Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.101898
Revised: December 31, 2024
Accepted: April 15, 2025
Published online: May 26, 2025
Processing time: 238 Days and 0.8 Hours
Skin is the largest organ in the human body and plays crucial roles in human health. Efficient and rapid healing of burn wounds is of great significance. While stem cell therapies have offered potential methods to treat burn wounds, re
To investigate the effect of the combined application of collagen III and adipose-derived stem cells (ASCs) on vascular regeneration in skin wound healing.
Burn wounds were created in 18 adult male Sprague-Dawley rats, and they were randomly divided into two groups. In the treatment group, each rat was injected with 4 × 106 Zs-Green-labeled autologous ASCs suspended in collagen III. In the control group, each rat was injected with collagen III. Each rat received six in
Multiple injections of autologous ASCs improved wound closure rate more efficiently compared to the control group. Moreover, autologous ASCs do not survive long-term during the skin wound healing process.
This study demonstrated that multiple injections of autologous ASCs combined with collagen III accelerated burn wound healing by increasing collagen deposition and improving angiogenesis.
Core Tip: This study demonstrates that multiple injections of autologous adipose-derived mesenchymal stem cells combined with collagen III accelerated burn wound healing by increasing collagen deposition and improving angiogenesis. Moreover, the autologous adipose-derived mesenchymal stem cells had no long-term survival in the skin wound healing process. This study provides insight into novel treatments of skin wound healing. Further detailed investigations are necessary to fully understand the therapeutic mechanism of this innovative strategy.
- Citation: Zhou XL, Wu B, Xie ZJ, Li HD. Collagen III combined with autologous adipose-derived mesenchymal stem cells accelerates burn wound healing in a rat model. World J Stem Cells 2025; 17(5): 101898
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/101898.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.101898
Skin wounds caused by various factors, such as fire, hot water or steam, trauma, noxious agents, ultraviolet radiation, diabetes and pathogens, are often seen in the clinic. There were 9 million burn cases (95% uncertainty interval, 6.8 to 11.2 million) and 111000 deaths from burns (95% uncertainty interval) globally in 2019[1]. Burn patients are highly susceptible to bacterial infections and metabolic imbalance[2], which most often result in amputation or death[3]. Therefore, accelerating the wound healing process remains a great challenge.
The process of skin wound healing consists of several stages: hemostasis, inflammation, remodeling and proliferation[4]. This process is characterized by the extracellular matrix (ECM) deposition, scar tissue development and wound contraction. Wound healing involves the interplay of several cell types, including keratinocytes, endothelial cells, fibroblasts, stem cells, and immune cells. Fibroblasts replace the ECM in the wound by secreting new fibronectin and collagen. Sebaceous glands, sweat glands, and hair follicles can also induce re-epithelialization[5]. However, the healing process is difficult when these dermal appendages are completely damaged. Furthermore, wound healing has high oxygen and nutrient requirements[6,7].
Skin grafting and regular dressing are conventional options for traditional wound management. However, traditional burn wound management is time-consuming, costly and has a high morbidity rate[8]. Infections lead to delayed wound healing and pain, and scarring harms patients[9,10]. Innovations in stem cell therapy bring new hope for the treatment of burn wounds. Stem cells, such as epidermal stem cells, hold great potential in wound healing treatments[11]. However, the source of epidermal stem cells is limited; therefore, other sources of stem cells are necessary for clinical use. Adipose-derived stem cells (ASCs) have gained increasing attention from scientists, in part because they are easy to isolate from subcutaneous adipose tissue. ASCs are a promising type of mesenchymal stem cell in regenerative medicine. Moreover, they can be used for autologous transplantation to minimize immunological rejection. Numerous studies have de
ASCs promote the wound healing process via paracrine effects, such as immune modulation, angiogenesis[13], cell proliferation, ECM remodeling, autophagy, and oxidative stress. ASCs also secrete many growth factors and cytokines[14]. MicroRNA expression in ASCs has been reported to regulate vascular endothelial growth factor via HOXA11-AS to facilitate proliferation[15]. Fibroblasts play a central role in the formation of granulation tissue, as they synthesize and deposit ECM proteins that provide structural support for its development[16]. Mesenchymal stem cell-derived extracellular vesicles also play important roles in skin wound healing[17,18].
In this study, we explored the efficacy of ASCs and collagen III in the skin wound healing process. Collagen III is an ECM that reportedly accelerates wound closure by enhancing angiogenesis or collagen deposition, indicating its significant application value in wound dressings for chronic wound repair[19].
To study skin wound healing and related mechanisms, eighteen male Sprague-Dawley rats were acquired (SLAC, Changsha). All rats were maintained at Changsha Medical University under standardized laboratory conditions (24 ± 1°C, 50%-60% relative humidity, 12-hour light/dark cycle) with free access to food and water. After a 12-month growth period, anesthesia was induced via intraperitoneal injection of pentobarbital sodium. The dosage and concentration were 50 mg/kg and 2% (w/v), respectively. A 4 cm² full thickness burn wound was created using a temperature-controlled instrument (Yiyan Technology Co., Ltd., China). The iron head (1000 g) was maintained on the skin at 100°C for 10 seconds. The animal experiment protocol was approved by the Institutional Animal Care and Use Committee of Changsha Medical University.
Thirty days prior to the induction of the burn, the inguinal fat pad (about 1.5 cm3) was excised from each rat, and autologous ASCs were isolated as described previously[20]. In a biosafety cabinet, the fat pad was minced into small pieces using sterile scissors. Then, the minced fat tissue was mixed with type 1 collagenase (1‰). This mixed solution was placed in a 37°C water bath and intermittently agitated the solution. When all adipose tissue was digested, the mixed liquid was subjected to centrifugation at 300 g for 3 minutes. The supernatant was removed, PBS was added, and the mixture was passed through a 70 μm filter. The solution was then centrifuged, and the cell pellet was resuspended in complete medium. The cells were cultured under normal conditions (5% CO2, 37°C).
When ASCs (passage 1) reached 50% confluence, they were transduced with lentiviral vectors (Zs-Green labeled). Two days after transduction, 4 μg/mL puromycin was added to the culture medium, and a fluorescence microscope was used to assess transduction efficacy. After the burn wound was created, 4 × 106 Zs-Green-labeled autologous ASCs (suspended in collagen III) or collagen III (1 mg/mL; pH = 7.0) were subcutaneously injected into the wound bed of each rat. The diameter of the injection area was 0.4 cm larger than that of the wound. The rats were treated every 3-4 days, and each rat received a total of 6 injections. Images of the wounds were acquired every 3 days.
All rats were euthanized on day 55 by intraperitoneal administration of pentobarbital sodium. The repaired skin from each rat was excised using microdissection scissors and divided into two parts. One part was fixed in 4% paraformaldehyde and 0.1 M phosphate buffer for 24 hours. Fixed tissues were processed through a graded ethanol-xylene series (70%-100% ethanol, xylene substitute), and the fixed tissues were embedded in paraffin with optimal cutting temperature and cut into 5 μm sections. Masson staining was performed according to the manufacturer’s protocol (Biosciences Inc.). Microvascular density within the wounded dermal tissue was assessed by immunohistochemical staining using an antibody against CD31 (1:2000 dilution, Ab182981; Abcam, United Kingdom).
The other portion of each specimen was cryo-embedded in OCT compound (Sakura Finetek, Japan) followed by rapid freezing in dry ice to minimize ice crystal formation. Samples were cryo-sectioned into 10 μm sections and incubated with 4,6-diamidino-2-phenylindole. Finally, green and blue fluorescence was detected via fluorescence microscopy.
Statistical analysis was performed via t-test (α = 0.05) using GraphPad Prism. The data are presented as the mean ± SD, and differences were considered significant when P < 0.05. Data that were not statistically significant are not shown.
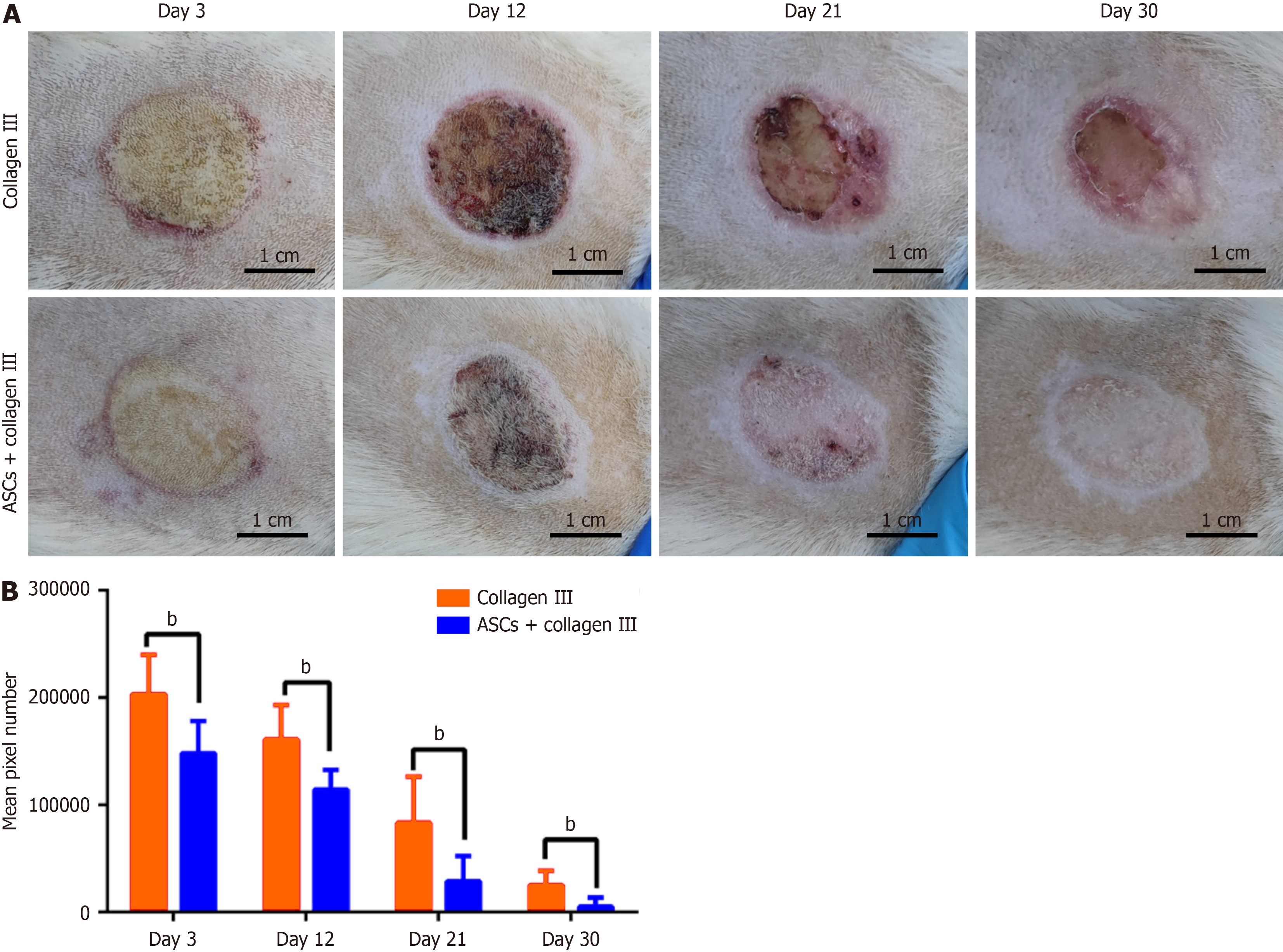
We measured the wound size over time and found that multiple injections of 4 × 106 autologous ASCs combined with collagen III (treatment group, n = 9) accelerated the healing process. As shown in Figure 1A, the mean wound size was significantly smaller in the treatment group compared to the control group (Figure 1B). Moreover, we assessed healing quality by evaluating softness, scar formation, and other relevant parameters. There was no hair in the healing area. The repaired skin was harder than normal skin, and no obvious scar formed.
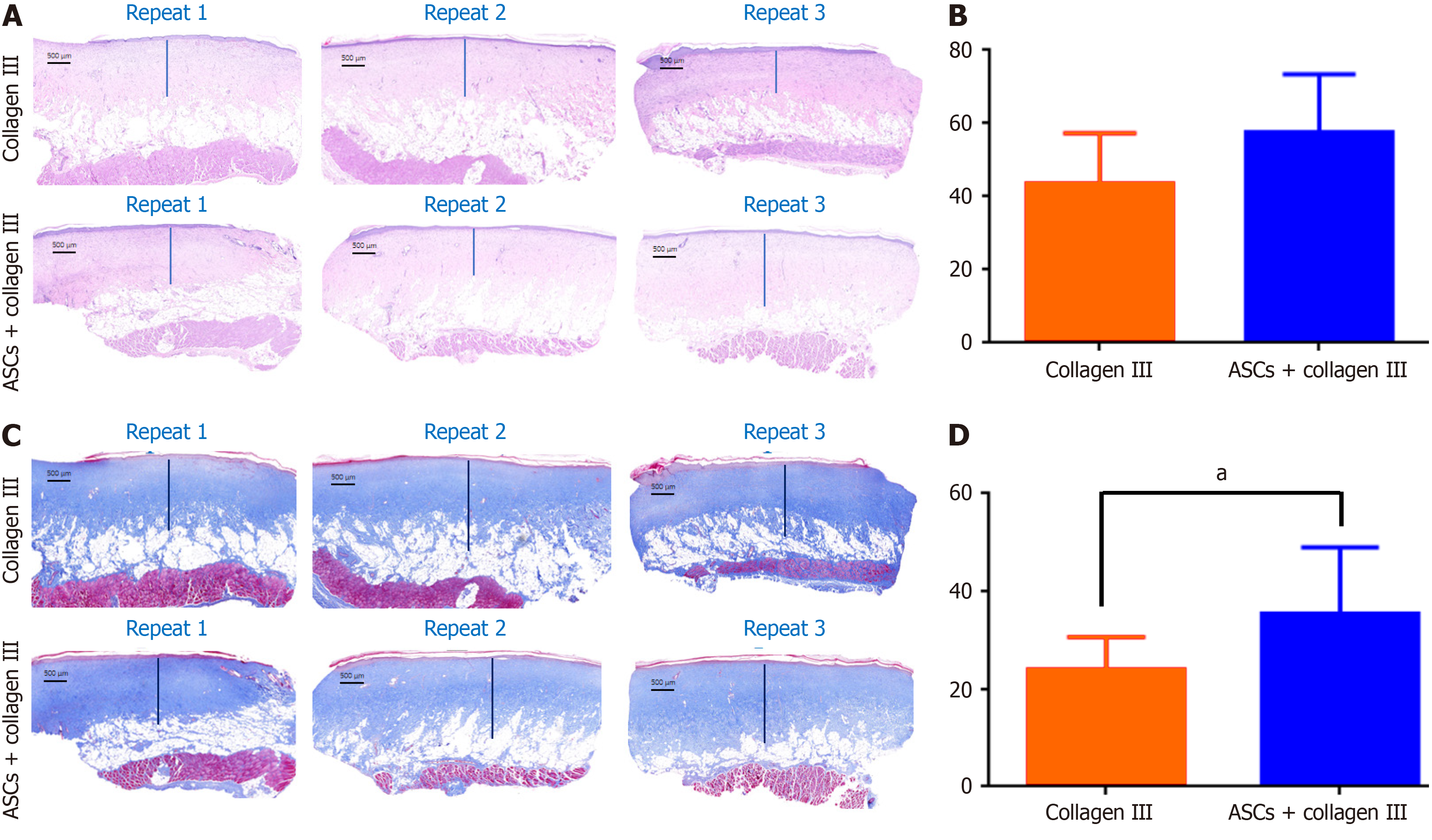
We used hematoxylin and eosin staining to detect dermal layer thickness of the recovered skin. The average thickness of the dermal layer was thinner in the control group than in the treated group and there was more loose subcutaneous tissue below the dermal layer (Figure 2A). Average dermal layer thickness was significantly different between the collagen III group (control) and the ASC combined with collagen III group (Figure 2B). More complex structures were found in the dermal layer of the treatment group, and Masson staining revealed increased collagen deposition in the treatment group (Figure 2C). The average collagen deposition thickness was significantly different between the control collagen III group and the treatment group (Figure 2D). These findings suggest that autologous ASCs increase the skin wound healing process by accelerating ECM deposition. Furthermore, some rats had no sebaceous gland-like or hair follicle-like structures in the healed wound area 55 days after transplantation.
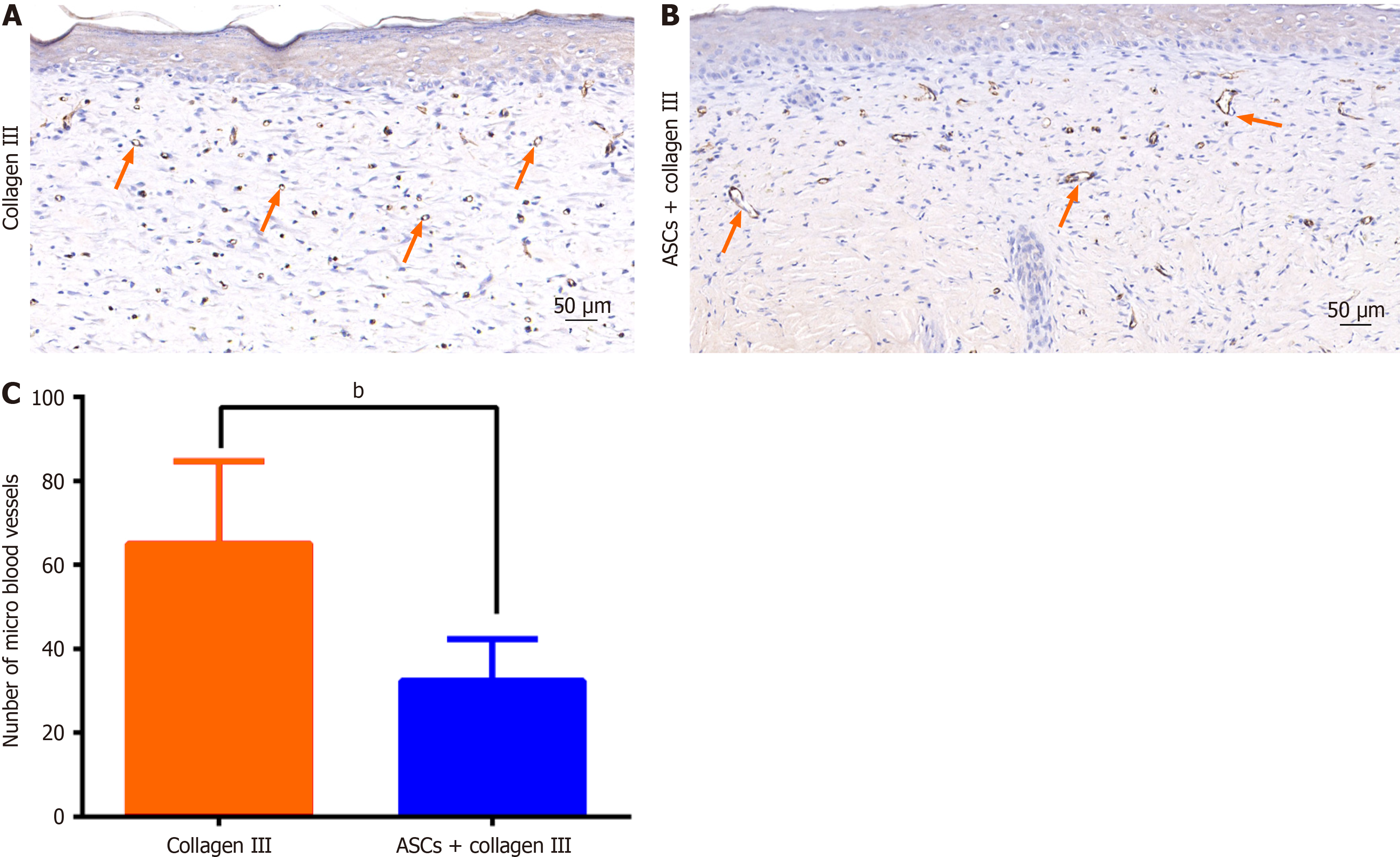
Blood vessel regeneration is crucial for wound healing. To evaluate the vascularity of the wound tissue, we performed immunohistochemical staining for CD31. The control group exhibited more microvessels (Figure 3A), whereas the treatment group showed larger blood vessels (Figure 3B). The average number of microvessels in the dermal layer was significantly different between the collagen III-only group and the ASC plus collagen III group (Figure 3C). These findings indicate that ASCs can improve blood vessel development in wounds.
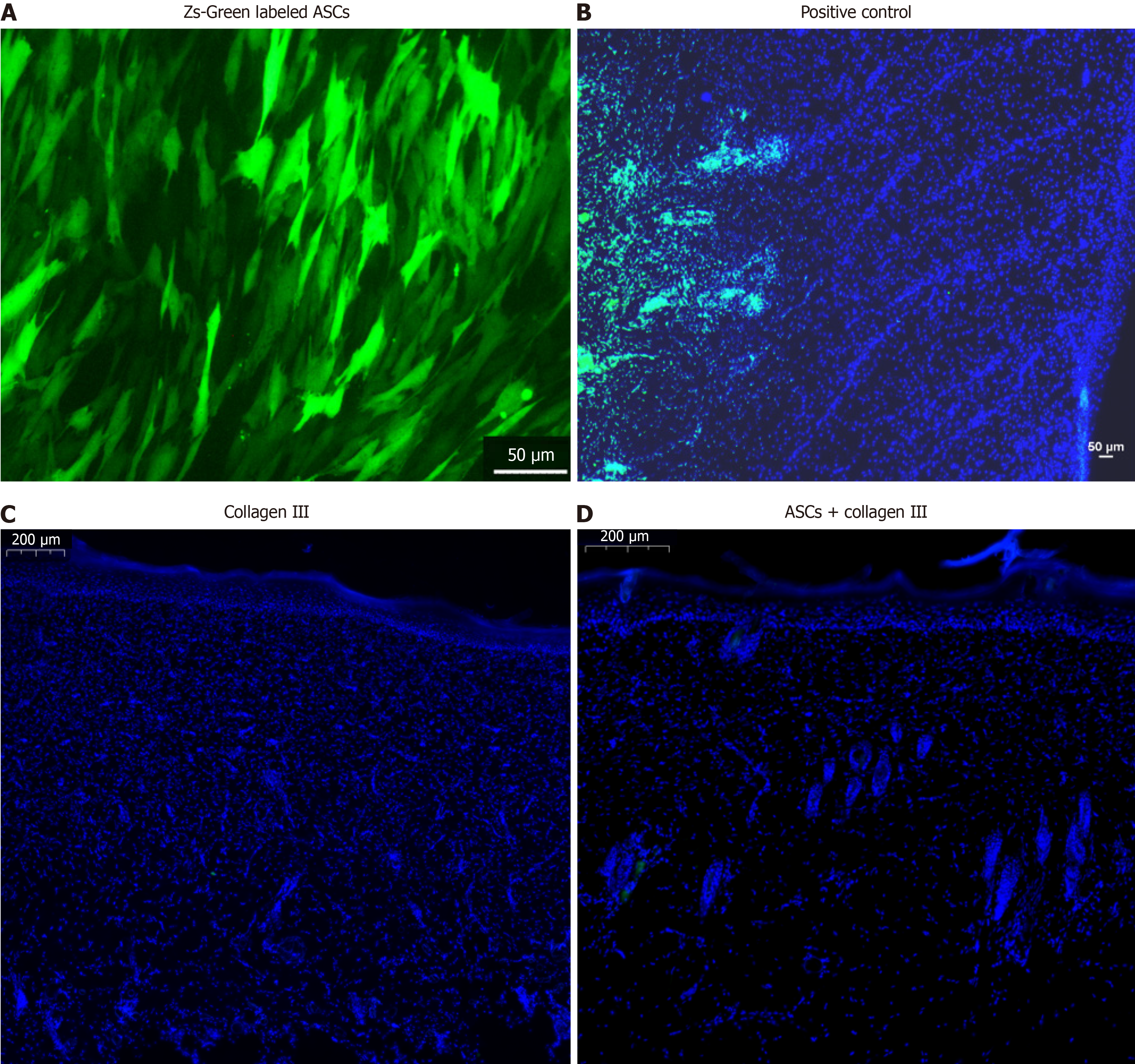
On day 55, we carefully excised the skin and used the Zs-Green signal to detect ASCs (Figure 4A). Autologous ASCs were present in the wound area for a few days (Figure 4B), but did not survive long-term in the dermal tissue (Figure 4D). These findings indicate that autologous ASCs may accelerate skin wound healing through a non-cell replacement mechanism.
In our previous study, we injected ASCs three times to accelerate the burn wound healing process. In this study, we selected a higher frequency of autologous ASC administration (six injections) for skin wound healing treatment. Although the wound closure rate was higher than baseline, it remained slower than anticipated. We propose three potential explanations for this phenomenon. First, the wounded skin may have reached its maximum intrinsic healing capacity. Second, injected ASCs may undergo apoptosis or migrate away from the wound site[21]. Third, other factors, such as bacterial infection, may be involved.
We tested collagen III in facilitating the migration of fibroblasts and ASCs to ultimately promote wound healing. Collagen III reportedly accelerates wound closure by reducing the inflammatory response and enhancing angiogenesis and collagen deposition[19]. Our results show that collagen III significantly increased the number of blood vessels. However, we observed fewer but larger blood vessels and more collagen deposition in the collagen III combined with ASC-treated group. The underlying mechanism is unknown. In our previous study, both ASC- and PBS-treated rats had small vessels and microvessels. We hypothesize that collagen III may suppress vessel formation, while ASCs can counteract this effect by degrading collagen III.
Finally, we did not find Zs-Green-labeled ASCs in the skin wound. We hypothesize that the injected ASCs underwent apoptosis within a few days after transplantation. This observation aligns with findings by Terrovitis et al[22], who reported that no human cells survived in the rat myocardium at 7 and 21 days after injection in histological sections despite the use of a robust genetic labeling technique and two independent methods for detecting transgene expression. Although we used autologous ASCs in this study, the cell culture medium contained xenogeneic animal proteins. These ASCs might have triggered immune-mediated clearance.
In conclusion, this study demonstrated that repeated administration of autologous ASCs combined with collagen III enhanced burn wound healing rates through accelerated collagen deposition and improved angiogenesis. Moreover, autologous ASCs did not promote hair follicle or sebaceous gland regeneration. There is no long-term survival of autologous ASCs during the skin wound healing process.
| 1. | Gerstl JVE, Ehsan AN, Lassarén P, Yearley A, Raykar NP, Anderson GA, Smith TR, Sabapathy SR, Ranganathan K. The Global Macroeconomic Burden of Burn Injuries. Plast Reconstr Surg. 2024;153:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Loder S, Peterson JR, Agarwal S, Eboda O, Brownley C, DeLaRosa S, Ranganathan K, Cederna P, Wang SC, Levi B. Wound healing after thermal injury is improved by fat and adipose-derived stem cell isografts. J Burn Care Res. 2015;36:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Li Q, Wang LF, Chen Q, Wang SJ, Li F, Ba T. Amputations in the burn unit: A retrospective analysis of 82 patients across 12 years. Burns. 2017;43:1449-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Janis JE, Harrison B. Wound Healing: Part I. Basic Science. Plast Reconstr Surg. 2016;138:9S-17S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020;21:1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 6. | Seth I, Lim B, Cevik J, Gracias D, Chua M, Kenney PS, Rozen WM, Cuomo R. Impact of nutrition on skin wound healing and aesthetic outcomes: A comprehensive narrative review. JPRAS Open. 2024;39:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 7. | Castilla DM, Liu ZJ, Velazquez OC. Oxygen: Implications for Wound Healing. Adv Wound Care (New Rochelle). 2012;1:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Abouzaid AM, El Mokadem ME, Aboubakr AK, Kassem MA, Al Shora AK, Solaiman A. Effect of autologous fat transfer in acute burn wound management: A randomized controlled study. Burns. 2022;48:1368-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, Lajevardi SS, Li Z, Maitz PKM. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 390] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 10. | Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 511] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 11. | Yang R, Liu F, Wang J, Chen X, Xie J, Xiong K. Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res Ther. 2019;10:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Kohlhauser M, Tuca A, Kamolz LP. The efficacy of adipose-derived stem cells in burn injuries: a systematic review. Cell Mol Biol Lett. 2024;29:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Zhou X, Ning K, Ling B, Chen X, Cheng H, Lu B, Gao Z, Xu J. Multiple Injections of Autologous Adipose-Derived Stem Cells Accelerate the Burn Wound Healing Process and Promote Blood Vessel Regeneration in a Rat Model. Stem Cells Dev. 2019;28:1463-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Klar AS, Zimoch J, Biedermann T. Skin Tissue Engineering: Application of Adipose-Derived Stem Cells. Biomed Res Int. 2017;2017:9747010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Zhou X, Lu J, Wu B, Guo Z. HOXA11-AS facilitates the proliferation, cell cycle process and migration of keloid fibroblasts through sponging miR-188-5p to regulate VEGFA. J Dermatol Sci. 2022;106:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 16. | Spielman AF, Griffin MF, Parker J, Cotterell AC, Wan DC, Longaker MT. Beyond the Scar: A Basic Science Review of Wound Remodeling. Adv Wound Care (New Rochelle). 2023;12:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Cheng L, Zhao H, Li Z, Chen J, Cen Y, Zhang Z. The Therapeutic Role of ADSC-EVs in Skin Regeneration. Front Med (Lausanne). 2022;9:858824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Saadh MJ, Ramírez-Coronel AA, Saini RS, Arias-Gonzáles JL, Amin AH, Gavilán JCO, Sârbu I. Advances in mesenchymal stem/stromal cell-based therapy and their extracellular vesicles for skin wound healing. Hum Cell. 2023;36:1253-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Long LY, Liu W, Li L, Hu C, He S, Lu L, Wang J, Yang L, Wang YB. Dissolving microneedle-encapsulated drug-loaded nanoparticles and recombinant humanized collagen type III for the treatment of chronic wound via anti-inflammation and enhanced cell proliferation and angiogenesis. Nanoscale. 2022;14:1285-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Zografou A, Papadopoulos O, Tsigris C, Kavantzas N, Michalopoulos E, Chatzistamatiou T, Papassavas A, Stavropoulou-Gioka C, Dontas I, Perrea D. Autologous transplantation of adipose-derived stem cells enhances skin graft survival and wound healing in diabetic rats. Ann Plast Surg. 2013;71:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Kim TW, Koo SY, Riessland M, Chaudhry F, Kolisnyk B, Cho HS, Russo MV, Saurat N, Mehta S, Garippa R, Betel D, Studer L. TNF-NF-κB-p53 axis restricts in vivo survival of hPSC-derived dopamine neurons. Cell. 2024;187:3671-3689.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, Schär M, Gerstenblith G, Weiss RG, Marbán E, Abraham MR. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |