Published online May 16, 2025. doi: 10.4253/wjge.v17.i5.106074
Revised: March 31, 2025
Accepted: April 17, 2025
Published online: May 16, 2025
Processing time: 77 Days and 20.8 Hours
Inflammatory fibroid polyps (IFPs) are generally considered as benign and re
A 43-year-old man, hospitalized with abdominal pain and distension, underwent his first gastroscopy in 2020, which revealed chronic superficial erosive gastritis. From 2021 to 2022, his condition progressed from antral ulcers to a 2.0 cm gastric antrum bulge of an unclear nature. After proton pump inhibitor treatment, the lesion shrank but did not heal completely. Following a thorough assessment using magnifying endoscopy with narrow-band imaging, gastric-enhanced computed tomography, and endoscopic ultrasonography, endoscopic submucosal dissection was performed on the identified lesion. A subsequent postoperative pathological examination conclusively diagnosed the lesion as an IFP. At 6 months follow-up, no recurrence or metastasis was observed, with good mucosal scar healing.
Through using multiple diagnostic and therapeutic test results, an IFP with an unusual morphology could be identified.
Core Tip: This article presents a rare case of inflammatory fibroid polyp (IFP) in the gastric antrum, tracking a dynamic change in the lesion from gastritis to a bulge. Advanced diagnostic methods such as magnifying endoscopy with narrow-band imaging, gastric-enhanced computed tomography, and endoscopic ultrasonography were combined for diagnosis and treatment. Postoperative pathological examination and immunohistochemistry findings confirmed the diagnosis. Endoscopic submucosal dissection is a safe and effective treatment with almost no recurrence. This case report may prompt clinicians to consider IFP when detecting bulge lesions during gastroscopy and to utilize multiple methods for diagnosis and evaluation.
- Citation: Yang HC, Qu W. Diagnostic and therapeutic review of a rare gastric inflammatory fibroid polyps case with distinctive features: A case report. World J Gastrointest Endosc 2025; 17(5): 106074
- URL: https://www.wjgnet.com/1948-5190/full/v17/i5/106074.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i5.106074
Inflammatory fibroid polyps (IFPs) are benign mesenchymal tumors of the gastrointestinal tract. While IFPs can occur at any site in the digestive system, including the stomach, small intestine, colon, gallbladder, esophagus, duodenum, appendix, and rectum, they are most prevalent in the stomach, particularly in the gastric antrum. Owing to their low incidence in clinical practice and lack of distinctive endoscopic features, the preoperative diagnosis rate of IFPs is disappointingly low, often leading to missed diagnosis or misdiagnosis. We report a patient with an IFP that presented as a polypoid bulge with erosion on the small curvature side of the gastric antrum. Postoperative pathological examination findings confirmed the final diagnosis. In this case report, we review the disease development, dynamic changes, and endoscopic characteristics of this particular case, with a perspective of enhancing clinical diagnosis and treatment proficiency for patients with this condition.
Upper abdominal pain for over 1 year, with gastric antrum bulging for over 2 months.
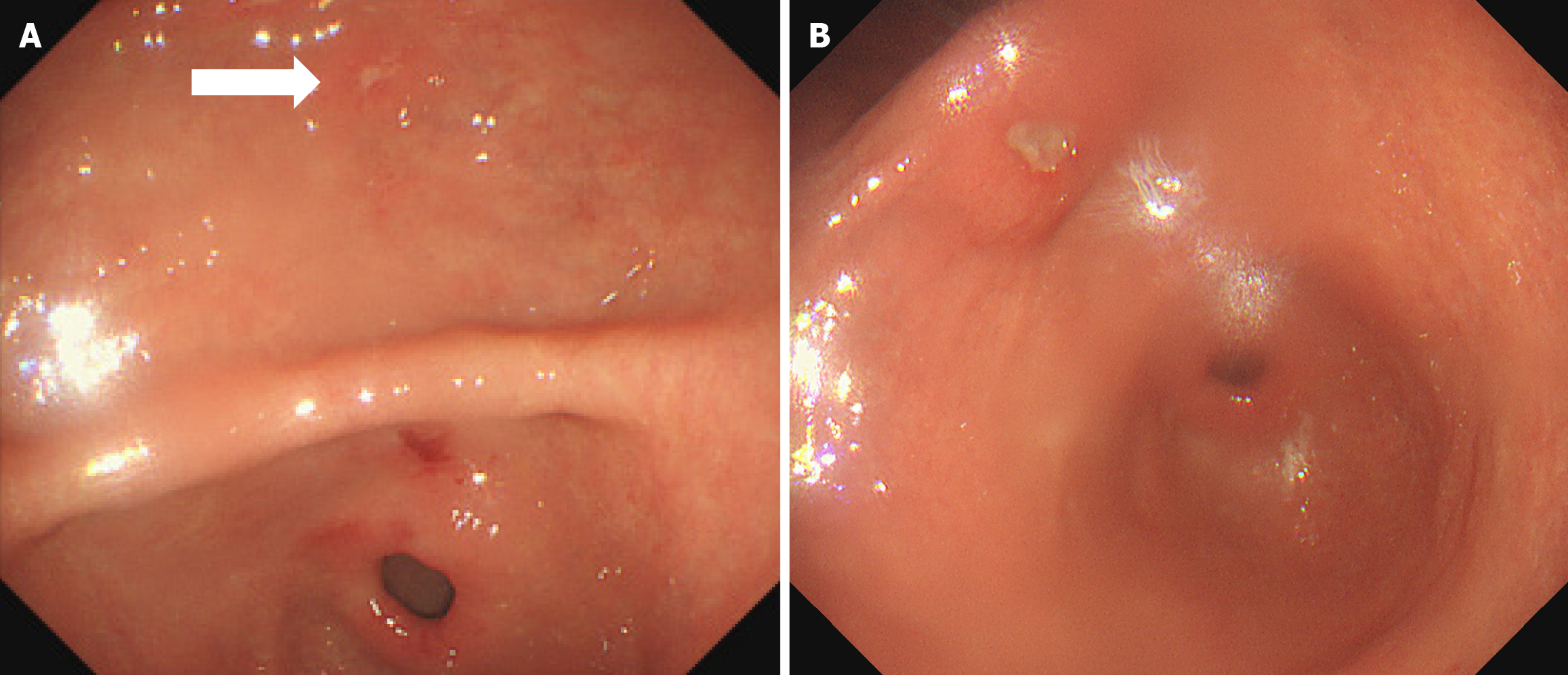
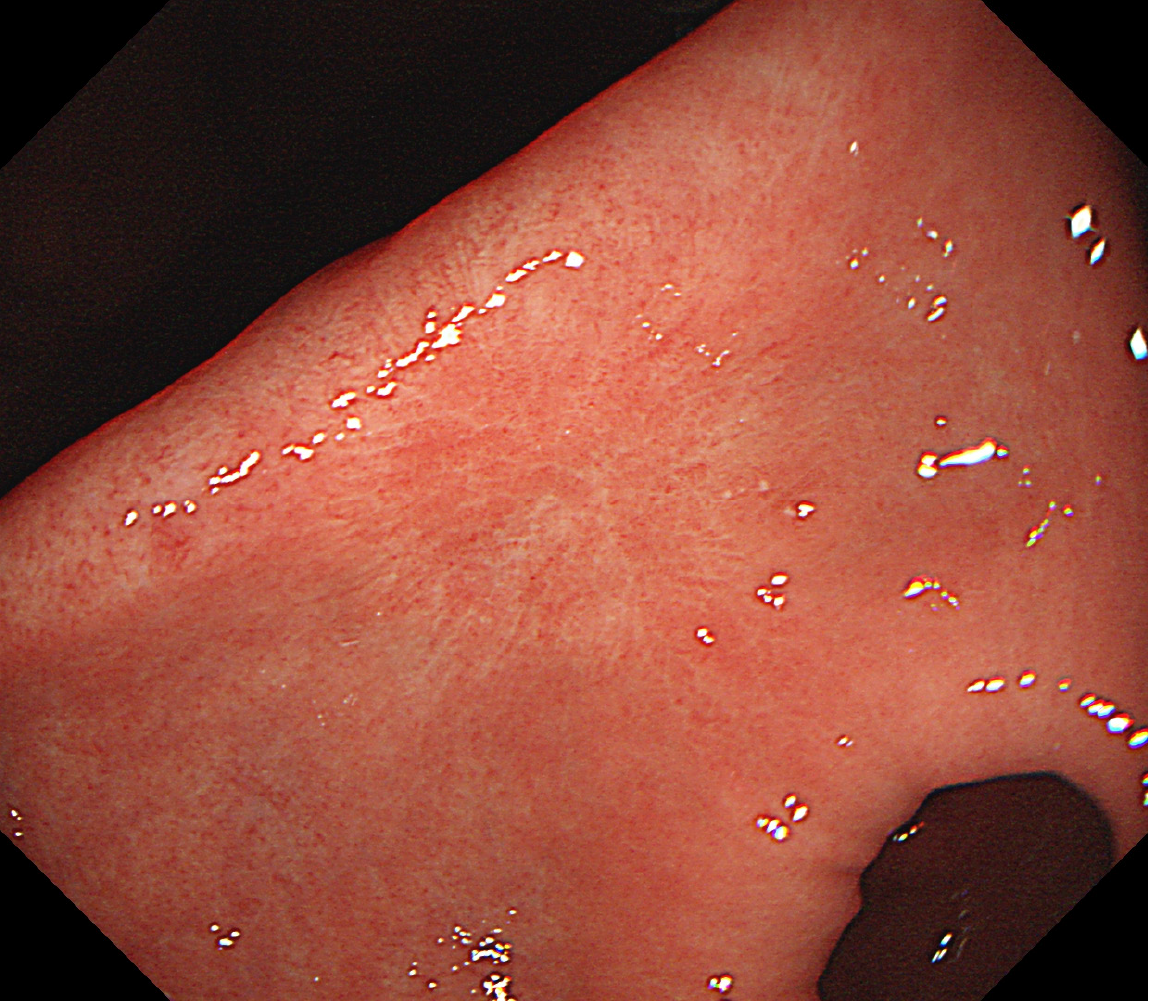
In September 2020, a 43-year-old man admitted to a local county-level hospital with abdominal pain and distension underwent his first gastroscopy, which indicated chronic superficial erosive gastritis. The pathological diagnosis was severe chronic non-atrophic gastritis of the antrum accompanied with acute inflammation and positive (+) adenopathy. In February 2021, he underwent a second gastroscopy, on which multiple microscopic antral ulcers were observed. The pathological diagnosis was moderate chronic non-atrophic gastritis of the antrum with severe acute inflammation and erosion. Ten months later, an ulcer was observed on the small curvature of the antrum. The pathological diagnosis was ulceration of the antrum with moderate chronic non-atrophic gastritis and severe acute inflammation, accompanied with a small region of ulcer necrosis (Figure 1).
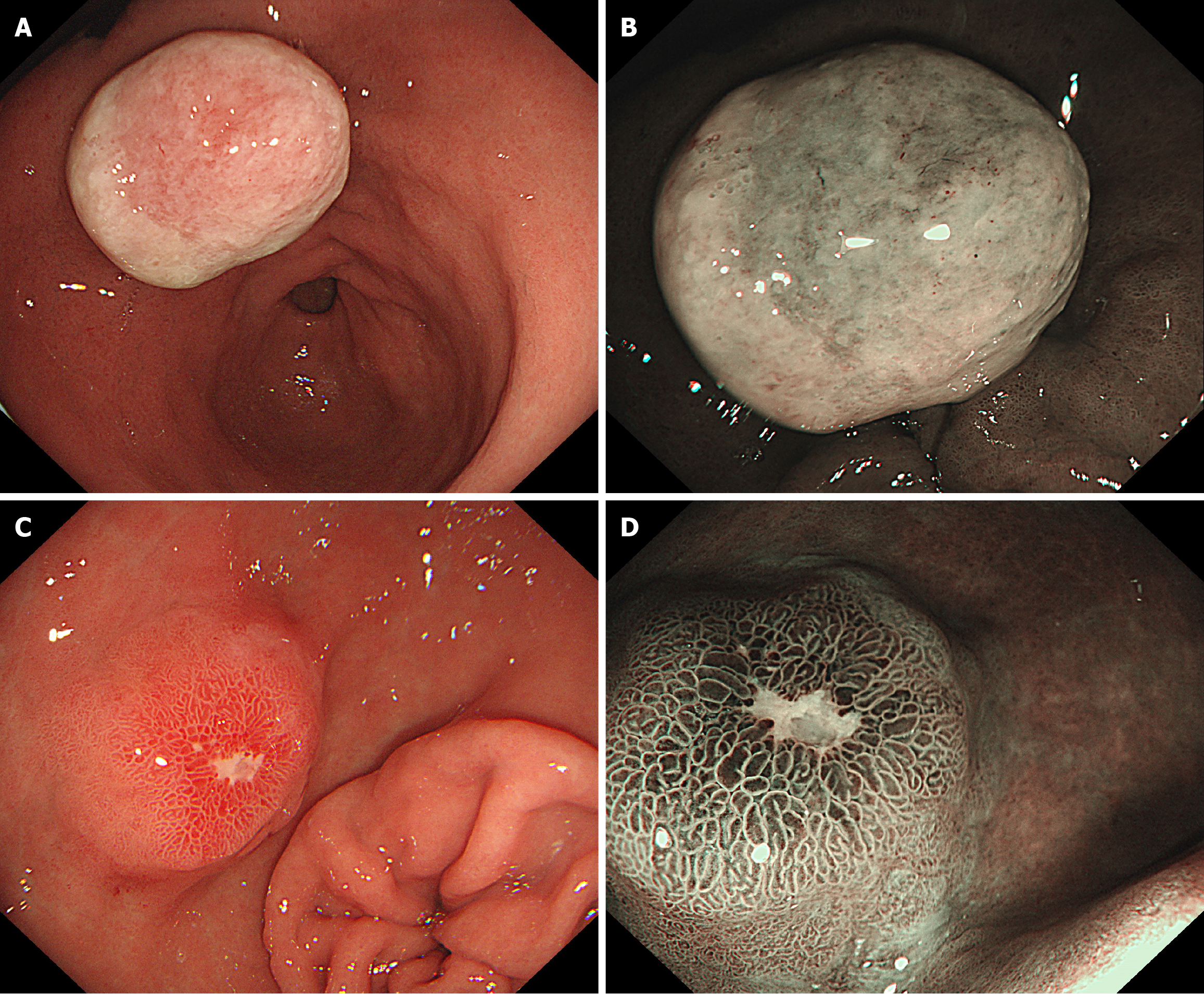
In March 2022, treatment was sought at a high-level hospital, and he underwent a third gastroscopy, which revealed an approximately 2.0 cm bulge on the small-curvature side of the gastric antrum. The bulge surface was covered with firmly adherent debris, and the surrounding mucosa appeared congested and edematous. A diagnosis of ulcerative–necrotic tissue was made, with no mucosal epithelium detected. Based on endoscopic and pathological findings, the nature of the gastric antrum bulge lesion was undetermined, and oral administration of a proton pump inhibitor (PPI) was recommended.
Two months later, a subsequent gastroscopy revealed a lesion (diameter, approximately 1.5 cm) close to the anterior wall of the gastric antrum. The surface of the lesion appeared congested, edematous, eroded, firm on palpation, and with poor mobility. Pathological diagnosis indicated high eosinophil, neutrophil, and lymphocyte counts. Erosion and significant hyperplasia of regional fibrous tissues were also observed (Figure 2).
In June 2022, the patient was admitted to our Gastroenterology Department for further diagnosis and treatment. On admission, he reported no symptoms of pain or discomfort.
His prior health status was good.
He had no remarkable personal history apart from a paternal history of esophageal cancer.
The physical examination findings were as follows: (1) Temperature: 36.2 ºC; (2) Heart rate: 70 beats per minute; (3) Respiratory rate: 18 beats/minute; and (4) Blood pressure: 112/74 mmHg. He reported mild tenderness in the upper abdomen on palpation.
Routine blood, urine, and stool tests, in addition to biochemical marker and coagulation function tests, were all normal. No abnormalities were detected in tumor markers, such as carcinoembryonic antigen, alpha-fetoprotein, cancer antigen (CA) 125, CA19-9, CA153, and CA724.
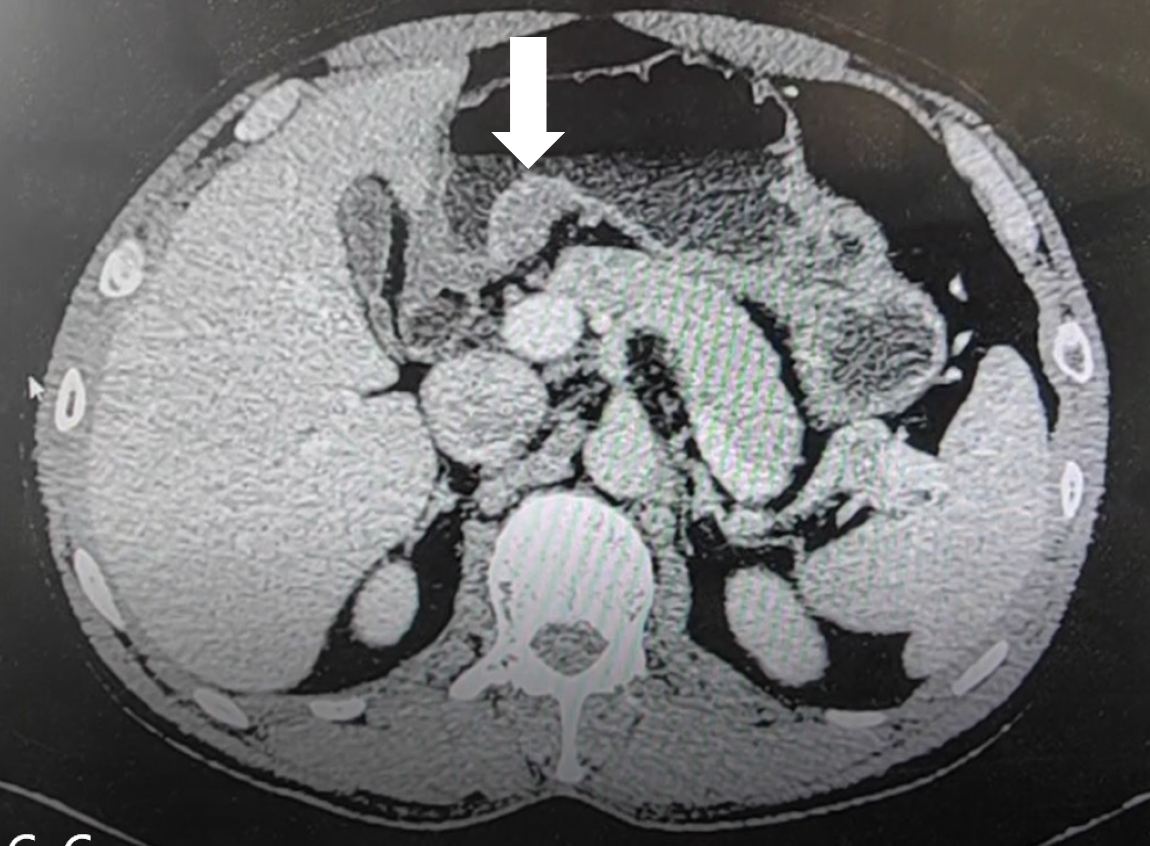
Gastric-enhanced computed tomography (CT) scans identified a soft tissue mass located beneath the mucosa on the small-curvature side of the antrum. The mass had a clear boundary, uniform internal density, and uniform enhancement after contrast administration. The mass size was approximately 26 mm × 16 mm. The serosal layer was intact, and no enlarged lymph nodes were observed within the vicinity (Figure 3). Ultrasound gastroscopy findings revealed a hypoechoic mass in the bulge of the gastric sinus, which was located within the mucosa and submucosa.
Based on the examination findings, the patient was diagnosed with a submucosal tumor of the gastric antrum. Postoperative pathological specimen was finally diagnosed as IFP.
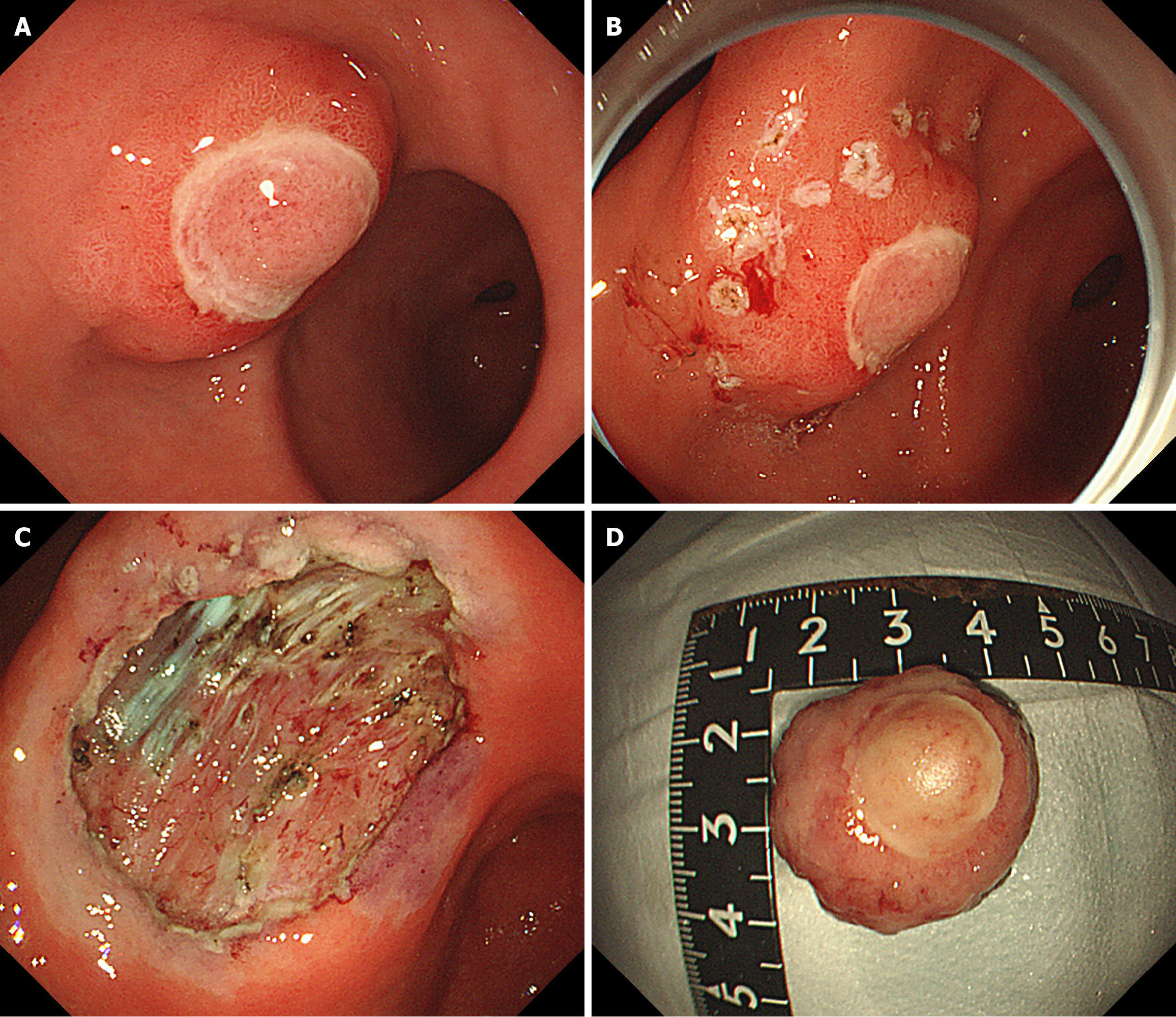
To further clarify the diagnosis and treatment, the patient underwent endoscopic submucosal dissection (ESD) of the submucosal tumor under general anesthesia. The lesion was completely removed, and the surgery proceeded uneventfully (Figure 4).
After 3 days of observation after surgery, the patient improved and was discharged. At 6 months follow-up, no recurrence or metastasis was observed. During the follow-up gastroscopy, a mucosal scar was observed on the gastric sinus, and the ulcer appeared to have healed well (Figure 5).
IFPs are rare gastrointestinal tract tumors of mesophyllogenic origin. The understanding and nomenclature of this disease has evolved over time. In 1937, Kaijser first proposed the concept of gastric acidophilic granuloma. In 1949, Vanek[1] named the condition as “gastrointestinal submucosal granuloma with eosinophil infiltration”, also known as Vanek’s tumor. In 1953, Helwig and Ranier[2] first used the term IFP and elaborated on its histological features, advocating that this condition should be regarded as an independent disease. Based on further investigation of its pathogenesis, it was revealed that IFPs typically exhibit platelet-derived growth factor receptor α (PDGFRα) gene mutations. PDGFRα mutations are a type of cancer-related mutation frequently detected in gastrointestinal stromal tumors (GISTs). Eventually, IFPs were confirmed as genuine tumors. In 2010, the World Health Organization classified IPFs as benign tumors derived from mesenchymal tissue[3]. The peak age at IFP onset is 40–70 years[4], with a higher prevalence in women. IFPs can occur in multiple parts of the digestive system, most commonly in the stomach and especially in the antrum.
The clinical manifestations of gastric IFP are diverse. Common symptoms include upper abdominal pain[5], dyspepsia, and gastrointestinal bleeding. Some patients may be asymptomatic. Endoscopically, IFP presents as a lumen-bulging lesion that is mostly solitary, although some cases have been reported to present with multiple lesions. The lesions vary in size and morphology. Wang et al[6] reported a case of an IFP with a polypoid bulge approximately 2 cm in diameter, accompanied with ulcerations on the raised surface and minor bleeding. Wang et al[7] reported a case of IFP showing a submucosal bulge with a smooth surface and a slightly indented center. Shim et al[8] identified a mass-like IFP approximately 4 cm in diameter accompanied with mucosal edema and superficial antral ulcers. Mori et al[9] reported a case of a woman who underwent surgical treatment for early gastric cancer. Postoperative pathology revealed extensive early-stage gastric cancer with two IFPs that had an inward growth pattern without a polypoid appearance. Andrzej[10] laparoscopically excised a massive gastric cardia IFP measuring 10 cm × 5.5 cm × 7.5 cm.
The clinical diagnosis of IFPs remains challenging, and is characterized with low preoperative diagnostic accuracy. This diagnostic ambiguity stems from significant overlaps in clinical manifestations, endoscopic features, and CT imaging findings among IFPs, GISTs, leiomyomas, and schwannomas. These entities typically present as submucosal masses within the gastrointestinal tract, particularly in the gastric wall, and may elicit nonspecific symptoms such as abdominal pain. Hemorrhagic complications may arise when mucosal integrity is compromised by surface erosion or ulceration. These mechanisms may manifest clinically as melena or hematemesis. Endoscopically, all four pathologies present as submucosal protrusions with smooth overlying mucosa. On non-contrast CT imaging, they similarly appear as nodular or mass-like soft tissue densities within the gastrointestinal wall. These overlapping features substantially complicate clinical differentiation. To address this diagnostic conundrum, a multimodal evaluation protocol integrating narrow-band imaging (NBI), contrast-enhanced CT, and endoscopic ultrasonography (EUS) is essential for comprehensive lesion characterization. Definitive diagnosis ultimately requires histopathological examination coupled with immunohistochemical profiling post-resection. The comparative analysis presented in Table 1 delineates key discriminators among IFPs, GISTs, leiomyomas, and schwannomas across diagnostic modalities.
| Disease type | Endoscopy (white light) | Narrow-band imaging | Contrast-enhanced computed tomography | Endoscopic ultrasonography | Pathological features |
| Inflammatory fibroid polyp | Solitary, broad-based submucosal protrusion with smooth or mildly eroded surface. Firm consistency, well-defined borders (1-5 cm) | Regular vascular pattern, normal pit pattern (type I/II) | Submucosal hypo-/iso-dense nodule. Mild-moderate homogeneous enhancement; "target sign" (central hypodensity + peripheral enhancement) | Originates from submucosa (3rd layer). Homogeneous hypoechoic, minimal vascularity | Spindle-shaped fibroblasts+ eosinophil infiltration, CD34 (+), CD117/DOG-1 (−) |
| Gastrointestinal stromal tumor | Submucosal mass, smooth or ulcerated surface. Hard texture, often large (> 5 cm) | Normal vessels (if mucosa intact), vascular interruption in ulcers | Heterogeneous enhancement with necrosis/cystic degeneration | Originates from muscularis propria (4th layer). Heterogeneous echogenicity, hypervascular | Spindle/epithelioid cells, CD117/DOG-1 (+) |
| Leiomyoma | Submucosal protrusion, smooth surface, small (1-3 cm) | Regular vascular pattern, normal pit pattern | Homogeneous moderate enhancement, no necrosis/calcification | Originates from muscularis propria (4th layer). Homogeneous hypoechoic, well-defined | Bundled spindle cells. Serum response factor-associated protein (+), desmin (+) |
| Schwannoma | Submucosal protrusion, smooth surface (rare ulceration | Normal or mildly distorted vessels | Mild homogeneous enhancement, rare cystic degeneration | Originates from submucosa or muscularis propria. Heterogeneous hypoechoic, occasional cysts | Palisaded spindle cells (Verocay bodies), S-100 (+), SRY-related HMG-box 10 protein (+) |
Our case involved a middle-aged man who presented with abdominal pain and distension. During the first gastroscopic examination performed in 2020, no obvious lesions were detected in the gastric antrum. However, from 2021 to 2022, over the course of a year, small gastric ulcer lesions progressed into a 2 cm bulging lesion. The surface of the bulge was completely covered by ulcer necrosis, and there was an evident inflammatory response. After 2 months of oral PPI treatment, the lesion size decreased, and the erosion and ulcers were reduced; however, a complete cure was not achieved, suggesting that the lesion was not merely an inflammatory, reactive, or proliferative lesion. The nature of the lesion remained undetermined after multiple gastroscopies and routine biopsies. To comprehensively characterize the lesion, a multimodal imaging protocol incorporating NBI, CT, and EUS was systematically implemented. This integrative approach enabled precise delineation of the lesion’s anatomical origin, spatial relationship with adjacent vascular structures, dimensional parameters (size and extent), and sonographic stratification. Diagnostic evaluation revealed a well-circumscribed hypoechoic mass confined to the submucosal layer, exhibiting clear demarcation from the muscularis propria. Based on these findings, ESD was determined to be technically feasible for achieving a complete en bloc resection. Following therapeutic intervention, confirmatory histopathological analysis with adjunctive immunohistochemical profiling was performed. Microscopic examination demonstrated lesion localization within the mucosal and submucosal layers, featuring characteristic histoarchitectural components: (1) Fibroblast-like spindle cells arranged in a storiform pattern; (2) Prominent vascular proliferation; and (3) Eosinophil infiltration—all pathognomonic of IFP. Immunophenotypic analysis revealed cluster of differentiation (CD) 34 positivity in neoplastic cells, while critical markers, including CD117, S-100, and discovered on GIST 1 (DOG-1), remained negative. This distinct immunoprofile conclusively established a diagnosis of IFP while effectively excluding differential considerations of GIST, leiomyoma, and schwannoma.
Currently, minimally invasive endoscopic surgery is the preferred treatment for IFP[11]. Compared with traditional surgical treatment, endoscopic treatment has the advantages of lower cost, minimal damage, faster recovery, and a significantly shorter hospital stay. Endoscopic treatment generally ensures a good prognosis, and recurrence and metastasis rarely occur. In this case, we accurately evaluated the lesion using CT and EUS. Finally, the lesion was dissected via ESD. Postoperative pathology revealed that the lesion was located in the mucosa and submucosa, and the pathological tissue consisted of fibroblast-like spindle cells, blood vessels, and eosinophils, which are characteristics of IFP. Immunophenotypically, the tumor cells expressed only CD34 but not CD117 or DOG-1, further supporting the diagnosis of IFP. The patient underwent a repeat gastroscopy at 6 months postoperatively, and no recurrence or metastasis was observed, indicating a good prognosis.
ESD has been widely recognized as a safe and effective therapeutic strategy for IFPs, with robust clinical validation from multiple case studies. In a seminal report by Wang et al[7], ESD was successfully employed to achieve complete resection of a 1.3 cm × 1.0 cm gastric IFP in a middle-aged woman. The procedure resulted in excellent clinical outcomes with no recurrence observed during longitudinal follow-up, underscoring the technique’s therapeutic reliability. Further corroborating evidence has been reported by Watahiki and Hikichi[12], who documented a 37-year-old woman presen
IFPs are rare gastrointestinal tumors that are easily overlooked in clinical practice, and there are certain challenges in both clinical and endoscopic diagnosis. When a raised lesion is detected during gastroscopy, careful observation of its morphology, microvascular patterns, and microsurface structural characteristics using white light and magnifying endoscopy with magnifying endoscopy-NBI, EUS, CT, and other diagnostic methods is recommended. The lesion’s origin, extent, and relationship with surrounding tissues should be examined to ensure accurate preoperative evaluation and select the optimal treatment approach. For submucosal lesions, IFPs should be considered. Differential diagnosis from GISTs, leiomyomas, and schwannomas should also be meticulously performed to reduce misdiagnosis and missed diagnoses. Minimally invasive endoscopic treatment has been proven to be a safe and effective approach for treating gastric IFPs. Moreover, postoperative pathology plays a crucial role in the differential diagnosis of this disease.
| 1. | Vanek J. Gastric submucosal granuloma with eosinophilic infiltration. Am J Pathol. 1949;25:397-411. [PubMed] |
| 2. | Helwig EB, Ranier A. Inflammatory fibroid polyps of the stomach. Surg Gynecol Obstet. 1953;96:335-367. [PubMed] |
| 3. | Li ZS, Li Q. [The latest 2010 WHO classification of tumors of digestive system]. Zhonghua Bing Li Xue Za Zhi. 2011;40:351-354. [PubMed] |
| 4. | Ozolek JA, Sasatomi E, Swalsky PA, Rao U, Krasinskas A, Finkelstein SD. Inflammatory fibroid polyps of the gastrointestinal tract: clinical, pathologic, and molecular characteristics. Appl Immunohistochem Mol Morphol. 2004;12:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Nonose R, Valenciano JS, da Silva CM, de Souza CA, Martinez CA. Ileal Intussusception Caused by Vanek's Tumor: A Case Report. Case Rep Gastroenterol. 2011;5:110-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Wang X, Ma R, Ma J, Tang N, Li R, Ma X. Endoscopic submucosal dissection for the treatment of a large inflammatory fibroid polyp in the gastric antrum prolapsing into the duodenum: A case report. Medicine (Baltimore). 2024;103:e37877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Wang H, Zhou T, Zhang C, Li H, Lü M. Inflammatory Fibroid Polyp: An Unusual Cause of Abdominal Pain in the Upper Gastrointestinal Tract a Case Report. Open Med (Wars). 2020;15:225-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Shim EJ, Ahn SE, Lee DH, Park SJ, Kim YW. Dynamic enhanced computed tomography imaging findings of an inflammatory fibroid polyp with massive fibrosis in the stomach. World J Gastroenterol. 2017;23:2090-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Mori M, Kakeji Y, Adachi Y, Korenaga D, Sugimachi K. Non-polypoid inflammatory fibroid polyps concomitant with early carcinoma in the stomach. Eur J Surg Oncol. 1992;18:632-635. [PubMed] |
| 10. | Kwiatkowski AP, Paśnik K. Large inflammatory fibroid polyp of cardia managed laparoscopically - a case report and review of the literature. Wideochir Inne Tech Maloinwazyjne. 2014;9:623-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Dias E, Marques M, Santos-Antunes J, Baldaque-Silva F, Moutinho-Ribeiro P, Macedo G. The role of endoscopic submucosal dissection in the management of gastric inflammatory fibroid polyps: a single-center experience. Rev Esp Enferm Dig. 2022;114:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Watahiki Y, Hikichi T, Watanabe K, Nakamura J, Kikuchi H, Hahimoto M, Takagi T, Suzuki R, Sugimoto M, Konno N, Sato Y, Irie H, Ohira H. A case of inflammatory fibroid polyp of the stomach with an "erect penis like appearance" successfully removed by endoscopic submucosal dissection. Clin J Gastroenterol. 2019;12:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Shimura T, Kataoka H, Sasaki M, Kubota E, Shiraki S, Matsusako K, Nakayama Y, Mizoshita T, Mizushima T, Joh T. Rectal inflammatory fibroid polyp resected with endoscopic submucosal dissection. Intern Med. 2008;47:2029-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |