Published online Sep 26, 2015. doi: 10.4252/wjsc.v7.i8.1127
Peer-review started: November 29, 2014
First decision: December 26, 2014
Revised: June 25, 2015
Accepted: July 16, 2015
Article in press: July 17, 2015
Published online: September 26, 2015
Processing time: 303 Days and 13.6 Hours
AIM: To investigate whether we could create natural autologous tissue patches in the subcutaneous space for organ repair.
METHODS: We implanted the following three types of inert foreign bodies in the subcutaneous tissue of rats to produce autologous tissue patches of different geometries: (1) a large-sized polyvinyl tube (L = 25 mm, internal diameter = 7 mm) sealed at both ends by heat application for obtaining a large flat piece of tissue patch for organ repair; (2) a fine polyvinyl tubing (L = 25 mm, internal diameter = 3 mm) for creating cylindrically shaped grafts for vascular or nerve repair; and (3) a slurry of polydextran particle gel for inducing a bladder-like tissue. Implantation of inert materials was carried out by making a small incision on one or either side of the thoracic-lumbar region of rats. Subcutaneous pockets were created by blunt dissection around the incision into which the inert bodies were inserted (1 or 2 per rat). The incisions were closed with silk sutures, and the animals were allowed to recover. In case of the polydextran gel slurry 5 mL of the slurry was injected in the subcutaneous space using an 18 gauge needle. After implanting the foreign bodies a newly regenerated encapsulating tissue developed around the foreign bodies. The tissues were harvested after 4-42 d of implantation and studied by gross examination, histology, and histochemistry for organization, vascularity, and presence of mesenchymal stem cells (MSCs) (CD271+CD34+ cells).
RESULTS: Implanting a large cylindrically shaped polyvinyl tube resulted in a large flat sheet of tissue that could be tailored to a specific size and shape for use as a tissue patch for repairing large organs. Implanting a smaller sized polyvinyl tube yielded a cylindrical tissue that could be useful for repairing nerves and blood vessels. This type of patch could be obtained in different lengths by varying the length of the implanted tube. Implanting a suspension of inert polydextran suspension gave rise to a bladder-like tissue that could be potentially used for repairing heart valves. Histologically, the three different types of tissue patches generated were organized similarly, consisting of three layers, increasing in thickness until day 14. The inner layer in contact with the inert material was avascular; a middle layer that was highly vascular and filled with matrix, and an outer layer consisting of loose connective tissue. MSCs identified as CD271+CD34+ cells were present in the medial layer and around major blood vessels at day 4 but absent at later time points. The early-harvested tissues, endowed with MSCs, could be used for tissue repair, while the later-harvested tissues, being less vascular but thicker and tougher, could be used as filler tissue for cosmetic purposes.
CONCLUSION: An autologous, vascularized tissue patch of desired shape and size can be created in the subcutaneous space by implanting different types of inert bodies.
Core tip: While much progress has been made in man-made tissue patches for organ repair, their clinical success has been bogged down by their inability to get promptly vascularized in vivo. We describe a methodology for creating tissue patches of several possible shapes/sizes in the subcutaneous tissue utilizing the foreign body reaction. These patches are endowed with a rich capillary network, abundant mesenchymal stem cells, and other accessory cells that support the viability of the tissue. Such in vivo generated patches for tissue repair promise to be safer, more readily vascularized, and stable for longer period in the body.
- Citation: Garcia-Gomez I, Gudehithlu KP, Arruda JAL, Singh AK. Autologous tissue patch rich in stem cells created in the subcutaneous tissue. World J Stem Cells 2015; 7(8): 1127-1136
- URL: https://www.wjgnet.com/1948-0210/full/v7/i8/1127.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i8.1127
There is an unmet need to develop biocompatible tissue patches for repair of various diseased organs such as ischemic heart, large open wounds, vascular defects, damaged nerves and many others. Much progress has been made in creating engineered tissue patches using polymer scaffolds (which mimic tissue matrix) or processed xenogeneic biomaterials seeded with functional cells, endothelial cells, paracrine growth factors, and fetal or adult stem cells[1-7]. The complexity of these constructs is necessitated by the need to have an immediate blood supply to the patch for survival of the seeded endothelial and functional cells. A myriad of growth factors (from stem cells) should also be available in the patch to rapidly form the new vasculature that would connect with the host blood vessels. Despite such advances the constructs have proven to be sub-optimal in performance. There is the problem of core degradation of the construct and the lack of integration of the seeded functional cells with the host tissue cells, probably arising from lack of adequate blood supply in the construct[3,8]. The failing patches could occasionally end up being a source of dying cells and thus causing adverse reactions rather than benefit. A multitude of accessory supporting cells other than endothelial cells are needed to form the desired new blood vessels and maintain the integrity of an avascular patch[9-14]. Because many of these cells are not readily available and several others remain unknown, these patches remain poorly perfused in vivo. A natural autologous tissue patch containing stem cells, other supporting cells, and more importantly, a functional vasculature, would be a good solution to the problem.
We present here a methodology for creating tissue patches in the subcutaneous space of different shapes and sizes that are richly endowed with mesenchymal stem cells (MSCs), microvessels and interstitial cells. The principle behind the technique is to implant an inert material in the subcutaneous space which elicits a “foreign-body reaction” leading to the formation of a highly vascular, newly regenerated tissue surrounding the foreign body. Using this approach we have created vascularized tissue patches of various shapes and identified the time after implantation when the MSC population is highest in the new tissue for the purpose of using them as grafts for tissue repair.
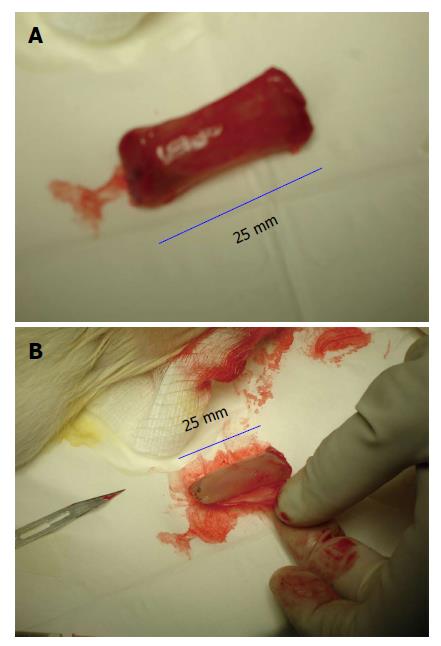
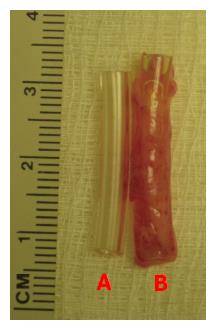
The following types of inert materials were implanted in the rat subcutaneous space: (1) large-sized polyvinyl tube (Polyvinyl chloride 180; Nalgene Nunc International, Rochester, New York) (L = 25 mm, internal diameter = 7 mm) sealed at both ends by heat application for obtaining a large flat piece of tissue patch for organ repair (Figure 1); (2) fine polyvinyl tubing (L = 25 mm, internal diameter = 3 mm) for creating cylindrically shaped grafts for vascular or nerve repair (Figure 2); and (3) a slurry of polydextran particle gel for inducing a bladder-like tissue. Polyvinyl chloride after polyvinyl bodies were sterilized by immersing them in 70% ethanol, then washed vigorously with sterile saline and air-dried before implantation in the rat. Polydextran slurry was made from Sephadex-25 (course; particle size 100-300 μm diameter; General Electric Healthcare Bio-Sciences, Pittsburgh, Pennsylvania, United States). The polydextran particles were pre-swollen in water and sterilized by suspending them in 70% ethanol for 2-3 h followed by removal of ethanol and re-suspension in 10 volumes of sterile saline. After allowing the particles to re-swell for several days in sterile saline, excess saline was removed and the slurry adjusted to a particle: saline ratio of 1:1 (vol/vol) for use.
All the animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the John H. Stroger, Jr. Hospital of Cook County. The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to the laboratory conditions for 2 wk prior to experimentation and given food and water ad libitum. All animals were euthanized by barbiturate overdose (150 mg/kg body weight pentobarbital).
Sprague-Dawley males rats (250-300 g) were anesthetized with intraperitoneal sodium nembutal (5 mg/100 g wt) and their thoracic-lumbar region was shaved and cleaned with alcohol/povidone. For implantation of inert materials a small incision was made on one or either side of the region. Subcutaneous pockets were created by blunt dissection around the incision into which the inert materials were inserted (1 or 2 per rat). The incisions were closed with silk sutures, and the animals were allowed to recover. In case of the polydextran gel slurry 5 mL of the slurry was injected in the subcutaneous space using an 18 gauge needle.
Rats were sacrificed at day 4, 7, 14, 21, 35 and 42 after implantation of the three types of inert materials (n = 3 rats at each time point for large and fine polyvinyl tube and n = 2 at each time point for polydextran; total rats used = 48).
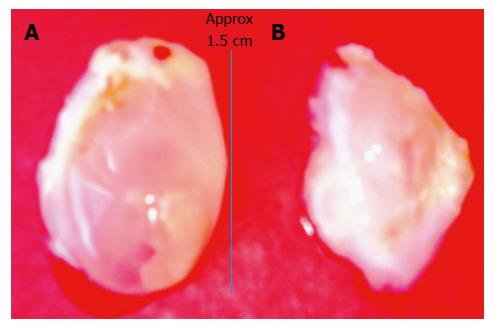
At day 4, 7, 14, 21, 35 and 42 after placement in the subcutaneous space the inert bodies were harvested aseptically. The tissue encapsulating the materials was removed according to the type of tissue required. For obtaining a flat piece of tissue, the tissue encapsulating the large polyvinyl tube was removed by cutting along the length of the tube as shown in Figure 1. For obtaining a cylindrical tissue as for a vascular graft, the encapsulating tissue from the finer polyvinyl tube was gently slipped off the tube as shown in Figure 2. The bladder-like tissue from the polydextran slurry injected rats was separated from the adhering connective tissue using blunt dissection (Figure 3).
For histological examination samples of the three tissues were fixed in 4% para-formaldehyde for 24 h with vigorous shaking. The fixed tissues were then paraffin embedded in the manner that the inner and outer aspects of the tissues were histologically recognizable. Approximately 5 μm thick sections were obtained on glass slides for routine histological staining (trichrome) and histochemistry described below.
Indirect immunofluorescence, double immunofluorescence or immune-phosphatase techniques were performed on 4-6 μm thickness sections of paraffin embedded tissue patches. Sections were deparaffinized before staining.
For indirect immunofluorescence, the sections were treated with citrate buffer (pH = 6) at 100 °C for 10 min for antigen retrieval followed by 10 min incubation with sodium borohydride (4 mg/mL) to minimize auto-fluorescence. Sections were incubated with the primary antibody (mouse anti-proliferating cell nuclear antigen (PCNA) (Millipore, Temecula, CA) or mouse anti- α-smooth muscle actin (α-SMA) (Sigma, Saint Louis, MO) at 4 °C overnight. Next day the sections were incubated for 1 h at room temperature with the appropriate secondary antibody [anti-mouse IgG linked to Alexa Fluor 633 (Invitrogen, Eugen, OR)] and mounted with Prolong Gold anti-fade reagent containing 4',6-diamidino-2-phenylindole (DAPI) to counterstain nuclei with blue fluorescence (Invitrogen, Eugen, OR).
For double immunofluorescence, the protocol followed was as described above with the difference that the overnight incubation was with both primary antibodies [goat anti-CD34 (R and D, Minneapolis, MN) and mouse the anti-CD271 (Abcam, Cambridge, MA)] at the same time. Next day, the sections were incubated with the secondary antibodies [anti-mouse IgG linked to Alexa Fluor 633 and also anti-goat IgG linked to Alexa Fluor 488 (Invitrogen, Eugen, OR)] at the same time for 1 h at room temperature followed by mounting with Prolong Gold anti-fade reagent containing DAPI (Invitrogen, Eugen, OR).
For immune-phosphatase, the sections were treated with 75 mmol/L borate buffer (pH = 10.0) at 121 °C for 10 min for antigen retrieval. Sections were incubation with goat anti-collagen type IV (Southern Biotechnology Associates, Birmingham, AL) overnight, followed by incubation with corresponding alkaline phosphatase conjugated secondary antibody. The reaction was detected with alkaline phophatase substrate (red) (Vector Laboratories Burlingham, CA) and finally mounted in 1:1 water: glycerol solution.
Negative controls consisted of sections in which the primary antibodies were omitted. All slides were examined and digitally photographed using a fluorescent/optical microscope (Nikon, New York, NY). Merge panels for immunofluorescence and double immunofluorescence were performed with Adobe Photoshop CS6 software (Adobe Systems, San Jose, CA).
When a large-sized polyvinyl tube implant was placed in the subcutaneous space and extracted after 4 d, the implant was completely encapsulated by a de novo tissue (Figure 1A). The de novo tissue could be conveniently stripped off the implant as shown in Figure 1B to obtain a flat piece of tissue patch. The patch could be tailored to the size and shape required. The size of the flat tissue patch depended on the size of the original inert material implanted, as long as the size and shape of the implant conformed to the available subcutaneous space.
A cylindrically shaped tissue patch could be obtained by implanting a fine polyvinyl tubing (Figure 2A) and removing the cylindrical tissue by slipping off the tissue from the implant like removing a “sock” (Figure 2B). This type of patch could be obtained in different lengths by varying the length of the implanted tube (not shown).
Injecting a slurry of poldextran gel particles in the subcutaneous space induced an unusual tissue patch in the form of a sealed bladder or pouch. While the day 4-7 bladder tissues were too delicate to handle, the day 14 tissue was strong enough to be obtained as an integral bladder (Figure 3A). The bladder-like tissue enclosed the injected gel particles. By piercing the balloon-like tissue the polydextran particles could be removed from the tissue to deflate the bladder (Figure 3B). The tissue could potentially be used in a variety of ways including as a cusp of a required curvature for repair of solid tissues or heart valves.
All the three types of tissue showed similar histology with regard to the inner and outer aspect of the tissue. The inner aspect of the tissue represented the surface in contact with the inert material and the outer aspect represented the surface away from the inert material and in contact with the surrounding connective tissue. We have chosen to describe the histology of the tissue obtained from the large-sized polyvinyl tube as representative of all others.
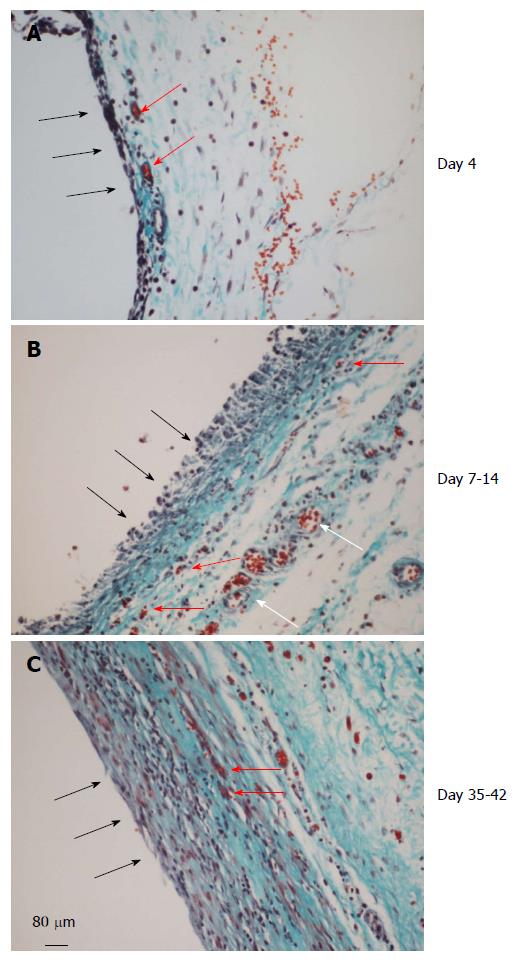
Implanting any of the three types of inert material in the subcutaneous tissue induced a de novo tissue which completely surrounded the foreign body as a capsule by day 4. The tissue at day 4 grossly appeared thin and filmy but well vascularized (Figure 4A). The inner layer consisted of one or two-cell thick mononuclear cells (20-40 μm) but devoid of blood vessels. The medial layer was 4-6 cell thick (100-150 μm) and contained microvessels and fibrous matrix. The outer layer (300-400 μm) consisted of loose connective tissue.
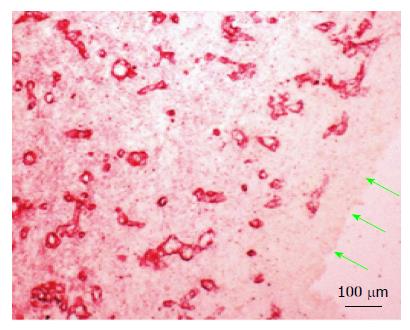
At day 7-14 the de novo tissue appeared thicker and stronger than at day 4 and histologically better organized into inner, medial and outer layers (Figure 4B). The inner layer in contact with the inert material was approx. 100 μm thick, consisted of mononuclear cells but devoid of extracellular matrix and blood vessels. The medial layer was thicker (200-300 μm), consisting of fibroblastic cells, rich network of microvessels and extracellular matrix. The outer layer remained loose but contained large blood vessels. The high vascularity of the medial layer at this time point is shown in Figure 5 by immune-staining sections for collagen type IV, shown in our previous study to reveal newly formed blood vessels[15].
Between day 35-42 the granulation tissue appeared slightly thicker than day 7-14 tissue but was tougher and more fibrous to touch. Histologically this tissue appeared more compact with the inner layer becoming less distinguished than at day 7-14 and merging with the medial layer. Also, this layer showed reduced number of blood vessels and increased amounts of extracellular matrix arranged in parallel sheets. The outer layer remained unchanged (Figure 4C).
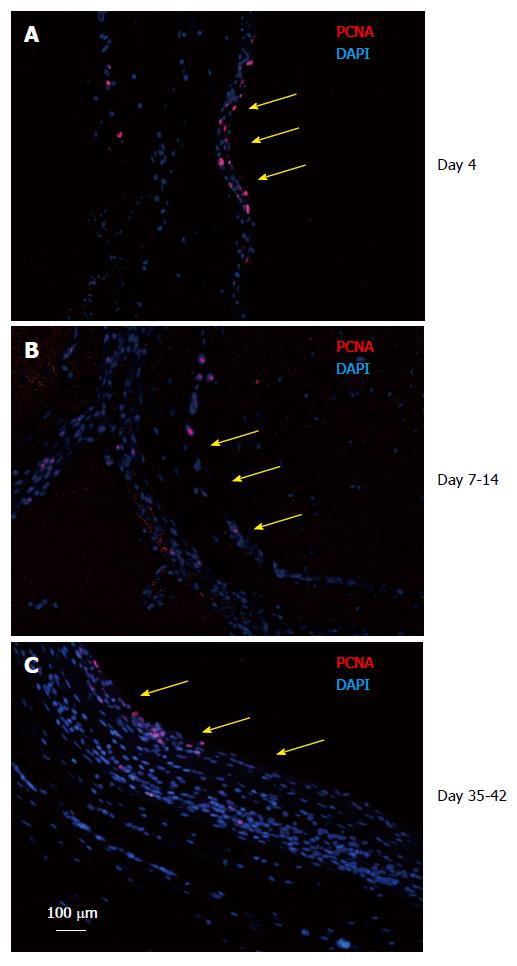
Because the tissue patch was found to be a dynamic tissue between day 4-42 after implantation of the inert body, it was important to determine the growing edge of the tissue. The tissues were immune- stained for PCNA to identify the proliferating cells in the patch. We found that the patch was a continually growing tissue with the inner aspect (side in contact with the inert body) and the medial layer staining positive for PCNA at all times tested (Figure 6), suggesting that the patch was growing from inside to outside.
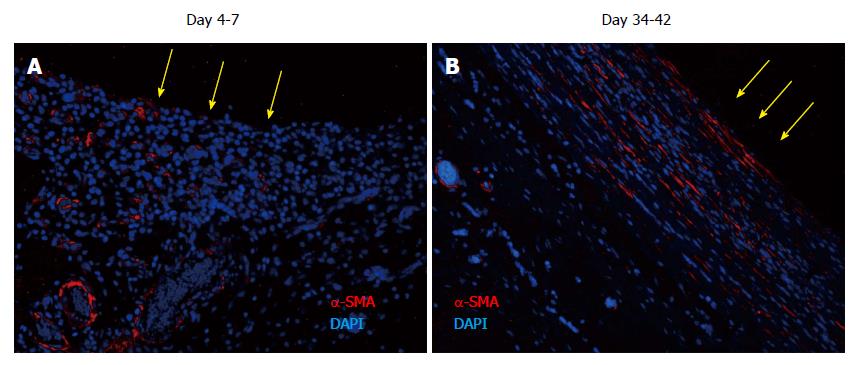
The tissues were immune-stained for α-SMA, a marker of myofibroblasts, to identify the source and organization of the fibrous material identified in the medial layer of the tissue patch. At day 4-7, α-SMA was found to be associated with blood vessels as expected, but little extracellular α-SMA was observed at these times (Figure 7). At later time points there was more and more of α-SMA observed in the extracellular areas (not shown), which by day 35-42 had stratified and compacted itself in parallel to the length of the implants (Figure 7), consistent with the tissue being tougher and fibrous to touch.
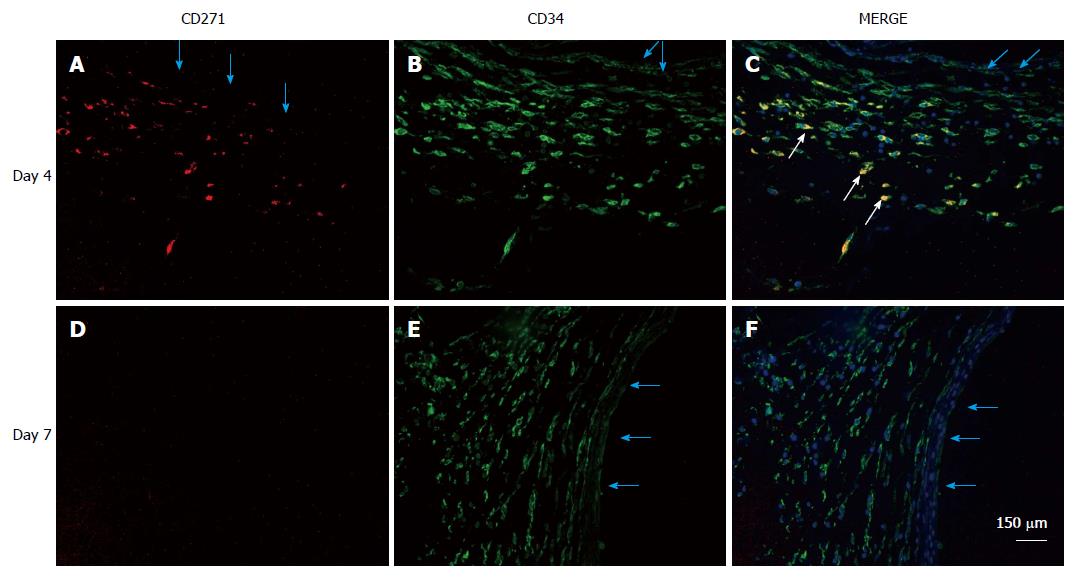
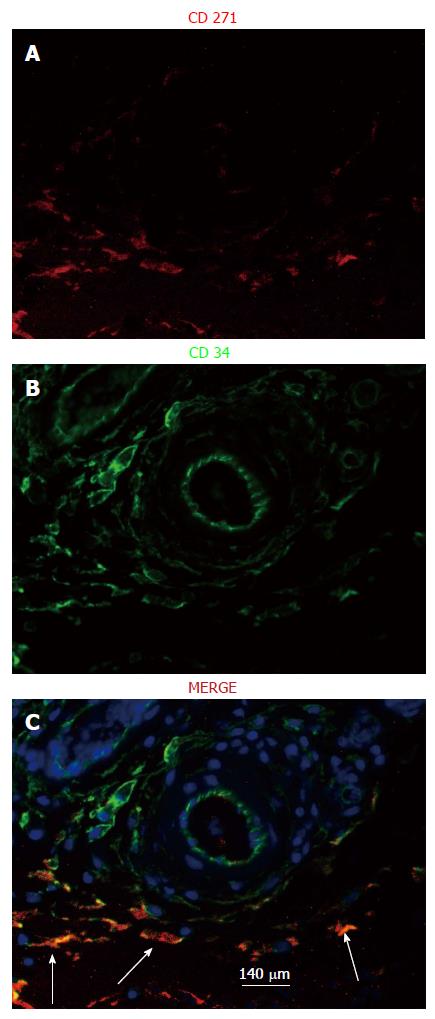
It has been recently recognized that CD34+CD271+ cells mark the presence of MSCs in a tissue[16-20]. Tissue patches from days 4-42 were immune-stained for CD34 and CD271 to identify the location of MSCs. We found that MSCs were clearly identifiable in the stroma of the medial layer of the tissue patch at day 4 after the implantation of the inert body but rarely seen at day 7 or after (Figure 8). Also, MSCs were found to be concentrated in the vicinity of major blood vessels in the medial layer at day 4 but never at day 7 or after (Figure 9).
We demonstrate that the subcutaneous space is an effective and convenient compartment to create an autologous tissue patch of desired shape and size for the purpose of repairing organs or tissues. By implanting inert material of different shapes and sizes we obtained newly regenerated tissues of corresponding shapes and sizes containing abundant blood vessels, multitude of other functional cells, and importantly, also MSCs in a matter of 4 d. These early-harvested tissues, which were highly vascular and abundant in MSCs, could potentially be used for tissue repair. When the de novo tissues were allowed to grow for longer times the tissues obtained were less vascular but thicker and tougher. Such tissues could be applied for structural support or as a filler tissue for cosmetic purposes.
There are several advantages of using subcutaneous tissue patches as described in this report compared to patches made of synthetic materials and seeded with cultured cells[1-7]. First and foremost is the autologous nature of the subcutaneous patch which can be created in the patient’s own body. Clearly, such patches will be safer because they will be better tolerated immunologically in the patient body than patches made from xenogenic or synthetic materials[21]. Second, the subcutaneous patches will have a ready-made vascular system for the perfusion of blood though the entire body of the patch. Man-made patches have the problem of core degeneration because of lack of vascularity which results in patch failure and the possibility of adverse reactions because of necrotic foci in the patch. Thirdly, these in vivo created patches contain other important accessory cells including interstitial cells which create matrix to provide body and integrity to the patch, and pericytes which provide support to the developing blood vessels during their integration with the host blood vessels.
The efficacy of autologously created in vivo patches in repairing functional organs is highlighted by the omentum which, either as an attached (with vascular connection intact) or detached tissue (severed vascular connection), has proven to be successful in repairing ischemic heart, spinal cord, and bony fractures[22]. More recently, we showed that the omentum is capable of being activated to a state in which its content of MSCs and blood vessels become several fold higher[23,24]. In this state it could be used for regenerating endocrine damaged pancreas, liver and kidney[25-27]. Notwithstanding the utility of the activated omentum in regenerating tissues, its use has been greatly restricted by the difficulty of obtaining the tissue from the abdominal cavity which necessitates laparotomy, a risky surgical procedure. A similar patch created in the subcutaneous tissue, as described here, is clearly less risky to the patient.
The presence of an intact and functional vascular system in a tissue implant is clearly a factor which favors its success in vivo with respect to survival and integration with the host tissue. Consistent with this premise, pre-vascularized man-made tissue patches for later implantation have proven to be longer-lasting than un-prevascularized patches[28,29]. Because of the preformed capillary network in such constructs, whether created in vitro or in vivo, they were able to anastamose with the host vasculature and thereby perfuse the construct promptly after in vivo implantation[12,28,30,31]. The strategies used for prevascularization of synthetic scaffolds involve the sandwiching of pro-endothelial cells and myoblasts on the scaffolds which are subsequently placed in tissue culture, resulting in capillary network formation[32]. Another alternative is to pre-implant the construct on the omentum or in the renal pouch[29,30], a tissue known to induce angiogenesis in tissue grafts, resulting in a rich network of capillaries penetrating the construct. However, there are drawbacks to these approaches which need to be overcome for better outcomes: (1) the technology to create such capillary network in vitro is labor intensive, expensive, and difficult in terms of controlling quality; (2) pre-implanting the construct on the omentum (or renal pouch) for pre-vascularization, is unsafe due to the risk of infections by the surgical procedures required, as mentioned above; and (3) such patches have yet the potential problem of safety because of the use of allogeneic cells and materials for their production. In light of the above considerations a tissue patch created in the subcutaneous tissue overcomes many of the problems inherent in man-made patches.
The finding of MSCs in the subcutaneous patch we describe herein is not surprising considering that the patch is a newly regenerated tissue and must involve such cells to create an organized tissue. Earlier we had shown that the subcutaneous patch also contained cells which expressed CXC chemokine receptor 4 (CXCR4), the receptor for stromal cell derived factor-1 (SDF-1), a protein which is released by injured tissues[15]. The presence of CXCR4 has been considered to be a stem cell marker based on the rationale that an injured tissue releasing SDF-1 would attract the CXCR4 bearing bone marrow cells for repair. One outstanding problem in the field of MSCs is the lack of validated markers to identify them, especially in an intact tissue. While many cell markers in blood cells have been described as representing MSCs there is a dearth of knowledge on such markers to identify MSCs in a solid tissue. We therefore believe there may be many other cell-specific stem cells in addition to the CD34+CD271+ we found in this tissue.
In summary, we demonstrate here that it is convenient to create a tissue patch of preferred shape and size in the subcutaneous space. A patch created in the subcutaneous tissue was highly vascularized, and contained MSCs and other accessory cells, which should endow it with the ability to successfully graft to an injured tissue and augment its healing.
The authors wish to thank Lev Rappoport, MD and Sethupathi Periannan, MD for help with histological processing and animal surgeries respectively. Ignacio Garcia-Gomez was supported during this period by a Fellowship from the Ramon Areces Foundation of Spain. The work was financially supported by an unrestricted grant from the Hektoen Institute of Medicine, Chicago IL, United States.
Despite much progress in the development of engineered tissue patches for organ repair these constructs often fail from lack of adequate blood supply. A natural autologous tissue patch containing a functional vasculature and stem cells would overcome such problems. The authors created such autologous tissue patches of different geometries by implanting three types of inert foreign bodies in the subcutaneous tissue of rats.
There is an unmet need to develop biocompatible tissue patches for repair of various diseased organs such as ischemic heart, large open wounds, vascular defects, damaged nerves and many others. Much progress has been made in creating engineered tissue patches using polymer scaffolds or processed xenogeneic biomaterials seeded with functional cells, endothelial cells, paracrine growth factors, and fetal or adult stem cells. Despite such advances the constructs have proven to be sub-optimal in performance. There is the problem of core degradation of the construct and the lack of integration of the seeded functional cells with the host tissue cells, probably arising from lack of adequate blood supply in the construct. A natural autologous tissue patch containing stem cells, other supporting cells, and more importantly, a functional vasculature, would be a good solution to the problem.
The authors demonstrate in this manuscript that it is possible to create an autologous tissue patch of preferred shape and size in the subcutaneous space. A patch created in the subcutaneous tissue is highly vascularized, contains mesenchymal stem cells (MSCs), and other accessory cells, which should endow it with the ability to successfully graft to an injured tissue and augment its healing.
Availability of an autologous tissue patch containing MSCs, as described herein, will open up the possibility of repairing diseased or injured organs of patients in a safe and effective manner.
Although creating man-made patches for tissue repair have been described, they contain synthetic materials and cells grown outside the body, which make such patches bio-incompatible and unsafe for implanting in patients. An autologous tissue patch on the other hand is one which is created in the patient itself making them completely bio-compatible and safer to use in the patient. A synthetic or natural tissue patch implanted in the patient’s body must be sustained by blood. If the patch lacks built-in blood vessels the patch will quickly die and fail. On the other hand if the patch already has blood vessels in it (functional vasculature) it has a better chance of survival and functionality.
Authors reported their successful challenge to create a vascularized, ready-to-use patch by an extremely feasible method of subcutaneous implantation of polyvinyl tubes. Their technique will contribute to therapeutic development for the treatment of acute tissue injures. The only one regrettable point is that they did not present a case where the implanted patch eventually improved the repair of injured tissues. It is best for the authors to prove the efficacy of their patches in their future works.
P- Reviewer: Kashani IR, Saeki K S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Simpson D, Liu H, Fan TH, Nerem R, Dudley SC. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25:2350-2357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Bursac N. Cardiac tissue engineering using stem cells. IEEE Eng Med Biol Mag. 2009;28:80, 82, 84-86, 88-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | O`Brien FJ. Biomaterials and scaffolds for tissue engineering. Materialstoday. 2011;14:88-95. [DOI] [Full Text] |
| 4. | Rouwkema J, Gibbs S, Lutolf MP, Martin I, Vunjak-Novakovic G, Malda J. In vitro platforms for tissue engineering: implications for basic research and clinical translation. J Tissue Eng Regen Med. 2011;5:e164-e167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, Marui A, Shimizu T, Ikeda T, Okano T. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Fujita J, Itabashi Y, Seki T, Tohyama S, Tamura Y, Sano M, Fukuda K. Myocardial cell sheet therapy and cardiac function. Am J Physiol Heart Circ Physiol. 2012;303:H1169-H1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Lam MT, Nauta A, Meyer NP, Wu JC, Longaker MT. Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing. Tissue Eng Part A. 2013;19:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Wendel JS, Ye L, Zhang P, Tranquillo RT, Zhang JJ. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng Part A. 2014;20:1325-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 524] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 10. | Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875-2888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Phelps EA, Garcia AJ. Update on therapeutic vascularization strategies. Regen Med. 2009;4:65-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci USA. 2009;106:16568-16573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 312] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 13. | Papavasiliou G, Cheng MH, Brey EM. Strategies for vascularization of polymer scaffolds. J Investig Med. 2010;58:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 14. | Sukmana I. Bioactive polymer scaffold for fabrication of vascularized engineering tissue. J Artif Organs. 2012;15:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Gudehithlu KP, Ahmed N, Wu H, Litbarg NO, Garber SL, Arruda JA, Dunea G, Singh AK. Antagonism of vascular endothelial growth factor results in microvessel attrition and disorganization of wound tissue. J Lab Clin Med. 2005;145:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Kuçi S, Kuçi Z, Kreyenberg H, Deak E, Pütsch K, Huenecke S, Amara C, Koller S, Rettinger E, Grez M. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Iso Y, Yamaya S, Sato T, Poole CN, Isoyama K, Mimura M, Koba S, Kobayashi Y, Takeyama Y, Spees JL. Distinct mobilization of circulating CD271+ mesenchymal progenitors from hematopoietic progenitors during aging and after myocardial infarction. Stem Cells Transl Med. 2012;1:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Maijenburg MW, Kleijer M, Vermeul K, Mul EP, van Alphen FP, van der Schoot CE, Voermans C. The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. Haematologica. 2012;97:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Quirici N, Scavullo C, de Girolamo L, Lopa S, Arrigoni E, Deliliers GL, Brini AT. Anti-L-NGFR and -CD34 monoclonal antibodies identify multipotent mesenchymal stem cells in human adipose tissue. Stem Cells Dev. 2010;19:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 627] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 21. | Tisato V, Cozzi E. Xenotransplantation: an overview of the field. Methods Mol Biol. 2012;885:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Vernik J, Singh AK. Omentum: power to heal and regenerate. Int J Artif Organs. 2007;30:95-99. [PubMed] |
| 23. | Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JA, Dunea G, Singh AK. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res. 2007;328:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Patel J, Gudehithlu KP, Dunea G, Arruda JA, Singh AK. Foreign body-induced granulation tissue is a source of adult stem cells. Transl Res. 2010;155:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Singh AK, Gudehithlu KP, Litbarg NO, Sethupathi P, Arruda JA, Dunea G. Transplanting fragments of diabetic pancreas into activated omentum gives rise to new insulin producing cells. Biochem Biophys Res Commun. 2007;355:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Singh AK, Pancholi N, Patel J, Litbarg NO, Gudehithlu KP, Sethupathi P, Kraus M, Dunea G, Arruda JA. Omentum facilitates liver regeneration. World J Gastroenterol. 2009;15:1057-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Garcia-Gomez I, Pancholi N, Patel J, Gudehithlu KP, Sethupathi P, Hart P, Dunea G, Arruda JA, Singh AK. Activated omentum slows progression of CKD. J Am Soc Nephrol. 2014;25:1270-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Luo X, Liu Y, Zhang Z, Tao R, Liu Y, He A, Yin Z, Li D, Zhang W, Liu W. Long-term functional reconstruction of segmental tracheal defect by pedicled tissue-engineered trachea in rabbits. Biomaterials. 2013;34:3336-3344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Martinez EC, Wang J, Lilyanna S, Ling LH, Gan SU, Singh R, Lee CN, Kofidis T. Post-ischaemic angiogenic therapy using in vivo prevascularized ascorbic acid-enriched myocardial artificial grafts improves heart function in a rat model. J Tissue Eng Regen Med. 2013;7:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Dvir T, Kedem A, Ruvinov E, Levy O, Freeman I, Landa N, Holbova R, Feinberg MS, Dror S, Etzion Y. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc Natl Acad Sci USA. 2009;106:14990-14995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 31. | Sun D, Yang Y, Wei Z, Xu Y, Zhang X, Hong B. Engineering of pre-vascularized urethral patch with muscle flaps and hypoxia-activated hUCMSCs improves its therapeutic outcome. J Cell Mol Med. 2014;18:434-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Muscari C, Giordano E, Bonafè F, Govoni M, Guarnieri C. Strategies affording prevascularized cell-based constructs for myocardial tissue engineering. Stem Cells Int. 2014;2014:434169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |