Published online Feb 15, 2003. doi: 10.3748/wjg.v9.i2.359
Revised: September 24, 2002
Accepted: October 18, 2002
Published online: February 15, 2003
AIM: To determine whether parenchymal cells or hepatic cytochrome P450 protein was changed in chronic liver diseases, and to compare the difference of CYP3A4 enzyme and its gene expression between patients with hepatic cirrhosis and obstructive jaundice, and to investigate the pharmacologic significance behind this difference.
METHODS: Liver samples were obtained from patients undergoing hepatic surgery with hepatic cirrhosis (n = 6) and obstructive jaundice (n = 6) and hepatic angeioma (controls, n = 6). CYP3A4 activity and protein were determined by Nash and western bloting using specific polychonal antibody, respectively. Total hepatic RNA was extracted and CYP3A4cDNA probe was prepared according the method of random primer marking, and difference of cyp3a4 expression was compared among those patients by Northern blotting.
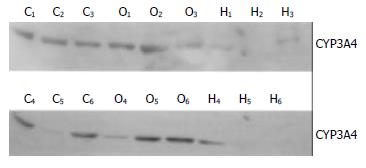
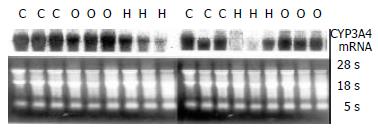
RESULTS: Compared to control group, the CYP3A4 activity and protein in liver tissue among patients with cirrhosis were evidently reduced. (P < 0.01) Northern blot showed the same change in its mRNA levels. In contrast, the isoenzyme and its gene expression were not changed among patients with obstructive jaundice.
CONCLUSION: Hepatic levels of P450s and its CYP3A4 isoform activity were selectively changed in different chronic liver diseases. CYP3A4 isoenzyme and its activity declined among patients with hepatic cirrhosis as expression of cyp3a4 gene was significantly reduced. Liver's ability to eliminate many clinical therateutic drug substrates would decline consequently, These findings may have practical implications for the use of drugs in patients with cirrhosis and emphasize the need to understand the metabolic fate of therapeutic compounds. Elucidation of the reasons for these different changes in hepatic CYP3A4 may provide insight into more fundamental aspects and mechanisms of imparied liver function.
- Citation: Yang LQ, Li SJ, Cao YF, Man XB, Yu WF, Wang HY, Wu MC. Different alterations of cytochrome P450 3A4 isoform and its gene expression in livers of patients with chronic liver diseases. World J Gastroenterol 2003; 9(2): 359-363
- URL: https://www.wjgnet.com/1007-9327/full/v9/i2/359.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i2.359
Hepatic cytochrome P450 enzymes constitute a superfamily of hemoproteins which play a major role in the metabolism of endogenous compounds and in the detoxification of xenobiotic molecules, includeing anesthetics and carcinogens[1-3]. About 200 CYPs have been found in the past 20 years, and many factors including age, gender, nutrition, hormone and general or local pathol ogic reaction affect CYPs, and the biotransformation of many clinical therapeutic drugs would be changed. P450 3A4 is one of the most important forms in human, mediating the metabolism of about 70% of therapeutic drugs and endogenous compounds[4-6].
Although the mechanism and consequences of regulation of P450s by drugs and chemicals have been intensively studied, the mechanisms by which P450s are changed by hepatic pathological factors still remained unclear[7-11]. Hepatic cirrhosis and obstructive jaundice are most common chronic hepatobiliary diseases among Chinese people, the change of CYPs with cirrhosis and jaundice provided us fundamental knowledge about the effect of pathological factors on P450s[12-15]. The aim of this study is to determine the alterations of CYP3A4 enzyme and its gene expression in patients with those chronic liver diseases, and to investigate the pharmacologic and clinical significance behind this alterations.
pBS M13 CYP3A4 plasmid was kindly provided by Prof Ying-Nian Yu (Zhe-jiang University, China). Rabbit anti-human CYP3A4 polyclonal antibody was purchased from Chemicon (San Diego, CA); HRP taged sheep anti-rabbit antibody was purchased from PharMingen (Mannheim, Germany); glucose 6- phosphoric acid, erythromycin, Lowry's phenol reagent, glucose 6-phosphoric transferase, acetic ammonium, acetyl-acetone, and NADP were purchased from Sigma Chemical (St. Louis, MO); and all other reagents used in this study were of analytical grade.
