Published online Jun 27, 2025. doi: 10.4254/wjh.v17.i6.104073
Revised: February 24, 2025
Accepted: May 21, 2025
Published online: June 27, 2025
Processing time: 192 Days and 22.4 Hours
Primary biliary cholangitis (PBC) is a chronic autoimmune-mediated cholestatic liver disease. Nanoparticles encapsulating rapamycin (ImmTOR) suppress ada
To investigate the effects of ImmTOR in PBC mouse models.
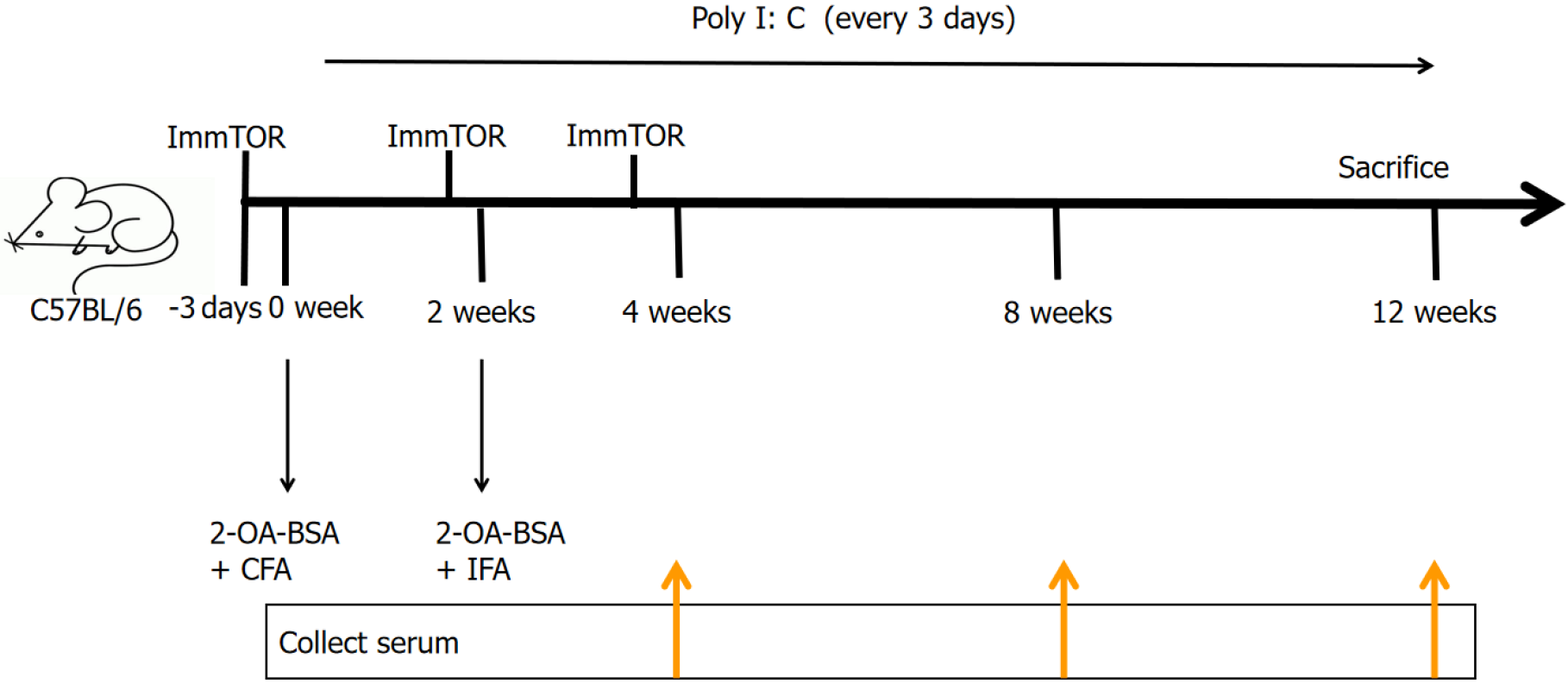
PBC models were induced in C57BL/6 mice by two immunizations of 2-octynoic acid-coupled bovine serum albumin at two-week intervals, and polycytidylic acid every three days. The PBC mouse models were separated into the treatment group and the control group. The levels of alkaline phosphatase (ALP) and alanine aminotransferase in the mice were detected using an automatic biochemical analyzer. Liver and spleen mononuclear cells were analyzed by flow cytometry, and serum anti-mitochondrial antibodies (AMA) and the related cytokines were analyzed by enzyme-linked immunosorbent assay. Liver histopathology was examined by hematoxylin and eosin staining and scored.
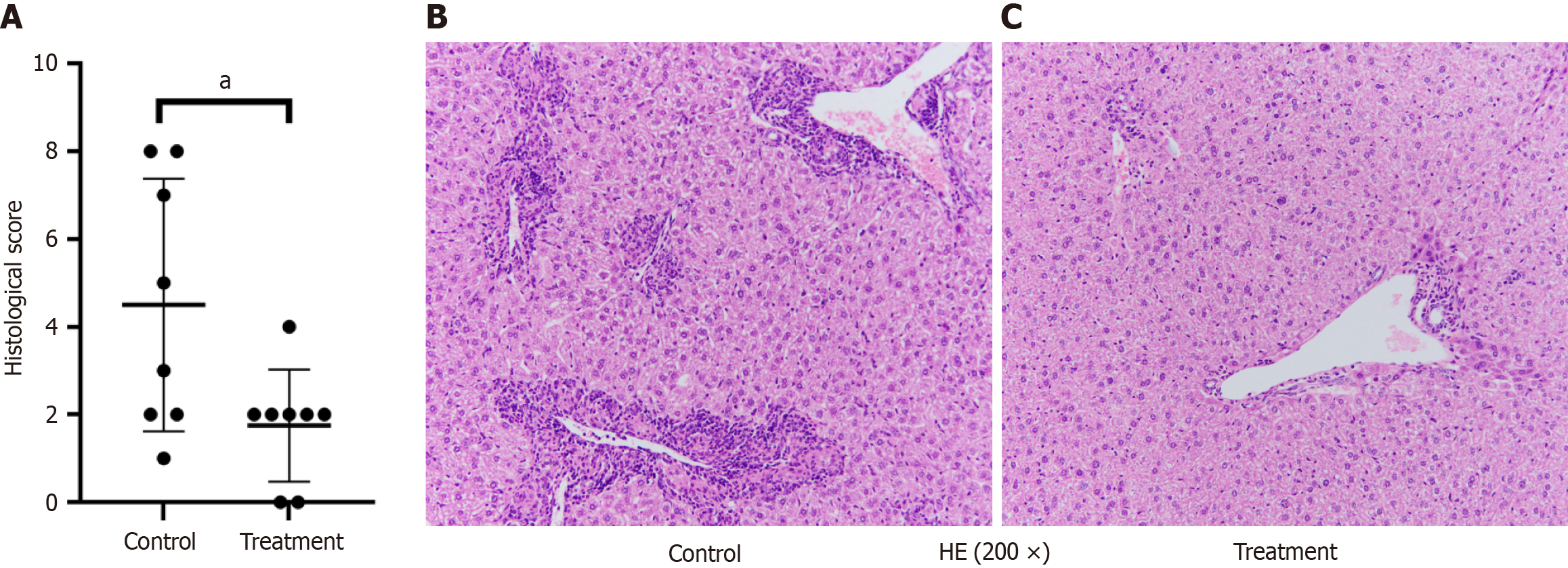
After treatment with ImmTOR, the ALP level was significantly decreased (189.60 U/L ± 27.25 U/L vs 156.00 U/L ± 17.21 U/L, P < 0.05), the level of AMA was reduced (1.28 ng/mL ± 0.27 ng/mL vs 0.56 ng/mL ± 0.07 ng/mL, P < 0.001) and the expression levels of interferon gamma and tumor necrosis factor α were significantly decreased (48.29 pg/mL ± 10.84 pg/mL vs 25.01 pg/mL ± 1.49 pg/mL, P < 0.0001) and (84.24 pg/mL ± 23.47 pg/mL vs 40.66 pg/mL ± 14.65 pg/mL, P < 0.001). The CD4+ T lymphocytes, CD8+ T lymphocytes and B lymphocytes in the liver were significantly reduced, with statistically significant differences (24.21% ± 6.55% vs 15.98% ± 3.03%, P < 0.05; 9.09% ± 1.91% vs 5.49% ± 1.00%, P < 0.001; 80.51% ± 2.96% vs 75.31% ± 4.34%, P < 0.05). The expression of CD8+ T lymphocytes and B lymphocytes in the ImmTOR treatment group also decreased (9.09% ± 1.91% vs 5.49% ± 1.00%, P < 0.001; 80.51% ± 2.96% vs 75.31% ± 4.34%, P < 0.05). The liver pathology of PBC mice in the treatment group showed reduced inflammation and a decreased total pathology score, and the difference in the scores was statistically significant (4.50 ± 2.88 vs 1.75 ± 1.28, P < 0.05).
ImmTOR can improve biochemistry and pathology of liver obvious by inhibiting the expression of CD8+ T cells and B cells, and reducing the titer of AMA.
Core Tip: Primary biliary cholangitis (PBC) is a chronic autoimmune-mediated cholestatic liver disease, which can gradually progress to liver fibrosis, cirrhosis. The global incidence of PBC has been increasing year by year in recent decades. But there are few effective drugs, the effect of nanoparticles encapsulating rapamycin on the treatment of PBC using an animal model is examined in this paper, targeting at new approaches for the treatment of PBC.
- Citation: Yang YS, Li XR, Wang ZM, Zheng L, Li JL, Cui XL, Song YB, Ma JJ, Guo HF, Gao LX, Zhou XH. Effect of rapamycin nanoparticles in an animal model of primary biliary cholangitis. World J Hepatol 2025; 17(6): 104073
- URL: https://www.wjgnet.com/1948-5182/full/v17/i6/104073.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i6.104073
Primary biliary cholangitis (PBC) is an organ-specific autoimmune liver disease of unknown etiology, and its pathological characteristic is chronic non-suppurative destructive cholangitis (CNSDC). PBC has an insidious onset and can gradually progress to liver fibrosis, cirrhosis, and even endanger life. Epidemiological studies have found that the global incidence of PBC has been increasing year by year in recent decades[1,2]. Although research on the treatment of PBC has been continuously expanding, ursodeoxycholic acid (UDCA) remains the only first-line drug for PBC currently. Rapamycin is a member of the phosphatidylinositol-3-kinase (PI3K) family, a conserved serine-threonine-protein kinase with a molecular weight of 289 kDa, which can prolong the lifespan of several animals and immunomodulation, and has been recommended for liver transplant patients[3]. This study examines the effects of nanoparticles encapsulating rapamycin (ImmTOR) on the treatment of a PBC mouse model, aiming at developing new approaches for the treatment of PBC.
C57BL/6 mice (6-8 weeks old) were purchased from Beijing Weitong Lihua Experimental Animal Technology [Beijing, China; license number: SCXK (Beijing) 2021-0006]. All mice were housed in the specific pathogen-free-grade breeding room of the Experimental Animal Center of The Second Hospital of Hebei Medical University (22 °C, 55% humidity, and 12-hours day-night cycle) and were raised in strict accordance with the animal ethics requirements of the Experimental Animal Center of The Second Hospital of Hebei Medical University. There were 16 mice, including 12 females and 4 males, with 8 mice in each group. The PBC animal model was appropriately modified based on the 2-octynoic acid-coupled bovine serum albumin (2-OA-BSA)-induced model. The modeling method was as follows: (1) The control group was injected with blank nanoparticles dissolved in phosphate-buffered saline (PBS) 3 days before immunization; and (2) Then 2-OA-BSA was coupled with complete Freund’s adjuvant and injected intraperitoneally (IP), and the final concentration of the reagent was 100 μg/100 μL. Two weeks later, 2-OA-BSA was coupled with incomplete Freund’s adjuvant (IFA) and injected IP once in the model mice. Polyinosinic polycytidylic acid (poly I: C) (5 mg/kg) was then injected once every 3 days until the mice were sacrificed. The treatment group was injected IP with ImmTOR dissolved in PBS, at three specified time points: Three days before immunization, at injection of IFA and at four weeks, for a total of 3 times, and the control group was given PBS at the same time points (Figure 1). PBC in C57BL/6 mice was induced by two IP immuni
ImmTOR were prepared according to the literature[4]. Rapamycin (North China Pharmaceutical Company, Ltd, China.) and Poly (lactic-co-glycolic acid) (PLGA) (Sigma, United States) were also prepared. First, 100 mg PLGA polymer was dissolved in methylene chloride (MC) at a ratio of 5% w/v. Then, rapamycin solution was dissolved in dimethyl sulfoxide, placed in the PLGA-MC solution, and 5% polyvinyl alcohol (PVA) was added at a PVA:MC ratio of 1:1. Subsequently, the mixed solution was homogenized at 35000 rpm for 180 seconds using a tissue homogenizer (IKA T 10 basic ULTRA-TURRAX®, Germany) to form a primary emulsion. After homogenization, this solution was added to 30 mL of 1% PVA solution, and the solution was stirred overnight using a magnetic stirrer to evaporate the residual MC. The following day, the remaining solution was centrifuged at 10000 g for 10 minutes to collect the nanoparticles, and then washed 3 times with double-distilled water. The centrifuged water was removed, and the sample was quickly frozen at
Determination of serum anti-mitochondrial antibodies (AMA) was carried out using the enzyme-linked immunosorbent assay (ELISA). This was carried out in strict accordance with the instructions of the ELISA kit (Mouse anti-mitochondrial antibody M2 subtype kit, ZCIBIO Technology Co., Ltd., Cat ZC-38391). ELISA was also used to detect cytokines. Levels of the cytokine interferon gamma (IFN-γ) were detected using a mouse IFN-γ ELISA kit (Elabscience, product number: E-MSEL-M0007). Tumor necrosis factor α (TNF-α) was detected using a mouse TNF-α ELISA kit (ZCIBIO Technology Co., Ltd., Cat ZC–39024). This was carried out in strict accordance with the instructions of the reagent supplier. Briefly, the diluted serum was added to the ELISA microplate and incubated at room temperature. After washing 5 times, horseradish peroxidase-coupled anti-mouse secondary antibody was added to each well and incubated at room temperature. After incubation, the substrate was added, and the absorbance was measured at 450 nm. The enzyme-labeled instrument was used for measurement, and the concentration of the sample was calculated. Flow cytometry analysis, after the mice were sacrificed, the liver and spleen mononuclear cells were isolated and collected for flow cytometry analysis[5]. All reagents were purchased from BioLegend and the optimal dilution was used as positive and negative controls. First, cell surface staining was performed. The cells were resuspended in a staining buffer (0.2% bovine serum albumin, 0.04% ethylene diamine tetraacetic acid and 0.05% sodium azide in PBS) at a concentration of 1 × 106 and incubated with anti-mouse FcR blocking reagent at 4 °C for 15 minutes. Followingcell washing, monoclonal antibodies conjugated with fluorescent dyes were added and incubated at 4 °C for 30 minutes. The markers used for cell surface staining included peridinin-chlorophyll-protein/Cyanine 5.5-labeled anti-CD3 monoclonal antibody, fluorescein isothiocyanate-labeled anti-CD4, Alexa Fluor 700-labeled anti-CD8a, and APC-labeled anti-CD19. The BD FACS Diva 9.0 flow cytometer was used for detection and analysis. The acquired data were analyzed using CellQuest software (BD Biosciences). Histopathology and hematoxylin and eosin (HE) staining.
Liver tissue was collected immediately after the animals were sacrificed, fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4 μm slices. The paraffin was removed, and HE staining was performed using standard protocols. The stained liver slices were scored for liver inflammation by a pathologist under a light microscope (Olympus). Liver histological scoring was performed as described previously[6,7]. Liver pathology was evaluated by analyzing inflammation in the portal area, lobular inflammation, bile duct structure, bile duct cell damage, and the occurrence and distribution range of granulomas, and corresponding scores were allocated.
The data were analyzed using the GraphPad Prism 8 software. Quantitative data are represented by mean ± SD, median (quartile range) [M (P25, P75)] depending on whether they conformed to a normal distribution, and count data are represented by percentage (%). The quantitative data were tested for normality, and according to the results, t-tests, Mann-Whitney U test or Kruskal-Wallis H test were used, respectively, and the Wilcoxon test was used for data that did not conform to a normal distribution. The χ² test or Fisher's exact test was used for the comparison of count data between groups. A P value < 0.05 was considered statistically significant.
The PBC mice were divided into the control group and the treatment group, and ImmTOR was injected at three different time points in the treatment group. During the entire model construction process, there were no significant differences in the general condition of the mice, including coat color, body weight, food intake, water intake, and activity. No jaundice was observed in the PBC mouse model.
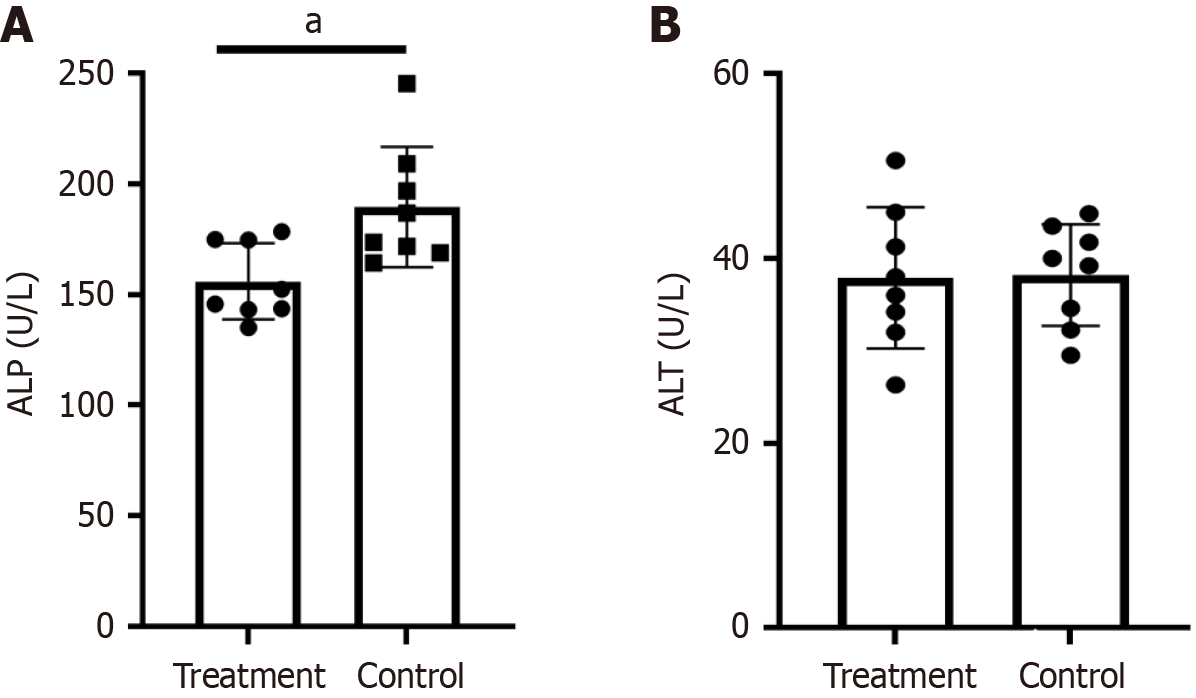
Compared with PBC mice in the treatment group, the ALP level in the PBC control group was significantly increased (189.60 U/L ± 27.25 U/L vs 156.00 U/L ± 17.21 U/L, P < 0.05) (Figure 2A). There was no significant difference in ALT (38.23 U/L ± 5.52 U/L vs 37.93 U/L ± 7.65 U/L, P > 0.05) (Figure 2B).
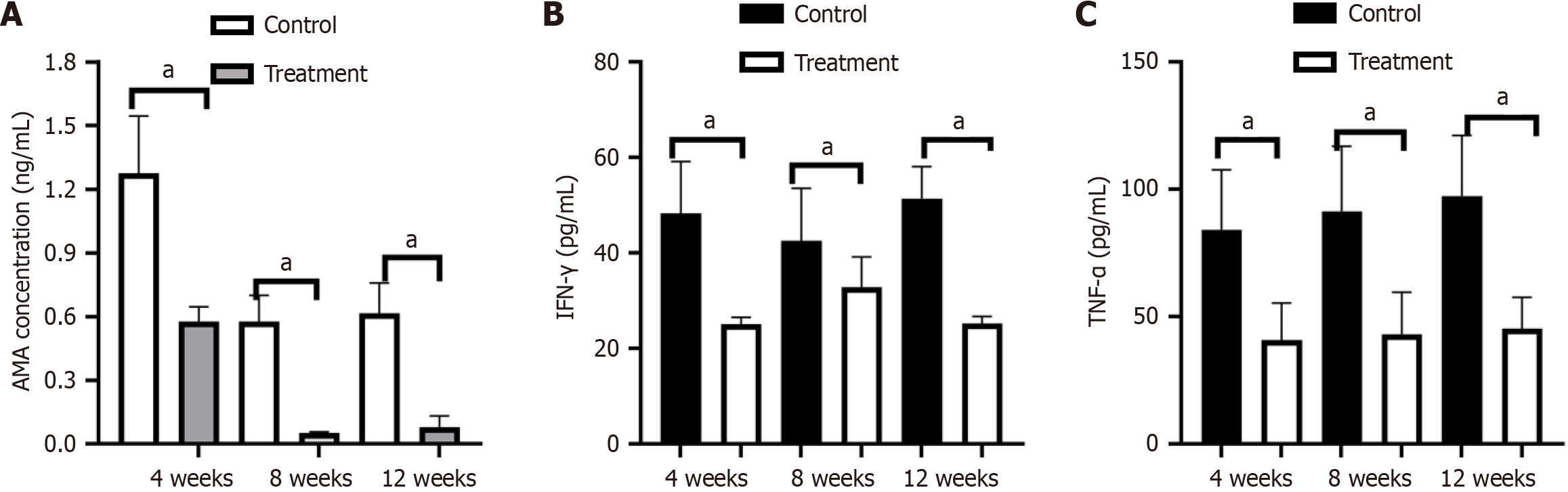
In terms of autoantibodies, pyruvate dehydrogenase complex (PDC)-E2 antibody was positively detected and significantly increased in serum 4 weeks after immunization, and the expression of AMA in the PBC control group was significantly higher than that in the treatment group. With the extension of time, the expression of AMA showed a downward trend. There were notable statistical differences between the control group and the treatment group in the expression of AMA at week 4 (1.28 ng/mL ± 0.27 ng/mL vs 0.56 ng/mL ± 0.07 ng/mL, P < 0.001). The expression of AMA in both groups gradually decreased at week 8 (0.58 ng/mL ± 0.12 ng/mL vs 0.05 ng/mL ± 0.01 ng/mL, P < 0.001), but was still statistically different. At week 12, there was still a significant difference in AMA between the two groups (0.62 ng/mL ± 0.43 ng/mL vs 0.08 ng/mL ± 0.05 ng/mL, P < 0.001), but the level of AMA did not show an obvious change from week 8 to week 12 (Figure 3A).
The expression level of IFN-γ in the control group significantly increased at week 4, and there was a significant difference between the two groups (48.29 pg/mL ± 10.84 pg/mL vs 25.01 pg/mL ± 1.49 pg/mL, P < 0.0001). At week 8, expression in the treatment group was lower, but was not statistically different (42.47 pg/mL ± 11.10 pg/mL vs 34.84 pg/mL ± 6.30 pg/mL, P = 0.051). At week 12, the level of IFN-γ in the treatment group continued to decrease, and there was a significant difference in IFN-γ levels between the two groups (51.46 pg/mL ± 6.61 pg/mL vs 25.26 pg/mL ± 1.42 pg/mL, P < 0.0001) (Figure 3B). The expression level of TNF-α in the control group was significantly increased at week 4, and there was a significant difference between the two groups (84.24 pg/mL ± 23.47 pg/mL vs 40.66 pg/mL ± 14.65 pg/mL, P < 0.001), and there was still a statistical difference between the two groups at week 8 (91.44 pg/mL ± 25.45 pg/mL vs 43.12 pg/mL ± 16.48 pg/mL, P < 0.001) and at week 12 (97.47 pg/mL ± 23.69 pg/mL vs 45.26 pg/mL ± 12.28 pg/mL, P < 0.001) (Figure 3C). During the entire experiment, neither the treatment group nor the control group showed any change compared to before.
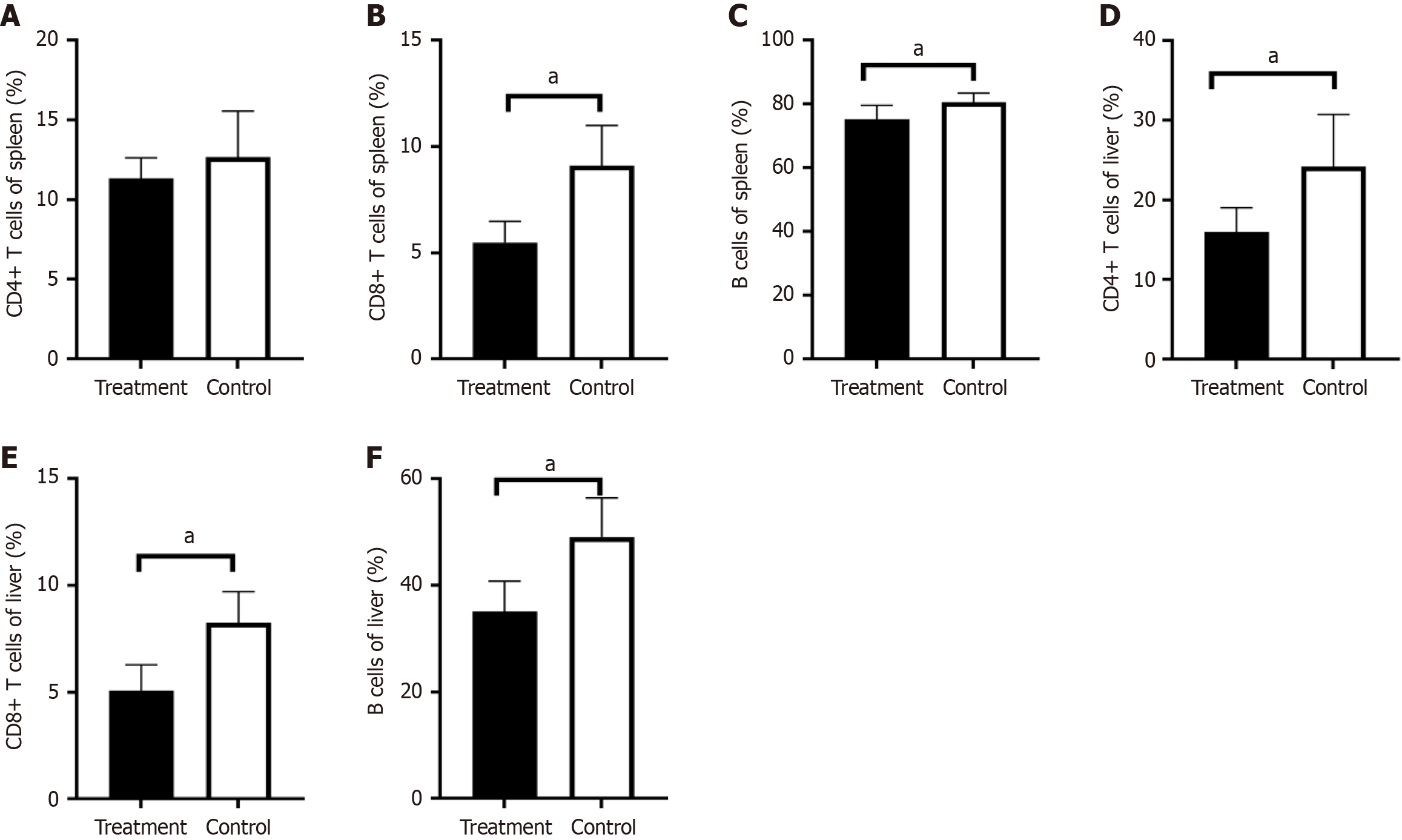
Lymphocyte subsets were analyzed, and the results showed that the expression of total lymphocytes, these results indicated a significant reduction in the expression of T lymphocytes (38.11% ± 8.96% vs 28.09% ± 4.12%, P < 0.05) and the expression of CD4+ T lymphocytes in the liver of treated mice (24.21% ± 6.55% vs 15.98% ± 3.03%, P < 0.05) (Figure 4A). The expression of CD8+ T lymphocytes (8.25% ± 1.45% vs 5.01% ± 1.20%, P < 0.001) (Figure 4B) and B lymphocytes (49.06% ± 7.38% vs 35.11% ± 5.65%, P < 0.001) (Figure 4C) showed a more obvious reduction in the ImmTOR treatment group compared with the control group.
The level of T lymphocytes and CD4+ T lymphocytes in the spleen of the two groups were not statistically different (Figure 4D). However, CD8+ T lymphocytes in the ImmTOR treatment group were significantly reduced, and there was a notable statistical difference in the expression of CD8+ T lymphocytes (9.09% ± 1.91% vs 5.49% ± 1.00%, P < 0.001 ) (Figure 4E). The expression of B lymphocytes in the ImmTOR treatment group also decreased, and there was a statistically significant difference between two groups (80.51% ± 2.96% vs 75.31% ± 4.34%, P < 0.05) (Figure 4F).
In terms of liver pathology, the PBC model showed obvious inflammatory infiltration, extensive bile duct destruction and obvious bile duct hyperplasia following injection of 2-OA-BSA and poly I: C (Figure 5B). When the treatment group was given ImmTOR, liver pathology in PBC mice (Figure 5C) showed reduced inflammation and a decreased total pathology score. The PBC control group and the treatment group had scores of 4.50 ± 2.88 and 1.75 ± 1.28, respectively (Figure 5A), with a statistically significant difference (P < 0.05).
PBC, an organ-specific autoimmune disease, is still difficult to treat. Following treatment with UDCA, 15% of patients still have cirrhosis and other related complications within 15 years[8]. Obeticholic acid, as a second-line treatment, shows unsatisfactory results in the clinic and is listed as a contraindication for use in patients with cirrhosis[9-11]. Rapamycin, as a classic mammalian target of rapamycin inhibitor (mTORi), has various activities and has been studied in organ transplantation, tumors, and various autoimmune diseases. In recent years, research on mTORi in liver diseases has gradually increased. Rapamycin not only has a protective effect on liver injury and liver fibrosis but has also been included in the treatment consensus for liver transplantation[12,13]. The main affected organ in PBC is the liver. Given the complex immune microenvironment of the liver, as a special immune organ, it can uptake a large number of nanoparticles with different characteristics[14]. Therefore, this study attempted to conduct research on ImmTOR in PBC, a liver-specific immune disease.
CNSDC is a typical pathological feature of PBC. In damaged bile duct cells in CNSDC of PBC, there is mitochondrial autophagy dysregulation. This dysregulated autophagy contributes to the abnormal expression of mitochondrial antigens and is involved in the pathogenesis of PBC. UDCA can reduce bile enzymes and improve the symptoms of PBC, and one of the mechanisms of UDCA is inhibition of autophagy. Our study found that ALP in the PBC group of mice significantly decreased after ImmTOR treatment, as rapamycin is a classic autophagy inhibitor of mammalian target of rapamycin (mTOR), which explains this result. However, whether other mechanisms are involved requires further research.
High levels of AMAs and antinuclear antibodies can be detected in the serum of PBC animal models and PBC patients, suggesting that B lymphocytes also participate in the autoimmune process of PBC. However, it has been reported in the literature that the elimination of B cells alone does not reduce liver pathology and the production of inflammatory cytokines in PBC mice[15]. Our study found that the expression of B lymphocytes in the liver and spleen of mice decreased after ImmTOR treatment, and the level of AMA also decreased accordingly. The mTOR inhibitor rapamycin can block the activation of mTORC1 in B cells and downregulate the co-stimulatory molecule to organize B cells to differentiate into plasmablasts, thereby reducing antibody secretion, suggesting that the mTOR pathway participates in the dysfunction and activation of B cells[16]. AMA, as a marker antibody of PBC, targets the PDC-E2 and plays an important role in the pathogenesis and diagnosis of PBC. In this study, the AMA titer decreased after ImmTOR treatment. Potential reasons for this include, on the one hand, ImmTOR reduces the expression of B cells and may have an inhibitory effect on the production of autoantibodies. On the other hand, mTOR, as a sensor of metabolic stress, monitors the available glucose content through mitochondrial activity and plays an important role in mitochondrial oxidative stress and metabolism. In vitro experiments have confirmed that rapamycin-related proteins can be integrated into the mito
Rapamycin can regulate Ca2+ flow to improve mitochondrial function and then affect the activation of T cells in autoimmune diseases[18]. Our study found that the expression of liver T lymphocytes in PBC mice decreased, and the expression levels of CD4+ T lymphocytes and CD8+ T lymphocytes also decreased after ImmTOR treatment. Rapamycin is a triene macrolide immunosuppressant that specifically binds to mTOR and phosphorylates downstream target proteins, inhibits interleukin (IL)-2-induced T cell protein and DNA synthesis, and blocks the progress of T cells from G1 phase to S phase, resulting in a decrease in T cell expression, thereby exerting its immunosuppressive effect[19]. The literature has consistently confirmed that CD8+ T cells are the main pathogenic cells of PBC. Transferring the spleen and liver CD8+ T cells of dnTGFbetaRII model mice into Rag1−/− mice can cause Rag1−/− mice to produce the typical liver pathology of PBC[20-22]. In biliary epithelial cells (BECs), CD8+ T cells are related to biliary duct damage associated with PBC, and CD8+ T cells are considered to be the main cells among various immune cells that cause liver inflammation and fibrosis[5]. Considering the above literature, rapamycin inhibits the production of liver CD8+ T cells and improves the relationship between CD4+ T and CD8+ T cells, which is expected to provide a direction for the treatment of PBC.
Our study found that the changes in T cells in mouse liver were different to those in the spleen. The expression of CD8+ T lymphocytes in the spleen of mice decreased, while the expression of CD4+ T cells did not change significantly. This may be due to the organ specificity of the liver and ImmTOR as reported in the literature[14]. Cells with a phagocytic phenotype, such as Kupffer cells, take up ImmTOR in large quantities, and hepatocytes and liver sinusoidal endothelial cells also significantly take up ImmTOR, while uptake in the spleen is relatively less. The phenotypes induced by ImmTOR in the liver and spleen are different. Many double-negative T cells can be observed in the liver of mice, while there is no such phenomenon in the spleen. The literature reports that the biological characteristics of ImmTOR distribution in the liver and its multiple effects on promoting antigen-presenting cells and T cells to a tolerant phenotype suggest that ImmTOR is beneficial for the treatment of autoimmune liver diseases. Individual or combined self-antigens, such as PDC-E2 in PBC, can help restore self-antigen immune tolerance, and our results also verify this.
Rapamycin can reduce the secretion of pro-inflammatory factors such as IFN- γ and has a dose-dependent effect. However, it can also inhibit the maturation of dendritic cells and promote the proliferation of regulatory T cells, inducing the recipient's immune tolerance state. Our study found that the levels of TNF-α and IFN-γ in the treatment group were lower than those in the control group after receiving ImmTOR. IFN-γ expression is critical for the pathogenesis of PBC[6], and TNF–α is elevated in patients with PBC and decreases after treatment with UDCA[23]. CD8+ T cells produce and release more IFN-γ and TNF–α. CD8+ T cells that produce effector cytotoxic effects in PBC have reached the end of the differentiation stage, especially the liver CD8aa T cell subgroup[21]. These cells can secrete a large amount of effector cytokines such as IFN-γ and TNF-α, and release perforin and granzyme B molecules to kill liver parenchymal cells and BECs. Combined with the decrease in the expression of liver CD8+ T cells, it is speculated that ImmTOR improved the cellular immunity and inflammation of PBC mice. There is already similar evidence in other autoimmune diseases, such as systemic lupus erythematosus, that rapamycin can adjust the imbalance of T cells, improve the expansion of naive T cell subgroups and the consumption of CD8+ memory T cells[24,25]. ImmTOR increased the treatment window of an artificially synthesized IL-2 mutant and drove the expansion of antigen-specific regulatory T cells, resulting in immune tolerance, which can help relieve or mitigate PBC[19]. Further research in this area should be conducted in the future.
In our study, compared with the control group, the liver pathology score in the treatment group decreased and inflammation decreased. Animal experiments have found that the PI3K/protein kinase B (AKT)/mTOR signal pathway participates in the pathogenesis of PBC[26]. The Ppp1r21 gene mutation can lead to the appearance of PBC-like features, and it has been found that there is an over-activation of the PI3K/AKT/mTOR pathway in the liver tissue of this model. Its pathway inhibitors LY294 and rapamycin can alleviate the symptoms of PBC[26]. Rapamycin can down-regulate the level of PDC-E2 and improve the pathological manifestations of PBC[26,27].
Nanotechnology has shown great potential in the treatment of autoimmune diseases. Compared with traditional methods, nanotechnology has unique advantages in drug delivery. Nanotechnology has provided references for the treatment of autoimmune diseases such as rheumatoid arthritis[28,29]. Given the anatomical characteristics and immunological specificity of PBC[30], the effect of this treatment may be ideal. However, nanotechnology is still a new and unknown field in the treatment of immune diseases, and its long-term effects still need to be determined.
The administration of nanoparticles containing rapamycin in an animal model of PBC was able to inhibit the expression of CD8+ T and B cells in the liver and spleen, thereby reducing the titer of anti-mitochondrial antibodies, improving liver enzymes, and alleviating liver pathological manifestations. However, its specific mechanism of action and the impact of nanotechnology still require further study.
We would like to thank all the staff of the Department of Rheumatology and Immunology in The Second Hospital of Hebei Medical University and College of Pharmacy in Hebei Medical University, and The Central Laboratory and Hebei Key Laboratory of Gastroenterology in The Second Hospital of Hebei Medical University for providing convenient conditions for implementing this study.
| 1. | Lv T, Chen S, Li M, Zhang D, Kong Y, Jia J. Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1423-1434. [PubMed] [DOI] [Full Text] |
| 2. | Faisal MS, Gonzalez HC, Gordon SC. Primary Biliary Cholangitis: Epidemiology, Diagnosis, and Presentation. Clin Liver Dis. 2024;28:63-77. [PubMed] [DOI] [Full Text] |
| 3. | Fan GH, Zhang CZ, Gao FQ, Wei XY, Ling SB, Wang K, Wang JG, Zheng SS, Nikfarjam M, Xu X. A mixed blessing for liver transplantation patients - Rapamycin. Hepatobiliary Pancreat Dis Int. 2023;22:14-21. [PubMed] [DOI] [Full Text] |
| 4. | Yang L, Bracho-Sanchez E, Fernando LP, Lewis JS, Carstens MR, Duvall CL, Keselowsky BG. Poly(2-propylacrylic acid)/poly(lactic-co-glycolic acid) blend microparticles as a targeted antigen delivery system to direct either CD4(+) or CD8(+) T cell activation. Bioeng Transl Med. 2017;2:202-211. [PubMed] [DOI] [Full Text] |
| 5. | Yang GX, Wu Y, Tsukamoto H, Leung PS, Lian ZX, Rainbow DB, Hunter KM, Morris GA, Lyons PA, Peterson LB, Wicker LS, Gershwin ME, Ridgway WM. CD8 T cells mediate direct biliary ductule damage in nonobese diabetic autoimmune biliary disease. J Immunol. 2011;186:1259-1267. [PubMed] [DOI] [Full Text] |
| 6. | Bae HR, Leung PS, Tsuneyama K, Valencia JC, Hodge DL, Kim S, Back T, Karwan M, Merchant AS, Baba N, Feng D, Park O, Gao B, Yang GX, Gershwin ME, Young HA. Chronic expression of interferon-gamma leads to murine autoimmune cholangitis with a female predominance. Hepatology. 2016;64:1189-1201. [PubMed] [DOI] [Full Text] |
| 7. | Shao T, Leung PSC, Zhang W, Tsuneyama K, Ridgway WM, Young HA, Shuai Z, Ansari AA, Gershwin ME. Treatment with a JAK1/2 inhibitor ameliorates murine autoimmune cholangitis induced by IFN overexpression. Cell Mol Immunol. 2022;19:1130-1140. [PubMed] [DOI] [Full Text] |
| 8. | Harms MH, Lammers WJ, Thorburn D, Corpechot C, Invernizzi P, Janssen HLA, Battezzati PM, Nevens F, Lindor KD, Floreani A, Ponsioen CY, Mayo MJ, Dalekos GN, Bruns T, Parés A, Mason AL, Verhelst X, Kowdley KV, Goet JC, Hirschfield GM, Hansen BE, van Buuren HR; Global PBC Study Group. Major Hepatic Complications in Ursodeoxycholic Acid-Treated Patients With Primary Biliary Cholangitis: Risk Factors and Time Trends in Incidence and Outcome. Am J Gastroenterol. 2018;113:254-264. [PubMed] [DOI] [Full Text] |
| 9. | Real-World Effectiveness of Obeticholic Acid in Patients With Primary Biliary Cholangitis. Gastroenterol Hepatol (N Y). 2021;17:7. [PubMed] |
| 10. | D'Amato D, De Vincentis A, Malinverno F, Viganò M, Alvaro D, Pompili M, Picciotto A, Palitti VP, Russello M, Storato S, Pigozzi MG, Calvaruso V, De Gasperi E, Lleo A, Castellaneta A, Pellicelli A, Cazzagon N, Floreani A, Muratori L, Fagiuoli S, Niro GA, Feletti V, Cozzolongo R, Terreni N, Marzioni M, Pellicano R, Pozzoni P, Baiocchi L, Chessa L, Rosina F, Bertino G, Vinci M, Morgando A, Vanni E, Scifo G, Sacco R, D'Antò M, Bellia V, Boldizzoni R, Casella S, Omazzi B, Poggi G, Cristoferi L, Gerussi A, Ronca V, Venere R, Ponziani F, Cannavò M, Mussetto A, Fontana R, Losito F, Frazzetto E, Distefano M, Colapietro F, Labanca S, Marconi G, Grassi G, Galati G, O'Donnell SE, Mancuso C, Mulinacci G, Palermo A, Claar E, Izzi A, Picardi A, Invernizzi P, Carbone M, Vespasiani-Gentilucci U; Italian PBC Registry and the Club Epatologi Ospedalieri (CLEO)/Associazione Italiana Gastroenterologi ed Endoscopisti Digestivi Ospedalieri (AIGO) PBC Study Group. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep. 2021;3:100248. [PubMed] [DOI] [Full Text] |
| 11. | Sylvia D, Tomas K, Marian M, Martin J, Dagmar S, Peter J. The treatment of primary biliary cholangitis: from shadow to light. Therap Adv Gastroenterol. 2024;17:17562848241265782. [PubMed] [DOI] [Full Text] |
| 12. | Kang HG, Park H, Myong GE, Kim WJ, Mun CE, Kim CR, You CY, Kim SK, Park MS, Park SI. Beneficial Effect of Rapamycin on Liver Fibrosis in a Mouse Model (C57bl/6 Mouse). Transplant Proc. 2024;56:701-704. [PubMed] [DOI] [Full Text] |
| 13. | di Francesco F, Di Lorenzo N, Gruttadauria S. Extra-hepatic epithelioid hemangioendothelioma: pushing the limit with sirolimus in combination with liver transplantation. Updates Surg. 2024;76:2091-2092. [PubMed] [DOI] [Full Text] |
| 14. | Ilyinskii PO, Roy CJ, LePrevost J, Rizzo GL, Kishimoto TK. Enhancement of the Tolerogenic Phenotype in the Liver by ImmTOR Nanoparticles. Front Immunol. 2021;12:637469. [PubMed] [DOI] [Full Text] |
| 15. | Dhirapong A, Lleo A, Yang GX, Tsuneyama K, Dunn R, Kehry M, Packard TA, Cambier JC, Liu FT, Lindor K, Coppel RL, Ansari AA, Gershwin ME. B cell depletion therapy exacerbates murine primary biliary cirrhosis. Hepatology. 2011;53:527-535. [PubMed] [DOI] [Full Text] |
| 16. | Xiang Y, Liu L, Hou Y, Du S, Xu S, Zhou H, Shao L, Li G, Yu T, Liu Q, Xue M, Yang J, Peng J, Hou M, Shi Y. The mTORC1 pathway participate in hyper-function of B cells in immune thrombocytopenia. Ann Hematol. 2023;102:2317-2327. [PubMed] [DOI] [Full Text] |
| 17. | Desai BN, Myers BR, Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2002;99:4319-4324. [PubMed] [DOI] [Full Text] |
| 18. | Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2983-2988. [PubMed] [DOI] [Full Text] |
| 19. | Kishimoto TK, Fournier M, Michaud A, Rizzo G, Roy C, Capela T, Nukolova N, Li N, Doyle L, Fu FN, VanDyke D, Traber PG, Spangler JB, Leung SS, Ilyinskii PO. Rapamycin nanoparticles increase the therapeutic window of engineered interleukin-2 and drive expansion of antigen-specific regulatory T cells for protection against autoimmune disease. J Autoimmun. 2023;140:103125. [PubMed] [DOI] [Full Text] |
| 20. | Kawata K, Yang GX, Ando Y, Tanaka H, Zhang W, Kobayashi Y, Tsuneyama K, Leung PS, Lian ZX, Ridgway WM, Ansari AA, He XS, Gershwin ME. Clonality, activated antigen-specific CD8(+) T cells, and development of autoimmune cholangitis in dnTGFβRII mice. Hepatology. 2013;58:1094-1104. [PubMed] [DOI] [Full Text] |
| 21. | Han Y, Bian ZH, Yang SY, Wang CB, Li L, Yang YQ, Ansari AA, Gershwin ME, Zeng X, Lian ZX, Zhao ZB. Single-Cell Characterization of Hepatic CD8(+) T Cells in a Murine Model of Primary Biliary Cholangitis. Front Immunol. 2022;13:860311. [PubMed] [DOI] [Full Text] |
| 22. | Yang GX, Lian ZX, Chuang YH, Moritoki Y, Lan RY, Wakabayashi K, Ansari AA, Flavell RA, Ridgway WM, Coppel RL, Tsuneyama K, Mackay IR, Gershwin ME. Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice. Hepatology. 2008;47:1974-1982. [PubMed] [DOI] [Full Text] |
| 23. | Bossen L, Lau TS, Nielsen MB, Nielsen MC, Andersen AH, Ott P, Becker S, Glerup H, Svenningsen L, Eivindson M, Kornerup L, Kjeldsen NB, Neumann A, Møller HJ, Jepsen P, Grønbæk H. The association between soluble CD163, disease severity, and ursodiol treatment in patients with primary biliary cholangitis. Hepatol Commun. 2023;7:e0068. [PubMed] [DOI] [Full Text] |
| 24. | Lai ZW, Kelly R, Winans T, Marchena I, Shadakshari A, Yu J, Dawood M, Garcia R, Tily H, Francis L, Faraone SV, Phillips PE, Perl A. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet. 2018;391:1186-1196. [PubMed] [DOI] [Full Text] |
| 25. | Fernandez DR, Crow MK. CD8 T cells and mTOR: new concepts and targets for systemic lupus erythematosus. Lancet. 2018;391:1126-1127. [PubMed] [DOI] [Full Text] |
| 26. | Wu C, Zhang W, Luo Y, Cheng C, Wang X, Jiang Y, Li S, Luo L, Yang Y. Zebrafish ppp1r21 mutant as a model for the study of primary biliary cholangitis. J Genet Genomics. 2023;50:1004-1013. [PubMed] [DOI] [Full Text] |
| 27. | Xu Y, Shen J, Ran Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy. 2020;16:3-17. [PubMed] [DOI] [Full Text] |
| 28. | Nune A, Barman B, Sapkota HR, Ish P, Chelliah EG, Diwan M, Chiphang A, Iyengar KP. Nanotechnology applications in rheumatology. Rheumatol Int. 2022;42:1883-1891. [PubMed] [DOI] [Full Text] |
| 29. | Li Y, Wang X, Gao Y, Zhang Z, Liu T, Zhang Z, Wang Y, Chang F, Yang M. Hyaluronic acid-coated polypeptide nanogel enhances specific distribution and therapy of tacrolimus in rheumatoid arthritis. J Nanobiotechnology. 2024;22:547. [PubMed] [DOI] [Full Text] |
| 30. | Gao L, Wang L, Woo E, He X, Yang G, Bowlus C, Leung PSC, Gershwin ME. Clinical Management of Primary Biliary Cholangitis-Strategies and Evolving Trends. Clin Rev Allergy Immunol. 2020;59:175-194. [PubMed] [DOI] [Full Text] |