Published online Jun 26, 2021. doi: 10.4252/wjsc.v13.i6.645
Peer-review started: February 5, 2021
First decision: March 17, 2021
Revised: March 29, 2021
Accepted: June 4, 2021
Article in press: June 4, 2021
Published online: June 26, 2021
Processing time: 140 Days and 15.2 Hours
Mesenchymal stem cells (MSCs) represent a promising therapy for the treatment of equine joint diseases, studied due to their possible immunomodulatory characteristics and regenerative capacity. However, the source of most suitable MSCs for producing cartilage for regenerative processes in conjunction with biomaterials for an enhanced function is yet to be established.
To compare the chondrogenicity of MSCs derived from synovial fluid, bone marrow, and adipose tissue of horses, using the aggrecan synthesis.
MSCs from ten horses were cultured, phenotypic characterization was done with antibodies CD90, CD44 and CD34 and were differentiated into chondrocytes. The 3D cell culture system in which biocompatible nanoparticles consisting of gold, iron oxide, and poly-L-lysine were added to the cells, and they were forced by magnets to form one microspheroid. The microspheroids were exposed to a commercial culture medium for 4 d, 7 d, 14 d, and 21 d. Proteoglycan extraction was performed, and aggrecan was quantified by enzyme-linked immunosorbent assay. Keratan sulfate and aggrecan in the microspheroids were identified and localized by immunofluorescence.
All cultured cells showed fibroblast-like appearance, the ability to adhere to the plastic surface, and were positive for CD44 and CD90, thus confirming the characteristics and morphology of MSCs. The soluble protein concentrations were higher in the microspheroids derived from adipose tissue. The aggrecan concentration and the ratio of aggrecan to soluble proteins were higher in microspheroids derived from synovial fluid than in those derived from bone marrow, thereby showing chondrogenic superiority. Microspheroids from all sources expressed aggrecan and keratan sulfate when observed using confocal immunofluorescence microscopy. All sources of MSCs can synthesize aggrecan, however, MSCs from synovial fluid and adipose tissue have demonstrated better biocompatibility in a 3D environment, thus suggesting chondrogenic superiority.
All sources of MSCs produce hyaline cartilage; however, the use of synovial liquid or adipose tissue should be recommended when it is intended for use with biomaterials or scaffolds.
Core Tip: As a method no yet studied in equine chondrocytes, chondrogenic differentiation was performed in three-dimensional plate culture, using technology with biocompatible nanoparticles consisting of gold, iron oxide and poly-L-lysine, forming microspheroids. It has been shown that this technique is advantageous, as it allows for aggregation and a lower number of cells, ensuring high cell density, providing an adequate microenvironment for the differentiation and phenotypic expression of chondrocytes.
- Citation: Fülber J, Agreste FR, Seidel SRT, Sotelo EDP, Barbosa ÂP, Michelacci YM, Baccarin RYA. Chondrogenic potential of mesenchymal stem cells from horses using a magnetic 3D cell culture system. World J Stem Cells 2021; 13(6): 645-658
- URL: https://www.wjgnet.com/1948-0210/full/v13/i6/645.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i6.645
Equines have excellent athletic abilities, and therefore often suffer injuries related to the musculoskeletal system, which can occur because of several factors, such as physical condition, preexisting individual vulnerability, poor conformation, genetics, inadequate shoeing, nutritional factors, and errors in training programs[1].
Among all injuries that comprise the musculoskeletal system, those related to the joints are frequent causes of performance loss. This performance loss is due to the mechanical stress caused by excessive loads that exceed physiological joint limits[2] and most often progress quickly to the destruction of the articular cartilage[3].
Thus, an increasing number of studies focus on therapies that aim the recover of hyaline cartilage integrity. Regenerative medicine can overcome many difficulties or partial failure of the current therapeutic approaches in promoting hyaline cartilage regeneration. The use of cell therapy and mesenchymal stem cells (MSCs) have promising applications[4].
MSCs offer a strategy for the repair of damaged cartilage because they can differentiate into chondrocytes. These cells emphasize the possibility of cartilage regene
Since there are some niches to obtain MSCs, several factors must be considered, such as ease of collection procedure, the possibility of related morbidity at the time of collection, quantity of cells obtained from different sources, and proliferative capacity and cell differentiation[6,7]. All these factors must be studied and understood to establish a beneficial MSC source, especially when the focus is on the treatment of specific tissues, such as articular cartilage.
The differentiation potential is one of the most discussed features of MSCs, as different sources can directly or indirectly influence the ability to differentiate[8].
Bone marrow, adipose tissue, and synovial tissue are some of the primary sources of MSCs in regenerative therapy for the treatment of joint diseases; however, there are differences in chondrogenic differentiation and cell proliferation results. Bone marrow has chondrogenic capacity in vivo, and in vitro[9-11]. However, these cells are capable of producing more significant amounts of collagen I, X, and alkaline phosphatase, thus indicating that they may evolve into a hypertrophic cartilage phenotype[12].
Adipose tissue-derived MSCs in co-culture with human chondrocytes inhibited chondrocyte apoptosis as well as aggrecan and SOX9 expression, with a decrease in hypertrophic chondrocyte markers such as metalloproteinase 13, alkaline phosphatase, and collagen I, III, IV, and X. However, the expression of collagen X decreased, suggesting chondrocyte protection and loss of the hypertrophic phenotype[13]. Moreover, synovial tissue-derived MSCs have better differentiation potential as they form less hypertrophic cartilage compared to that of bone marrow- and adipose tissue-derived MSCs[14].
This study investigated MSCs isolated from synovial fluid, adipose tissue, and bone marrow for their specific biological activities related to extracellular matrix synthesis after chondrogenic differentiation. Besides, we sought to establish whether such differences could justify preferential use in scaffold applications specific to joint disease treatment.
Ten clinically healthy horses aged three to five years were included in the experiment after clinical evaluation, as donors of synovial fluid, bone marrow, and adipose tissue.
All collections were performed after sedation of the animals, trichotomy of the anatomical regions, and antisepsis with 2% chlorhexidine digluconate and alcoholic chlorhexidine solution. An anesthetic block was performed with lidocaine hydrochloride without a vasoconstrictor for bone marrow and adipose tissue collections.
Synovial fluid was obtained by arthrocentesis of the tibiotarsal joint, from medial access to the saphenous vein below the tibial malleolus on the dorsomedial face of the tarsal joint, as described previously[15].
The bone marrow was obtained from the fifth sternum, and the puncture was performed using a Jamshidi 8G needle, 10 cm in the ventrodorsal direction, perpendicular to the skin. The medullary content was obtained using a 20 mL syringe containing 1 mL of heparin[16,17].
Finally, the adipose tissue was obtained from the lateral region at the base of the tail above the dorsal gluteal muscle. The adipose tissue was removed with scissors and surgical forceps after skin incision[18,19]. The sample was stored in 50 mL conical tubes containing 30 mL of DMEM/F12 culture medium supplemented with 1% penicillin 10000 U/mL, 10 mg/mL streptomycin, and 25 µg/mL amphotericin B (LGC, Biotechnology, Brazil).
The synovial fluid was transferred to 25 cm3 culture flasks and cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum, 1% penicillin 10000 U/mL, streptomycin 10 mg/mL, amphotericin B 25 µg/mL, and glutamine 200 mmol/L. The flasks were incubated at 37 °C (98.6 ºF) with relative humidity close to 100% and a gaseous atmosphere of 5% CO2. After cell adhesion, the culture medium was changed every 48 h, until 70%–80% cellular confluence[20].
The medullary fraction was diluted 1:1 in phosphate-buffered saline (PBS) and gently layered onto Ficoll Histopaque® solution 1077 g/mL (Sigma, St. Louis, MO, United States, ref 10771). Then, the sample was centrifuged at 400 r/min for 30 min at 24 °C (75.2 ºF). After centrifugation, the layer containing the mononuclear cell fraction was resuspended in PBS and centrifuged at 720 r/min for 10 min, followed by another washing step under the same conditions. The pellet was resuspended in 25 cm3 culture flasks filled with supplemented DMEM/F12 culture medium and incubated at 37 °C (98.6 ºF) with a relative humidity close to 100% and a gaseous atmosphere of 5% CO2[21].
The adipose tissue was washed with PBS, fragmented, and incubated overnight in collagenase type I enzyme solution (GIBCO Thermo Fisher ScientificTM, Carlsbad, CA, United States, ref 17100017) at a concentration of 2 mg/mL, diluted in DMEM/F12 culture medium supplemented with 1% penicillin 10000 U/mL, streptomycin 10 mg/mL, and amphotericin B 25 µg/mL, and incubated at 37 °C (98.6 ºF) with a relative humidity close to 100% and a gaseous atmosphere of 5% CO2. After incubation, the sample was transferred to conical tubes, and the supplemented DMEM/F12 culture medium was added, and the mixture centrifuged for 10 min at 260 r/min. The supernatant containing the fat fraction was removed, and the pellet was resuspended in culture medium and centrifuged twice. After washing and centrifugation, the pellet was resuspended in culture medium and transferred to 25 cm3 culture flasks, incubated at 37 °C (98.6 ºF) with a relative humidity close to 100% and a gaseous atmosphere of 5% CO2. The culture medium was changed every 48 h until 70%-80% confluency[22].
The cell samples between the third (P3) and the fourth passage (P4) were washed with a PBS and distributed at 2 × 105 cells by tube. The cells were centrifuged (300 r/min, 5 min) and the pellets were resuspended in PBS containing of the antibodies [mouse anti-rat CD90-phycoerythrin - PE (clone OX-7; BD, San Jose, CA, United States)/mouse anti-horse CD44-fluorescein isothiocyanate - FITC (clone CVS18; AbD Serotec, Oxford, United Kingdom)] and mouse anti-human CD34-FITC (clone 581; BD)]. Anti- IgG1-PE and anti-IgG1-FITC were used as control isotypes to calibrate the cytometer. After 45 min incubation (4 °C, in the dark), the cells were washed with PBS and centrifugated (300 r/min, 5 min). Pellets were resuspended in 300 μL of PBS and tubes analyzed to flow cytometer (FACSCalibur® - Becton Dickinson, San Jose, CA, United States) and Cell-Quest software (Becton Dickin- son, San Jose, CA, United States). The protocols were performed according to the manufacturer’s instructions.
Viable cells in third passage (P3) (1 × 104 cells/well) were plated in microplates (Greiner Bio-One), and 100 µL of supplemented DMEM/F12 was added to 0.3 µL of nanoparticles (NanoshuttleTM Greiner Bio-One). The experiment was performed in triplicate, and the plates were incubated at 37 °C (98.6 ºF) with a relative humidity close to 100% and a gaseous atmosphere of 5% CO2. After 24 h, the nanoparticles were incorporated into the cell membranes, and the plate was then incubated under a magnet, subjecting the cells to three-dimensional culture. The chondrogenic differentiation process was initiated using a commercial inducing medium (StemPro chondrogenesis kit; GIBCO ThermoFisher CientificTM, Carlsbad, California, United States, ref A1007101), which was changed every 48 h. The formed microspheroids were removed from the culture on days 4, 7, 14, and 21 after the onset of chondrogenic differentiation.
The microspheroids were digested with guanidine hydrochloride (GuHCl) using the methodology as previously described[23], followed by the extraction of proteoglycans and subsequent evaluation of the extracellular matrix synthesized by chondrocytes.
After chondrogenic differentiation, the microspheroids were incubated with 500 µL of 4 mol/L GuHCl solution in 0.05 mol/L sodium acetate buffer (pH 4.5) and 5 µL protease inhibitor under agitation at 4 °C (39.2 ºF), overnight. Then, the samples were centrifuged for 5 min at 260 r/min, and the supernatant was stored in 15 mL conical tubes and frozen at -20 ºC (-4 ºF). Then, 500 µL of 4 mol/L GuHCl solution in 0.05 mol/L sodium acetate buffer (pH 4.5) and 5 µL of protease inhibitor was added to the pellet and incubated under the same conditions described above. After incubation, the samples were centrifuged for 5 min at 260 r/min, the supernatant was preserved in conical tubes, and the pellet was discarded. The macromolecules present in the supernatant were precipitated by the slow addition of 3 volumes (3 mL) of methanol and incubated at -20 °C (-4°F) overnight. Following incubation, the samples were centrifuged for 15 min at 1500 r/min, the supernatant was discarded, and the precipitate was collected and dried under a vacuum. The samples were resuspended in 50 µL of distilled water to quantify the total soluble proteins and aggrecan. The aggrecan standard was extracted from equine cartilage to measure the concentration of aggrecan at samples.
The ratio of aggrecan to soluble proteins was established as a correction factor to compensate for possible differences in the weight or size of microspheroids.
The soluble protein concentration was measured using a modified biuret test (Pierce BCA Protein Assay Thermo CientificTM Kit, ref 23225). The reagents were diluted according to the manufacturer’s instructions, and the absorbance was measured at 560 nm on a microplate reader (Versa max microplate reader) using the SoftMax Pro program.
The aggrecan concentration was measured using an enzyme-linked immunosorbent assay as described previously[24-26]. The standard curve was produced from horse aggrecan extracted from cartilage.
The addition of the primary monoclonal antibody, horse anti-aggrecan (mouse – Immuny, lote14070), diluted 1:1000 in PBS containing 1% BSA, was followed by secondary anti-peroxidase-conjugated mouse IgG (HRP) antibody diluted 1:50000 in PBS containing 1% BSA. The absorbance was measured at 492 nm on a Vermax microplate reader using the SoftMax Pro program.
The microspheroids were incubated with 0.1 mol/L glycine solution in PBS for 10 min and incubated for 60 min at 24 °C (75.2 ºF) in a blocking solution composed of 5% fetal bovine serum and 0.1% saponin diluted in PBS. The blocking solution was then removed, and the microspheroids were washed thrice with PBS. Next, the primary anti-aggrecan antibody (mouse - Immuny) diluted 1:200 with 0.1% saponin solution in PBS containing 1% BSA was added and incubated for 60 min at 24 °C (75.2 ºF). The microspheroids were washed thrice with PBS and incubated for 60 min with goat anti-mouse IgG secondary antibody (2 mg/mL) (Alexa Fluor™ 633, Invitrogen) diluted 1:200 with 0.1% saponin solution in PBS containing 1% BSA. The microspheroids were washed with PBS and incubated with 4',6-diamidino-2-phenylindole (DAPI) (diluted 1:10000) in the same dilution solution as the antibodies for 40 min. The microspheroids were washed thrice with PBS, the slides were prepared, and the images were captured using a Leica TCS SP8 confocal microscope. A sample prepared without the primary antibody was used as the control for laser configuration and excitation range of the primary antibody.
The cells from all three sources showed fibroblastoid morphology and adhesion to plastic, thus confirming the characteristics and morphology of MSCs.
The MSCs from the synovial fluid began adhering to the plastic within approximately four days of cultivation and reached about 70% confluency within 25.5 ± 3.8 d. The bone marrow cells started adhering to plastic after approximately five days of cultivation and reached approximately 70% confluency within 18.5 ± 1.89 d.
On the other hand, MSCs obtained from adipose tissue showed adherence to plastic one day after culturing and reached 70% confluency within 3.8 ± 0.89 d.
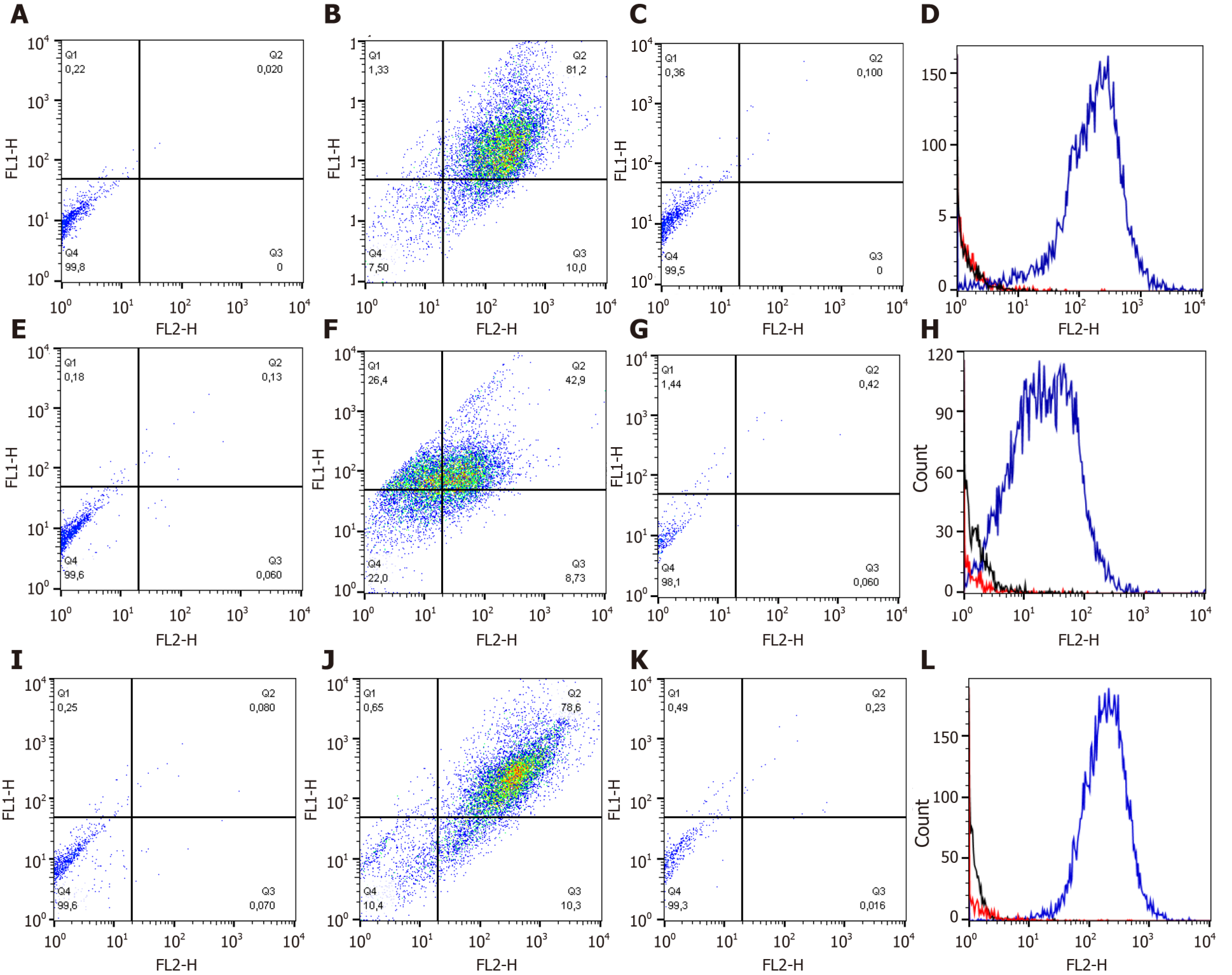
Synovial fluid (SF)-MSCs, bone marrow (BM)-MSCs and adipose tissue (AT)-MSCs were positive for CD44 and CD90, besides, the cells were negative for hematopoietic marker like CD34 (Figure 1 and Table 1).
| Source | Expression (%) | ||||||
| CD44-CD90+ | CD44+CD90- | CD44+CD90+ | CD44-CD90- | CD34+ | CD90+ | CD44+ | |
| SF | 21.33 ± 12.89 | 5.12 ± 8.19 | 57.92 ± 11.71 | 15.62 ± 7.10 | 1.49 ± 2.02 | 79.26 ± 9.69 | 63.04 ± 15.16 |
| BM | 19.09 ± 16.38 | 15.80 ± 20.95 | 45.55 ± 26.65 | 23.37 ± 12.92 | 1.98 ± 2.83 | 64.64 ± 27.99 | 61.34 ± 22.13 |
| AT | 19.21 ± 19.50 | 5.32 ± 8.31 | 54.53 ± 21.83 | 20.43 ± 16.26 | 1.90 ± 3.04 | 73.74 ± 21.23 | 59.85 ± 20.26 |
MSCs from all sources were able to form microspheroids, which showed a three-dimensional shape after two days of chondrogenic induction. After 4 d, 7 d, 14 d, and 21 d, the microspheroids were removed and stored in PBS for analysis.
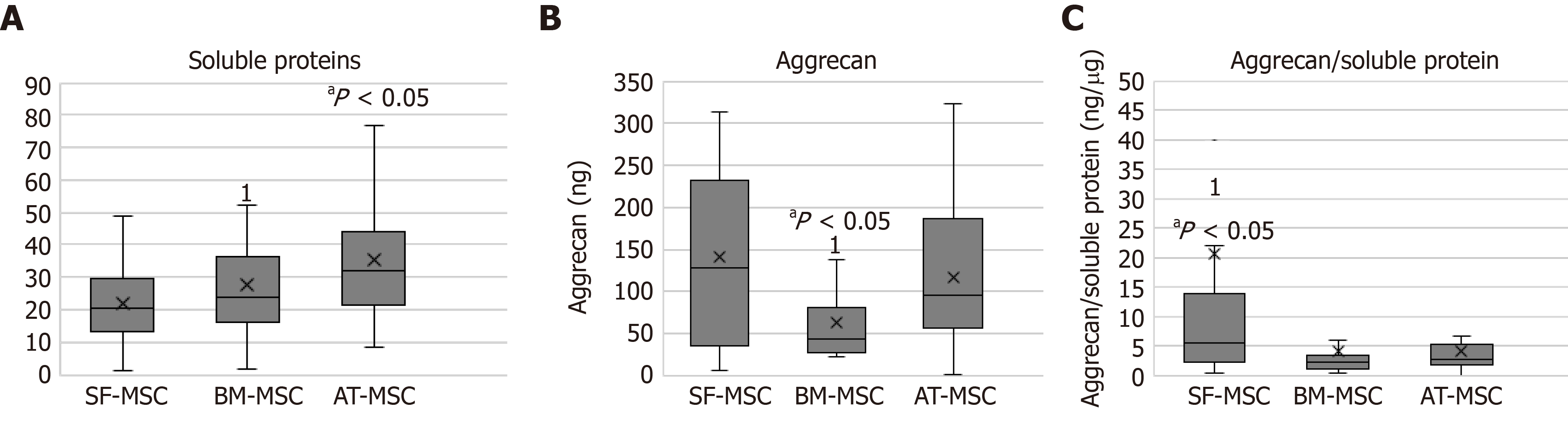
Soluble proteins were obtained from the microspheroids formed from all three sources. The concentrations of soluble proteins in the microspheroids from adipose tissues were higher than those from bone marrow and synovial fluid (P > 0.05) (Figure 2A).
The microspheroids from synovial fluid and adipose tissue showed a higher concentration of aggrecan when compared to those from bone marrow (Figure 2B).
The ratio of aggrecan to soluble proteins was higher in the microspheroids derived from synovial fluid than in those derived from bone marrow (Figure 2C).
The time of differentiation into MSCs from different sources did not interfere with the results of soluble protein, aggrecan concentration, or aggrecan to soluble protein ratio.
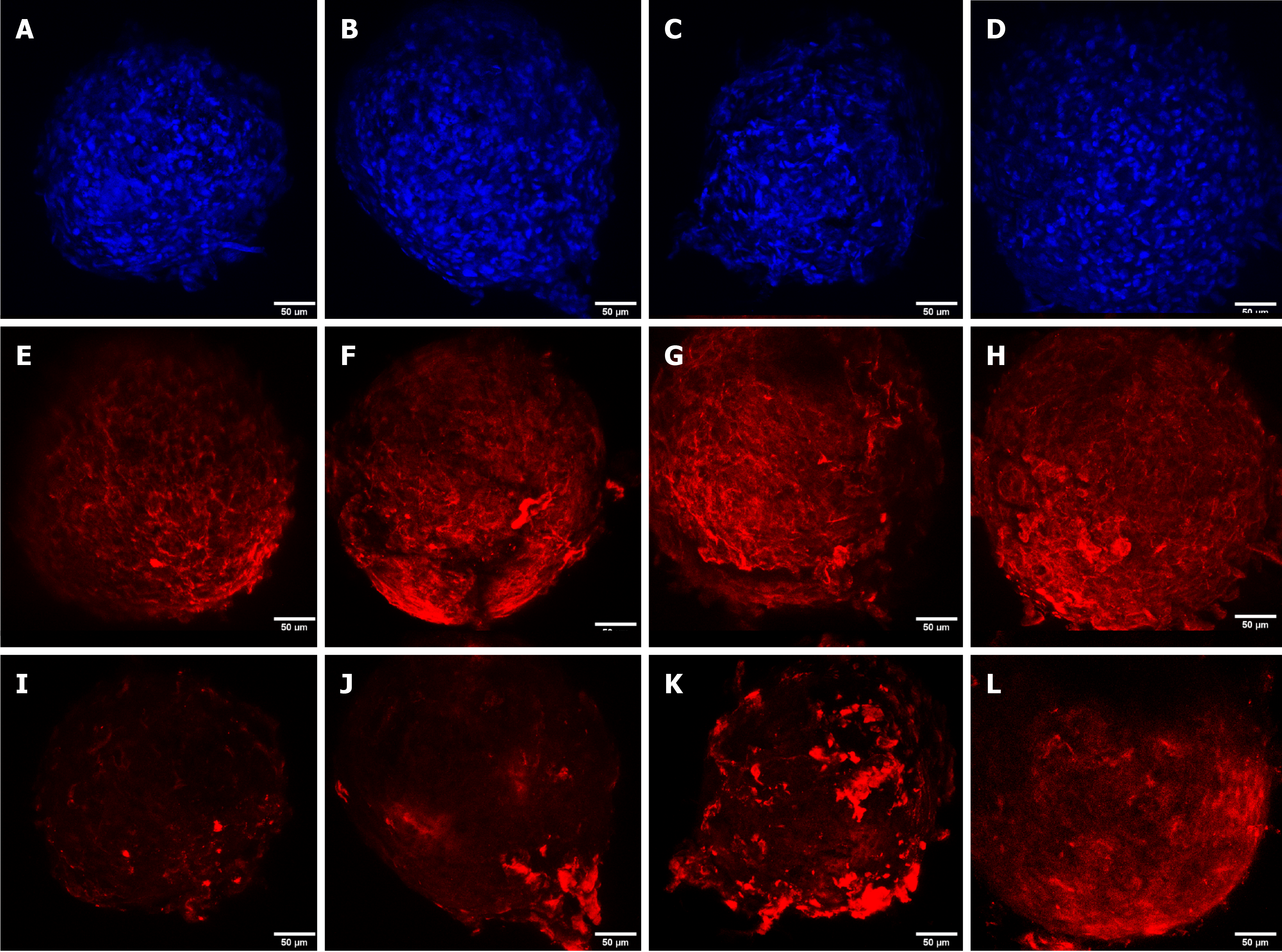
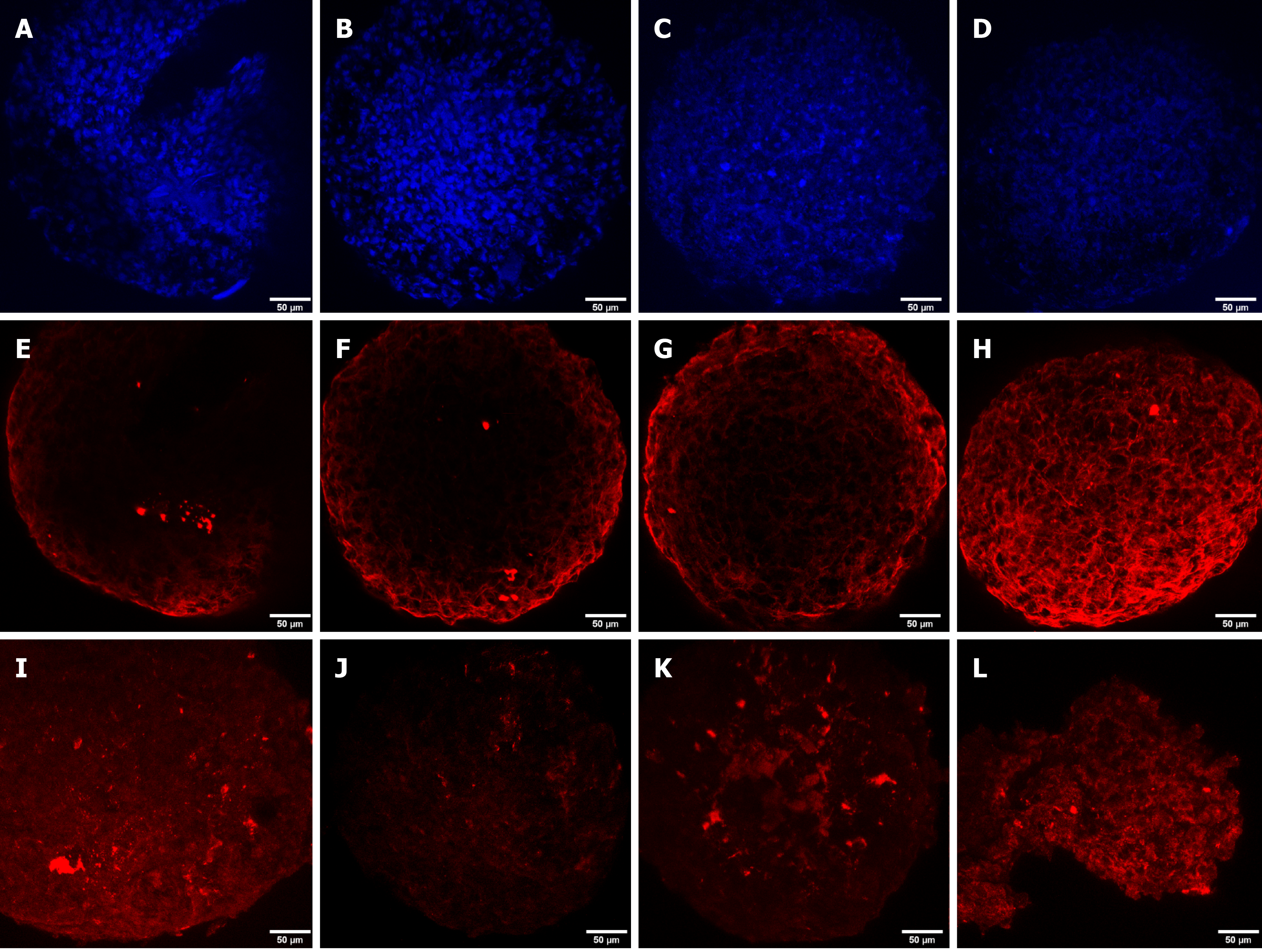
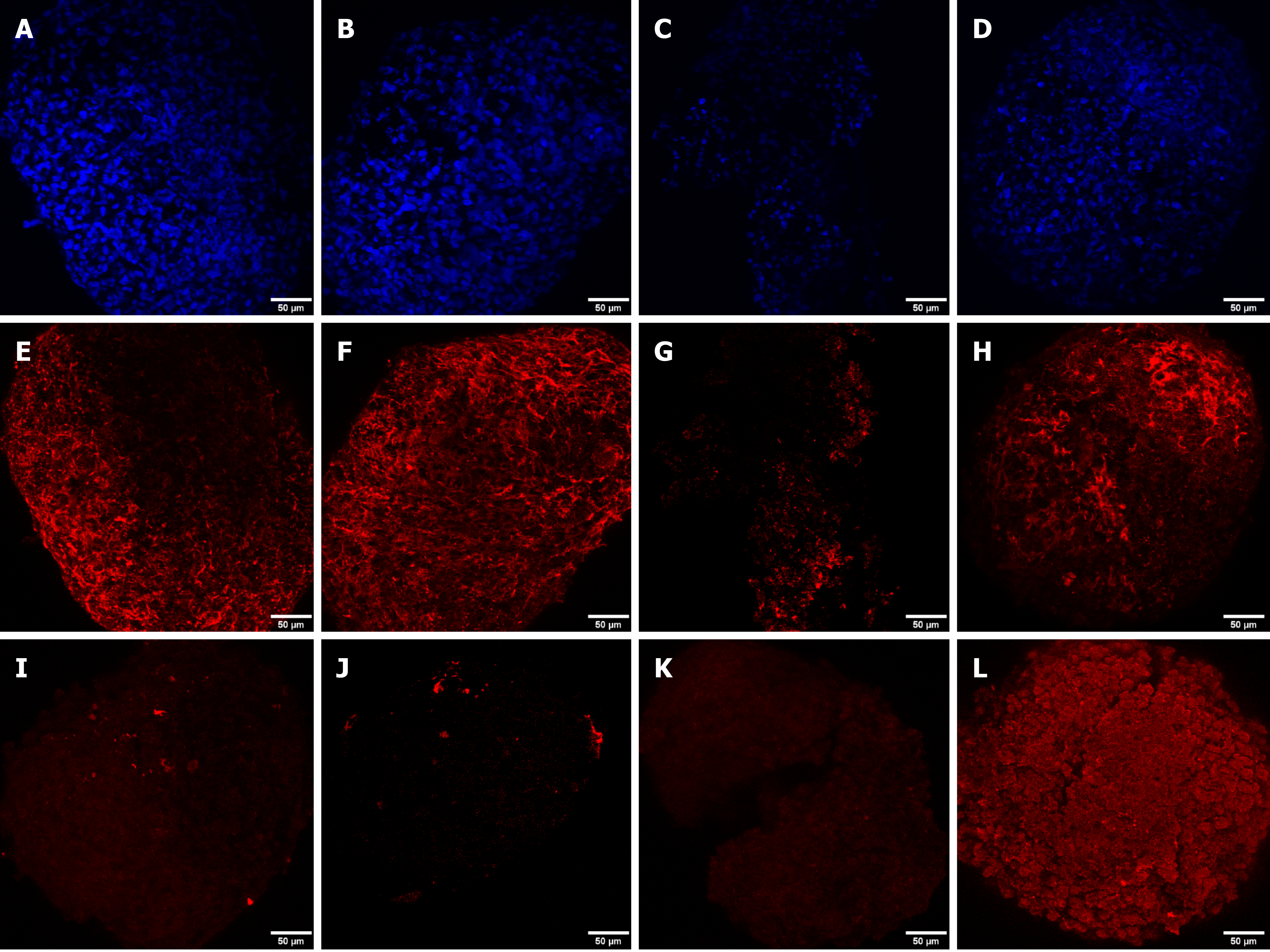
DAPI staining was performed to observe the cell nucleus in all the microspheroid slides. Aggrecan and keratan sulfate were identified in the microspheroid matrix of all sources of MSCs (SF-MSCs, BM-MSCs, and AT-MSCs) using a confocal immunofluorescence assay. We noticed better tissue quality over time with deposition of extracellular matrix and synthesis of aggrecan and keratan sulfate in the MSCs from synovial fluid than in those from adipose tissue and bone marrow (Figures 3, 4 and 5, respectively).
Tissue bioengineering studies have demonstrated the importance of the environment in which MSCs can express their phenotype. The application of MSCs directly to the desired location does not appear to be the best therapeutic method for promoting tissue regeneration, as MSCs can migrate to sites other than the lesion. They have been known to assume immunomodulatory and paracrine roles that are beneficial[27]. Therefore, the use of scaffolds is a suitable method for MSCs because it provides ideal characteristics for avoiding differentiation and favors the permanence of MSCs at the site of the lesion, thereby preventing the migration of cells through the body of the animal.
The magnetic 3D culture technology mimics an environment similar to the scaffolds. MSCs are cultured with biocompatible nanoparticles consisting of gold, iron oxide, and poly-L-lysine, which aids in cell modeling and the formation of microspheroids, which allows high cell density and better aggregation, providing better conditions for the synthesis and secretion of extracellular matrix. Also, the magnetic 3D microplate culture technology is beneficial because the formation of nanoparticles requires fewer cells and allows chondrocytes to express their phenotype by providing the necessary environment for adequate cell differentiation.
Chondrocytes cultured at high density demonstrated a more significant amount of extracellular matrix with proteoglycans and keratan sulfate when compared to cultures at low cell density. In other words, culturing at high density stabilizes the phenotype of chondrocytes, thereby enhancing their function[28,29]. Thus, the importance of high cell density for differentiation into chondrocytes is accounted for by the effect on the morphology and intercellular communication, by cell-cell contact, or through factors secreted by the cells.
This differentiation technique is advantageous because creates the possibility of achieving high cell densities, even with a small number of cells. In this aspect, it differs from the chondrogenic induction technique in conical tubes that requires a larger number of cells and higher energy demand to create a cartilaginous spheroid. The chondrogenic differentiation technique in conical tubes occurs around days 14-21 of chondrogenic induction and compares the results of MSCs extracted from different sources in horses[30,31].
Chondrogenic differentiation time is another essential feature since we observed synthesis of the extracellular matrix by chondrocytes from day 4 onwards, regardless of the source. MSCs are arbitrarily induced to remain in the three-dimensional culture. The microspheroids demonstrated firm characteristics and synthesis of extracellular matrix earlier than those observed through induction techniques in conical tubes in which the chondrogenic differentiation process was shown on days 7[32], 14[33], and 21[20,30,31].
MSCs have common characteristics; however, they demonstrate distinct properties depending on their origin[34-37]. The chondrogenic potential is one property that represents the differences between various sources of MSCs; therefore, the type of cell used for cartilage engineering is critical for the effective treatment of joint diseases and establishing long-term results.
The chondrogenic potential of equine MSCs has already been reported using simple monolayer cultures[38,39], 3D cultures with fibrin[40], and pellet cultures[41-44] with agarose hydrogels with and without transforming growth factor -1 (TGFβ1)[45], TGFβ3, and bone morphogenic protein-6[46]. Our results indicate that SF-MSCs and AT-MSCs show chondrogenic superiority in comparison to BM-MSCs, as they demonstrated higher synthesis of aggrecan, the main proteoglycan in articular cartilage. During the analysis of the aggrecan to soluble protein ratio, the SF-MSCs demonstrated greater aggrecan synthesis in comparison to BM-MSCs. Still, AT-MSCS showed similar aggrecan synthesis in comparison to BM-MSCs.
The chondrogenic superiority of SF-MSCs corroborates the hypothesis that the metabolic capacity of cells derived from the synovial fluid is better due to differences in the number of ancestral cells. It seems that the chondrogenesis is a consequence of the propensity for these cells to follow a chondrogenic pathway, thus suggesting that the local tissue microenvironment can influence this pre-determined lineage[36].
In horses, Kisiday et al[45] demonstrated that BM-MSCs had a lower capacity to produce aggrecan in comparison to AT-MSCs. This finding contrasts to the results published by Vidal et al[46] which demonstrated that BM-MSCs have superior chondrogenic potential in comparison to AT-MSCs.
Based on bioengineering and the use of animal models, MSCs derived from synovial tissue encapsulated in scaffolds produced from gels composed of hyaluronic acid, collagen, and fibrinogen demonstrate tissue repair ability similar to hyaline cartilage in osteochondral defects induced in rabbits[47].
Ichinose et al[48] reported the chondrogenic superiority of SF-MSCs over BM-MSCs after demonstrating greater expression of platelet derived growth factor in human synovial cells. Furthermore, aggrecan expression was extremely low in BM-MSCs when compared with SF-MSCs in humans[49].
In addition to these results, it was shown that BM-MSCs result in a hypertrophic chondrogenic phenotype in horses, thereby limiting the repair of cartilage by promoting mineralization, as they exhibit less collagen II and aggrecan marking, and increased type X collagen detection and Runx2 signaling pathway in comparison to SF-MSCs[50].
In contrast to Sakaguchi et al[34] and Shirasawa et al[51] who used histologic appearance along with toluidine blue staining for the evaluation of the extracellular cartilage matrix produced by MSCs from synovial, bone marrow, and human adipose tissue, in this study we used confocal immunofluorescence in accordance to Zayed et al[52]. All sources showed positive expression for aggrecan and keratan sulfate when analyzed by confocal immunofluorescence at four-time points (4 d, 7 d, 14 d, and 21 d), confirming the chondrogenic differentiation and synthesis of an extracellular cartilage matrix.
Confocal immunofluorescence was used for the evaluation of tissue quality. We observed a higher synthesis of aggrecan and keratan sulfate after chondrogenic differentiation of MSCs from synovial fluid, with evident improvement in the expression and filling of the extracellular matrix from day 4 of the formation of the microspheroids with gradual improvement as time increased. MSC microspheroids from adipose tissue exhibited higher synthesis of aggrecan on day 4 of chondrogenic differentiation than MSCs from the bone marrow, which revealed higher expression on day 21. MSCs from adipose tissue and bone marrow showed similarity in the synthesis of keratan sulfate over time, with evident improvement on day 21.
Tissue engineering or scaffold development involves the production of a three-dimensional tissue that functions as a mechanical support to culture cells. Scaffolds formed by chitosan, polylactic acid, and hyaluronic acid show excellent biocompatibility, high stability, and in vitro chondrogenic differentiation, and confirm the ability of chondrocytes to attach to the scaffold. Thus, they can be applied as a therapeutic strategy for improving the regeneration of cartilage tissue[53]. Three-dimensional cell culture technology mimics an environment similar to scaffolds; however, there are no studies that compare the chondrogenic potential of MSCs using a magnetic three-dimensional cell culture system. Most studies have shown that MSCs from bone marrow are superior to adipose tissue for chondrogenic quality. Thus, new therapeutic strategies based on the bone marrow have appeared more and more in the literature, mainly those based on tissue bioengineering and scaffold development. However, our study shows that MSCs from synovial fluid and adipose tissue develop better in the three-dimensional environment, suggesting the feasibility of future in vivo applications, including in the other species and humans. Furthermore, the use of the cell niche associated with scaffolds may favor the differentiation of MSCs into chondrocytes in vivo and contribute to cartilage repair.
In summary, we established a simple three-dimensional culture method that promoted the differentiation of MSCs into chondrocytes capable of synthesizing extracellular matrix compatible with articular cartilage molecules.
There are no studies about differentiation in chondrocytes in vitro using three-dimensional technology with nanoparticles; therefore, these results represent essential tools for choosing the niche of MSCs for future intra-articular treatment, mainly as it relates to biomaterials. Therefore, niches that have shown repair of chondrogenic impairment can be used with scaffolds, biomaterials, or hydrogels.
MSCs derived from synovial fluid, bone marrow, and adipose tissue are capable of chondrogenic differentiation and aggrecan synthesis.
MSCs derived from synovial fluid and adipose tissue cultured under three-dimensional technology system conditions demonstrated better biocompatibility, suggesting chondrogenic superiority. The use of MSCs from synovial fluid or adipose tissue should be recommended when it is intended for use in conjunction with biomaterials or scaffolds for intra-articular treatment of joint disease.
Osteoarthritis is the main cause of economic loss in equine industry as its prompt lameness and limits or ends the equine athletic career. Fewer studies involve comparison tests of mesenchymal stem cells (MSCs) for the chondrogenic lineage in order to find a consistent and long-term therapeutic solution.
Nanoparticle’s technology applied in tridimensional cell culture was an innovative method, as it had not yet been studied in MSCs for chondrocyte differentiation.
To study the three sources of MSCs in order to find out which niche has the best commitment with the cartilage lineage, to use them in joint diseases in the future.
We investigate the chondrogenic differentiation in vitro with tridimensional technology with cell exposure to the nanoparticles, evaluate and quantified the aggrecan, the main proteoglycan present in the hyaline cartilage, and identified aggrecan and keratan sulfate.
Cell culture from the three sources exhibited expression of MSC markers and, after chondrocytes differentiation, demonstrated aggrecan and keratan sulfate.
MSCs derived from synovial fluid and adipose tissue cultured under three-dimensional technology system conditions demonstrated better biocompatibility, suggesting chondrogenic superiority.
Future tests involve clinical application of MSCs in equine diseases and, in vitro and in vivo test based on the development of biomaterial in conjunction with the MSCs of synovial fluid for the evaluation of in vitro efficacy and in vivo safety.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gunel-Ozcan A S-Editor: Liu M L-Editor: A P-Editor: Xing YX
| 1. | Barrey E. Biomechanics of locomotion in the athletic horse. In: Hinchcliff K, Kaneps AJ, Geor RJ. Equine Sports Medicine and Surgery Basic and clinical of the equine athlete, New York: Saunders Elsevier, 2014: 189. |
| 2. | Riggs CM. Osteochondral injury and joint disease in the athletic horse. Equine Vet Educ. 2006;18:100-112. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Van Weeren. Joint physiology: responses to exercise and training. In: Hinchcliff K, Kaneps AJ, Geor RJ. Equine Sports Medicine and Surgery Basic and clinical of the equine athlete, New York: Saunders Elsevier, 2014: 213. |
| 4. | Orth P, Rey-Rico A, Venkatesan JK, Madry H, Cucchiarini M. Current perspectives in stem cell research for knee cartilage repair. Stem Cells Cloning. 2014;7:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54:1418-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 309] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 6. | Frisbie DD, Smith RK. Clinical update on the use of mesenchymal stem cells in equine orthopaedics. Equine Vet J. 2010;42:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Toupadakis CA, Wong A, Genetos DC, Cheung WK, Borjesson DL, Ferraro GL, Galuppo LD, Leach JK, Owens SD, Yellowley CE. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am J Vet Res. 2010;71:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8:886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 731] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 9. | Park JS, Shim MS, Shim SH, Yang HN, Jeon SY, Woo DG, Lee DR, Yoon TK, Park KH. Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-β3. Biomaterials. 2011;32:8139-8149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Pievani A, Scagliotti V, Russo FM, Azario I, Rambaldi B, Sacchetti B, Marzorati S, Erba E, Giudici G, Riminucci M, Biondi A, Vergani P, Serafini M. Comparative analysis of multilineage properties of mesenchymal stromal cells derived from fetal sources shows an advantage of mesenchymal stromal cells isolated from cord blood in chondrogenic differentiation potential. Cytotherapy. 2014;16:893-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Honarpardaz A, Irani S, Pezeshki-Modaress M, Zandi M, Sadeghi A. Enhanced chondrogenic differentiation of bone marrow mesenchymal stem cells on gelatin/glycosaminoglycan electrospun nanofibers with different amount of glycosaminoglycan. J Biomed Mater Res A. 2019;107:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | White JL, Walker NJ, Hu JC, Borjesson DL, Athanasiou KA. A Comparison of Bone Marrow and Cord Blood Mesenchymal Stem Cells for Cartilage Self-Assembly. Tissue Eng Part A. 2018;24:1262-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Maumus M, Manferdini C, Toupet K, Peyrafitte JA, Ferreira R, Facchini A, Gabusi E, Bourin P, Jorgensen C, Lisignoli G, Noël D. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013;11:834-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Fernandes TL, Kimura HA, Pinheiro CCG, Shimomura K, Nakamura N, Ferreira JR, Gomoll AH, Hernandez AJ, Bueno DF. Human Synovial Mesenchymal Stem Cells Good Manufacturing Practices for Articular Cartilage Regeneration. Tissue Eng Part C Methods. 2018;24:709-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Moyer W, Schumacher J, Schumacher J. A Guide to equine Joint Injection and Regional Anesthesia. Veterinary Learning Systems, 2007. |
| 16. | Fortier LA, Smith RK. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet Clin North Am Equine Pract. 2008;24:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Ortved KF, Nixon AJ. Cell-based cartilage repair strategies in the horse. Vet J. 2016;208:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Carvalho AM, Alves ALG, De Oliveira PGG, Cisneros Álvarez LE, Amorim RL, Hussni CA, Deffune E. Use of Adipose Tissue-Derived Mesenchymal Stem Cells for Experimental Tendinitis Therapy in Equines. J Equine Vet Sci. 2011;31:26-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Alipour F, Parham A, Kazemi Mehrjerdi H, Dehghani H. Equine adipose-derived mesenchymal stem cells: phenotype and growth characteristics, gene expression profile and differentiation potentials. Cell J. 2015;16:456-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 20. | Fülber J, Maria DA, da Silva LC, Massoco CO, Agreste F, Baccarin RY. Comparative study of equine mesenchymal stem cells from healthy and injured synovial tissues: an in vitro assessment. Stem Cell Res Ther. 2016;7:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Smith RK, Korda M, Blunn GW, Goodship AE. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. 2003;35:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | de Mattos Carvalho A, Alves AL, Golim MA, Moroz A, Hussni CA, de Oliveira PG, Deffune E. Isolation and immunophenotypic characterization of mesenchymal stem cells derived from equine species adipose tissue. Vet Immunol Immunopathol. 2009;132:303-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Hassell JR, Newsome DA, Hascall VC. Characterization and biosynthesis of proteoglycans of corneal stroma from rhesus monkey. J Biol Chem. 1979;254:12346-12354. [PubMed] |
| 24. | Saxne T, Heinegård D, Wollheim FA. Therapeutic effects on cartilage metabolism in arthritis as measured by release of proteoglycan structures into the synovial fluid. Ann Rheum Dis. 1986;45:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 91] [Reference Citation Analysis (0)] |
| 25. | Heinegård D, Inerot S, Wieslander J, Lindblad G. A method for the quantification of cartilage proteoglycan structures liberated to the synovial fluid during developing degenerative joint disease. Scand J Clin Lab Invest. 1985;45:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Skiöldebrand E, Lorenzo P, Zunino L, Rucklidge GJ, Sandgren B, Carlsten J, Ekman S. Concentration of collagen, aggrecan and cartilage oligomeric matrix protein (COMP) in synovial fluid from equine middle carpal joints. Equine Vet J. 2001;33:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Santos VHD, Pfeifer JPH, Souza JB, Stievani FC, Hussni CA, Golim MA, Deffune E, Alves ALG. Evaluation of alginate hydrogel encapsulated mesenchymal stem cell migration in horses. Res Vet Sci. 2019;124:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Solursh M, Meier S. Effects of cell density on the expression of differentiation by chick embryo chondrocytes. J Exp Zool. 1974;187:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Watt FM. Effect of seeding density on stability of the differentiated phenotype of pig articular chondrocytes in culture. J Cell Sci. 1988;89:373-378. [PubMed] |
| 30. | Burk J, Ribitsch I, Gittel C, Juelke H, Kasper C, Staszyk C, Brehm W. Growth and differentiation characteristics of equine mesenchymal stromal cells derived from different sources. Vet J. 2013;195:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Adam EN, Janes J, Lowney R, Lambert J, Thampi P, Stromberg A, MacLeod JN. Chondrogenic differentiation potential of adult and fetal equine cell types. Vet Surg. 2019;48:375-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Zubillaga V, Alonso-Varona A, Fernandes SCM, Salaberria AM, Palomares T. Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering. Int J Mol Sci. 2020;21:1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Sekiya I, Ojima M, Suzuki S, Yamaga M, Horie M, Koga H, Tsuji K, Miyaguchi K, Ogishima S, Tanaka H, Muneta T. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res. 2012;30:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1089] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 35. | Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 223] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 36. | Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 514] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 37. | Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, Sekiya I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 38. | Fortier LA, Nixon AJ, Williams J, Cable CS. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res. 1998;59:1182-1187. [PubMed] |
| 39. | Worster AA, Nixon AJ, Brower-Toland BD, Williams J. Effect of transforming growth factor beta1 on chondrogenic differentiation of cultured equine mesenchymal stem cells. Am J Vet Res. 2000;61:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Worster AA, Brower-Toland BD, Fortier LA, Bent SJ, Williams J, Nixon AJ. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Arnhold SJ, Goletz I, Klein H, Stumpf G, Beluche LA, Rohde C, Addicks K, Litzke LF. Isolation and characterization of bone marrow-derived equine mesenchymal stem cells. Am J Vet Res. 2007;68:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Giovannini S, Brehm W, Mainil-Varlet P, Nesic D. Multilineage differentiation potential of equine blood-derived fibroblast-like cells. Differentiation. 2008;76:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Hegewald AA, Ringe J, Bartel J, Krüger I, Notter M, Barnewitz D, Kaps C, Sittinger M. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004;36:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Stewart AA, Byron CR, Pondenis H, Stewart MC. Effect of fibroblast growth factor-2 on equine mesenchymal stem cell monolayer expansion and chondrogenesis. Am J Vet Res. 2007;68:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Kisiday JD, Kopesky PW, Evans CH, Grodzinsky AJ, McIlwraith CW, Frisbie DD. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008;26:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 46. | Vidal MA, Robinson SO, Lopez MJ, Paulsen DB, Borkhsenious O, Johnson JR, Moore RM, Gimble JM. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 2008;37:713-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Lee JC, Lee SY, Min HJ, Han SA, Jang J, Lee S, Seong SC, Lee MC. Synovium-derived mesenchymal stem cells encapsulated in a novel injectable gel can repair osteochondral defects in a rabbit model. Tissue Eng Part A. 2012;18:2173-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Ichinose S, Muneta T, Koga H, Segawa Y, Tagami M, Tsuji K, Sekiya I. Morphological differences during in vitro chondrogenesis of bone marrow-, synovium-MSCs, and chondrocytes. Lab Invest. 2010;90:210-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford). 2008;47:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 50. | McCarthy HE, Bara JJ, Brakspear K, Singhrao SK, Archer CW. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J. 2012;192:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Shirasawa S, Sekiya I, Sakaguchi Y, Yagishita K, Ichinose S, Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97:84-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 52. | Zayed MN, Schumacher J, Misk N, Dhar MS. Effects of pro-inflammatory cytokines on chondrogenesis of equine mesenchymal stromal cells derived from bone marrow or synovial fluid. Vet J. 2016;217:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Mallick SP, Rastogi A, Tripathi S, Srivastava P. Strategies on process engineering of chondrocyte culture for cartilage tissue regeneration. Bioprocess Biosyst Eng. 2017;40:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |