Published online Jul 18, 2017. doi: 10.4254/wjh.v9.i20.884
Peer-review started: November 8, 2016
First decision: November 30, 2016
Revised: May 12, 2017
Accepted: May 30, 2017
Article in press: May 31, 2017
Published online: July 18, 2017
Processing time: 255 Days and 3.7 Hours
To determine the impact of Charlson comorbidity index (CCI) on waiting list (WL) and post liver retransplantation (LRT) survival.
Comparative study of all adult patients assessed for primary liver transplant (PLT) (n = 1090) and patients assessed for LRT (n = 150), 2000-2007 at our centre. Demographic, clinical and laboratory variables were recorded.
Median age for all patients was 53 years and 66% were men. Median model for end stage liver disease (MELD) score was 15. Median follow-up was 7-years. For retransplant patients, 84 (56%) had ≥ 1 comorbidity. The most common comorbidity was renal impairment in 66 (44.3%). WL mortality was higher in patients with ≥ 1 comorbidity (76% vs 53%, P = 0.044). CCI (OR = 2.688, 95%CI: 1.222-5.912, P = 0.014) was independently associated with WL mortality. Patients with MELD score ≥ 18 had inferior WL survival (Log-Rank 6.469, P = 0.011). On multivariate analysis, CCI (OR = 2.823, 95%CI: 1.563-5101, P = 0.001), MELD score ≥ 18 (OR 2.506, 95%CI: 1.044-6.018, P = 0.04), and requirement for organ support prior to LRT (P < 0.05) were associated with reduced post-LRT survival. Donor/graft parameters were not associated with survival (P = NS). Post-LRT mortality progressively increased according to the number of transplanted grafts (Log-Rank 18.455, P < 0.001). Post-LRT patient survival at 1-, 3- and 5-years were significantly inferior to those of PLT at 88% vs 73%, P < 0.001, 81% vs 71%, P = 0.018 and 69% vs 55%, P = 0.006, respectively.
Comorbidity increases WL and post-LRT mortality. Patients with MELD ≥ 18 have increased WL mortality. Patients with comorbidity or MELD ≥ 18 may benefit from earlier LRT. LRT for ≥ 3 grafts may not represent appropriate use of donated grafts.
Core tip: The prevalence and impact of comorbidity on waiting list (WL) and post-transplant survival is unknown in patients who had liver retransplantation. This study identified comorbidity(ies) were common (56%) in this cohort, most with renal impairment. WL mortality was higher in patients with ≥ 1 comorbidity and model for end stage liver disease (MELD) score ≥ 18. Post-transplant survival was inferior in patients with ≥ 1 comorbidity, MELD score ≥ 18 and in patients who required organ support prior to retransplantation. Comorbidity increases WL and post-transplant mortality. Patients with comorbidity or MELD ≥ 18 may benefit from earlier retransplantation.
- Citation: Al-Freah MAB, Moran C, Foxton MR, Agarwal K, Wendon JA, Heaton ND, Heneghan MA. Impact of comorbidity on waiting list and post-transplant outcomes in patients undergoing liver retransplantation. World J Hepatol 2017; 9(20): 884-895
- URL: https://www.wjgnet.com/1948-5182/full/v9/i20/884.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i20.884
Liver retransplantation (LRT) represents the only viable option for survival for some patients who develop graft failure following primary liver transplant (PLT). Published reports on cohorts of patients who underwent LRT indicate inferior post-transplant survival in these patients[1-5]. There has been an increase in the number of patients awaiting PLT which was not associated with increase in donated organs[6]. Although transplant programmes have tried to compensate for this increase in demand by more liberal use of marginal grafts, there is evidence that death on the waiting list (WL) for patients listed for PLT remains high[7]. Therefore, the combination of increased WL mortality with increasing demand for PLT coupled with the known inferior outcomes of LRT; raises concerns and generates ethical debate in the transplant community on the use of scarce resource of donated organs for LRT[8].
This debate has motivated researchers to identify predictors of survival following LRT to improve the selection of patients who might benefit most from LRT. Model for end-stage liver disease (MELD) score > 25, recipient age, creatinine level, bilirubin level, indication for retransplantation, the urgency for LRT, coma episodes, haemoglobin (Hb) level and the number of fresh frozen plasma units transfused were identified as factors associated with reduced post-LRT survival in a number of studies[3,5,9,10]. Death or graft loss was shown to increase gradually following LRT according to the timing of LRT with marked increase in risk between 4-38 d following LRT[11-13]. Inferior survival was also observed according to increasing number of transplanted graft[13].
Comorbidity as defined by the Charlson comorbidity index (CCI) was found to adversely affect post-transplant survival in patients who underwent PLT[14]. Thuluvath et al[7] analysed the data of the scientific registry of transplant recipients (SRTR) in the United States from 1999 to 2008. The prevalence of comorbidity such as diabetes mellitus (DM), renal impairment (RI) and obesity was found to have steadily increased in candidates listed for liver transplantation over the ten year period[7]. However, the prevalence and impact of comorbidity on WL and post-transplant survival in patients listed for LRT have not been studied previously.
The aims of this study were three fold, firstly, to identify the prevalence of comorbidity according to CCI in patients listed for LRT, secondly, to study the impact of comorbidity on WL and post-LRT mortality, and finally, to identify other factors associated with reduced WL and post-LRT survival.
This is a retrospective study of all patients referred to the liver unit at King’s for LRT assessment between January 2000 and December 2007. There were 151 assessments for LRT on 137 patients over the 8 year period. One patient was excluded because of incomplete information. Data analysis was performed on 150 LRT assessments. We utilized a cohort of patients who underwent PLT over the same time period for comparison of outcomes of PLT and LRT (n = 1332). Patients assessed for acute liver failure (n = 175), familial amyloid polyneuropathy (43) and 24 with incomplete information were excluded. We analysed data on 1090 patients with end stage liver disease (ESLD) who were assessed for PLT.
All patients assessed for liver transplantation at our centre had their clinical, laboratory, radiological and histological data as well as the outcome of transplant assessment entered at the time of liver transplant assessment into a prospective electronic database. This database was analysed in addition to electronic patient records and clinical notes to record demographic, clinical and laboratory variables of this cohort. Prognostic scores such as MELD and United Kingdom model for end- stage liver disease (UKELD) scores were calculated at the time of assessment and at the time of transplantation. MELD was calculated according to the UNOS adjustment[15]. The UKELD score was calculated according to Barber et al[16]. Donor and graft variables were collected and donor risk index was calculated according to Feng et al[17]. Patient survival was recorded according to their survival status in our hospital information system and further confirmed using the National Health System electronic portal. This is a United Kingdom wide national database, where patient survival status is updated according to the generation of death certificates in the United Kingdom.
WL outcome was defined for this study by death on WL or delisting because of significant deterioration or hepatocellular carcinoma (HCC) progression beyond Milan criteria whilst awaiting LT. To study the influence of comorbidity and other variables on listing outcome, we used the transplant free survival (defined as time from listing to death, time to delisting or time to transplant) to eliminate the artificial impact of transplantation on survival of this cohort. Post-transplant patient survival was defined as time from transplantation to death, and if alive, censored on 01/11/2011. Graft survival was defined as time from transplantation to retransplantation or death, and if alive censored on 01/11/2011. Patients who were lost to follow-up were censored as being alive at the date of their last follow-up. Post-LRT patient survival was defined as time from second or subsequent transplant to death, and if alive, censored on 01/11/2011. Post-LRT graft survival was defined as time from second or subsequent transplant to further retransplantation or death, and if alive censored on 01/11/2011. One-year post transplant patient survival was defined as time from LRT to death, and if alive, censored at 12 mo following transplantation. One-year post transplant graft survival was defined as time from transplantation to retransplantation or death, and if alive censored at 12 mo following transplantation. Marginal grafts were defined as graft with Donor Risk Index > 1.8[7]. Cut off values for MELD score of 18 and 25 were chosen according to Rosen et al[18] and Edwards and Harper[19].
Nine comorbidities were prospectively defined according to Volk et al[14]. These included congestive heart failure, coronary artery disease, DM, peripheral vascular disease, cerebro-vascular disease, chronic pulmonary disease, connective tissue disease, RI and malignancy. DM was defined as a chronic hyperglycaemia requiring medication use at any time during the month preceding transplant assessment. RI was defined as serum creatinine of ≥ 1.5 mg/dL (≥ 132 μmol/L) on transplant assessment, being on renal replacement therapy or history of renal transplantation. Congestive heart failure was defined as documented decrease in left ventricular function on echocardiogram or left ventricle angiogram; or increased pulmonary artery pressure of ≥ 25 mmHg on echocardiogram or on invasive pulmonary artery pressure study, including patients with porto-pulmonary hypertension. Coronary artery disease was defined as documented history of myocardial infarction or abnormal coronary angiography. All patients underwent a functional cardiac assessment of ischemia either with Bruce protocol exercise tolerance test or cardio-pulmonary exercise test. Those with positive functional test but negative coronary angiogram were not considered as having coronary artery disease. Peripheral vascular disease was defined as documented history of peripheral ischaemia on angiography, abnormal ankle-brachial index or history of vascular bypass surgery. Cerebrovascular disease was defined as a history of stroke with residual neurological deficit. Chronic pulmonary disease was defined as chronic pulmonary disease requiring medication, a forced expiratory volume of < 1.5 L or history of intubation for respiratory failure. Connective tissue disease was defined as a rheumatologist diagnosis of rheumatoid arthritis, systemic lupus erythematosis, scleroderma or spondyloarthropathies excluding patients with arthralgia without evidence of inflammatory arthritis or those with osteoarthritis. Malignancy was defined as documented history of any malignancy excluding HCC or non melanoma skin cancers. To calculate the CCI, each comorbidity was assigned 1 point when present and was assigned 0 points when absent. The CCI was calculated as the sum of points of all 9 comorbidities. CCI was calculated at the time of assessment and at the time of transplantation.
Continuous variables were presented as median (range) and analysed using non-parametric methods (Mann Whitney-U or Kruskall Wallis test) for non-normally distributed data as appropriate. Categorical variables were presented as numbers (percentages) and analysed using χ2 test or Fisher’s exact test as appropriate. Cox proportional hazard analysis was used to identify factors associated with listing and transplant outcomes. Factors associated with outcome (P-value < 0.05) were entered into multivariate analysis. Collinearity diagnostics were used to determine whether variables entered into a model were collinear. MELD, UKELD, Child-Turcotte-Pugh (CTP), renal impairment and sodium level showed high collinearity (variance inflation factor - VIF > 5). Once MELD and UKELD were removed of the model, CTP, sodium level and renal impairment and all other individual comorbidities showed no collinearity (VIF < 3). Kaplan-Meier analysis was performed to assess survival outcomes. Statistical analyses were performed with SPSS software (SPSS® 17.0 for Windows ®SPSS Inc, Chicago, IL, United States).
One hundred and fifty assessments for LRT were examined and compared to a control group of 1090 patients assessed for PLT. Median follow-up was 7 years (3-12). There were 124 assessments for a second transplant, 21 assessments for a third transplant, 3 assessments for a fourth transplant, and 1 assessment each for a fifth and sixth transplant out of 150 LRT assessments. Out of these150 assessments for LRT, six were not listed for LRT (two because of early referral, 1 because of alcohol abuse, 1 declined re-listing, 1 with complete porto-mesenteric thrombosis and 1 died during the assessment process. Only121 patients received LRT of the144 listed patients. Twenty three patients were delisted for the following reasons: 12 died awaiting a graft, 6 had significant clinical improvement and 5 were delisted because of significant clinical deterioration, whilst on WL. Information regarding mechanical ventilation, renal replacement therapy, vasopressor support and location of patient [home, hospital or intensive care unit (ICU)] prior to LRT was available on 113 patients. Thirty two patients (28%) received renal replacement therapy, 21 (19%) received mechanical ventilation and 20 (18%) received vasopressor support prior to LRT. Forty four patients (36%) were transplanted from the hospital ward, 40 (33%) were transplanted from ICU and 28 (23%) were transplanted from home.
Table 1 summarises baseline characteristics according to PLT and LRT. LRT patients were significantly younger and were less likely to have ascites. However, this group were more likely to have higher median serum sodium levels (Na), creatinine values, bilirubin levels, MELD and UKELD scores (P < 0.05). There were no significant differences in proportion of patients with encephalopathy or median INR level between groups (P = NS).
| Variables | PLT (n = 1090) | LRT (n = 150) | P value |
| Demographic | |||
| Age | 54 (18-82) | 46 (18-72) | < 0.001 |
| Gender (male, %) | 736 (67.5) | 80 (53.3) | 0.001 |
| Etiology | |||
| ALD (%) | 345 (31.7) | 18 (12.0) | < 0.001 |
| Viral (%) | 303 (27.8) | 50 (33.3) | 0.159 |
| Cholestatic and autoimmune (%) | 227 (20.8) | 34 (22.7) | 0.604 |
| Clinical | |||
| Na, mmol/L | 135 (116-151) | 138 (118-150) | < 0.001 |
| Creatinine, mg/dL | 1.0 (0.4-6.8) | 1.3 (0.7-8.3) | < 0.001 |
| Bilirubin, mg/dL | 2.7 (0.2-68.4) | 4.7 (0.4-56.3) | < 0.001 |
| INR | 1.3 (0.8-5.0) | 1.2 (0.8-13.0) | 0.078 |
| MELD | 14 (6-40) | 20 (6-40) | < 0.001 |
| UKELD | 55 (43-77) | 56 (44-79) | 0.041 |
| Ascites (%) | 669 (62.9) | 38 (25.5) | < 0.001 |
| Encephalopathy (%) | 350 (33.0) | 52 (34.9) | 0.637 |
The most common indication for LRT was vascular complications (thrombotic and non-thrombotic infarction of the graft) in 49 (33%) followed by graft rejection in 40 (27%), disease recurrence in 35 (23%), early graft dysfunction in 18 (12%) and 8 for other indications (5%). There were 30 patients (20%) who had biliary complications; however, only 3 (10%) patients developed graft failure secondary to biliary complications. Biliary strictures following PLT (anastomotic, hilar, papillary stenosis) were managed endoscopically, except for 2 patients who required per cutaneous biliary interventions. Eventually, 8 patients (27%) underwent biliary reconstruction for definitive management of post-transplant biliary complications. Thirty seven (78%) of patients with vascular-related complications had hepatic artery thrombosis, 9 (18%) had non thrombotic graft infarction, 1 (2%) had veno-occlusive disease and 1 (2%) had portal vein thrombosis resulting in graft infarction.
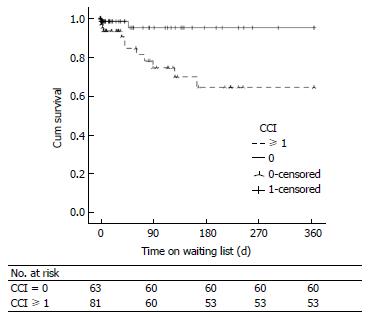
There were 84 patients (56%) who had ≥ 1 comorbidity as defined by CCI. The most common comorbidity was RI in 66 (44.3%), followed by DM in 25 (16.8), chronic pulmonary disease in 2 (1.3%) and 1 patient (0.7%) for each of cerebrovascular disease, connective tissue disease and history of previous malignancy. None of the patients had coronary artery disease, congestive heart failure or peripheral vascular disease according to CCI definitions. There was higher percentage of patients who died on the WL or delisted with ≥ 1 comorbidity compared to those without any comorbidity (76% vs 53%, P = 0.044). There was a higher percentage of patients with ≥ 1 comorbidity in those assessed for LRT compared to those assessed for PLT (56% vs 43%, P = 0.002). The CCI (HR = 2.688, 95%CI: 1.222-5.912, P = 0.014) and the presence of any comorbidity (HR = 5.475, 95%CI: 1.177-25.646, P = 0.030) were independently associated with WL mortality on Cox proportional hazard analysis (Table 2). Only DM and RI as individual comorbidities were included in the Cox model because of the infrequency of other comorbidities in this cohort. RI (HR = 3.802, 95%CI: 1.147-12.603, P = 0.029) was independently associated with WL mortality. WL mortality in patients with any comorbidity was higher compared to those without comorbidities as shown in Figure 1.
| Variable | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age > 60 yr | 2.959 | 0.550-3.896 | 0.048 | 3.102 | 1.015-9.484 | 0.047 |
| DM | 1.587 | 0.499-5.042 | 0.434 | |||
| Renal impairment | 4.771 | 1.496-15.217 | 0.008 | 3.802 | 1.147-12.603 | 0.029 |
| CCI continuous | 3.121 | 1.589-6.130 | 0.001 | 2.688 | 1.222-5.912 | 0.014 |
| CCI dichotomous | 6.528 | 1.472-28.962 | 0.014 | 5.475 | 1.177-25.464 | 0.030 |
| Hb, g/dL | 0.755 | 0.545-1.047 | 0.092 | |||
| Platelet count, × 109/mL | 0.994 | 0.986-1.001 | 0.090 | |||
| Bilirubin, mg/dL | 1.012 | 0.979-1.045 | 0.481 | |||
| Creatinine, mg/dL | 3.200 | 1.888-5.421 | < 0.001 | 2.691 | 1.261-5.740 | 0.010 |
| INR | 1.489 | 1.055-2.102 | 0.024 | 1.406 | 0.967-2.044 | 0.075 |
| Encephalopathy | 2.049 | 0.620-6.770 | 0.239 | |||
| Ascites | 2.781 | 1.006-7.682 | 0.049 | |||
| MELD | 1.154 | 1.067-1.248 | < 0.001 | 2.691 | 1.261-5.740 | 0.01 |
| MELD ≥ 18 | 3.827 | 1.190-12.315 | 0.024 | 4.369 | 1.255-15.215 | 0.021 |
| Na, mmol/L | 0.945 | 0.870-1.027 | 0.180 | |||
| UKELD | 1.121 | 1.029-1.220 | 0.009 | 1.117 | 1.037-1.204 | 0.003 |
With regards to post-transplant outcomes, the CCI (HR = 2.823, 95%CI: 1.563-5101, P = 0.001) and the presence of comorbidity (HR = 2.870, 95%CI: 1.306-6.307, P = 0.009) were independently associated with 12-mo patient and graft survival post-LRT on Cox proportional hazard analysis (Table 3).
| Variable | Patient survival | Graft survival | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Univariate analysis | ||||||
| Age | 0.796 | 0.973-1.021 | 0.997 | 0.992 | 0.969-1.016 | 0.515 |
| Early graft dysfunction | 2.143 | 0.919-4.998 | 0.078 | 1.788 | 0.776-4.123 | 0.173 |
| DM | 2.242 | 0.961-5.228 | 0.062 | 2.004 | 0.869-4.618 | 0.103 |
| Renal impairment | 4.385 | 2.133-9.017 | < 0.001 | 3.494 | 1.759-6.941 | < 0.001 |
| CCI continuous | 3.344 | 1.949-5.738 | < 0.001 | 2.755 | 1.638-4.633 | < 0.001 |
| CCI dichotomous | 3.56 | 1.691-7.493 | 0.001 | 2.751 | 1.377-5.494 | 0.004 |
| Pre-LRT mechanical ventilation | 3.044 | 1.461-6.342 | 0.003 | 2.456 | 1.210-4.983 | 0.013 |
| Pre-LRT vasopressor support | 4.714 | 2.239-9.928 | < 0.001 | 3.618 | 1.758-7.443 | < 0.001 |
| Pre-LRT renal replacement therapy | 4.233 | 2.029-8.829 | < 0.001 | 3.271 | 1.630-6.562 | 0.001 |
| Transplant from ICU | 2.744 | 1.318-5.712 | 0.007 | 2.101 | 1.049-4.206 | 0.036 |
| MELD score ≥ 18 | 4.714 | 2.239-9.928 | 0.009 | 3.105 | 1.399-6.890 | 0.005 |
| Encephalopathy at LRT | 2.593 | 1.213-5.544 | 0.014 | 2.28 | 1.121-4.639 | 0.023 |
| Hb, g/dL | 0.791 | 0.629-0.994 | 0.044 | 0.792 | 0.636-0.985 | 0.037 |
| ABO mismatch | 2.37 | 1.015-5.532 | 0.046 | 2.338 | 1.053-5.190 | 0.037 |
| Cold ischemia time (h) | 1.113 | 0.987-1.255 | 0.082 | 1.081 | 0.962-1.216 | 0.191 |
| DRI | 0.68 | 0.236-1.963 | 0.476 | 0.693 | 0.250-1.918 | 0.476 |
| DRI > 1.8 | 1.736 | 0.772-3.902 | 0.180 | 1.67 | 0.747-3.737 | 0.212 |
| Multivariate analysis | ||||||
| Renal impairment | 3.215 | 1.147-12.603 | 0.005 | 2.543 | 1.160-5.573 | 0.020 |
| CCI Continuous | 2.823 | 1.563-5.101 | 0.001 | 2.350 | 1.331-4.148 | 0.003 |
| CCI Dichotomous | 2.87 | 1.306-6.307 | 0.009 | 2.223 | 1.067-4.633 | 0.033 |
| Pre-LRT mechanical ventilation | 2.52 | 1.126-5.637 | 0.024 | 2.099 | 0.968-4.552 | 0.060 |
| Pre-LRT vasopressor support | 4.004 | 1.554-10.314 | 0.004 | 3.023 | 1.216-7.514 | 0.017 |
| Pre-LRT renal replacement therapy | 2.691 | 1.261-5.740 | 0.01 | 2.441 | 1.107-5.383 | 0.027 |
| Transplant from ICU | 1.859 | 0.794-4.354 | 0.153 | 1.437 | 0.640-3.230 | 0.380 |
| MELD score ≥ 18 | 2.506 | 1.044-6.018 | 0.04 | 2.512 | 1.098-5.743 | 0.029 |
| Encephalopathy at LRT | 1.922 | 0.856-4.315 | 0.113 | 1.626 | 0.752-3.515 | 0.217 |
| Hb, g/dL at LRT | 0.883 | 0.694-1.125 | 0.314 | 0.883 | 0.698-1.116 | 0.297 |
| ABO mismatch | 1.795 | 0.739-4363 | 0.197 | 1.827 | 0.791-4.220 | 0.158 |
Sixteen out of 144 patients (11%) died awaiting a graft. Eight had disease recurrence (of which 5 had HCV recurrence), 3 had vascular complications, 4 had graft rejection and 1 had other indication for LRT. None of the patients with early graft dysfunction died awaiting a graft. WL mortality for PLT was significantly higher compared to LRT (24% vs 11%, P < 0.001). However, median waiting time was significantly shorter for LRT compared to PLT (16 d, range: 0-1118 d vs 100 d, range: 1-922, P < 0.001).
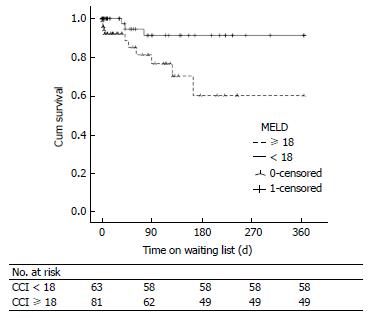
Table 2 summarises variables associated with WL mortality on univariate and multivariate analysis. Only age > 60 years and the presence of ascites were included as fixed variables in the multivariate model to prevent interaction of variables with similar clinical relevance (such as creatinine, MELD, RI, comorbidity). Factors which were independently associated with WL mortality were age > 60 years, RI, creatinine level, the presence of comorbidity, CCI, MELD score and UKELD score. Figure 2 illustrates significantly increased 12 mo WL mortality in patients with MELD score ≥ 18 on Kaplan Meier survival analysis (Log-Rank: 6.741, P = 0.009). Similar findings observed with MELD cut-off value of 25 (Log-Rank: 8.195, P = 0.004). WL mortality was not increased when comparing patients listed for their second graft (n = 118) to those listed for their third or more grafts (n = 26) (Log-Rank: 0.156, P = 0.693).
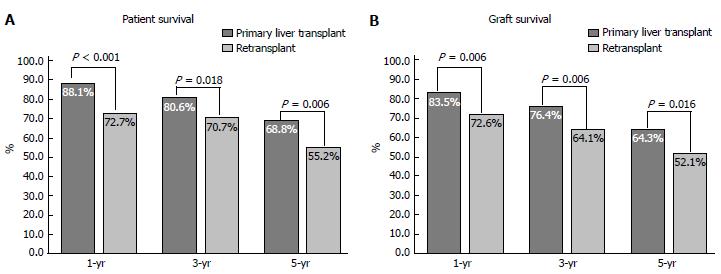
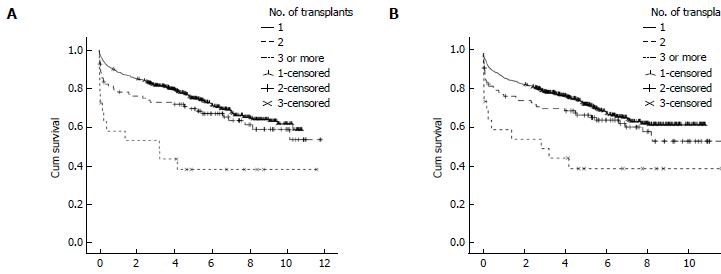
The 1-, 3- and 5-year post-transplant patient and graft survival were significantly lower for patients who had LRT compared to those who had PLT. Figure 3 summarise these findings. In retransplanted patients, patient and graft survival were significantly different according to the number of grafts transplanted as analysed by Kaplan Meier survival method (Figure 4). The difference is mainly attributed to the inferior post-transplant survival of patients who had ≥ 3 transplants. There was no significant difference in patient or graft survival between patients who had PLT and patients who had a second transplant, Log-Rank = 1.741, P = 0.187 and Log-Rank = 2.225, P = 0.136, respectively. Patients who received ≥ 3 grafts had significantly decreased 5-year survival of 40% compared to 72% in those who received 1 graft and 64% in patients who received 2 previous grafts (Log-Rank test: 13.737, P < 0.001).
With regards to the time interval between liver transplantation and repeat transplant, patients who were transplanted between day 8-30 had the worse 1-year post transplant survival followed by those transplanted within the first 7 d. Patients who were transplanted > 30 d had significantly improved 1-year post-LRT survival (Log-Rank test: 6.952, P = 0.031). Table 4 summarises prognostic variables and indications for LRT according to time interval between transplants. Age, MELD at transplantation and the presence of comorbidity were not significantly different between the groups. Majority of patients who had LRT within 7 d of index transplant had early graft dysfunction. Majority of patients who had LRT 8-30 d of index transplant had vascular complications.
| Time between transplants (d) | 0-7 (n = 19) | 8-30 (n = 16) | > 30 (n = 86) | P value |
| Age | 54 (18-67) | 44 (20-63) | 43 (19-70) | 0.086 |
| Transplant MELD | 22 (10-40) | 17 (8-36) | 17 (6-31) | 0.104 |
| CCI ≥ 1, n (%) | 10 (71) | 11 (61) | 44 (49) | 0.095 |
| Indication, n (%) | ||||
| Early graft dysfunction | 14 (74) | 2 (13) | 2 (2) | < 0.001 |
| Graft rejection | 0 (0) | 1 (6) | 30 (35) | < 0.001 |
| Vascular | 5 (26) | 13 (81) | 23 (27) | < 0.001 |
| Disease recurrence | 0 (0) | 0 (0) | 24 (28) | 0.001 |
| Other indications | 0 (0) | 0 (0) | 7 (8) | 0.106 |
We performed Cox proportional hazard analysis to identify factors associated with post-transplant patient and graft survival in retransplanted patients. CCI (OR = 2.048, 95%CI: 1.294-3.241, P = 0.002), the presence of any comorbidity (HR = 1.920, 95%CI: 1.092-3.373, P = 0.023) and requirement for RRT (HR = 1.890, 95%CI: 1.044-3.424, P = 0.036) were associated with post-LRT patient survival on univariate analysis. Liver prognostic models (MELD, UKELD), donor or graft variables were not associated with patient survival in retransplanted patients. With regards to graft survival, only vasopressor support prior to LRT was associated with increased graft loss on univariate analysis (HR = 1.974, 95%CI: 1.033-3.744, P = 0.04).
Table 3 summarises variables associated with 1-year post-LRT patient and graft survival on Cox proportional hazard analysis. Only the presence of encephalopathy at LRT, Hb level at LRT and ABO mismatch were chosen as fixed variables in the model to avoid cross interaction between variables of similar clinical significance. The CCI, RI, pre-LRT mechanical ventilation, requirement for renal replacement therapy, vasopressor support and listing MELD ≥ 18 were independently associated with 1-year patient survival. Similar findings were found for graft survival except for mechanical ventilation which was not associated with outcome.
Median donor age was 44 years (10-76), 55 (45.5%) of donors were males. Donor - recipient gender mismatch was seen in 45 cases (37.2%). Donor ethnicity was Caucasian in 117 (96.7%). Donor cause of death was trauma related in 20 donors (16.5%). Median donor height was 170 cm (147-196), median donor weight was 70 kg (28-110) and BMI was 24 kg/m2 (16-34). Only 4 patients received split organs and 1 patient received a graft donated after cardiac death (DCD). Blood group mismatch was seen in 17 cases (14%). Median cold ischemia time was 10.55 h (0.92-19.53) and median DRI was 1.511 (1.0-2.8). There were 24 patients (20.2%) who received marginal grafts (DRI > 1.8). The DRI, or components of DRI in isolation, and DRI > 1.8 were not associated with 12-mo or long-term post-transplant patient or graft survival on Cox proportional hazard analysis.
The CCI was originally developed and validated as a tool to predict hospital outcome in general medical patients[20]. Composed of medical conditions with varying assigned weights, versions of CCI were found to predict outcomes in multiple clinical settings[21-26]. In this study, we reported on 150 episodes of assessment for LRT from a single centre. We demonstrated that comorbidity as defined by CCI is common (56%) in patients assessed for LRT, and higher than that reported for PLT (40%)[14]. This high prevalence of comorbidity is mainly attributed to the high prevalence of renal impairment (44%) in this cohort. It is difficult to estimate the rate of renal dysfunction in LRT patients from previously published studies[3,4,12,18]. RI was seen in 33% of candidates listed for PLT according to the data of the SRTR[7].
Renal dysfunction is a well recognised complication in patients with ESLD, critical illness and in PLT[27-30]. Renal impairment is known to have detrimental impact on survival of patients with ESLD[31,32]. Therefore, the increased prevalence of RI in our cohort can be explained by the fact that patients listed for LRT have more severe liver dysfunction, reflected by higher MELD scores compared to PLT patients and also by the large proportion of patients who were transplanted from ICU (33%) reflecting the severity of their illness. Furthermore, standard immunosuppression agents with Calcineurin inhibitors such as Ciclosporin or Tacrolimus which is routinely used following liver transplantation to prevent rejection are known to cause or at least contribute to renal impairment following liver transplantation[33]. Other comorbidities, apart from DM, were rare which may be explained by the relatively young median age of patients listed for LRT compared to PLT. The younger age of LRT patients compared to PLT is consistent with previous reports[18,34].
This is the first study to demonstrate the impact of comorbidity on WL mortality in LRT patients. The presence of any comorbidity defined by the CCI was independently associated with a greater than 5 times the risk of death on the wait list. Furthermore, this study has shown that the presence of any comorbidity was associated with twice the risk of post-LRT patient death. Similarly, comorbidity was associated with a three-fold increased risk of patient death and two fold increased risk of graft loss within 12 mo post-LRT. The only study to date which investigated the effect of comorbidity on post liver transplant outcome showed that the presence of any comorbidity was associated with 21% increase in patient death following PLT[14]. Comorbidity was also found to predict post-transplant outcome in patients who received renal and allogeneic stem cell transplantation[35-38].
We have demonstrated in this study that the median MELD score for patients assessed for LRT was significantly higher compared to PLT patients. We have also shown that the increase in MELD among LRT candidates was attributable to the high median bilirubin and creatinine levels but not to an increase in INR which is consistent with UNOS data (Table 1)[34]. We have also shown that the already established models to assess the severity of hepatic impairment (MELD and UKELD) were independently associated with WL mortality. Furthermore, MELD score at a cut-off as low as 18 was associated with WL mortality which was increased by more than 4 fold. This suggests that patients listed for LRT with MELD score ≥ 18 may benefit from prioritization on WL and earlier transplantation to improve LRT outcome.
Our data showed increased WL mortality in LRT patients with MELD score of 18 or higher. In a report from The University of Nebraska, Watt et al[39] showed that MELD score was predictive of WL mortality in 63 patients listed for a second transplant. WL mortality was also shown to increase with increasing MELD scores, especially at the lower range of MELD[34]. None of the other previously reported studies examined the performance of MELD in predicting WL mortality in LRT patients. Instead, these reports focused on factors predictive of post-LRT outcomes[2,3,5,11,18,40,41]. Surprisingly, WL mortality was lower for LRT patients (11% vs 24%, P < 0.001), discordant to previous reports[34]. This can be explained by the fact that patients listed for LRT had significantly shorter median waiting time (16 d vs 100 d, P < 0.001) which may indicate an informal prioritization mechanism for patients listed for LRT in our hospital. Our report also suggests that UKELD score retains its predictive capacity of WL mortality in patients listed for LRT with a 12% rise in WL mortality with every point increase in the UKELD score. Another important finding of the current study is that recipient age > 60 years was independently associated with death on the WL in LRT patients, consistent with previous studies that identified advanced recipient age as a risk factor for WL mortality in patients listed for PLT[28,42,43].
We have shown that 1-, 3- and 5-year patient and graft survival were inferior in patients who underwent retransplantation, consistent with previously published reports[5,13]. This inferior post-transplant survival in our cohort is mainly attributed to the poor post-LRT survival in patients who received ≥ 3 grafts. Patients who had a second graft had slightly lower patient survival compared to PLT. Although these findings contrast with the outcome of patients who underwent retransplantation 1984-2001 at The University of California Los Angeles, improved survival of patients who had a second transplant in our cohort may be explained by both a different era of transplantation, advances made in immunosupression and local patient selection processes[13]. Our findings also suggest that a second liver transplant may represent an acceptable use of donated organs in selected patients. However, if we take into consideration the rule of 50% survival benefit at 5 years post-transplant, according to our findings a third or subsequent grafts may not represent an appropriate use of donated organs, except in rare instances[6].
Published reports suggested that the time interval between PLT and LRT has an influence on post-transplant outcome. Reports from 2 transplant programs indicated that LRT 4-30 d or 8-30 d following first transplant carries a worse post-transplant survival[11,13,40]. Our data showed inferior survival in patients who were transplanted within 30 d from previous liver transplantation, irrespective of whether LRT occurred in the first 7 d or between 8-30 d. In our cohort, the most common indication for LRT within the first 7 d following a previous transplant was early graft dysfunction whilst vascular complications (thrombotic and non-thrombotic graft infarction) were the primary indication in patients who had LRT 8-30 d following a previous transplant. This increased post-LRT mortality in patients who receive early LRT may be explained by severity of illness, intense immunosuppression, hence increased risk of infections[2,44]. Our findings are consistent with those of Rosen et al[18] who reported significantly inferior long term survival in patients who had LRT for PNF and vascular complications. In both United States and the United Kingdom, in recognition of the severity of illness and the high mortality associated with PNF and early HAT without LRT, an urgent priority for LRT is given[45,46].
Regarding post-LRT survival, we demonstrated that the CCI, RI, MELD score ≥ 18 and requirement for organ support were independent factors associated with 1-year post-LRT patient and graft survival consistent with the reported literature in which MEDL, or individual components of MELD, were associated with post-LRT outcome[2,3,9,10,12,40,41]. Similarly, requirement for mechanical ventilation and renal replacement therapy were found to negatively impact on post-LRT outcome in agreement with the reported literature[2,5,12,40]. Interestingly, we identified pre-LRT vasopressor support as the only factor associated with long term graft outcome. Vasopressor use was also an independent factor associated with 12 mo post-transplant patient and graft survival. This finding has never been reported in previous studies. The requirement for vasopressors may therefore reflect the severity of recipient illness with hemodynamic instability and it may indirectly suggest the negative impact of graft ischemia on patient and graft survival.
Despite our detailed analysis of donor and graft variables, we found no association between graft quality and post-LRT outcomes. This is likely to reflect our local donor-recipient matching practices demonstrated by the limited use of marginal grafts in this cohort and a low median donor age of 44 years which is well within the confines of non-extended criteria donor parameters. Few studies analyzed the impact of graft and donor factors on post-LRT survival. Whilst Pfitzmann et al[5] found no correlation between graft survival and donor variables, others identified donor age, ethnicity and warm ischemia time as factors independently associated with inferior outcome[5,10,40].
Limitations of this study were that it represents a single centre experience; therefore, applicability of the findings on other cohorts may be limited. Secondly, data on immunosuppression were not included in our analysis, although standard immunosuppression was used in all cases except for patients with eGFR < 50 mL/min, a renal sparing regimen of low dose Tacrolimus and interleukin-2 (IL-2) blocker and prednisolone was used preferentially. Indeed, the choice of immunosuppression not only can influence post-transplant outcomes in patients underwent PLT, it can influence the rate of complications related to immunosuppression such as RI which we found as an important factor associated with inferior patient and graft survival[33,47,48]. Thirdly, we used a version of the CCI tested in liver transplant cohort[14]. Therefore, the impact of other comorbidities on post-transplant survival such as inflammatory bowel disease, peptic ulcer disease, valvular heart disease or obesity, which were found to affect patient survival, remains unknown given they were not incorporated in the model[20,49]. Lastly, although the definitions of individual comorbidities were consistent with previous reports, clinical applicability of these definitions maybe limited.
In conclusion, our data indicates that the presence of comorbidity in liver retransplant candidates increases mortality on the WL and following LRT. The severity of recipient liver disease was associated with WL mortality. MELD score was able to discriminate between survival and death whilst on the WL at a lower cut-off value of 18 which suggests that patients undergoing LRT should be transplanted at lower MELD scores. Post-transplant mortality progressively increased according to the number of transplanted grafts; however, the greatest adverse impact was seen after transplanting ≥ 3 grafts with only 40% 5-year survival seen in this group. Graft and donor variables were not found to influence patient or graft survival in this study which may reflect centre-related donor-recipient matching.
The prevalence and impact of comorbidities on waiting list (WL) and post-transplant survival in patients undergoing liver retransplantation (LRT) is not known. This study evaluates the impact of comorbidity on the above parameters.
Model for end-stage liver disease (MELD) score > 25, recipient age, creatinine level, bilirubin level, indication for retransplantation, the urgency for LRT, coma episodes, haemoglobin level and the number of fresh frozen plasma units transfused were identified as factors associated with reduced post-LRT survival in a number of studies.
Comorbidity in liver retransplant patients increases mortality on the WL and following LRT. MELD score of ≥ 18 was associated with increased risk of death on WL and within 12 mo following retransplantation. Post-transplant mortality progressively increased according to the number of transplanted grafts.
Patients undergoing LRT should be transplanted at lower MELD scores. Assessment of comorbidity in LRT candidates can provide important prognostic information. A third or subsequent grafts may not represent an appropriate use of donated organs, except in rare instances.
ALD: Alcohol-related liver disease; CCI: Charlson comorbidity index; CI: Confidence interval; CIT: Cold ischemia time; DBD: Donation after brain death; DCD: Donation after cardiac death; DM: Diabetes mellitus; DRI: Donor risk index; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus infection; ICU: Intensive care unit; IL-2: Interleukin-2; INR: International normalized ratio; LRT: Liver retransplantation; MELD: Model for end-stage liver disease; Na: Sodium; NHS: National Health Services; OR: Odds ratio; PLT: Primary liver transplantation; RI: Renal impairment; SRTR: Scientific Registry of Transplant Recipients; UCLA: University of California Los Angeles; UKELD: United Kingdom End-stage Liver Disease; UNOS: United Network of Organ Sharing; WL: Waiting list.
Very well written paper.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Facciorusso A, Karthik SV, Nicolas CT S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Ghobrial RM, Farmer DG, Baquerizo A, Colquhoun S, Rosen HR, Yersiz H, Markmann JF, Drazan KE, Holt C, Imagawa D. Orthotopic liver transplantation for hepatitis C: outcome, effect of immunosuppression, and causes of retransplantation during an 8-year single-center experience. Ann Surg. 1999;229:824-831; discussion 831-833. [PubMed] |
| 2. | Markmann JF, Markowitz JS, Yersiz H, Morrisey M, Farmer DG, Farmer DA, Goss J, Ghobrial R, McDiarmid SV, Stribling R. Long-term survival after retransplantation of the liver. Ann Surg. 1997;226:408-418; discussion 418-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 172] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Rosen HR, Madden JP, Martin P. A model to predict survival following liver retransplantation. Hepatology. 1999;29:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Ghobrial RM. Retransplantation for recurrent hepatitis C. Liver Transpl. 2002;8:S38-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Pfitzmann R, Benscheidt B, Langrehr JM, Schumacher G, Neuhaus R, Neuhaus P. Trends and experiences in liver retransplantation over 15 years. Liver Transpl. 2007;13:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 6. | Neuberger J, Gimson A, Davies M, Akyol M, O’Grady J, Burroughs A, Hudson M. Selection of patients for liver transplantation and allocation of donated livers in the UK. Gut. 2008;57:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1003-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Biggins SW. Futility and rationing in liver retransplantation: when and how can we say no? J Hepatol. 2012;56:1404-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Watt KD, Lyden ER, McCashland TM. Poor survival after liver retransplantation: is hepatitis C to blame? Liver Transpl. 2003;9:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Ghabril M, Dickson R, Wiesner R. Improving outcomes of liver retransplantation: an analysis of trends and the impact of Hepatitis C infection. Am J Transplant. 2008;8:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Powelson JA, Cosimi AB, Lewis WD, Rohrer RJ, Freeman RB, Vacanti JP, Jonas M, Lorber MI, Marks WH, Bradley J. Hepatic retransplantation in New England--a regional experience and survival model. Transplantation. 1993;55:802-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Doyle HR, Morelli F, McMichael J, Doria C, Aldrighetti L, Starzl TE, Marino IR. Hepatic Retransplantation--an analysis of risk factors associated with outcome. Transplantation. 1996;61:1499-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 118] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, Saab S, Han S, Durazo F, Weaver M. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. 2005;241:905-916; discussion 916-918. [PubMed] |
| 14. | Volk ML, Hernandez JC, Lok AS, Marrero JA. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transpl. 2007;13:1515-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1865] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 16. | Barber K, Madden S, Allen J, Collett D, Neuberger J, Gimson A. Elective liver transplant list mortality: development of a United Kingdom end-stage liver disease score. Transplantation. 2011;92:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1489] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 18. | Rosen HR, Prieto M, Casanovas-Taltavull T, Cuervas-Mons V, Guckelberger O, Muiesan P, Strong RW, Bechstein WO, O’grady J, Zaman A. Validation and refinement of survival models for liver retransplantation. Hepatology. 2003;38:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Edwards E, Harper A. Does MELD work for relisted candidates? Liver Transpl. 2004;10:S10-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 38321] [Article Influence: 1008.4] [Reference Citation Analysis (0)] |
| 21. | Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 319] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Wang CY, Lin YS, Tzao C, Lee HC, Huang MH, Hsu WH, Hsu HS. Comparison of Charlson comorbidity index and Kaplan-Feinstein index in patients with stage I lung cancer after surgical resection. Eur J Cardiothorac Surg. 2007;32:877-881. [PubMed] |
| 24. | Christensen S, Johansen MB, Christiansen CF, Jensen R, Lemeshow S. Comparison of Charlson comorbidity index with SAPS and APACHE scores for prediction of mortality following intensive care. Clin Epidemiol. 2011;3:203-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Thompson HJ, Rivara FP, Nathens A, Wang J, Jurkovich GJ, Mackenzie EJ. Development and validation of the mortality risk for trauma comorbidity index. Ann Surg. 2010;252:370-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Hines RB, Chatla C, Bumpers HL, Waterbor JW, McGwin G, Funkhouser E, Coffey CS, Posey J, Manne U. Predictive capacity of three comorbidity indices in estimating mortality after surgery for colon cancer. J Clin Oncol. 2009;27:4339-4345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2069] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 28. | Luca A, Angermayr B, Bertolini G, Koenig F, Vizzini G, Ploner M, Peck-Radosavljevic M, Gridelli B, Bosch J. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Silvester W, Bellomo R, Cole L. Epidemiology, management, and outcome of severe acute renal failure of critical illness in Australia. Crit Care Med. 2001;29:1910-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 458] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 31. | Fernández-Esparrach G, Sánchez-Fueyo A, Ginès P, Uriz J, Quintó L, Ventura PJ, Cárdenas A, Guevara M, Sort P, Jiménez W. A prognostic model for predicting survival in cirrhosis with ascites. J Hepatol. 2001;34:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Cooper GS, Bellamy P, Dawson NV, Desbiens N, Fulkerson WJ, Goldman L, Quinn LM, Speroff T, Landefeld CS. A prognostic model for patients with end-stage liver disease. Gastroenterology. 1997;113:1278-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Lucey MR, Abdelmalek MF, Gagliardi R, Granger D, Holt C, Kam I, Klintmalm G, Langnas A, Shetty K, Tzakis A. A comparison of tacrolimus and cyclosporine in liver transplantation: effects on renal function and cardiovascular risk status. Am J Transplant. 2005;5:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Kim HJ, Larson JJ, Lim YS, Kim WR, Pedersen RA, Therneau TM, Rosen CB. Impact of MELD on waitlist outcome of retransplant candidates. Am J Transplant. 2010;10:2652-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Sorror ML, Maris MB, Storer B, Sandmaier BM, Diaconescu R, Flowers C, Maloney DG, Storb R. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004;104:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 36. | Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912-2919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 2347] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 37. | Jassal SV, Schaubel DE, Fenton SS. Baseline comorbidity in kidney transplant recipients: a comparison of comorbidity indices. Am J Kidney Dis. 2005;46:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, Sandmaier BM, Storb R. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Watt KD, Menke T, Lyden E, McCashland TM. Mortality while awaiting liver retransplantation: predictability of MELD scores. Transplant Proc. 2005;37:2172-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Hong JC, Kaldas FM, Kositamongkol P, Petrowsky H, Farmer DG, Markovic D, Hiatt JR, Busuttil RW. Predictive index for long-term survival after retransplantation of the liver in adult recipients: analysis of a 26-year experience in a single center. Ann Surg. 2011;254:444-448; discussion 444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Yao FY, Saab S, Bass NM, Hirose R, Ly D, Terrault N, Lazar AA, Bacchetti P, Ascher NL, Roberts JP. Prediction of survival after liver retransplantation for late graft failure based on preoperative prognostic scores. Hepatology. 2004;39:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Ripoll C, Bañares R, Rincón D, Catalina MV, Lo Iacono O, Salcedo M, Clemente G, Núñez O, Matilla A, Molinero LM. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD Era. Hepatology. 2005;42:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 43. | Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 524] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 44. | Marudanayagam R, Shanmugam V, Sandhu B, Gunson BK, Mirza DF, Mayer D, Buckels J, Bramhall SR. Liver retransplantation in adults: a single-centre, 25-year experience. HPB (Oxford). 2010;12:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | OPTN. Organ distribution: allocation of livers. [Accessed 02/02/2011]. Available from: http://optntransplanthrsagov/policiesandbylaws2/policies/pdfs/policy_8pdf. |
| 46. | NHS OD. NHS Blood and Transplant Liver Advisory Group. Protocols and guidelines for adults undergoing deceased donor liver transplantation in the UK. 4.1.1 Super-urgent liver transplantation. 2012;NHS Blood and Transplant Liver Advisory Group. Protocols and guidelines for adults undergoing deceased donor liver transplantation in the UK. 4.1.1 Super-urgent liver transplantation (Accessed 24/09/2012) Available from: http://www.organdonation.nhs.uk/ukt/about_transplants/organ_allocation/liver/liver_organ_sharing_principles/liver_organ_sharing_principles.asp#b1. |
| 47. | O’Grady JG, Burroughs A, Hardy P, Elbourne D, Truesdale A. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet. 2002;360:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 48. | Wiesner RH. A long-term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation. 1998;66:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 156] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Martins M, Blais R. Evaluation of comorbidity indices for inpatient mortality prediction models. J Clin Epidemiol. 2006;59:665-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |