Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2261
Revised: July 25, 2003
Accepted: August 2, 2003
Published online: October 15, 2003
AIM: To investigate the effect of epidermal growth factor (EGF) on mucosal healing in rats with duodenal ulcer.
METHODS: Male Sprague-Dawley rats were randomly divided into sham operation without EGF, sham operation with EGF, duodenal ulcer without EGF, or duodenal ulcer with EGF groups. Additionally, normal rats without operation served as the control group. Duodenal ulcer was induced in rats by 300 mL/L acetic acid. Rats with EGF were orally administered at a dose of 60 μg/kg/day in drinking water on the next day of operation (day 1). Healing of duodenal ulcer was detected by haematoxylin and eosin staining. Cell growth of damaged mucosa was determined by the contents of nucleic acids and proteins. The level of EGF in duodenal mucosa was measured by ELISA.
RESULTS: The pathological results showed that duodenal ulcer rats with EGF improved mucosal healing compared with those without EGF after day 5. Duodenal ulcer rats with EGF significantly increased duodenal DNA content compared with those without EGF on day 15 (6.44 ± 0.54 mg/g vs 1.45 ± 0.52 mg/g mucosa, P < 0.05). Duodenal RNA and protein contents did not differ between duodenal ulcer rats with and without EGF during the experimental period. Sham operation and duodenal ulcer rats with EGF significantly increased duodenal mucosal EGF content compared with those without EGF on day 5 (76.0 ± 13.7 ng/g vs 35.7 ± 12.9 ng/g mucosa in sham operation rats, and 68.3 ± 10.9 ng/g vs 28.3 ± 9.2 ng/g mucosa in duodenal ulcer rats, P < 0.05).
CONCLUSION: Oral EGF can promote mucosal healing of the rats with duodenal ulcer by stimulating mucosal proliferation accompanied by an increase in mucosal EGF content.
- Citation: Chao JC, Liu KY, Chen SH, Fang CL, Tsao CW. Effect of oral epidermal growth factor on mucosal healing in rats with duodenal ulcer. World J Gastroenterol 2003; 9(10): 2261-2265
- URL: https://www.wjgnet.com/1007-9327/full/v9/i10/2261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i10.2261
Epidermal growth factor (EGF) is present in various body fluids and tissues, and is continuously secreted into the gastrointestinal lumen in humans by submandibular glands, mucous neck cells of the stomach, Brunner’s glands of the duodenum, Paneth cells of the small intestine, and ulcer-associated cell lineage (a recently identified glandular structure induced at the sites of injury)[1-3]. EGF and EGF family of related peptides are involved as key constituents in the maintenance and repair of gastrointestinal mucosa[4]. There has been evidence that increases in the EGF receptor and EGF producing cells around acetic acid-induced gastric ulcer in rats, and a novel cell lineage in human mucosal ulceration secreting EGF adjacent to peptic ulcer[3,5]. The results suggest that EGF plays an important role in ulcer healing.
Previous studies showed that EGF administration regulated the healing of ulcers in rats[6,7] and humans[8]. Oral administration of EGF, given at 30 μg/kg/day in the drinking water for 25 or 50 d, promoted the healing of cysteamine HCl-induced duodenal ulcer in rats to the same extent as cimetidine, a H2-receptor antagonist[6] . However, Kuwahara et al[7] demonstrated human EGF, given orally twice daily at 30 and 100 μg/kg for 2 weeks or at 100 μg/kg for 4 weeks, had no effect on natural healing of acetic acid-induced gastric ulcer in rats. It has been controversial if orally administered EGF, a feasible and easy way in clinical therapy, is effective to promote the healing of duodenal ulcer. Therefore, the purpose of the study was to investigate the effect of orally administered EGF on the healing of intestinal mucosa and the content of EGF in acetic acid-induced duodenal ulcer rats.
Male Sprague-Dawley rats (-200 g) were purchased from the National Laboratory Animal Center (National Science Council, Taipei, Taiwan). The rats were housed in individual cages and had free access to food (powdered laboratory autoclavable rodent diet 5010, PMI Nutrition International Inc., Brentwood, MO), except for the fasting period. The light cycle was 12 h and the room temperature was kept at 22-24 °C. The rats were randomly divided into four operated groups: sham operation without EGF, sham operation with EGF, duodenal ulcer without EGF, and duodenal ulcer with EGF groups (n = 10 on each sacrificed day, 6 rats for biological analysis, and 4 rats for pathological examination and photography). Additionally, normal rats (n = 10) without operation served as the control group. Duodenal ulcer was induced in rats by acetic acid according to the modified method of Konturek et al[9]. Prior to operation, the rats were fasted overnight, anesthetized by intraperitoneal injection with 50 mg/kg body weight thiopental sodium (Abbott Australia Pty.Ltd., Kurnell, Australia), and the abdomen was then opened. A plastic tube (4.5 mm inner diameter) filled with 70 μL of 300 mL/L acetic acid was applied tightly to the surface of the duodenum for 10 sec. Due to different tolerance to acetic acid in various layers of the duodenum, it only caused immediate necrosis in the mucosal and submucosal layers exactly within the area (4.5 mm diameter) of acetic acid application without penetration or perforation to the surrounding organs. Normal saline instead of 300 mL/L acetic acid was applied to sham operation rats. After operation, the rats were allowed to recover from anesthesia. The operated rats received only water on the day of operation (day 0), and were fed a normal chow diet ad libitum next day (day 1). Body weight, food intake, and water intake of the rats were routinely recorded. All protocols were conducted under the guidelines of Animal Care and Use Committee, Taipei Medical University.
The next day after operation at approximately 15:00, the rats were orally administered recombinant human EGF (60 μg/kg body weight) (Biosource International, Camarillo, CA) in 35 mL (minimal intake during the adaptation period) sterile deionized drinking water, and remaining water was exactly recorded to determine actual intake of EGF. The rats without oral EGF were given the same amount of sterile deionized drinking water. The operated rats were killed on day 1 to identify the formation of duodenal ulcer, and on days 5 and 15 at 15:00 for pathological and biological analyses. The duodenum (5 × 5 mm) was excised, preserved in 10% formaldehyde, and stained with haematoxylin and eosin. The diameter of ulcer size was measured in sectioned samples by microscopy. Coded mucosal specimens were evaluated under a light microscope at × 100 or × 200 magnification by a pathologist in a blinded fashion.
The ulcer area of duodenal mucosa in duodenal ulcer rats and a similar area of intact duodenal mucosa in sham operation rats were excised. Duodenal mucosa was entirely scraped off, weighed, and immediately frozen at -80 °C for further analysis. DNA, RNA, and protein in duodenal mucosa were purified using a commercial TRIZOL reagent (Life Technologies, Inc., Rockville, MD) to evaluate mucosal growth[10]. Duodenal mucosa (0.2-0.5 g wet weight) was homogenized in 1 mL of TRIZOL reagent followed by the addition of 200 μL chloroform. After centrifugation, RNA remained exclusively in the aqueous phase, and DNA and protein were then recovered by sequential precipitation. RNA was precipitated with isopropanol. DNA was precipitated with ethanol from the interphase, and protein was precipitated with isopropanol from the organic phase after separation from DNA. DNA, RNA, and protein pellets were resuspended in 8 mmol/L NaOH, diethylpyrocarbonate-water, or 1 mol/L NaOH, respectively. DNA and RNA were quantitated spectrophotometrically at 260 nm. Protein content was determined spectrophotometrically at 690 nm by a Bio-Rad Dc protein assay kit (Bio-Rad Laboratories, Hercules, CA).
Duodenal mucosal EGF content was measured by a commercial EGF immunoassay kit (QuantikineTM, DEG00, Research and Diagnostics Systems, Inc., Minneapolis, MN)[11]. Duodenal mucosa (0.2-0.3 g wet weight) was homogenized with RD1 reagent. The mucosal homogenate (200 μL) was incubated with EGF antibody coated in a 96-well plate for 2 h at room temperature, washed 3 times with 400 μL wash buffer, and then incubated with 200 μL polyclonal EGF antibody conjugated to horseradish peroxidase for 2 h. After several washes, samples were incubated with 200 μL substrate (tetramethylbenzidine:H2O2 = 1:1) for 20 min. The reaction was terminated by 50 μL of 1 mol/L sulfuric acid. Mucosal EGF content was determined at 450 nm and corrected at 540 nm using an ELISA reader (Multiskan RC, Labsystems, Helsinki, Finland).
All values were expressed as mean ± SE. Data were analyzed by three-way ANOVA to determine the main effects of duodenal ulcer, oral EGF, and time using SAS 6.12 (SAS Institute, Cary, NC). Post hoc multiple comparisons between two groups were performed either by Fisher’s least significant difference or Dunnett’s test. Differences were considered significant at P < 0.05.
Body weight of sham operation and duodenal ulcer rats with or without EGF on different sacrificed days is shown in Table 1. At the beginning of the experiment, body weight of the rats was similar in all operated groups and the control group (189.0 ± 4.5 g). Body weight of sham operation and duodenal ulcer rats with or without EGF significantly increased (P < 0.05) on day 15 compared with that of the control group on day 0 and the corresponding group on day 5. Oral EGF did not affect body weight.
| Group | Sham operation | Duodenal ulcer | ||
| -EGF | +EGF | -EGF | +EGF | |
| Body weight before operation (g)2 | ||||
| Day 5 | 192.0 ± 3.9 | 199.8 ± 3.8 | 200.6 ± 5.0 | 195.2 ± 5.0 |
| Day 15 | 192.4 ± 4.8 | 198.9 ± 4.2 | 194.9 ± 4.7 | 195.9 ± 6.0 |
| Body weight before sacrifice (g)2 | ||||
| Day 5 | 203.6 ± 4.0* | 209.9 ± 3.6* | 208.1 ± 4.7* | 200.5 ± 4.9 |
| Day 15 | 271.4 ± 5.5*† | 279.8 ± 7.7*† | 267.1 ± 7.7*† | 278.8 ± 5.3*† |
Exact oral EGF intake of the rats was close to 60 μg/kg/day, and not significantly different between sham operation and duodenal ulcer groups, and between different days in the same group (Table 2). The initial food intake of the rats before operation was similar to that in the control group (26.7 ± 3.1 g/day), and not significantly different among the groups. On day 15, decreased food intake was only observed in duodenal ulcer rats without EGF compared with that in sham operation rats. Neither EGF nor time in sham operation or duodenal ulcer rats affected food intake.
| Group | Sham operation | Duodenal ulcer | ||
| -EGF | +EGF | -EGF | +EGF | |
| EGF intake (μg/kg/day)2 | ||||
| Day 5 | -3 | 59.9 ± 3.8 | - | 57.8 ± 0.4 |
| Day 15 | - | 57.3 ± 0.6 | - | 58.1 ± 0.3 |
| Food intake before operation (g/day)2 | ||||
| Day 5 | 24.5 ± 1.1 | 25.2 ± 0.8 | 26.5 ± 0.5 | 25.4 ± 0.9 |
| Day 15 | 25.1 ± 0.5 | 25.3 ± 0.4 | 26.6 ± 0.8 | 24.8 ± 0.6 |
| Food intake before sacrifice (g/day) | ||||
| Day 5 | 27.9 ± 4.3a | 27.4 ± 6.4a | 24.2 ± 3.6a | 25.6 ± 5.4a |
| Day 15 | 27.2 ± 1.8b | 26.7 ± 1.0b | 25.1 ± 1.4a | 25.2 ± 1.8ab |
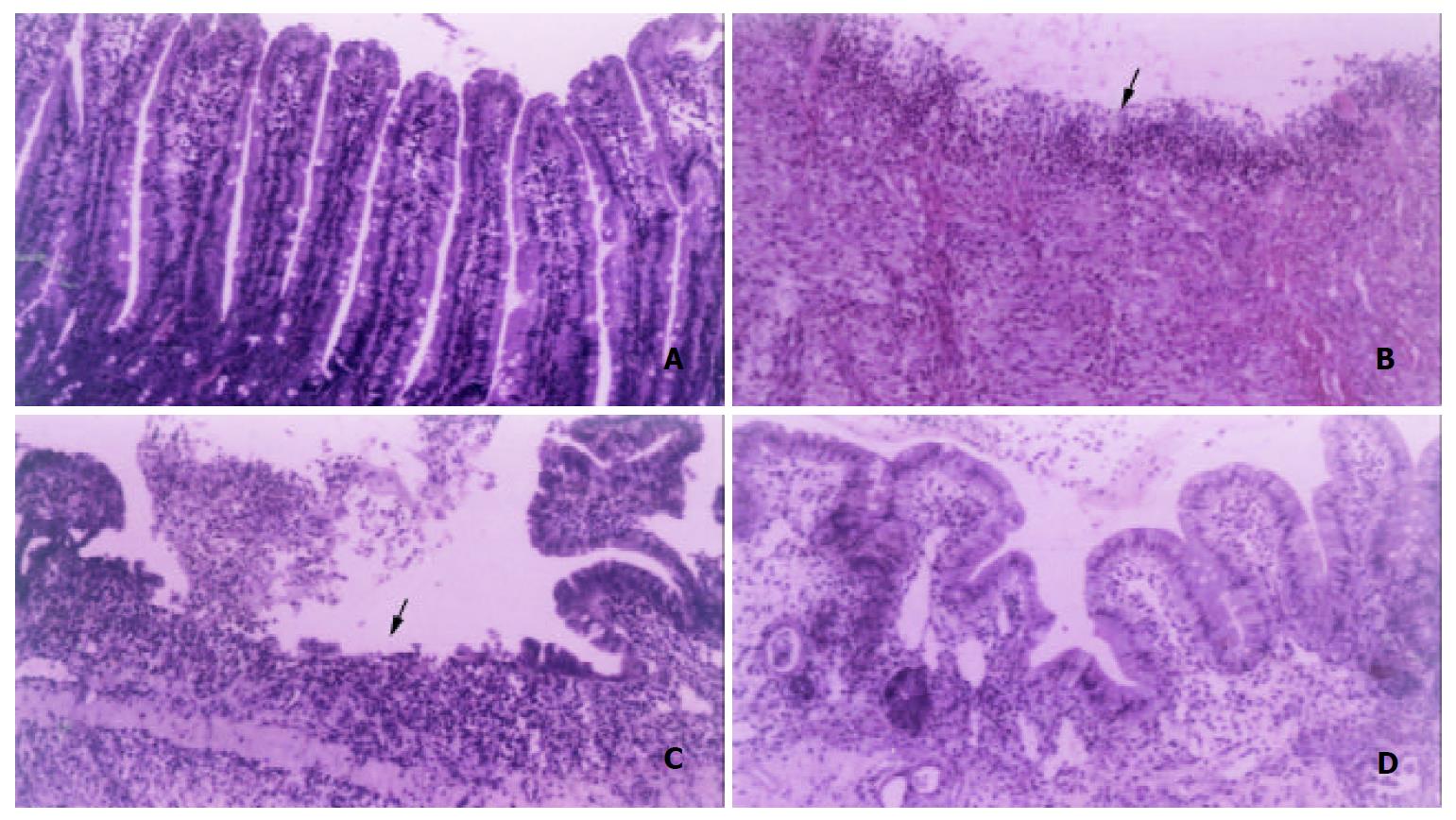
To identify the formation and healing of duodenal ulcer in rats after operation, the morphological appearance magnified by × 100 or × 200 is shown in Figure 1. Compared with the control group (Figure 1A), duodenal ulcer rats on day 1 had the discontinuous lining of the mucosal and submucosal layers, and serious inflammation (Figure 1B). The mean diameter of ulcer damage was 2 mm. Sham operation rats had similar morphology as the control rats (data not shown). The morphology was not different between sham operation and the control rats on days 5 and 15. On day 5, the mean diameter of ulcer damage reduced to 1 mm in duodenal ulcer rats without EGF. Although some microvilli proliferated, but the discontinuous lining of the mucosal layer and inflammation were still observed in the ulcer area of duodenal ulcer rats without EGF (Figure 1C). Duodenal ulcer rats with EGF had obvious mucosal healing and proliferation (Figure 1D), and the mean diameter of ulcer damage was undetectable on day 5. On day 15, the morphology was not different between duodenal rats with and without EGF (data not shown).
The results of mucosal DNA, RNA, and protein contents in the duodenum are shown in Table 3 to evaluate the growth of duodenal mucosa in rats after operation. Mucosal DNA content in the duodenum significantly increased (P < 0.05) in sham operation rats without EGF (2.96 ± 1.27 mg/g mucosa) and duodenal ulcer rats with EGF (6.44 ± 0.54 mg/g mucosa) on day 15 compared with that in the control rats (0.86 ± 0.06 mg/g mucosa) on day 0 and the corresponding group on day 5 (Table 3). Duodenal ulcer rats with EGF had higher duodenal DNA content (P < 0.05) on day 15 compared with other groups. Three-way ANOVA showed both oral EGF and time significantly increased duodenal DNA content (P < 0.05).
| Group | Sham operation | Duodenal ulcer | ||
| -EGF | +EGF | -EGF | +EGF | |
| Duodenal mucosal DNA (mg/g mucosa) | ||||
| Day 5 | 1.06 ± 0.20a | 1.51 ± 0.57a | 1.31 ± 0.45a | 0.70 ± 0.25a |
| Day 15 | 2.96 ± 1.27a*† | 1.77 ± 0.64a | 1.45 ± 0.52a | 6.44 ± 0.54b*† |
| Duodenal mucosal RNA (mg/g mucosa) | ||||
| Day 5 | 4.89 ± 0.42a | 7.10 ± 0.20b | 4.71 ± 0.35a* | 3.32 ± 0.82a* |
| Day 15 | 5.24 ± 1.60a | 6.00 ± 0.30a | 5.38 ± 0.44a | 4.79 ± 0.91a |
| Duodenal mucosal protein (mg/g mucosa) | ||||
| Day 5 | 49.8 ± 2.9a* | 20.5 ± 5.4b | 22.7 ± 2.8b | 25.5 ± 3.3b |
| Day 15 | 25.2 ± 2.8a† | 19.3 ± 2.7a | 24.3 ± 1.6a | 26.2 ± 1.3a |
Mucosal RNA content in the duodenum did not change with EGF treatment and time in sham operation and duodenal ulcer rats (Table 3). However, duodenal ulcer significantly decreased mucosal RNA content (P < 0.05). Duodenal ulcer rats had significantly lower RNA content (P < 0.05) in duodenal mucosa on day 5 compared with the control rats (15.9 ± 9.3 mg/g mucosa) on day 0. Sham operation rats with EGF had higher duodenal RNA content (P < 0.05) than other operated groups on day 5. On day 15, duodenal RNA content did not differ among four operated groups.
Three-way ANOVA showed that oral EGF and time affected duodenal protein content (P < 0.05). Duodenal protein content in sham operation rats without EGF significantly decreased (P < 0.05) with time, but significantly increased (P < 0.05) compared with that in the control group (25.1 ± 1.4 mg/g mucosa) on day 0 and other operated groups on day 5 (Table 3). On day 15, duodenal protein content did not differ among four operated groups.
Duodenal EGF content significantly increased (P < 0.05) with time in sham operation rats without EGF, but significantly decreased (P < 0.05) in duodenal ulcer rats with EGF (Table 4). Sham operation or duodenal ulcer rats with EGF significantly increased duodenal EGF content (P < 0.05) compared with those without EGF on day 5. However, duodenal EGF content did not differ between sham operation and duodenal ulcer rats with or without EGF on day 5. On day 15, duodenal EGF content significantly decreased (P < 0.05) in duodenal ulcer rats without EGF (12.9 ± 3.9 ng/g mucosa) compared with that in the control rats (61.1 ± 5.4 ng/g mucosa) on day 0 and sham operation rats without EGF (84.8 ± 22.8 ng/g mucosa). However, duodenal EGF content did not differ between sham operation or duodenal ulcer rats with and without EGF. Three-way ANOVA showed only duodenal ulcer significantly decreased mucosal EGF content (P < 0.05).
| Group | Sham operation | Duodenal ulcer | ||
| -EGF | +EGF | -EGF | +EGF | |
| Duodenal mucosal EGF (ng/g mucosa) | ||||
| Day 5 | 35.7 ± 12.9ab | 76.0 ± 13.7c | 28.3 ± 9.2a | 68.3 ± 10.9bc |
| Day 15 | 84.8 ± 22.8a† | 47.5 ± 19.3ab | 12.9 ± 3.9b* | 28.3 ± 10.5b† |
Duodenal ulcer per se and oral EGF at a dose of 60 μg/kg/day did not obviously affect body weight of the rats in this study. However, Majumdar[12] found administration of EGF (20 μg/kg/day, ip. injection) to undernourished weanling rats for 7 d significantly reversed the decreased weight of whole body, small intestine, and oxyntic gland in the stomach caused by undernutrition. Similar to the results of body weight, oral EGF did not affect food intake. Because food intake was similar, body weight of the rats did not differ among four operated groups.
From the pathological observation, the mean diameter of ulcer area was undetectable in duodenal ulcer rats with EGF on day 5, and the damaged mucosa apparently recovered. However, the damaged mucosa was still found in duodenal ulcer rats without EGF on day 5. The data revealed that oral EGF reversed the damaged mucosa of duodenal ulcer on day 5, but duodenal ulcer could be self-recovered after 15 d. The results for cell growth of duodenal ulcer evaluated by mucosal DNA, RNA, and protein levels in the duodenum showed that mucosal DNA content did not significantly increase in duodenal ulcer rats with EGF until day 15 compared with those without EGF. Although we only measured DNA, RNA, and protein contents in the ulcer area of duodenal mucosa in duodenal ulcer rats and a similar area of intact duodenal mucosa in sham operation rats, these levels could be overestimated due to mixed cell types while collecting the samples. According to the results of pathological observation, the healing of damaged mucosa obviously occurred after day 5 in duodenal ulcer rats with EGF. Mucosal DNA content could reflect cell proliferation or cell division, but not measure DNA synthesis in the real time. While the pathological observation in the healing of damaged mucosa included the overall results of both cell proliferation and DNA synthesis. Oral EGF did not affect mucosal RNA and protein contents in duodenal ulcer rats. The data suggested that oral EGF had hyperplastic rather than hypertrophic effect on duodenal mucosa in duodenal ulcer rats. Although oral EGF did not influence cell proliferation in sham operation rats, EGF supplementation might temporarily cause cell hypertrophy due to increased ratio of RNA to protein caused by an increase in RNA and a decrease in protein on day 5. However, a previous study[7] demonstrated that orally administered EGF twice daily at 30 and 100 μg/kg body weight for 2 weeks had no effect on ulcer area and healing rate in Donryu rats with gastric ulcer induced by a submucosal injection of 20 mL/L acetic acid into the antrum. Different strain of animals, method of ulcer induction, severity of ulcer, and duration of EGF treatment could cause different results.
Our data showed that sham operation and duodenal ulcer rats with EGF significantly elevated mucosal EGF to 2.1- and 2.4-fold, respectively, compared with those without EGF on day 5. However, mucosal EGF did not differ between sham operation and duodenal ulcer rats with or without EGF on day 5. The results indicated that exogenous EGF increased mucosal EGF in the duodenum of both sham operation and duodenal ulcer rats to the similar extent on day 5. Therefore, exogenous EGF could be directly uptaken by the mucosa, and increased mucosal EGF in the duodenum of sham operation and duodenal ulcer rats could be derived from both exogenous and endogenous EGF. Increased mucosal EGF in sham operation and duodenal ulcer rats with EGF reduced to the similar level as in those without EGF on day 15, probably because of the adaptation to exogenous EGF through down-regulation of endogenous EGF. The EGF receptor was localized in the apical membrane of the enterocytes of rat duodenum[13,14], therefore the hypertrophic and hyperplastic effect of EGF on the mucosa of sham operation and duodenal ulcer rats, respectively, in this study could be induced via the interaction with the EGF receptor. Whereas if and how exogenous EGF regulates endogenous production of EGF or the interaction with the EGF receptor in the gastrointestinal tract of sham operation or duodenal ulcer rats, a further study to measure EGF mRNA and EGF receptors in the duodenum is required.
Our study showed that oral EGF significantly increased EGF content in the duodenal mucosa on day 5, accompanied by grossly improved healing in duodenal ulcer rats after day 5, and followed by elevated mucosal DNA content on day 15. The data suggested that oral EGF improved mucosal healing in duodenal ulcer rats by increasing EGF content in the duodenal mucosa to accelerate cell proliferation. Although EGF could be cleaved to smaller, less active forms in acidic gastric juice, the proportion of intact EGF increased to about 60% if the pH was maintained above 4[15], which allowed it to survive passage through the intestinal tract. After administration of 125I-labeled EGF, reversed-phase HPLC identified > 90% and 46%-51% of C18-extracted radioactivity from gastric and midjejunal luminal contents as intact 125I-EGF, whereas < 3% of C18-extracted radioactivity in extracts of duodenal, jejunal, and ileal luminal contents was intact 125I-EGF in adult mice[16]. The result indicated that EGF, given by oral administration, in the gut lumen, more or less, was still intact. Additionally, Tsujikawa et al[17] suggested that the luminal EGF might play a role only under tissue damage, where enhanced permeability allowed passage of luminal EGF to its receptor on the membranes. The mechanism for the healing effect of EGF on the damaged intestine is now uncertain. Most studies have focused on the mitogenic and antisecretory actions of EGF[6,9,18-21]. Konturek et al[18] suggested that the mechanism for protective and ulcer healing effects of EGF involved the activation of ornithine decarboxylase, the key enzyme in the biosynthesis of polyamines, which play a crucial role in the growth-promoting action of EGF. Additionally, EGF administration (50 μg/kg) accelerated the healing of acetic acid-induced duodenal ulcer in rats via an increase in collagen proliferation and secretion without affecting gastric acid secretion[19]. A previous study[9] reported that subcutaneous injection of EGF increased duodenal DNA and RNA contents in rats with duodenal ulcer after 7-day treatment, but oral administration of EGF did not. In addition, they indicated that oral dose (10 μg/kg) of EGF had no influence on gastric secretion in rats with chronic gastroduodenal ulcer but subcutaneous injection of EGF (10 μg/kg) decreased gastric acid output by 59% compared with the control rats without EGF administration. They suggested that the ulcer-healing effects of EGF were mediated by factors other than the inhibition of acid secretion, because oral EGF did not have any influence on gastric secretion. However, decreased gastric acid secretion in rats with chronic duodenal ulcer was observed after intravenous administration of EGF at a dose of 36 μg/kg but not at doses of 0.36 or 3.6 μg/kg[6]. Furthermore, EGF has been reported to inhibit gastric acid and stimulate duodenal bicarbonate secretion[20]. The physiological effect of EGF on acid secretion was mediated by induction of gastric H+, K+-ATPase gene expression[21,22].
In conclusion, oral administration of EGF (60 μg/kg/day) can increase EGF content in the duodenal mucosa and promote the healing of the rats with duodenal ulcer by its mitogenic action.
Edited by Wang XL
| 1. | Marti U, Burwen SJ, Jones AL. Biological effects of epidermal growth factor, with emphasis on the gastrointestinal tract and liver: an update. Hepatology. 1989;9:126-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 197] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Poulsen SS, Nexø E, Olsen PS, Hess J, Kirkegaard P. Immunohistochemical localization of epidermal growth factor in rat and man. Histochemistry. 1986;85:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 156] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990;343:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 320] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Jones MK, Tomikawa M, Mohajer B, Tarnawski AS. Gastrointestinal mucosal regeneration: role of growth factors. Front Biosci. 1999;4:D303-D309. [PubMed] |
| 5. | Konturek PC, Bielanski W, Bobrzynski A, Hahn EG, Konturek SJ. Gastric mucosal expression and luminal release of growth factors in gastric carcinoma and duodenal ulcer patients be-fore and after eradication of Helicobacter pylori. J Physiol Pharmacol. 1997;48:375-382. |
| 6. | Skov Olsen P, Poulsen SS, Therkelsen K, Nexø E. Oral administration of synthetic human urogastrone promotes healing of chronic duodenal ulcers in rats. Gastroenterology. 1986;90:911-917. [PubMed] |
| 7. | Kuwahara Y, Sunagawa Y, Imoto Y, Okabe S. Effects of orally administered human epidermal growth factor on natural and delayed healing of acetic acid-induced gastric ulcers in rats. Jpn J Pharmacol. 1990;52:164-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Palomino A, Hernández-Bernal F, Haedo W, Franco S, Más JA, Fernández JA, Soto G, Alonso A, González T, López-Saura P. A multicenter, randomized, double-blind clinical trial examining the effect of oral human recombinant epidermal growth factor on the healing of duodenal ulcers. Scand J Gastroenterol. 2000;35:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Konturek SJ, Dembinski A, Warzecha Z, Brzozowski T, Gregory H. Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology. 1988;94:1300-1307. [PubMed] |
| 10. | Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532-534, 532-534. [PubMed] |
| 11. | Sizemore N, Dudeck RC, Barksdale CM, Nordblom GD, Mueller WT, McConnell P, Wright DS, Guglietta A, Kuo BS. Development and validation of two solid-phase enzyme immunoassays (ELISA) for quantitation of human epidermal growth factors (hEGFs). Pharm Res. 1996;13:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Majumdar AP. Postnatal undernutrition: effect of epidermal growth factor on growth and function of the gastrointestinal tract in rats. J Pediatr Gastroenterol Nutr. 1984;3:618-625. [PubMed] [DOI] [Full Text] |
| 13. | Montaner B, Pérez-Tomás R. Epidermal growth factor receptor (EGF-R) localization in the apical membrane of the enterocytes of rat duodenum. Cell Biol Int. 1999;23:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Montaner B, Asbert M, Pérez-Tomás R. Immunolocalization of transforming growth factor-alpha and epidermal growth factor receptor in the rat gastroduodenal area. Dig Dis Sci. 1999;44:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Playford RJ, Marchbank T, Calnan DP, Calam J, Royston P, Batten JJ, Hansen HF. Epidermal growth factor is digested to smaller, less active forms in acidic gastric juice. Gastroenterology. 1995;108:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Rao RK. Luminal processing of epidermal growth factor in mouse gastrointestinal tract in vivo. Peptides. 1995;16:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Tsujikawa T, Itoh A, Yasuoka T, Fukunaga T, Satoh J, Uda K, Ihara T, Sasaki M, Fujiyama Y. Mucosal permeability regulates receptor binding of luminal epidermal growth factor in the adult rat intestine. Int J Mol Med. 2003;11:349-352. [PubMed] |
| 18. | Konturek JW, Brzozowski T, Konturek SJ. Epidermal growth factor in protection, repair, and healing of gastroduodenal mucosa. J Clin Gastroenterol. 1991;13 Suppl 1:S88-S97. [PubMed] |
| 19. | Perez Aisa A, Sopena Biarge F, Arceiz Gonzalo E, Sainz Samitier R, Ortego Diez De Retana J, Lanas Arbeloa A. Effect of exog-enous administration of platelet-derived growth factor and epi-dermal growth factor on duodenal ulcer healing in rats treated with indomethacin. Gastroenterol Hepatol. 2002;25:299-305. |
| 20. | Szabo S, Vincze A. Growth factors in ulcer healing: lessons from recent studies. J Physiol Paris. 2000;94:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Kaise M, Muraoka A, Yamada J, Yamada T. Epidermal growth factor induces H+,K+-ATPase alpha-subunit gene expression through an element homologous to the 3' half-site of the c-fos serum response element. J Biol Chem. 1995;270:18637-18642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Kusayanagi S, Takeuchi Y, Todisco A, Mitamura K. Extracellular signal-regulated protein kinases mediate H(+),K(+)-ATPase alpha-subunit gene expression. Biochem Biophys Res Commun. 2002;290:1289-1294. [PubMed] |