Human liver samples (30-50 g) were taken from patients undergoing hepatic surgery. Patients had not receive medication of CYPs activator and inhibitor (rifampicin, dexamethasone, propofol, etc.) before the surgery. None of the patients were habitual consumers of alcohol or other drugs. A total of 18 liver samples from 15 men and 3 women were used. They were all cases admitted from 2000 to 2001 in Eastern Hepatobiliary Surgery Hospital in Shanghai, China. Informed content was obtained from all patients for subsequent use of their specimen tissues. These specimens were immediately dissected into small pieces under aseptic condition within half an hour, quickly frozen and preserved in liquid nitrogen before subsequent procedure. Patients’ characters and liver function are shown in Table 1.
| Groups | Median Age (yrs) | Gender F/M | Smoking (n) | Ethanol (n) | Pugh Class A,B,C |
| C (n = 6) | 42 (21-56) | 2/4 | 2 | 1 | A (6) |
| H (n = 6) | 38 (28-61) | 1/5 | 1 | 1 | A (4), B (2) |
| O (n = 6) | 44 (27-65) | 0/6 | 1 | 2 | B (6) |
Liver tissues were subsequently homogenized in ice-cold 0.1 mol/L Tris-HCl buffer containing 1.15% KCl (pH7.4) and to yield a liver homogenate tissue concentration of 0.33 g/mL. Microsomal fractions were prepared by differential ultracentrifugation. After tissue homogenization in 20 mM Tris-HCl buffer, pH7.4, containing 0.15 M KCl, the microsomal fraction was isolated from the supernatant of a 20-min 9000 × g spin by ultracentrifugation. The microsomal precipitate was suspended in 100 mM potassium phosphate buffer, pH7.4, and recentrifuged at 105000 × g for an additional 60 min. The final precipitate was suspended in 10 Mm Tris-HCl buffer (pH7.4) containing 10 mM EDTA and 20% (v/v) glycerol. Liver microsomal protein contents were determined following the methods of Lowry et al[16], using bovine serum albumin as standard.
CO-bound total cytochrome P450 content was determined by the method of Omura et al[17]. Spectra were recorded using a Shimadzu UV-250 double-beam spectrophotometer. CYP3A4 specific activity was determined by N-demethylation of erythromycin using the Nash method as previously described[18].
Hepatic microsomal proteins were resolved by SDS-PAGE with vertical mini-gel electrophoresis equipment. Samples of liver microsomal protein (10 μg/lane) were denatured in 10 μL loading buffer (4 mL distilled water, 1 mL 0.5M Tris-HCl, pH6.8, 0.8 mL glycerol, 1.6 mL 10% w/v SDS, 0.4 mL mercaptoethanol, 0.05 mL 0.05% w/v Pyronin Y) and were separated on a 10% w/v resolving gel. Proteins were transferred from the polyacrylamide gel to the nitrocellulose sheets by an electrophoretic method, and probed with rabbit anti-human CYP3A4 polyclonal antibody (not cross-reactive with other rat P450s) according to supplied protocol. CYP3A4 protein was detected by secondary conjugation to the primary antibody by a HRP-linked sheep anti-rabbit second antibody using diaminobenzidine as substrate.
Total RNA was isolated from frozen human liver tissues by the acid guanidinium thiocyanate-phenol-chloroform one step extraction method as previously described[19], 20 mg of RNA was size-fractionated on a 1.0% agarose gel containing 2.2 mol/L formaldehyde, and then transferred into nitrocellulose membrane (BA85, Schleicher Schuell, Germany). The membrane wes dried in a vacuum drying over at 80 °C for 2 h and sealed in a plastic bag for use. CYP3A4 probe wes cut from pBS M13 CYP3A4 plasmid by Hand III. Hybridization was performed in the presence of the appropriate 32P-labeled probes. The membrane was washed twice at room temperture in 2 × SSC, 0.1% SDS for 30 min, once at 65 °C in 1 × SSC, 0.1% SDS for 30 min and once at 65 °C in 0.1 × SSC, 0.1% SDS for 30 min. Membranes was then exposed to X-ray films (Fujifilms, Tokyo, Japan) at -70 °C for a week and analyzed by Phosphor Image (FLA 2000, Fujifilm, Japan). The difference of CYP3A4mRNA was compared among three groups.
Data was analyzed using the χ2 test. A P < 0.05 was considerd significant.
As shown in Table 2, compared with controls, the hepatic microsome protein and total P450 content remained unchange in the patients with hepatic cirrhosis and obstructive jaundice, but CYP3A4 activity in the liver tissue of patients with cirrhosis liver was evidently reduced. This change was not seen in the obstructive jaundice group.
| C | H | O | |
| Microsome protein (g/L) | 10.32 ± 3.98 | 9.57 ± 3.72 | 9.42 ± 3.26 |
| P450 content (nmol/mg protein) | 0.99 ± 0.16 | 0.94 ± 0.151 | 0.89 ± 0.18 |
| CYP3A4 activity (nmol/min/mg protien) | 3.01 ± 0.74 | 1.78 ± 0.653a | 2.89 ± 0.65 |
Hepatic CYP3A4 protein expression was shown in Figure 1 by western blot analysis. CYP3A4 protein in liver tissues was also reduced in the patients with cirrhosis liver, but in obstructive jaundice, there was no change of as compared with controls.
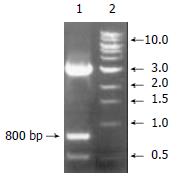
As shown in Figure 2, CYP3A4 probe was cut from pBS M13 CYP3A4 plasmid by Hand III, we got a 800 bp cDNA fragment as expected.
Northern blot analysis showed that CYP3A4 was expressed well in human liver tissues, which agreed with other reports[20-23]. In patients with cirrhosis (shown in Figure 3), CYP3A4mRNA reduced significantly as compared with controls, but no change happened in the jaundice group.
CYP3A appears to be one of the most important human enzymes as approximately 60% of oxidised drugs are biotransformed. The isoforms of CYP3A in humans include 3A3, 3A4, 3A5 and 3A7, each of these enzymes shared at least 85% amino acid sequence homology[24]. CYP3A4 is the predominant isoform of CYP3A in adult humans. It can catalyse a remarkable number of metabolic processes including aliphatic oxidation, aromatic hydroxylation, N-dealkylation, O-demethylation, S-demethylation, oxidative deamination, sulfoxide formation, N-oxidation and N-hydroxylation. This usually produced inactivation and elimination of most pharmaceuticals. A number of drugs from a broad range of therapeutic categories are CYP3A4 substrates. The change of CYP3A4 isform was the main reason for enhancement or reduction of drug elimination[25-27].
In our studies, total P450 contents of 18 Chinese patients were obviously lower than results those reported about Caucasian. (1 pmol/mg vs 5-6 pmol/mg), and the activity of CYP3A4 isoform was also lower[28-30]. Although CYP3A4 drug metabolizing activity varied widely among individuals, it had a unimodal population distribution and did not appear to be subject to genetic polymorphism as seen with other CYP isoforms (2D6, 2C9 and 2C19)[31-34]. The wide inter races variability was likely, in part, to be caused by ethnic or cultural differences, which might be related to an interaction between habit and diet. Therefore we could not draw any conclusion about the normal distribution character of CYPs in Chinese because of the limited sample number and experimental conditions. More detailed and complete studies should be performed for analysising the distribution of CYPs in Chinese in the near future[35].
Most information on drug metabolism impairment at pathologic status has been obtained in rodent in vivo or in vitro models, and most of these studies have focused on the effects of IFNs and the major inflammatory cytokines, namely, IL-6, IL1 and TNFα[36-39], but relatively few studies have examined the effect of liver disease on human CYP expression. Hepatic cirrhosis and obstructive jaundice are most common chronic hepatobiliary disease in Chinese, the change of CYPs with cirrhosis and jaundice can provide us basic knowledge about the effect of pathological factors on P450s. The present study demonstrated that, in patients with cirrhosis, CYP3A4-mediated erythromycin N-demethylation activity and 3A4 protein were significantly less than in controls, but the total P450 content and hepatic microsome protein still remained unchanged. These results suggest that family ingredients of P450s have changed in the cirrhosis. That is, CYP family 1, 2 may enhance following with CYP3A reduced, since CYP1 and CYP2 families play a major role in biotransformation of most carcinogens, but few studies described whether high morbidity of hepatic cancer in cirrhosis is correlated with these changes of drug metabolic enzymes[40].
Although many factors including age, gender, nutrition, hormone and general or local pathologic reaction affect drug elimination, the enzymatic activity as well as content of P450s is still a basic reason for change of drug metabolism, and the biotransformation of many clinical therapeutic drugs either enhanced or reduced[41-43]. This study is for the first time to examine simultaneously in patients with liver diseases the hepatic P450 protein level, isoform activity as well as its mRNA expression. Significant correlations with CYP3A4 protein level, isoform activity and mRNA expression were observed, suggesting that with the decrease of CYP3A4 mRNA expression, RNA encoded CYP3A4 isoform protein reduced, which would cause the decrease of CYP3A4-mediated erythromycin N-demethylation activity. Since CYP3A4 is the predominant isoform of CYP3A in adult humans, the Change of hepatic CYP3A4 activity will change the metabolism of most clinical therapeutic drugs. Firstly, a large number of intravenous anesthetic and sedative agents (including diazepam, midozolam, fentanyl, lidocaine, etc.) are substrates of CYP3A4 isoform, N-hydroxylation and N-dealkylation reactions of anesthetics reduced in cirrhosis patiens will cause drug raccumulation, oversedative and postoperative awake delay[44,45]. Secondly, Amiodarone, quinidine, nifedipine, berhomine and Cyclosporin were also eliminated through CYP3A4, thus competitive inhibition should be noticed and avoided especially when more than one drugs must be administrated in patients with cirrhosis. These findings are in agreement with pharmacokinetics studies that have shown reduced clearance of midozolam when combined with fentanyl in cirrhosis, but over-dosage condition of anti-irrhythmia drugs had more clinical signifiance than that of other therapeutic drugs[46,47]. Thirdly, as CYP3A4 also plays an important role in the biotransformation and detoxification of many endogenetic substrates, reduction of CYP3A4 activity may result in inactivation disorder of endogenetic substance including cholesterol, bile acid and sex steroids, thus causing more extensive physiopathologic changes in patients with cirrhosis, these changes, on contrary, will affect the drug metabolic enzymes[48,49].
In summary, the present study demonstrated that, hepatic levels of individual P450s and its CYP3A4 isoform activity can selectively change in different chronic liver diseases. The hepatic microsome proteins and total P450 content remained unchanged in patients with hepatic cirrhosis and obstructive jaundice , but CYP3A4 activity and its protein level in liver tissue among patients with cirrhosis were evidently lowered. This change was not seen in obstructive jaundice group, and the cause of this change may be the lowered expression of CYP3A4 mRNA. These findings may have practical implications for the use of drugs in patients with liver diseases and emphasize the need to understand the metabolic fate of therapeutic compounds[50,51]. Elucidation of the reasons for these different changes in hepatic P450s may provide insight into more fundamental aspects and mechanisms of impaired liver function in patients with chronical liver diseases.
Surported by Military Medical Science Found of China, No.98Q050
Edited by Ma JY
| 1. | Kostrubsky VE, Ramachandran V, Venkataramanan R, Dorko K, Esplen JE, Zhang S, Sinclair JF, Wrighton SA, Strom SC. The use of human hepatocyte cultures to study the induction of cytochrome P-450. Drug Metab Dispos. 1999;27:887-894. [PubMed] |
| 2. | Nebert DW, Nelson DR, Coon MJ, Estabrook RW, Feyereisen R, Fujii-Kuriyama Y, Gonzalez FJ, Guengerich FP, Gunsalus IC, Johnson EF. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991;10:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 733] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 3. | Capdevila JH, Harris RC, Falck JR. Microsomal cytochrome P450 and eicosanoid metabolism. Cell Mol Life Sci. 2002;59:780-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 934] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 5. | Raucy JL, Allen SW. Recent advances in P450 research. Pharmacogenomics J. 2001;1:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Zhu-Ge J, Yu YN, Qian YL, Li X. Establishment of a transgenic cell line stably expressing human cytochrome P450 2C18 and identification of a CYP2C18 clone with exon 5 missing. World J Gastroenterol. 2002;8:888-892. [PubMed] |
| 7. | Goodwin B, Redinbo MR, Kliewer SA. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu Rev Pharmacol Toxicol. 2002;42:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 246] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 8. | Quattrochi LC, Guzelian PS. Cyp3A regulation: from pharmacology to nuclear receptors. Drug Metab Dispos. 2001;29:615-622. [PubMed] |
| 9. | Waxman DJ. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys. 1999;369:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 515] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Nicholson TE, Renton KW. Modulation of cytochrome P450 by inflammation in astrocytes. Brain Res. 1999;827:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Dogra SC, Whitelaw ML, May BK. Transcriptional activation of cytochrome P450 genes by different classes of chemical inducers. Clin Exp Pharmacol Physiol. 1998;25:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257:59-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 258] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Paintaud G, Bechtel Y, Brientini MP, Miguet JP, Bechtel PR. Effects of liver diseases on drug metabolism. Therapie. 1996;51:384-389. [PubMed] |
| 14. | Brockmöller J, Roots I. Assessment of liver metabolic function. Clinical implications. Clin Pharmacokinet. 1994;27:216-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Murray M. P450 enzymes. Inhibition mechanisms, genetic regulation and effects of liver disease. Clin Pharmacokinet. 1992;23:132-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 102] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Lowry OH, Passonneau JV. Some recent refinements of quantitative histochemical analysis. Curr Probl Clin Biochem. 1971;3:63-84. [PubMed] |
| 17. | Omura T. Forty years of cytochrome P450. Biochem Biophys Res Commun. 1999;266:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Hover CG, Kulkarni AP. Lipoxygenase-mediated hydrogen peroxide-dependent N-demethylation of N,N-dimethylaniline and related compounds. Chem Biol Interact. 2000;124:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40518] [Cited by in RCA: 39100] [Article Influence: 1028.9] [Reference Citation Analysis (0)] |
| 20. | Goodwin B, Hodgson E, D'Costa DJ, Robertson GR, Liddle C. Transcriptional regulation of the human CYP3A4 gene by the constitutive androstane receptor. Mol Pharmacol. 2002;62:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Lin YS, Dowling AL, Quigley SD, Farin FM, Zhang J, Lamba J, Schuetz EG, Thummel KE. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol Pharmacol. 2002;62:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Finnström N, Thörn M, Lööf L, Rane A. Independent patterns of cytochrome P450 gene expression in liver and blood in patients with suspected liver disease. Eur J Clin Pharmacol. 2001;57:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Rodríguez-Antona C, Donato MT, Pareja E, Gómez-Lechón MJ, Castell JV. Cytochrome P-450 mRNA expression in human liver and its relationship with enzyme activity. Arch Biochem Biophys. 2001;393:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Patel J, Mitra AK. Strategies to overcome simultaneous P-glycoprotein mediated efflux and CYP3A4 mediated metabolism of drugs. Pharmacogenomics. 2001;2:401-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet. 1999;37:485-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 411] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Hakkola J, Pelkonen O, Pasanen M, Raunio H. Xenobiotic-metabolizing cytochrome P450 enzymes in the human feto-placental unit: role in intrauterine toxicity. Crit Rev Toxicol. 1998;28:35-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 152] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Inaba T, Nebert DW, Burchell B, Watkins PB, Goldstein JA, Bertilsson L, Tucker GT. Pharmacogenetics in clinical pharmacology and toxicology. Can J Physiol Pharmacol. 1995;73:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | El-Sankary W, Bombail V, Gibson GG, Plant N. Glucocorticoid-mediated induction of CYP3A4 is decreased by disruption of a protein: DNA interaction distinct from the pregnane X receptor response element. Drug Metab Dispos. 2002;30:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Hijazi Y, Boulieu R. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2002;30:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Shu Y, Cheng ZN, Liu ZQ, Wang LS, Zhu B, Huang SL, Ou-Yang DS, Zhou HH. Interindividual variations in levels and activities of cytochrome P-450 in liver microsomes of Chinese subjects. Acta Pharmacol Sin. 2001;22:283-288. [PubMed] |
| 31. | Tennezé L, Tarral E, Ducloux N, Funck-Brentano C. Pharmacokinetics and electrocardiographic effects of a new controlled-release form of flecainide acetate: comparison with the standard form and influence of the CYP2D6 polymorphism. Clin Pharmacol Ther. 2002;72:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Cai L, Yu SZ, Zhan ZF. Cytochrome P450 2E1 genetic polymorphism and gastric cancer in Changle, Fujian Province. World J Gastroenterol. 2001;7:792-795. [PubMed] |
| 33. | Zheng YX, Chan P, Pan ZF, Shi NN, Wang ZX, Pan J, Liang HM, Niu Y, Zhou XR, He FS. Polymorphism of metabolic genes and susceptibility to occupational chronic manganism. Biomarkers. 2002;7:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Dahl ML. Cytochrome p450 phenotyping/genotyping in patients receiving antipsychotics: useful aid to prescribing. Clin Pharmacokinet. 2002;41:453-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Bertilsson L. Geographical/interracial differences in polymorphic drug oxidation. Current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin Pharmacokinet. 1995;29:192-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 288] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Renton KW. Alteration of drug biotransformation and elimination during infection and inflammation. Pharmacol Ther. 2001;92:147-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | Warren GW, van Ess PJ, Watson AM, Mattson MP, Blouin RA. Cytochrome P450 and antioxidant activity in interleukin-6 knockout mice after induction of the acute-phase response. J Interferon Cytokine Res. 2001;21:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | De Smet K, Loyer P, Gilot D, Vercruysse A, Rogiers V, Guguen-Guillouzo C. Effects of epidermal growth factor on CYP inducibility by xenobiotics, DNA replication, and caspase activations in collagen I gel sandwich cultures of rat hepatocytes. Biochem Pharmacol. 2001;61:1293-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Okamoto T, Okabe S. Minimal effect of cytokine-independent hepatitis induced by anti-Fas antibodies on hepatic cytochrome P450 gene expression in mice. Int J Mol Med. 2000;6:459-462. [PubMed] |
| 40. | Shoda T, Mitsumori K, Onodera H, Toyoda K, Uneyama C, Takada K, Hirose M. Liver tumor-promoting effect of beta-naphthoflavone, a strong CYP 1A1/2 inducer, and the relationship between CYP 1A1/2 induction and Cx32 decrease in its hepatocarcinogenesis in the rat. Toxicol Pathol. 2000;28:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Tanaka E. Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther. 1999;24:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Morgan ET, Sewer MB, Iber H, Gonzalez FJ, Lee YH, Tukey RH, Okino S, Vu T, Chen YH, Sidhu JS. Physiological and pathophysiological regulation of cytochrome P450. Drug Metab Dispos. 1998;26:1232-1240. [PubMed] |
| 43. | Imaoka S, Funae Y. [The physiological role of P450-derived arachidonic acid metabolites]. Nihon Yakurigaku Zasshi. 1998;112:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Vuyk J. Clinical interpretation of pharmacokinetic and pharmacodynamic propofol-opioid interactions. Acta Anaesthesiol Belg. 2001;52:445-451. [PubMed] |
| 45. | Oda Y, Mizutani K, Hase I, Nakamoto T, Hamaoka N, Asada A. Fentanyl inhibits metabolism of midazolam: competitive inhibition of CYP3A4 in vitro. Br J Anaesth. 1999;82:900-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Nims RW, Prough RA, Jones CR, Stockus DL, Dragnev KH, Thomas PE, Lubet RA. In vivo induction and in vitro inhibition of hepatic cytochrome P450 activity by the benzodiazepine anticonvulsants clonazepam and diazepam. Drug Metab Dispos. 1997;25:750-756. [PubMed] |
| 47. | Iribarne C, Dréano Y, Bardou LG, Ménez JF, Berthou F. Interaction of methadone with substrates of human hepatic cytochrome P450 3A4. Toxicology. 1997;117:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Ourlin JC, Handschin C, Kaufmann M, Meyer UA. A Link between cholesterol levels and phenobarbital induction of cytochromes P450. Biochem Biophys Res Commun. 2002;291:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos. 2001;29:1467-1472. [PubMed] |
| 50. | Hung DY, Chang P, Cheung K, McWhinney B, Masci PP, Weiss M, Roberts MS. Cationic drug pharmacokinetics in diseased livers determined by fibrosis index, hepatic protein content, microsomal activity, and nature of drug. J Pharmacol Exp Ther. 2002;301:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 872] [Article Influence: 37.9] [Reference Citation Analysis (0)] |