Published online May 26, 2019. doi: 10.4252/wjsc.v11.i5.254
Peer-review started: February 20, 2019
First decision: March 15, 2019
Revised: March 29, 2019
Accepted: April 9, 2019
Article in press: April 9, 2019
Published online: May 26, 2019
Processing time: 95 Days and 19.3 Hours
Articular cartilage damage and osteoarthritis (OA) are common orthopedic diseases in both humans and dogs. Once damaged, the articular cartilage seldom undergoes spontaneous repair because of its avascular, aneural, and alymphatic state, and the damage progresses to a chronic and painful situation. Dogs have distinctive characteristics compared to other laboratory animal species in that they share an OA pathology with humans. Dogs can also require treatment for naturally developed OA; therefore, effective treatment methods for OA are desired in veterinary medicine as well as in human medicine. Recently, interest has grown in regenerative medicine that includes the use of mesenchymal stem cells (MSCs). In cartilage repair, MSCs are a promising therapeutic tool due to their self-renewal capacity, ability to differentiate into cartilage, potential for trophic factor production, and capacity for immunomodulation. The MSCs from dogs (canine MSCs; cMSCs) share various characteristics with MSCs from other animal species, but they show some deviations, particularly in their differentiation ability and surface epitope expression. In vivo studies of cMSCs have demonstrated that intraarticular cMSC injection into cartilage lesions results in excellent hyaline cartilage regeneration. In clinical situations, cMSCs have shown great therapeutic effects, including amelioration of pain and lameness in dogs suffering from OA. However, some issues remain, such as a lack of regulations or guidelines and a need for unified methods for the use of cMSCs. This review summarizes what is known about cMSCs, including their in vitro characteristics, their therapeutic effects in cartilage lesion treatment in preclinical in vivo studies, their clinical efficacy for treatment of naturally developed OA in dogs, and the current limitations of cMSC studies.
Core tip: Mesenchymal stem cells (MSCs) are promising therapeutic tools for treatment of cartilage damage and osteoarthritis (OA). This review summarizes the current knowledge of MSCs from dogs, including in vitro characteristics, in vivo cartilage regenerative potential, and therapeutic effects for naturally developed OA in dogs. MSCs from dogs share many in vivo characteristics with MSCs from other animal species and are reported to have excellent cartilage repair potential in experimental and clinical situations. The article also describes the current limitations of MSC use in dogs that remain to be resolved in the future.
- Citation: Sasaki A, Mizuno M, Mochizuki M, Sekiya I. Mesenchymal stem cells for cartilage regeneration in dogs. World J Stem Cells 2019; 11(5): 254-269
- URL: https://www.wjgnet.com/1948-0210/full/v11/i5/254.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i5.254
Articular cartilage is critical for the normal functioning of the synovial joint. The ability of cartilage to withstand high cyclic loads and to minimize surface friction on articular surfaces allows it to act as a shock absorber and a lubricator in synovial joints[1-3]. Nevertheless, articular cartilage is still subject to damage by a variety of factors, including acute trauma, degeneration, or joint diseases. Once damaged, hyaline articular cartilage shows very limited intrinsic repair potential because of its’ avascular, aneural, and alymphatic state. The unrepaired cartilage defects lead to pain, swelling, and deterioration in mobility and will eventually progress to osteo-arthritis (OA)[4,5].
OA is a common degenerative, progressive, and painful disease in both humans[6] and dogs[7]. Nevertheless, an adequate therapy for the repair of cartilage lesions has yet to be established. A variety of surgical interventions are available in human medicine, including microfracture[8], osteochondral autografts[9], and autologous chondrocyte implantation[10]. Following microfracture, the regenerated tissue occurs as a mixed fibrocartilage tissue that is inferior in its mechanical properties to native hyaline cartilage and has poor long-term results[11]. Similarly, osteochondral autografts have limitations of graft availability and risk of donor site morbidity[12,13]. Autologous chondrocyte implantation, although it can achieve hyaline-like cartilage repair, also has some issues, including loss of the chondrocytic phenotype in monolayer culture, limited availability of chondrocytes and inadequate chondrocyte proliferative poten-tial[14,15].
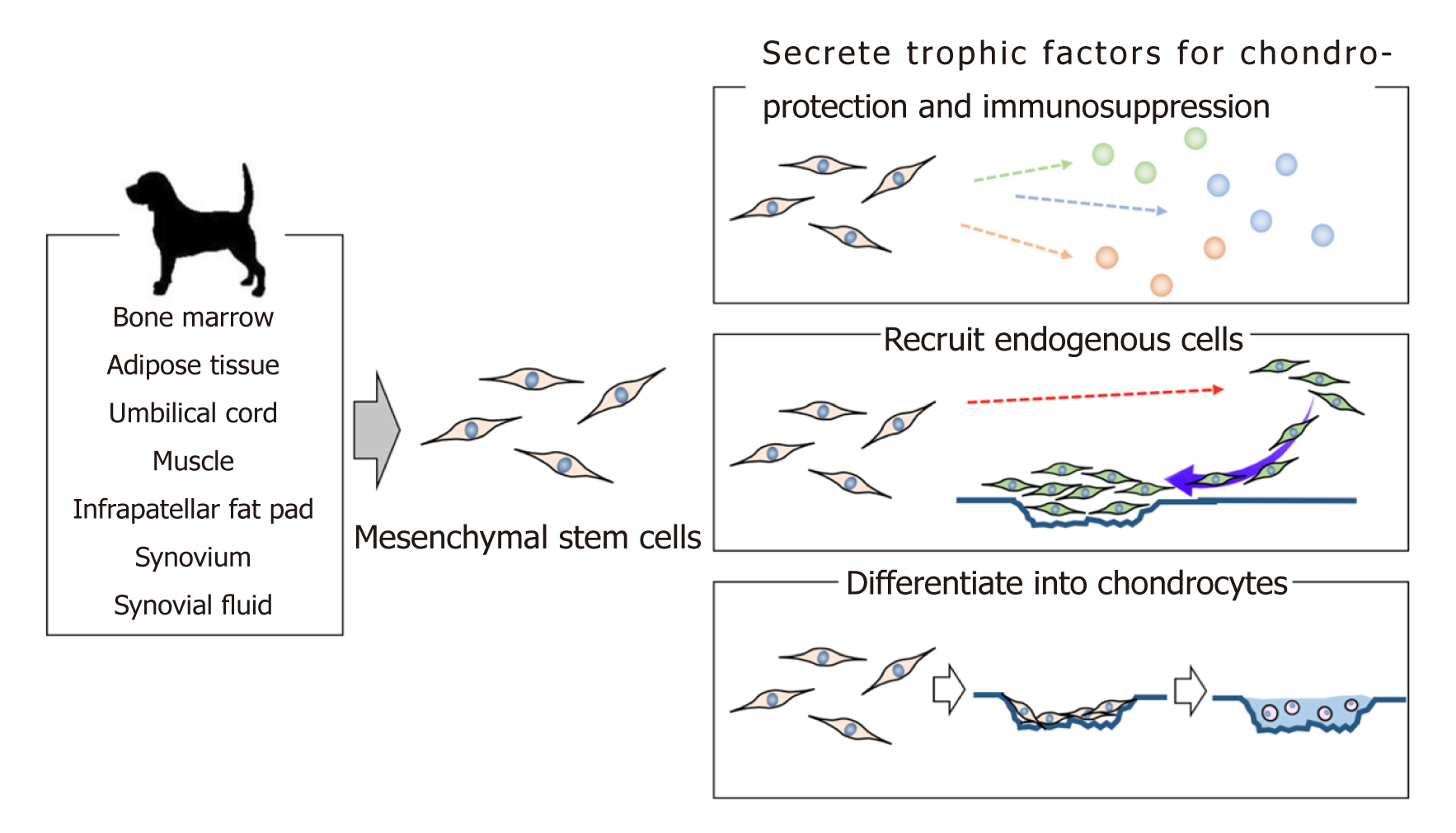
One attractive alternative source of cells for cartilage regeneration is mesenchymal stem cells (MSCs), which are readily available, possess great proliferation ability, and can differentiate into many different cell types in the body[16,17]. MSCs have been used in various applications in both human and veterinary medicine. For cartilage lesion studies, dogs are a particularly distinctive animal species as they can serve as an experimental animal model, a clinical model for human medicine, and a treatment subject. This review summarizes the in vitro data, preclinical in vivo studies, and clinical studies involving the use of MSCs for cartilage regeneration in dogs (Figure 1).
Dogs can serve as experimental or clinical models for studies on cartilage repair in humans. Although the articular cartilage is thinner in dogs than that in humans (range in humans: 1.0-2.6 mm)[18-20], this thickness is greater in medium to large breed dogs (range: 0.95-1.3 mm) than in rodent and rabbit models. This greater thickness allows the experimental creation of articular cartilage defects without causing detrimental changes in the subchondral bone[20]. In addition, unlike the other experimental animals, dogs can accept various human-type postoperative mana-gements, including bandaging, splinting, or exercise involving (leash) walking or training on treadmills[20-22], which can prevent OA or regulate its progression after the experimental interventions.
However, the use of companion animals such as dogs as experimental models for human medicine raises ethical considerations. By contrast, the use of farm animals, such as goats, sheep and pigs, as experimental animal models is less controver-sial[20,23,24]. These farm animals have the further advantage that their articular cartilage thicknesses are closer to those of humans and are adequate for the creation of partial- and full-thickness defects[20,23,24]. For these reasons, dogs might be superseded by goats or pigs as experimental animal models.
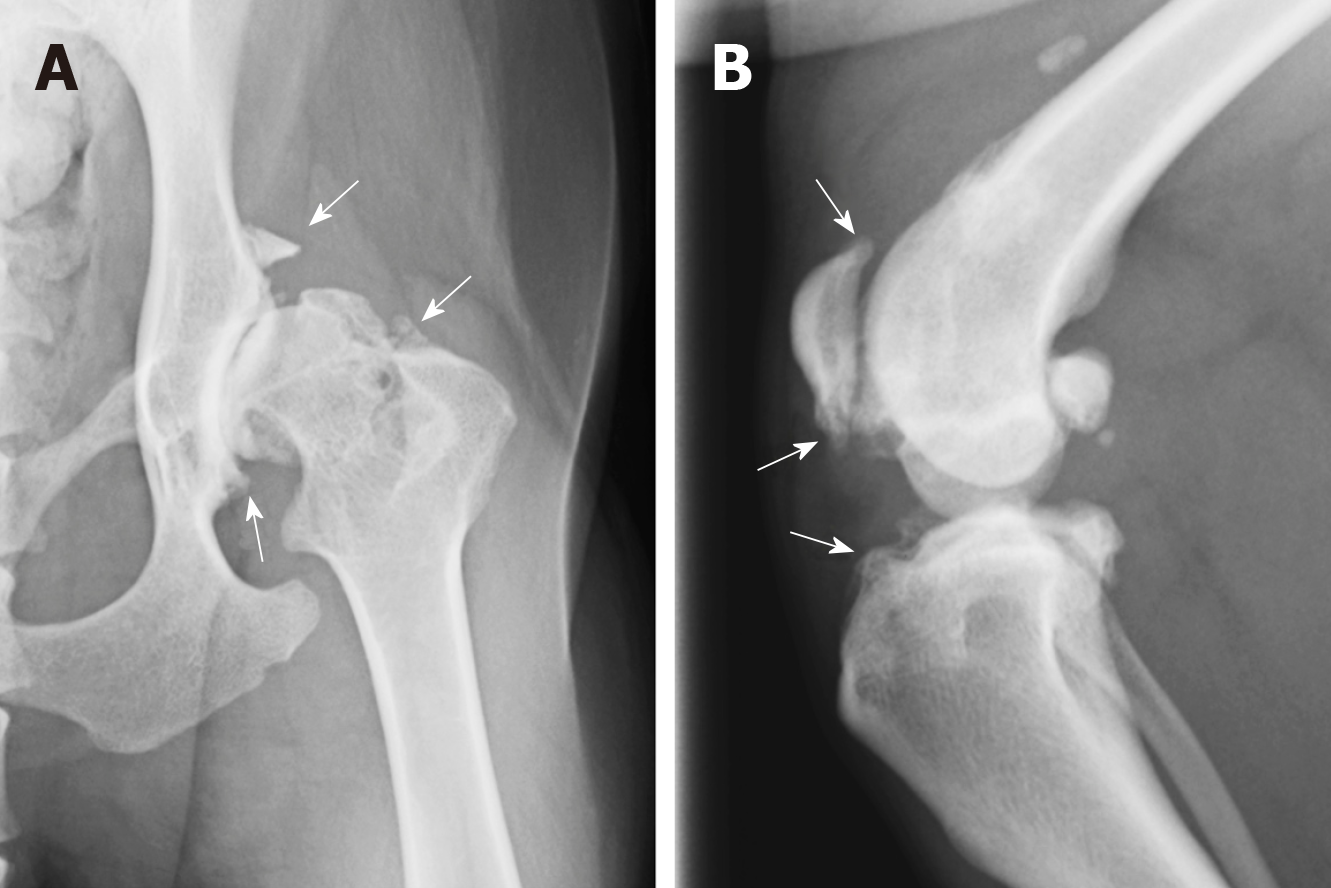
Nevertheless, despite the disadvantages of using dogs as experimental animal models for preclinical studies of cartilage repair, dogs have one distinctive feature that differentiates them from other animals as they naturally develop diseases that share a close analogy with human diseases, including OA[25,26] (Figure 2). Therefore, clinical studies that are performed in dogs with naturally occurring OA can provide valuable information for the treatment of human OA patients. A variety of ante mortem diagnostic monitoring protocols, such as gait and kinematic analyses[27], arthroscopic evaluation[28], and magnetic resonance imaging[29], are feasible in dogs and allow evaluation of the therapeutic effect from a variety of viewpoints. In addition, while sharing cartilage pathologies with humans, dogs also share the lifestyles of their owners, including home and exercise habits. The life expectancy of dogs is also relatively long, and dogs are subject to similar natural and environmental factors that lead to the occurrence of OA in humans, such as traumatic injuries, obesity, and aging. Many adult dogs suffer from OA, which creates a large pool of client-owned dogs that could be available for enrollment in clinical trials of potential OA treatments[7]. Although considerable interindividual variability is evident in these clinical cases when compared to purpose-bred dogs, the results are still suitable for extrapolation to human patients.
The clinical study of dogs with OA is worthwhile for both human and veterinary medicine. Dogs suffer from many cartilage pathologies that can lead to OA, such as osteochondritis dissecans and cartilage injury caused from cranial cruciate ligament failure[30,31]; therefore, the need is increasing for cartilage lesion treatment methods that can regenerate hyaline cartilage and allow recovery of the normal mechanical function of the articular cartilage. Thus, studies on cartilage regeneration in dogs have important roles in the improvement of cartilage repair treatments and can have great significance in both human and veterinary medicine.
MSCs are cells that display self-renewal capacity and have the potential for diffe-rentiation under specific conditions into other cell types, including cartilage, bone, and adipose tissue. MSCs were first identified in bone marrow derived from mice[32], but were subsequently isolated from other tissues, including adipose tissue[33], umbilical cord[34], dental pulp[35], infrapatellar fat pad[36], and synovial membrane[37]. MSCs can also be isolated and cultured from tissues obtained from various animal species, including rats[38], rabbits[39], dogs[40], cats[41], pigs[42], and horses[43], as well as humans[44].
The International Society for Cellular Therapy (ISCT) proposed minimal criteria to define the characteristics of human MSCs[45]: (1) Adherence to plastic in standard culture conditions; (2) Specific surface antigen expression of CD105, CD73, CD90 and lack of expression of other hematopoietic cell surface markers, including CD45, CD34, CD14 or CD11b, CD79α or CD19, HLA-DR; and (3) Multipotent differentiation potential under specific in vitro culture conditions. These criteria also serve, to a certain extent, as parameters for MSCs derived from various animal species; however, the characteristics of MSCs differ somewhat among various animal species or tissues. Therefore, the criteria for human MSCs cannot be completely extrapolated to MSCs from veterinary species.
MSCs from dogs (canine MSCs: cMSCs) can be isolated from wide variety of tissue sources, including bone marrow[46,47], adipose tissue[48,49], umbilical cord[50,51], muscle[52], infrapatellar fat pad[40,53], synovium[40,53,54], and synovial fluid[55]. For research and therapeutic purposes, MSCs are needed in large numbers, so MSCs must have a high proliferation ability. Some studies have demonstrated a higher proliferation ability for cMSCs derived from adipose tissue (AD-cMSCs) than from bone marrow (BM-cMSCs)[50,56,57]. Furthermore, the colony-forming potential and proliferation ability is higher in cMSCs from synovium (Sy-cMSCs) and from infrapatellar fat pads (IF-cMSCs) than in AD-cMSCs and BM-cMSCs[40,53]. Notably, further differences are observed depending on the age of the donor dogs or the passage number of cMSCs in terms of proliferation capacity[58,59]. For example, Bertolo et al[60] have shown a greater tendency for senescence in cMSCs than in human MSCs when grown in monolayer culture. These findings suggest that the optimal culture conditions for cMSCs should be reconsidered; for instance, the inclusion of supplemental growth factors, such as fibroblast growth factor-2 (FGF-2), could increase the proliferation ability of cMSCs[61].
Table 1 shows the surface marker expression of cMSCs derived from various sources. The expression of cell surface markers is one of the identification criteria for MSCs[45]; however, the criteria used for human MSCs may not be completely compa-tible with those required for cMSCs because of interspecies differences and the lack of validated antibodies and controls for cMSCs. The definitive expression of surface antigens by cMSCs has not been demonstrated, and results for cMSC surface antigen expression have been contradictory among various studies. For example, high expression (> 90%) of CD90 was reported in cMSCs derived from adipose tissue[53,56,57,62] and bone marrow[47,53,56,63] in some studies, whereas other studies have shown low expression (< 40%) of CD90 for cMSCs derived from adipose tissue[49,64,65] and bone marrow[49,64]. Surface epitope expression has a known correlation with differentiation potential and proliferation ability as well as with stemness[66,67]; therefore, further studies are needed to identify appropriate antibody sets for cMSC characterization.
| Source | Positive marker | Negative marker | Ref. |
| Adipose tissue | CD90, CD44, CD73, CD105 | CD45, CD34, CD14 | Kang et al[50], 2012 |
| Bone marrow | CD90, CD44, CD73, CD105 | CD45, CD34, CD14 | |
| Umbilical cord | CD90, CD44, CD73, CD105 | CD45, CD34, CD14 | |
| Wharton's jelly | CD90, CD44, CD73, CD105 | CD45, CD34, CD14 | |
| Adipose tissue | CD90, CD44 | CD45, CD34, CD146 | Kisiel et al[52], 2012 |
| Bone marrow | CD90, CD44 | CD45, CD34, CD146 | |
| Muscle | CD90, CD44 | CD45, CD34, CD146 | |
| Periosteum | CD90, CD44 | CD45, CD34, CD146 | |
| Adipose tissue | CD90, CD44 | CD45, CD11b | Sasaki et al[40], 2018 |
| Bone marrow | CD90, CD44 | CD45, CD11b | |
| Synovium | CD90, CD44 | CD45, CD11b | |
| Infrapatellar fat pad | CD90, CD44 | CD45, CD11b | |
| Adipose tissue | CD90, CD44, CD105, CD9 | CD45, CD34, Stro-1 | Bearden et al[53], 2017 |
| Bone marrow | CD90, CD44, CD105, CD9 | CD45, CD34, Stro-1 | |
| Synovium | CD90, CD44, CD105, CD9 | CD45, CD34, Stro-1 | |
| Adipose tissue | CD90 | - | Reich et al[56], 2012 |
| Bone marrow | CD90 | - | |
| Adipose tissue | CD90, CD44, CD29 | CD45, CD34 | Takemitsu et al[64], 2012 |
| Bone marrow | CD90, CD44, CD29 | CD45, CD34 | |
| Adipose tissue | CD90, CD44, MHCI | CD34, CD29, CD14, MHCII | Screven et al[49], 2014 |
| Bone marrow | CD90, CD44, MHCI | CD34, CD29, CD14, MHCII | |
| Adipose tissue | CD90, CD44 | CD45, CD34, CD14 | Sullivan et al[118], 2016 |
| Bone marrow | CD90, CD44 | CD45, CD34, CD14 | |
| Adipose tissue | CD90, CD44, CD29, CD73, CD4, CD8, MHCI | CD45, CD34, CD14, MHCII | Russell et al[57], 2016 |
| Bone marrow | CD90, CD44, CD29, CD73, CD4, CD8, MHCI | CD45, CD34, CD14, MHCII | |
| Adipose tissue | CD90, CD44, MHCI | CD45, CD34, CD14, CD3, CD4, CD8, CD172a, CD11c, HLA-DR, sIgM | Kang et al[62], 2008 |
| Adipose tissue | CD90, CD44, CD29 | CD45, CD34, CD14, CD117, CD13 CD105, CD73 | Vieira et al[48], 2010 |
| Adipose tissue | CD90, CD44, CD140, CD117 | CD45, CD34 | Martinello et al[65], 2011 |
| Bone marrow | CD90, MHCI | CD45, CD34, MHCII | Kamishina et al[119], 2006 |
| Bone marrow | CD90, CD105 | CD45, CD34 | Csaki et al[46], 2007 |
| Bone marrow | CD44, Stro-1 | CD45, CD34 | Hodgkiss-Geere et al[120], 2012 |
| Bone marrow | CD90, CD44 | CD14 | Bertolo et al[73], 2015 |
| Bone marrow | CD90, CD44, CD105, CD73, CD166, vimentin | CD31 | Zhang et al[47], 2018 |
| Bone marrow | CD90, CD44, CD29, CD105, CD166 | CD45, CD34 | Li et al[63], 2018 |
| Synovium | CD90, CD44 | - | Wijekoon et al[54], 2017 |
| Synovial fluid | CD90 | CD45, CD34 | Krawetz et al[55], 2012 |
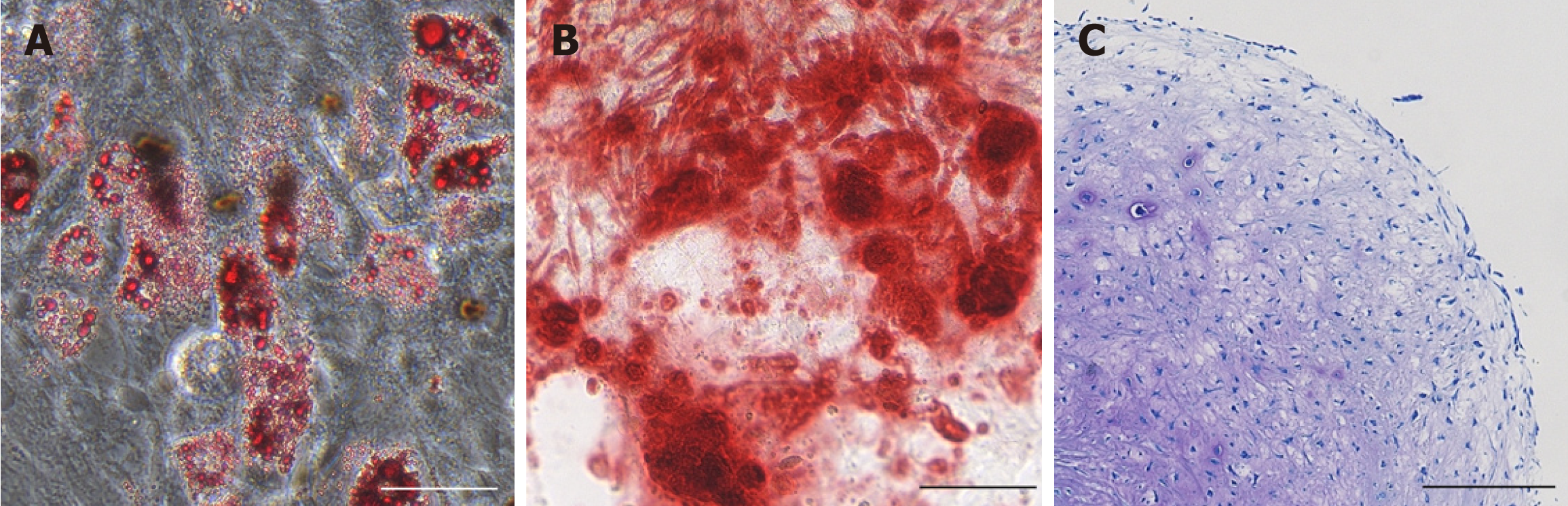
Multipotent differentiation potential is one of the defined criteria proposed by ISCT and is a crucial characteristic of MSCs used in regenerative therapy. Adipogenesis, osteogenesis, and chondrogenesis in cMSC studies are validated by specific staining or analysis of mRNA expression (Figure 3 and Table 2).
| Source | Differentiation potential | Ref. |
| Adipose tissue | Adipogenesis, osteogenesis, chondrogenesis | Csaki et al[46], 2007 |
| Adipose tissue | Adipogenesis, osteogenesis, neurogenesis | Kang et al[62], 2008 |
| Adipose tissue | Adipogenesis, osteogenesis, chondrogenesis | Neupane et al[68], 2008 |
| Adipose tissue | Adipogenesis, osteogenesis, chondrogenesis, myogenesis | Vieira et al[48], 2010 |
| Adipose tissue | Adipogenesis, osteogenesis, myogenesis | Martinello et al[65], 2011 |
| Adipose tissue | Chondrogenesis | Reich et al[56], 2012 |
| Bone marrow | ||
| Adipose tissue | Osteogenesis | Kang et al[50], 2012 |
| Bone marrow | ||
| Umbilical cord | ||
| Wharton's jelly | ||
| Adipose tissue | Adipogenesis, osteogenesis | Kisiel et al[52], 2012 |
| Bone marrow | ||
| Muscle | ||
| Periosteum | ||
| Adipose tissue | Adipogenesis, osteogenesis | Takemitsu et al[64], 2012 |
| Bone marrow | ||
| Bone marrow | Chondrogenesis, cardiogenesis | Hodgkiss-Geere et al[120], 2012 |
| Synovial fluid | Chondrogenesis | Krawetz et al[55], 2012 |
| Bone marrow | Adipogenesis, osteogenesis, chondrogenesis | Volk et al[59], 2012 |
| Adipose tissue | Adipogenesis, osteogenesis, chondrogenesis | Guercio et al[58], 2013 |
| Adipose tissue | Adipogenesis, osteogenesis, chondrogenesis | Screven et al[49], 2014 |
| Bone marrow | ||
| Bone marrow | Adipogenesis, osteogenesis, chondrogenesis | Bertolo et al[60], 2015 |
| Adipose tissue | Osteogenesis, chondrogenesis | Sullivan et al[118], 2016 |
| Bone marrow | ||
| Adipose tissue | Adipogenesis, osteogenesis | Russell et al[57], 2016 |
| Bone marrow | ||
| Synovium | Adipogenesis, osteogenesis, chondrogenesis, osteoclast differentiation | Wijekoon et al[54], 2017 |
| Adipose tissue | Adipogenesis, osteogenesis, chondrogenesis | Bearden et al[53], 2017 |
| Bone marrow | ||
| Synovium | ||
| Bone marrow | Adipogenesis, osteogenesis, chondrogenesis Hepatocyte and functional insulin-secreting cell differentiation | Zhang et al[47], 2018 |
| Adipose tissue | Adipogenesis, osteogenesis, chondrogenesis | Sasaki et al[40], 2018 |
| Bone marrow | ||
| Synovium | ||
| Infrapatellar fat pad | ||
| Bone marrow | Chondrogenesis | Endo et al[61], 2018 |
In vitro adipogenesis of cMSCs has been confirmed for AD-cMSCs, BM-cMSCs, Sy-cMSCs, and IF-cMSCs (Table 2). The formation of oil lipid droplets is verified with oil red-O staining; however, cMSCs sometimes cannot sufficiently differentiate into adipocytes in the induction medium used for adipogenic differentiation of human MSCs, so the induction media need to be optimized for cMSCs. Neupane et al[68] utilized rabbit serum, rosiglitazone, and a higher glucose concentration in their medium to promote the adipogenic differentiation of AD-cMSCs. The optimized induction medium increased the numbers and sizes of fat globules and the expression of adipogenic transcription factors, such as PPARγ2, when compared to the conventional adipogenic differentiation medium used for human MSCs[68]. A higher adipogenic differentiation ability has been reported for AD-cMSCs and IF-cMSCs than for BM-cMSCs and Sy-cMSCs[40,49,53].
During osteogenic differentiation, the morphology of cMSCs progresses from a fibroblast-like shape to a cuboidal-like appearance, together with the formation of aggregates that stain with von Kossa or alizarin red stains[46,52,58,68]. Unlike human MSCs, cMSCs have been known to roll up into a sheet and become easily detached from culture dishes during mineralization assays[40,53,58,68]. BM-cMSCs have shown superiority in osteogenic differentiation in some studies, when compared to AD-cMSCs, Sy-cMSCs, and IF-cMSCs[40,49,53]. However, Kang et al[50] showed a greater osteogenic ability of AD-cMSCs when compared with BM-cMSCs and Wharton’s jelly-derived cMSCs.
The chondrogenic differentiation potency is a crucial characteristic of cMSCs intended for use in cartilage regenerative therapy. In vivo cartilage formation during embryogenesis is initiated by condensation of cells, and the cell-to-cell contacts induced by condensation are crucial for the onset of chondrogenesis[69]. The growth factor transforming growth factor-β (TGF-β) is crucial for the formation of three-dimensional aggregates during chondrogenesis in human MSCs[70], whereas cMSCs intrinsically aggregate in micromass culture even in the absence of TGF-β[52,68]. Nevertheless, some studies have reported difficulties in attaining substantial chondrogenic differentiation of cMSCs[52,56,57,60].
Since no definitive methods have been established for chondrogenic differentiation of cMSCs, the various studies have all used different tissue source origins, growth factors, and culture methods. The chondrogenic differentiation potential of cMSCs varies with the tissue source origin, as indicated by the larger cartilage pellets obtained from differentiating Sy-cMSCs and IF-cMSCs than from differentiating BM-cMSCs and AD-cMSCs[40,53]. The cartilage pellets that differentiate from BM-cMSCs are small, but they show intense staining with toluidine blue, alcian blue, or collagen type II antibody when compared to cartilage pellets differentiated from Sy-cMSCs, IF-cMSCs, or AD-cMSCs[53,56]. Conversely, AD-cMSCs, despite their abundance and ready availability, form only small cartilage pellets that show low stainability with toluidine blue, alcian blue, or collagen type II antibody and that sometimes show signs of necrosis[40,56]. Human Sy-MSCs and BM-MSCs are also known to have higher chondrogenic differentiation capacity when compared with AD-MSCs[44,71,72]. Inter-estingly, Bertolo et al[73] showed that cMSCs from different dog breeds can also vary in their differentiation potential.
Chondrogenic differentiation can be enhanced in vitro by supplementing the culture medium with growth factors, such as TGF-β and bone morphogenic protein (BMP). TGF-β, which is crucial for chondrogenic differentiation of MSCs, exists as three isoforms: TGF-β1, TGF-β2, and TGF-β3. Of these, TGF-β1[48,52,56,61] or TGF-β3[47,49,57] have been the most commonly used in studies of chondrogenesis in cMSCs. TGF-β3 is reported to result in higher and more rapid chondrogenic differentiation in human MSCs[74], but the optimal TGF-β isoform for chondrogenesis in cMSCs remains to be elucidated.
BMPs have been reported to promote chondrogenesis[75], and a greater stimulation of chondrogenesis in human MSCs has been reported for BMP-2 than for BMP-4 or BMP-6[76]. In addition to promoting chondrogenesis, BMPs can also stimulate endochondral ossification[77]. Many studies of cMSCs have shown the efficacy of BMPs for osteogenesis[53,78], although one study reported no improvement in chondrogenesis by BM-cMSCs or AD-cMSCs[56] in response to BMP-2. Therefore, the effectiveness of BMPs at inducing chondrogenesis in cMSCs remains unclear.
Chondrogenic differentiation ability decreases with the loss of CD90 in human MSCs[79,80] and cMSCs[55], indicating the importance of surface epitope expressions. Therefore, the initial cell selection may improve the chondrogenic potential of MSCs. The in vitro expansion of human MSCs, rabbit MSCs, and cMSCs in culture also impairs chondrogenic differentiation potential[44,81-83]. Moreover, senescence is reportedly more rapid in cMSCs than in human MSCs, particularly after passage 3[60]. Sekiya et al[84] reported that cartilage pellets were larger, stained more extensively for proteoglycans, and expressed higher levels of mRNA for type II procollagen when prepared from cultures enriched for small and rapidly self-renewing cells than from cultures containing more mature and slowly replicating cells. The smaller and more rapidly proliferating cells can be produced using FGF-2 during monolayer culture of cMSCs[61]. FGF-2 preconditioning of cMSCs also improved their chondrogenic differentiation potential[61] in a similar fashion to that observed for human MSCs[85]. Therefore, it is worthwhile to consider the use of FGF-2 preconditioning for cMSC expansion, as well as the use of cMSCs at early passages, for cartilage regenerative therapy.
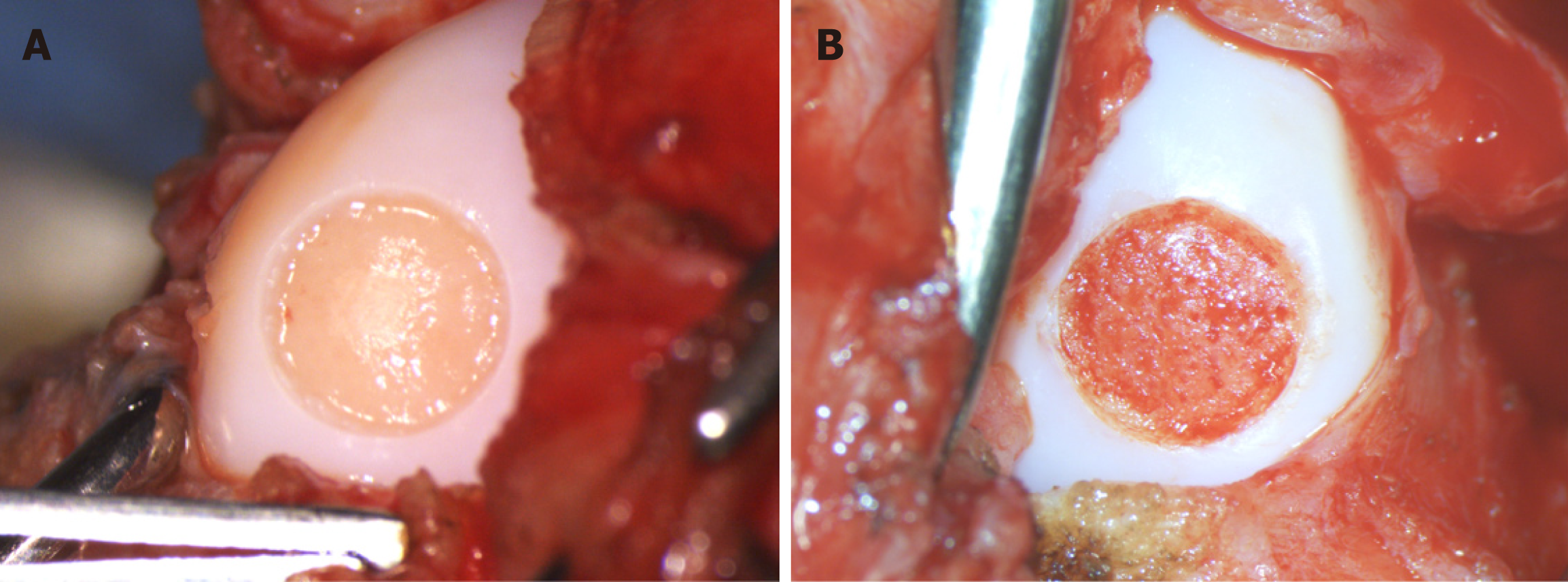
While in vitro studies provide a substantial amount of information about the potential of MSCs for cartilage repair, evaluation of the in vivo behavior and function of these cells is also required. In vivo studies of cMSCs for cartilage regeneration have involved the use of surgically induced cartilage defect models, including partial-thickness cartilage defect models[62,85-87] (Figure 4A), full-thickness cartilage defect models[88,89] (Figure 4B) and cranial cruciate ligament transection models[90]. (Table 3) In every model, intraarticular autologous[86-88] or allogeneic[51,63,89] cMSC injection demonstrated excellent regenerative effects, including hyaline-like cartilage regeneration, when compared with control groups.
| Source | Injection / trans-plantation | Combination use | Cell number (million) | Model | Evaluation method | Ref. |
| Bone marrow | Single intraarticular, autologous | - | 7-8 | Partial thickness articular cartilage defect | Histology | El-Tookhy et al[86], 2008 |
| Adipose tissue | Single intraarticular, autologous | - | 7-8 | Partial thickness articular cartilage defect | Morphology, histology, fluorescence analysis | Mokbel et al[87], 2011 |
| Adipose tissue | Four times intraarticular, allogeneic | - | 5 (three times), 66 (once) | Intact | Pain and lameness scoring, immunohistochemistry | Park et al[95], 2013 |
| Bone marrow | Transplantation with scaffold, allogeneic | Scaffold | 0.01 | Full-thickness cartilage defect | Histology, immunohistochemistry, micro CT | Duan et al[89], 2013 |
| Synovium | Single intraarticular, autologous | Hyaluronic acid | 0.05, 5 or 50 | Partial thickness articular cartilage defect | Histology | Miki et al[88], 2015 |
| Adipose tissue | Single intraarticular, allogeneic | Platelet-rich plasma | 10 | Cranial cruciate ligament transection | Lameness scoring, focal compression strength, extracellular matrix composition, histopathology, real-time PCR | Yun et al[90], 2016 |
| Bone marrow | Single intraarticular, allogeneic | Hyaluronic acid | 10 | Partial thickness articular cartilage defect | Gross appearance, magnetic resonance imaging, histology, immunohistochemistry | Li et al[63], 2018 |
| Umbilical cord | Single intraarticular, allogeneic | - | 1 | Surgical manipulation of articular cartilage | Magnetic resonance imaging, radiography, ultrasonography, blood test, scanning electron microscope | Zhang et al[51], 2018 |
El-Tookhy et al[86] reported that earlier injections of BM-cMSCs (i.e., one day after creating the chondral defect) resulted in hyaline cartilage regeneration in the defect area, whereas the therapeutic effect of a later injection (i.e., one month after creating the chondral defect) was limited to fibro-hyaline cartilage regeneration[86]. Similar results were reported for AD-cMSCs[87]. These findings suggest that injured tissue may express specific receptors or ligands that promote the adhesion or infiltration of MSCs to the defect site in the early phase of injury and that these receptors or ligands may be down-regulated as time progresses after the injury.
Mokbel et al[87] showed the homing of cMSCs in dog stifle joints with a partial thickness chondral defect after intraarticular injection of autologous AD-cMSCs labelled with green fluorescent protein. They detected labelled cells in the neocartilage at 8 wk after the cMSC injection[87]. Koga et al[91] showed that human synovial MSCs attached to the chondral defect in knee joints of humans and rabbits after placement of an MSC suspension on the defect for 10 min, and the MSCs that attached to the chondral defect expressed several adhesion molecules[91]. Injected MSCs could also be detected in the neocartilage tissue at 8 wk after injection in a rabbit osteochondral defect model, however, no labeled MSCs could be detected at 12 wk[92]. Most of the MSCs injected into synovial joints migrate into the synovial membrane, in addition to the articular cartilage, in rat OA models[93] and in normal or OA horses[94]. After migration to the synovium, the MSCs maintain their MSC properties, without differentiating into another lineage, for at least 28 d. Meanwhile, they secrete several trophic factors, such as PRG-4, BMPs, and TSG-6, which are the key trophic factors for chondroprotection and immunosuppression[93]. Thus, in early phase of cartilage injury, intraarticularly injected MSCs may migrate into the chondral defect and regenerate neocartilage tissue, while most of the residual MSCs migrate into the synovium in the joints and produce trophic factors that provide chondro-protective and immunosuppressive effects. The immunomodulatory effects of cMSCs in dog joints have also been confirmed in several studies[51,90,95].
Some studies have demonstrated greater repair effects, determined by histological evaluation of chondral defects, for intraarticular injections of cMSCs in combination with either hyaluronic acid[63,88] or platelet-rich plasma[90] when compared with injection of cMSCs alone. Therefore, some synergistic effects of cMSCs are expected when used in combination with chondroprotective materials.
Conventional OA therapies in dogs include drug therapy, weight loss regimens, exercise programs, nutraceuticals, and supplements[96]. The objectives of these treatment methods are to minimize joint pain, reduce inflammation, and restrain the progression of the cartilage damage; however, they often do not provide complete pain relief[27,97,98]. MSCs are a promising therapeutic tool for OA as they can secrete trophic factors[93,99], recruit endogenous cells to the damaged lesion[100,101], and differentiate into chondrocytes[102], thereby ameliorating the cartilage injury. Several clinical studies have utilized cMSCs for OA in dogs, with most treating OA with AD-cMSCs (Table 4).
| Source | Injection | Cell number (million) | Target joint | Observation period | Evaluation points | Ref. |
| Adipose tissue | Single intraarticular, autologous | 4.2-5 | Hip | 30, 60, 90 d | Lameness, pain, range of motion, functional disability, owner questionnaire | Black et al[121], 2007 |
| Adipose tissue | Single intraarticular, autologous | 3-5 | Elbow | 30, 60, 90, 180 d | Lameness, pain, range of motion, functional disability, owner questionnaire | Black et al[107], 2008 |
| Adipose tissue | Single intraarticular with platelet-rich plasma or hyaluronic acid, autologous | 3-5 | Elbow | 1 wk, 1 mo | Not described | Guercio et al[103], 2012 |
| Adipose tissue | Single intraarticular with platelet-rich plasma, autologous | Over 30 | Hip | 0, 30, 90, 180 d | Gait analysis | Vilar et al[104], 2013 |
| Adipose tissue | Single intraarticular, autologous | 30 | Hip | 1, 3, 6 mo | Pain, functional disability, range of motion, owner questionnaire | Cuervo et al[122], 2014 |
| Adipose tissue | Single intraarticular, autologous | 15 | Hip | 0, 30, 90, 180 d | Gait analysis | Vilar et al[108], 2014 |
| Adipose tissue | Acupoint injection, allogeneic | 0.2-0.8 | Hip | 7, 15, 30 d | Pain, functional disability, range of motion | Marx et al[106], 2014 |
| Adipose tissue | Single intraarticular, allogeneic | 12 | Hip, elbow, stifle, shoulder | 60 d | Pain, owner questionnaire | Harman et al[123], 2016 |
| Bone marrow | Single intraarticular and single intravenous, autologous | 5 for intraarticular, 2 for intravenous | Stifle | 4, 8 wk | Circulating T lymphocyte, C reactive protein and cytokine concentration, total cell count in synovial fluid | Muir et al[110], 2016 |
| Adipose tissue | Single intraarticular with hyaluronic acid, allogeneic | 12 | Elbow | 6, 9, 12 mo | Owner questionnaire, arthroscopic image, histology | Kriston-Pál et al[105], 2017 |
| Adipose tissue | Single intraarticular and/or single intravenous, allogeneic | Not described | Various joints | 10 wk | Lameness, pain, range of motion, functional disability | Shah et al[124], 2018 |
Dogs in actual conditions of OA can serve as vulnerable translational animal models for human medicine in terms of the use of MSCs. Each cMSC study has demonstrated improvement in OA symptoms after single intraarticular, intravenous, or acupuncture point injections of cMSCs. Some studies have injected cMSCs in combination with platelet-rich plasma[103,104] or hyaluronic acid[105]. Improvements in pain, lameness, and range of motion were observed from 1 week[103,106] after the cMSC injection up to 180 d after cMSC injection alone[107] or up to 12 mo after cMSC injection in combination with hyaluronic acid[105]. However, one report showed a decrease in the effects of cMSCs between 30 and 90 d after the cMSC intraarticular injection[108]. Since the number of cells is known to decrease rapidly after administration of MSCs into the stifle joint[93], longer lasting effects of cMSCs might be obtained by periodic injections.
Most of the clinical studies have used subjective evaluation methods, including pain, lameness, range of motion, and functional disability scoring or owner questionnaires, while two studies provided objective evaluations with gait analysis using a force platform[104,108]. Assessing pain in an objective manner is important, especially in nonverbal patients, and gait analysis with a force platform is a valuable method that offers repeatable and quantitative measurements of ground reaction force from the feet of the dog[109]. However, the objective measures cannot provide information on some features, such as activity level, reluctance to run in dogs, and regeneration of damaged articular cartilage. Therefore, multi-aspect evaluation is needed for the measurement of cMSC effects. One study by Kriston-Pál et al[105] utilized arthroscopic and histological evaluation in addition to owners’ questionnaires and demonstrated that the repaired articular cartilage in the cMSC-treated joint consisted of hyaline-like cartilage.
The immunomodulatory effect of BM-cMSCs was demonstrated by Muir et al[110] for dog stifles with cranial cruciate ligament rupture. Intraarticular and intravenous injection of autologous BM-cMSCs in dogs with partial rupture of the cranial cruciate ligament suppressed systemic and stifle joint inflammation, including C-reactive protein concentrations and circulating CD8+ T lymphocyte number.
No serious adverse events have been reported in the clinical studies using intraarticular injection, acupuncture point injection, and intravenous injection of cMSCs. Nevertheless, Kang et al[111] indicated that the development of pulmonary edema and hemorrhage are possible adverse reactions following intravenous injections of cMSCs. Therefore, dogs treated with cMSC transplantation should be observed cautiously after injection.
This is the first review of cMSC isolation, characterization, differentiation, and use in in vivo models for cartilage repair and clinical trial for cartilage diseases. The cMSCs can be isolated from various tissues in the body, and the cMSCs derived from each tissue have specific characteristics. Although the cMSCs used in most studies at least partially meet the criteria for human MSCs declared by ISCT, suitable surface epitopes for cMSCs have not yet been established. cMSCs can differentiate into adipocytes, osteocytes, and chondrocytes in vitro; however, the differentiation methods differ among studies, and some studies have reported difficulties in inducing chondrogenic differentiation. Thus, suitable differentiation protocols for cMSCs should be identified to ensure sufficient differentiation in in vitro studies.
Dogs can serve as an experimental animal model for studies on cartilage repair, and cMSCs can lead to hyaline cartilage repair of experimentally created cartilage defects in vivo. Although dogs have a variety of unique characteristics compared to other experimental animals, they also share some problems, including body size and ethical concerns. For these reasons, farm animals, including pigs, sheep, and goats, can supersede dogs as experimental animal models for human medicine.
One distinctive feature of dogs is that many dogs naturally develop OA during their lives, which means that clinical trials can be performed. The results of clinical studies have proved the great cartilage regenerative and immunomodulatory effects of cMSCs in veterinary patient dogs with OA. Since the current therapeutic objective for OA treatment in both human and veterinary patients is management of pain and functional recovery of joints[112], the results of clinical studies in dogs can provide significant information for the treatment of cartilage lesions in both human and veterinary medicine. However, it should be noted that cell therapies in veterinary patients are not strictly supervised by regulatory agencies in most countries[113]. Regulations and guidelines for the use of MSCs in veterinary medicine are still urgently required. In addition, dogs cannot communicate the presence or degree of pain in diagnostic or therapeutic effect measurement phases, so caution is required in interpreting the results of studies using veterinary MSCs.
In stifle joints, meniscus damage is also known to induce and progress carti-laginous damage in dogs and humans[114-116], and the meniscal tear is one of the most prevalent injuries in the stifle joint[115,117]. Therefore, the effectiveness of cMSC treatments for meniscal injury as well as chondral lesions is expected to be elucidated in the future.
In recent years, expectations have increased regarding the use of MSCs in both human and veterinary medicine, and many in vitro or in vivo studies support the therapeutic effects of cMSCs for treatment of cartilage lesions and OA in dogs. Dogs can serve as clinical models as well as experimental animal models for human medicine, while they themselves, as veterinary patients, require effective treatment methods for cartilage lesions and OA. Thus, despite several limitations and problems, including ethical issues and difficulty of effect measurement, studies of MSCs in dogs have great significance from various points of view. Regulations and guidelines for cMSCs should be established in the future, and standardized methods for cMSC usage would provide more unified and reliable results from the studies. More data on cMSC characteristics and their use as an OA treatment in dogs will be needed, and they must be meaningful for the improvement of cartilage repair treatment in both human and veterinary medicine.
We thank Ms. Ellen Roider for English editing.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bolshakova GB, Ding J S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | Bonnevie ED, Galesso D, Secchieri C, Bonassar LJ. Degradation alters the lubrication of articular cartilage by high viscosity, hyaluronic acid-based lubricants. J Orthop Res. 2018;36:1456-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Shaktivesh, Malekipour F, Lee PVS. Shock absorbing ability in healthy and damaged cartilage-bone under high-rate compression. J Mech Behav Biomed Mater. 2019;90:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Armiento AR, Stoddart MJ, Alini M, Eglin D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018;65:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 398] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 4. | Lo Monaco M, Merckx G, Ratajczak J, Gervois P, Hilkens P, Clegg P, Bronckaers A, Vandeweerd JM, Lambrichts I. Stem cells for cartilage repair: preclinical studies and insights in translational animal models and outcome measures. Stem Cells Int. 2018;2018:9079538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Hunziker EB, Lippuner K, Keel MJ, Shintani N. An educational review of cartilage repair: precepts & practice--myths & misconceptions--progress & prospects. Osteoarthritis Cartilage. 2015;23:334-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 6. | Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015-2040. Arthritis Rheumatol. 2016;68:1582-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 395] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 7. | Anderson KL, O'Neill DG, Brodbelt DC, Church DB, Meeson RL, Sargan D, Summers JF, Zulch H, Collins LM. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci Rep. 2018;8:5641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Orth P, Gao L, Madry H. Microfracture for cartilage repair in the knee: a systematic review of the contemporary literature. Knee Surg Sports Traumatol Arthrosc. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 9. | Wang D, Chang B, Coxe FR, Pais MD, Wickiewicz TL, Warren RF, Rodeo SA, Williams RJ. Clinically meaningful improvement after treatment of cartilage defects of the knee with osteochondral grafts. Am J Sports Med. 2019;47:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Zhang C, Cai YZ, Lin XJ. Autologous chondrocyte implantation: is it likely to become a saviour of large-sized and full-thickness cartilage defect in young adult knee? Knee Surg Sports Traumatol Arthrosc. 2016;24:1643-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Green CJ, Beck A, Wood D, Zheng MH. The biology and clinical evidence of microfracture in hip preservation surgery. J Hip Preserv Surg. 2016;3:108-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Redondo ML, Naveen NB, Liu JN, Tauro TM, Southworth TM, Cole BJ. Preservation of knee articular cartilage. Sports Med Arthrosc Rev. 2018;26:e23-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Andrade R, Vasta S, Pereira R, Pereira H, Papalia R, Karahan M, Oliveira JM, Reis RL, Espregueira-Mendes J. Knee donor-site morbidity after mosaicplasty - a systematic review. J Exp Orthop. 2016;3:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Mennan C, Garcia J, McCarthy H, Owen S, Perry J, Wright K, Banerjee R, Richardson JB, Roberts S. Human articular chondrocytes retain their phenotype in sustained hypoxia while normoxia promotes their immunomodulatory potential. Cartilage. 2018;1947603518769714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Davies RL, Kuiper NJ. Regenerative medicine: a review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering (Basel). 2019;6:pii: E22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 16. | Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, Tenen A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Musculoskelet Disord. 2016;17:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 17. | Reissis D, Tang QO, Cooper NC, Carasco CF, Gamie Z, Mantalaris A, Tsiridis E. Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert Opin Biol Ther. 2016;16:535-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Shepherd DE, Seedhom BB. Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis. 1999;58:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 364] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Eckstein F, Reiser M, Englmeier KH, Putz R. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging--from image to data, from data to theory. Anat Embryol (Berl). 2001;203:147-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Moran CJ, Ramesh A, Brama PA, O'Byrne JM, O'Brien FJ, Levingstone TJ. The benefits and limitations of animal models for translational research in cartilage repair. J Exp Orthop. 2016;3:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Verpaalen VD, Baltzer WI, Smith-Ostrin S, Warnock JJ, Stang B, Ruaux CG. Assessment of the effects of diet and physical rehabilitation on radiographic findings and markers of synovial inflammation in dogs following tibial plateau leveling osteotomy. J Am Vet Med Assoc. 2018;252:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Gustås P, Pettersson K, Honkavaara S, Lagerstedt AS, Byström A. Kinematic and spatiotemporal assessment of habituation to treadmill walking in Labrador retrievers. Acta Vet Scand. 2016;58:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Music E, Futrega K, Doran MR. Sheep as a model for evaluating mesenchymal stem/stromal cell (MSC)-based chondral defect repair. Osteoarthritis Cartilage. 2018;26:730-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Cope PJ, Ourradi K, Li Y, Sharif M. Models of osteoarthritis: the good, the bad and the promising. Osteoarthritis Cartilage. 2019;27:230-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 25. | Knazovicky D, Helgeson ES, Case B, Gruen ME, Maixner W, Lascelles BD. Widespread somatosensory sensitivity in naturally occurring canine model of osteoarthritis. Pain. 2016;157:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Hoffman AM, Dow SW. Concise Review: Stem Cell Trials Using Companion Animal Disease Models. Stem Cells. 2016;34:1709-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Gagnon A, Brown D, Moreau M, Lussier B, Otis C, Troncy E. Therapeutic response analysis in dogs with naturally occurring osteoarthritis. Vet Anaesth Analg. 2017;44:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Brimmo OA, Bozynski CC, Cook CR, Kuroki K, Sherman SL, Pfeiffer FM, Stoker AM, Cook JL. Subchondroplasty for the treatment of post-traumatic bone marrow lesions of the medial femoral condyle in a pre-clinical canine model. J Orthop Res. 2018;36:2709-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Ren J, Ma J, Zhang X, Aimaiti A, Saiyiti M, Chen Y, Cao L. Diagnostic value of combined serum marker changes and quantitative MRI evaluation of cartilage volume of tibial plateau in a surgically-induced osteoarthritis dog model. J Int Med Res. 2017;45:2023-2035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Egan P, Murphy S, Jovanovik J, Tucker R, Fitzpatrick N. Treatment of osteochondrosis dissecans of the canine stifle using synthetic osteochondral resurfacing. Vet Comp Orthop Traumatol. 2018;31:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Cachon T, Frykman O, Innes JF, Lascelles BDX, Okumura M, Sousa P, Staffieri F, Steagall PV, Van Ryssen B; COAST Development Group. Face validity of a proposed tool for staging canine osteoarthritis: Canine OsteoArthritis Staging Tool (COAST). Vet J. 2018;235:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-390. [PubMed] |
| 33. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5763] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 34. | Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 613] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 35. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3365] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 36. | Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003;196-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 37. | De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 38. | Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 514] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 39. | Khalilifar MA, Baghaban Eslaminejad MR, Ghasemzadeh M, Hosseini S, Baharvand H. In vitro and in vivo comparison of different types of rabbit mesenchymal stem cells for cartilage repair. Cell J. 2019;21:150-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Sasaki A, Mizuno M, Ozeki N, Katano H, Otabe K, Tsuji K, Koga H, Mochizuki M, Sekiya I. Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS One. 2018;13:e0202922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 41. | Quimby JM, Borjesson DL. Mesenchymal stem cell therapy in cats: Current knowledge and future potential. J Feline Med Surg. 2018;20:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Bharti D, Shivakumar SB, Subbarao RB, Rho GJ. Research Advancements in Porcine Derived Mesenchymal Stem Cells. Curr Stem Cell Res Ther. 2016;11:78-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Zayed M, Caniglia C, Misk N, Dhar MS. Donor-matched comparison of chondrogenic potential of equine bone marrow- and synovial fluid-derived mesenchymal stem cells: implications for cartilage tissue regeneration. Front Vet Sci. 2017;3:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Fellows CR, Matta C, Zakany R, Khan IM, Mobasheri A. Adipose, bone marrow and synovial joint-derived mesenchymal stem cells for cartilage repair. Front Genet. 2016;7:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 45. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 46. | Csaki C, Matis U, Mobasheri A, Ye H, Shakibaei M. Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study. Histochem Cell Biol. 2007;128:507-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Zhang S, Zhao C, Liu S, Wang Y, Zhao Y, Guan W, Zhu Z. Characteristics and multilineage differentiation of bone marrow mesenchymal stem cells derived from the Tibetan mastiff. Mol Med Rep. 2018;18:2097-2109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010;19:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 49. | Screven R, Kenyon E, Myers MJ, Yancy HF, Skasko M, Boxer L, Bigley EC, Borjesson DL, Zhu M. Immunophenotype and gene expression profile of mesenchymal stem cells derived from canine adipose tissue and bone marrow. Vet Immunol Immunopathol. 2014;161:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Kang BJ, Ryu HH, Park SS, Koyama Y, Kikuchi M, Woo HM, Kim WH, Kweon OK. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton's jelly for treating bone defects. J Vet Sci. 2012;13:299-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Zhang BY, Wang BY, Li SC, Luo DZ, Zhan X, Chen SF, Chen ZS, Liu CY, Ji HQ, Bai YS, Li DS, He Y. Evaluation of the curative effect of umbilical cord mesenchymal stem cell therapy for knee arthritis in dogs using imaging technology. Stem Cells Int. 2018;2018:1983025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Kisiel AH, McDuffee LA, Masaoud E, Bailey TR, Esparza Gonzalez BP, Nino-Fong R. Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vet Res. 2012;73:1305-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 53. | Bearden RN, Huggins SS, Cummings KJ, Smith R, Gregory CA, Saunders WB. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: a donor-matched comparative study. Stem Cell Res Ther. 2017;8:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 54. | Wijekoon HMS, Toyota K, Kim S, Fang J, Bwalya EC, Hosoya K, Okumura M. Differentiation potential of synoviocytes derived from joints with cranial cruciate ligament rupture and medial patella luxation in dogs. Res Vet Sci. 2017;114:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Krawetz RJ, Wu YE, Martin L, Rattner JB, Matyas JR, Hart DA. Synovial fluid progenitors expressing CD90+ from normal but not osteoarthritic joints undergo chondrogenic differentiation without micro-mass culture. PLoS One. 2012;7:e43616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Reich CM, Raabe O, Wenisch S, Bridger PS, Kramer M, Arnhold S. Isolation, culture and chondrogenic differentiation of canine adipose tissue- and bone marrow-derived mesenchymal stem cells--a comparative study. Vet Res Commun. 2012;36:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Russell KA, Chow NH, Dukoff D, Gibson TW, LaMarre J, Betts DH, Koch TG. Characterization and immunomodulatory effects of canine adipose tissue- and bone marrow-derived mesenchymal stromal cells. PLoS One. 2016;11:e0167442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 58. | Guercio A, Di Bella S, Casella S, Di Marco P, Russo C, Piccione G. Canine mesenchymal stem cells (MSCs): characterization in relation to donor age and adipose tissue-harvesting site. Cell Biol Int. 2013;37:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | Volk SW, Wang Y, Hankenson KD. Effects of donor characteristics and ex vivo expansion on canine mesenchymal stem cell properties: implications for MSC-based therapies. Cell Transplant. 2012;21:2189-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Bertolo A, Schlaefli P, Malonzo-Marty C, Baur M, Pötzel T, Steffen F, Stoyanov J. Comparative characterization of canine and human mesenchymal stem cells derived from bone marrow. Int J Stem Cell Res Ther. 2015;2:1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Endo K, Fujita N, Nakagawa T, Nishimura R. Effect of fibroblast growth factor-2 and serum on canine mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 63. | Li L, Duan X, Fan Z, Chen L, Xing F, Xu Z, Chen Q, Xiang Z. Mesenchymal stem cells in combination with hyaluronic acid for articular cartilage defects. Sci Rep. 2018;8:9900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 64. | Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet Res. 2012;8:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 65. | Martinello T, Bronzini I, Maccatrozzo L, Mollo A, Sampaolesi M, Mascarello F, Decaminada M, Patruno M. Canine adipose-derived-mesenchymal stem cells do not lose stem features after a long-term cryopreservation. Res Vet Sci. 2011;91:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Kocan B, Maziarz A, Tabarkiewicz J, Ochiya T, Banaś-Ząbczyk A. Trophic activity and phenotype of adipose tissue-derived mesenchymal stem cells as a background of their regenerative potential. Stem Cells Int. 2017;2017:1653254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 67. | Mo M, Wang S, Zhou Y, Li H, Wu Y. Mesenchymal stem cell subpopulations: phenotype, property and therapeutic potential. Cell Mol Life Sci. 2016;73:3311-3321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 68. | Neupane M, Chang CC, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng Part A. 2008;14:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Allas L, Boumédiene K, Baugé C. Epigenetic dynamic during endochondral ossification and articular cartilage development. Bone. 2019;120:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Omar SNC, Goh BS, Ubaidah MA, Khairoji KA, Sulaiman S, Saim L, Chua KH. Transforming growth factor beta 3 induced human adipose-derived stem cells for auricular chondrogenesis. Sains Malays. 2018;47:2349-2358. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 71. | Garcia J, Mennan C, McCarthy HS, Roberts S, Richardson JB, Wright KT. Chondrogenic potency analyses of donor-matched chondrocytes and mesenchymal stem cells derived from bone marrow, infrapatellar fat pad, and subcutaneous fat. Stem Cells Int. 2016;2016:6969726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1089] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 73. | Bertolo A, Steffen F, Malonzo-Marty C, Stoyanov J. Canine mesenchymal stem cell potential and the importance of dog breed: implication for cell-based therapies. Cell Transplant. 2015;24:1969-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 74. | Van de Walle A, Faissal W, Wilhelm C, Luciani N. Role of growth factors and oxygen to limit hypertrophy and impact of high magnetic nanoparticles dose during stem cell chondrogenesis. Comput Struct Biotechnol J. 2018;16:532-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Augustyniak E, Trzeciak T, Richter M, Kaczmarczyk J, Suchorska W. The role of growth factors in stem cell-directed chondrogenesis: a real hope for damaged cartilage regeneration. Int Orthop. 2015;39:995-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 76. | Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 77. | Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016;12:203-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 609] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 78. | Volk SW, Diefenderfer DL, Christopher SA, Haskins ME, Leboy PS. Effects of osteogenic inducers on cultures of canine mesenchymal stem cells. Am J Vet Res. 2005;66:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Nagase T, Muneta T, Ju YJ, Hara K, Morito T, Koga H, Nimura A, Mochizuki T, Sekiya I. Analysis of the chondrogenic potential of human synovial stem cells according to harvest site and culture parameters in knees with medial compartment osteoarthritis. Arthritis Rheum. 2008;58:1389-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Ogata Y, Mabuchi Y, Yoshida M, Suto EG, Suzuki N, Muneta T, Sekiya I, Akazawa C. Purified human synovium mesenchymal stem cells as a good resource for cartilage regeneration. PLoS One. 2015;10:e0129096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 81. | Shen C, Jiang T, Zhu B, Le Y, Liu J, Qin Z, Chen H, Zhong G, Zheng L, Zhao J, Zhang X. In vitro culture expansion impairs chondrogenic differentiation and the therapeutic effect of mesenchymal stem cells by regulating the unfolded protein response. J Biol Eng. 2018;12:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Jiang T, Xu G, Wang Q, Yang L, Zheng L, Zhao J, Zhang X. In vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis. 2017;8:e2851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 83. | Bwalya EC, Wijekoon HS, Fang J, Kim S, Hosoya K, Okumura M. Independent chondrogenic potential of canine bone marrow-derived mesenchymal stem cells in monolayer expansion cultures decreases in a passage-dependent pattern. J Vet Med Sci. 2018;80:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Sekiya I, Colter DC, Prockop DJ. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun. 2001;284:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 85. | Solchaga LA, Penick K, Goldberg VM, Caplan AI, Welter JF. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2010;16:1009-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 86. | El-Tookhy, Abou Elkheir W, Mokbel A, Osman A. Intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Egypt Rheumatol. 2008;30:1-10. |
| 87. | Mokbel A, El-Tookhy O, Shamaa AA, Sabry D, Rashed L, Mostafa A. Homing and efficacy of intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Clin Exp Rheumatol. 2011;29:275-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Miki S, Takao M, Miyamoto W, Matsushita T, Kawano H. Intra-articular injection of synovium-derived mesenchymal stem cells with hyaluronic acid can repair articular cartilage defects in a canine model. J Stem Cell Res Ther. 2015;5:1000314. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Duan X, Zhu X, Dong X, Yang J, Huang F, Cen S, Leung F, Fan H, Xiang Z. Repair of large osteochondral defects in a beagle model with a novel type I collagen/glycosaminoglycan-porous titanium biphasic scaffold. Mater Sci Eng C Mater Biol Appl. 2013;33:3951-3957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Yun S, Ku SK, Kwon YS. Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. J Orthop Surg Res. 2016;11:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 91. | Koga H, Shimaya M, Muneta T, Nimura A, Morito T, Hayashi M, Suzuki S, Ju YJ, Mochizuki T, Sekiya I. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther. 2008;10:R84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 92. | Harada Y, Nakasa T, Mahmoud EE, Kamei G, Adachi N, Deie M, Ochi M. Combination therapy with intra-articular injection of mesenchymal stem cells and articulated joint distraction for repair of a chronic osteochondral defect in the rabbit. J Orthop Res. 2015;33:1466-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 93. | Ozeki N, Muneta T, Koga H, Nakagawa Y, Mizuno M, Tsuji K, Mabuchi Y, Akazawa C, Kobayashi E, Matsumoto K, Futamura K, Saito T, Sekiya I. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage. 2016;24:1061-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 94. | Grady ST, Britton L, Hinrichs K, Nixon AJ, Watts AE. Persistence of fluorescent nanoparticle-labelled bone marrow mesenchymal stem cells in vitro and after intra-articular injection. J Tissue Eng Regen Med. 2019;13:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Park SA, Reilly CM, Wood JA, Chung DJ, Carrade DD, Deremer SL, Seraphin RL, Clark KC, Zwingenberger AL, Borjesson DL, Hayashi K, Russell P, Murphy CJ. Safety and immunomodulatory effects of allogeneic canine adipose-derived mesenchymal stromal cells transplanted into the region of the lacrimal gland, the gland of the third eyelid and the knee joint. Cytotherapy. 2013;15:1498-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 96. | Bland SD. Canine osteoarthritis and treatments: a review. Vet Sci Dev. 2015;5:84-89. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 97. | Scott RM, Evans R, Conzemius MG. Efficacy of an oral nutraceutical for the treatment of canine osteoarthritis. A double-blind, randomized, placebo-controlled prospective clinical trial. Vet Comp Orthop Traumatol. 2017;30:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 98. | Belshaw Z, Asher L, Dean RS. The attitudes of owners and veterinary professionals in the United Kingdom to the risk of adverse events associated with using non-steroidal anti-inflammatory drugs (NSAIDs) to treat dogs with osteoarthritis. Prev Vet Med. 2016;131:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 99. | Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther. 2016;7:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 263] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 100. | Toh WS. The emerging role of mesenchymal stem cell secretome in cartilage regeneration. Front Stem Cell Regen Med Res. 2017;4:3-25. [DOI] [Full Text] |
| 101. | Nakagawa Y, Muneta T, Kondo S, Mizuno M, Takakuda K, Ichinose S, Tabuchi T, Koga H, Tsuji K, Sekiya I. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthritis Cartilage. 2015;23:1007-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 102. | Atesok K, Fu FH, Sekiya I, Stolzing A, Ochi M, Rodeo SA. Stem cells in degenerative orthopaedic pathologies: effects of aging on therapeutic potential. Knee Surg Sports Traumatol Arthrosc. 2017;25:626-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 103. | Guercio A, Di Marco P, Casella S, Cannella V, Russotto L, Purpari G, Di Bella S, Piccione G. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int. 2012;36:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 104. | Vilar JM, Morales M, Santana A, Spinella G, Rubio M, Cuervo B, Cugat R, Carrillo JM. Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet Res. 2013;9:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 105. | Kriston-Pál É, Czibula Á, Gyuris Z, Balka G, Seregi A, Sükösd F, Süth M, Kiss-Tóth E, Haracska L, Uher F, Monostori É. Characterization and therapeutic application of canine adipose mesenchymal stem cells to treat elbow osteoarthritis. Can J Vet Res. 2017;81:73-78. [PubMed] |
| 106. | Marx C, Silveira MD, Selbach I, da Silva AS, Braga LM, Camassola M, Nardi NB. Acupoint injection of autologous stromal vascular fraction and allogeneic adipose-derived stem cells to treat hip dysplasia in dogs. Stem Cells Int. 2014;2014:391274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 107. | Black LL, Gaynor J, Adams C, Dhupa S, Sams AE, Taylor R, Harman S, Gingerich DA, Harman R. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | Vilar JM, Batista M, Morales M, Santana A, Cuervo B, Rubio M, Cugat R, Sopena J, Carrillo JM. Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet Res. 2014;10:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 109. | Sharkey M. The challenges of assessing osteoarthritis and postoperative pain in dogs. AAPS J. 2013;15:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 110. | Muir P, Hans EC, Racette M, Volstad N, Sample SJ, Heaton C, Holzman G, Schaefer SL, Bloom DD, Bleedorn JA, Hao Z, Amene E, Suresh M, Hematti P. Autologous bone marrow-derived mesenchymal stem cells modulate molecular markers of inflammation in dogs with cruciate ligament rupture. PLoS One. 2016;11:e0159095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 111. | Kang MH, Park HM. Evaluation of adverse reactions in dogs following intravenous mesenchymal stem cell transplantation. Acta Vet Scand. 2014;56:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Cimino Brown D. What can we learn from osteoarthritis pain in companion animals? Clin Exp Rheumatol. 2017;35 Suppl 107:53-58. [PubMed] |
| 113. | Fortier LA, Travis AJ. Stem cells in veterinary medicine. Stem Cell Res Ther. 2011;2:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 114. | Kahn D, Mittelstaedt D, Matyas J, Qu X, Lee JH, Badar F, Les C, Zhuang Z, Xia Y. Meniscus induced cartilaginous damage and non-linear gross anatomical progression of early-stage osteoarthritis in a canine model. Open Orthop J. 2016;10:690-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Krier EM, Johnson TA, Breiteneicher AH, Peycke LE, Hulse DA. Articular cartilage lesions associated with complete lateral meniscal tears in the dog. Vet Surg. 2018;47:958-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 116. | Hart HF, Crossley KM, Felson D, Jarraya M, Guermazi A, Roemer F, Lewis CE, Torner J, Nevitt M, Stefanik JJ. Relation of meniscus pathology to prevalence and worsening of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2018;26:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 117. | Katano H, Koga H, Ozeki N, Otabe K, Mizuno M, Tomita M, Muneta T, Sekiya I. Trends in isolated meniscus repair and meniscectomy in Japan, 2011-2016. J Orthop Sci. 2018;23:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 118. | Sullivan MO, Gordon-Evans WJ, Fredericks LP, Kiefer K, Conzemius MG, Griffon DJ. Comparison of mesenchymal stem cell surface markers from bone marrow aspirates and adipose stromal vascular fraction sites. Front Vet Sci. 2016;2:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 119. | Kamishina H, Deng J, Oji T, Cheeseman JA, Clemmons RM. Expression of neural markers on bone marrow-derived canine mesenchymal stem cells. Am J Vet Res. 2006;67:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 120. | Hodgkiss-Geere HM, Argyle DJ, Corcoran BM, Whitelaw B, Milne E, Bennett D, Argyle SA. Characterisation and differentiation potential of bone marrow derived canine mesenchymal stem cells. Vet J. 2012;194:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 121. | Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA, Harman R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;8:272-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 122. | Cuervo B, Rubio M, Sopena J, Dominguez JM, Vilar J, Morales M, Cugat R, Carrillo JM. Hip osteoarthritis in dogs: a randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. Int J Mol Sci. 2014;15:13437-13460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 123. | Harman R, Carlson K, Gaynor J, Gustafson S, Dhupa S, Clement K, Hoelzler M, McCarthy T, Schwartz P, Adams C. A prospective, randomized, masked, and placebo-controlled efficacy study of intraarticular allogeneic adipose stem cells for the treatment of osteoarthritis in dogs. Front Vet Sci. 2016;3:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 124. | Shah K, Drury T, Roic I, Hansen P, Malin M, Boyd R, Sumer H, Ferguson R. Outcome of allogeneic adult stem cell therapy in dogs suffering from osteoarthritis and other joint defects. Stem Cells Int. 2018;2018:7309201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |