Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.168
Revised: October 16, 2001
Accepted: November 15, 2001
Published online: February 15, 2002
AIM: To investigate the mechanism underlying intestinal barrier function damage after severe trauma and the therapeutic effect of glutamine.
METHODS: Burned patients, and animal models of severe trauma replicated by hemorrhagic shock combined with endotoxin infusion and burn injury, were included in a serial experiment. Effects of oral glutamine on intestinal barrier function were observed in scalded rats. Parameters measured in these experiments were as follows: plasma levels of diamine oxidase (DAO), tumor necrosis factor (TNFα ), endotoxin (LPS), and lactate as well as D-lactate by biochemical methods, lactose/mannitol (L/M) ratio in urine by SP-3400, and pathological examination of intestinal mucosa under light microscopy.
RESULTS: Plasma DAO activity was significantly increased after injury. There was a negative correlation between plasma DAO and intestinal mucosal DAO or pHi (r = -0.93, plasma 0.80 ± 0.93, 2.83 ± 1.71, 1.14 ± 0.64, 2.36 ± 2.06 and 2.49 ± 1.67 vs intestinal 0.52 ± 0.12, 0.34 ± 0.03, 0.45 ± 0.18, 0.37 ± 0.26 and 0.41 ± 0.07; r = -0.533, plasma 0.87 ± 0.75, 1.89 ± 1.13, 1.21 ± 0.23, 3.03 ± 2.61 and 4.70 ± 1.22 vs pHi 7.03 ± 0.05, 7.05 ± 0.06, 7.14 ± 0.096, 7.20 ± 0.08 and 7.05 ± 0.07; P < 0.01-0.05). Positive correlations were found between DAO activity and plasma TNFα , LPS, lactate, L/M and D-lactate (r = 0.817, 0.842, 0.872, and 0.951; plasma DAO 0.87 ± 0.75, 1.89 ± 1.13, 1.21 ± 0.23, 3.03 ± 2.61 and 4.70 ± 1.22 vs TNFα 0.08 ± 0.02, 0.03 ± 0.25, 0.17 ± 0.09, 0.34 ± 0.15 and 0.33 ± 0.18; vs LPS 0.14 ± 0.03, 0.16 ± 0.04, 0.21 ± 0.02, 0.18 ± 0.16 and 0.37 ± 0.10; vs lactate 9.03 ± 2.19, 18.30 ± 2.56, 9.81 ± 2.83, 12.01 ± 6.83, 12.01 ± 6.84 and 43.61 ± 11.27; vs L/M 0.03 ± 0.01, 0.41 ± 0.27, 0.62 ± 0.20, 1.70 ± 0.60; r = 0.774, plasma DAO 1.25 ± 0.41, 2.17 ± 0.71, 2.29 ± 0.87, 1.23 ± 0.55 and 1.11 ± 0.47 vs D-lactate 8.37 ± 2.48, 18.25 ± 6.18, 13.96 ± 4.94, 8.93 ± 3.00 and 12.39 ± 4.94; all P < 0.01), repestively. Damage of intestinal mucosa was found by pathological examination. Intestinal barrier function was improved to a certain extent by oral glutamine in scalded rats.
CONCLUSION: Intestinal barrier function was damaged in the early stage after trauma. Plasma DAO activity, D-lactate content, intestinal pHi and urine L/M may be sensitive markers of intestinal mechanical injury, and glutamine may protect against intestinal barrier dysfunction after severe trauma.
- Citation: Li JY, Lu Y, Hu S, Sun D, Yao YM. Preventive effect of glutamine on intestinal barrier dysfunction induced by severe trauma. World J Gastroenterol 2002; 8(1): 168-171
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/168.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.168
It is generally accepted that the intestine may serve as an important organ in the development of severe complications under critically ill conditions, including trauma, burns, shock, etc. [1-3]Hemorrhagic shock and/or gut ischemia-reperfusion injury commonly occur in the early stage after acute insults, leading to gut-derived sepsis as a result of gut barrier dysfunction[4-10]. In order to investigate the mechanism underlying intestinal barrier function damage and its potential interventional measures, burned patients and animal models of severe trauma were employed in our current experiments[6,11-15].
Animal models of severe trauma were replicated by hemorrhagic shock combined with endotoxin infusion. Male Wistar rats, weighing 190 g-230 g, were anaesthetized with intraperitoneal injection of 30 g·L-1 barbitone sodium (35 mg·kg-1), and the femoral artery and jugular vein were cannulated under aseptic conditions. The rats were then bled via the jugular vein catheter until a mean arterial pressure of 30-35 mmHg (4.6 kPa) was reached. At the end of shock, endotoxin (E.coliO55 B5, Sigma) was infused through tail vein at a dose of 2 mg·kg-1. A goat model of hemorrhagic shock combined with endotoxin challenge was established according to the previous report(n = 20)[5]. Animals received E.coliO26 B6 endotoxin via portal vein 24 h after the recovery from shock, and the dosage was 30 ng·kg-1·min-1, which was given in a continuous infusion lashing for 5 d. Wistar rats were divided randomly into three groups: normal controls, early feeding with standard feed plase Gln 0.5 g after scalding, and animals (except control group) sustained a 30% TBSA full-thickness scald covering the back and flanks[6]. Determination of plasma diamine oxidase in 21 burned patients (17 male and 4 female) at the age of 33 ± 10 years, with burn area (64% ± 21%), and (35% ± 20%) III0. Plasma DAO activity was determined on day 1, 3, 7, 14 and 21 postburn. Blood and intestinal DAO levels were tested according to our previous report[17]. Plasma lactate and D-lactate concentrations were determined by biochemical methods as described by Brandt et al[18]. Microassay for quantitation of endotoxin in blood was made with new PCA treatment using chromogenic limulus amebocyte lysate[19].
Tumor necrosis factor (TNFα) assay. Plasma TNF content was measured by radioimmunoassay.Lactulose/mannitol (L/M) ratio tests in urine were made by SP-3400[20].
Pathological examination. Tissue samples were examined under light microscopy.
Data were expressed as the mean ± standard error, and were statistically evaluated by Students t test and correlation analysis. Differences were considered to be significant with P < 0.05.
Plasma DAO levels were elevated in double-peak patterns, one at early stage after trauma and another during invading infection in animal models. Similar results were also obtained in burned patients. Meanwhile, intestinal DAO levels were decreased to certain extent after trauma in animal model. There was a significantly negative correlation between plasma and intestinal DAO activity (Table 1; Figures 1 and 2).
| Animal model | Before injury | T (after injury)/h | ||||
| 2 | 6 | 24 | 48 | 72 | ||
| Goat | 0.9 ± 0.8 | 1.9 ± 1.1a | 1.2 ± 0.2 | 3.0 ± 2.6 | 4.7 ± 1.2b | |
| Rats | 1.3 ± 0.4 | 2.2 ± 0.7b | 2.3 ± 0.87b | 1.2 ± 0.6 | 1.1 ± 0.5 | |
| Scalded rats | 0.8 ± 0.9 | 0.8 ± 1.8b | 1.1 ± 0.6 | 2.4 ± 2.1a | ||
| Burnd pigs | 4.1 ± 1.4 | 4.7 ± 1.5 | 4.7 ± 1.4 | 4.8 ± 1.1 | 5.8 ± 1.4a | |
| Gun shooting | 1.5 ± 0.6 | 1.2 ± 0.5 | 2.0 ± 0.6b | 1.9 ± 0.2b | ||
Plasma DAO concentrations and the level of the related index, and plasma TNFα significantly increased at various intervals after trauma in goats. Plasma endotoxin levels notably increased at 24 h and 72 h after injury. Blood lactic acid significantly increased from 2 h to 72 h after trauma. The plasma DAO activity was obviously correlated with plasma TNFα, LPS and lactate (P < 0.01; Tables 2 and 3). Changes in DAO activity were significantly related with plasma TNFα and LPS levels in scalded rats. Plasma DAO activity and plasmaD-lactate were significantly correlated in rats secondary to hemorrhage followed by endotoxin challenge (r = 0.774; P < 0.01).
| Before injury | T (after injury)/h | ||||
| 2 | 24 | 48 | 72 | ||
| TNF/ μg·L-1 | 0.08 ± 0.02 | 0.03 ± 0.25a | 0.17 ± 0.09a | 0.34 ± 0.15a | 0.33 ± 0.18a |
| LPS/ × 103Eu·L-1 | 0.14 ± 0.03 | 0.16 ± 0.04 | 0.21 ± 0.02a | 0.18 ± 0.16 | 0.37 ± 0.10b |
| Laclate/nmol·L-1 | 9.03 ± 2.19 | 18.30 ± 2.56a | 9.81 ± 2.83 | 12.01 ± 6.84 | 43.61 ± 11.27b |
| L/M rat | 0.03 ± 0.01 | 0.41 ± 0.27 | 0.62 ± 0.20 | 1.70 ± 0.60b | |
| Intestinal pHi | 7.03 ± 0.05 | 7.05 ± 0.06b | 7.14 ± 0.09b | 7.20 ± 0.08a | 7.05 ± 0.07b |
| X | Y | r | P |
| DAO | TNFα | 0.817 | < 0.01 |
| DAO | LPS | 0.842 | < 0.01 |
| DAO | Laclate | 0.872 | < 0.01 |
| DAO | l/m | 0.951 | < 0.01 |
| DAO | Intestinal pHi | -0.553 | < 0.05 |
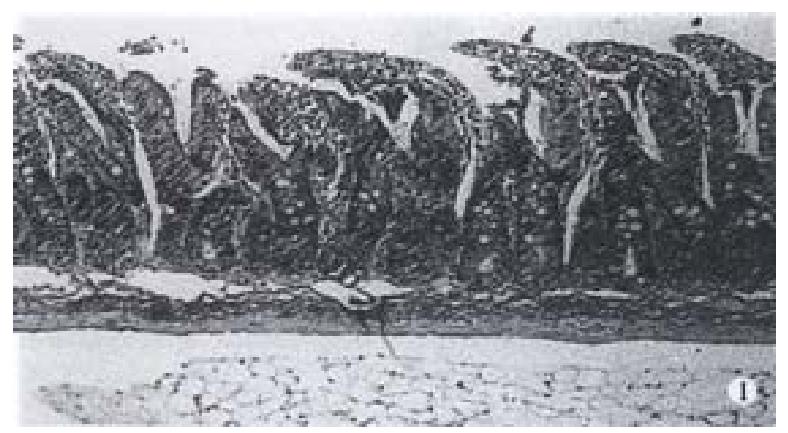
Glutamine could protect against intestinal barrier function damage. The results indicated that plasma DAO activity was decreased in animals with early glutamine supplementation compared with those without glutamine treatment (10 h after trauma P < 0.05). Results of intestinal pathological examination. The pathologic examination of the intestine showed that the damage of epithelial cells of intestinal mucosa, hemorrhage and necrosis, accompanied by the inflammatory cell infiltration in intestinal wall in goats suffering from hemorrhagic shock combined with endotoxin infusion. It was revealed that there was disruption of intestinal mucosa after scald and gut ischemia-reperfusion combined with endotoxin challenge in rats, whereas oral glutamine supplementation could markedly improve intestinal mucosa following acute insults (Figure 2).
DAO is located in the upper part of intestinal mucosa in human as well as in mammals, and is a highly active intracelluar enzyme. Under certain circumstances, intestinal mucosa cells became necrosed and dropped into the intestinal cavity, leading to decrease in intestinal mucosal DAO, and increase in DAO activity inside the intestinal cavity. DAO can also outer into the mucosal space between cells, lymphatic vessel[21-27] and blood flow, making plasma DAO markedly elevated. Gut as an important organ, may play an important role in the pathogenesis of serious complications. Intestinal mucosal surface layer with tight epithelial cells is an important component for intestinal barrier function, thus it can be seen that intestinal epithelial tissue integrated property is a key part to preserve intestinal barrier function. The changes in DAO activity is an ideal index to investigate intestinal barrier function damage after trauma, specially the changes in plasma DAO activity[2,6,10,11]. Therefore, intestinal barrier function injury after severe trauma could result in bacterial/toxin translocation, in turn evoke systemic inflammatory response syndrome and multiple organ dysfunction syndrome[3,28-29].
The intestinal blood flow might keep relatively low despite of systemic circulation recovery during trauma, which was evident by significant decrease in intestinal mucosal pHi[30-33]. This may be the pathological basis for intestinal origin sepsis and multiple organ dysfunction syndrome. The changes in intestinal permeability were reflected by blood D-lactate and L/M rate in urine. Secretory IgA is an important component part for regulation intestinal immune function, it can prevent the bacteria from adhering to intestinal epithelial cells, and prevent gut-derived bacteria from invading through the intestinal barrier, which might reduce the toxicity of bacterial products to epithelial cells. Therefore, IgA may possess the beneficial effect on the preservation of intestinal mechanical barrier[34-37]. From our data that changes in several indexes of intestinal barrier function in various animal models after trauma, it was shown that DAO was released to increase in blood, and decreased in intestinal tissues. Thus, determination of DAO activity might reflect the condition of intestinal injury and repaired process.
The change in plasma D-lactate, lactulose, mannitol and ratio of L/M could reflect the increased intestinal permeability. The intestinal IgA levels appear to be associated with the local immunological dysfunction[38-41]. The change in intestinal pHi showed that intestinal hemorrhagic injury may result in the release of intestinal mucosal enzyme, subsequently leading to significant elevation of plasma DAO activity. This study showed that there is a close relationship between plasma DAO and TNFα, LPS, D-lactate, lactate, L/M, and the change of intestinal pathology and intestinal barrier function index was similar, indicating that intestinal barrier function was damaged after trauma.
We also observed that the plasma DAO activity was increased 2 h and 72 h after trauma, especially at 72 h, and change of plasma TNFα and LPS was similar to the former. These results suggest that stress injury can cause changes of intestine barrier function index following intestinal ischemia-reperfusion injury, repair or endo/inextra protection, but the trends and degrees of changes were varied. Therefore, it is important to reduce development of SIRS to MODS by protection of intestinal barrier function in early trauma, and prevention of translation of intestinal origin bacteria and toxins[6,10,14,42-45].
Recent studies have shown that GLN may provide protection against intestinal barrier function injury after trauma[46-52]. Blood DAO activity was reduced to different extents at 10h,5d and 8d after early stage of oral GLN in scalded rats. Intestinal pathogical examination showed that the damage of epithetial cells of intestinal mucosa could be markedly improved, and the close correlation between plasma DAO and LPS and TNFα could also be changed after scalding[6]. The results indicate that GLN can protect against the intestinal barrier function damage after trauma.
Edited by Ma JY
| 1. | Li JY, Hu S, Sun XQ, Yan M, Jin H, Jiang XG, Zhou BT, Sheng ZY. Study on pathaphysiogical changes during early stage of sepsis after trauma. Zhongguo Weizhongbing Jijiu Yixue. 2001;291-294. |
| 2. | Li JY, Sun SR, Xue LB, Lu Y, Jing H, Gu ZR. Sequential changes in diamine oxidase activity after burns. Zhonghua Zhengxing Shaoshang Zazhi. 1997;13:40-42. |
| 3. | Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 424] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Chen HL, Wu JZ, Pei DK, Guan FL. Damage of gut barrier in the pathogenesis of mutiple organ dysfunction syndrome. Zhonghua Putong Waike Zazhi. 1998;13:50-53. |
| 5. | Hu S, Sheng ZY, Xue XB, Zhou BT, Lu JY, Jin H, Yu Y, Sun XQ, Li JY, Zheng YQ. A serilized studies of animal models of posttraumatic multiple organ dysfunction syndrome. Jiefangjun Yixue Zazhi. 1996;21:5-9. |
| 6. | Li JY, Lu Y, Xue LB, Wan SY, Sun SR, Yu Y, Xu HJ, Sheng ZY. The effect of oral glutamine gut mucosa Function and structure in scalded rat. Jiefangjun Yixue Zazhi. 1996;21:91-93. |
| 7. | Yao YM, Sheng ZY, Wu Y, Yu Y, Lu LR. Relationship between plasma D-lactate levels and acute intestinal injury in rats following ischemia-reperfusion. Zhonghua Zhengxing Shaoshang Zazhi. 1998;14:266-269. |
| 8. | Lu Y, Sheng ZY, Li JY, Sun XQ, Jin H, Jiang XG. The relationship between the ICAM-1 expression of hemangioen dotheliocyte and the dysfunction of murine small bowel in an intestinal ischemia-reperfusion model. Zhonggua Putong Waike Zazhi. 2000;15:145-147. |
| 9. | Cui XL, Sheng ZY, Guo ZR, He LX, Zao J, Ren XW, Sun SR, Jiang LX. Mechanisms of early gastro-intestinal ischemia after burn: hemo-dynamic and hemorrheologic features. Zhonghua Zhengxing Shaoshang Zazhi. 1998;14:262-265. |
| 10. | Li JY, Lu Y, Fu XB, Jin H, Hu S, Sun XQ, Sheng ZY. The significance of changes in diamine oxidase activity in intestinal injury after trauma. Zhongguo Weizhongbing Jijiu Yixue. 2000;12:482-484. |
| 11. | Li JY, Lu Y, Hu S, Jin H. An experimental study on monitoring of barrier function of small intestine. Chuangshang Waike Zazhi. 2001;3:109-112. |
| 12. | Li JY, Lu Y, Jin H, Hu S, Sun XQ, Jiang XG, Sheng ZY. Sequential changes of diamine oxidase activity after trauma. Anjisuan He Shengwu Ziyuan. 1999;21:17-19. |
| 13. | Fang WH, Yao YM, Shu HG, Yu Y, Wu Y, Zheng YQ. The time course and tissue distribution of endotoxin in rats after thermal injury. Zhonghua Zhengxing Shaoshang Zazhi. 1999;15:298-300. |
| 14. | Fu XB, Yang YH, Sun XQ, Gu XM, Sheng ZY. Protective effects of endogenous basic fibroblast growth factor activated by 2.3 butanedion monoxime of on functional changes of ischemic intestine, liver and kidney in rats. Zhongguo Weizhongbing Jijiu Yixue. 2000;12:69-72. |
| 15. | Fu X, Sheng Z, Wang Y, Ye Y, Xu M, Sun T, Zhou B. Basic fibroblast growth factor reduces the gut and liver morphologic and functional injuries after ischemia and reperfusion. J Trauma. 1997;42:1080-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Wu CT, Li ZL. Effect of DAO on intestinal damage in acute necrotiz-ing panereatitis in dogs. Shijie Huaren Xiaohua Zazhi. 1999;7:64-65. |
| 17. | Li JY, Yu Y, Hao J, Jin H, Xu HJ. Determination of diamine oxdase activity in intestinal tissue and blood using spectrophotometry. Anjisuan He Shengwu Ziyuan. 1996;18:28-30. |
| 18. | Sun XQ, Fu XB, Zhang R, Lu Y, Deng Q, Zhang C, Jiang XG, Sheng ZY. Plasma D-lactate levels as a useful marker of increased intestinal permeability after severe injuries. Zhongguo Weizhongbing Jijiu Yixue. 2000;12:476-478. |
| 19. | Yao YM, Tian HM, Wang YP, Yu Y, Shi ZG, Sheng ZY. Microassay for quantitation of endotoxin in blood with new PCA treatment using chromogenic limulus amebocyte lysate. Shanghai Yixue Jianyan Zazhi. 1993;8:31-33. |
| 20. | Zhu XF, Xiong DX, Sheng ZY. Establish measuring Method of alter-ations in intestinal permeability. Zhongguo Weishengtai Zazhi. 1994;6:36-38. |
| 21. | Hosoda N, Nishi M, Nakagawa M, Hiramatsu Y, Hioki K, Yamamoto M. Structural and functional alterations in the gut of parenterally or enterally fed rats. J Surg Res. 1989;47:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Yan BG, Yang ZC, Huang YS, Liu ZY, Li A. Effect of delayed rapid fluid resuscitation on plasma diamine oxidase in early postburn dogs. Shijie Huaren Xiaohua Zazhi. 2000;8:178-180. |
| 23. | Lu Y, Li JY, Sun SR, Jin H, Jiang XG, Sun XQ, Sheng ZY. Relation-ship between change of plasma diamine oxidase activity and gut injury in rats during gut ischemia-reperfusion. Anjisuan He Shengwu Ziyuan. 2000;22:50-53. |
| 24. | Liu MH, Gao LX. The effect of oral glutamine suppentation on small intestinal function in burned rats. Yingyang Xuebao. 1995;17:17-21. |
| 25. | Dong HL, Cai JW, Si ZX. Effects of radiation on intestinal and plasma DAO activety in rats. Shijie Xiaohua Zazhi. 1998;6:698. |
| 26. | Bragg LE, Thompson JS, West WW. Intestinal diamine oxidase levels reflect ischemic injury. J Surg Res. 1991;50:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Ci XL, Wang BS, Zhang SW, Zhang NN. Experimental treatment with berbal medicines for impaired intestinal mucosal barrier in-duced by endotoxin plus tumornecrosis factor-alpha. Zhongguo Weizhongbing Jijiu Yixue. 1999;11:262-265. |
| 28. | Rush BF, Sori AJ, Murphy TF, Smith S, Flanagan JJ, Machiedo GW. Endotoxemia and bacteremia during hemorrhagic shock. The link between trauma and sepsis. Ann Surg. 1988;207:549-554. [PubMed] |
| 29. | Yao YM, Sheng ZY, Tian HM, Wang YP, Yu Y, Fu XB, Lu LR, Wang DW, Ma YY. Gut-derived endotoxemia and multiple system organ failure following gunshot wounds combined with hemorrhagic shock: an experimental study in the dog. J Trauma. 1995;38:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Hu S, Jin H, Lu Y, Li JY, Zhou BT. The changes of PH on gastroduode-nal mucosa after hemorrhage shock and resuscitation in goats. Zhongguo Weizhongbing Jijiu Yixue. 1997;9:708-710. |
| 31. | Murray MJ, Gonze MD, Nowak LR, Cobb CF. Serum D(-)-lactate levels as an aid to diagnosing acute intestinal ischemia. Am J Surg. 1994;167:575-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 154] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Sun XQ, Lu Y, Jin H, Li JY, Jiang XG, Hu S, Fu XB. Change of intestine mucosal barrier induced by intestine ischmia-reperfusion injury. Chuangshang Waike Za zhi. 1999;1:208-210. |
| 33. | Murray MJ, Barbose JJ, Cobb CF. Serum D(-)-lactate levels as a predictor of acute intestinal ischemia in a rat model. J Surg Res. 1993;54:507-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Doe WF. The intestinal immune system. Gut. 1989;30:1679-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Yu Y, Shi ZG, Chai JK, Guo ZY, Yao YM, Li G, Ho XX, Jiang W, Wang HB, Sheng ZY. The clinical significance of changes in IgA levels in intestinal tract after major burns. Zhongguo Weizhongbing Jijiu Yixue. 1998;10:725-727. |
| 36. | Yu Y, Sheng ZY, Tian HM, Wang YP, Yu Y, Lu LR, Chang GY, Ma RS. An experimental study on injury of intestinal immuno-barrier in rat after scald. Zhonghua Zhengxing Shaoshang Waike Zazhi. 1996;12:86-89. |
| 37. | Biesbrock AR, Reddy MS, Levine MJ. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect Immun. 1991;59:3492-3497. [PubMed] |
| 38. | Harmatz PR, Carter EA, Sullivan D, Hatz RA, Baker R, Breazeale E, Grant K, Bloch KJ. Effect of thermal injury in the rat on transfer of IgA protein into bile. Ann Surg. 1989;210:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Cappeller WA, Bloch KJ, Hatz RA, Carter EA, Fagundes J, Sullivan DA, Harmatz PR. Reduction in biliary IgA after burn injury. Role of diminished delivery via the thoracic duct and of enhanced loss from the systemic circulation. Ann Surg. 1992;215:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Alverdy JC, Aoys E. The effect of dexamethasone and endotoxin administration on biliary IgA and bacterial adherence. J Surg Res. 1992;53:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Yu Y, Chai JK, Li JY, Jin H, Dong N. A preliminary study on the relationship between level of IgA in intestinal content and urine after burns. Anjisuan He Shengwu Ziyuan. 2001;23:49-51. |
| 42. | Diebel LN, Liberati DM, Brown WJ, Dulchavsky SA, Painter TM, Diglio CA, Montgomery PC. Secretory immunoglobulin A blocks hypoxia-augmented bacterial passage across Madin-Darby canine kidney cell monolayers. J Trauma. 1997;43:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Noguchi Y, James JH, Fischer JE, Hasselgren PO. Increased glutamine consumption in small intestine epithelial cells during sepsis in rats. Am J Surg. 1997;173:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Yu B, Wang SL, You ZY, Li A, Zhao Y. Effects of L-Glutamine on ghe tut nutrient metabolism in severe burnedminiswines. Changnei Yu Changwai Yingyang. 1995;2:7-10. |
| 45. | Li N, Liu FN, Li YS, Kang J, Li FJ, Li JS. The Changes of plasma glutamine and its influence on intestinal permeability after abdomi-nal Surgery. Changnei Changwai Yingyang. 1998;5:3-6. |
| 46. | Rhoads JM, Keku EO, Quinn J, Woosely J, Lecce JG. L-glutamine stimulates jejunal sodium and chloride absorption in pig rotavirus enteritis. Gastroenterology. 1991;100:683-691. [PubMed] |
| 47. | Yu B, Wang SL, You ZY, Zhao Y, Li A. Enhancement of gut absorp-tive function by early enteral feeding enriched with L-Glutamine in severe burned miniswines. Zhonghua Waike Zazhi. 1995;33:742-744. |
| 48. | Wu F, Jin XQ, Wu SX, Li J, Xu JL. The experimental study of cholestasis due to parenteral nutrition. Changnei Changwai Yingyang. 1998;5:96-98. |
| 49. | Yang JT, Wang ZG, Zhu PF. The Effects of glutamine supplementa-tion on the cellular immunity in traumatized rats. Yingyang Xuebao. 1999;21:8-12. |
| 50. | Dai DW, Wu SM, Li M, Chen HJ, Cai W. Protective effect of Glutamine in the anosia/reoxygenation induced injury of human intestinal epi-thelial cells in vitro. Anjisuan He Shengwu Ziyuan. 1997;19:1-3. |
| 51. | Tu WF, Li JS, Zhu WM, Li ZD, Liu FN, Chen YM, XU JG, Shao HF, Xiao GX, LI A. Infiluence of glutamine on gut bacteria/endotoxin translocation in acute severe pancreatites. Changwai Yu Changnei Yingyang. 1999;6:29-32. |
| 52. | Yu B, Wang SL, You ZY, Zhao Y, Li A. Protection against intestinal injury with oral feeding L-glutamine in severe burned mini-swines. Zhonghua Waike Zazhi. 1998;14:19-21. |