Published online Feb 15, 2001. doi: 10.3748/wjg.v7.i1.74
Revised: July 23, 2000
Accepted: July 30, 2000
Published online: February 15, 2001
AIM: To evaluate a new balloon-expandable stainless steel stent (Cordis stent) in a transjugular intrahepatic portosystemic shunt (TIPS) porcine model and compared with Wallstent.
METHODS: TIPS was performed in 26 normal domestic pigs weighing 20 kg-30 kg using a Cordis stent or Wallstent (13 pigs in each stent). All pigs were sacrificed at the 14th day after TIPS. The stent deployment delivery system, stent patency, and stent recoil after placement were evaluated.Proliferative response in representative histological sections from the center, hepatic and portal regions of the two stent designs were quantified.
RESULTS: The shunt was widely patent in 4 pigs in the Cordis stent group (4/12, premature dead in 1 pig), and in 5 pigs in the Wallstent group (5/13). All remaining stents of both designs were occluded or stenotic. The mean quantified proliferation including thickness of the proliferation and the ratio of proliferation: total area in three assayed regions in Cordis stent and Wallstent was 2.18 mm2 2:00 mm, and 59.18 mm2. 51.66 mm2, respectively (P < 0.05). The delivery system and mechanical properties of the Cordis stent fuctioned well.
CONCLUSION: The new Cordis stent is appropriate for TIPS procedure.
- Citation: Teng GJ, Bettmann MA, Hoopes PJ, Yang L. Comparison of a new stent and Wallstent for transjugular intrahepatic portosystemic shunt in a porcine model. World J Gastroenterol 2001; 7(1): 74-79
- URL: https://www.wjgnet.com/1007-9327/full/v7/i1/74.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i1.74
The high incidence of stenosis following transjugular intrahepatic portosystemic shunt (TIPS) remains the major barrier in this relatively new technique which is effective in controlling variceal hemorrhage secondary to portal hypertension and utilized in refractory ascites[1,2]. The exact mechanism for TIPS restenosis is not well understood and seems multifactorial. One relevant factor may be stent design. Wallstent is the most common stent currently used for TIPS procedures, but various stent designs may be effective. To evaluate a new balloon-expandable stent (Cordis stent) in TIPS, a comparative study of the Cordis stent and Wallstent was performed in a TIPS porcine model.
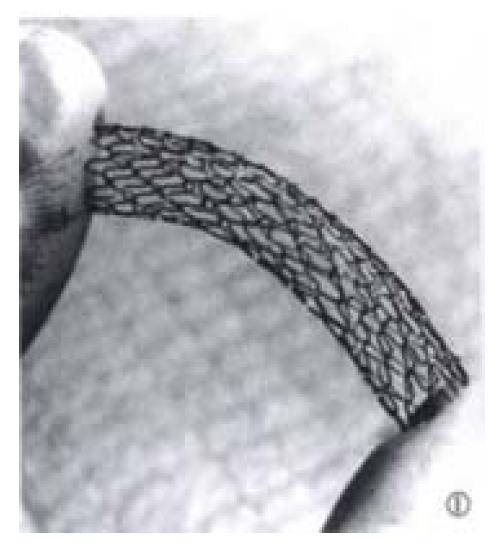
TIPS was performed in 26 domestic swine weighing 20 kg-30 kg, with two stent designs: balloon-expandable stainless steel stents (Cordis Co., Miami, FL) in 13 pigs and Wallstents (Schneider, Minneapolis, MN) in 13 pigs, respectively. The Cordis stent is made up of one stainless steel wire, which is formed into a sinusoidal wave pattern, wrapped in a tubular helical fashion and held in place by a series of welded joints (Figure 1). Either Cordis stent or Wallstent with an diameter of 8 mm and 4 cm in length was used in each pig.
TIPS was performed with a 22 G, 80 cm long puncture needle with a 5-F,70 cm long coaxial Teflon catheter (AngioDynamic, Queenshury, NY), a 9 F sheath set and a 14 G metal cannula with a curved tip (Cook, Bloomington, IN).
The animals in this study had normal livers and did not have portal hypertension. All protocols were approved by the Dartmouth Animal Care and Use Committee. Animals were sedated with intramuscular Ketamine (20 mg/kg) injection, intubated and maintained with 1%-3% halothane and oxygen.
The sheath was assembled with a 9-F Teflon catheter with a tapered tip and was introduced into the IVC by a standard percutaneous Seldinger method from the right jugular vein. A 7-F NIH catheter was advanced into the right lateral hepatic vein. The sheath was then placed into the hepatic vein and followed by the metal cannula containing the coaxial puncture needle. Through the metal cannula, the puncture needle together with the coaxial catheter was advanced into the hepatic parenchyma in an anterior direction targeted toward the right branch of portal vein. Following confirmation of suitable portal vein puncture by contrast injection, a 0.035 inch steerable guidewire (Cordis Co., Miami, FL) was advanced into the portal vein and followed by the 5-F catheter. Following portography, the floppy-tip guide wire was exchanged for an Amplatz super stiff guide wire, and a hepatic parenchymal tract was dilated using an 8 mm balloon catheter. The Cordis balloon catheter-stent delivery system was introduced into the sheath by advancing the insertion tool over the wire through the hemostasis valve, until a hand stop was reached. A stent mounted balloon was introduced through the insertion tool and advanced over the guidewire until the stent was in the desired location for deployment. The stent was deployed by inflating the balloon with a 50% saline and 50% Renografin 76 solution using a standard manual technique (Le Veen balloon inflator). A 10 mm diameter balloon catheter was used with 6 atm pressure for 2 minutes with 3 repetitions. The Wallstent was deployed in a standard fashion and inflated using equivalent inflation pressures and technique for Cordis stent deployment.
Since the coagulation process is much faster in pigs than in humans based on our previous experience, 500 U/kg heparin was routinely administered in each procedure. No anticoagulants or other medicines were used after the procedure.
A numerical rating system was developed in the evaluation of the following deployment factors at implantation: stent insertion through the sheath, insertion tool function, stent tracking, balloon inflation and deflation, stent expansion, ease of positioning, balloon catheter removal, and stent architecture and visibility under fluoroscopy. The rating system was scored as follows: excellent = 1; satisfactory = 2; poor = 3; and not achieved = 4.
Portal venography was performed following TIPS placement and prior to sacrifice. Plain radiography was taken and the following factors were assessed: stent migration, distortion of stent architecture and changes of diameter and length of the stent.
Animals were euthanized 2 weeks after TIPS placement by intravenous injection of 15 mL saturated KCL. All of the shunts were examined by portography via transjugular immediately prior to euthanasia.
Necropsy was performed immediately following euthanasia. The TIPS shunt and involved adjacent tissues were evaluated by gross examination at necropsy and preserved for histologic assessment. Representative crossections from the central, hepatic and portal vein regions of the stent were cut after fixation in buffered 4% formalin for at least 24 hours. The metal of Wallstent was removed before embedding while the metal of Cordis stent had to be removed using a hand saw after embedding and then reembedded in JB-4. The tissues were then cut at 5 microns and stained with modified Giemsa and basic fuchsin.
At least 3 sections in each region of stent were prepared and reviewed. Histologic quantification including thickness, the area of the proliferative response and the ratio of the proliferative area/total area of the stent was achieved using standard planimetry techniques and Nikon microscopy and Camera Lucita with Micro-Plan II Image Analysis (Laboratory Computer Systems, INC).
All quantitated data were expressed as the mean value ± SD. Stent patency and quantified proliferative reaction within the stent were compared using Chi-Square test and Student’s t test, respectively. Statistical significance was defined as P < 0.05.
TIPS was successfully performed in all pigs with both stent designs (100%). No difficulties were encountered in delivering and deploying the Cordis stents. All pigs remained healthy until the scheduled date of euthanasia except one. This pig died 2 d following Cordis stent placement from introgenic hemorrhage secondary to inadvertent gastric puncture during the TIPS procedure. This early death was excluded from the numerical results.
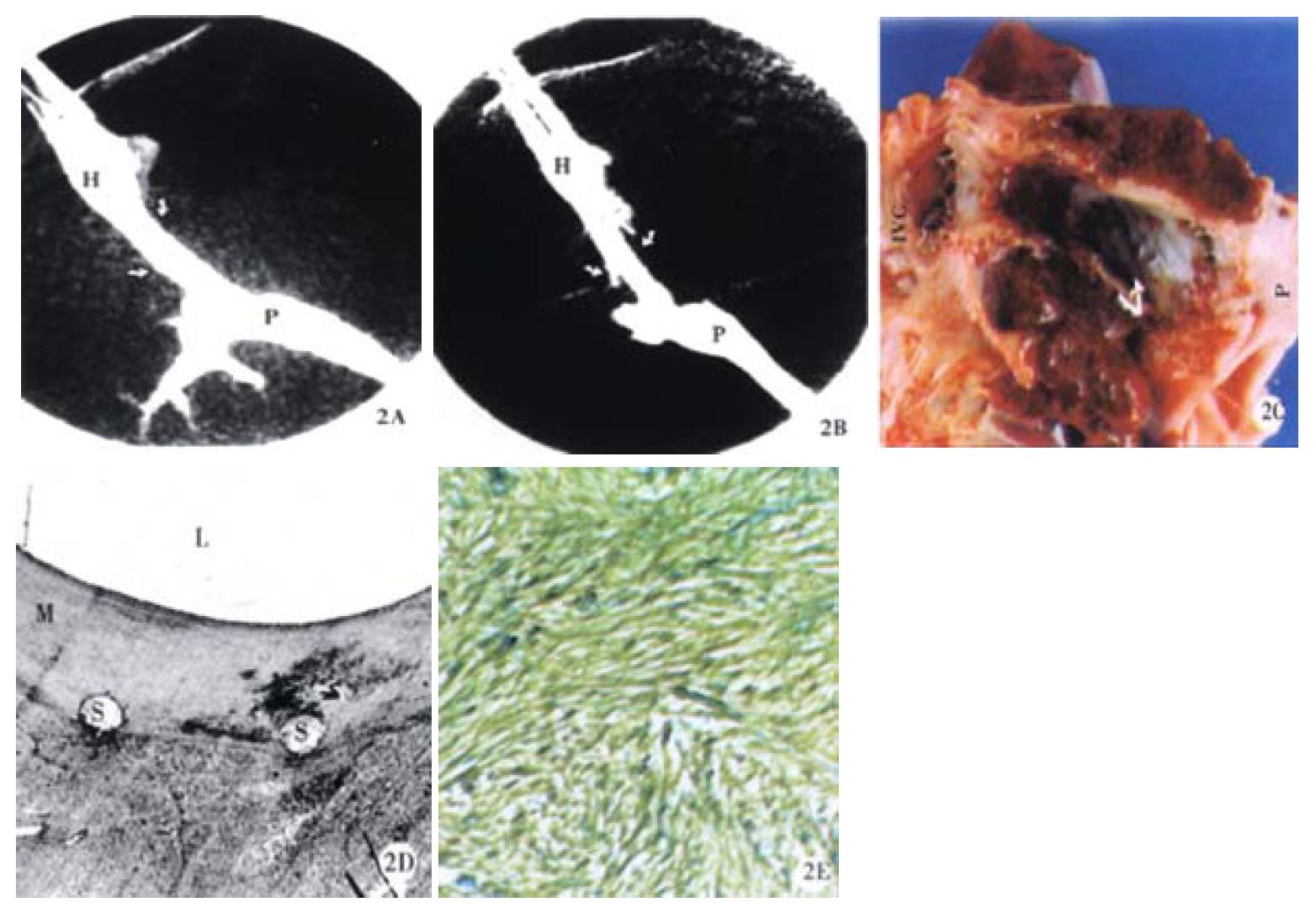
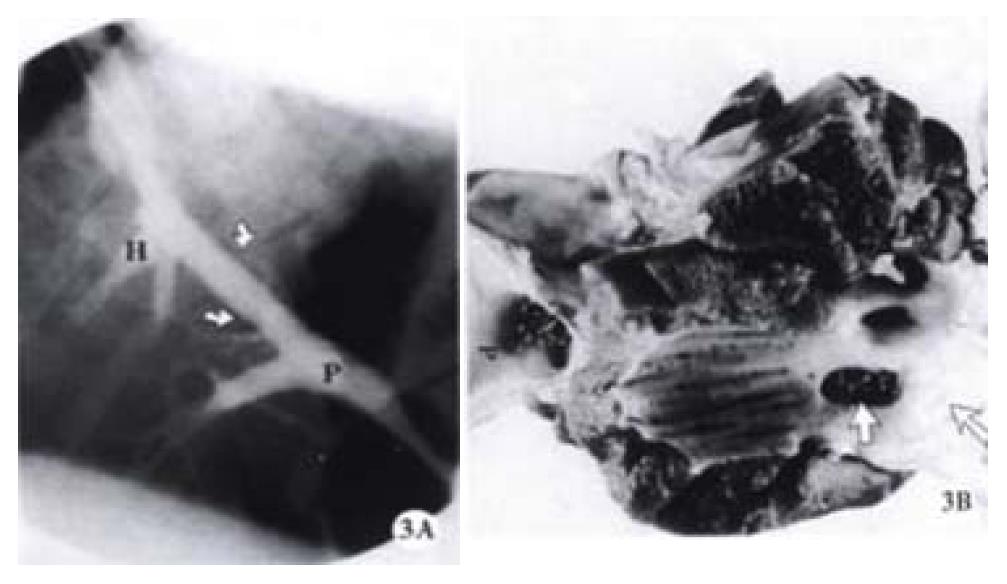
Stent patency was determined by venography and pathohistological examinations, 33.3% (4/12) of the Cordis stent group and 38.5% (5/13) of the Wallstent group were patent at 2 weeks post-deployment (Figure 2). This difference did not reach a statistical significance (P > 0.05, Chi-Square test). One pig had an occluded Cordis stent because its proximal end wedged into the liver parenchyma due to its stiff property (Figure 3). Likewise, one pig had an occluded Wallstent due to longitudinal recoiling of the stent causing the distal end to withdraw from the portal vein into the liver parenchyma.
The mean score for stent deployment factors are listed in Table 1. Stent insertion through the sheath and stent expansion were scored excellent in all procedures performed with both Cordis stent and Wallstent. Cordis stent had a higher score than Wallstent in stent tracking, positioning, and visibility by fluoroscopy. The factors of tool function, balloon in/de-flation and catheter removal which are unique to Cordis stent, were scored excellent for all procedures.
| Stent types | Insert thru sheath | Tool function | Stent tracking | Balloon in/de flation | Stent expansion | Position | Catheter removal | Architecture & visibility |
| Cordis | 1 ± 0.0 | 1 ± 0.0 | 1.07 ± 0.27 | 1 ± 0.0 | 1.14 ± 0.53 | 1.29 ± 0.61 | 1 ± 0.0 | 1.86 ± 0.54 |
| Wallstent | 1 ± 0.0 | * | 2.17 ± 0.84 | * | 1.00 ± 0.00 | * | 1.75 ± 0.87 | 2.42 ± 0.52 |
No stent migration or stent distortion was demonstrated in either Cordis stent or Wallstent groups. Stent diameter and length immediately after TIPS placement and 2 weeks after TIPS are shown in Table 2 and Table 3. The interval change in the Wallstent group at 2 weeks after TIPS is larger than in the Cordis stent group. The Wallstent demonstrated an average shortening of 5.18% as compared with 0.69% with Cordis stent.
| Interval | Diameter (mm) | Length (CM) | |||
| Proximal | Middle | Distal | Overall | ||
| Imm post-TIPS | 7.46 ± 0.80 | 7.32 ± 0.70 | 7.43 ± 0.73 | 7.40 ± 0.73 | 4.35 ± 0.33 |
| 2 weeks | 7.51 ± 0.79 | 7.25 ± 0.73 | 7.50 ± 0.74 | 7.42 ± 0.74 | 4.32 ± 0.32 |
| Change (%) | +0.67 | -0.96 | +0.94 | +0.27 | -0.69 |
| Interval | Diameter (mm) | Length (CM) | |||
| Proximal | Middle | Distal | Overall | ||
| Imm post-TIPS | 9.17 ± 0.94 | 7.42 ± 0.60 | 7.72 ± 0.71 | 7.98 ± 1.05 | 4.83 ± 0.42 |
| 2 weeks | 9.28 ± 0.83 | 7.67 ± 0.53 | 7.94 ± 0.52 | 8.22 ± 0.90 | 4.58 ± 0.48 |
| Change (%) | +1.2 | +3.37 | +2.77 | +3.01 | -5.18 |
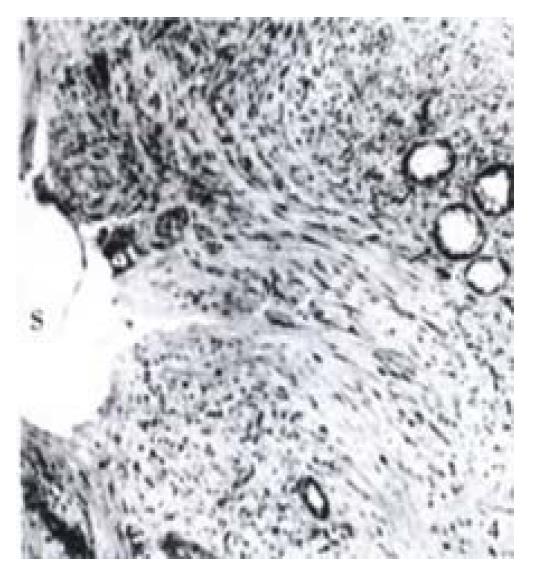
Pseudointimal proliferation (PIH) formed within the shunt with both stent designs, and histology was similar in all three assayed areas. PIH was primarily composed of myofibroblasts (Figure 2, Figure 4), organized thrombus, and collagen. Infla mmatory cells were mainly lymphocytes and mononuclear cells commonly located around the stent wires. Organized thrombus was characterized by palisading and herringbone deposition of the fibrin and the invasion of blood vessels (neovascularization). A single-cell lining of endothelial-like cells covering the lumen was observed only in specimens with a patent shunt. The liver tissue adjacent to the stent showed minimal injury characterized by sinusoidal dilatation, hemorrhage, focal necrosis, mononuclear infla mmation and fibrosis. There was no major difference in histological characteristics between the two groups with the different stent designs.
In 10 specimens (5 in each stent group), bile leak was identified by bile staining of thrombus. Histologic sections demonstrated extensive neo-bile duct proliferation within the PIH or on the surface of the lumen in some specimens with bile leak (Figure 4). This partially contributed to occlusion of the stent.
PIH thickness and the ratio of proliferative area/total area within both stent designs are shown in Table 4. The portal vein aspect of the stents had the highest proliferative response, the hepatic region the second highest and the middle region of the stents the least proliferative. There was no statistically significant difference among the three assayed regions between Cordis stent and Wallstent specimens (P > 0.05).
| Region | Thickness (mm) | Proliferation area/total area (%) | ||||
| Cordis stent | Wallstent | P value* | Cordis stent | Wallstent | P value* | |
| Hepatic | 2.04 ± 1.28 | 1.85 ± 0.96 | 0.66 | 57.49 ± 23.60 | 51.43 ± 24.61 | 0.74 |
| Middle | 1.42 ± 0.82 | 1.31 ± 0.87 | 0.63 | 49.89 ± 17.31 | 47.91 ± 20.12 | 0.60 |
| Portal | 2.46 ± 0.98 | 2.38 ± 0.23 | 0.61 | 71.88 ± 21.18 | 69.97 ± 29.45 | 0.57 |
| Average | 2.18 ± 1.07 | 2.00 ± 0.02 | 0.73 | 59.18 ± 23.06 | 51.66 ± 25.11 | 0.78 |
Various stent designs including Palmaz stent, Gianturco-Rosch Z stent, Strecker stent and Memotherm stent have been utilized in TIPS procedures[3-6]. Wallstent is the most co mmon stent used for TIPS currently within the United States due to its flexibility which allows easy negotiation of the angles bridging a hepatic vein and a portal vein[7]. Unfortunately, TIPS stent stenosis rates are high with all stent designs, although PTFE covered stent seems to decrease the stenosis of TIPS shunt in pigs[8,9]. The mechanism and process of restenosis in TIPS remains poorly understood. However, it is well accepted that stent design including mechanical properties are important factors influencing stent patency.
Since multiple interdependent mechanical characteristics of stents may play a role in stent restenosis[10-19], the ideal stent appears to be non-existent. To produce a satisfactory stent, the following factors should be considered: stent wire material and diameter, longitudinal flexibility, hoop strength (circumferential strength), stent texture and stent surface area.
A layer of metal oxide provides the ultimate interface between stent and host following stent implantation. This is independent of metal content[10]. Similar thrombogenicity and patency rate have been observed in stented arteries using different stent metals[16,20]. Hehrlein et al[18] evaluated stent biocompatibility as related to surface texture and charge. Palmaz-Schatz stents were coated either by electrochemical metal deposition (platinum, gold, copper) or coated with a metallic film implanted onto the stent surface by argon ion bombardment. These stents were implanted and evaluated in a rabbit iliac arterial model. The results demonstrated that the most electropositive coating (platinum or gold) induced markedly less neointima formation than the least electropositive (copper). The authors therefore concluded that stent surface texture was the most important factor determining biocompatibility of the stent while the charge on stents appeared to be less important[18]. Strength, elasticity, and plasticity of various stents co mmonly used were recently investigated in an in vitro model[12,13]. The results suggested that the Palmaz stent is appropriate for insertion into highly resistant obstructions due to its superior resistance to deformation. Strecker stents and Wallstents required good wall contact to achieve adequate strength and Wallstent may not function well if implanted into eccentric stenoses of tough consistency due to its unique deformation. The Gianturco stent showed the lowest resistance and deformed more readily. We did not evaluate such criteria regarding the Cordis stent in this study. However, the higher longitudinal rigidity and hoop strength of this new stent may have merit for placement within tough and highly resistant cirrhotic liver tissue. Strong mechanical support by a stent is important to prevent proliferation as demonstrated by Ikari et al[17]. They demonstrated significantly higher proliferation at the articulation of Palmaz-Schats stents in stented conorary arteries and partially attributed this to lack of mechanical support in the articulation area of the stent. Surface area occupied by the metal stent mesh also influences restenosis.
A predominant difference between Cordis stent and Wallstent evaluated in this study is the rigid characteristic of Cordis stent and the flexible characteristic of Wallstent. The benefits of the Palmaz stent and Wallstent in TIPS were compared in a prospective randomized study[21]. The authors illustrated that early shunt thrombosis was more frequent with Wallstent (4/45 vs 0/45). Palmaz suggests that the relatively more rigid Palmaz stent could provide a stable, nonshifting surface for endothelial growth, whereas dimensional change in a prosthetic surface causes increased endothelial slough and platelet proliferation[10]. However, late observation in the same study[21] showed more frequent shunt insufficiency with Palmaz stent than Wallstent (6/45 vs 2/45). This indicates that the process of TIPS stenosis should be multifactorial.
Wallstent is frequently associated with unpredictable shortening in TIPS at follow-up due to the self-expanding force from elasticity[6,22]. Wallstent shortened 5.18% in our study at 2 weeks as compared with 0.69% shortening of the Cordis stent. Stent flexibility is required to negotiate curves when delivering the stent by catheter through tortuous vessels leading to the target area. Is it possible to compromise flexibility and rigidity without the drawback of shortening like a Wallstent in TIPS Several other stent designs have been utilized in TIPS. Gianturco-Rosch stent was the first stent designed in experimental TIPS, but it seems to be unco mmonly used. Strecker stent is highly radiopaque, flexible and shortens minimally but tends to dislodge and is difficult to be recatheterized[23]. Preliminary experience in TIPS using the Memotherm stent (Angiomed, Germany) showed promising results, but the number of samples was small[6]. The new Cordis stent used in this study is relatively more flexible than the Palmaz stent, and has the added advantage of being highly radiopaque with minimal shortening. From a technical perspective, the Cordis stent deployment system consistently works well and is easy to use, compared with Wallstent. In our opinion, the Cordis stent is an appropriated compromise between the rigid Palmaz and the flexible but foreshortening Wallstent.
The similar histologic characteristics of proliferative response were observed in the two groups using different designs of stent, which is also very similar to the findings described previously by us and other authors[24-26].
To date, there are few experimental studies or clinical trials available to compare different stent designs in TIPS. It is difficult to directly compare different stent designs following deployment. For instance, although equal stent diameters and balloons with equal inflation pressures were used in our study, TIPS stent length was not equivalent following deployment. Stent diameter following TIPS completion in the Wallstent group was larger than the Cordis stent group (7.98 mm vs 7.40 mm). This unexpected size variation may contribute to the relative rigidity of Cordis stents compared with the relative flexibility of Wallstents and the stronger circumferential strength of Cordis stents compared with the relatively decreased hoop strength of Wallstents. Perhaps the difference in luminal diameter contributed to the increased PIH in the pigs with Cordis stents (not statistically significant). Such an explanation is favored by our recent study (unpublished data, 1996) which demonstrated significantly thicker pseduointimal formation within the lumen (2.07 mm vs 1.25 mm, P = 0.006) when comparing a smaller diameter Wallstent (6.50 mm) to larger Wallstent (7.25 mm) in a TIPS porcine model. Nevertheless, the short-term patency rate using the Cordis balloon-expandable stainless steel stent in a TIPS porcine model in this study is comparable to that found using Wallstent.
There were interesting findings in two occluded stents in this study: one stent probably occluded because of Cordis stent rigidity, and the other probably occluded because of Wallstent longitudinal recoil. This probably reflects the inherent drawbacks of Cordis stent and Wallstent respectively. However, Cordis stent rigidity may be an advantage when deployed in stiff cirrhotic liver parenchyma in TIPS rather than in healthy, compliant normal pig livers, although Cordis stent should not be deployed where sharp curves are required to bridge the portal and hepatic veins. Use of a longer Wallstent than the exact length of the tract required in TIPS may overcome the shortening disavantage of Wallstent[22]. However, it is important to accurately place the stent in the hepatic vein to ensure patency and not to compromise future opportunity for liver transplantation[27]. The easy to use, reliable Cordis deployment system and increased radiopacity allow for accurate manipulation and precise positioning under fluoroscopy.
Therefore, our study suggests that the Cordis stent merits further investigation for potential use in TIPS patients.
Edited by Ma JY
| 1. | Cello JP, Ring EJ, Olcott EW, Koch J, Gordon R, Sandhu J, Mor-gan DR, Ostroff JW, Rochey DC, Bacchetti P. Endo-scopic sclerotherapy compared with percutaneous transjugular intrahepatic portosystemic shunt after initial sclerotherapy in patients with acute variceal hemorrhage. A randomized, controlled trial. Ann Intern. 1997;126:907-910. [RCA] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Ochs A, Rössle M, Haag K, Hauenstein KH, Deibert P, Siegerstetter V, Huonker M, Langer M, Blum HE. The transjugular intrahepatic portosystemic stent-shunt procedure for refractory ascites. N Engl J Med. 1995;332:1192-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 273] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Noeldge G, Richter GM, Roessle M, Haag K, Katzen BT, Becker GJ, Palmaz JC. Morphologic and clinical results of the transjugular intrahepatic portosystemic stent-shunt (TIPSS). Cardiovasc Intervent Radiol. 1992;15:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Matsuzaka S, Kanazawa H, Kobayashi M, Kumazaki T. [An experience of transjugular intrahepatic portosystemic shunt in the treatment of gastroesophageal varices]. Nihon Shokakibyo Gakkai Zasshi. 1994;91:257-266. [PubMed] |
| 5. | Echenagusia AJ, Camúñez F, Simó G, Peiró J, Garay MG, Rodriguez Laiz JM, Bañares R. Variceal hemorrhage: efficacy of transjugular intrahepatic portosystemic shunts created with Strecker stents. Radiology. 1994;192:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Tesdal IK, Jaschke W, Bühler M, Adamus R, Filser T, Holm E, Georgi M. Transjugular intrahepatic portosystemic shunting (TIPS) with balloon-expandable and self-expanding stents: technical and clinical aspects after 3 1/2 years' experience. Cardiovasc Intervent Radiol. 1997;20:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kerlan RK, LaBerge JM, Gordon RL, Ring EJ. Transjugular intrahepatic portosystemic shunts: current status. AJR Am J Roentgenol. 1995;164:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Nishimine K, Saxon RR, Kichikawa K, Mendel-Hartvig J, Timmermans HA, Shim HJ, Uchida BT, Barton RE, Keller FS, Rösch J. Improved transjugular intrahepatic portosystemic shunt patency with PTFE-covered stent-grafts: experimental results in swine. Radiology. 1995;196:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Haskal ZJ. Improved patency of transjugular intrahepatic portosystemic shunts in humans: creation and revision with PTFE stent-grafts. Radiology. 1999;213:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Palmaz JC. Intravascular stents: tissue-stent interactions and design considerations. AJR Am J Roentgenol. 1993;160:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 140] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Fontaine AB, Spigos DG, Eaton G, Das Passos S, Christoforidis G, Khabiri H, Jung S. Stent-induced intimal hyperplasia: are there fundamental differences between flexible and rigid stent designs? J Vasc Interv Radiol. 1994;5:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Lossef SV, Lutz RJ, Mundorf J, Barth KH. Comparison of mechanical deformation properties of metallic stents with use of stress-strain analysis. J Vasc Interv Radiol. 1994;5:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Flueckiger F, Sternthal H, Klein GE, Aschauer M, Szolar D, Kleinhappl G. Strength, elasticity, and plasticity of expandable metal stents: in vitro studies with three types of stress. J Vasc Interv Radiol. 1994;5:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Tominaga R, Kambic HE, Emoto H, Harasaki H, Sutton C, Hollman J. Effects of design geometry of intravascular endoprostheses on stenosis rate in normal rabbits. Am Heart J. 1992;123:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Tominaga R, Harasaki H, Sutton C, Emoto H, Kambic H, Hollman J. Effects of stent design and serum cholesterol level on the restenosis rate in atherosclerotic rabbits. Am Heart J. 1993;126:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Hausegger KA, Lammer J, Hagen B, Flückiger F, Lafer M, Klein GE, Pilger E. Iliac artery stenting--clinical experience with the Palmaz stent, Wallstent, and Strecker stent. Acta Radiol. 1992;33:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Ikari Y, Hara K, Tamura T, Saeki F, Yamaguchi T. Luminal loss and site of restenosis after Palmaz-Schatz coronary stent implantation. Am J Cardiol. 1995;76:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Hehrlein C, Zimmermann M, Metz J, Ensinger W, Kübler W. Influence of surface texture and charge on the biocompatibility of endovascular stents. Coron Artery Dis. 1995;6:581-586. [PubMed] |
| 19. | Schürmann K, Vorwerk D, Kulisch A, Stroehmer-Kulisch E, Biesterfeld S, Stopinski T, Günther RW. Experimental arterial stent placement. Comparison of a new Nitinol stent and Wallstent. Invest Radiol. 1995;30:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Scott NA, Robinson KA, Nunes GL, Thomas CN, Viel K, King SB, Harker LA, Rowland SM, Juman I, Cipolla GD. Comparison of the thrombogenicity of stainless steel and tantalum coronary stents. Am Heart J. 1995;129:866-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Hauenstein KH, Vinee P, Haag K, Ochs A, Rossle M. Palmaz stent versus Wallstent for transjugular intrahepatic portosystemic shunts: Results of a prospective randomized study in 90 patients (abstr.). Radiology. 1994;193:166. |
| 22. | Hausegger KA, Sternthal HM, Linbichler F, Ascharuer M, Karaic R, Stauber R. Progressive shortening of the Wallstent after TIPS: A co mmon cause for shunt occlusion (abstr.). Eur Radiol. 1995;5:219. |
| 23. | Ugolotti U, Larini P, Marcato C, Saccani A, Puccianti F, Pedretti G. Is the tantalum Strecker stent suitable for TIPS creation? Short- and mid-term results in 20 consecutive patients. Cardiovasc Intervent Radiol. 1997;20:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Rösch J, Uchida BT, Putnam JS, Buschman RW, Law RD, Hershey AL. Experimental intrahepatic portacaval anastomosis: use of expandable Gianturco stents. Radiology. 1987;162:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Teng GJ, Bettmann MA, Hoopes PJ, Ermeling BL, Yang L, Wagner RJ. Transjugular intrahepatic portosystemic shunt in a porcine model: histologic characteristics at the early stage. Acad Radiol. 1998;5:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Teng GJ, Bettmann MA, Hoopes PJ, Wagner RJ, Park BH, Yang L, Baxter BR. Transjugular intrahepatic portosystemic shunt: effect of bile leak on smooth muscle cell proliferation. Radiology. 1998;208:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Wilson MW, Gordon RL, LaBerge JM, Kerlan RK, Radosevich PM, Roberts JP, Ring EJ. Liver transplantation complicated by malpositioned transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1995;6:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |