Published online Feb 15, 1999. doi: 10.3748/wjg.v5.i1.38
Revised: November 27, 1998
Accepted: December 16, 1998
Published online: February 15, 1999
AIM To assess the possible roles of cytokines (TNF-α, IFN-γ, IL-6 and IL-8) in liver damage of hepatitis B.
METHODS The serum TNF-α, IFN-γ, IL-6 and IL-8 were detected by ELISA in 66 patients with hepatitis B and 20 healthy blood donors.
RESULTS TNF-α and IL-6 in all types of clinical hepatitis B were significantly higher than those in healthy blood donors (P < 0.05 ); meanwhile the levels of TNF-α, IFN-γ, IL-6 and IL-8 in the patients with fulminant hepatitis B were much higher than those in the patients with acute hepatitis B(P < 0.05); the level of TNF-α was positively correlated with the levels of IFN-γ, Il-6 and IL-8 in all types of hepatitis B (rIFN = 0.24, rIL-6 = 0.35, rIL-8 = 0.44) and the TNF-α, IFN-γ, IL-6 and IL-8 were positively correlated with serum bilirubin (P < 0.05). Dynamic changes of these cytokines were observed in the course of acute and fulminant hepatitis. The level of IFN-γ peaked in the initial period of acute hepatitis and early stage of hepatic coma in fulminant hepatitis; TNFα, IL-6 and IL-8 increased with exacer-bation, and reached a peak when the liver da-mage was most serious, then decreased when patient conditions were improved.
CONCLUSION The increased cytokines were re-lated to the inflamm ation of liver cells and multi-ple factors may play certain roles in liver dam-age.
- Citation: Wang JY, Wang XL, Liu P. Detection of serum TNF-α, IFN-γ, IL-6 and IL-8 in patients with hepatitis B. World J Gastroenterol 1999; 5(1): 38-40
- URL: https://www.wjgnet.com/1007-9327/full/v5/i1/38.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i1.38
Since Muto[1] reported that TNF-α and IL-1 were re-lated to fulminant hepatitis, the studies on the rela-tionship between cytokines and liver damage have been paid more and more attention especially in re-cent years. Most scholars now agree that TNF and IFN are related to liver damage, so are IL-6 and IL-8. We detected the serum TNF-α, IFN-γ, IL-6 and IL-8 in the patients with different clinical types of hepatitis B by ELISA for assessing the relationship between the cytokines and the liver damage.
A total of 66 patients with HBV infection and 20 healthy blood donors were studied. They were ad-mitted to this college between 1993 and 1997. The patients (48 men and 18 women) ranged in age from 21 to 56 years. There were 22 cases of acute hepati-tis (AH), 25 cases of chronic hepatitis (CH) and 19 cases of fulminant hepatitis (FH). The serological markers of HAV, HCV, HEV, CMV and EBV were negative, and HBsAg and other markers of HBV were positive in all the patients.
Healthy blood donors, aged from 25 to 43 years, in-cluded 16 men and 4 women. They had no serologi-cal markers of HAV-HEV, CMV, EBV infection and liver functions were normal.
The kits of the four cytokines were produced by the Genzyme Company, U. S. A. No. 1 9970214. The four cytokins were detected by ELISA according to the manufacturer’s instructions. The first antibody was biotin-labelled and the second one was connect-ed with horse radish peroxidase.
The serum TNF-α, IFN-γ, IL-6 and IL-8 in patients with different types of hepatitis B are shown in Table 1.
The TNF-α and IL-6 in various clinical types of hepatitis B were significantly higher than those in healthy blood donors (P < 0.05). Except acute hep-atitis B, the levels of IFN-γ and IL-8 were obviously higher in other types than those in healthy blood donors. The levels of four cytokines of patients with FH were much higher than those of patients with AH (P < 0.05).
The correlations between TNF-α and IFN-γ, IL-6 and IL-8 in HBV infections were analyzed. The re-sults suggested that TNF-α and the other cytokines were positively correlated (rIFN = 0.24, rIL-6 = 0.35, rIL-8 = 0.44).
The correlation between serum billirubin and four cytokines was analyzed in HBV infections. The results suggested that bilirubin was positively related to the levels of four cytokines (P < 0.05).
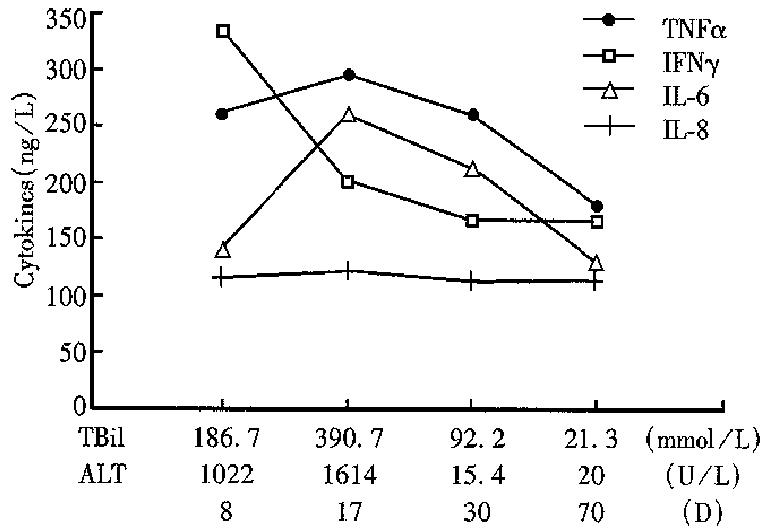
The dynamic changes of four cytokines in the course of AH and FH are shown in Figures 1 and 2. At the early stage of AH, IFN-α peaked and decreased rapidly. TNF-α and IL-6 increased with exacerba-tion, reached a highest level when jaundice became most severe, and then decreased gradually. IL-8 level did not change during the course.
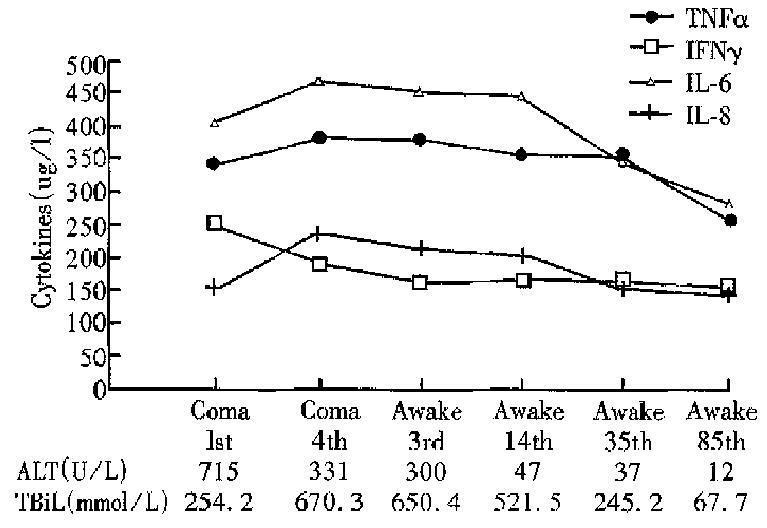
On the first day of hepatic encephalopathy of FH, IFN-γ reached a peak, then decreased rapidly. TNF-α, IL-6 and IL-8 increased when condition of the patients worsened. They reached the highest level during the peak of jaudice, then decreased gradually, and maintained abnormal for a long time.
Since it was reported that TNF and IL-1 in super-natant of cultured monocytes of peripheral blood in patients with FH were obviously higher than those with AH, the studies on cytokines related to liver damage advanced rapidly. Pei Liu[2] proved that TNF could cause liver necrosis in corynebacterium sensitized animals and the necrosis could be blocked by anti-TNF monoclonal antibody[3]. At present, the studies of hepatitis focused on the roles of cy-tokines in cell-mediated injury of tissues. Ferluga et al[4] reported that the liver injury of animal model induced by corynebacterium-endotoxin could be caused by the soluble factors produced by monocytes gathering at the hepatic lobules. Luca[5] proved that activation of CTL induced by IFN-γ resulted in CTL-mediated hepatocytic injury in the study of HBV transgenic mouse. IL-6 may induce the activa-tion, differentiation and maturation of NK cells[6] and expression of monocytic IL-8 gene[7]. The IL-8 may cause degranulation of nutrophile granulocyte, leading to DIC within the liver[8].
The serum levels of TNF-α, IFN-γ, IL-6 and IL-8 in patients with HBV infection were higher than those in healthy blood donors. The difference was obvious between the levels of cytokines in FH and those in AH. TNF-α and IFN-γ, IL-6 and IL-8 were positively correlated in various types of hepati-tis B. The bilirubin was also positively related to the four cytokines. In the course of AH and FH, IFN-γ peaked in the early stage of AH and the 1st day of hepatic coma of FH. TNF-α, IL-6 and IL-8 increased with exacerbation of condition of the patients with AH and FH except for IL-8 in AH. These suggested that the four cytokines were related to liver injury.
The roles of cytokines in the liver damage are complex. They affect each other to form a cytokine network, in which IFN-γ may be the chief cytokine and induce the immune cells to release other cytokines and improve cytotoxic activities mediated by immune cells.
Edited by Jing-Yun Ma
| 1. | Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988;2:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 203] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Liu P, Ohnishi H, Moriwaki H, Muto Y. Enhanced tumor necrosis factor and interleukin-1 in an experimental model of massive liver cell necrosis/fatal hepatitis in mice. Gastroenterol Jpn. 1990;25:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Pei L. The protective effects of Anti mouse TNF monoclonal antibody in experimental massive hepatic cell necrosis model. Chin J Digestion. 1994;14:150. |
| 4. | Ferluga J, Allison AC. Role of mononuclear infiltrating cells in pathogenesis of hepatitis. Lancet. 1978;2:610-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764-3768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 319] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Kato K, Yokoi T, Takano N, Kanegane H, Yachie A, Miyawaki T, Taniguchi N. Detection by in situ hybridization and phenotypic characterization of cells expressing IL-6 mRNA in human stimulated blood. J Immunol. 1990;144:1317-1322. [PubMed] |
| 7. | Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung HF, Leonard EJ, Oppenheim JJ. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883-1893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 759] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Larsen CG, Anderson AO, Oppenheim JJ, Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989;68:31-36. [PubMed] |