Published online Apr 15, 2003. doi: 10.3748/wjg.v9.i4.788
Revised: January 6, 2003
Accepted: January 13, 2003
Published online: April 15, 2003
AIM: To investigate Tie-2 expression during the repair of acetic acid-induced gastric ulcers in rats treated with recombinant human IL-11 (rhIL-11) and in untreated control animals.
METHODS: Gastric ulcers were induced in male Wistar rats by applying acetic acid to the fundus of the stomach. RhIL-11 (100 µg/kg twice daily, subcutaneously) was administered from two days before ulcer induction and continued for five days after the induction. Control rats received bovine serum albumin. Gastric specimens were collected at 3 and 5 d after the induction of ulcer for immunohistochemical observation, Western blotting, and reverse transcription polymerase chain reaction (RT-PCR).
RESULTS: Immunohistochemical and Western blot analysis demonstrated that Tie-2 expression was enhanced in the rhIL-11-treated rats compared with the control animals at both intervals.
CONCLUSION: These findings suggested that IL-11 could accelerate ulcer healing, in part, by up-regulating Tie-2 expression and promoting angiogenesis.
- Citation: Wen CY, Ito M, Wang H, Chen LD, Xu ZM, Matsuu M, Shichijo K, Nakayama T, Nakashima M, Sekine I. IL-11 up-regulates Tie-2 expression during the healing of gastric ulcers in rats. World J Gastroenterol 2003; 9(4): 788-790
- URL: https://www.wjgnet.com/1007-9327/full/v9/i4/788.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i4.788
IL-11 is a kind of pleiotropic cytokine that stimulates stem cell proliferation and affects multiple types of cells[1]. RhIL-11 may be useful in accelerating the recovery of both hematopoietic cells and gastrointestinal mucosal cells after cytoablative therapies[2]. RhIL-11 is in Phase II clinical trials for the treatment of thrombocytopenia that occurs secondary to chemotherapy[3]. Gross and microscopic evidence suggested that rhIL-11 treatment could improve acute colitis caused by both chemically-induced damage and chronic inflammatory bowel disease[4]. Bruce et al[5] recently reported that short-term treatment with rhIL-11 was well tolerated in patients with active Crohn’s disease. Our previous study demonstrated that rhIL-11 facilitates gastric ulcer healing in rats[6].
Angiogenesis is critical to ulcer healing since it regulates nutrient and oxygen delivery to the injured site and, thus, controls the healing rate. Tie-2 (tek) is a member of the endothelial cell-specific receptor tyrosine kinase family[7,8], and is essential for the formation of the embryonic vasculature[9]. Our recent study suggests that Tie-2 plays an important role in the angiogenesis associated with the healing of gastric ulcers[10]. The present study examined the effect of IL-11 on Tie-2 expression in acetic acid-induced gastric ulcers by comparing with in the rhIL-11-treated and control rats.
This study was approved by the Animal Care Committee of Nagasaki University. Male Wistar rats, purchased from Charles River Japan (Atsugi, Japan) at 7 weeks of age, were housed 3 or 4 per cage in an air-conditioned room(24 °C, 12 hr light cycle) at the Laboratory Animal Center of Nagasaki University. The animals were fed with laboratory chow (F2, Japan CLEA, Tokyo, Japan) and tap water ad libitum.
Gastric ulcers were induced by luminal application of a 40% acetic acid solution as reported previously[11]. Under ether anesthesia, the stomach was exposed via a midline incision and the anterior and posterior walls of the gastric fundus were clamped together with ring forceps (ID, 6 mm). The acetic acid solution was injected into the clamped portion through the forestomach using 21 gauge needles. After forty-five seconds, the acid solution was removed, the abdomen closed and the animals fed and housed as above.
RhIL-11 (Genetics Institute, Andover, MA, USA), courtesy of Yamanouchi Pharmaceutical Co., Ltd. (Tokyo, Japan), was diluted in 0.1% bovine serum albumin (BSA) and administered subcutaneously (100 µg/kg/twice daily) for seven consecutive days beginning two days before ulcer induction as described previously (n = 16)[12]. Control animals (n = 16) received the same volume of 0.1% BSA twice daily. Eight rhIL-11-treated and eight untreated rats were sacrificed 3 and 5 d after the induction of gastric ulcers. Gastric tissues were collected for Western blot analysis, and reverse transcription polymerase chain reaction (RT-PCR). Tissues were fixed in 4% paraformaldehyde solution for immunohistochemical examination of Tie-2 expression.
Paraformaldehyde-fixed and paraffin-embedded tissues were cut into 4 µm sections, deparaffinized in xylene and rehydrated in phosphate-buffered saline. The tissues were subsequently preincubated in 3% H2O2 for 30 minutes, followed by incubation in bovine serum to prevent nonspecific binding, and then incubated overnight at 4 °C (2 µg/mL) with rabbit anti-mouse Tie-2 (C-20, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The slides were subsequently incubated in biotinylated anti-rabbit immunoglobulin G followed by avidin-horseradish peroxidase and the reaction product was resolved using DAB (Vectastain ABC kit; Vector Laboratories, Burlingame, CA).
Fresh gastric tissues obtained from ulcerated areas were immediately frozen, suspended in RIPA buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.05% SDS, pH7.4), broken into pieces on ice and subjected to three freeze-thaw cycles. Insoluble cell debris was removed by centrifugation at 14000 × g at 0 °C for 10 minutes. The protein concentrations in the resultant supernates were quantified using a protein assay reagent (Bio-Rad Laboratories, Hercules, CA). Data from four rats were recorded at each time point and the assays were performed in duplicate. The proteins (30 µg) were separated by polyacrylamide gel electrophoresis (PAGE) under denaturing and reducing conditions, and transferred to a Hybond ECL Nitrocellulose Membrane (Amersham Life Science, Buckinghamshire, U.K.). The membranes were rinsed in TBS, blocked with 5% low-fat dried milk in TBS containing 0.1% Tween 20 (TTBS), and incubated for 2 hours at room temperature in a 1:500 dilution of mouse anti-rat Tie-2 antibodies. After extensive washing with TTBS, the membranes were incubated for 1 hour in TTBS with 1:2000 dilution of horseradish-peroxidase-conjugated goat anti-mouse immunoglobulin G containing 3% low-fat dried milk. The membranes were washed, developed with a horseradish peroxidase chemiluminescence detection reagent (ECL Plus System, Amersham, N.D.), and exposed to Hyperfilm ECL (Amersham).
Total RNA was prepared from gastric tissue using the acid guanidine phenol method. RNA (1 µg) was incubated at 37 °C for 1 hour in 50 µl reverse transcriptase buffer containing 20 units RNAsin (Promega Corp., Madison, WI), 100 pmol of random hexamer primers (Boehringer Mannheim, Germany), and 400 units Moloney murine leukemic virus reverse transcriptase (GIBCO/BRL). Reverse transcription was terminated by heating to 95 °C for 10 minutes, and 20% of the resulting cDNA was used for PCR. PCR samples were incubated with 50 pmol of each primer and 2.5 units of Taq DNA polymerase. The rat Tie-2 PCR primers were as followings: 5’-TGTTCCTGTGCCACAGGCTG-3’ (sense) and 5’-CACTGTCCCATCCGGCTTCA-3’ (antisense). The human β-actin PCR primers were as followings: 5’-TCCTCCCTGGAGAAGAGCTA-3’ (sense) and 5’-AGTACTTGCGCTCAGGAGGA-3’ (antisense). The Tie-2 and β-actin primers were predicted to amplify 317 and 313 bp DNA products, respectively. Primer pairs were chosen to span introns of their respective rat genes. Samples were subjected to 40 cycles of PCR amplification, each cycle consisting of denaturation at 95 °C for 3 minutes, annealing at 50 °C for 1 minute, and primer extension at 72 °C for 1 minute. An aliquot of each amplification mixture was subjected to electrophoresis on 2% agarose gel, and DNA was visualized by ethidium bromide staining.
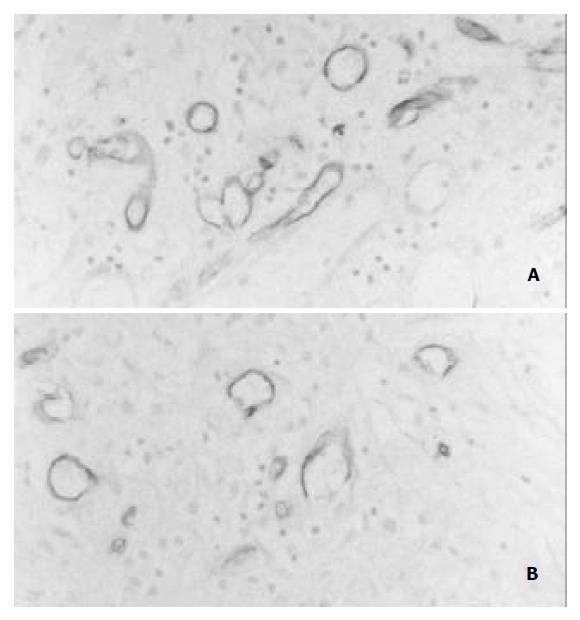
Immunohistochemical staining of Tie-2 was weakly positive in the endothelial cells of pre-existing vessels in the gastric wall. Tie-2 expression in the endothelial cells of new capillaries was enhanced in the rhIL-11-treated rats (Figure 1A) compared with the control rats (Figure 1B) after 3 and 5 d of the induction of ulcers.
A major band of 125 KD representing Tie-2 protein was detected in the Western blots. Tie-2 expression was increased significantly after the appearance of ulcers in the rhIL-11-treated rats compared with the untreated control animals on day 3 and day 5 (Figure 2).
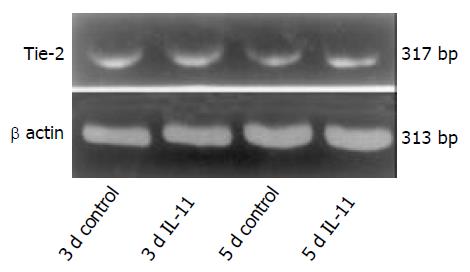
Tie-2 mRNA was detected between rhIL-11-treated rats and the control animals after 3 and 5 d of the induction of gastric ulceration. β-actin mRNA, a constitutively expressed transcript, was detected in all of the samples from both the rhIL-11 and untreated rats (Figure 3).
Angiogenesis occurs in many physiological and pathological processes, including embryonic development, wound healing, and tumor growth[13,14]. Ulcer healing consists of two processes, epithelial regeneration and mesenchymal reconstruction. The process of mesenchymal reconstruction consists of angiogenesis, fibrosis and smooth muscle regeneration. Therefore, angiogenesis is central to the formation of granulation tissue since newly formed vessels are required to supply oxygen and nutrients to the regenerating tissue. Tie-2 is a receptor tyrosine kinase expressed by endothelial cells[15,16] and it has been reported to play an important role in embryonic angiogenesis[17]. Furthermore, our own recent study revealed that Tie-2 was important in the angiogenesis that occurs during the healing of gastric ulcers[10].
Our previous study was the first to demonstrate that IL-11 could promote gastric ulcer healing in rat model[6]. This effect of IL-11 on the repair of mucosal injury most likely reflects its trophic action on mucosal epithelial and smooth muscle cells. RhIL-11 also exhibits an anti-apoptotic effect on the gastric mucosa. RhIL-11 most likely acts on epithelial cells via the regeneration of epithelial cell facilitated by IL-11 receptor. Concomitant smooth muscle hyperplasia may induce tissue contraction, thus promoting healing. The importance of smooth muscle contraction at the base of the ulcer has been previously demonstrated[18]. Interestingly, IL-11 inhibits the production of nitric oxide, an agent that can relaxs smooth muscle cells[19].
Our immunohistochemical and Western blot data showing that rhIL-11 enhanced Tie-2 expression suggest that Il-11 accelerates healing by promoting angiogenesis. The results of this study demonstrate that rhIL-11 up-regulates Tie-2 expression during the healing of gastric ulcer in rats and suggest that rhIL-11 may have clinical benefits in the treatment of gastric ulcers.
Edited by Xu XQ
| 1. | Du XX, Williams DA. Interleukin-11: a multifunctional growth factor derived from the hematopoietic microenvironment. Blood. 1994;83:2023-2030. [PubMed] |
| 2. | Gordon MS, McCaskill-Stevens WJ, Battiato LA, Loewy J, Loesch D, Breeden E, Hoffman R, Beach KJ, Kuca B, Kaye J. A phase I trial of recombinant human interleukin-11 (neumega rhIL-11 growth factor) in women with breast cancer receiving chemotherapy. Blood. 1996;87:3615-3624. [PubMed] |
| 3. | Gordon MS. Thrombopoietic activity of recombinant human interleukin-11 in cancer patients receiving chemotherapy. Can-cer Chemother Pharmacol. 1996;38:S96-S98. |
| 4. | Keith JC, Albert L, Sonis ST, Pfeiffer CJ, Schaub RG. IL-11, a pleiotropic cytokine: exciting new effects of IL-11 on gastrointestinal mucosal biology. Stem Cells. 1994;12 Suppl 1:79-89; discussion 89-90. [PubMed] |
| 5. | Bruce ES, Simmy B, Charles AS, Malcolm R, Seymour K, John WS, Philip BM Jr, Michael AS, Susan G, Stephen BH. Preliminary evaluation of safety and activity of recombinant human interleukin 11 in patients with active Crohn's disease. Gastroenterology. 1999;177:58-64. |
| 6. | Wen CY, Ito M, Matsuu M, Fukuda E, Shichijo K, Nakashima M, Nakayama T, Sekine I. Mechanism of the antiulcerogenic effect of IL-11 on acetic acid-induced gastric ulcer in rats. Life Sci. 2002;70:2997-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Schnürch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119:957-968. [PubMed] |
| 8. | Maisonpierre PC, Goldfarb M, Yancopoulos GD, Gao G. Distinct rat genes with related profiles of expression define a TIE receptor tyrosine kinase family. Oncogene. 1993;8:1631-1637. [PubMed] |
| 9. | Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1319] [Cited by in RCA: 1251] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 10. | Wen CY, Ito M, Chen LD, Matsuu M, Shichijo K, Nakayama T, Nakashima M, Xu ZM, Ohtsuru A, Hsu CT. Expression of Tie-2 and angiopoietin-1 and -2 in early phase of ulcer healing. J Gastroenterol. 2003;38:431-435. [PubMed] |
| 11. | Tsukimi Y, Okabe S. Changes in gastric function and healing of chronic gastric ulcers in aged rats. Jpn J Pharmacol. 1995;68:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Potten CS. Interleukin-11 protects the clonogenic stem cells in murine small-intestinal crypts from impairment of their reproductive capacity by radiation. Int J Cancer. 1995;62:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Millauer B, Longhi MP, Plate KH, Shawver LK, Risau W, Ullrich A, Strawn LM. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;56:1615-1620. [PubMed] |
| 14. | Nakayama T, Ito M, Ohtsuru A, Naito S, Nakashima M, Fagin JA, Yamashita S, Sekine I. Expression of the Ets-1 proto-oncogene in human gastric carcinoma: correlation with tumor invasion. Am J Pathol. 1996;149:1931-1939. [PubMed] |
| 15. | Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci USA. 1993;90:9355-9358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 325] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Maisonpierre PC, Goldfarb M, Yancopoulos GD, Gao G. Distinct rat genes with related profiles of expression define a TIE receptor tyrosine kinase family. Oncogene. 1993;8:1631-1637. [PubMed] |
| 17. | Korhonen J, Polvi A, Partanen J, Alitalo K. The mouse tie receptor tyrosine kinase gene: expression during embryonic angiogenesis. Oncogene. 1994;9:395-403. [PubMed] |
| 18. | Tsukimi Y, Okabe S. Acceleration of healing of gastric ulcers induced in rats by liquid diet: importance of tissue contraction. Jpn J Pharmacol. 1994;66:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |