Published online Sep 16, 2012. doi: 10.4253/wjge.v4.i9.387
Revised: June 14, 2012
Accepted: September 12, 2012
Published online: September 16, 2012
Narrow band imaging (NBI) is a new image enhancement system employing optic digital methods to enhance images of blood vessels on mucosal surfaces, allowing improved visualization of mucosal surface structures. Studies have progressed over the last several years, and the clinical usefulness has been demonstrated. NBI has become frequently applied for preoperative diagnosis before endoscopic submucosal dissection (ESD) of digestive tract cancers, as well as for assessment of the range of ESD for en-bloc resection of large lesions. Consensus has been reached with regard to the usefulness of NBI for detecting micro-lesions of esophageal squamous cell carcinoma indicated for ESD, for the diagnosis of the range and depth. NBI has also been attracting attention for diagnosing gastric cancer based on the observation of micro blood vessels on the mucosal surface and mucosal surface microstructures. The usefulness of NBI has been reported in relation to various aspects of colon cancer, including diagnoses of the presence, quality, range, and depth of lesions. However, as NBI has not surpassed diagnostic methods based on magnifying observation combined with the established and widely employed dye method, its role in ESD is limited at present. Although NBI is very useful for the diagnosis of digestive tract cancers, comprehensive endoscopic diagnosis employing the combination of conventional endoscopy including dye spraying, EUS, and NBI may be important and essential for ESD.
- Citation: Nonaka K, Nishimura M, Kita H. Role of narrow band imaging in endoscopic submucosal dissection. World J Gastrointest Endosc 2012; 4(9): 387-397
- URL: https://www.wjgnet.com/1948-5190/full/v4/i9/387.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i9.387
Endoscopic submucosal dissection (ESD) was first reported in the late 1990s in Japan as a treatment for early-stage gastric cancer, and it has rapidly spread, surpassing conventional endoscopic mucosal resection (EMR)[1-4]. The advantage of ESD it that en-bloc resection employing ESD is possible even if the lesion is large and pathological evaluation of en-bloc resected specimen is accurate. Therefore, the preoperative diagnosis of not only cancer but also its range is very important before ESD.
Recently, diagnoses based on the observation of micro blood vessels on the mucosal surface and mucosal microstructures by narrow band imaging (NBI) have rapidly become common. Previous studies reported that this procedure was useful for the diagnosis of tumors of the digestive tract, suggesting its usefulness in screening, the differentiation between epithelial and non-epithelial tumors, differentiation between benign and malignant tumors, and evaluation of the infiltration of digestive tract cancer[5-10].
The role of NBI in ESD has become increasingly important, and NBI has been employed for not only detecting early neoplastic lesions indicated for treatment and the differentiation of benign and malignant lesions, but also for diagnosis of the range of lesions.
In this report, the usefulness of NBI for ESD and current problems are described by digestive organ (esophagus, stomach, and colon).
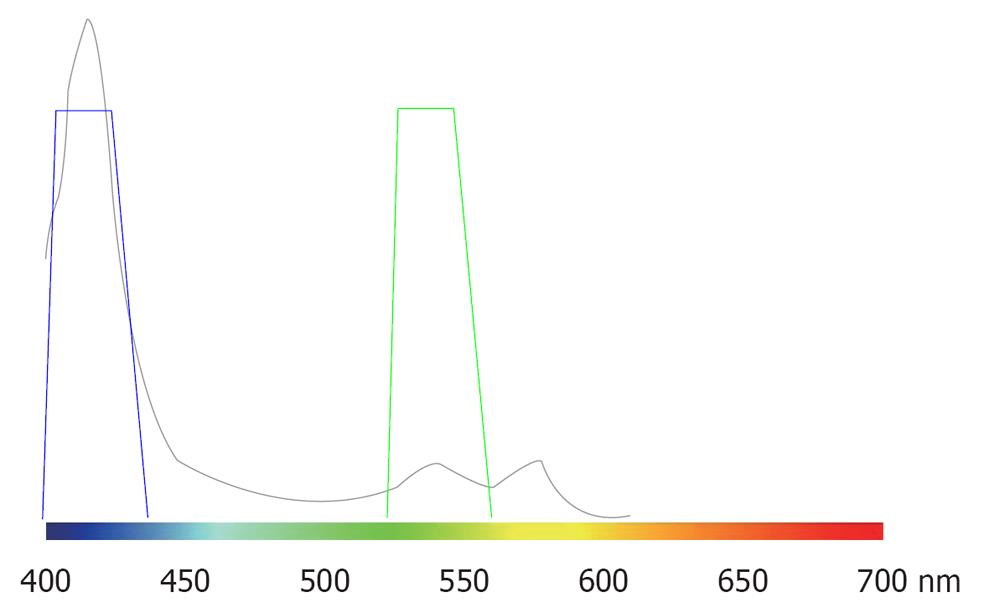
In vivo, pigments absorb a specific wavelength of visible light. Under an endoscope, a pigmental protein contained in blood, hemoglobin, can be observed. Figure 1 shows the absorption characteristics of hemoglobin. Strong peaks are noted at approximately 415 and 540 nm, suggesting that red light with a long wavelength is not absorbed. Therefore, to display capillaries in the superficial mucosal layer at a high contrast, blue and green light at 415 and 540 nm respectively, should be employed for illumination.
NBI is an image enhancement technology using the optical digital method developed by Olympus Co. Light is employed with central wavelengths of 415 and 540 nm, the same as those strongly absorbed by blood and strongly reflected and scattered by the mucosa. The spectrum widths are changed to narrow bands, through which images of micro blood vessels on the mucosal surface and mucosal micro patterns are enhanced at a higher contrast than those observed under white light[11].
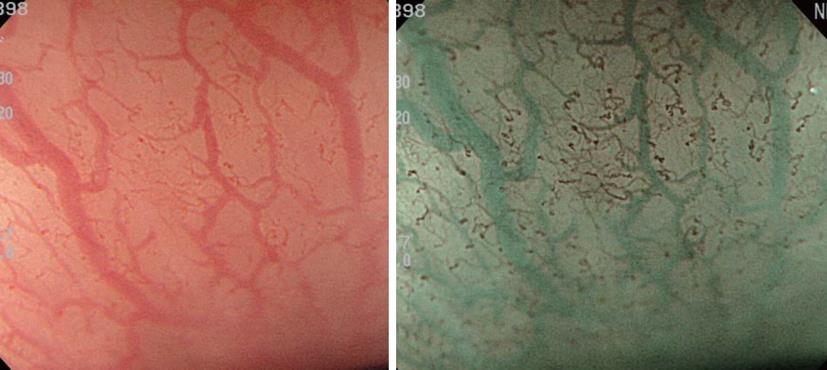
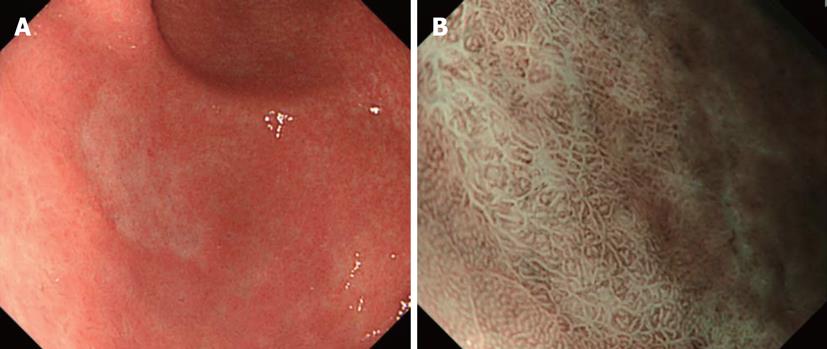
Typical images are shown in Figure 2. On standard endoscopy, fine blood vessels in the superficial layer and thick blood vessels in the deep layer are visualized as red areas. In addition, NBI facilitates the observation of capillaries in the superficial layer, which cannot be observed on standard endoscopy. Furthermore, it is possible to identify blood vessels at different depths by differences in the color tone. As a result, NBI may improve the visualization of capillaries in the superficial mucosal layer, which is important for the early diagnosis of cancer. In addition, the absorption/diffraction of narrow band light with a central wavelength of 415 nm in the stomach and colon, as well as the visualization of fine structures on the mucosal surface as a white pattern in contrast to enhanced capillaries, are important. Microvascular features may be a useful independent factor in cancer diagnosis.
In the treatment of esophageal squamous cell carcinomas (ESCCs), early detection in the phase where endoscopic treatment, less invasive than surgery, is possible, is essential to improving the prognosis.
Noninvasive carcinoma (carcinoma in situ, m1; EP) and intramucosal invasive carcinoma limited to the lamina propria mucosae (m2; LPM) without vessel infiltration, have proved to have no lymph node or distant metastases. This has been shown by a large number of retrospective histopathological analyses of surgically resected esophageal squamous cell neoplasms (SCNs) and, as a result, endoscopic therapy may be considered as a treatment option for these lesions. EMR and ESD have been accepted widely for localized SCNs as an alternative to surgical therapy, especially in Japan, because of the considerable rates of surgical mortality and postsurgical complications related to esophagectomy (range 2.1% to 13.7%) that result in poor patient quality-of-life[11-13].
There is a considerable increase in the number of esophageal SCNs indicated for local treatment thanks to recent developments in endoscopy, including magnifying endoscopy by using NBI.
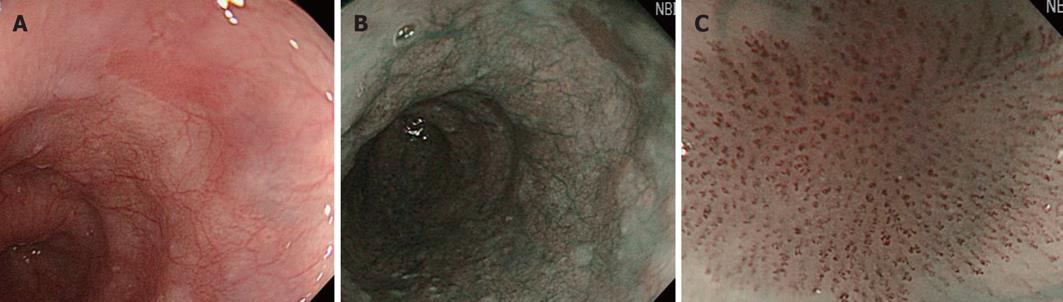
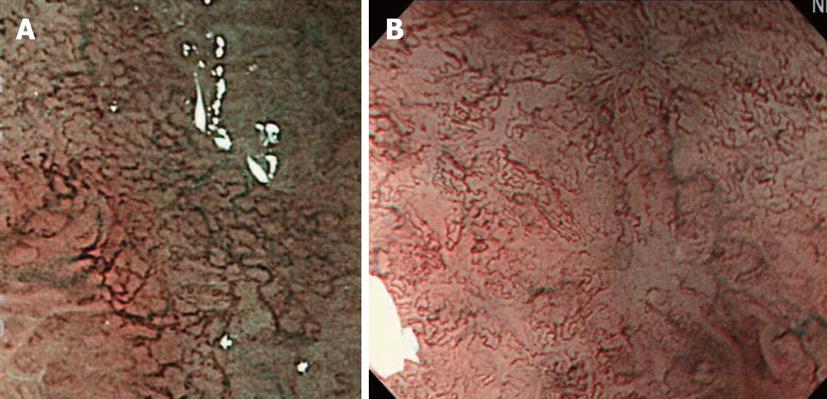
The superficial esophageal vascular network consists of thick blood vessels in the submucosal layer, a branch-like vascular network existing immediately above the mucosal myotome, and the intra-epithelial papillary capillary loop (IPCL) vertical to the branch-like vascular network. On standard endoscopy, branch-like or larger blood vessels can be observed. When performing magnifying observation using NBI, which facilitates the visualization of hemoglobin, i.e., blood vessels, the IPCL may be visualized, as shown in Figure 2. Inoue et al[14] reported that changes in the IPCL pattern were very useful for the qualitative diagnosis of cancer/non-cancerous lesions and evaluation of the degree of infiltration. NBI is advantageous for IPCL-pattern recognition (Figure 3).
A basic diagnostic method for ESCCs is to observe the mucosa under a standard endoscope and find flare points/surfaces. When finding them, this method should be switched to NBI observation by pressing a switch, facilitating the visualization of the lesion site as a brownish area. Under magnifying observation, the qualitative diagnosis of cancer/non-cancerous lesions and evaluation of the degree of infiltration are performed based on the IPCL pattern. Currently, microcarcinoma can be relatively readily detected by making a diagnosis in this way (Figure 3).
Muto et al[5] compared conventional endoscopic white light imaging (WLI) with NBI in high-risk patients in a prospective, randomized study of the capacity to diagnose ESCCs, and reported that NBI was more useful for the early detection of superficial esophageal cancer.
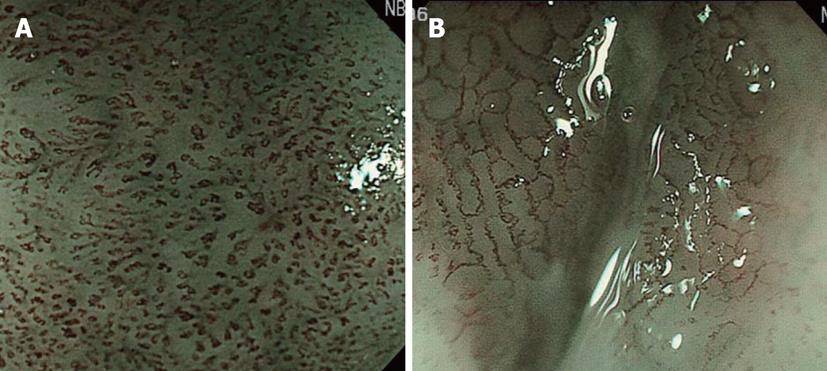
Before the development of NBI, the preoperative depth of lesions indicated for ESD, i.e., lesions without lymph node metastasis at a depth up to that of intramucosal invasive carcinoma limited to the lamina propria mucosae (m2; LPM), was comprehensively diagnosed based on conventional observation under white light, EUS, and X-ray. However, it was difficult to reliably diagnose M2 and M3 prior to ESD. The development of NBI facilitated the visual observation of IPCL, revolutionizing the diagnosis of depth (Figure 4). However, it is necessary to demonstrate improved diagnosis of depth by adding NBI to the current diagnostic tools, and the further accumulation and investigation of cases is expected.
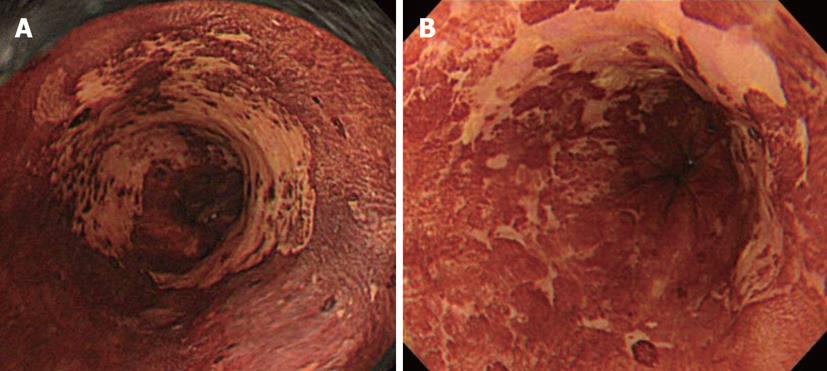
For ESD, the range of superficial esophageal SCNs is diagnosed by Lugol spraying, and its usefulness remains unchanged. However, this method causes unpleasant adverse effects such as severe chest pain and discomfort, and attention must be paid to possible patient allergy to iodine. Moreover, not only normal but also cancerous epithelium is partially desquamated by Lugol spraying because of its strong irritability. When Lugol is sprayed immediately before treatment, the size and morphology of regions not stained on the previous examination may be markedly changed and it should, therefore, be applied carefully (Figure 5). We require at least a 4-wk interval after the final Lugol spraying before ESD. When diagnosis of range is necessary immediately before ESD, we confirm the brownish area in NBI as the lesion. NBI can be introduced by pressing a switch, and it is very advantageous that this procedure is non-invasive.
NBI for esophageal superficial squamous cell carcinoma is the most advanced field, and consensus concerning the usefulness of NBI has been reached with regard to the detection of lesions and diagnoses of the quality, range, and depth.
Barrett’s esophageal adenocarcinoma, which is frequent in Europe and the United States, is a type of adenocarcinoma. Therefore, the diagnostic method for this carcinoma differs from that for squamous cell carcinoma of the esophagus. On NBI, there are no characteristic findings. A diagnosis of Barrett’s esophagus and Barrett’s esophageal adenocarcinoma is made based on the fine mucosal structure and microvascular features, as described below in relation to the stomach. In patients with Barrett’s esophagus, the risk of adenocarcinoma is 30 to 125 times higher than in the general population[15,16]. Therefore, close follow-up is needed. However, a recent meta-analysis showed that chromoendoscopy was less useful than random biopsy[17]. For the regular surveillance of Barrett’s esophagus, a large number of random biopsies are still always performed.
In 2010, Mannath reporting the results of a meta-analysis[18], found that the sensitivity and specificity of NBI for the diagnosis of high-grade dysplasia were high in patients with Barrett’s esophagus, suggesting its usefulness. In future, spot biopsy with NBI-combined magnifying observation at sites where high-grade dysplasia is suspected may decrease the number of unnecessary biopsies. This procedure may also be useful for diagnosis on endoscopic treatment for early neoplastic lesions.
In contrast to the esophagus and colon, NBI is not ideal for screening in the stomach due to the presence of mucus/regurgitated bile and wide cavity-related light volume insufficiency. Even when examining normal mucosa, NBI findings differ between sites. In addition, chronic food-associated stimuli and H. pylori-related gastritis further complicate the understanding of magnifying NBI findings and diagnosis. However, with recent advances in research on magnifying NBI of the stomach, many studies have reported that NBI-combined magnifying observation is useful for differentiating malignant from benign epithelial tumors, evaluating the extent of gastric cancer, and predicting the histological type of gastric cancer[9,19-21]. NBI-combined magnifying endoscopy of the gastric mucosa facilitates the visualization of various microvessels below the mucosal epithelium and fine structures on the mucosal surface. For magnifying NBI-guided assessment of the stomach, it is necessary to analyze microvascular features and fine mucosal structures separately and make a diagnosis based on these findings.
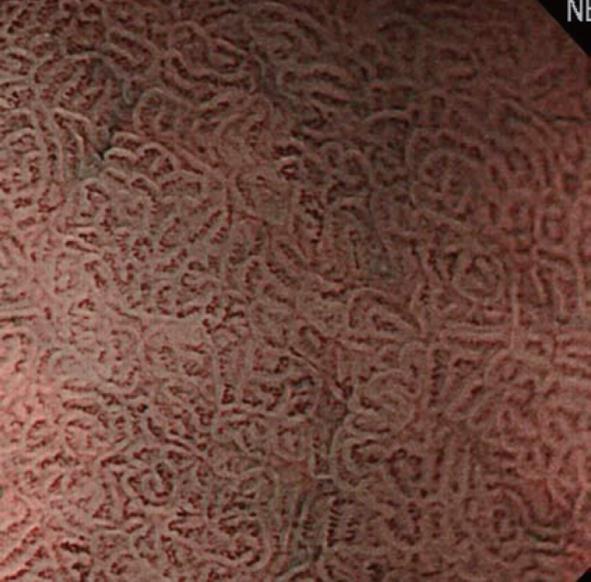
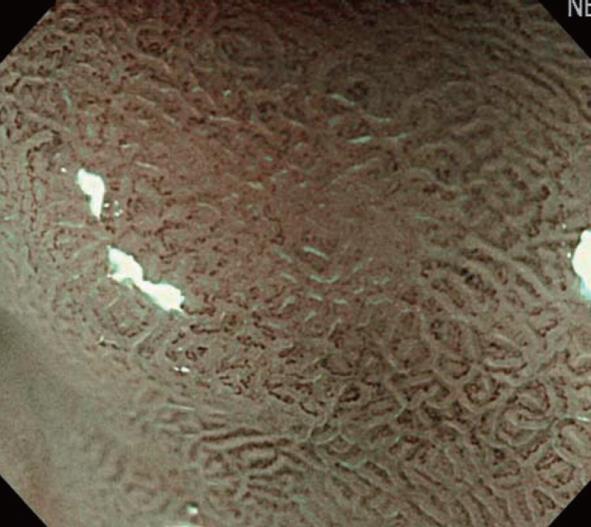
Initially, we will explain the microsurface pattern. On NBI-combined magnifying endoscopy, the marginal crypt epithelium (MCE) is visualized as a white line, as shown in Figure 6. When the morphology of the white line is similar to that of the peripheral mucosa, the microsurface pattern is regarded as “regular”. When the morphology, width, and arrangement are irregular in comparison with the peripheral mucosa, the microsurface pattern is regarded as “irregular”. When the white line shows a tendency toward disappearance or completely disappears, the microsurface pattern is regarded as showing “disappearance tendency or disappearance”. Many patients with an irregular pattern have cancer. However, it is often difficult to differentiate cancer from gastritis. In those with “disappearance tendency or disappearance”, undifferentiated carcinoma involving the glandular cervical region and atrophic gastritis with mucosal atrophy must be considered. Therefore, it is important to make a diagnosis based on comprehensive findings including microvascular features.
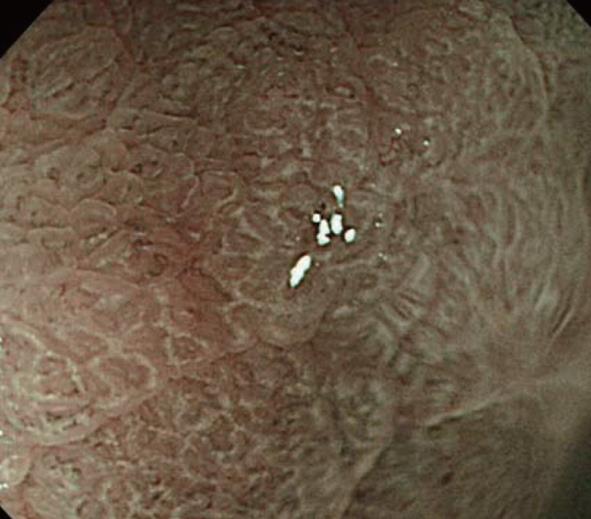
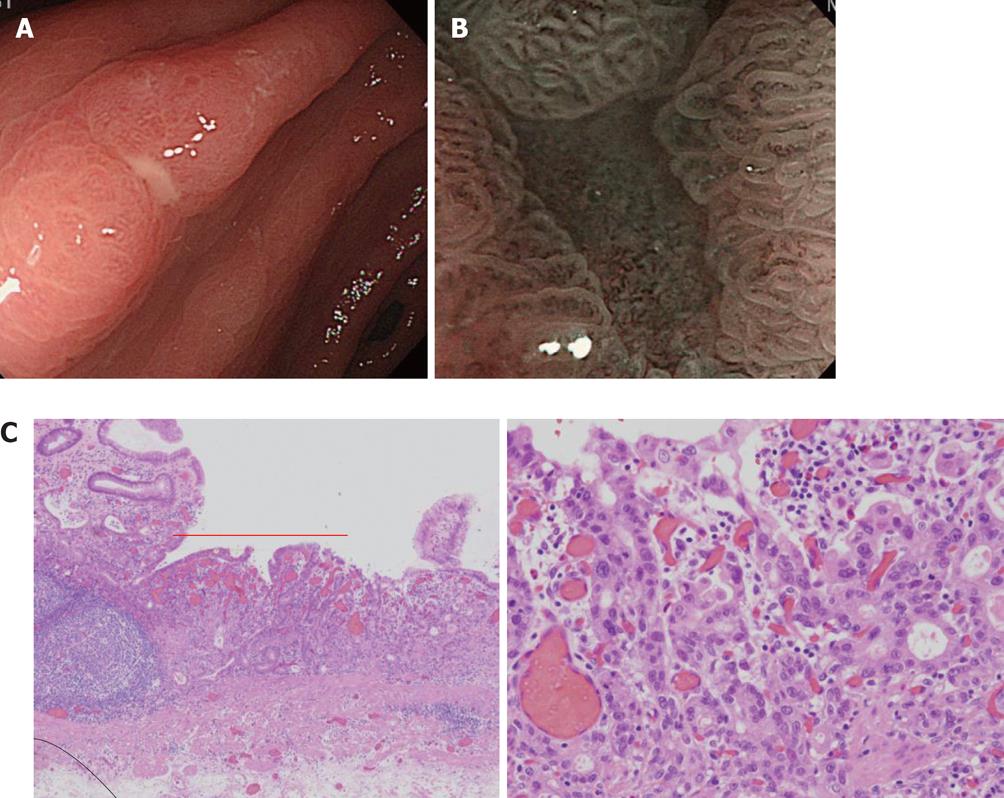
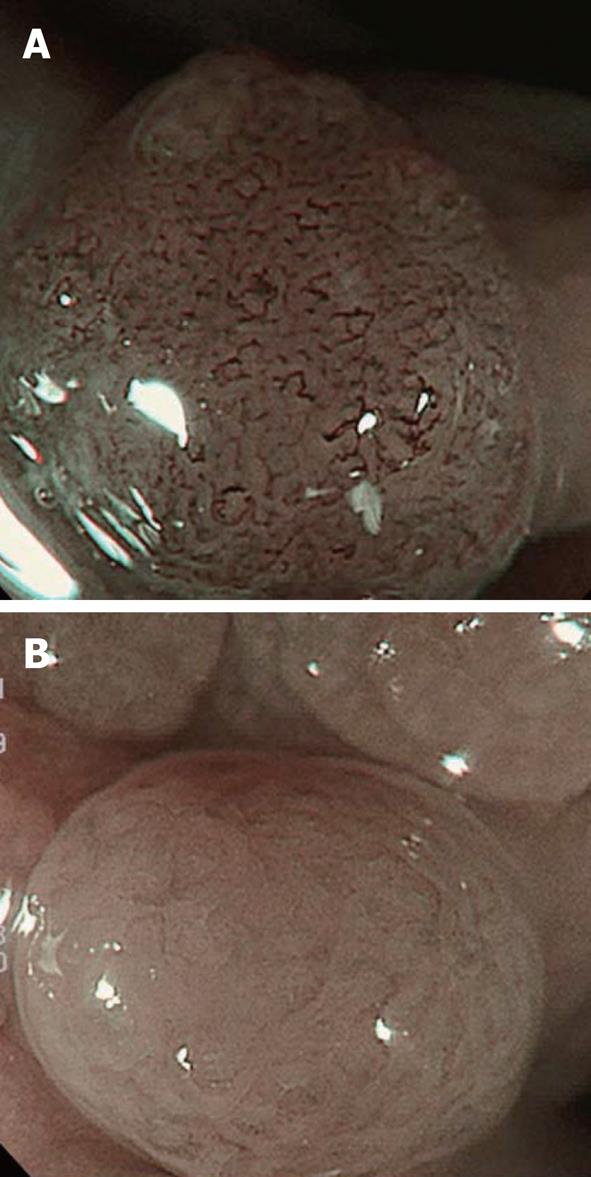
In addition, a white opaque substance (WOS) and light blue crest (LBC) were reported by Yao et al[9] and Uedo et al[22], respectively, as indices of fine mucosal structures. WOS (Figure 7) is a white substance existing in the superficial mucosal layer. Its presence reduces mucosal permeability, making the visualization of microvessels immediately below the epithelium difficult. The characteristics of this substance remain to be clarified. WOS appears in the presence of gastric adenoma or cancer, in most cases. In particular, its appearance is more frequent in patients with gastric adenoma and previous studies suggested its usefulness for the diagnosis of this condition[9,23]. WOS is visualized on white light or NBI-combined magnifying observation. However, it is more clearly visualized on NBI-combined magnifying observation. LBC (Figure 8) is a specific phenomenon visualized only on NBI. When performing NBI-combined magnifying endoscopy of the gastric mucosa in patients with Helicobacter pylori-related chronic gastritis, light blue, linear reflex light is sometimes observed at the epithelial margin. This is termed LBC and is reportedly useful for the objective, endoscopic diagnosis of intestinal metaplasia of the gastric mucosa[22].
Next, we will explain the microvascular pattern. When conducting NBI-combined magnifying endoscopy of the gastric mucosa in a physiological state, microvessels such as subepithelial capillaries and collecting venules can be observed. In a morbid state, blood vessels appearing in the healing process of inflammations such as ulcers, as well as tumor microvessels, can be observed. In particular, it is necessary to recognize, by NBI-combined magnifying observation, the presence or absence of abnormal blood vessels appearing in tumors and make a diagnosis based on comprehensive findings including fine mucosal structures. However, the absence of visualized microvascular features must also be regarded as a significant finding.
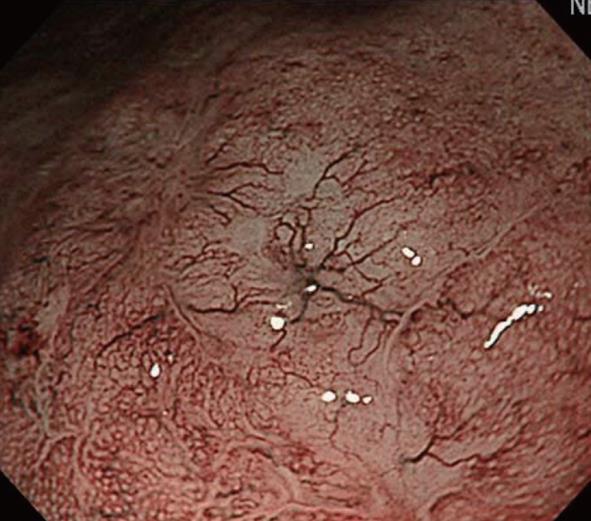
Next, we will describe abnormal microvessels. Nakayoshi et al[21] reported that, on NBI-combined magnifying endoscopy in gastric cancer patients, microvessels could be classified into 2 patterns: fine network and corkscrew patterns (Figure 9). They indicated that 68.4% of differentiated adenocarcinoma patients showed the fine network pattern, whereas 85.3% of undifferentiated carcinoma patients showed the corkscrew pattern. Based on this, it became possible to predict the histological type of gastric cancer to some extent by employing NBI-combined magnifying endoscopy in the absence of biopsy. Currently, although this procedure is utilized in clinical practice, it is an auxiliary diagnostic method and biopsy is still the gold standard.
In Japan, ESD for gastric cancer is generally indicated for differentiated adenocarcinoma lesions in the mucosa, and caution is necessary when deciding a therapeutic policy when it is applied for the undifferentiated type. At present, for the undifferentiated type of cancer, ESD is performed for 2-cm or smaller undifferentiated intramucosal carcinoma without lymph node metastasis and ulcerative changes as an extended indication at a limited number of facilities for patients who give consent. For lesions suspected to be a mixture of differentiated and undifferentiated types, it is necessary to perform NBI-combined magnifying observation, biopsy of both types of lesions, and thereafter decide on a therapeutic policy based on the findings.
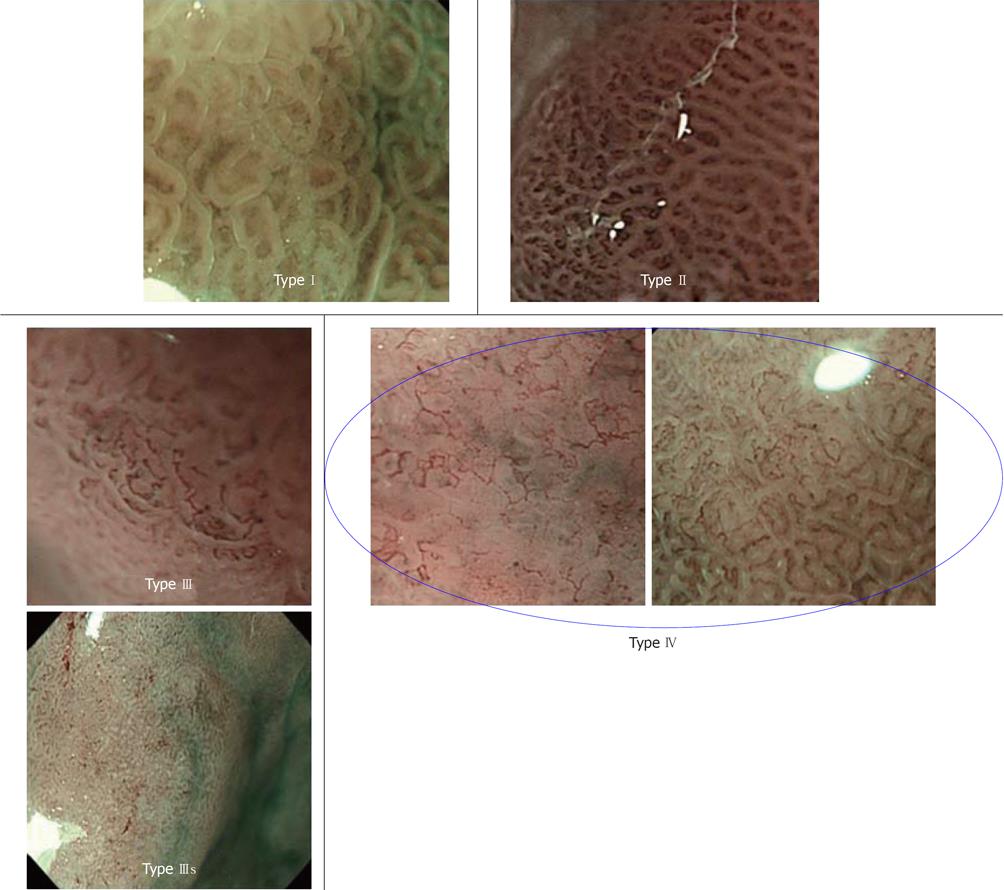
We will briefly explain microsurface and microvascular pattern-based diagnosis. We performed NBI-combined magnifying endoscopy on protruding lesions in which differentiation between gastric adenoma and differentiated adenocarcinoma was necessary, and reported that the two lesions could be simply and accurately differentiated by classifying them into 4 types, as shown in Figure 10. According to our classification, 79% of type I/II patients had gastric adenoma, and 93% of type III/IV patients had differentiated adenocarcinoma[23]. Gastric adenoma is a benign tumor, and the selection of course observation is not problematic in most cases. When no changes are observed in the morphology or size on periodic endoscopy of the upper digestive tract, and adenoma is diagnosed by biopsy, course observation may normally be acceptable. However, high-risk cases, such as patients who are under oral anticoagulant treatment and require heparin substitution only for biopsy, have been increasing in the currently aging society. If gastric adenoma and differentiated adenocarcinoma can be distinguished by endoscopy alone without biopsy, this may be very useful as a diagnostic system. Considering that the diagnostic accuracy of gastric adenoma by biopsy is only about 50%-70%[24], our classification by NBI may be equally or more useful than biopsy.
Another study also indicated that a definitive diagnosis of cancer could be made at a high probability on the basis of microsurface and microvascular patterns, as described in our study[9,19]. Ezoe et al[10] conducted a prospective study, and reported that the accurate diagnosis rate for the combination of WLI and NBI was higher than that for WLI alone in small, depressive gastric lesions, including gastritis and cancerous lesions.
Here, we present one case for which NBI-combined magnifying observation was useful for the differentiation of benignity and malignancy of a small depressed lesion (erosion) (Figure 11). Solitary erosion was present in the antrum on the small curvature side. Diagnosis by conventional observation was limited, but when close NBI magnifying observation was applied, the glandular structure of the erosion surface was clearly different from that of the surrounding region, and a few abnormal micro blood vessels with an abnormal distribution and irregular width were observed. After ESD, moderately differentiated adenocarcinoma (tub2) was diagnosed.
Recently, the widespread application of ESD has contributed to marked advances in the treatment of early gastric cancer. In the stomach, it has become possible to perform ESD regardless of the lesion size. However, border diagnosis becomes more important with increasing lesion size. In differentiated adenocarcinoma lesions, the border can usually be recognized employing standard endoscopy and the chromoendoscopy. However, there are many superficial-type lesions whose borders are difficult to recognize. In this case, NBI-combined magnifying endoscopy is very useful and the demarcation line (DL), recognized on the basis of differences in the microsurface or microvascular patterns between the gastric cancer lesion and non-lesion site, is very useful for evaluating the extent of gastric cancer.
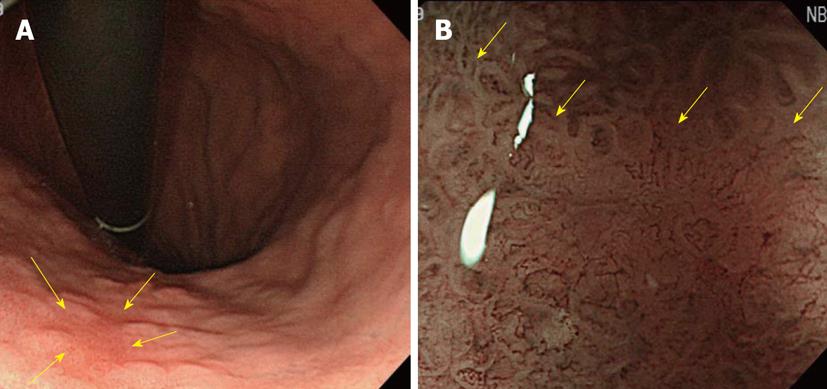
As shown in Figure 12, a small flat-type differentiated early-stage gastric cancer of about 6-mm with an unclear boundary was present in the small curvature of the lower gastric body. The lesion was undetectable by conventional observation, giving considerably difficulty in diagnosing the range. Employing NBI magnifying endoscopy, the observation of tumor blood vessels and an apparent tumor/non-tumor boundary is possible.
On the other hand, caution is needed for border diagnosis in undifferentiated carcinoma lesions. Undifferentiated carcinoma transversely infiltrates the deep mucosal and submucosal layers; cancer does not appear on the surface in many cases.
As described above, on NBI magnifying endoscopy of undifferentiated cancer, abnormal blood vessels showing a cork screw pattern are observed in some cases, whereas only an increased interval between white lines due to swelling of the intervening part of glands without abnormal blood vessels is observed in others, as shown in Figure 13. These findings may serve as a clue to decide on the range of undifferentiated cancer although only micro blood vessels directly below the microstructure of the mucosal surface are visually observed by NBI magnifying endoscopy. Therefore, the boundary of undifferentiated cancer advancing in the deep mucosal or submucosal layer should not be diagnosed by NBI.
In undifferentiated carcinoma lesions, ESD and surgical resection lines should be established by verifying cancer-negative reactions using biopsy specimens.
Finally, we describe findings on NBI-combined magnifying observation of non-epithelial tumors, such as MALT lymphoma, which may require differentiation from early-stage gastric cancer. We previously reported that, when performing NBI-combined magnifying endoscopy of non-epithelial tumors such as mucosa-associated lymphoid tissue lymphomas and Mantle lymphomas of the stomach, some patients showed a tree-like appearance, as presented in Figure 14[7,8]. Since collecting venules are present in the mucosa of an H. pylori-negative normal stomach body as a branching vascular network, differentiation from abnormal blood vessels branching like trees in MALT lymphoma is necessary, and these can be distinguished based on the microstructure of the background mucosa of abnormal branching blood vessels. In MALT lymphoma, the gland structure is destroyed by lymphoma cells infiltrating the proper lamina and the mucosal microstructure is lost, giving the mucosa a glossy appearance. In contrast, the background mucosal microstructure shows a normal regular pattern of collecting venules. In addition, the collecting venules are seen as a greenish-blue color on NBI because venules are present in a slightly deeper region of the mucosa, another point of differentiation.
Furthermore, this procedure may be useful for differentiating non-epithelial tumors from gastric cancer and gastritis when standard endoscopic findings are similar. In the future, this should be further investigated in a larger number of patients.
ESD for the colon has not surpassed EMR and it is performed by only a limited number of facilities because of its technical difficulty and a high complication rate[25-29]. Moreover, the role of NBI in the colon ESD is very limited. In the diagnosis of the range for ESD, the mucosal thickness of colorectal tumor lesions is different from that of the normal mucosa. Therefore, the boundary is clear on conventional observation (including dye spraying) even after local injection. Accordingly, diagnosis of the boundary is not problematic, unlike in esophageal and gastric ESD, and no mark is applied around lesions for ESD, as is characteristic of colon ESD. NBI-combined magnifying observation before ESD is useful for the diagnosis of lesion depth because it can be simply applied by pressing a button. However, it has not yet surpassed the dye sprayed magnifying endoscopic diagnosis (pit pattern diagnostics) established by Kudo et al[30].
Kudo et al[30,31] reported that, in the diagnosis of colon polyps, magnifying chromoendoscopy of pit appearance on the polyp surface was useful for differentiating tumors from non-tumorous lesions and evaluating the degree of infiltration. Currently, their pit-pattern classification is employed throughout the world. Two NBI-based diagnostic methods for colon lesions have been proposed: one is to examine microvascular features on the mucosal surface[6], and the other is to make a diagnosis based on fine mucosal structure and microvascular features[32], as reported for the stomach. According to East et al[33], a fine mucosal structure observed on NBI-combined magnifying endoscopy, of pit-like appearance, may differ from a pit pattern observed on magnifying chromoendoscopy. This may be because microvessels in which hemoglobin is present are emphasized on NBI-combined endoscopy, and the visualization of a fine mucosal structure as a secondary finding, whereas the pit pattern is a direct observation in chromoendoscopy.
The clinical significance of NBI-combined magnifying endoscopy in tumorous lesions of the colon is its ability to pick up lesions. However, a consensus has not yet been reached on this[6,34,35]. On the other hand, an international consensus regarding the usefulness of this procedure for the differential diagnosis of tumors from non-tumorous lesions has been reached[36,37]. Sano et al[6] reported that NBI-combined magnifying endoscopy involving the observation of meshed capillary vessels on the polyp surface was useful for differentiating tumorous from non-tumorous colon polyps (Figure 15).
Furthermore, this procedure provides a simple method for the indirect evaluation of a pit-like appearance without dye spray. Tanaka et al[38] reported that the degree of infiltration of early colorectal cancer could be assessed by comprehensively evaluating the microvascular structure and surface pattern (pit-like appearance). According to their classification, lesions are classified into 3 types: A, B, and C. In addition, type C lesions are divided into 3 subtypes: C1 to C3. They observed that 94% of C3 lesions showed massive SM infiltration (P < 0.01[32]).
Although the role of NBI in the colon ESD is limited, the simplicity of its application makes it useful for the differentiation between tumors and non-tumors and diagnosis of the depth of early-stage colorectal cancer, as described above. The addition of evaluation of microvascular construction by NIB-combined magnifying observation to pit pattern diagnostics may further increase the accuracy of colonoscopic diagnosis.
The use of ESD has markedly spread over the last several years with the development of devices, and is becoming established as a minimally-invasive treatment for tumors. In parallel with this, clinical studies on NBI have markedly progressed and spread over the last several years, demonstrating its usefulness and increasing its role in ESD.
However, not all problems have been completely resolved, and because only the mucosal surface is observed and diagnosed on NBI this limits its value in the diagnoses of the range of undifferentiated gastric cancer and depth of gastric cancer. In the colon, NBI has not surpassed pit pattern diagnostics by magnifying observation employing dye spraying. In the esophagus, the use NBI magnifying observation of Barett’s esophageal adenocarcinoma in Barett’s esophagus, frequently observed in Western countries, is still under development.
For the endoscopic diagnosis of ESD, NBI-combined comprehensive diagnosis by conventional endoscopy including dye spraying and EUS is important and essential.
Peer reviewer: Noriya Uedo, Director, Endoscopic Training and Learning Center; Vice Director, Department of Gastrointestinal Oncology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan
S- Editor Song XX L- Editor Hughes D E- Editor Zheng XM
| 1. | Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis. 2005;6:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Yamamoto H, Kita H. Endoscopic therapy of early gastric cancer. Best Pract Res Clin Gastroenterol. 2005;19:909-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3:S71-S73. [PubMed] |
| 4. | Fujishiro M. Endoscopic submucosal dissection for stomach neoplasms. World J Gastroenterol. 2006;12:5108-5112. [PubMed] |
| 5. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 525] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 6. | Sano Y, Ikematsu H, Fu KI, Emura F, Katagiri A, Horimatsu T, Kaneko K, Soetikno R, Yoshida S. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009;69:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 7. | Nonaka K, Ishikawa K, Shimizu M, Sakurai T, Nakai Y, Nakao M, Yoshino K, Arai S, Kita H. Education and Imaging. Gastrointestinal: gastric mucosa-associated lymphoma presented with unique vascular features on magnified endoscopy combined with narrow-band imaging. J Gastroenterol Hepatol. 2009;24:1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Nonaka K, Ishikawa K, Arai S, Shimizu M, Sakurai T, Nishimura M, Nakao M, Sasaki Y, Kita H. Magnifying endoscopic observation of mantle cell lymphoma in the stomach using the narrow-band imaging system. Endoscopy. 2010;42 Suppl 2:E94-E95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Yao K, Iwashita A, Tanabe H, Nishimata N, Nagahama T, Maki S, Takaki Y, Hirai F, Hisabe T, Nishimura T. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008;68:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Ezoe Y, Muto M, Horimatsu T, Minashi K, Yano T, Sano Y, Chiba T, Ohtsu A. Magnifying narrow-band imaging versus magnifying white-light imaging for the differential diagnosis of gastric small depressive lesions: a prospective study. Gastrointest Endosc. 2010;71:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Makuuchi H. Endoscopic mucosal resection for mucosal cancer in the esophagus. Gastrointest Endosc Clin N Am. 2001;11:445-458. [PubMed] |
| 12. | McCulloch P, Ward J, Tekkis PP. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327:1192-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 270] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Karl RC, Schreiber R, Boulware D, Baker S, Coppola D. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg. 2000;231:635-643. [PubMed] |
| 14. | Inoue H. [Endoscopic diagnosis of tissue atypism (EA) in the pharyngeal and esophageal squamous epithelium; IPCL pattern classification and ECA classification]. Kyobu Geka. 2007;60:768-775. [PubMed] |
| 15. | van der Burgh A, Dees J, Hop WC, van Blankenstein M. Oesophageal cancer is an uncommon cause of death in patients with Barrett's oesophagus. Gut. 1996;39:5-8. [PubMed] |
| 16. | Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249-1256. [PubMed] |
| 17. | Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett's esophagus: a meta-analysis. Gastrointest Endosc. 2009;69:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Mannath J, Subramanian V, Hawkey CJ, Ragunath K. Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett's esophagus: a meta-analysis. Endoscopy. 2010;42:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Kaise M, Kato M, Urashima M, Arai Y, Kaneyama H, Kanzazawa Y, Yonezawa J, Yoshida Y, Yoshimura N, Yamasaki T. Magnifying endoscopy combined with narrow-band imaging for differential diagnosis of superficial depressed gastric lesions. Endoscopy. 2009;41:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Kadowaki S, Tanaka K, Toyoda H, Kosaka R, Imoto I, Hamada Y, Katsurahara M, Inoue H, Aoki M, Noda T. Ease of early gastric cancer demarcation recognition: a comparison of four magnifying endoscopy methods. J Gastroenterol Hepatol. 2009;24:1625-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 23. | Nonaka K, Arai S, Ban S, Kitada H, Namoto M, Nagata K, Ochiai Y, Togawa O, Nakao M, Nishimura M. Prospective study of the evaluation of the usefulness of tumor typing by narrow band imaging for the differential diagnosis of gastric adenoma and well-differentiated adenocarcinoma. Dig Endosc. 2011;23:146-152. [PubMed] |
| 24. | Muehldorfer SM, Stolte M, Martus P, Hahn EG, Ell C. Diagnostic accuracy of forceps biopsy versus polypectomy for gastric polyps: a prospective multicentre study. Gut. 2002;50:465-470. [PubMed] |
| 25. | Ono S, Fujishiro M, Goto O, Kodashima S, Omata M. Submerging endoscopic submucosal dissection leads to successful en bloc resection of colonic laterally spreading tumor with submucosal fat. Gut Liver. 2008;2:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 27. | Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Mori H, Kawabe M, Nagahara A, Otaka M, Ogihara T. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc. 2009;69:1370-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Oka S, Tanaka S, Kanao H, Ishikawa H, Watanabe T, Igarashi M, Saito Y, Ikematsu H, Kobayashi K, Inoue Y. Current status in the occurrence of postoperative bleeding, perforation and residual/local recurrence during colonoscopic treatment in Japan. Dig Endosc. 2010;22:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 30. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [PubMed] |
| 31. | Kudo S, Rubio CA, Teixeira CR, Kashida H, Kogure E. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy. 2001;33:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 32. | Oba S, Tanaka S, Oka S, Kanao H, Yoshida S, Shimamoto F, Chayama K. Characterization of colorectal tumors using narrow-band imaging magnification: combined diagnosis with both pit pattern and microvessel features. Scand J Gastroenterol. 2010;45:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: a pilot study. Gastrointest Endosc. 2007;66:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Adler A, Aschenbeck J, Yenerim T, Mayr M, Aminalai A, Drossel R, Schröder A, Scheel M, Wiedenmann B, Rösch T. Narrow-band versus white-light high definition television endoscopic imaging for screening colonoscopy: a prospective randomized trial. Gastroenterology. 2009;136:410-6.e1; quiz 715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 35. | Uraoka T, Saito Y, Matsuda T, Sano Y, Ikehara H, Mashimo Y, Kikuchi T, Saito D, Saito H. Detectability of colorectal neoplastic lesions using a narrow-band imaging system: a pilot study. J Gastroenterol Hepatol. 2008;23:1810-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Tischendorf JJ, Schirin-Sokhan R, Streetz K, Gassler N, Hecker HE, Meyer M, Tacke F, Wasmuth HE, Trautwein C, Winograd R. Value of magnifying endoscopy in classifying colorectal polyps based on vascular pattern. Endoscopy. 2010;42:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Wada Y, Kashida H, Kudo SE, Misawa M, Ikehara N, Hamatani S. Diagnostic accuracy of pit pattern and vascular pattern analyses in colorectal lesions. Dig Endosc. 2010;22:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Tanaka S, Sano Y. Aim to unify the narrow band imaging (NBI) magnifying classification for colorectal tumors: current status in Japan from a summary of the consensus symposium in the 79th Annual Meeting of the Japan Gastroenterological Endoscopy Society. Dig Endosc. 2011;23 Suppl 1:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |