Published online Jan 26, 2018. doi: 10.4252/wjsc.v10.i1.1
Peer-review started: August 14, 2017
First decision: September 25, 2017
Revised: October 26, 2017
Accepted: November 27, 2017
Article in press: November 27, 2017
Published online: January 26, 2018
Processing time: 164 Days and 13.3 Hours
To establish a rat model of anal sphincter injury and test different systems to provide stem cells to injured area.
Adipose-derived stem cells (ASCs) were isolated from BDIX rats and were transfected with green fluorescent protein (GFP) for cell tracking. Biosutures (sutures covered with ASCs) were prepared with 1.5 x 106 GFP-ASCs, and solutions of 106 GFP-ASCs in normal saline were prepared for injection. Anorectal normal anatomy was studied on Wistar and BDIX female rats. Then, we designed an anal sphincter injury model consisting of a 1-cm extra-mucosal miotomy beginning at the anal verge in the anterior middle line. The sphincter lesion was confirmed with conventional histology (hematoxylin and eosin) and immunofluorescence with 4', 6-diamidino-2-phenylindole (commonly known as DAPI), GFP and α-actin. Functional effect was assessed with basal anal manometry, prior to and after injury. After sphincter damage, 36 BDIX rats were randomized to three groups for: (1) Cell injection without repair; (2) biosuture repair; and (3) conventional suture repair and cell injection. Functional and safety studies were conducted on all the animals. Rats were sacrificed after 1, 4 or 7 d. Then, histological and immunofluorescence studies were performed on the surgical area.
With the described protocol, biosutures had been covered with at least 820000-860000 ASCs, with 100% viability. Our studies demonstrated that some ASCs remained adhered after suture passage through the muscle. Morphological assessment showed that the rat anal anatomy is comparable with human anatomy; two sphincters are present, but the external sphincter is poorly developed. Anal sphincter pressure data showed spontaneous, consistent, rhythmic anal contractions, taking the form of “plateaus” with multiple twitches (peaks) in each pressure wave. These basal contractions were very heterogeneous; their frequency was 0.91-4.17 per min (mean 1.6980, SD 0.57698), their mean duration was 26.67 s and mean number of peaks was 12.53. Our morphological assessment revealed that with the aforementioned surgical procedure, both sphincters were completely sectioned. In manometry, the described activity disappeared and was replaced by a gentle oscillation of basal line, without a recognizable pattern. Surprisingly, these findings appeared irrespective of injury repair or not. ASCs survived in this potentially septic area for 7 d, at least. We were able to identify them in 84% of animals, mainly in the muscular section area or in the tissue between the muscular endings. ASCs formed a kind of “conglomerate” in rats treated with injections, while in the biosuture group, they wrapped the suture. ASCs were also able to migrate to the damaged zone. No relevant adverse events or mortality could be related to the stem cells in our study. We also did not find unexpected tissue growths.
The proposed procedure produces a consistent sphincter lesion. Biosutures and injections are suitable for cell delivery. ASCs survive and are completely safe in this clinical setting.
Core tip: Fecal incontinence is very frequent and associated with severe consequences for patients. Surgical treatment outcomes are not as good as they should be, mainly in the long term. Stem cells could improve these results, as demonstrated in other clinical settings. We report a simple rat model for experimental anal sphincter injury (a surgical section), and characterize it from morphological (conventional histology and immunofluorescence) and functional (anal manometry) points of view. Then, we describe two approaches for adipose-derived stem cell administration to the injured area (injection and biosutures) and demonstrate stem cell survival during at least 7 d, as well as their safety.
- Citation: Trébol J, Georgiev-Hristov T, Vega-Clemente L, García-Gómez I, Carabias-Orgaz A, García-Arranz M, García-Olmo D. Rat model of anal sphincter injury and two approaches for stem cell administration. World J Stem Cells 2018; 10(1): 1-14
- URL: https://www.wjgnet.com/1948-0210/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v10.i1.1
Fecal incontinence is a very prevalent nonfatal illness associated with considerable embarrassment, very relevant psychosocial repercussions (disability, anxiety, depression, social isolation and job loss; it is the second cause of institutionalization, etc.) and poor quality of life. It is estimated to affect 11%-15% of adults[1] as well as 2.2% of the general population and 47% of institutionalized people[2], but its real prevalence is probably much higher[3].
This condition also has a very important economic impact, consisting of direct (diagnostic test, treatments, etc.) and indirect (labor production, secondary treatments, etc.) costs. In a Seattle study, annual healthcare costs augmented significantly in multivariate analysis, up to $2897 of 2005 (pads, barriers or institutionalization costs were not included)[4]. In a Dutch study, global costs grew €2169 yearly for each patient[5].
Fecal incontinence is a multifactorial disease. The most frequent morphological alteration is a sphincter lesion, found in almost 60% of patients, most of them obstetric (30%-40%). Sphincter lesions during delivery ranges from 11%[6] to 26.9%[7] and cause incontinence in 76.8%-82.8% of patients[7].
Although in the last years sacral neuromodulation has been growing exponentially, surgery remains the treatment of choice for the most severe or refractory cases, mainly if sphincter lesions are present. Sphincter repair is the most frequently performed and successful technique for traumatic lesions. Sphincter repair has good results in the short term, being excellent to good in 66%, moderate in 22% and poor in 12% of patients[8]. But this outcome does not persist in the long term[9] for reasons not well understood.
Stem cell (SC) therapy has demonstrated promising results in a wide variety of clinical settings. Application of adipose-derived stem cells (ASCs), one type of mesenchymal stem/stromal cells (MSCs), is a novel approach for enhancing regeneration or repair of damaged tissues[10-12]. ASCs have been tried in environments particularly unfavorable for healing, such as experimental colitis[13], sepsis[14], anal fistula[15] and Crohn´s[16], with promising results. ASCs have important proliferation and differentiation capabilities but also immunoregulatory and antiinflammatory properties[17]. ASCs are isolated easily from subcutaneous fat, in a process that yields 100 times more SCs than bone marrow aspirates[18].
Our research group has been working with ASCs since 2002 in the clinical and experimental setting and has been a pioneer on their use in digestive fistulizing diseases[15] and other conditions. We have conducted three clinical trials (phases I, II and III)[19-21] with autologous ASCs and one with allogeneic (phase I-IIa)[22], and participated in two with allogeneic[23,24]. All these trials included more than 400 treated patients. Sutures covered with ASCs (named “biosutures”) were designed and tested on colonic[25-26] and tracheal experimental anastomosis[27]. All these studies, and many others, have proven ASCs to be safe and possibly effective.
Our aim was to test ASCs for fecal incontinence in a murine model prior to study of their use in humans. The first step was to design an easy and reproducible model for sphincter injury, and the second to test two different ASCs administration systems.
We used 50 adult female BDIX rats (selected for cell therapy because they are syngenic to mimic an autologous application), weighing 170–260 g and aged 16-24 wk (provided by Charles River Laboratories International, Inc., Wilmington, MA, United States), and 10 adult female Wistar rats (bred at the animal facility in the University Hospital La Paz, Madrid, Spain). Two days before surgery, animals were transferred to individual cages. They had free access to water and a standard diet (Panlab S.L.) and were housed in a restricted access room, with controlled temperature (23 °C) and a 12-h light/12-h dark cycle.
Ten Wistar and fourteen BDIX rats were employed for anatomy studies, surgical damage model creation, obtaining ASCs, functional assays, and anesthetic dosage adjustments. Thirty-six BDIX rats were used for cell therapy and functional studies.
The present study was performed in accordance with the European Union guidelines for reducing pain and discomfort to experimental animals (86/609/CEE). Animals surpassed all sanitary controls. The study protocol was approved by the Ethical Committee for Animal Welfare of University Hospital La Paz, Madrid, Spain.
For anesthesia induction, we used isoflurane (Forane®; Abbott Laboratories, Abbott Park, IL, United States) in oxygen (3 lpm O2 and 5 lpm Forane® for induction and 1.5–2 lpm for maintenance). For functional studies, an intraperitoneal mixture of ketamine (Ketalar®; Pfizer, New York, NY, United States) and xylazine 2% (Xilagesic®; Calier, Buenos Aires, Argentina) was selected following Wang et al[28] and Zutshi et al[29]. We applied lower doses than those used in the mentioned studies: 50 mg/kg ketamine and 5 mg/kg xylazine for surgery with functional registry, and 30 mg/kg plus 3 mg/kg for functional study alone.
For surgery, standard microsurgical equipment, a 10 × to 40 × magnifying lens and 0.5 mL syringes with 30 G (0.3 mm) and 8 mm needles (BD Micro-FineTM; Becton Dickinson, Franklin Lakes, NJ, United States) for injecting ASCs were used. For suturing, 6/0 polyglactin 910 sutures with cylindrical needle (½ circumference and 17 mm length) were used (Vycril®; Ethicon, Johnson and Johnson, Somerville, NJ, United States).
We performed basal anal manometry, modifying the model pioneered by Vinograd et al[30] and used by Wang et al[28] and Zutshi et al[29]. A 0.4 mL latex balloon (Kent Scientific Corp., Torrington, CT, United States) was connected through a rigid tube to two 3-way stopcocks; one of them had a free opening to set 0 values, and the other one was connected through one side to an infusion pump (that filled the system with normal saline solution) and to a Millar SPR-524 pressure transducer (Millar Inc., Houston, TX, United States) through the other side. The transducer was then connected to an amplifier (ML224) and this to a digital pulse detecting system (Powerlab 4/30) with real time recording software (Chart v5.5); these three elements were from ADInstruments, Sidney, Australia.
ASCs were obtained from the subcutaneous fat tissue according to a previously described protocol in humans by Zuk et al[31], based on their adherence to culture, with some minor modifications. Stromal vascular fraction (SVF) was obtained and digested with type I collagenase (0.075%; Gibco®Thermo Fisher Scientific, Waltham, MA, United States). The digested tissue was centrifuged and the pellet was resuspended in 0.16 M NH4Cl for erythrocyte lysis. Then, the cells were separated by filtering the product through a 70-μm nylon mesh, plated in culture dishes, and cultured at 37 °C in a humid atmosphere (90%-95%) with 5% carbon dioxide in the Gibco™ Dulbecco’s Modified Eagle’s Medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, United States) containing 10% phosphate buffered saline (PBS), 2 mmol/L glutamine, and 1% penicillin–streptomycin. Nonadherent cells were removed and subcultured 24 h after seeding; these were the ASCs.
In order to identify ASCs in animal tissue samples, we marked them. In the third subculture at 60% to 70% confluence, ASCs were infected with enhanced green fluorescent protein (eGFP) transducing lentivirus (CNIC, Madrid, Spain). Sorting of selected eGFP-ASCs (99.6% positive) was applied after another 2 to 3 passages.
Cell cultures were analyzed by four-color flow cytometry using a FACS Calibur (Becton Dickinson Biosciences–BDB, San José, CA, United States) after staining with fluorochrome (Alexa Fluor 647; Thermo Fisher Scientific) conjugated with two positive markers: anti-CD90 (BDB) and anti-CD29 (Millipore, Burlington, MA, United States) and two negative markers: anti-CD45 (BDB) and anti-CD11b (BDB). With these four markers and the confirmed plastic adherent capability, our cells fulfill at least two out of three minimal internationally-decided defining criteria[32,33]. Differentiation capability was proven in prior experiments with the same isolation protocol.
Biosutures were obtained by culturing 30 cm 6/0 polyglactin 910 sutures with 1.5 × 106 eGFP-ASCs on ultra-low attachment plates (P6 ULA, Costar®; Corning, Corning, NY, United States) over 72 h, according to the published protocol with minor changes[25]. Then, the sutures were washed with saline and used. Cell viability was evaluated by trypan blue. Cell adherence to suture and needle was previously confirmed by fluorescence and electron microscopy[25,27], and cell density significantly decreased after two stitches in muscle tissue[27]. Thus, we decided to use each biosuture for only two stitches.
We performed some studies to calculate the real cell dose adhered to sutures and studied with immunofluorescence for several biosutures after their use, looking for cells, and then counted adhered cells in the last centimeter of the remaining suture.
eGFP-ASCs (106 cells) diluted in 50 μL normal saline were applied in each case.
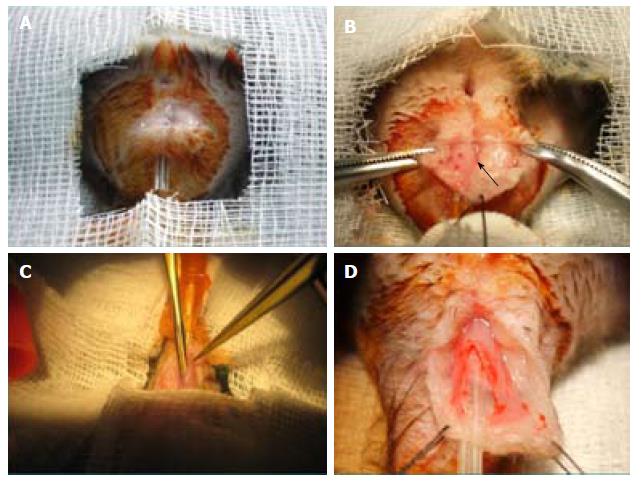
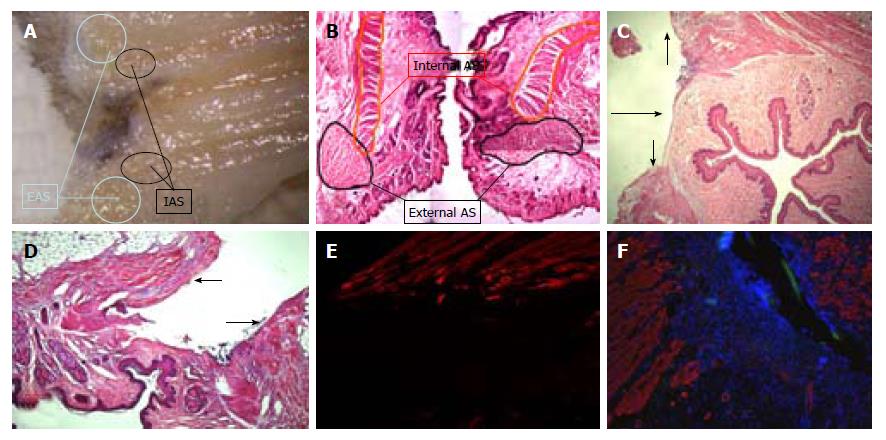
After detailed study of the anorectal morphology on three Wistar and three BDIX rats, we designed a simple procedure, as follows (Figure 1).
Firstly, the animal was placed over a heating blanket, in a supine position. Perineal antisepsis was performed. The anal canal was emptied and a probe (Abbocath® 14G or similar) was inserted through it.
An arciform 10 mm to 15 mm anterior perianal incision (3 mm from verge) was performed. A 6/0 stitch at the inferior border to the tail was knotted. Adipose tissue was dissected sideways (with scissors or gauze) to expose approximately 20 mm of anorectal conduit until visualization of a thin darker line (external sphincter) or up to the anal verge.
Then, the muscular layer was held with clamps and a small incision was made until herniation of the submucosa or the probe was visualized by transparency. Submucosal dissection was continued longitudinally and the muscular layer was sectioned in a longitudinal fashion up to the anal verge and near the stitch caudally and proximally until completing 10 mm. If a mucosal perforation occurred, an interrupted suture was performed, leaving knots on the parietal side.
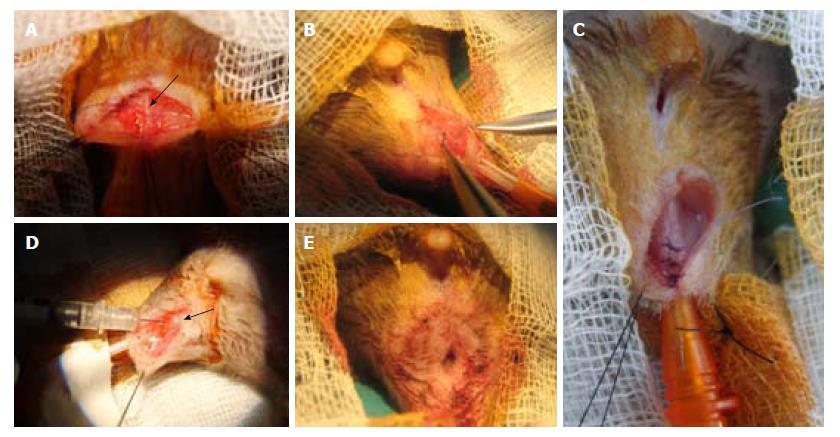
Immediate repair was performed with three to four interrupted 6/0 stitches. In the biosuture group, one suture was used for two stitches and on the second one, a back and forth motion was used three to four times, trying to deposit more ASCs. For ASC injections, two injections were given on each side of the muscle incision (Figure 2).
Finally, the skin incision was closed with interrupted stitches, burying the knots in the depth (to keep the animals from biting the suture). We washed the wound with iodine solution and optionally applied an aerosol plastic dressing (Nobecutan®; Inibsa, Barcelona, Spain). Analgesia (petidine) was injected subcutaneously immediately afterwards and on the following day.
The system was purged to avoid air bubbles, and the latex balloon was filled to 20-90 mmHg (to remain turgescent). The rectal ampulla was emptied, and the balloon was inserted until the silk knot that secures it to the tube reached the verge. The registry took between 30-60 min to reach a stable and regular activity. The system was configured to detect 10 pulses per second, registered as mmHg, and at least 30 min of stable activity was registered.
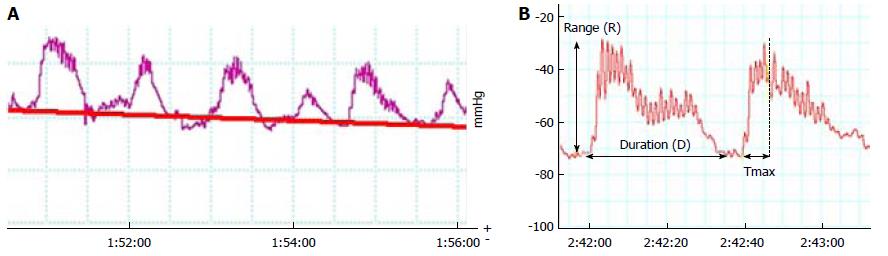
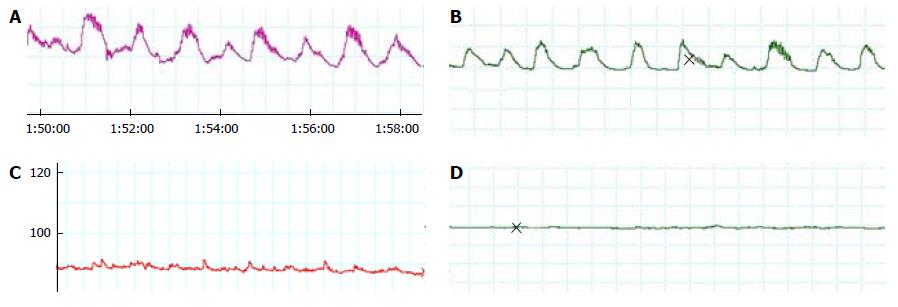
Based on our experience and previously published papers, we picked the 12 min most stable registry selections as highly representative to study such. The area-under-the-contraction waveforms vs time curve (AUC), related to the selection baseline and not to 0 to correct for basal value (related to balloon inflation) variability, was obtained from these tracings. Total number of contractions (defined as a rise of at least 10 mmHg) and their frequency were determined for each selection. Since AUC could not reveal minor changes in every contraction, we selected the six best contractions in the investigator´s viewpoint. For them, we calculated the mean values in each registry of the following: total duration (D), time to peak (Tmax), difference between maximal and minimal pressure value (range, R), and number of peaks (NP) (Figure 3).
Animals were sacrificed at days 1, 4 or 7. Anorectal conduits were extracted, fixed in 4% formaldehyde, and embedded in paraffin.
Histopathology: Slices (5-μm thick) were stained with hematoxylin and eosin and examined under a Leica DM LS2 microscope.
Immunofluorescence: Incubation of slices with primary antiGFP (SC-8334; Santa Cruz Biotechnology, Dallas, TX, United States) and antiα-actin (MAB-1501; Millipore) antibodies was carried out overnight and then for 1 h with the secondary, antirabbit Alexa Fluor 488 (Molecular Probes, Eugene, OR, United States) and antimouse Alexa Fluor 544 (Molecular Probes). Sections were mounted with antifade reagent, with 4′, 6-diamidino-2-phenylindole (DAPI) (Prolong Gold; Molecular Probes) and viewed under a fluorescent microscope (Leica DMI6000B). To reduce the effect of spontaneous natural fluorescence, we used primary antibody omitted sections as negative controls and detected GFP presence and not GFP fluorescence (sometimes too faint).
Animals were examined for any local or systemic abnormality. If death occurred, an autopsy and an exam of the operated region were performed. If adverse events appeared, they were recorded and exhaustively analyzed. If any abnormal tissue was observed, a biopsy was done.
We divided 36 BDIX rats into three groups, with three subgroups of 4 animals based on time to sacrifice (1, 4 or 7 d): (1) group 1, no repair and ASCs injection; (2) group 2, repair with biosutures; (3) group 3, repair with conventional sutures and ASCs injection.
We used Kolmogórov-Smirnov test to assess if normal distribution criteria were met. In descriptive analyses, means, standard deviation and range were applied. To compare groups, we used ANOVA or Kruskal-Wallis test. P-value significance was 0.05. All statistics were performed using SPPS version 15.0 (IBM Corp., Armonk, NY, United States).
Flow cytometry confirmed eGFP expression > 95% until the 15th passage and mesenchymal phenotype by the wide expression (> 95%) of CD90 and CD29 and the absence (< 5%) of CD45 and CD11b.
Sometimes, we found some clots in the supernatant that contained grouped ASCs. Similar structures appeared occasionally when using biosutures on the outer side of the muscular layer and less frequently with injections.
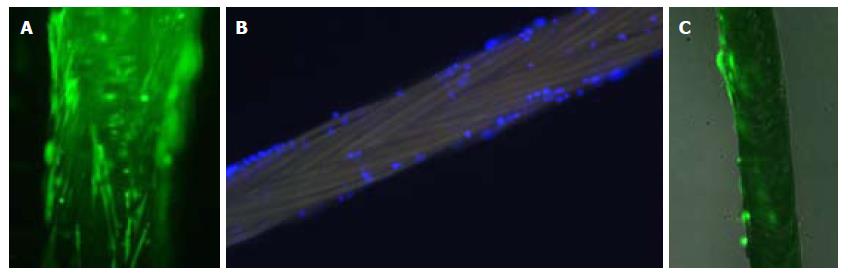
Cellular distribution over the suture was heterogeneous (Figure 4).
Studies of cell count and viability showed the following: (1) in culture medium (seven determinations): 100000 to 280000 (mean 171428.57 with standard deviation (SD) 64627.14, viability with blue trypan was 98%; (2) adhered cells (detached with trypsin, three sutures): 820000 to 860000 (mean 840.000 with SD 20,000), viability was 100%.
We studied seven biosutures under microscopic immunofluorescence, prior to and after surgical use, and counted the remaining cells in their distal centimeter (Figure 4). (1) Some ASCs persistently adhered after its use, mainly in the proximal two-thirds of the suture. (2) cell count of the last centimeter ranged from zero to two ASCs per millimeter, so the majority of ASCs on the distal end were left on the animal tissue.
These results confirmed that the maximal dosage of ASCs that we could supply was 820000 to 860000, which was less than published data with the same charge of ASCs (80% of total, 1.2 × 106)[27]. Maybe, it could be a bit higher because with 98% to 100% cell viability some ASCs could divide.
From a surgery viewpoint, it is frequently difficult to identify both sphincters-the external one because it is poorly developed (it has a slightly darker color), and the internal one because there is no clear continuity solution with rectal musculature (Figure 5). To identify anatomy, we made multiple coronal and sagittal sections and a 10 × reconstruction of hematoxylin and eosin imprints. It was very similar to that of humans, with the anal canal being 3 to 5 mm long and surrounded by two sphincters. The internal one consisted of smooth muscle, was an enlargement of the circular enteric musculature, and ended about 1 mm proximal to the anal verge. Immediately external to it, there are some longitudinal smooth fibers, and external to them the striated muscle bundles of the external sphincter, which ends close to the anal verge and has two to three different parts. A transition zone marks the change from rectum to anus, and there are some anal glands closer to it.
With the designed procedure (1 cm section), both sphincters were always sectioned, as confirmed by morphological studies (Figure 5).
Regarding functional assays, spontaneous, rhythmic contractions in the form of “plateaus” with multiple fasciculation-producing peaks appeared (Figure 6). These basal contractions were very heterogeneous, as we can see in Table 1, with a frequency of 0.91 to 4.17 per min (mean 1.6980 with SD 0.57698), a mean duration of 26.67 seconds, and a mean number of peaks of 12.53. Mean registered amplitude was 25.95 mmHg and the Tmax was 8.88 seconds with SD 2.93. Probably the most important component of this activity was the internal sphincter, but both could contribute. Postoperatively, findings were consistent with total sphincter lesion, irrespective of the applied treatment; the described activity disappeared and was substituted by a gentle oscillation of the basal line without a recognizable pattern (Figure 6). Only in 4 out of 36 animals did some small contractions appear (Table 1). There were no differences between groups at the immediate postoperative measurements (P = 0.329–0.73 on ANOVA). AUC decreased to less than one-tenth in the lesion group, without significant differences between groups (P = 0.134).
| Number of contractions | Frequency, n /min | Duration in s | Time to max in s | Peaks number, n | Range of mmHg | AUC | |
| Preoperative | |||||||
| Number | 36 | 36 | 36 | 36 | 36 | 36 | 36 |
| Mean | 19.489 | 1.6980 | 26.674752 | 8.882553 | 12.5323 | 25.9462 | 4884.9013 |
| Standard deviation | 7.1593 | 0.57698 | 6.6690300 | 2.9265056 | 3.52514 | 16.68935 | 3181.86004 |
| Minimum | 9.0 | 0.91 | 13.0000 | 4.2667 | 7.33 | 6.27 | 1227.46 |
| Maximum | 50.0 | 4.17 | 41.2800 | 15.6333 | 21.67 | 111.93 | 15790.69 |
| Postoperative | |||||||
| Number | 36 | 36 | 4 | 4 | 4 | 4 | 32 |
| Mean | 0.535 | 0.0446 | 26.425000 | 9.056250 | 7.5000 | 12.1950 | 418.2139 |
| Standard deviation | 1.5016 | 0.12513 | 5.8105507 | 4.2522727 | 2.48328 | 2.09572 | 361.06246 |
| Minimum | 0 | 0.00 | 20.4000 | 6.0750 | 5.00 | 10.39 | 12.91 |
| Maximum | 6.0 | 0.50 | 31.6000 | 15.1500 | 10.50 | 15.21 | 1610.80 |
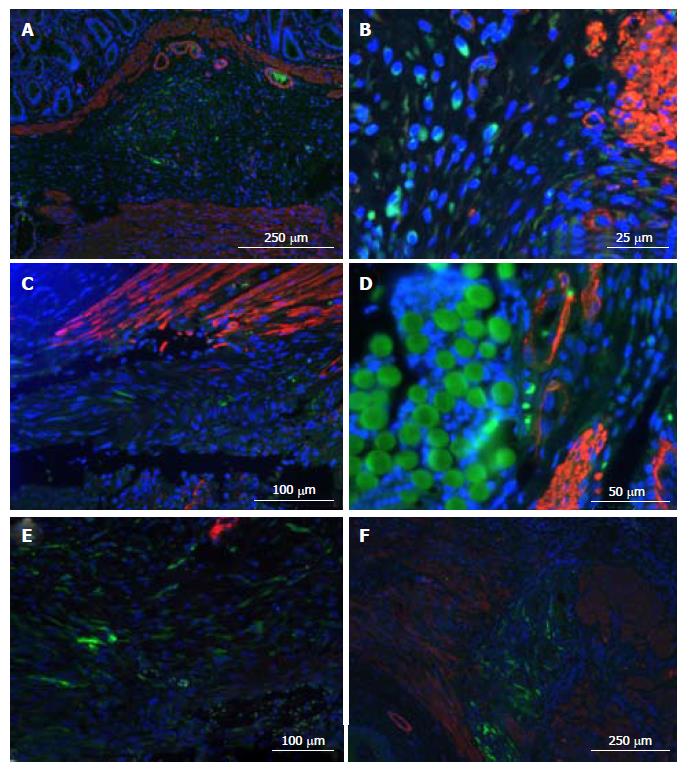
We analyzed the presence of GFP+ cells in 19 rats (Figure 7), finding them in 16 (84.21%) and in 100% of the 7 sacrificed at 7 d.
Regarding GFP+ cells’ location, the majority of animals had many simultaneously. The most frequent were muscular section endings and the tissue between them (10/16 animals), then submucosa (9/16 animals), and surrounding sutures (6/16 animals; 100% of the 5 animals with biosutures). We could not find them at the mucosa. Concerning spatial disposition, the most frequent was in small groups (10/16) or making “conglomerates” (8/16; 5/6 of group 1 and 3/5 of group 3). Three animals presented with perivascular disposition.
We could not identify cells simultaneously expressing GFP and α-actin.
Five deaths took place (13.88%), without relevant findings on postmortem studies and unrelated to the treatment received; eighty percent were probably related to anesthetic drugs.
We found 17 adverse events, affecting 13 rats (incidence 36.11%). None of them were considered severe. In order of frequency, they were: fecal impaction; hypotonic anus and anal laceration (3 cases of each); perianal laceration, perianal phlegmon, and cutaneous suture dehiscence (2 cases of each) and 1 case of anal stenosis. We considered 2 cases attributable to received treatment (muscular suture), 3 cases doubtfully related, and 12 cases non-related. None seemed attributable to ASCs.
No significant differences were found between groups in the incidence of mortality or adverse events. So, SC therapy on this model was completely safe.
The anatomy of rat anal sphincters is similar to that of humans. The most remarkable differences are that the external sphincter is poorly developed and the internal sphincter is difficult to distinguish from the rectal muscular layer. Using the 1-cm section from the anal verge, we can include both, even though they are not well identified.
Related to function, several publications reflect that the external sphincter’s contribution to basal tone in rats seems higher than in humans[29,30]. We decided to use the anesthetic ketamine/xylazine mixture, because it was the one we found to provide more stable registries. This mixture was also used by Zutshi and colleagues[29,34-36], but other authors have employed ketamine with better results than ours[37].
Our results were similar to other publications with regard to detected activity. However, numeric values were in some cases very dissimilar, probably due to the different rat species employed (BDIX vs Sprague-Dawley) or slight methodological variations. Our frequency was similar to that of Vinograd (0.91–4.17 per min compared with 1–3[30]) or Zutshi et al[29], also duration (26.67 with SD 6.67 compared to 35.10 ± 2.53[29] or 26.23 ± 1.68[36]) and Tmax (8.88 second, SD 2.93, compared to 10[29] or 12.24 ± 0.078[36]). But our mean NP was higher (12.53 SD 3.53 compared to 8.9 ± 0.5[29] or to 7.22 ± 0.45[36]) and our range was also much wider (25.95, SD 16.69 compared to 10.01 ± 0.66[29] or to 12.0 ± 0.1[37]).
In our experiments, practically all spontaneous activity disappeared after surgery (irrespective of the reparation or SC presence). Nevertheless, Zutshi et al[29] and others[34] found some pressure waves, despite muscle disruption. The only possible explanation for this phenomenon is the difference in the injury, as they practice “a precise 3-mm to 4-mm incision” for selective sphincter section that might not be complete.
Compared with other published works using SCs for fecal incontinence in rats, our lesion is one of the most extensive. Lorenzi et al[38] described a left lateral selective sphincterotomy without specifying the length used later by Mazzanti et al[39]. Kang et al[40] performed cryoinjury without specifying the damaged volume; later, Bisson et al[41] published that cryoinjury must be at least 90º to be significant. Zutshi´s model[29] was also employed in other publications[34]. White et al[42] and Pathi et al[43] performed a total section of 7 mm, followed by rectal mucosa repair. Going further, Lane et al[37] performed a more aggressive injury (“proctoepisiotomy”) without describing technical details and extension; Salcedo et al[36] applied an even more aggressive procedure involving an excision of 25% of both sphincters, but they did not perform an immediate postoperative control so we could not compare our functional results with theirs.
Aiming to minimize the effects of the poorly developed sphincters, some authors have tested bigger animals (rabbits[44-46] and dogs[47,48]), but there are only five published papers compared to ten for rats.
We can discuss clinical relevance of our injury model. Human obstetric trauma is more complex than a simple section during episiotomy or a perineum tear, it combines muscle injury, regional hypoxia, denervation, etc. Other factors could be added later in life, such as ageing, hormonal changes, surgeries, etc.
On the other hand, in the clinical setting, immediate repair offers better results, but the most frequent scenario is a repair indicated years later. This could modify some confounding factors that we have observed with immediate repair (mucosal tears, fecal contamination, etc.) that could compromise ASCs survivorship or effects.
In an effort to reproduce these complex effects, simulated childbirth injury models have been designed, mainly for urinary incontinence. It was first described by Resplande et al[49], who inserted an inflated 10F Foley catheter inside the vagina over 3 to 4 h to simulate labor; an episiotomy and extraction of inflated Foley was performed. Later, Healy et al[50] published a model for fecal incontinence using two intrapelvic, retrouterine balloons (6 Fr urinary catheters) placed through a 3-cm laparotomy over 1 h. We tested the model of Resplande, but we decided not to use it anymore because we observed high variability in injuries. We think that our model results in a more homogeneous injury. More studies on simulated childbirth models may be needed and also consideration of a delayed repair, as some authors have performed[51].
Regarding SC vehicles, we have employed biosutures and injections, finding GFP+ cells during at least 7 d with both. Biosutures can be used in a similar fashion to conventional sutures. The pioneer model with biosutures and colonic anastomoses studied in the short-term found fewer adherences on days 4 and 7 (preserving anastomotic resistance[25]), which was significantly greater on day 4 in a poor adhesion environment[26]. Later, tracheal anastomoses were studied for 60 d and a swift inflammatory response was found: on postoperative days 1 and 4, acute inflammation was substituted by chronic inflammation[27]. Yao et al[52] used embryonic SCs, added molecules to improve cell adherence (poly-L-lysine and fibronectin)[53], and applied them to rat tendons. Horváthy et al[54] employed bone marrow-MSCs and observed better cell adherence if the suture was covered with albumin and the SC surviving in implanted tissues during 5 wk. They recommended a 48 h culture as the best for clinical usage (over 168 h, 910 polyglactin loses resistance)[55]. No evidence exists about the best dose. However, we found that some cells tend to form “clusters” over the suture, in the culture medium and also remaining ASCs adhered to the sutures after their usage. These findings make us wonder about the suitability of this vehicle, so more studies on suture preparation are needed. Delivered cell dose could be more controlled by injection, but similar clusters can be observed sometimes, losing ASCs.
Now the questions that arise are how to get better SC delivery, survival, and function in tissues. An interesting approach is molecule addition to sutures, such as vascular endothelial growth factor (VEGF) by Bigalke et al[56]. Cytokines could also be of high relevance, mostly in models with delayed repair because they could already be normalized; some examples are stromal-derived factor-1 (SDF-1) and monocyte chemotactic protein-3 (MCP-3)[57]. As an example, Sun et al[58] performed a 50% excision of the anal sphincter, and they compared SDF-1 alone or combined with MSCs 3 wk later with interesting results.
Published animal investigations in this field have employed muscle progenitors in 10 papers and BM-MSCs in 5 papers. All except one confirm the safety. Concerning results, generally good morphological and functional responses have been observed with questions about cell survival and only one long-term study[46]. In human research, there is one study unrelated to fecal incontinence that finds improvement with ASCs[20], three incontinence studies involving 38 patients with promising results[59-62], and seven ongoing clinical trials.
There are many questions remaining about the mechanism of action of ASCs, which is not totally understood nowadays. Their multi-lineage differentiation potential coupled with their immune-privilege and their ability to stimulate resident progenitor cells through paracrine secretion, as well as their angiogenic potential, are important. There is growing evidence about their immunomodulation capability. It is thought to be largely based on inhibition of T cell and B cell proliferation and dendritic cell maturation[63] and in the secretion of cytokines[64]. For example, Nemeth et al[65] observed MSCs’ sepsis attenuation by macrophages reprogramming to increase IL-10, a cytokine that decreases neutrophil migration to tissues. There is much interest in identifying secretome and immunosuppressive properties of ASCs. Our research team has added some contributions. Georgiev-Hristov et al[27] showed an early switch from acute to chronic inflammation in the presence of ASCs after tracheal anastomosis. And, Riera del Moral et al[66] observed less acute and chronic inflammation and increasing fibrosis of aneurysm sacs in pigs.
Finally, our present results show that SC therapy on this model is safe, as no serious adverse reactions or neoplastic processes were observed. In this field, there is a worrisome paper in which 2/24 rats receiving 5 × 106 myogenic SCs developed local abnormal foci of growth that were benign[67].
Our proposed surgical procedure produces a consistent lesion of both sphincters and could be a model for posttraumatic fecal incontinence. Biosutures and injections are both suitable for cell delivery. Biosutures do not change the surgical technique or suture manageability. ASCs are able to survive in the complex area of anal sphincters, at least 7 d, and are safe in this clinical setting.
Fecal incontinence is a very prevalent (11% to 15% of adults), nonfatal illness associated with devastating consequences mainly in the psychosocial sphere and quality of life. Although in the last years sacral neuromodulation has been improving the poor results of available treatments, surgery remains the choice for the most severe or refractory cases, mainly if sphincter lesions are present (the most frequently observed anatomic alteration). Sphincter repair is the most successful technique used for traumatic fecal incontinence, but its results are not very satisfactory, mainly in the long term. Stem cells (SCs) and adipose-derived stem cells (ASCs) have demonstrated promising results in a wide variety of clinical settings, including particularly unfavorable environments for wound healing, such as anal fistulas and Crohn´s disease.
To test if SC therapy could improve postoperative healing mechanisms in patients with fecal incontinence. If this hypothesis is correct, surgical outcomes could be improved and more patients would benefit from surgery in the short and long term.
The first objective was obtaining an in-depth knowledge of rat anal region anatomy so as to design an easy and reproducible model for fecal incontinence or sphincter injury. The second objective was to establish a method for studying rat anal sphincter function, defining the best anesthetic method and physiological test to be used with low morbidity. Finally, the main objective was to study the feasibility and safety of ASC administration to rat anal sphincters via different methods. All of the previously mentioned objectives were accomplished. This furnishes future investigations with the proposed animal model for study of potential SC efficacy; and, if the expected results are obtained, they will support trying this therapy on humans.
Rat anal region normal anatomy was studied on BDIX and Wistar female rats. Once anatomy was well known, the authors studied a system capable of detecting the low-pressure waves supposed to be created in this area, and the capability of this system to detect sphincter lesions. Simultaneously, the authors needed to select a model for fecal incontinence. Since simulated childbirth injury models are complex, associate morbidity in animals and generate highly variable injuries, the authors decided to create a simple model of sphincter injury. The authors tested different injury models and finally selected an anterior extra-mucosal longitudinal myotomy of 1 cm. Researchers studied its morphology and physiology to verify if this procedure injured both sphincters in a constant way. Going further, to decide the best system to detect sphincter pressures, the authors tested different anesthetic drugs (inhaled isoflurane, intraperitoneal ketamine, intraperitoneal ketamine plus xylazine) and different systems to detect pressure, including an endorectal balloon that was retired slowly (similar to human manometry), a normal saline infusion through a mini-laparotomy in the rectum (until anus became opened by rectal increasing pressure), and basal anal manometry with a stationary endoanal balloon. Preliminary studies led the authors to select intraperitoneal ketamine plus xylazine (provided the most stable functional registries) and basal anal manometry with a stationary endoanal balloon as the best options. ASCs were obtained from subcutaneous fat from two BDIX rat males and later were marked with eGFP. Later, preparations of 106 ASCs in 50 μL of normal saline or biosutures of polyglactin 910 6/0 suture covered with 1.5 × 106 ASCs were prepared. Finally, those preparations were applied to 36 BDIX virgin female adult rats that underwent functional studies prior to and after the surgery. Then, safety and cell tracking studies were performed during a follow-up period lasting 7 d. Animals were distributed among three groups: biosuture repair; cell injection without repair; and conventional suture repair and cell injection. Moreover, some quality studies were performed with biosutures, trying to establish the real cell dose administered during their use.
A dose of 820000 to 860000 ASCs adhered to the suture, but not all of them remained on the animal tissue. The described lesion produced a constant injury to both anal sphincters. Rat anal sphincter spontaneous function is composed of heterogeneous, spontaneous, consistent, and rhythmic contractions in the form of “plateaus”, with multiple fasciculations that disappeared consistently with the described surgical damage. ASCs are able to survive in this potentially septic area for at least 7 d. We were able to identify them in 84% of the studied animals, mainly in the muscular section area or in the tissue that appeared between both muscular endings. ASCs form a kind of “conglomerate” in rats treated with injections, while they wrapped around the suture in the biosuture group. ASCs were also able to migrate to the damaged zone, and the most frequent disposition was formation of small groupings. No relevant adverse events or mortality could be related to the SCs in our study. We also did not find unexpected tissue growths. So, this cell therapy was deemed as safe, at least in the short term. As unresolved issues, safety must be studied in the long term, ASC survival must be confirmed in longer follow-ups, systems to improve SC function and survival could be tested, and biosuture cell dosing must be studied thoroughly.
The authors propose an easy, reproducible and safe method for rat sphincter injury that could represent a model for posttraumatic fecal incontinence. ASC administration through cell injections or biosutures is feasible and safe. Both systems are suitable for cell delivery, and biosutures do not change the surgical technique or suture manageability. Applied ASCs are able to survive in this complex area. By this approach, ASCs could offer a benefit for postoperative healing in fecal incontinence.
The model for rat anal sphincter injury can be used in future experiments for testing potential SC efficacy and obtaining long-term results. Anesthetic and functional study methodologies may need some minor changes. Areas prone to be studied deeply are: obtaining knowledge about the potential mechanism of action of ASCs; improving SC delivery, survival and function in the receiving animals (cytokine or molecule addition, etc.); and, supplying SCs through minimally invasive methods. This study allows continuing studies on animal models prior to human use, and, if the expected results are obtained, support trying this therapy on humans (only in empirical and highly controlled settings).
The authors gratefully acknowledge the Animal Housing Unit at University Hospital La Paz’s personnel for their collaboration, Carlota Largo Aramburu (veterinary of Experimental Surgery Unit, University Hospital La Paz) for her continuous support, and Fernando de Miguel Pedrero and Susana Olmedillas for their scientific support and collaboration.
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gazouli M, Garg P, Liu L, Sanal MG, Tsuchiya A S- Editor: Cui LJ L- Editor: Filipodia E- Editor: Li RF
| 1. | Macmillan AK, Merrie AE, Marshall RJ, Parry BR. The prevalence of fecal incontinence in community-dwelling adults: a systematic review of the literature. Dis Colon Rectum. 2004;47:1341-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Nelson R, Norton N, Cautley E, Furner S. Community-based prevalence of anal incontinence. JAMA. 1995;274:559-561. [PubMed] [DOI] [Full Text] |
| 3. | Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91:33-36. [PubMed] |
| 4. | Dunivan GC, Heymen S, Palsson OS, von Korff M, Turner MJ, Melville JL, Whitehead WE. Fecal incontinence in primary care: prevalence, diagnosis, and health care utilization. Am J Obstet Gynecol. 2010;202:493.e1-493.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Deutekom M, Dobben AC, Dijkgraaf MG, Terra MP, Stoker J, Bossuyt PM. Costs of outpatients with fecal incontinence. Scand J Gastroenterol. 2005;40:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Dudding TC, Vaizey CJ, Kamm MA. Obstetric anal sphincter injury: incidence, risk factors, and management. Ann Surg. 2008;247:224-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 8. | Madoff RD. Surgical treatment options for fecal incontinence. Gastroenterology. 2004;126:S48-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Halverson AL, Hull TL. Long-term outcome of overlapping anal sphincter repair. Dis Colon Rectum. 2002;45:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 503] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 11. | Trebol Lopez J, Georgiev Hristov T, García-Arranz M, García-Olmo D. Stem cell therapy for digestive tract diseases: current state and future perspectives. Stem Cells Dev. 2011;20:1113-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Ma T, Sun J, Zhao Z, Lei W, Chen Y, Wang X, Yang J, Shen Z. A brief review: adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res Ther. 2017;8:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 14. | Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 497] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 15. | García-Olmo D, García-Arranz M, García LG, Cuellar ES, Blanco IF, Prianes LA, Montes JA, Pinto FL, Marcos DH, García-Sancho L. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn’s disease: a new cell-based therapy. Int J Colorectal Dis. 2003;18:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, Brush M, Verda L, Kowalska B, Krosnjar N. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology. 2005;128:552-563. [PubMed] [DOI] [Full Text] |
| 17. | Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566-2573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 409] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 18. | Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 507] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 19. | García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 571] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 20. | Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 566] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 21. | Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D; FATT Collaborative Group. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Dis Colon Rectum. 2012;55:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 22. | García-Arranz M, Herreros MD, González-Gómez C, de la Quintana P, Guadalajara H, Georgiev-Hristov T, Trébol J, Garcia-Olmo D. Treatment of Crohn’s-Related Rectovaginal Fistula With Allogeneic Expanded-Adipose Derived Stem Cells: A Phase I-IIa Clinical Trial. Stem Cells Transl Med. 2016;5:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 24. | Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 730] [Article Influence: 81.1] [Reference Citation Analysis (1)] |
| 25. | Pascual I, de Miguel GF, Gómez-Pinedo UA, de Miguel F, Arranz MG, García-Olmo D. Adipose-derived mesenchymal stem cells in biosutures do not improve healing of experimental colonic anastomoses. Br J Surg. 2008;95:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Pascual I, Fernández de Miguel G, García Arranz M, García-Olmo D. Biosutures improve healing of experimental weak colonic anastomoses. Int J Colorectal Dis. 2010;25:1447-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Georgiev-Hristov T, García-Arranz M, García-Gómez I, García-Cabezas MA, Trébol J, Vega-Clemente L, Díaz-Agero P, García-Olmo D. Sutures enriched with adipose-derived stem cells decrease the local acute inflammation after tracheal anastomosis in a murine model. Eur J Cardiothorac Surg. 2012;42:e40-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Wang EQ, Soda DM, Fung HL. Nitroglycerin-induced relaxation of anorectal smooth muscle: evidence for apparent lack of tolerance development in the anaesthetized rat. Br J Pharmacol. 2001;134:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Zutshi M, Salcedo LB, Zaszczurynski PJ, Hull TL, Butler RS, Damaser MS. Effects of sphincterotomy and pudendal nerve transection on the anal sphincter in a rat model. Dis Colon Rectum. 2009;52:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Vinograd I, Hanani M, Hadary A, Merguerian P, Nissan S. Animal model for the study of internal anal sphincter activity. Eur Surg Res. 1985;17:259-263. [PubMed] |
| 31. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5761] [Article Influence: 240.0] [Reference Citation Analysis (0)] |
| 32. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12678] [Article Influence: 704.3] [Reference Citation Analysis (2)] |
| 33. | Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1370] [Article Influence: 114.2] [Reference Citation Analysis (2)] |
| 34. | Salcedo L, Damaser M, Butler R, Jiang HH, Hull T, Zutshi M. Long-term effects on pressure and electromyography in a rat model of anal sphincter injury. Dis Colon Rectum. 2010;53:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Salcedo L, Mayorga M, Damaser M, Balog B, Butler R, Penn M, Zutshi M. Mesenchymal stem cells can improve anal pressures after anal sphincter injury. Stem Cell Res. 2013;10:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Salcedo L, Penn M, Damaser M, Balog B, Zutshi M. Functional outcome after anal sphincter injury and treatment with mesenchymal stem cells. Stem Cells Transl Med. 2014;3:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Lane FL, Jacobs SA, Craig JB, Nistor G, Markle D, Noblett KL, Osann K, Keirstead H. In vivo recovery of the injured anal sphincter after repair and injection of myogenic stem cells: an experimental model. Dis Colon Rectum. 2013;56:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Lorenzi B, Pessina F, Lorenzoni P, Urbani S, Vernillo R, Sgaragli G, Gerli R, Mazzanti B, Bosi A, Saccardi R. Treatment of experimental injury of anal sphincters with primary surgical repair and injection of bone marrow-derived mesenchymal stem cells. Dis Colon Rectum. 2008;51:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Mazzanti B, Lorenzi B, Borghini A, Boieri M, Ballerini L, Saccardi R, Weber E, Pessina F. Local injection of bone marrow progenitor cells for the treatment of anal sphincter injury: in-vitro expanded versus minimally-manipulated cells. Stem Cell Res Ther. 2016;7:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Kang SB, Lee HN, Lee JY, Park JS, Lee HS, Lee JY. Sphincter contractility after muscle-derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis Colon Rectum. 2008;51:1367-1373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Bisson A, Fréret M, Drouot L, Jean L, Le Corre S, Gourcerol G, Doucet C, Michot F, Boyer O, Lamacz M. Restoration of anal sphincter function after myoblast cell therapy in incontinent rats. Cell Transplant. 2015;24:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | White AB, Keller PW, Acevedo JF, Word RA, Wai CY. Effect of myogenic stem cells on contractile properties of the repaired and unrepaired transected external anal sphincter in an animal model. Obstet Gynecol. 2010;115:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Pathi SD, Acevedo JF, Keller PW, Kishore AH, Miller RT, Wai CY, Word RA. Recovery of the injured external anal sphincter after injection of local or intravenous mesenchymal stem cells. Obstet Gynecol. 2012;119:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Aghaee-Afshar M, Rezazadehkermani M, Asadi A, Malekpour-Afshar R, Shahesmaeili A, Nematollahi-mahani SN. Potential of human umbilical cord matrix and rabbit bone marrow-derived mesenchymal stem cells in repair of surgically incised rabbit external anal sphincter. Dis Colon Rectum. 2009;52:1753-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Kajbafzadeh AM, Elmi A, Talab SS, Esfahani SA, Tourchi A. Functional external anal sphincter reconstruction for treatment of anal incontinence using muscle progenitor cell auto grafting. Dis Colon Rectum. 2010;53:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Kajbafzadeh AM, Kajbafzadeh M, Sabetkish S, Sabetkish N, Tavangar SM. Tissue-Engineered External Anal Sphincter Using Autologous Myogenic Satellite Cells and Extracellular Matrix: Functional and Histological Studies. Ann Biomed Eng. 2016;44:1773-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Oh HK, Lee HS, Lee JH, Oh SH, Lim JY, Ahn S, Hwang JY, Kang SB. Functional and histological evidence for the targeted therapy using biocompatible polycaprolactone beads and autologous myoblasts in a dog model of fecal incontinence. Dis Colon Rectum. 2015;58:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Oh HK, Lee HS, Lee JH, Oh SH, Lim JY, Ahn S, Kang SB. Coadministration of basic fibroblast growth factor-loaded polycaprolactone beads and autologous myoblasts in a dog model of fecal incontinence. Int J Colorectal Dis. 2015;30:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Resplande J, Gholami SS, Graziottin TM, Rogers R, Lin CS, Leng W, Lue TF. Long-term effect of ovariectomy and simulated birth trauma on the lower urinary tract of female rats. J Urol. 2002;168:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Healy CF, O’Herlihy C, O’Brien C, O’Connell PR, Jones JF. Experimental models of neuropathic fecal incontinence: an animal model of childbirth injury to the pudendal nerve and external anal sphincter. Dis Colon Rectum. 2008;51:1619-1626; discussion 1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Sun L, Yeh J, Xie Z, Kuang M, Damaser MS, Zutshi M. Electrical Stimulation Followed by Mesenchymal Stem Cells Improves Anal Sphincter Anatomy and Function in a Rat Model at a Time Remote From Injury. Dis Colon Rectum. 2016;59:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Yao J, Korotkova T, Riboh J, Chong A, Chang J, Smith RL. Bioactive sutures for tendon repair: assessment of a method of delivering pluripotential embryonic cells. J Hand Surg Am. 2008;33:1558-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Yao J, Korotkova T, Smith RL. Viability and proliferation of pluripotential cells delivered to tendon repair sites using bioactive sutures--an in vitro study. J Hand Surg Am. 2011;36:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Horváthy DB, Vácz G, Cselenyák A, Weszl M, Kiss L, Lacza Z. Albumin-coated bioactive suture for cell transplantation. Surg Innov. 2013;20:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Horváthy D, Vácz G, Szabó T, Renner K, Vajda K, Sándor B, Lacza Z. Absorption and tensility of bioactive sutures prepared for cell transplantation. Materials. 2013;6:544-550. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Bigalke C, Luderer F, Wulf K, Storm T, Löbler M, Arbeiter D, Rau BM, Nizze H, Vollmar B, Schmitz KP. VEGF-releasing suture material for enhancement of vascularization: development, in vitro and in vivo study. Acta Biomater. 2014;10:5081-5089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Salcedo L, Sopko N, Jiang HH, Damaser M, Penn M, Zutshi M. Chemokine upregulation in response to anal sphincter and pudendal nerve injury: potential signals for stem cell homing. Int J Colorectal Dis. 2011;26:1577-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Sun L, Xie Z, Kuang M, Penn M, Damaser MS, Zutshi M. Regenerating the Anal Sphincter: Cytokines, Stem Cells, or Both? Dis Colon Rectum. 2017;60:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Frudinger A, Kölle D, Schwaiger W, Pfeifer J, Paede J, Halligan S. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: pilot study with 1 year follow-up. Gut. 2010;59:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 60. | Frudinger A, Pfeifer J, Paede J, Kolovetsiou-Kreiner V, Marksteiner R, Halligan S. Autologous skeletal-muscle-derived cell injection for anal incontinence due to obstetric trauma: a 5-year follow-up of an initial study of 10 patients. Colorectal Dis. 2015;17:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Romaniszyn M, Rozwadowska N, Malcher A, Kolanowski T, Walega P, Kurpisz M. Implantation of autologous muscle-derived stem cells in treatment of fecal incontinence: results of an experimental pilot study. Tech Coloproctol. 2015;19:685-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Sarveazad A, Newstead GL, Mirzaei R, Joghataei MT, Bakhtiari M, Babahajian A, Mahjoubi B. A new method for treating fecal incontinence by implanting stem cells derived from human adipose tissue: preliminary findings of a randomized double-blind clinical trial. Stem Cell Res Ther. 2017;8:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [PubMed] [DOI] [Full Text] |
| 64. | Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1199] [Cited by in RCA: 1177] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 65. | Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1919] [Cited by in RCA: 1818] [Article Influence: 113.6] [Reference Citation Analysis (1)] |
| 66. | Riera del Moral L, Largo C, Ramirez JR, Vega Clemente L, Fernández Heredero A, Riera de Cubas L, Garcia-Olmo D, Garcia-Arranz M. Potential of mesenchymal stem cell in stabilization of abdominal aortic aneurysm sac. J Surg Res. 2015;195:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Jacobs SA, Lane FL, Pham QA, Nistor G, Robles R, Chua C, Boubion B, Osann K, Keirstead H. Safety assessment of myogenic stem cell transplantation and resulting tumor formation. Female Pelvic Med Reconstr Surg. 2013;19:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |