Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.116
Revised: February 1, 1999
Accepted: February 12, 1999
Published online: April 15, 1999
AIM To study the angiogenesis induced by liver cancer with different metastatic potentials using corneal micropocket model in nude mice.
METHODS Corneal micropockets were created in nude mice. Tumor tissues and liver tissues were implanted into the corneal micropockets. Angiogenesis was observed using a digital camera under slit-lamp biomicroscope, and compared among different grafts and incision alone. Vascular responses were recorded in regard to the range, number and length of new blood vessels toward the grafts or incisions.
RESULTS Vascular responses induced by tumor tissues were grea ter than those by incision alone and liver tissue grafts. LCI-D20 induced more intensive angiogenesis than LCI-D35.
CONCLUSION Highly metastatic liver cancer LCI D20 was more angiogenic than low metastatic cancer LCI D35 and liver tissue. Micropocket was a useful model to study dynamic process of angiogenesis in vivo.
- Citation: Sun HC, Li XM, Xue Q, Chen J, Gao DM, Tang ZY. Study of angiogenesis induced by metastatic and non-metastatic liver cancerby corneal micropocket model in nude mice. World J Gastroenterol 1999; 5(2): 116-118
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/116.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.116
Angiogenesis is a tumor growth and metastasis dependent process. It has been proved that subtype of tumor with metastatic potential is more angiogenic than non-metastatic one[1]. Corneal micropocket is an ideal model to observe the dynamic change in angiogenesis in vivo[2]. Therefore, this model was created in nude mice to study the angiogenesis induced by different grafts.
Animals: Six-week-old male BALB/c nu/nu mice were provided by Shanghai Pharmaceutical Institute. The eyes of each mouse were treated with aureomycin ointment everyday for one week before operation to prevent any potential infection. A slit-light biomicroscope (Wuxi Optical Instrument Factory, Jiangsu Province, China) was used to oberve the eyes of mice. Highly metastatic LCI D20 and lowly metastatic LCI D35 human hepatoma models were established by orthotopic implantation of surgical specimens of liver cancer patients in nude mice[3]. In LCI D20 model, metastasis to the lung, peripheral liver and celiac lym phnodes was found 20 days after transplantation to the liver. Rapid deterioration and death occurred in nude mice. On the contrary, in LCI D35 model, 35 days af ter orthotopic transplantation, no metastatic lesion appeared, the nude mice sur vived for more than two months without distant metastasis[4].
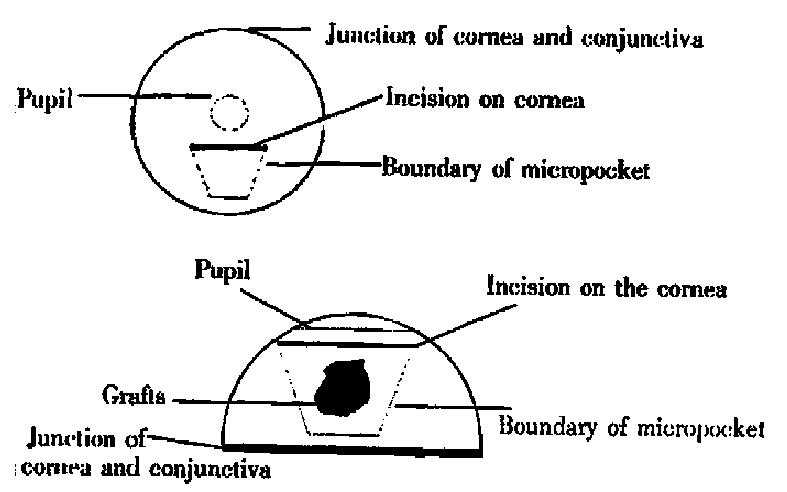
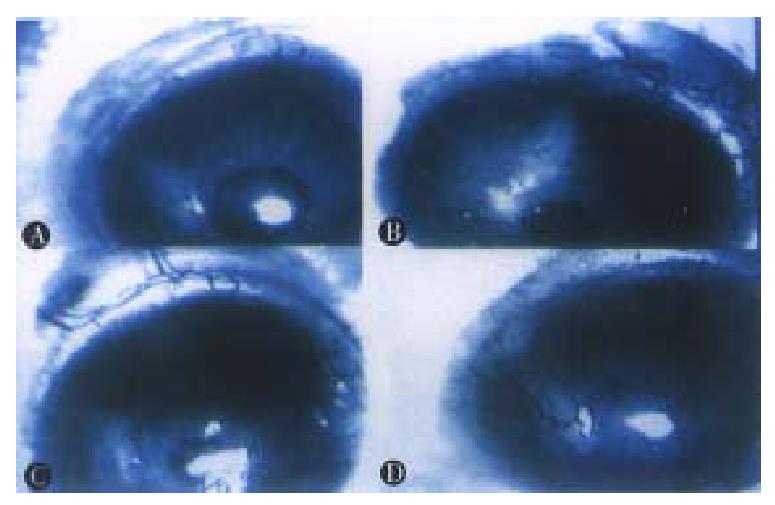
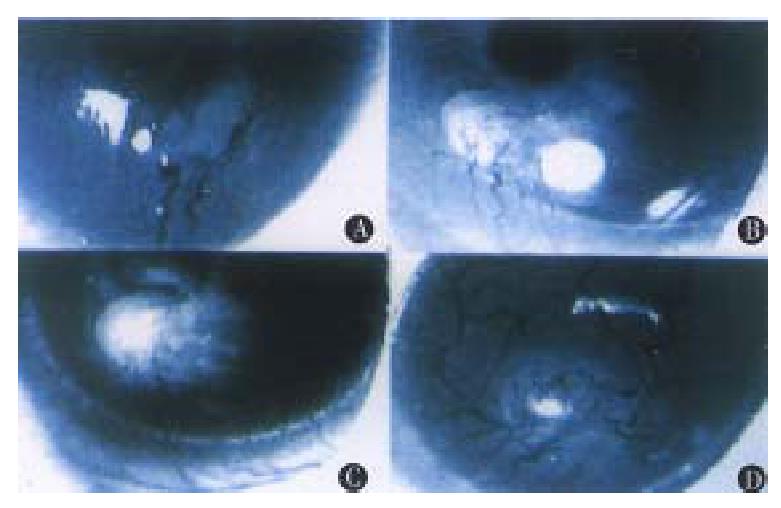
Operations were performed in aseptic condition under anesthesia with 0.06 mL 3% pentobarbital and 0.9% natural solution was used to wash the eyes. Under an anatomic microscope, the eyes were fixed and moved forward by two sutures through the conjunctiva near cornea. With a modified blade (Gillette Limited, Shanghai, China), a superficial incision about 1.5 mm long not through the cornea was made on the corneal dome near the pupil. A modified spa tula made from a sterilized 27gauge needle was used to make a 1.5 mm × 0.8 mm-micropocket in the corneal stroma from incision toward the cornea-sc leral junction. A graft, about 0.1 mm × 0.1 mm × 0.5 mm, was put into the bottom of the micropocket using the modified spatula. The incision could be self-closed (Figure 1). Aureomycin ointment was applied to the operated eyes to prevent postoperative infection for 3 days. On the 3rd, 5th, 7th, 10 th, 15th and 20th days after operation, the operated eyes were observed under slit-lamp biomicroscope with their images recorded in computer via a digital camera (Figures 2 and 3). Angiogenesis induced by the implanted tissues was defi ned as those blood vessels originating at the adjacent limbal plexus toward the implants. The methods of quantitative study followed those reported in the literature[5]. Briefly, the circle of the cornea was evenly divided into 24 p arts to present the range of angiogenesis. For example, if the range of angiogenesis was one-fourth of circle, it was 6. The distance from the cornea-scleral junction to the pupil was divided evenly into 5 parts. The length of blood vessels could be calculated from one to five. The number of blood vessels was counted from the pictures.
The angiogenesis on the cornea of nude mice could be separated into two phases. In the first phase, the blood vessels originating from the limbal plexus at the cornea-scleral junction and pointing to the incision in the operated areas were caused by the surgical manipulations. If there was no graft, the blood vessels disappeared 3 days after operation. However, the angiogenesis on the fourth day after operation was different in case of different grafts (Table 1). Vascular response was described in detail as follows.
| Angiogenesis | LCI D20 (n = 4) | LCI D35 (n = 4) | Liver tissues (n = 4) | Incision alone (n = 4) | P value |
| Range (-x±s) | 24.00 ± 0.00 | 11.50 ± 1.29 | 7.25 ± 0.96 | 4.50 ± 0.58 | < 0.01 |
| Number(-x±s) | 24.75 ± 2.63 | 16.50 ± 2.98 | 12.50 ± 1.29 | < 0.01 | |
| Length (-x) | 5.1 | 4.4 | 4.3 | > 0.05 |
On the 1st day after operation, the new blood vessels developed from the limbal plexus and pointed to the operating site. But on the 2nd day, the number of blood vessels decreased, and no blood vessel was found on the cornea on the 3rd day.
On the 7th day after operation, the blood vessels reached the implants, which were the peak of angiogenesis induced by liver tissues. Its range was 7.25. Its average length and number were 4.4 and 12. 5 respectively. On the following days, the size of grafts was decreased, with the length and number of new blood vessels reduced as well.
On the 7th day after operation, the blood vessels reached the tumor implants. The tumor was larger than that when it was implanted. The range of an-giogenesis was 11.5, and the average length and number of new blood vessels were 4.4 and 16.5 respectively.
On the third day after operation, the blood vessels reached the tumor implants. On the 7th day, the blood vessels were penetrating to the tumor tissues with the tumor implants enlarged. On the 15th day, the blood vessels originating from the counterpart of the operative limbal plexus could be found. The range was 24, the average length and number of blood vessels were 5.1 and 24.75 respectively. The tumor graft was 2-3 times larger than its original size. Hemorrhagic necros is often was presented at the central part of the tumor. The sizes of blood vessels were obviously bigger than those of others.
The human hepatoma metastatic model LCI D20 and LCI D35 were good tools for the study of metastasis. We have observed that the LCI D20 tumor was ruddier than LCI D35 tumor, and there were more blood vessels in LCI D20, suggesting LCI D20 is more angiogenic than LCI D35.
To study angiogenesis in vivo, we established a corneal micropocket model in nude mice to show the angiogenic reaction induced by human hepatoma tissues in vivo. Because in vivo study can dynamically demonstrate angiogenesis, it is more reliable than in vitro study.
It was demonstrated that the blood vessels on cornea induced by incision alone disappeared 3 days after operation, which was considered as a normal blood vessel reaction caused by trauma. Liver graft induced mild reaction on the cornea of nude mice, which was greater than incision alone. It may result from the slight difference in their histological compatibility between the donor mice and recipient mice in our corneal micropocket model. Folkman et al[2] also reported the rejection caused by xenogenic implantation in the corneal micropocket model. However, the angiogenesis induced by xenogenic tissue implantation and tumor tissues were very different. Tumor angiogenesis was more potent and durable than others, and the grafts grew rapidly instead of disappearing in case of liver grafts.
Our experiment also indicated that tumor tissues with different metastatic potentials had different angiogenecities. LCI D20 induced a relatively stronger angiogenesis than LCI D35 did. In addition, the growth rate of LCI D20 tumor was greater than that of LCI D35. A peak of angiogenesis was observed in LCI D35 implants. Our hypothesis is that proliferation of tumor was balanced by apoptosis due to lack of enough blood supply. However, no peak of angiogenesis was found in LCI D20 grafts, indicating angiogenesis induced by LCI D20 became more intensive at the end of the observation period.
In preparation of the corneal micropocket model in nude mice, the depth of incision into the cornea, the size of micropockets and grafts are vitally important for success.
Observation of dynamic changes in angiogenesis shown by our model is very helpful for screening anti-angiogenic drugs or other anti-tumor drugs.
Edited by Xian-Lin Wang
| 1. | Folkman J. Tumor angiogenesis. In: Holland JF, Bast RC, Morton DL, Frei E, Kufe DW,Weichselbaum RR, eds. Cancer medicine, 4th Edition. Baltimore, Maryland: Williams & Wilkens. 1996;181-204. |
| 2. | Gimbrone MA, Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974;52:413-427. [PubMed] |
| 3. | He B, Liu KD, Tang ZY, Xue Q. Different mutation and expres-sion patterns in LCI D20 and LCI D35 models. Chinese Hepatology. 1997;5:245-246. |
| 4. | Sun FX, Tang ZY, Lui KD, Ye SL, Xue Q, Gao DM, Ma ZC. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer. 1996;66:239-243. [PubMed] |
| 5. | Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 495] [Article Influence: 16.5] [Reference Citation Analysis (0)] |