Published online Dec 15, 2002. doi: 10.3748/wjg.v8.i6.1112
Revised: June 19, 2002
Accepted: June 26, 2002
Published online: December 15, 2002
AIM: To study the effect of matrine on activation of Kupffer cell during cold ischemia and reperfusion injury in rat orthotopic liver transplantation (OLT).
METHODS: 168 syngeneic SD rats were randomly divided into four groups: untreated group, small-dose treated group, large-dose treated group and sham operation group. After 5 h of preservation in Ringer’s (LR) solution, orthotopic implantation of the donor liver was performed. At 1 h, 2 h, 4 h and 24 h after reperfusion of the portal vein, 6 rats were killed in each group to collect the serum and the liver for assay and pathology.
RESULTS: Matrine markedly inhibited the activation of Kupffer cells and their release of tumor necrosis factor (TNF). TNF cytotoxicity level at 2 h decreased significantly by matrine treatment (7.94 ± 0.42, 2.39 ± 0.19 and 2.01 ± 0.13 U/mL, respectively; P < 0.01), so did the other three time points. The level of hylluronic acid (HA) and alanine transaminase (ALT) decreased significantly in both treated groups, and matrine treatment markedly ameliorated focal necrosis of hepatocytes, inflammatory cells aggregating, rounding and detachment of sinusoidal endothelial cells (SEC). And no significant difference was observed between the treated groups.
CONCLUSION: Matrine can inhibit the activation of Kupffer cell and prevent the donor liver from cold preservation and reperfusion injury in rat orthotopic liver transplantation.
- Citation: Zhu XH, Qiu YD, Shen H, Shi MK, Ding YT. Effect of matrine on Kupffer cell activation in cold ischemia reperfusion injury of rat liver. World J Gastroenterol 2002; 8(6): 1112-1116
- URL: https://www.wjgnet.com/1007-9327/full/v8/i6/1112.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i6.1112
Preservation injury continues to be a major clinical problem in orthotopic liver transplantation (OLT) with a 10% incidence of primary nonfunction[1,2]. Additionally, there is clinical evidence that severe preservation injury is associated with an increase in liver graft rejection[3]. Therefore, it is clinically and pathophysiologically important to elucidate the mechanism and prevention of cellular injury during hepatic ischemia and the subsequent reperfusion. Kupffer cells become activated during cold preservation and subsequent reperfusion, as has been demonstrated by enhanced endocytosis of carbon particles[4], an increased release of TNF-α[5-8] and ultrastructural changes[9]. When activated, they generate a plethoric inflammatory cytokines and oxygen-derived free radicals, which play a particularly important part in the pathogenesis of hepatic ischemia and reperfusion injury[10].
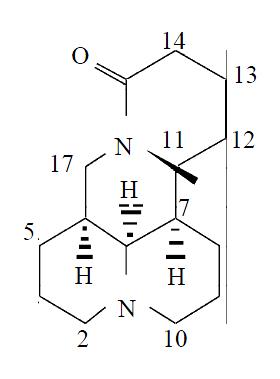
Matrine (matridin-15-one) is a typical lupine alkaloid along with lupinine, sparteine and cytisine, and has an absolute structure of 5S, 6S, 7R, 11R[11] (Figure 1). This alkaloid, isolated from kinds of Sophora plants in Leguminosae, shows pharmacological effects as anti-inflammation[12], immunity-inhibition[13], B and C hepatitis virus inhibition[14], anti-tumor[15] and anti-arrhythmia activity[16], and has been used in treatment of chronic viral hepatitis. Pharmacological studies revealed no obvious side-effect of matrine[17]. The present study was designed to evaluate the effects of matrine on Kupffer cell activation caused by preservation and reperfusion with an orthotopic liver transplantation model.
Marine, a parenteral solution (50 mg/5 mL), was purchased from Ming Xing Pharmaceutical Factory, Guangzhou, China. HA RIA kit was from Shanghai Ocean Research Biomedical Technology Center, China. Recombinant human TNF (1000 units/mL) was from Genzyme Co. in Boston, America. Rat TNF-α ELISA kit was purchased from Bender MedSystems Co. in Vienna, Austria. Endotoxin quantitation kit was from Shanghai Medical Test Center, China.
168 male inbred SD rats weighing 200 to 220 g were purchased from the Animal Center of Jin Ling Hospital (Nanjing, China). All rats were provided with standard laboratory chow and water and housed in accordance with institutional animal care policies. Prior to being used in the study, the rats were fasted for 12 h and were allowed free access to water.
Rat orthotopic liver transplantation was performed using the technique of Kamada and Calne[18] under ether anesthesia, and the hepatic artery was not reconstructed. Ringer’s (LR) solution was used for perfusion. The liver graft was preserved for 5 h in Ringer’s solution at 4 °C, then transplanted orthotopically. The explantation of the recipient liver required < 10 minutes and the rewarming time of the graft (i.e, clamping of the portal vein and the subhepatic vena cava during implantation) did not exceed 18 minutes.
The animals were randomly assigned into four experimental groups as follows: (1) a control group in which the recipients were injected ip Normal Saline (N.S, 1ml) before laparotomy. (2) a small-dose treated group (ST) in which the recipients were injected ip matrine (40 mg·Kg-1) before surgery. (3) a large-dose treated group (LT), in which the transplantation was performed following the injection of matrine (80 mg·Kg-1) as above. (4) a sham operation group (Normal), in which the liver was mobilized as the others without hepatectomy to exclude the influence of surgery.
At 1 h, 2 h, 4 h and 24 h after reperfusion of the portal vein, 6 rats were killed in each group to collect the blood sample via the infrahepatic vena cava and the median lobe of liver for assay. The serum was separated and stored at -70 °C until analysis. Washed with cold Saline solution, the liver samples were stored immediately in liquid nitrogen until analysis.
Alanine aminotransferase (ALT) activity of the serum samples collected at 4 h and 24 h after reperfusion was determined by automatic biochemistry analyzer (HITACHI 7600).
HA plasma levels of those samples collected at 1 h, 2 h, and 4 h after reperfusion were determined in duplicate using a HA RIA kit according to the manufacturer’s protocols.
Endotoxin plasma levels of the samples collected at 1h and 2h were determined in duplicate using a limulus amebocyte lysate assay kit according to the manufacturer’s instruction.
Plasma TNF cytotoxicity of all samples were determinated with TNF cytotoxicity L929 assays as described[19,20]. Each sample was assayed in duplicate wells.
Coomassie Light Blue assay was used for protein quantitation of all hepatic samples. Hepatic levels of TNF-α were assayed with a rat TNF-α ELISA kit from the Bender MedSystems corporation. This was an enzyme-linked immunoabsorbent assay for the quantitation of natural or recombinant rat TNF-α levels. The assay was carried out according to the manufacturer’s instructions.
Six hepatic specimens were collected at 4 hr after reperfusion of portal vein in each group. The liver specimens for light microscopy were fixed with 10% formalin and then embedded in paraffin. The sections were stained with hematoxylin and eosin for histologic examination.
For transmission electron microscopy, liver fragments of approximately 1 mm3 were fixed in 2% glutaraldehyde containing 0.1 M phosphate buffer for 3 h. After washing in phosphate buffer, specimens were postfixed with osmium tetroxide, dehydrated in graded alcohols, and embedded in Epon 812. Ultrathin sections were stained with uranyl acetate and lead citrate and examined under an electron microscope (JEM-1200EX).
The results are expressed as mean ± SD. Data were analyzed using Statistical Analysis System (SAS). One-way analysis of variance (ANOVA) was used for multiple comparisons with Student-Newman-Keuls (snk) test. A P value of < 0.05 was considered statistically significant.
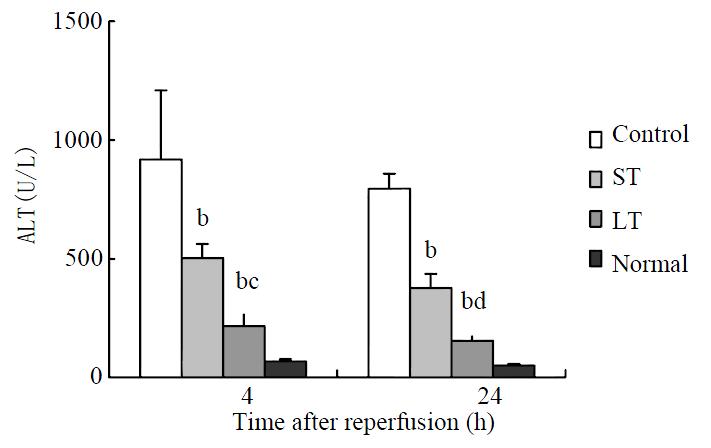
ALT serum levels in both treated groups decreased significantly compared with control values at different time points after reperfusion, and two folds-dose matrine provided the large-dose treated group with a significant decrease in ALT. (Figure 2).
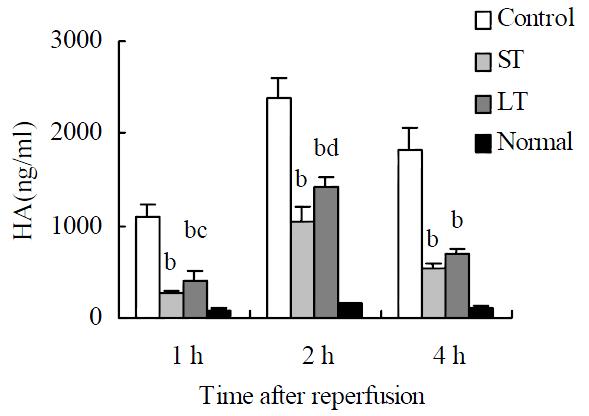
Compared with the sham operation group, a significant elevation of serum HA was observed in the other three groups at different time points, with the peak level at 2 h after surgery. HA levels at different time points were ameliorated markedly by matrine treatment, and two folds-dose matrine provided the large-dose treated group with a significant elevation in HA. (Figure 3).
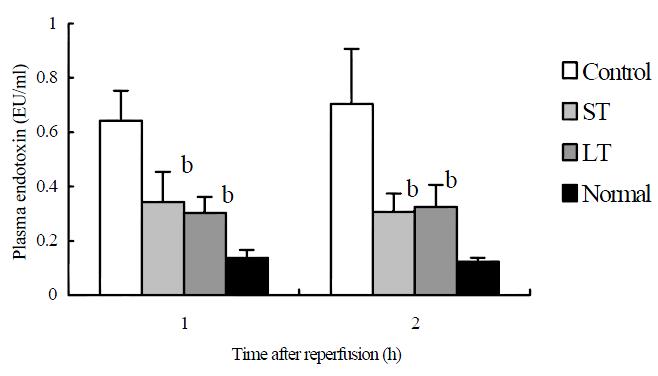
In both treated groups, plasma endotoxin levels increased significantly compared with control values at 1 h and 2 h after reperfusion. No significant difference was noted between the two treated groups (Figure 4).
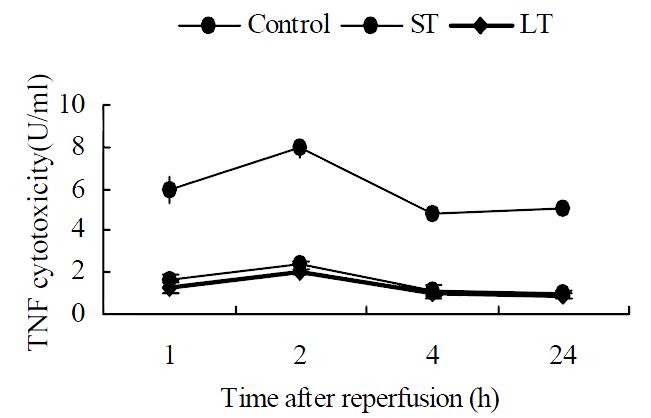
Almost no plasma TNF cytotoxicity was detected in the sham operation group. In the other three groups, the maximum TNF values were obtained 2 h after surgery as shown in Figure 5. TNF cytotoxicity level at 2 h decreased significantly by matrine treatment (7.94 ± 0.42, 2.39 ± 0.19 and 2.01 ± 0.13 U/mL, respectively; P < 0.01), so did the other three time points. No significant difference was noted between the two treated groups.
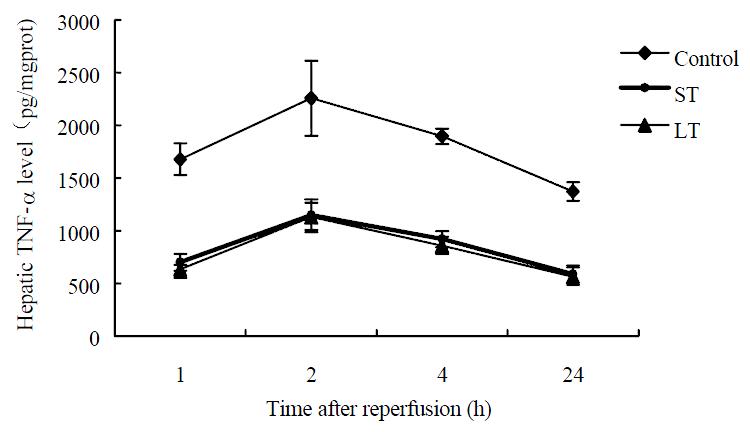
The hepatic TNF-α level of sham operation group was low (56.8 ± 13.2 pg/mgprot). In the other three groups, the maximum TNF-α levels were obtained at 2 h after surgery as shown in Figure 6, and TNF-α levels at different time points post reperfusion were significantly decreased by matrine treatment, but no significant difference was noted between the two treated groups.
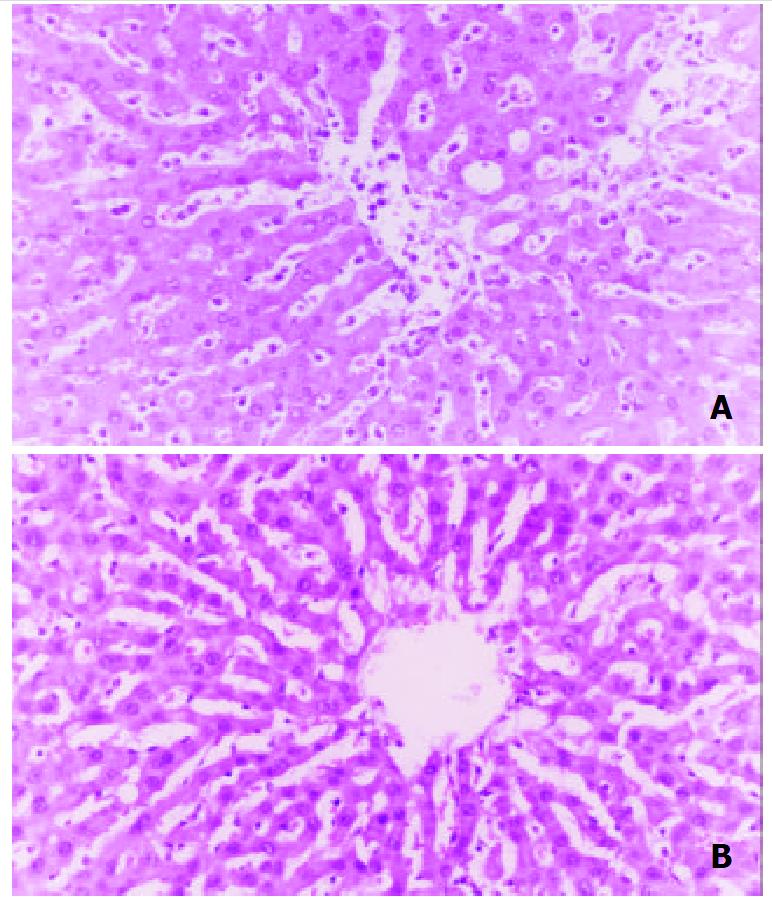
In sham operation group, the histological findings indicated that the degree of liver cell injury, Kupffer cell hyperplasia, and inflammatory cell infiltration in portal areas and sinusoids were mild. As shown in Figure 7, histological examination revealed some focal necrosis of hepatocytes, extensive congestion, and inflammatory cells aggregating in hepatic sinusoid lumen in the control group, and the obvious Kupffer cell hyperplasia and rounding and detachment of SEC were observed, too. These were ameliorated markedly in both treated groups, and no significant difference was observed between the two treated groups.
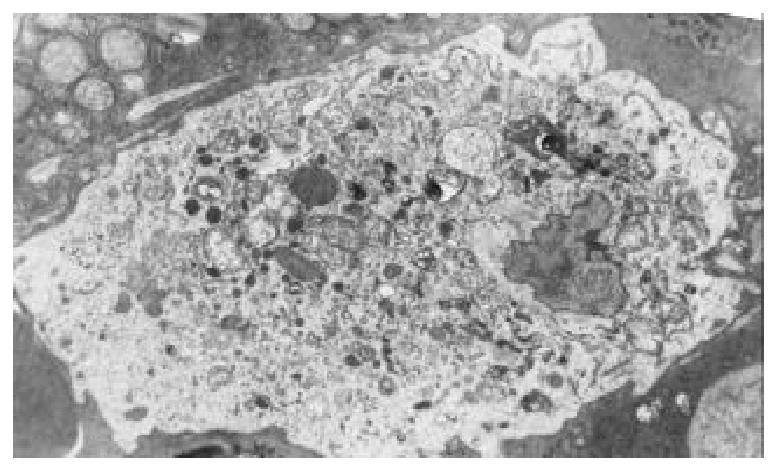
Samples taken from the control group showed obvious activation of Kupffer cells, including increase of cytoplasmic processes, irregular nucleus and many phagolysosomes, hyaline vacuoles, and granules in cytoplasm. (Figure 8) The treated groups showed a nearly normal ultrastructure of Kupffer cells in the samples examined compared with the control. This was reflected in rounding of Kupffer cell, regular nucleus, and a reduction in the amount of cytoplasmic processes. No significant differences were noted between the two treated groups.
With donor shortage becoming worse, preservation injury continues to be a major clinical problem with a 10% incidence of primary nonfunction[1,2]. Kupffer cells play an important part in mediating ischemia and reperfusion injury after hepatic transplantation. It is likely that they are primed during cold preservation and then activated at the time of reperfusion. The addition of an inhibitor of Kupffer cell activation to liver preservation solutions may further increase the duration of cold storage times and also improve the outcome of grafts[21-23].
In the present investigation, rat livers were kept for 5 h in cold Ringer’s solution (4 °C), exceeding the safe limit of 4 h[24].Under these preservation and transplantation conditions (i.e., a portal vein clamping time of less than 20 min), a postoperative survival rate of about 40% was obtained. Thus, 5 h in cold Ringer’s solution, although a severely compromising condition, should allow an adequate assessment of the mechanisms of cold ischemia and reperfusion injury that lead to primary nonfunction. Usually, the rats without matrine treatment recovered well from anesthesia; however, their clinical status began to deteriorate within 4 to 6 h, and nonsurvivors died within 24 h, mostly between 10 to 20 h.
After reperfusion, serum endotoxins immediately bind to lipopolysaccharides binding protein (LPSBP) and are ususlly trapped by Kupffer cells[25]. Kupffer cells exhibited progressive rounding, ruffling of the cell surface, polarization, appearance of wormlike densities, vacuolization, and degranulation. After activation, Kupffer cells produce TNF-α and induce neutrophil chemotaxis and activation[5,26]. TNF-α has potent proinflammatory actions, which can induce IL-8 synthesis and up-regulate the expression of adhesion molecules giving rise to increased leukocyte-sinusoidal endothelial cell interactions[23,27], which result in further cytokine production. In our study, serum endotoxin levels at different time points post transplantation were ameliorated markedly by matrine treatment, and the results have confirmed matrine markedly inhibited the activation of Kupffer cells and their release of TNF, inflammatory cell infiltration and injury of sinusoidal endothelial cell.
The serum ALT and HA levels were determined as functional indices of hepatocyte and sinusoidal endothelial cell damage, respectively. ALT and HA levels at different time points post-transplantation improved markedly by matrine treatment, and their pathological changes of liver graft ameliorated, too. Two folds-dose matrine treatment can’t give rats the better therapeutic effect, and no obvious side effect was noted in our study.
In conclusion, the results of this study demonstrated the inhibition effect of matrine on Kupffer cell activation and its protective effect against the cold ischemia reperfusion injury of the graft in liver transplantation.
Edited by Wu XN
| 1. | Greig PD, Woolf GM, Sinclair SB, Abecassis M, Strasberg SM, Taylor BR, Blendis LM, Superina RA, Glynn MF, Langer B. Treatment of primary liver graft nonfunction with prostaglandin E1. Transplantation. 1989;48:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 185] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 804] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 3. | Howard TK, Klintmalm GB, Cofer JB, Husberg BS, Goldstein RM, Gonwa TA. The influence of preservation injury on rejection in the hepatic transplant recipient. Transplantation. 1990;49:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 218] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Lindert KA, Caldwell-Kenkel JC, Nukina S, Lemasters JJ, Thurman RG. Activation of Kupffer cells on reperfusion following hypoxia: particle phagocytosis in a low-flow, reflow model. Am J Physiol. 1992;262:G345-G350. [PubMed] |
| 5. | Shibuya H, Ohkohchi N, Tsukamoto S, Satomi S. Tumor necrosis factor-induced, superoxide-mediated neutrophil accumulation in cold ischemic/reperfused rat liver. Hepatology. 1997;26:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Zhang SC, Dai Q, Wang JY, He BM, Zhou K. Gut-derived endotoxemia: One of the factors leading to production of cytokines in liver diseases. World J Gastroenterol. 2000;6:16. |
| 7. | Arii S, Monden K, Adachi Y, Zhang W, Higashitsuji H, Furutani M, Mise M, Fujita S, Nakamura T, Imamura M. Pathogenic role of Kupffer cell activation in the reperfusion injury of cold-preserved liver. Transplantation. 1994;58:1072-1077. [PubMed] |
| 8. | Xu MQ, Xue L, Gong JP. Significance of Kupffer cell NF-kB acti-vation during hepatic ischemia/reperfusion in rats. Shijie Huaren Xiaohua Zazhi. 2001;9:1250-1253. |
| 9. | Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Kupffer cell activation and endothelial cell damage after storage of rat livers: effects of reperfusion. Hepatology. 1991;13:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Shiratori Y, Kiriyama H, Fukushi Y, Nagura T, Takada H, Hai K, Kamii K. Modulation of ischemia-reperfusion-induced hepatic injury by Kupffer cells. Dig Dis Sci. 1994;39:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Okuda S, Yoshimoto M, Tsuda K, Utzugi N. [On the absolute configuration of matrin]. Chem Pharm Bull (Tokyo). 1966;14:314-318. [PubMed] |
| 12. | Hu ZL, Zhang JP, Yu XB, Lin W, Qian DH, Wan MB. Effect of matrine on lipopolysaccharides/D-galactosamine-induced hepatitis and tumor necrosis factor release from macrophages in vitro. Zhongguo Yaoli Xuebao. 1996;17:351-353. [PubMed] |
| 13. | Liang P, Bo AH, Xue GP, Han R, Li HF, Xu YL. Study on the mechanism of matrine on immune liver injury in rats. Shijie Huaren Xiaohua Zazhi. 1999;7:104-108. |
| 14. | Chen XS, Wang GJ, Cai X, Yu HY, Hu YP. Inhibition of hepatitis B virus by oxymatrine in vivo. World J Gastroenterol. 2001;7:49-52. [PubMed] |
| 15. | Si WK, Pan J, Lu H, Li ZQ. Study on matrine inhibiting prolif-eration of HepG2 cell and the relation between its dosage and inhibiting style. Shijie Huaren Xiaohua Zazhi. 2001;9:185-189. |
| 16. | Ai J, Gao HH, He SZ, Wang L, Luo DL, Yang BF. Effects of matrine, artemisinin, tetrandrine on cytosolic [Ca2+]i in guinea pig ventricular myocytes. Acta Pharmacol Sin. 2001;22:512-515. [PubMed] |
| 17. | Ye M. Pharmacological study of matrine injection. Guangdong Yixue. 1997;18:793. |
| 18. | Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 533] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Chang NS. Transforming growth factor-beta 1 induction of novel extracellular matrix proteins that trigger resistance to tumor necrosis factor cytotoxicity in murine L929 fibroblasts. J Biol Chem. 1995;270:7765-7772. [PubMed] |
| 20. | Chang NS. Hyaluronidase induces murine L929 fibrosarcoma cells resistant to tumor necrosis factor and Fas cytotoxicity in the presence of actinomycin D. Cell Biochem Biophys. 1996;27:109-132. [PubMed] |
| 21. | Shiratori Y, Kiriyama H, Fukushi Y, Nagura T, Takada H, Hai K, Kamii K. Modulation of ischemia-reperfusion-induced hepatic injury by Kupffer cells. Dig Dis Sci. 1994;39:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Marzi I, Cowper K, Takei Y, Lindert K, Lemasters JJ, Thurman RG. Methyl palmitate prevents Kupffer cell activation and improves survival after orthotopic liver transplantation in the rat. Transpl Int. 1991;4:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Li XK, Matin AF, Suzuki H, Uno T, Yamaguchi T, Harada Y. Effect of protease inhibitor on ischemia/reperfusion injury of the rat liver. Transplantation. 1993;56:1331-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Sumimoto R, Jamieson NV, Wake K, Kamada N. 24-hour rat liver preservation using UW solution and some simplified variants. Transplantation. 1989;48:1-5. [PubMed] |
| 25. | Vajdová K, Smreková R, Kukan M, Jakubovský J, van Rooijen N, Horecký J, Lutterová M, Wsólová L. Endotoxin-induced aggravation of preservation-reperfusion injury of rat liver and its modulation. J Hepatol. 2000;32:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Takei Y, Marzi I, Kauffman FC, Currin RT, Lemasters JJ, Thurman RG. Increase in survival time of liver transplants by protease inhibitors and a calcium channel blocker, nisoldipine. Transplantation. 1990;50:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 112] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Arii S, Monden K, Adachi Y, Zhang W, Higashitsuji H, Furutani M, Mise M, Fujita S, Nakamura T, Imamura M. Pathogenic role of Kupffer cell activation in the reperfusion injury of cold-preserved liver. Transplantation. 1994;58:1072-1077. [PubMed] |