Hydra Jagwire (Boston Scientific) | 0.035 | 260, 450 | Nitinol | Endo-Glide™ | Tungsten, two hydrophilic tips (5 cm, 10 cm) | Straight, angled; round | Kink resistant; two tips of varying stiffness on a single guidewire | 536/box of 2 |
| NaviPro (Boston Scientific) | 0.018, 0.025, 0.035 | 260 | Nitinol | Endo-Glide™ | Hydrophilic (fully) | Straight, angled | Fully hydrophilic; 0.035-in also available in stiff | 1124/box of 5 |
| Visiglide (Olympus) | 0.025, 0.035 | 270, 450 | Superelastic alloy | Fluorine | Hydrophilic (7 cm) | Straight; angled | 0.025-in has same stiffness as 0.035-in guidewire | 255 |
| XWire (ConMed) | 0.025, 0.035 | 260, 450 | Regiliant™ Nitinol | PTFE | Nitinol and Tungsten and PTFE, hydrophilic (5 cm) | Straight; angled | 5 cm radiopaque tip; 0.035-in also available in stiff | 460/box of 3 (260 cm) 583/box of 3 (450 cm) |
Stricture dilation (i.e., stricturoplasty) is primarily performed using a dilating balloon or bougie-like tapered catheter. Typical dilating balloon sizes range from 4 to 12 mm, and selection can generally be guided by upsizing 1-2 mm from the diameter of the distal bile duct. In the case of post-liver transplantation (LT) anastomotic biliary strictures (ABSs), dilating to the size of the adjacent donor or recipient duct, whichever is smaller, can be used as a guide[8]. Particular caution should be taken, however, when dilating ABSs during the early post-operative period (< 30 d after surgery) or while a patient is still on high dose immunosuppression, as both of these scenarios may be associated with a higher risk for anastomotic injury or disruption[8-12]. In such instances, less aggressive dilation using a smaller balloon or alternatively a tapered dilating catheter is advisable. With respect to duration of dilation intraprocedurally, most endoscopists adhere to 30 to 60 s of dilation, or until the stricture waist is fractured, before balloon deflation.
Balloon dilation alone, although immediately effective, is associated with a high rate of stricture recurrence (up to 47%) depending on the underlying nature of the BBS[13]. Therefore, insertion of biliary stents is frequently required to maintain stricture patency while permitting ductal remodeling. Moreover, placement of several, large-bore plastic stents side-by-side (i.e., multiple or “maximal” endoscopic stenting[8,14]) for up to 1 year has been shown to be superior than inserting only a single stent; this is therefore the currently recommended approach for the majority of BBSs[8,14-18].
The main limitation of endoscopic stenting in this setting is the need to undergo multiple ERCPs for stent exchange. This stems from the relatively short patency time of plastic biliary stents, although there is evidence to support that occlusion rates are similar between stents with dwell times shorter and longer than 6 mo[19]. In addition, and as alluded to above, placement of maximal stents may lessen the need for frequent stent exchange, as biliary drainage can continue to occur even after stent occlusion via the inter-stent spaces (i.e., “wick effect”)[8]. Avoiding multiple ERCPs can also be facilitated by placement of one or more (covered) self-expandable metal stents (SEMSs) instead of plastic stents. SEMSs offer an attractive alternative because of innate properties that allow them to self-expand to diameters 3 times that of 10-Fr plastic stents, thus resulting in longer duration of patency. SEMSs can also be delivered using smaller deployment systems (i.e., 8-8.5-Fr) that do not require as aggressive dilation at the time of stent placement or biliary sphincterotomy. SEMSs of various configurations and properties are currently available[1]; to date, however, none are approved by the United States Food and Drug Administration for the treatment of BBSs. The three major categories of stents, uncovered, partially-covered, and fully-covered, are briefly reviewed below.
Uncovered SEMSs are plagued by the ingrowth of reactive tissue (i.e., epithelial hyperplasia), which can lead to stent occlusion as well as irretrievable embedding of a stent in the ductal wall[20]. As a result, uncovered SEMSs should not be used in the treatment of BBSs[17]. Partially-covered stents, which leave proximal and distal ends bare, are consequently less prone to becoming embedded in issue and thus have improved ease of retrieval. In the largest study of partially-covered SEMSs used to treat BBSs of various etiologies (n = 79), Kahaleh et al[21] reported a stricture resolution rate of 90% following a 4-mo stenting period and 12-mo follow-up time. Although all attempted stent retrievals were successful in this study, the potential for tissue hyperplasia involving the bare ends, as reported in other studies, still exists[22,23]. In an effort to further reduce the risk of stent ingrowth and improve removability, fully-covered SEMSs (lined with silicone, polyether polyurethane, polyurethane, expanded polytetrafluoroethylene, or other materials) have been developed and investigated in the treatment of BBSs (Table 3). Most studies of fully-covered SEMSs, barring those with a predominance of patients with particularly refractory strictures (e.g., chronic pancreatitis), have reported favorable clinical success rates, ranging 80% to 90%, as well as low recurrence rates (≤ 10%)[24-33]. A tradeoff of this stent design, however, is their predilection for migration, with several studies reporting fully-covered SEMS migration rates between 20% to 40%[24,25,28,31-33]. Of particular concern is the potential for a migrated SEMS to complicate stent removal (proximal migration) or cause bowel obstruction (distal migration). Recent studies investigating anti-migratory modifications to fully-covered SEMSs (e.g., anchoring fins) have reported reduced albeit not clinically insignificant rates of migration[27,29,30]. The role of fully-covered and partially-covered SEMSs is described further in forthcoming sections.
| Stent name(manufacturer) | Covering | Core material | Diameter (mm) | Length (cm) | Delivery system (Fr) | Features |
| Wallflex RX (Boston Scientific) | Partial | Platinol | 8, 10 | 4, 6, 8 | 8.5 | Closed cell construction; retrieval loop; looped and flared ends; restrainable |
| Full | Platinol | 8, 10 | 4, 6, 8 | 8.5 | ||
| Wallstent (Boston Scientific) | Partial | Elgiloy | 8, 10, 12 | 2, 4, 4.2, 6, 6.8, 8, 9, 9.4 | 6, 7, 9 | Closed cell construction; restrainable |
| Niti-S ComVi (Taewoong Medical) | Partial | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 9, 10, 12 | 8 | Open cell; triple layered construction: mesh, membrane, and mesh to reduce migration |
| Full | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 9, 10, 12 | 8 | ||
| Niti-S Kaffes (Taewoong Medical) | Full | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8 | 9 | Long retrieval string |
| Niti-S (Taewoong Medical) | Partial | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 9, 10, 12 | 8 | Retrieval string at proximal end |
| Full | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 9, 10, 12 | 8.5 | ||
| Niti-S Bumpy (Taewoong Medical) | Full | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 9, 10, 12 | 8.5 | Irregular cell sizes; retrieval string at proximal end; flared ends |
| Nitinella Plus (ELLA-CS) | Partial | Nitinol | 8, 10 | 4, 6, 8, 10 | 9 | Reconstrainable; kink-resistant |
| Full | Nitinol | 8, 10 | 4, 6, 8, 10 | 9 | ||
| Hanarostent (M.I. Tech) | Full | Nitinol | 8, 10 | 4, 6, 8, 10 | 8 | Larger flared ends |
| Micro-Tech (Micro-Tech) | Partial | Nitinol | 10 | 4, 6, 8, 10 | 9 | |
| Full | Nitinol | 10 | 4, 6, 8 | 9 | ||
| Gore Viabil (CONMED) | Full (with sideholes) | Nitinol | 8, 10 | 6, 8, 10 | 8.5 | Sideholes allow branch drainage; anchoring fins |
| Full (without sideholes) | Nitinol | 8, 10 | 6, 8, 10 | 8.5 | ||
| Allium BIS (Allium Medical) | Full | Nitinol | 8, 10 | 6, 8, 10, 12 | 10 | Anchoring segment; non-shortening |
Post-cholecystectomy: Cholecystectomy remains a common etiology of BBSs, with an incidence of 0.2% to 0.7% among patients undergoing laparoscopic cholecystectomy[34]. Post-cholecystectomy BBSs develop as a consequence of bile duct injury that may occur intraoperatively (dissection, electrocautery, clip or suture placement, ligation) and/or post-operatively (adhesion formation)[35]. Long-term data of post-operative BBSs treated with multiple plastic stents and intermittent stent exchange (approximately every 3 mo) over the course of a year have demonstrated promising success rates ranging from 80% to 100%[15,18,36,37]. This approach has thus become the current standard of care when treating post-operative BBSs[38]. It should be noted, however, that post-operative strictures located at the hepatic ductal confluence may be less responsive to endoscopic stenting than strictures located more distally (25% vs 80% resolution rate)[15].
There are limited data regarding the use of fully-covered and partially-covered SEMSs in the treatment of post-cholecystectomy strictures. These data are derived from a small subset of patients with post-cholecystectomy strictures included in SEMSs studies. For example, in a large, multicenter study of fully-covered SEMS (n = 187), 18 patients with post-cholecystectomy strictures (14 of which were previously treated with plastic biliary stents) underwent SEMS placement. After 10-12 mo of stenting, 13 patients (72%) experienced stricture resolution without need for immediate re-stenting. Two-thirds, however, experienced stent migration by 12 mo, and 6 patients (33%) experienced cholangitis, fever or pancreatitis[39]. Based on these findings, SEMSs cannot be routinely recommended for treatment of post-cholecystectomy strictures.
Post-LT: Among patients who have undergone LT, BBSs are among the most common post-operative complications, with their incidence ranging from 5% to 15% and 28% to 32% following deceased donor and living donor LT, respectively, and even higher rates in cardiac death donor LT[12,40,41]. Post-LT BBSs can manifest early (< 30-90 d) or late (> 90 d) in the post-LT course and may occur at the anastomosis (i.e., ABS) or elsewhere in the biliary tree (i.e., non-anastomotic biliary stricture, NABS). Endoscopic therapy is the first line management approach for ABSs and for select NABSs, with percutaneous intervention and surgical revision or redo-LT being reserved for endoscopic treatment failures. ABSs and NABSs are further discussed below.
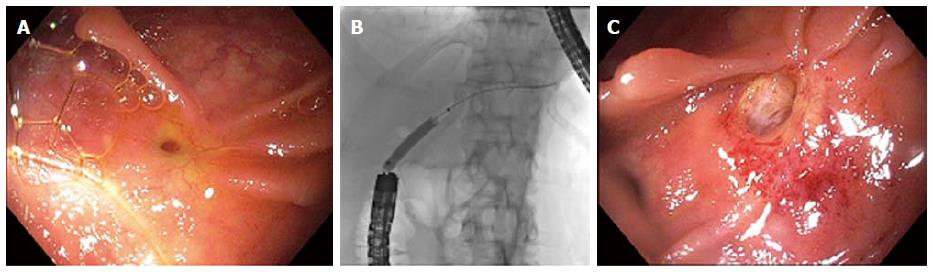
ABSs are a consequence of local trauma at the surgical juncture between the recipient’s and donor’s extrahepatic ducts (most commonly CBD-CBD choledocho-choledochostomy) and account for 80% of post-LT biliary strictures[42]. They appear as a short, single stricture localized to the anastomosis. Earlier presentations (< 30-90 d) generally respond well to endoscopic dilation (Figure 2) and a relatively brief period of plastic stenting (approximately 3 to 6 mo), whereas later presentations may require up to 1-2 years of stenting to avoid stricture recurrence based on the few available published series[42-44]. Unfortunately, most studies regarding management of ABSs are retrospective and heterogeneous (e.g., in stricture etiology, severity, and other variables), yet several have shown consistent long-term success rates of approaching 90% to 100% with balloon dilation and multiple or maximal plastic stent therapy[8,45-49]. ABSs may also be treated with SEMSs, but this has been less studied and seldom practiced for a variety of reasons[23-26]. For example, a multicenter trial of partially-covered SEMSs was associated with a modest long-term success rate of 53%, and removal of the stent was technically demanding in 6 out of 21 (29%) patients due to embedding of the bare ends[23]. Conversely, studies using fully-covered SEMSs have reported more promising success rates (ostensibly due to longer dwell times), ranging 92% to 100%, but with higher stent migration rates (as high as 24%)[24-26].
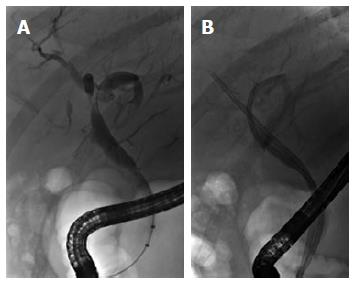
NABSs account for 10%-25% of post-LT biliary strictures[50,51] and are typically a sequela of donor-recipient ABO incompatibility, prolonged graft ischemic time peri-LT, or post-LT hepatic artery thrombosis[52]. NABSs are often referred to as ischemic strictures, although it should be noted that not all NABSs have a clearly ischemic etiology. In contrast to ABSs, NABSs may be either unifocal or distributed diffusely throughout the extra- and/or intrahepatic biliary tree (Figure 3), are more technically challenging to access and treat, and have lower long-term endoscopic treatment success rates (50% to 75%)[45,53]. Nevertheless, maximal stenting, as with ABS, may result in graft preservation and overall favorable outcomes in a considerable proportion of patients with NABSs[14,45,53-55], although some will ultimately require re-transplantation[10,45,56].
BBSs develop in approximately 25% of patients with chronic pancreatitis and represent a major clinical challenge[1]. These strictures occur in the distal CBD, and their refractory nature is largely attributable to robust periductal fibrosis secondary to the underlying chronic inflammatory process[57]. It is important to rule out underlying malignancy in this context, as it can have an initial presentation similar to BBSs and pancreatic cancer can occur in the setting of established chronic pancreatitis. With respect to treatment of chronic pancreatitis-associated BBS, biliary decompression is indicated in patients who are symptomatic (e.g., cholangitic, deeply jaundiced), and as with post-operative BBSs, insertion of multiple plastic stents with 3-4 exchanges over a year appears to offer the highest likelihood of long-term benefit. Studies range in overall success of endoscopic therapy from 44% to 92%, with lower rates among those with dystrophic calcification of the pancreatic head[15,58-60]. Surgical intervention (e.g., Puestow pancreaticojejunostomy, Traverso-Longmire pancreaticoduodenectomy[61]) is indicated in patients who fail endoscopic management and are fit for surgery[57,60].
A number of studies have investigated the role of fully as well as partially-covered SEMSs in chronic pancreatitis. Fully-covered SEMSs have demonstrated success rates ranging from 43% to 77% in patients with chronic pancreatitis-associated BBSs, but stent migration have historically been a common problem, as is the case with post-operative BBSs[21,27,62,63]. A recent, multicenter study of 118 patients with chronic pancreatitis-associated BBSs, however, found that fully-covered SEMS placement was associated with an 80% stricture resolution rate (median stent dwell time 11 mo) and a more acceptable stent migration rate (19% at 12 mo)[39]. Studies using fully-covered SEMSs with antimigratory modifications, or partially-covered SEMSs, have also reported encouraging stricture resolution rates (approximately 90%), and with even lower rates of stent migration[29,63,64].
Primary sclerosing cholangitis (PSC) is an idiopathic disorder characterized by periductal inflammation and fibrosis involving the intrahepatic and/or extrahepatic biliary tree. Up to 50% of patients with PSC will develop “dominant” strictures, which are loosely defined as a CBD stenosis of ≤ 1.5 mm in diameter or hepatic duct stenosis ≤ 1 mm in diameter, during their disease course[65,66]. A major challenge in the setting of a PSC-associated dominant stricture is excluding underlying malignancy (i.e., cholangiocarcinoma), which develops in up to 20% of patients with PSC[67-70]. At a minimum, brush cytology and/or intraductal biopsies, are required. If available, advanced cytologic and imaging methods should also be considered.
The overarching goal of endoscopic therapy in PSC-associated dominant BBSs is to improve signs, symptoms and sequelae of biliary obstruction; when performed appropriately (including both patient selection and procedural technique), endoscopic therapy can improve Mayo PSC risk score, which has been shown to translate into improved survival[68,71-74]. Biliary (balloon) dilation alone is the preferred therapeutic approach, as stenting has been shown to result in slightly higher rates of complications (i.e., stent occlusion and cholangitis) in some series[75,76]. Repeated dilation (i.e., multiple ERCP sessions) may be necessary in some patients to achieve maximal clinical benefit[77]. If dilation is unsuccessful (i.e., persistent stricture waist), short-term stentings with plastic biliary stents has been shown to be safe and effective with durable benefit[78]. Prophylactic antibiotics should also be administered periprocedurally to reduce the risk of ERCP-related cholangitis unless full biliary drainage is highly anticipated[79,80].
Biliary-enteric strictures can occur following pancreaticoduodenectomy (Whipple procedure), partial liver resection, and liver transplantation with Roux-en-Y hepaticojejunostomy in 12%-28% of patients[81,82]. Endoscopic therapy of these strictures was once felt to be impossible due to surgical alterations in intestinal anatomy that precluded access via conventional endoscopic methods. However, the use of colonoscopes and more recently, device-assisted enteroscopes (single, double, and short double balloon), combined with more widespread training of advanced endoscopists have brought these strictures within reach[83]. In patients post-standard Whipple, the hepaticojejunostomy is almost always reachable, whereas pylorus preserving Whipple, and choledocho- and hepaticojejunostomy Roux-en-Y render more challenging, but often still conquerable anatomy in the hands of an experienced endoscopist with balloon-enteroscopes. A recent meta-analysis included 15 studies and 461 patients with surgically altered pancreaticobiliary anatomy (Roux-en-Y bypass, Roux-en-Y reconstruction, and standard and pylorus preserving Whipple) undergoing single-balloon enteroscopy-assisted ERCP. The pooled enteroscopy, diagnostic, and procedural success rates were 81%, 69%, and 62%, though a high degree of heterogeneity was reported[84]. Limiting analysis to patients with Roux-en-Y reconstruction or Whipple yielded higher diagnostic and procedural success rates at 79% and 63% with much lower heterogeneity[85]. In a retrospective study of patients with biliary-enteric strictures following surgical repair of iatrogenic cholecystostomy injuries (n = 32), Lee et al[86] reported balloon dilation alone to be successful in 66% of patients with only 1 (5%) recurrence over a mean 13.1 years of follow-up.
An endoscopic approach can be limited by time, availability, and endoscopist expertise. When unsuccessful, percutaneous transhepatic access (with or without rendezvous techniques)[86], percutaneous drains, and surgical revision remain alternative therapeutic options.
Even in expert hands, attempts at therapeutic ERCP for BBSs may fail in 2% to 10% of cases due to inability to cannulate the bile duct (e.g., surgically altered anatomy, tumor infiltration) or traverse a tight bile duct stricture. In select cases, endoscopic ultrasound (EUS) may serve as ancillary therapeutic techniques prior to proceeding with options such as percutaneous or surgical intervention. EUS can be employed in a rendezvous technique that establishes transpapillary guidewire access, thereby allowing conventional ERCP with balloon dilation of a BBS followed by stent placement (if indicated).
EUS-guided biliary access and drainage can also be performed by needle puncture of the gastric wall and advancement into the left hepatic duct tributaries (i.e., hepaticogastrostomy)[87-90] or through the duodenal wall into the CBD (i.e., choledochoduodenostomy)[91,92]. Thereafter, drainage can be internalized through the papilla without requiring a rendezvous approach (although combination approaches can be useful as well)[93,94]. As alluded to before, this technique is particularly useful when biliary cannulation or access to the papilla cannot be achieved due to duodenal obstruction or other causes[95,96].
Adverse events related to endoscopic management of biliary strictures may occur secondary to stricture access or dilation, and stent placement or dwell time (early or late). Sphincterotomy can be associated with pancreatitis, luminal perforation, or bleeding, as seen in patients undergoing ERCP for other indications. Stricture dilation (particularly in the setting of a fresh surgical anastomosis) and stent deployment also run the risk of perforation. Stent-related adverse events include early or late migration, impaction or embedment (metal stents), or occlusion with the potential for cholangitis. Plastic stents therefore necessitate removal or exchange in 3 mo with concurrent removal of all stones and sludge.
Endoscopic therapy provides a minimally invasive, safe, and reliable first-line management option for most BBSs. An approach involving multiple plastic stent placement and intermittent stent exchanges works well in post-cholecystectomy strictures and ABSs, whereas other stricture types, such as NABSs and chronic pancreatitis-associated strictures, tend to be more challenging, with some patients ultimately requiring surgical intervention. The recent and rapid evolution of SEMSs may provide an alternative means to treat some BBSs while reducing the need for frequent ERCPs, but additional studies that better define their application, complications, and cost-effectiveness remain needed. Lastly, applications of therapeutic EUS for biliary disease are becoming increasingly recognized and implemented, and continued advancements in both ERCP and EUS are anticipated.
P- Reviewer: Albert JG, Deng B, Koivusalo A S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Baron TH, Davee T. Endoscopic management of benign bile duct strictures. Gastrointest Endosc Clin N Am. 2013;23:295-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Reynolds BM, Dargan EL. Acute obstructive cholangitis; a distinct clinical syndrome. Ann Surg. 1959;150:299-303. [PubMed] |
| 3. | Davids PH, Tanka AK, Rauws EA, van Gulik TM, van Leeuwen DJ, de Wit LT, Verbeek PC, Huibregtse K, van der Heyde MN, Tytgat GN. Benign biliary strictures. Surgery or endoscopy? Ann Surg. 1993;217:237-243. [PubMed] |
| 4. | McCune WS. ERCP at thirty years: an interview with Dr. William S. McCune (1909-1998). Gastrointest Endosc. 1998;48:643-644. [PubMed] |
| 5. | Tabibian JH, Macura SI, O’Hara SP, Fidler JL, Glockner JF, Takahashi N, Lowe VJ, Kemp BJ, Mishra PK, Tietz PS. Micro-computed tomography and nuclear magnetic resonance imaging for noninvasive, live-mouse cholangiography. Lab Invest. 2013;93:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Anderson MA, Ben-Menachem T, Gan SI, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 352] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 7. | Guido Costamagna IB, Pietro Familiari. Benign Biliary Strictures. Baron RAK, David L. Carr-Locke, editor ERCP. Philadelphia: Elsevier 2013; 383-388. |
| 8. | Tabibian JH, Asham EH, Han S, Saab S, Tong MJ, Goldstein L, Busuttil RW, Durazo FA. Endoscopic treatment of postorthotopic liver transplantation anastomotic biliary strictures with maximal stent therapy (with video). Gastrointest Endosc. 2010;71:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Zoepf T, Maldonado-Lopez EJ, Hilgard P, Malago M, Broelsch CE, Treichel U, Gerken G. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl. 2006;12:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Thuluvath PJ, Pfau PR, Kimmey MB, Ginsberg GG. Biliary complications after liver transplantation: the role of endoscopy. Endoscopy. 2005;37:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Tabibian JH, Yeh HC, Singh VK, Cengiz-Seval G, Cameron AM, Gurakar A. Sirolimus may be associated with early recurrence of biliary obstruction in liver transplant patients undergoing endoscopic stenting of biliary strictures. Ann Hepatol. 2011;10:270-276. [PubMed] |
| 12. | Tabibian JH, Girotra M, Yeh HC, Segev DL, Gulsen MT, Cengiz-Seval G, Singh VK, Cameron AM, Gurakar A. Sirolimus based immunosuppression is associated with need for early repeat therapeutic ERCP in liver transplant patients with anastomotic biliary stricture. Ann Hepatol. 2013;12:563-569. [PubMed] |
| 13. | Costamagna G, Boškoski I. Current treatment of benign biliary strictures. Ann Gastroenterol. 2013;26:37-40. [PubMed] |
| 14. | Tabibian JH, Asham EH, Goldstein L, Han SH, Saab S, Tong MJ, Busuttil RW, Durazo FA. Endoscopic treatment with multiple stents for post-liver-transplantation nonanastomotic biliary strictures. Gastrointest Endosc. 2009;69:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Draganov P, Hoffman B, Marsh W, Cotton P, Cunningham J. Long-term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest Endosc. 2002;55:680-686. [PubMed] |
| 16. | Costamagna G, Pandolfi M, Mutignani M, Spada C, Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54:162-168. [PubMed] |
| 17. | van Boeckel PG, Vleggaar FP, Siersema PD. Plastic or metal stents for benign extrahepatic biliary strictures: a systematic review. BMC Gastroenterol. 2009;9:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Bergman JJ, Burgemeister L, Bruno MJ, Rauws EA, Gouma DJ, Tytgat GN, Huibregtse K. Long-term follow-up after biliary stent placement for postoperative bile duct stenosis. Gastrointest Endosc. 2001;54:154-161. [PubMed] |
| 19. | Lawrence C, Romagnuolo J, Payne KM, Hawes RH, Cotton PB. Low symptomatic premature stent occlusion of multiple plastic stents for benign biliary strictures: comparing standard and prolonged stent change intervals. Gastrointest Endosc. 2010;72:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Kahaleh M, Tokar J, Le T, Yeaton P. Removal of self-expandable metallic Wallstents. Gastrointest Endosc. 2004;60:640-644. [PubMed] |
| 21. | Kahaleh M, Behm B, Clarke BW, Brock A, Shami VM, De La Rue SA, Sundaram V, Tokar J, Adams RB, Yeaton P. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video). Gastrointest Endosc. 2008;67:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Cantù P, Hookey LC, Morales A, Le Moine O, Devière J. The treatment of patients with symptomatic common bile duct stenosis secondary to chronic pancreatitis using partially covered metal stents: a pilot study. Endoscopy. 2005;37:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Chaput U, Scatton O, Bichard P, Ponchon T, Chryssostalis A, Gaudric M, Mangialavori L, Duchmann JC, Massault PP, Conti F. Temporary placement of partially covered self-expandable metal stents for anastomotic biliary strictures after liver transplantation: a prospective, multicenter study. Gastrointest Endosc. 2010;72:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | García-Pajares F, Sánchez-Antolín G, Pelayo SL, Gómez de la Cuesta S, Herranz Bachiller MT, Pérez-Miranda M, de La Serna C, Vallecillo Sande MA, Alcaide N, Llames RV. Covered metal stents for the treatment of biliary complications after orthotopic liver transplantation. Transplant Proc. 2010;42:2966-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Haapamäki C, Udd M, Halttunen J, Lindström O, Mäkisalo H, Kylänpää L. Endoscopic treatment of anastomotic biliary complications after liver transplantation using removable, covered, self-expandable metallic stents. Scand J Gastroenterol. 2012;47:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Hu B, Gao DJ, Yu FH, Wang TT, Pan YM, Yang XM. Endoscopic stenting for post-transplant biliary stricture: usefulness of a novel removable covered metal stent. J Hepatobiliary Pancreat Sci. 2011;18:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Mahajan A, Ho H, Sauer B, Phillips MS, Shami VM, Ellen K, Rehan M, Schmitt TM, Kahaleh M. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video). Gastrointest Endosc. 2009;70:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Moon JH, Choi HJ, Koo HC, Han SH, Lee TH, Cho YD, Park SH, Kim SJ. Feasibility of placing a modified fully covered self-expandable metal stent above the papilla to minimize stent-induced bile duct injury in patients with refractory benign biliary strictures (with videos). Gastrointest Endosc. 2012;75:1080-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Park do H, Lee SS, Lee TH, Ryu CH, Kim HJ, Seo DW, Park SH, Lee SK, Kim MH, Kim SJ. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos). Gastrointest Endosc. 2011;73:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Park JK, Moon JH, Choi HJ, Min SK, Lee TH, Cheon GJ, Cheon YK, Cho YD, Park SH, Kim SJ. Anchoring of a fully covered self-expandable metal stent with a 5F double-pigtail plastic stent to prevent migration in the management of benign biliary strictures. Am J Gastroenterol. 2011;106:1761-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Sauer P, Chahoud F, Gotthardt D, Stremmel W, Weiss KH, Büchler M, Schemmer P, Weitz J, Schaible A. Temporary placement of fully covered self-expandable metal stents in biliary complications after liver transplantation. Endoscopy. 2012;44:536-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Tarantino I, Mangiavillano B, Di Mitri R, Barresi L, Mocciaro F, Granata A, Masci E, Curcio G, Di Pisa M, Marino A. Fully covered self-expandable metallic stents in benign biliary strictures: a multicenter study on efficacy and safety. Endoscopy. 2012;44:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Traina M, Tarantino I, Barresi L, Volpes R, Gruttadauria S, Petridis I, Gridelli B. Efficacy and safety of fully covered self-expandable metallic stents in biliary complications after liver transplantation: a preliminary study. Liver Transpl. 2009;15:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Zepeda-Gómez S, Baron TH. Benign biliary strictures: current endoscopic management. Nat Rev Gastroenterol Hepatol. 2011;8:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Davidoff AM, Pappas TN, Murray EA, Hilleren DJ, Johnson RD, Baker ME, Newman GE, Cotton PB, Meyers WC. Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg. 1992;215:196-202. [PubMed] |
| 36. | Costamagna G, Tringali A, Mutignani M, Perri V, Spada C, Pandolfi M, Galasso D. Endotherapy of postoperative biliary strictures with multiple stents: results after more than 10 years of follow-up. Gastrointest Endosc. 2010;72:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Kuzela L, Oltman M, Sutka J, Hrcka R, Novotna T, Vavrecka A. Prospective follow-up of patients with bile duct strictures secondary to laparoscopic cholecystectomy, treated endoscopically with multiple stents. Hepatogastroenterology. 2005;52:1357-1361. [PubMed] |
| 38. | Dumonceau JM, Tringali A, Blero D, Devière J, Laugiers R, Heresbach D, Costamagna G. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44:277-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 39. | Devière J, Nageshwar Reddy D, Püspök A, Ponchon T, Bruno MJ, Bourke MJ, Neuhaus H, Roy A, González-Huix Lladó F, Barkun AN. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology. 2014;147:385-395; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 40. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 41. | Maheshwari A, Maley W, Li Z, Thuluvath PJ. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Thethy S, Thomson BNj, Pleass H, Wigmore SJ, Madhavan K, Akyol M, Forsythe JL, James Garden O. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Verdonk RC, Buis CI, Porte RJ, van der Jagt EJ, Limburg AJ, van den Berg AP, Slooff MJ, Peeters PM, de Jong KP, Kleibeuker JH. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl. 2006;12:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 44. | Alazmi WM, Fogel EL, Watkins JL, McHenry L, Tector JA, Fridell J, Mosler P, Sherman S, Lehman GA. Recurrence rate of anastomotic biliary strictures in patients who have had previous successful endoscopic therapy for anastomotic narrowing after orthotopic liver transplantation. Endoscopy. 2006;38:571-574. [PubMed] |
| 45. | Graziadei IW, Schwaighofer H, Koch R, Nachbaur K, Koenigsrainer A, Margreiter R, Vogel W. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl. 2006;12:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Holt AP, Thorburn D, Mirza D, Gunson B, Wong T, Haydon G. A prospective study of standardized nonsurgical therapy in the management of biliary anastomotic strictures complicating liver transplantation. Transplantation. 2007;84:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Kulaksiz H, Weiss KH, Gotthardt D, Adler G, Stremmel W, Schaible A, Dogan A, Stiehl A, Sauer P. Is stenting necessary after balloon dilation of post-transplantation biliary strictures? Results of a prospective comparative study. Endoscopy. 2008;40:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Morelli J, Mulcahy HE, Willner IR, Cunningham JT, Draganov P. Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest Endosc. 2003;58:374-379. [PubMed] |
| 49. | Pasha SF, Harrison ME, Das A, Nguyen CC, Vargas HE, Balan V, Byrne TJ, Douglas DD, Mulligan DC. Endoscopic treatment of anastomotic biliary strictures after deceased donor liver transplantation: outcomes after maximal stent therapy. Gastrointest Endosc. 2007;66:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Pfau PR, Kochman ML, Lewis JD, Long WB, Lucey MR, Olthoff K, Shaked A, Ginsberg GG. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Koneru B, Sterling MJ, Bahramipour PF. Bile duct strictures after liver transplantation: a changing landscape of the Achilles’ heel. Liver Transpl. 2006;12:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 53. | Rizk RS, McVicar JP, Emond MJ, Rohrmann CA, Kowdley KV, Perkins J, Carithers RL, Kimmey MB. Endoscopic management of biliary strictures in liver transplant recipients: effect on patient and graft survival. Gastrointest Endosc. 1998;47:128-135. [PubMed] |
| 54. | Rerknimitr R, Sherman S, Fogel EL, Kalayci C, Lumeng L, Chalasani N, Kwo P, Lehman GA. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc. 2002;55:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 225] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 55. | Zoepf T, Maldonado de Dechêne EJ, Dechêne A, Malágo M, Beckebaum S, Paul A, Gerken G, Hilgard P. Optimized endoscopic treatment of ischemic-type biliary lesions after liver transplantation. Gastrointest Endosc. 2012;76:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Pascher A, Neuhaus P. Biliary complications after deceased-donor orthotopic liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Familiari P, Boškoski I, Bove V, Costamagna G. ERCP for biliary strictures associated with chronic pancreatitis. Gastrointest Endosc Clin N Am. 2013;23:833-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Catalano MF, Linder JD, George S, Alcocer E, Geenen JE. Treatment of symptomatic distal common bile duct stenosis secondary to chronic pancreatitis: comparison of single vs. multiple simultaneous stents. Gastrointest Endosc. 2004;60:945-952. [PubMed] |
| 59. | Pozsár J, Sahin P, László F, Forró G, Topa L. Medium-term results of endoscopic treatment of common bile duct strictures in chronic calcifying pancreatitis with increasing numbers of stents. J Clin Gastroenterol. 2004;38:118-123. [PubMed] |
| 60. | Regimbeau JM, Fuks D, Bartoli E, Fumery M, Hanes A, Yzet T, Delcenserie R. A comparative study of surgery and endoscopy for the treatment of bile duct stricture in patients with chronic pancreatitis. Surg Endosc. 2012;26:2902-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Traverso LW, Longmire WP. Preservation of the pylorus in pancreaticoduodenectomy a follow-up evaluation. Ann Surg. 1980;192:306-310. [PubMed] |
| 62. | Cahen DL, Rauws EA, Gouma DJ, Fockens P, Bruno MJ. Removable fully covered self-expandable metal stents in the treatment of common bile duct strictures due to chronic pancreatitis: a case series. Endoscopy. 2008;40:697-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Perri V, Boškoski I, Tringali A, Familiari P, Mutignani M, Marmo R, Costamagna G. Fully covered self-expandable metal stents in biliary strictures caused by chronic pancreatitis not responding to plastic stenting: a prospective study with 2 years of follow-up. Gastrointest Endosc. 2012;75:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Behm B, Brock A, Clarke BW, Ellen K, Northup PG, Dumonceau JM, Kahaleh M. Partially covered self-expandable metallic stents for benign biliary strictures due to chronic pancreatitis. Endoscopy. 2009;41:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 65. | Stiehl A, Rudolph G, Klöters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151-156. [PubMed] |
| 66. | Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 67. | Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, Fleming TR, Fisher LD, Beaver SJ, LaRusso NF. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 68. | O’Hara SP, Gradilone SA, Masyuk TV, Tabibian JH, LaRusso NF. MicroRNAs in Cholangiopathies. Curr Pathobiol Rep. 2014;2:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Tabibian JH, Enders F, Imam MH, Kolar G, Lindor KD, Talwalkar JA. Association between serum IgE level and adverse clinical endpoints in primary sclerosing cholangitis. Ann Hepatol. 2014;13:384-389. [PubMed] |
| 70. | Boberg KM, Bergquist A, Mitchell S, Pares A, Rosina F, Broomé U, Chapman R, Fausa O, Egeland T, Rocca G. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 71. | Baluyut AR, Sherman S, Lehman GA, Hoen H, Chalasani N. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointest Endosc. 2001;53:308-312. [PubMed] |
| 72. | Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 73. | Gluck M, Cantone NR, Brandabur JJ, Patterson DJ, Bredfeldt JE, Kozarek RA. A twenty-year experience with endoscopic therapy for symptomatic primary sclerosing cholangitis. J Clin Gastroenterol. 2008;42:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 74. | Stiehl A, Rudolph G, Sauer P, Benz C, Stremmel W, Walker S, Theilmann L. Efficacy of ursodeoxycholic acid treatment and endoscopic dilation of major duct stenoses in primary sclerosing cholangitis. An 8-year prospective study. J Hepatol. 1997;26:560-566. [PubMed] |
| 75. | Linder S, Söderlund C. Endoscopic therapy in primary sclerosing cholangitis: outcome of treatment and risk of cancer. Hepatogastroenterology. 2001;48:387-392. [PubMed] |
| 76. | Kaya M, Petersen BT, Angulo P, Baron TH, Andrews JC, Gostout CJ, Lindor KD. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Chan CH, Telford JJ. Endoscopic management of benign biliary strictures. Gastrointest Endosc Clin N Am. 2012;22:511-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Ponsioen CY, Lam K, van Milligen de Wit AW, Huibregtse K, Tytgat GN. Four years experience with short term stenting in primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:2403-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 79. | Bangarulingam SY, Gossard AA, Petersen BT, Ott BJ, Lindor KD. Complications of endoscopic retrograde cholangiopancreatography in primary sclerosing cholangitis. Am J Gastroenterol. 2009;104:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 80. | Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (2)] |
| 81. | Saidi RF, Elias N, Ko DS, Kawai T, Markmann J, Cosimi AB, Hertl M. Biliary reconstruction and complications after living-donor liver transplantation. HPB (Oxford). 2009;11:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Icoz G, Kilic M, Zeytunlu M, Celebi A, Ersoz G, Killi R, Memis A, Karasu Z, Yuzer Y, Tokat Y. Biliary reconstructions and complications encountered in 50 consecutive right-lobe living donor liver transplantations. Liver Transpl. 2003;9:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Azeem N, Tabibian JH, Baron TH, Orhurhu V, Rosen CB, Petersen BT, Gostout CJ, Topazian MD, Levy MJ. Use of a single-balloon enteroscope compared with variable-stiffness colonoscopes for endoscopic retrograde cholangiography in liver transplant patients with Roux-en-Y biliary anastomosis. Gastrointest Endosc. 2013;77:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Inamdar S, Slattery E, Sejpal DV, Miller LS, Pleskow DK, Berzin TM, Trindade AJ. Systematic review and meta-analysis of single-balloon enteroscopy-assisted ERCP in patients with surgically altered GI anatomy. Gastrointest Endosc. 2015;82:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 85. | Abu Dayyeh B. Single-balloon enteroscopy-assisted ERCP in patients with surgically altered GI anatomy: getting there. Gastrointest Endosc. 2015;82:20-23. [PubMed] |
| 86. | Lee AY, Gregorius J, Kerlan RK, Gordon RL, Fidelman N. Percutaneous transhepatic balloon dilation of biliary-enteric anastomotic strictures after surgical repair of iatrogenic bile duct injuries. PLoS One. 2012;7:e46478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Shami VM, Kahaleh M. Endoscopic ultrasonography (EUS)-guided access and therapy of pancreatico-biliary disorders: EUS-guided cholangio and pancreatic drainage. Gastrointest Endosc Clin N Am. 2007;17:581-593, vii-viii. [PubMed] |
| 88. | Ang TL, Teo EK, Fock KM. EUS-guided transduodenal biliary drainage in unresectable pancreatic cancer with obstructive jaundice. JOP. 2007;8:438-443. [PubMed] |
| 89. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-59. [PubMed] |
| 90. | Park do H. Endoscopic ultrasonography-guided hepaticogastrostomy. Gastrointest Endosc Clin N Am. 2012;22:271-80, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Perez-Miranda M, Barclay RL, Kahaleh M. Endoscopic ultrasonography-guided endoscopic retrograde cholangiopancreatography: endosonographic cholangiopancreatography. Gastrointest Endosc Clin N Am. 2012;22:491-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Yamao K, Bhatia V, Mizuno N, Sawaki A, Ishikawa H, Tajika M, Hoki N, Shimizu Y, Ashida R, Fukami N. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy. 2008;40:340-342. [PubMed] |
| 93. | Kahaleh M, Artifon EL, Perez-Miranda M, Gupta K, Itoi T, Binmoeller KF, Giovannini M. Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013;19:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | Gupta K, Perez-Miranda M, Kahaleh M, Artifon EL, Itoi T, Freeman ML, de-Serna C, Sauer B, Giovannini M. Endoscopic ultrasound-assisted bile duct access and drainage: multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. 2014;48:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 95. | Khashab MA, Fujii LL, Baron TH, Canto MI, Gostout CJ, Petersen BT, Okolo PI, Topazian MD, Levy MJ. EUS-guided biliary drainage for patients with malignant biliary obstruction with an indwelling duodenal stent (with videos). Gastrointest Endosc. 2012;76:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 96. | Sarkaria S, Sundararajan S, Kahaleh M. Endoscopic ultrasonographic access and drainage of the common bile duct. Gastrointest Endosc Clin N Am. 2013;23:435-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Somogyi L, Chuttani R, Croffie J, Disario J, Liu J, Mishkin D, Shah R, Tierney W, Wong Kee Song LM, Petersen BT. Guidewires for use in GI endoscopy. Gastrointest Endosc. 2007;65:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |