Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.949
Peer-review started: June 16, 2020
First decision: August 22, 2020
Revised: September 5, 2020
Accepted: September 22, 2020
Article in press: September 22, 2020
Published online: November 27, 2020
Processing time: 160 Days and 19.7 Hours
Aceclofenac (ACF), a widely used nonsteroidal anti-inflammatory drug, has been associated with a number of severe cases of clinical hepatotoxicity. Terminalia bellirica, an evergreen tree, is known to have several ethnomedicinal uses including antioxidant and hepatoprotective effects. Hence T. bellirica fruit extracts and its phytoconstituent ellagic acid (EA) are expected to provide protection against oxidative stress and liver damage produced by long-term use of ACF.
To evaluate the antioxidant and hepatoprotective activities of T. bellirica fruit extracts and EA against ACF-induced toxicity in albino Wistar rats.
The in vitro antioxidant activities of T. bellirica fruit ethyl acetate and aqueous extracts were measured by metal ion chelation and nitric oxide radical scavenging assays. The in vivo antioxidant and hepatoprotective effects of T. bellirica extracts (200 mg/kg) and EA (40 mg/kg) in ACF-induced hepatotoxic rats were assessed in serum and liver tissue after oral administration for 21 d. Silymarin (40 mg/kg) was used as a standard control. Oxidative stress markers in the blood (ferric reducing ability of plasma and lipid peroxidation inhibition) and liver tissues (superoxide dismutase, catalase and malondialdehyde) were analyzed using standard protocols. Liver function markers such as alkaline phosphatase, glutamic pyruvic transaminase, glutamic oxaloacetic transaminase, lactate dehydrogenase, γ-glutamyl transferase, creatinine, total protein, and uric acid were evaluated in rat serum.
The T. bellirica fruit ethyl acetate extract exhibited superior metal ion chelating and nitric oxide radical scavenging abilities during in vitro antioxidant assays as compared to aqueous extracts. Oral administration of ACF in rats (15 mg/kg) for 21 d produced oxidative stress and adversely affected liver function suggesting liver injury. Treatment with extracts (ethyl acetate and aqueous), EA and silymarin accounted for a significant reduction in the adverse effects of ACF on oxidative stress and liver function markers in serum and hepatic tissue in rats. Histopathological evaluation of the liver indicated that the extracts and EA significantly decreased the degree of liver damage. The in vivo efficacy of EA was higher than T. bellirica fruit extracts. Of these extracts, ethyl acetate extract revealed comparatively better antioxidant and hepatoprotective activity.
Ellagic acid and T. bellirica fruit extracts exhibited considerable hepatoprotective and antioxidant activities in long-term ACF-treated rats.
Core Tip: Hepatotoxicity is the most serious adverse effects of aceclofenac (ACF). In this study, ACF-induced hepatic damage in rats was investigated. ACF administration (15 mg/kg/d) for 21 d produced severe hepatotoxicity and oxidative stress as demonstrated by abnormal elevations in serum and tissue markers. Co-administration of Terminalia bellirica fruit extracts (200 mg/kg) and ellagic acid (40 mg/kg) significantly attenuated ACF-induced hepatotoxicity. These results showed that supplementation with the test compounds led to restoration of serum liver function markers (SGOT, GPT, GGT, LDH, ALP, total protein, urea, uric acid, creatinine) and hepatic antioxidant status (superoxide dismutase, catalase, TBARS). Hence T. bellirica fruit extracts and ellagic acid have the potential to act as a hepatoprotectant and antioxidant in the treatment of drug-induced hepatotoxicity and oxidative stress. To the best of our knowledge, this is the first study to evaluate the therapeutic efficacy of T. bellirica fruit extracts and ellagic acid as hepatoprotective agents against ACF-induced hepatotoxicity.
- Citation: Gupta A, Pandey A. Aceclofenac-induced hepatotoxicity: An ameliorative effect of Terminalia bellirica fruit and ellagic acid. World J Hepatol 2020; 12(11): 949-964
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/949.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.949
Oxidative stress is characterized as disparity between the free radical generation and antioxidant defense mechanisms. As a consequence, free radicals attack biomolecules including lipids, proteins and DNA, thus leading to the development of various ailments at cellular and organ levels which ultimately precipitate in a disease etiology viz., hepatotoxicity, inflammation, cancer, diabetes, cardiovascular, and neurodegenerative disorders etc.[1,2]. Oxidative stress not only causes DNA damage, lipid peroxidation, and protein oxidation but also produces interference in the physiologic adaptation phenomenon and regulation of intracellular signal transduction mechanisms[3]. Antioxidants (enzymatic and non-enzymatic) existing in the living system are typically effective in neutralizing the adverse effects of free radicals. Numerous synthetic antioxidants are presently used in several food and pharmaceutical sectors although they are reported to produce toxicity. Hence, there is a growing demand from consumers for the utilization of natural antioxidants due to their virtuous efficacy and fewer side effects on health[4,5].
Nonsteroidal anti-inflammatory drugs (NSAIDs) are frequently recommended for the management of pain and inflammatory conditions. They obstruct the activity of cyclooxygenase-1 and -2 enzymes[6]. Aceclofenac (ACF), an established NSAID, is a phenylacetic acid derivative and chemically termed 2-[(2´,6´-dichlorophenyl) amino] phenylacetoxyacetic acid. In humans, ACF is metabolized to 4’-hydroxyaceclofenac via cytochrome P450 2C9b (CYP2C9)[7]. ACF has been shown to arouse selective inhibition of COX-2 as a consequence of limited and continued biotransformation to diclofenac[8]. Prolonged administration of ACF harms gastrointestinal mucosa by irritant action, affecting mucosal permeability and/or prevention of prostaglandin synthesis. Moreover, it exhibits analgesic, antipyretic, and anti-inflammatory activities, and inhibits the arachidonic acid pathway. In addition, prolonged consumption of ACF is associated with upper gastrointestinal complications, mainly perforated and bleeding peptic ulcer[9]. Previous studies on NSAIDs documented that they have adverse effects on the liver; but the incidence of these side effects is inconclusive. However, studies related to ACF-induced liver injuries are very limited.
Silymarin (SLM) is a polyphenolic flavonoid extracted from the fruit and seed of Silybum marianum (milk thistle). It is a well-recognized therapeutic agent for hepatic injury and has been used in the treatment of liver cirrhosis and severe hepatitis. SLM is also useful in the mitigation of damage inflicted by toxic compounds[10]. SLM has been shown to provide protection against hepatic, renal, neuronal, and gastric injury[11]. The hepatoprotective potential of SLM is related to its stabilizing action on cytoplasmic membranes[12]. Studies on different animal models have unveiled the notable therapeutic action of SLM on hepatic injury of diverse etiology[13].
Terminalia bellirica Roxb. (Combretaceae) is a perennial plant widely found in tropical regions and frequently observed in South-East Asia[14]. Its fruit has been used in various ailments in the indigenous medical system to cure cough, asthma, diarrhea, dyspepsia, anemia, cancer, fever and inflammation, and to promote rejuvenation[15,16]. It is one of the ingredients in “Triphala”, an ayurvedic formulation rich in antioxidants which is believed to promote health, immunity and longevity[17], and is used for the treatment of various disorders including fever, constipation, chronic ulcers, anemia, asthma and jaundice[18]. Chemical profiling of T. bellirica fruit revealed that gallic acid was one of the major active components of the fruit (2.6 mg/g of total polyphenols). However, other phytochemicals such as ellagic acid, ethyl gallate, chebulagic acid and β-sitosterol have also been reported to be present in noteworthy concentrations[19,20]. T. bellirica fruit has been scientifically proven to possess antibacterial, antifungal, antioxidant, antidiabetic and hepatoprotective effects[21,22]. The combination of three lignans and a flavan from T. bellirica fruit extract showed significant anti-HIV, anti-malarial and antifungal activity in vitro[23]. Ellagic acid (EA) is a polyphenolic compound present in T. bellirica fruit in a considerable amount (1.3-2.2 mg/g of total polyphenols), and has been studied extensively due to its medicinal attributes[24,25]. The antioxidant effect of EA is attributed to its free radical scavenging and metal ion chelating abilities, along with enhancement of cellular antioxidant defense. EA also protects cells from free radical-mediated DNA damage[26,27]. However, there are no reports on the therapeutic potential of T. bellirica fruit extracts and EA as a hepatoprotectant against ACF-induced toxicity. The present study reports the antioxidant and hepatoprotective activities of T. bellirica fruit ethyl acetate (Eth) and aqueous (AQ) extracts as well as EA against ACF-induced oxidative stress and hepatotoxicity. To the best of our knowledge, this is the first study to assess the antioxidant and hepatoprotective effects of T. bellirica fruit extracts (Eth and AQ) and EA against ACF-induced liver injury in albino Wistar rats.
Silymarin (Sigma), ellagic acid (Himedia Laboratories Pvt Ltd.), aceclofenac (Ipca Laboratory), ferrozine, ferric chloride, sodium nitroprusside, sulfanilamide, napthyldiamine, phosphoric acid, potassium chloride, and ferrous sulfate (Sisco Research Laboratory (SRL) Pvt. Ltd.) were procured from scientific suppliers. Biochemical kits for estimation of creatinine, uric acid, total protein, and serum enzymes (GPT, ALP, GOT, LDH, GGT) were purchased from Erba Transasia Bio-medicals Ltd. All other laboratory chemicals such as dimethyl sulfoxide, trichloroacetic acid, thiobarbituric acid, bovine serum albumin, butylated hydroxyanisole, sodium hydroxide, and hydrogen peroxide were also procured from SRL, India.
Fruits of T. bellirica were bought from Prayagraj local market and ground into a fine powder. A total of 100 g fruit powder was sequentially extracted with ethyl acetate and water in Soxhlet apparatus[28]. The extract was dried under reduced pressure.
Wistar rats (either sex) of a similar age group (weight 150-200 g) were used in the experiments. They were maintained in a temperature (24 ± 2°C) and humidity (40% ± 5%) controlled environment with a 12 h light/dark cycle. Rats were provided with a standard rodent diet and water ad libitum. The study was carried out according to the Guidelines of Institutional Animal Ethics Committee, University of Allahabad, India in agreement with the Committee for the Purpose of Control and Supervision of Experiments on Animals.
The rats were distributed into six groups with five rats in each group. These groups were as follows: Group-I - normal rats; Group-II - ACF treated (15 mg/kg); Group-III - ACF treated rats administered with the standard drug (Silymarin 40 mg/kg); Group-IV- ACF treated rats administered with ellagic acid (40 mg/kg); Group-V- ACF treated rats co-administered with the aqueous extract (200 mg/kg) and Group-VI- ACF treated rats co-administered with the ethyl acetate extract (200 mg/kg) of T. bellirica fruit. During the experimental period, all the rats received a single oral dose of the drugs or extracts or the combinations thereof for 21 d.
The weight of the rats was measured before administration of the drugs and test compounds every day until sacrifice. The relative liver weight was also determined after sacrifice by the formula: Relative liver weight = [liver weight/body weight] × 100.
4 mL blood was drawn by puncturing the rat’s heart. Of which, 2.5 mL blood was used for clot formation and serum was separated at 2500 rpm for 10 min. The remaining blood was placed into anticoagulant ampoules and kept in a cold environment before processing. The plasma was separated after centrifugation at 3000 rpm for 10 min. Both the serum and plasma were stored at -70 °C for further analysis.
Metal ion chelating activity: Ferrous ion chelation by the T. bellirica fruit AQ and Eth extracts was assessed using the method described by Dinis et al[29] with minor modifications[30]. Extracts were dissolved in distilled water instead of methanol. A small amount (200 µL) of extract samples was combined with ferric chloride (50 µL, 2 mmol/L). Ferrozine (200 µL, 5 mmol/L) was added to start the reaction followed by vigorous shaking, and then left for 10 min at room temperature. Butylated hydroxytoluene was used as a positive control. Absorbance was recorded at 562 nm. Chelating activity was estimated by the following formula:
% Metal ion chelating ability = [(A0-A1)/A1] × 100.
Where A0 and A1 are absorbance of the control and test samples, respectively.
Nitric oxide radical scavenging activity: Nitric oxide (NO) radical scavenging activity was determined by the method of Green et al[31]. To test extracts (0.5 mL), 1.0 mL of sodium nitroprusside (0.01 mol/L in PBS) was added and incubated at 25°C for 3 h followed by the addition of an equal volume of Griess reagent and left for 30 min at room temperature. The concentration of test compounds ranged between 10-100 µg/mL in the final reaction mixture. Ascorbic acid was used as a standard and absorbance was measured at 546 nm. The NO radical scavenging activity was determined using the formula:
% NO scavenging = [(Ac-As)/Ac] × 100.
Ac and As designate absorbance values of the control and test samples, respectively.
Lipid peroxidation inhibition assay: Lipid peroxidation inhibition (LPOI) by the T. bellirica fruit extracts was measured by the method of Halliwell et al[32] in 10% rat liver homogenate. BHA was used as a control. The % LPOI was determined using the following formula:
% Lipid peroxidation inhibition = [(A0-A1)/A0] × 100.
Where A0 and A1 are the absorbance of the control and test samples, respectively at 532 nm.
Estimation of total antioxidant activity by the ferric reducing antioxidant power assay: The ferric reducing antioxidant power (FRAP) assay[33] is a method for measuring total antioxidant potential of test compounds. To 0.05 mL plasma, FRAP reagent (4.5 mL) was added and absorbance was recorded at 593 nm after 5 min. The final concentration of T. bellirica fruit extracts in the reaction mixture was 44.44 μg/mL, while the concentration of EA and SLM was 8.89 μg/mL. Ferrous sulfate (100-1000 µmol/mL) was used to create a calibration curve and the result was expressed as the FRAP value (µM FeSO4·7H2O equivalent/L plasma).
Assessment of liver function markers in serum: The biochemical parameters including SGPT, SGOT, GGT, LDH, ALP, total protein, uric acid, and creatinine were assayed using commercially available kits (Erba Diagnostics Kits).
Preparation of liver tissue homogenate: The liver tissue homogenate (10% w/v) was prepared in phosphate buffer (0.1 mol/L, pH-7.4 with 0.15 mol/L KCl). Crude homogenate was centrifuged (1000 × g for 30 min, 4°C) and the supernatant was used for estimation of antioxidant enzymes and other biochemical analytes.
Estimation of malondialdehyde (MDA) in liver homogenate: Lipid peroxidation was assayed in tissue homogenate using the method of Niehaus and Samuelsson[34]. Thiobarbituric acid reagent (2 mL) was added to tissue homogenate (100 μL) and the content was boiled for 1 h followed by measurement of absorbance at 532 nm. The peroxidation product was represented as nM MDA/mg protein using the extinction coefficient of 1.56 × 105 M-1 cm-1.
Determination of total protein in liver tissue homogenate: The total protein present in the liver homogenate was measured by the method of Lowry et al[35].
Superoxide dismutase activity: The superoxide dismutase (SOD) activity was assayed by the method of Marklund and Marklund[36]. One unit of enzyme activity represents 50% inhibition of pyrogallol autooxidation per min.
Catalase activity: The catalase (CAT) activity was measured by assessing the reduction in the absorbance of H2O2 at 240 nm for 3 min at the interval of 30 s[37]. One unit of CAT activity is defined as micromoles of H2O2 disintegrated per min using the molar absorbance of H2O2 (43.6 M-1 cm-1).
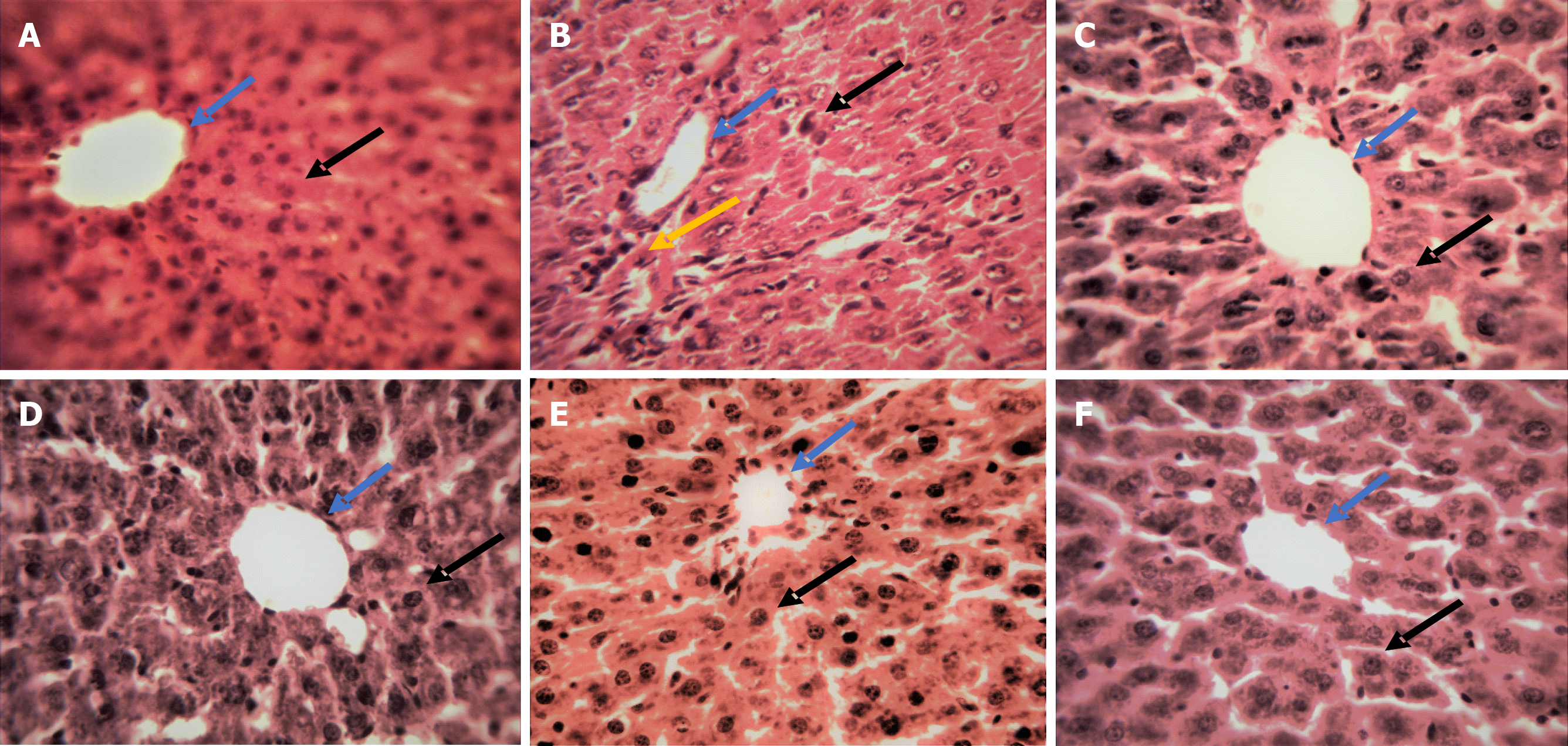
Histological analysis of liver: The liver biopsies from rats were fixed in 10% formalin, dehydrated in graded alcohol, and then embedded in paraffin wax blocks. The paraffin-block was sliced (5 μm) successively using a rotary microtome. The liver slices were stained with hematoxylin and eosin (H and E) on albumin-coated sterilized glass slides[38]. After mounting in DPX, the sections were studied for histological changes under a light microscope (× 40 magnification).
All the experiments were performed in triplicate. Results are represented as mean ± SD. GraphPad Prism software was used to create the graphs. P values (< 0.05) were considered significant.
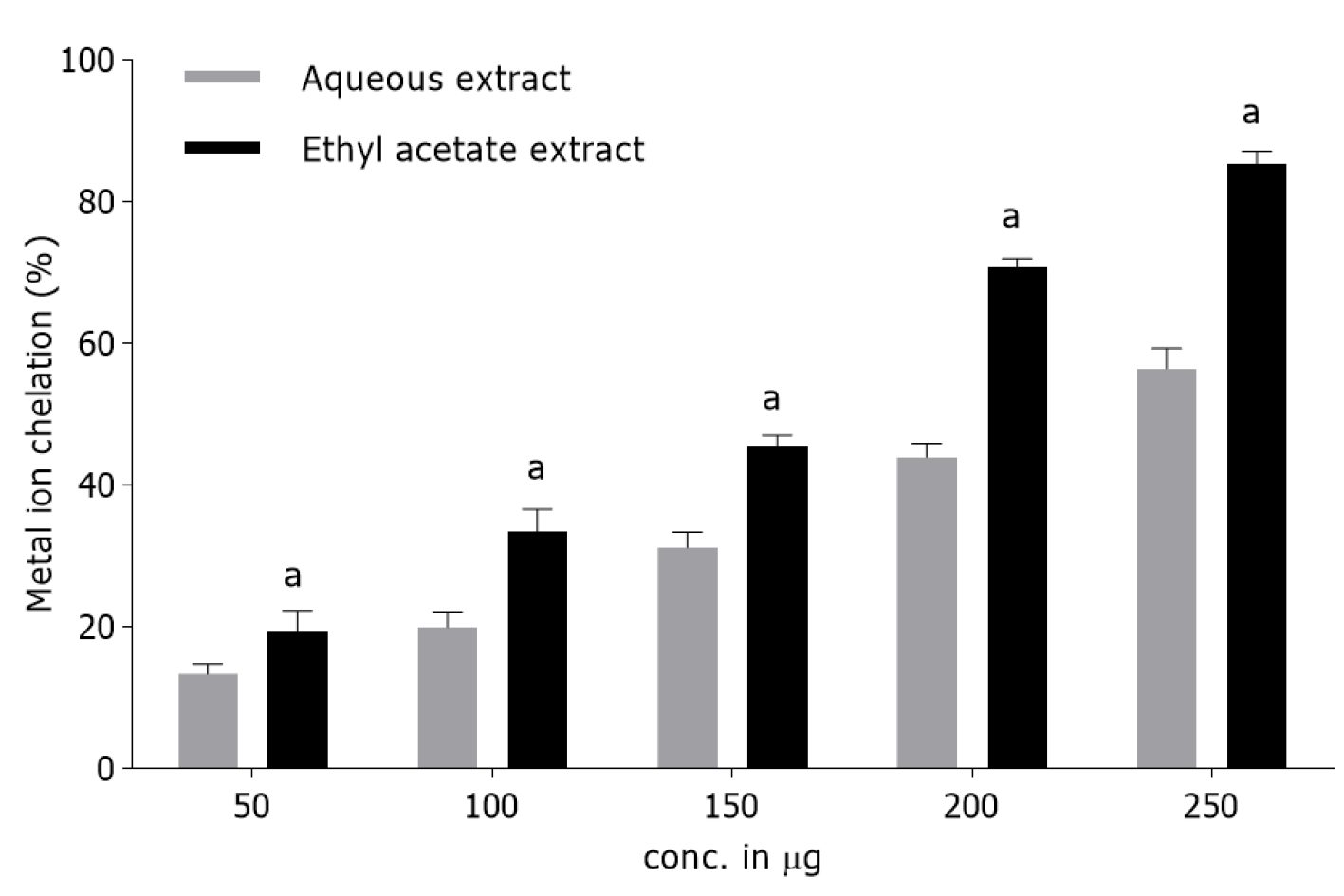
Metal ion chelation activity: T. bellirica fruit extracts (AQ and Eth) exhibited marked concentration-dependent metal ion chelating activity (13%-85%) (Figure 1). The degree of discoloration showed the chelating efficacy of the fruit extracts. Highest chelation potential was observed for the Eth extract (85.38%, IC50 168 µg/mL) followed by the AQ extract (56.42%, IC50 220 µg/mL). Butylated hydroxytoluene showed 90% metal ion chelation activity at a concentration of 100 µg/mL.
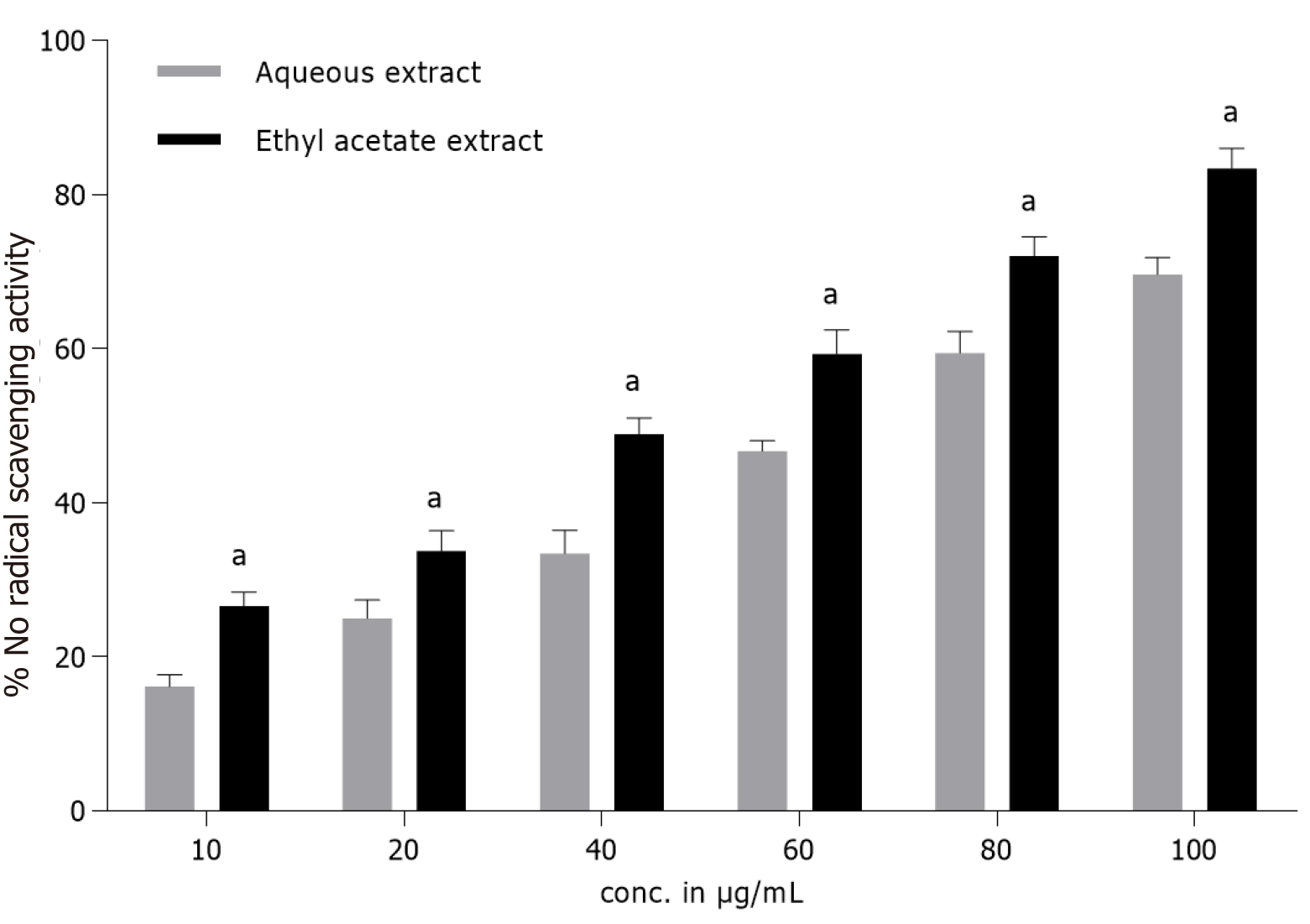
Nitric oxide radical scavenging activity: NO radical scavenging activity of T. bellirica fruit extracts was evaluated at different concentrations (10-100 µg/mL) and the results were expressed in terms of % NO radical scavenging activity (Figure 2). Considerable radical scavenging activity was observed in the test compounds during in vitro assay. The AQ extract exhibited comparatively lower NO radical scavenging activity (16%-66%, IC50 70 µg/mL) than the Eth extract (26%-83%, IC50 48 µg/mL) at all test concentrations. BHA (0.33-3.3 µg/mL) accounted for 37%-84% activity.
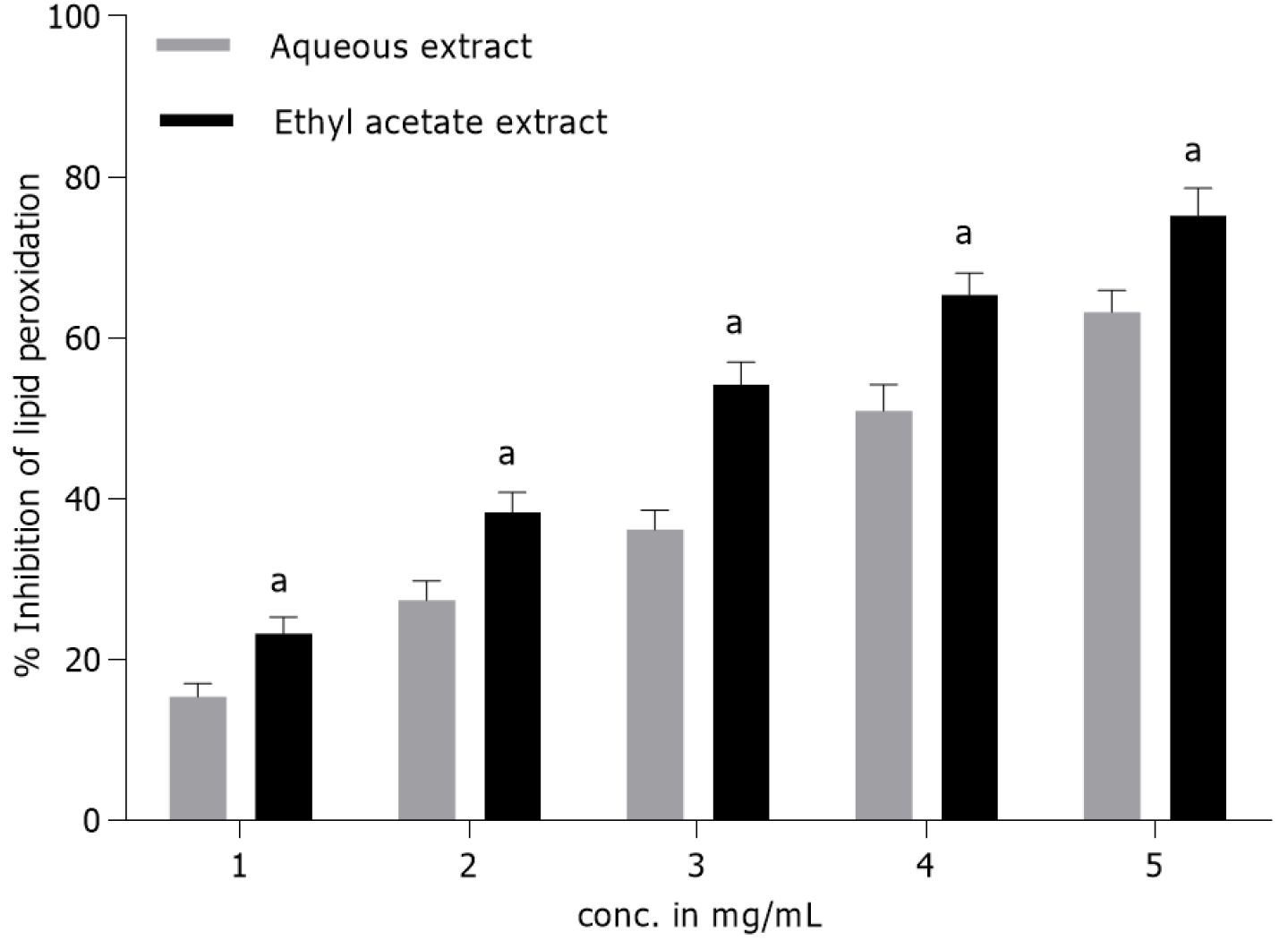
Lipid peroxidation inhibition activity: Fruit extracts of T. bellirica displayed dose-dependent anti-lipid peroxidative activity during in vitro assay. The Eth extract exhibited comparatively higher inhibitory response against Fe2+-triggered lipid peroxidation in liver homogenate signifying its lipo-protective efficacy. The LPOI values for the Eth and AQ extracts at a concentration of 5 mg/mL were 75% and 63%, respectively (Figure 3). However at lower concentration (1 mg/mL), the LPOI values for the Eth and AQ extracts were about 23% and 15%, respectively. Standard antioxidant BHA (2 mg/mL) under similar experimental conditions produced about 85% inhibition of lipid peroxidation.
Assessment of change in body weight and relative liver weight: A noteworthy decline in body weight was observed in ACF-treated rats (group II) in comparison with untreated rats (group I) (Table 1). Group I rats showed approximately 18.83% gain in body weight during the same time period. The percentage loss in body weight in group II rats (20.50%) was markedly higher than that in group I (P < 0.0001) and groups III-V (P < 0.005). Co-administration of EA and T. bellirica fruit extracts (AQ and Eth) with ACF exhibited a restorative effect (81%-89% recovery) on body weight. Standard drug SLM showed maximum recovery potential (90.83%) followed by EA (88.94%), Eth (85.17%) and AQ (81.50%). Furthermore, an inverse correlation was observed between body weight and relative liver weight. Relative liver weight increased from 2.85% in the control to 3.95% in ACF treated rats (P < 0.0001), while treatment with EA and T. bellirica fruit extracts (Eth and AQ) showed a restorative effect on the liver weight of rats. In comparison to group II rats, the recovery following administration of EA and T. bellirica fruit extracts was statistically significant (P < 0.005) (Table 1).
| Groups | Body weight, change (%) | Absolute liver, weight (g) | Relative liver, weight (%) |
| Group I | 18.83 ± 1.15 | 4.62 ± 0.16 | 2.85 ± 0.29 |
| Group II | -20.50 ± 6.87a | 6.56 ± 0.41a | 3.95 ± 1.29a |
| Group III | -09.17 ± 3.78b | 4.82 ± 0.19b | 3.05 ± 0.35b |
| Group IV | -11.06 ± 3.77b | 5.17 ± 0.87b | 3.13 ± 0.46b |
| Group V | -18.50 ± 6.26b | 5.70 ± 1.09b | 3.44 ± 0.58b |
| Group VI | -14.83 ± 3.21b | 5.03 ± 0.95b | 3.21 ± 0.64b |
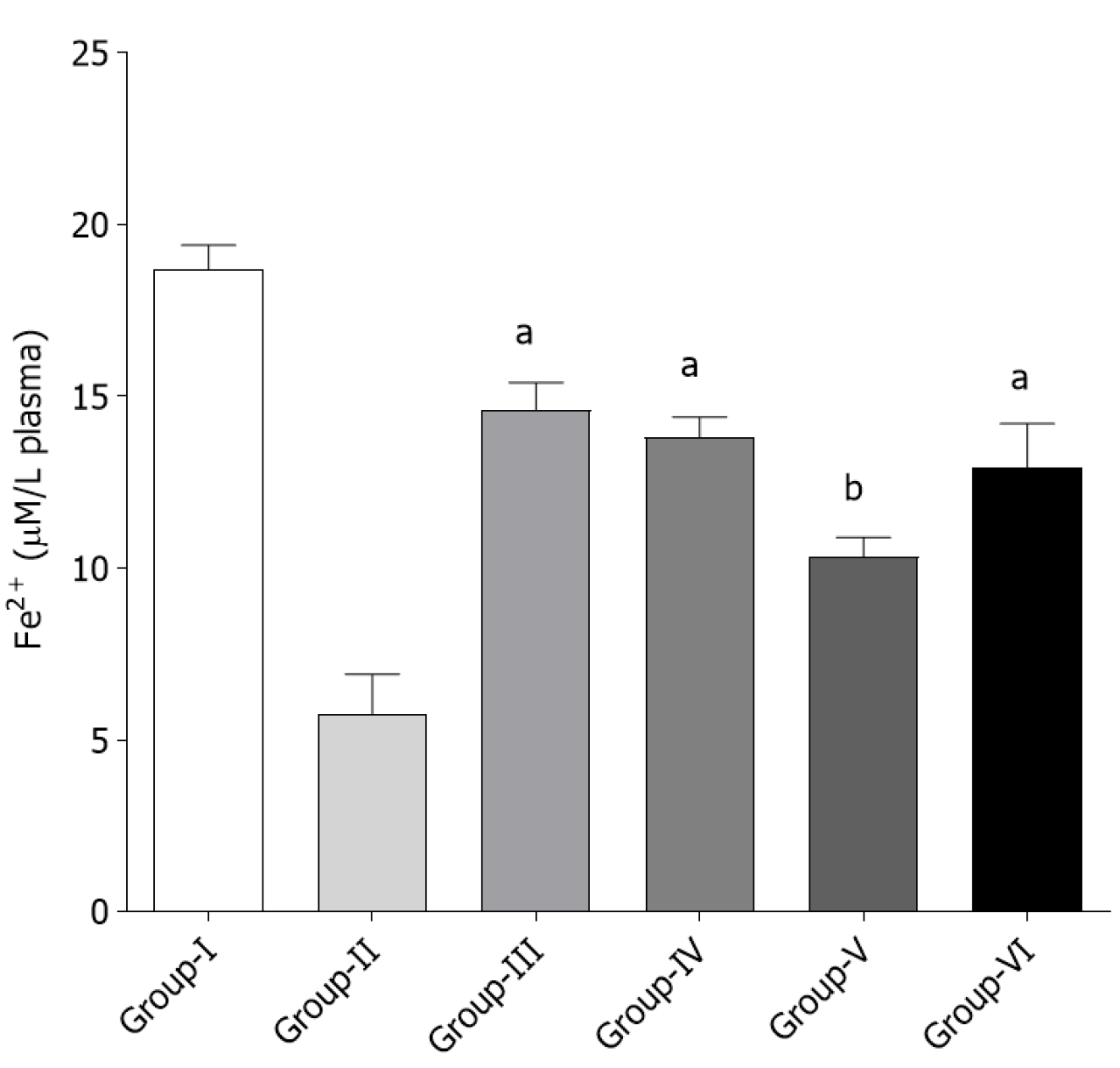
Assessment of total antioxidant activity by FRAP Assay: The therapeutic effect of T. bellirica fruit extracts (Eth and AQ) and EA on plasma FRAP are shown in Figure 4. In group II rats, the administration of ACF resulted in a marked decrease (P < 0.05) in plasma FRAP (5.76 µmol/L) as compared to group I. This indicated a reduction in the antioxidant potential of plasma with a simultaneous rise in oxidative stress. Co-administration of EA (group IV), AQ (group V) and Eth (group VI) with ACF caused a significant improvement (P < 0.05) in plasma antioxidant capacity.
Assessment of change in serum markers: The measurement of various markers of hepatic function is used in the diagnosis and treatment of a variety of diseases. The effects of ACF and test compound combination (T. bellirica fruit extracts and EA) on serum biomarkers including total protein, creatinine, urea, SGOT, SGPT, LDH, GGT, ALP and uric acid are shown in Table 2. Oral administration of ACF led to an elevation in serum creatinine, uric acid, SGOT, SGPT, ALP, LDH, and GGT (group II) (P < 0.05). Co-administration of extracts/EA/SLM with ACF resulted in marked restoration of these biochemical indices.
| Groups | Total protein (g/dL) | ALP (IU/L) | SGOT (IU/L) | SGPT (IU/L) | Uric acid (mg/dL) | Creatinine (mg/dL) | LDH (IU/L) | GGT (IU/L) |
| Group I | 6.05 ± 0.45 | 87.16 ± 14.19 | 69.37 ± 10.24 | 58.19 ± 09.13 | 1.71 ± 0.37 | 0.80 ± 0.33 | 304.76 ± 10.23 | 4.27 ±1.08 |
| Group II | 3.64 ± 0.14a | 157.32 ± 26.16a1 | 165.40 ± 12.51a1 | 158.43 ± 8.24a1 | 6.87 ± 0.22a | 3.12 ± 1.31a | 689.34 ± 11.21a1 | 9.29 ± 2.15a1 |
| Group III | 5.72 ± 0.17b1 | 93.49 ± 9.08b1 | 85.21 ± 16.34b1 | 69.09 ± 4.71b1 | 2.32 ± 0.13b1 | 1.37 ± 0.18b1 | 401.19 ± 3.89b1 | 5.87 ± 0.24b |
| Group IV | 5.52 ± 0.31b1 | 96.65 ± 11.25b1 | 89.56 ± 9.13b1 | 74.98 ± 11.38b1 | 2.34 ± 0.24b1 | 1.49 ± 0.17b1 | 449.54 ± 15.09b1 | 6.18 ± 0.51b1 |
| Group V | 4.75 ± 0.38b2 | 121.49 ± 16.13b2 | 140.17 ± 4.75b1 | 132.11 ± 14.17b1 | 3.38 ± 0.52b2 | 2.72 ± 0.18b2 | 587.37 ± 7.94b2 | 7.69 ± 0.27b2 |
| Group VI | 5.60 ± 0.11b2 | 107.17 ± 12.37b1 | 113.6 ± 4.89b1 | 109.25 ± 04.85b1 | 2.53 ± 0.19b2 | 2.16 ± 0.06b1 | 505.18 ± 11.45b1 | 6.68 ± 0.39b2 |
Assessment of MDA in liver tissue: ACF administration for three weeks caused an approximate seven-fold rise in MDA level (18.63 nmol/mg protein) in liver tissues as compared to the control (2.68 nmol/mg protein) (Table 2). SLM treatment reduced the level of MDA by up to 1.7-fold (4.5 nmol/mg protein) in group III rats. T. bellirica fruit AQ and Eth extract-treated groups also accounted for a noteworthy reduction in hepatic tissue MDA level (12.28 and 9.46 nmol/mg protein, respectively). Furthermore, co-administration of EA with ACF resulted in a comparatively better recovery in hepatic MDA level (6.74 nmol/mg protein) as compared to T. bellirica fruit extracts.
Assessment of antioxidant enzyme in liver tissue homogenate: ACF treatment in group II rats caused a noteworthy reduction (P < 0.05) in hepatic antioxidant enzyme activity i.e., SOD (12.04 U/mg protein) and catalase (3.21 U/mg protein) as compared to the control group (SOD-31.09 U/mg protein and catalase-8.45 U/mg protein). Appreciable restoration (P < 0.05) in hepatic tissue catalase activity was observed in the EA-treated groups (7.19 U/mg protein) followed by the Eth (6.23 U/mg protein) and AQ (5.77 U/mg protein) groups as compared with group II. Moreover, co-administration of EA and Eth and AQ extracts with ACF also caused appreciable enhancement (P < 0.05) in SOD enzyme activity (27.24, 21.15, 19.80 U/mg protein, respectively). It was observed that EA showed comparatively similar enzymatic activity to SLM treatment (29.11 U/mg protein) (Table 2).
Histopathological changes in liver: Histological sections of the normal rat liver slices showed intact hepatocytes with sinusoidal spaces and evenly distributed cytoplasm (Figure 5A). Oral administration of ACF resulted in severe hepatic damage as confirmed by immense hepatocellular deterioration, necrosis, sinusoidal dilatation, infiltration of inflammatory cells and cytoplasmic vacuolation (Figure 5B). However, treatment with EA and T. bellirica fruit extracts in ACF treated rats reduced hepatic damage and associated alterations and thereby improved liver structure and function (Figure 5D-F). Administration of SLM displayed relatively higher hepatoprotective efficacy (Figure 5C). The histopathological improvement observed in liver sections with EA, T. bellirica extracts and SLM in ACF treated rats had a direct correlation with liver weight, body weight and serum liver function markers along with tissue antioxidants.
The antagonistic properties of drugs and synthetic antioxidants have drawn the attention of scientists to explore new sources of natural antioxidants and hepatoprotectants which are more potent in mitigating oxidative stress and averting the initiation of disease[4,39]. Antioxidants hinder the oxidation of critical biomolecules by preventing the cascade of oxidizing chain reactions[5]. Interestingly, few studies on the antioxidant properties of T. bellirica fruit have been carried out[16,22,40]. However, this is the first study to assess the antioxidant and hepatoprotective attributes of T. bellirica fruit extracts and its constituent EA against ACF-induced liver injury in albino Wistar rats.
Antioxidant evaluation of T. bellirica fruit Eth and AQ extracts showed significant radical scavenging activities during in vitro metal ion chelation and NO radical scavenging assays. It was measured by a pink color complex formed due to ferrozine and Fe2+ interaction. Metal chelating ability showed the potential of the test compounds to protect lipids from oxidative damage[2]. In the present study, T. bellirica fruit Eth extract (IC50 168 µg/mL) showed higher ion chelating ability as compared to the AQ extract (IC50 220 µg/mL) (Figure 1). The chelation process promotes the lipophilicity of the metal ion and thereby favoring its penetration through the lipoid membrane. This action diminishes the production of OH• radical and thus averts the beginning of lipid peroxidation[41]. Previous studies have also confirmed the positive association between metal chelation and lipoprotective activities[42]. Moreover, it has been recognized that chelating agents act as secondary antioxidants by forming bonds with metals thus lowering their redox potential and stabilizing the oxidized state of the metal ion[43].
Nitric oxide is required during inflammatory processes but higher concentrations are toxic to tissues including vascular damage and other ailments. Sodium nitroprusside in the presence of oxygen generates nitrite ions at physiological pH, which is analyzed by a specific method[44]. The nitrite radical undergoes diazotization reaction with sulfanilamide and subsequent coupling with naphthyl ethylene diamine generates pink chromophore. The radical scavenging activity of fruit extracts may be attributed to its competition with oxygen to react with nitric oxide[45]. In the experiment, the Eth extract (83%, IC50 48 µg/mL) showed comparatively better NO scavenging activity than the AQ extract (74%, IC50 57 µg/mL) (Figure 2). The occurrence of p-hydroxyl groups in the aromatic ring structure and conjugated double bonds that make the electrons more delocalized are structural prerequisites for potent radical scavenging action by the extracts and BHA. The p-hydroxy system possesses electron-donating properties and is a radical target. The number, positions of OH-groups and the type of group replacements are mainly accountable for phenylpropanoids functioning as effective antioxidant[46], anti-inflammatory, enzyme modulator or antiproliferative agents[47].
Lipid peroxidation is a free radical-triggered redox process associated with inflammation and biochemical changes in the lipids[42,48]. It rapidly starts with the action of hydroxyl radicals generated during the Fenton reaction in the presence of iron (II)[49]. In this study, the Eth extract (5 mg/mL) exhibited a 75% decrease in peroxidation product suggesting its lipoprotective ability, while comparatively less activity was observed with the AQ extract (63%) (Figure 3). The protection accorded by the test extracts could be ascribed to the metal ion (Fe3+) chelation which is crucial for the production of hydroxyl radicals[41]. The antioxidants break the oxidation chain reaction initiated by free radicals through transfer of reducing equivalents (H+) from the phenolic hydroxyl groups, thus producing a stable end product that does not promote further lipid oxidation. T. bellirica fruit has been shown to possess potent chelating ability and therefore it may exhibit appreciable inhibitory action on lipid peroxidation[40]. These results are corroborated by a recently published study from our laboratory, which advocated that T. bellirica fruit Eth extract had comparatively higher antioxidant activity than the AQ extract during in vitro analysis[16].
FRAP provides a direct assessment of the antioxidant or reducing capacity of the samples. Reduction of Fe3+-TPTZ complex to Fe2+-TPTZ complex by the test compounds is the basis for measurement of this ability producing a blue color which is measured at 593 nm[33]. The absorbance of the reaction mixture is directly correlated with the reducing ability of the sample. The FRAP value is an indicator of the hydrogen or electron-donating ability of test samples[50]. The FRAP value shown by the EA treated rat group was significantly higher (P < 0.05) than that in the T. bellirica fruit extract treated groups (Figure 4).
Phytoconstituents isolated from T. bellirica fruit were previously reported to be antioxidant, anti-inflammatory and hepatoprotective agents. Triterpenoidal compounds (e.g., oleanolic and ursolic acids) are extensively found in food and therapeutic herbs[51]. The hepatoprotective efficacy of oleanolic acid against carbon tetrachloride (CCl4) and ursolic acid against ethanol-induced liver injury has been confirmed[52,53]. Both compounds individually exhibited significant in vitro antioxidant and anti-inflammatory activities in PC12 cell lines exposed to 1-methyl-4-phenylpyridinium ion or H2O2[54]. Moreover, oleanoic acid triggered expression of phase II response genes and stimulated the antioxidant enzymes and transcription factor (Nrf2)[55]. It also blocked the NF-κB pathway which was further substantiated by a high binding affinity towards NF-κB subunits (p50 and p52), TNF-α and COX-2 during in silico experiments[55,56].
The pharmacological activity of T. bellirica fruit extracts evaluated in this study can be accredited mainly to the higher amount of phenolic compounds and flavonoids. Tannins are phenolic compounds responsible for the bitter taste of foods and beverages[57]. A significant correlation between tannin content and total antioxidant activity has been established previously[58]. Additionally, tannins extracted from the acetone extract of natural products showed potent antioxidant properties compared with the low molecular-weight phenolic compounds derived from the same plant samples[57]. Recently, researchers evaluated the hepatoprotective and antioxidant attributes of gallic acid and EA against CCl4-induced hepatic injury in mice[59,60]. Gallic acid produced superior DPPH scavenging activity than EA[59] and methyl gallate[61]. This activity could be ascribed to the availability of a free carboxyl group.
Administration of ACF for 21 d led to a fall in body weight demonstrating the adverse effect of long-term treatment in Wistar rats. It has been reported that AFC caused gastric ulcers in an animal model leading to difficulty in food intake that culminated in malnourishment[62]. The unusual rise in liver weight in ACF treated rats seems to be due to its toxic behavior. Previous studies suggested that a decline in body weight during hepatic injury is associated with the interplay of adiponutrin and abdominal fat[63]. However, treatment with T. bellirica fruit extracts (Eth and AQ) and EA suppressed the toxic effect of ACF in rats and improved body weight. This result was more pronounced in SLM treated rats.
The activity of reactive oxygen species (ROS), mitochondrial stress, immune response, and idiosyncratic reactions are the prime causes of hepatic injury resulting from prolonged use of ACF. However, the precise mechanism of its toxic behavior is still unclear[7]. Altered serum and tissue biomarkers along with histological changes are clinical indicators of liver damage. Abnormally high levels of SGOT, SGPT, ALP, uric acid, creatinine, LDH and GGT in the circulation are directly correlated with severe liver injury[6,11]. In the current study, prolonged ACF intake caused hepatic damage in rats, as evidenced by a marked rise in serum and tissue markers as compared to the control rats. These study results are in line with previous studies[7,64]. The findings suggested that T. bellirica fruit Eth and AQ extract (200 mg/kg) and EA (40 mg/kg) treatment reduced ACF-induced elevations in the levels of these parameters towards the normal range revealing improvement in hepatic function (Table 2). Similar results have been observed in SLM treated rats.
The intracellular antioxidant enzymes such as SOD and CAT act as the first line of defense against oxidative damage in hepatic tissue. SOD catalyzes the dismutation of superoxide (O2ˉ) radical into H2O2 and oxygen, which is one of the chief cellular defense mechanisms[3]. Reduced SOD and CAT activities in ACF administered rats indicated the diminished potential of ROS scavenging action. In ACF fed rats, treatment with T. bellirica fruit extracts and EA enhanced the level of SOD in hepatic tissue (Table 3). A similar trend was also observed in the SLM treated group. Additionally, CAT also oozes into the extracellular fluid as a result of tissue damage. Inside the cell it has the potential to act as a strong antioxidative agent, and thus increases cell survival[1]. Decreased CAT activity in ACF-fed rats revealed lowered tissue protection ability. Co-administration of T. bellirica fruit extracts and EA in ACF-treated rats significantly enhanced the activity of CAT (Table 3). Previous studies on EA suggested that the hepatoprotective activity may be associated with its antioxidant and radical scavenging properties. EA might be responsible for increasing enzymatic protein synthesis or reducing the ROS-mediated loss of CAT activity[65]. It seems possible that CAT might act as an essential autocrine antioxidant and survival element. Furthermore, ROS produced as an offshoot of ACF treatment, also destroy liver tissues by stimulating lipid peroxidation. Increased level of MDA confirmed the higher rate of lipid peroxidation causing tissue damage and breakdown of cellular antioxidant defense systems[66-68]. Co-administration of T. bellirica fruit extract (200 mg/kg) and EA (40 mg/kg) with ACF appreciably reduced lipid peroxidation in rats.
| Groups | MDA (nmol/mg protein) | SOD (U/mg protein) | Catalase (U/mg protein) |
| Group I | 2.68 ± 0.08 | 31.09 ± 0.71 | 8.45 ± 0.17 |
| Group II | 18.63 ± 1.43a | 12.04 ± 1.49a | 3.21 ± 0.84a |
| Group III | 4.50 ± 0.18b1 | 29.11 ± 0.35b1 | 7.59 ± 0.41b1 |
| Group IV | 6.74 ± 0.14b1 | 27.24 ± 0.57b1 | 7.19 ± 0.37b1 |
| Group V | 12.28 ± 0.12b2 | 19.80 ± 0.62b2 | 5.77 ± 1.19b2 |
| Group VI | 9.46 ± 0.19b1 | 21.15 ± 0.49b2 | 6.23 ± 0.92b2 |
Histological analysis of ACF-treated rat liver slices also corroborated abnormal alterations observed in serum markers and tissue antioxidants. ACF produced notable impairment in the anatomical features of liver tissue encompassing pathological irregularities such as inflammatory infiltration, the formation of vacuoles and focal necrosis. T. bellirica fruit extracts (Eth and AQ) and EA treatment significantly lowered the number of damaged hepatocytes. Furthermore, the hepatoprotective potential of extracts and EA was also confirmed by the dearth of necrotic and inflammatory lesions in rat tissue sections. Comparatively better hepatoprotective efficacy was observed in SLM and EA treated rats.
The results of the current study suggest that the administration of T. bellirica fruit extracts and EA have considerable hepatoprotective efficacy against ACF-induced oxidative stress and hepatic damage in Wistar rats. ACF adversely altered the levels of serum liver function markers and tissue antioxidants. Abnormal levels of biomarkers might be the result of peroxidation reactions and biotransformation of ACF in liver. These reactions are accountable for oxidative damage to cellular components. T. bellirica fruit extracts and EA treatment significantly ameliorated the hepatic injury inflicted by ACF. However, further evaluation is warranted to reveal the complete mechanism of action of different phytoconstituents present in T. bellirica fruit which might be helpful in the development of a new therapeutic agent of natural origin.
Hepatotoxicity is one of the common side effects of nonsteroidal anti-inflammatory drugs (NSAIDs). Aceclofenac , a prodrug in the aryl-acetic acid class, is an oral NSAID effective in the treatment of painful inflammatory diseases. Chronic use of aceclofenac damages gastrointestinal mucosa by irritant action, causing an alteration in mucosal permeability and/or suppression of prostaglandin synthesis.
Previous studies on Terminalia bellirica fruit revealed that it possesses a wide range of bioactive compounds that are accountable for its antioxidant and radical scavenging potential against various types of experimental models. Moreover, its fruit has a positive impact on NSAIDs-induced liver injury. Therefore, in this study we explored the therapeutic attributes of T. bellirica fruit and ellagic acid against aceclofenac-induced hepatotoxicity and oxidative stress.
The major objectives were to evaluate the antioxidant and hepatoprotective activities of T. bellirica fruit ethyl acetate (Eth) and aqueous (AQ) extracts and its constituent ellagic acid against aceclofenac-induced hepatotoxicity in albino Wistar rats.
The antioxidant activities of T. bellirica fruit extracts were measured in vitro by metal ion chelation and nitric oxide radical scavenging assays. The in vivo antioxidant and hepatoprotective effects of T. bellirica extracts (200 mg/kg) and ellagic acid (40 mg/kg) in aceclofenac-induced hepatotoxic rats were evaluated in serum and liver tissue. The ferric reducing ability of plasma and lipid peroxidation inhibition were measured in blood. Liver function markers such as ALP, GPT, GOT, LDH, γ-glutamyl transferase, creatinine, total protein, and uric acid were evaluated in rat serum and superoxide dismutase, catalase and malondialdehyde were analyzed in liver tissues using standard protocols.
T. bellirica fruit Eth extract (IC50 168 µg/mL) showed higher ion chelating ability than the AQ extract (IC50 220 µg/mL). Similarly, the Eth extract (IC50 48 µg/mL) showed comparatively better NO scavenging activity than the AQ extract (IC50 57 µg/mL). The Eth extract also decreased lipid peroxidation by 75% indicating its lipoprotective ability. The ferric reducing ability of plasma value in the EA treated rat group was significantly higher (P < 0.05) than that in the T. bellirica fruit extract treated groups in vivo. T. bellirica fruit extracts and ellagic acid treatment suppressed the toxic effect of aceclofenac in rats and improved the body weight coupled with restoration of serum liver function markers and tissue specific antioxidants.
The results of the current study suggest that the administration of T. bellirica fruit extracts and ellagic acid exhibited considerable hepatoprotective efficacy against aceclofenac-induced oxidative stress and hepatic damage in Wistar rats. Abnormal levels of biomarkers may have occurred due to peroxidation reactions and biotransformation of aceclofenac in liver. These reactions were responsible for oxidative damage to cellular components. T. bellirica fruit extracts and ellagic acid treatment significantly ameliorated the hepatic injury induced by aceclofenac.
T. bellirica fruit extracts and its phytoconstituent ellagic acid exhibited appreciable radical scavenging, antioxidant and hepatoprotective activity in aceclofenac-induced liver injury. However, further evaluation is warranted to reveal the complete mechanism of action of different phytoconstituents present in T. bellirica fruit which might be helpful in the development of a new therapeutic agent of natural origin.
Both authors gratefully acknowledge UGC-SAP and DST-FIST facilities of the Department of Biochemistry, University of Allahabad, Prayagraj.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carrier P S-Editor: Zhang L L-Editor: Webster JR P-Editor: Wang LL
| 1. | Sharma UK, Kumar R, Gupta A, Ganguly R, Pandey AK. Renoprotective effect of cinnamaldehyde in food color induced toxicity. 3 Biotech. 2018;8:212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Kumar S, Gupta A, Pandey AK. Calotropis procera Root Extract Has the Capability to Combat Free Radical Mediated Damage. ISRN Pharmacol. 2013;2013:691372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Sharma UK, Kumar R, Gupta A, Ganguly R, Singh AK, Ojha AK, Pandey AK. Ameliorating efficacy of eugenol against metanil yellow induced toxicity in albino Wistar rats. Food Chem Toxicol. 2019;126:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 4. | Sharma UK, Sharma AK, Gupta A, Kumar R, Pandey A, Pandey AK. Pharmacological activities of cinnamaldehyde and eugenol: antioxidant, cytotoxic and anti-leishmanial studies. Cell Mol Biol (Noisy-le-grand). 2017;63:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Kumar R, Gupta A, Singh AK, Bishayee A, Pandey AK. The Antioxidant and Antihyperglycemic Activities of Bottlebrush Plant (Callistemon lanceolatus) Stem Extracts. Medicines (Basel). 2020;7 . [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Simon JP, Evan Prince S. Aqueous leaves extract of Madhuca longifolia attenuate diclofenac-induced hepatotoxicity: Impact on oxidative stress, inflammation, and cytokines. J Cell Biochem. 2018;119:6125-6135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Darbar S, Bhattacharya A, Chakraborty MR, Chattopadhyay S. Livina, a polyherbal preparation protects liver against aceclofenac-induced hepatic insult in sprague-dawley rats: a comparison with silymarin. Pharmacologyonline. 2010;2:889-907. [DOI] [Full Text] |
| 8. | Soumendra D, Anirbandeep B, Uttam B, Nilendra C, Bikash R, Kumar CT, Das A, Kumar PT. Antioxidant and hepatoprotective effect of Azadirachta indica leaf extract on aceclofenac induced hepatotoxicity in rats. J Pharm Res. 2009;8:116-121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Zaragoza Marcet A, Alfonso Moreno V, Roig Catalá E. NSAID-induced hepatotoxicity: aceclofenac and diclofenac. Rev Esp Enferm Dig. 1995;87:472-475. [PubMed] |
| 10. | Shalan MG, Mostafa MS, Hassouna MM, El-Nabi SE, El-Refaie A. Amelioration of lead toxicity on rat liver with Vitamin C and silymarin supplements. Toxicology. 2005;206:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Wang L, Huang QH, Li YX, Huang YF, Xie JH, Xu LQ, Dou YX, Su ZR, Zeng HF, Chen JN. Protective effects of silymarin on triptolide-induced acute hepatotoxicity in rats. Mol Med Rep. 2018;17:789-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Baradaran A, Samadi F, Ramezanpour SS, Yousefdoust S. Hepatoprotective effects of silymarin on CCl4-induced hepatic damage in broiler chickens model. Toxicol Rep. 2019;6:788-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Gillessen A, Schmidt HH. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv Ther. 2020;37:1279-1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 14. | Kuriakose J, Lal Raisa H, A V, Eldhose B, M S L. Terminalia bellirica (Gaertn.) Roxb. fruit mitigates CCl4 induced oxidative stress and hepatotoxicity in rats. Biomed Pharmacother. 2017;93:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Lobo VC, Phatak A, Chandra N. Antioxidant availabiltiy of beheda Terminalia bellerica (Roxb.) in relation to its medicinal uses. Phcog J. 2010;2:338-344. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Gupta A, Kumar R, Pandey AK. Antioxidant and antidiabetic activities of Terminalia bellirica fruit in alloxan induced diabetic rats. S Afr J Bot. 2020;130:308-315. [DOI] [Full Text] |
| 17. | Kaur S, Arora S, Kaur K, Kumar S. The in vitro antimutagenic activity of Triphala--an Indian herbal drug. Food Chem Toxicol. 2002;40:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Gupta A, Kumar R, Bhattacharyya P, Bishayee A, Pandey AK. Terminalia bellirica (Gaertn.) roxb. (Bahera) in health and disease: A systematic and comprehensive review. Phytomedicine. 2020;77:153278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Fahmy NM, Al-Sayed E, Singab AN. Genus Terminalia: A phytochemical and Biological Review. Med Aromat Plants. 2015;4:5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Gupta A, Singh AK, Kumar R, Ganguly R, Rana HK, Pandey PK, Sethi G, Bishayee A, Pandey AK. Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Cock IE. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacology. 2015;23:203-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Rashed K, Potocnjak I, Giacometti J, Skoda M, Domitrovic R. Terminalia bellerica aerial parts ethyl acetate extract exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. J Funct Foods. 2014;8:319-330. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Valsaraj R, Pushpangadan P, Smitt UW, Adsersen A, Christensen SB, Sittie A, Nyman U, Nielsen C, Olsen CE. New anti-HIV-1, antimalarial, and antifungal compounds from Terminalia bellerica. J Nat Prod. 1997;60:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Dhanani T, Shah S, Kumar S. A validated high-performance liquid chromatography method for determination of tannin-related marker constituents gallic acid, corilagin, chebulagic acid, ellagic acid and chebulinic Acid in four Terminalia species from India. J Chromatogr Sci. 2015;53:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Pfundstein B, El Desouky SK, Hull WE, Haubner R, Erben G, Owen RW. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochemistry. 2010;71:1132-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | García-Niño WR, Zazueta C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol Res. 2015;97:84-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 27. | Abdelkader NF, Elyamany M, Gad AM, Assaf N, Fawzy HM, Elesawy WH. Ellagic acid attenuates liver toxicity induced by valproic acid in rats. J Pharmacol Sci. 2020;143:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Mishra AK, Mishra A, Kehri HK, Sharma B, Pandey AK. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann Clin Microbiol Antimicrob. 2009;8:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1684] [Cited by in RCA: 1482] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 30. | Kumar S, Sharma UK, Sharma AK, Pandey AK. Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: phytochemical analysis and antioxidant effect. Cell Mol Biol (Noisy-le-grand). 2012;58:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8653] [Cited by in RCA: 9023] [Article Influence: 209.8] [Reference Citation Analysis (0)] |
| 32. | Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119:598-620. [PubMed] |
| 33. | Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12606] [Cited by in RCA: 12134] [Article Influence: 418.4] [Reference Citation Analysis (0)] |
| 34. | Niehaus WG Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 917] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 35. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 36. | Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6497] [Cited by in RCA: 6563] [Article Influence: 128.7] [Reference Citation Analysis (1)] |
| 37. | Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133-140. [PubMed] |
| 38. | Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014;2014:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 521] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 39. | Sharma UK, Kumar R, Ganguly R, Gupta A, Sharma AK, Pandey AK. Cinnamaldehyde, an active component of cinnamon provides protection against food colour induced oxidative stress and hepatotoxicity in albino Wistar rats. Vegetos. 2018;31(2): :123-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Nampoothiri SV, Prathapan A, Cherian OL, Raghu KG, Venugopalan VV, Sundaresan A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem Toxicol. 2011;49:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Shreaz S, Sheikh RA, Rimple B, Hashmi AA, Nikhat M, Khan LA. Anticandidal activity of cinnamaldehyde, its ligand and Ni(II) complex: effect of increase in ring and side chain. Microb Pathog. 2010;49:75-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Kumar S, Kumar R, Dwivedi A, Pandey AK. In vitro antioxidant, antibacterial, and cytotoxic activity and in vivo effect of Syngonium podophyllum and Eichhornia crassipes leaf extracts on isoniazid induced oxidative stress and hepatic markers. Biomed Res Int. 2014;2014:459452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. |
Gordon MH.
The Mechanism of Antioxidant Action |
| 44. | Johnson EI. The quantitative analysis of drugs. J Pharm Pharmacol. 1964;16:772-772. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol. 2002;40:1213-1232. [PubMed] |
| 46. | Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5980] [Cited by in RCA: 5212] [Article Influence: 179.7] [Reference Citation Analysis (0)] |
| 47. | Sartor L, Pezzato E, Dell'Aica I, Caniato R, Biggin S, Garbisa S. Inhibition of matrix-proteases by polyphenols: chemical insights for anti-inflammatory and anti-invasion drug design. Biochem Pharmacol. 2002;64:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Sharma AK, Kumar S, Pandey AK. Ferric reducing, anti-radical and cytotoxic activities of Tinospora cordifolia stem extracts. Biochem Anal Biochem. 2014;3:153. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 981] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 50. | Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40: :945-948. [DOI] [Full Text] |
| 51. | Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 961] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 52. | Kim NY, Lee MK, Park MJ, Kim SJ, Park HJ, Choi JW, Kim SH, Cho SY, Lee JS. Momordin Ic and oleanolic acid from Kochiae Fructus reduce carbon tetrachloride-induced hepatotoxicity in rats. J Med Food. 2005;8:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Saravanan R, Viswanathan P, Pugalendi KV. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006;78:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 54. | Tsai SJ, Yin MC. Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells. J Food Sci. 2008;73:H174-H178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 55. | Castellano JM, Guinda A, Delgado T, Rada M, Cayuela JA. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes. 2013;62:1791-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 56. | Maurya A, Khan F, Bawankule DU, Yadav DK, Srivastava SK. QSAR, docking and in vivo studies for immunomodulatory activity of isolated triterpenoids from Eucalyptus tereticornis and Gentiana kurroo. Eur J Pharm Sci. 2012;47:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Amarowicz R. Tannins: The new natural antioxidants? Eur J Lipid Sci Tech. 2007;109:549-551. [RCA] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Amarowicz R, Troszynska A, Baryłko-Pikielna N, Shahidi F. Polyphenolics extracts from legume seeds: Correlations between total antioxidant activity, total phenolics content, tannins content and astringency. J Food Lipids. 2004;11:278-286. [RCA] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Wang J, Tang L, White J, Fang J. Inhibitory effect of gallic acid on CCl4-mediated liver fibrosis in mice. Cell Biochem Biophys. 2014;69:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Girish C, Pradhan SC. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J Pharmacol Pharmacother. 2012;3:149-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 61. | Kaur R, Singh BP, Arora S. Amelioration of oxidative damage by methyl gallate in different in vitro models. Phytopharmacology. 2011;1:82-94. |
| 62. | Bort R, Ponsoda X, Carrasco E, Gómez-Lechón MJ, Castell JV. Comparative metabolism of the nonsteroidal antiinflammatory drug, aceclofenac, in the rat, monkey, and human. Drug Metab Dispos. 1996;24:969-975. [PubMed] |
| 63. | Marzuillo P, Grandone A, Perrone L, del Giudice EM. Weight loss allows the dissection of the interaction between abdominal fat and PNPLA3 (adiponutrin) in the liver damage of obese children. J Hepatol. 2013;59:1143-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Darbar S, Bose A, Chattaraj TK, Pal TK. Protective role of Zingiber officinale Roscoe on aceclofenac induced oxidative stress in rat liver. Int J Pharmtech Res. 2010;2:495-501. |
| 65. | Chao PC, Hsu CC, Yin MC. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab (Lond). 2009;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 66. | Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1411] [Article Influence: 141.1] [Reference Citation Analysis (0)] |
| 67. | Aust SD, Chignell CF, Bray TM, Kalyanaraman B, Mason RP. Free radicals in toxicology. Toxicol Appl Pharmacol. 1993;120:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Guillén-Sans R, Guzmán-Chozas M. The thiobarbituric acid (TBA) reaction in foods: a review. Crit Rev Food Sci Nutr. 1998;38:315-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |