Published online Aug 14, 2025. doi: 10.3748/wjg.v31.i30.108680
Revised: June 16, 2025
Accepted: July 16, 2025
Published online: August 14, 2025
Processing time: 98 Days and 22.5 Hours
Dyslipidemia, a complex disorder characterized by systemic lipid profile ab
Core Tip: Dyslipidemia’s multifactorial pathogenesis involves gene-environment-microbiota crosstalk, with gut microbiota (GM) emerging as a master regulator of lipid homeostasis through lipopolysaccharide-induced very low-density lipoprotein overproduction, short-chain fatty acid-G protein-coupled receptor signaling, bile acid-farnesoid X receptor/Takeda G protein-coupled receptor 5 axis modulation, and microbiota-host non-coding RNA crosstalk. This review delineates GM-dyslipidemia interactions, molecular mechanisms, and interventions such as probiotics, prebiotics and fecal microbiota transplantation. Key challenges involve establishing causal GM-lipid pathway links and optimal intervention timing. Advancing precision therapies require longitudinal multi-omics integration coupled with gnotobiotic validation models, ultimately enabling machine learning-driven prediction of personalized microbial signatures for targeted cardiovascular prevention.
- Citation: Lv J, Zhao HP, Yu Y, Wang JH, Zhang XJ, Guo ZQ, Jiang WY, Wang K, Guo L. From gut microbial ecology to lipid homeostasis: Decoding the role of gut microbiota in dyslipidemia pathogenesis and intervention. World J Gastroenterol 2025; 31(30): 108680
- URL: https://www.wjgnet.com/1007-9327/full/v31/i30/108680.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i30.108680
Lipid metabolism governs fundamental biological processes, including lipid assimilation, lipoprotein trafficking, and steroid hormone synthesis. Dyslipidemia is a pathological state characterized by elevated triglyceride (TG), total cholesterol (TC), and low-density lipoprotein (LDL) cholesterol (LDL-C) levels, and/or reduced high-density lipoprotein cholesterol (HDL-C) levels. In > 50% of the global adult population, this disorder accounts for approximately 33% of the annual ischemic heart disease mortality and is frequently comorbid with obesity and metabolic syndrome (MetS)[1]. The persistent residual cardiovascular risk in statin-treated patients underscores the urgent need for innovative therapeutic paradigms[1].
The gut microbiota (GM), an evolutionarily conserved ecosystem harboring trillions of microorganisms, has recently been recognized as a master conductor of lipid metabolism[1]. Phylogenetic analyses revealed Firmicutes and Bacteroidetes as the dominant phyla in healthy adults, with developmental trajectory studies demonstrating stage-specific colonization patterns: Neonatal dominance of Enterobacteriaceae and Staphylococcus transitions to Bifidobacterium enrichment until weaning, ultimately maturing into a geography- and diet-modulated steady state[2]. Enterotype classification (e.g., Bacteroides- vs Prevotella-dominant clusters) further correlates with metabolic phenotypes, whereas dyslipidemia-associated dysbiosis manifests as reduced α-diversity and altered Firmicutes/Bacteroidetes (F/B) ratios, particularly Bacteroides depletion, impairing cholesterol catabolism[3,4].
In addition to their taxonomic composition, the GM exerts systemic endocrine regulation through bioactive metabolites that interact with host physiology, including pro-inflammatory lipopolysaccharide (LPS)[1], short-chain fatty acids (SCFAs) that modulate hepatic lipogenesis[5], trimethylamine/trimethylamine-N-oxide (TMAO), which influences reverse cholesterol transport (RCT)[6,7], secondary bile acids (BAs) that regulate farnesoid X receptor (FXR)/Takeda G protein-coupled receptor 5 (TGR5) signaling[8], and cross-kingdom non-coding RNA (ncRNA) communication. Notably, emerging studies have demonstrated bidirectional crosstalk between the GM and circadian rhythms in the regulation of lipid metabolism[9-12]. Specifically, circadian disruption induces GM dysbiosis characterized by the loss of rhythmic taxa, altered BA rhythms, and impaired SCFA cycling, whereas high-fat diets (HFDs) suppress microbial oscillations as well. Causal relationships are evidenced by the resistance of germ-free (GF) mice to diet-induced dyslipidemia and the ability of fecal microbiota transplantation (FMT) to transfer atherogenic phenotypes[13]. These findings underscore the dual role of the GM as both a metabolic modulator and a therapeutic target[1,14]. While 16S rRNA sequencing establishes taxonomic associations, integrated multi-omics (metatranscriptomics-metaproteomics-metabolomics) now decodes functional microbial contributions to lipid dysregulation[15,16]. However, certain critical challenges persist: (1) Causality delineation: Overreliance on observational human studies; (2) Confounder control: Host genetics, diet, and medications masking microbial signals; and (3) Therapeutic translation: Strain-specific effects and microbiota engineering safety.
This review focuses on bidirectional GM-lipid axis interactions, molecular mechanisms linking microbial metabolites to lipidogenic pathways, and emerging interventions spanning precision probiotics, prebiotics, and metabolically optimized FMT. By bridging microbial ecology with host pathophysiology, we propose a roadmap for developing microbiota-centric strategies that complement conventional therapies, thereby addressing unmet needs in residual cardiovascular risk management through targeted microbial reprogramming.
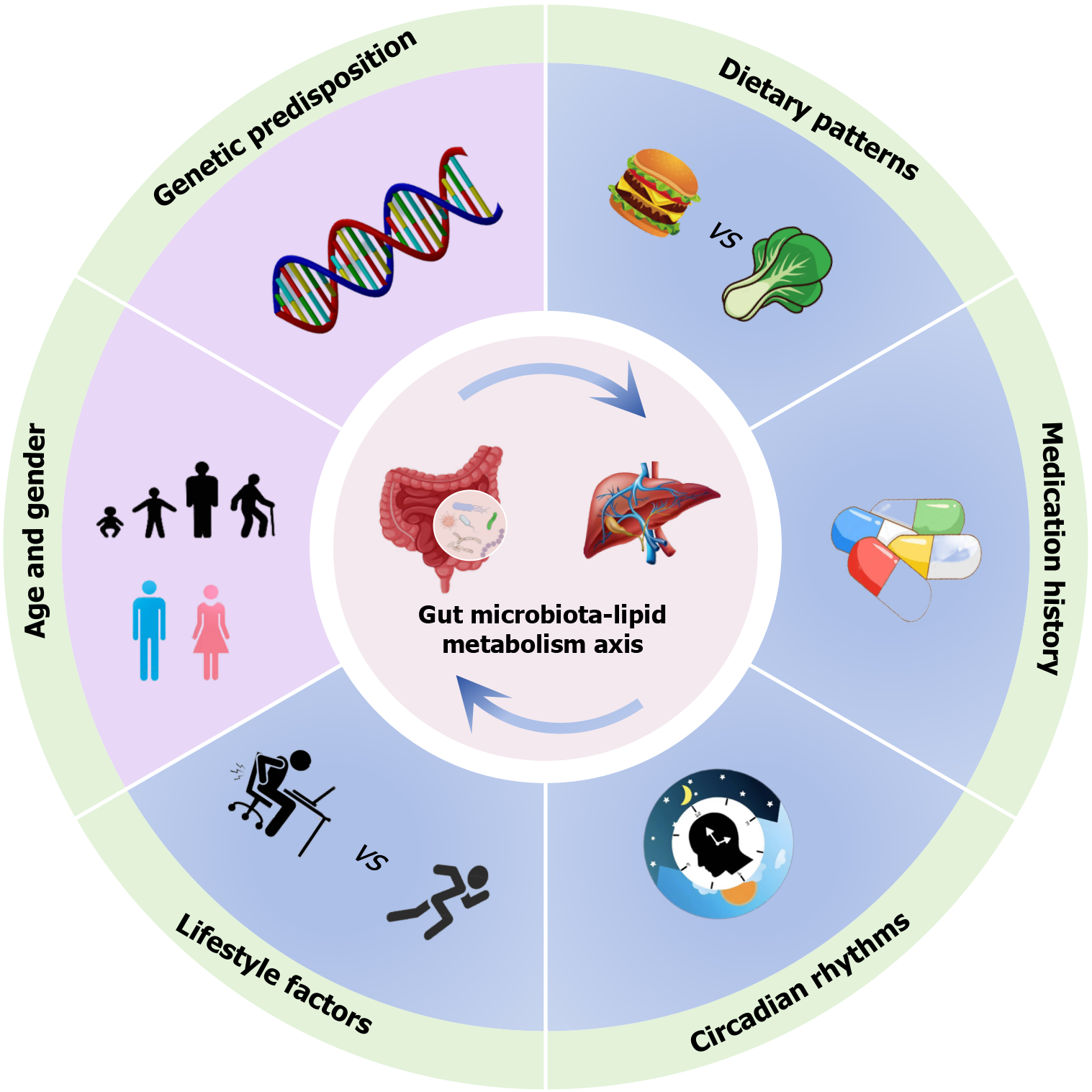
The GM is as a dynamically balanced supraorganism shaped by host-environmental co-dependencies, with its taxonomic architecture and metabolic output governed by host genetics, developmental stage, dietary patterns, lifestyle factors, pharmacological exposures, and ecological memory from prior microbial perturbations (Figure 1)[15,17]. Crucially, these confounders exhibit hierarchical interactions, necessitating multivariate adjustment in study designs to isolate authentic microbiota-dyslipidemia associations. This paradigm-shifting discovery centers on the GM’s mediation of obesity-dyslipidemia crosstalk. Central adiposity (waist-to-hip ratio > 0.9 in men/0.85 in women), independent of body mass index (BMI), elevates dyslipidemia risk, potentially through adipose-derived inflammatory cytokines that impair lipid homeostasis; however, the GM actively modulates this pathophysiology via the diet-microbiota-adipose axis, transgenerational metabolic memory, and ecological resilience loss[18]. Notably, the disease specificity of these microbial alterations remains enigmatic, e.g., causal vs compensatory; thus, resolution requires longitudinal multi-omics cohorts tracking GM trajectories from dyslipidemia initiation through progression.
Dietary patterns exert profound enterotype-specific influences on the gut microbial architecture and metabolic function. Chronic consumption of high-fat/Low-fiber diets favors Bacteroides-dominant ecosystems optimized for mucin degradation and saturated fatty acid (SFA) metabolism, whereas fiber-rich regimens promote Prevotella-enriched consortia specialized in complex polysaccharide fermentation[19]. This nutritional program demonstrates temporal stratification: Short-term dietary interventions (e.g., 5-day animal-based protocols) induce transient blooms of bile-tolerant genera (Alistipes, Bilophila, Bacteroides) accompanied by depletion of fiber-utilizing taxa, yet these shifts largely revert within several days of intervention cessation[20]. However, nutritional modifications during critical developmental windows (prenatal to early postnatal stages) can persistently reshape the microbial community[21]. This restructuring occurs through epigenetic imprinting mechanisms affecting both the host intestinal epithelia and the resident microbiota[21].
HFDs and their multifaceted impact on the GM and lipid metabolism: HFDs function as dual metabolic regulators, serving both as essential nutritional substrates and potent disruptors of lipid homeostasis. While dietary lipids constitute fundamental components of the cellular architecture and signaling networks, chronic overconsumption of SFAs and trans fatty acids and cholesterol directly perturbs systemic lipid homeostasis, establishing a causal link to dyslipidemia through microbiota-mediated and independent pathways[22,23]. The GM emerges as a central biological transducer in this process, orchestrating bidirectional crosstalk between dietary lipid intake and host metabolic regulation. Sustained HFD exposure drives substantial ecological restructuring of the GM ecosystem, characterized by a marked reduction in microbial diversity alongside selective enrichment of bile-tolerant taxa and depletion of fiber-fermenting consortia[20]. Functional metagenomic analyses revealed that this compositional shift precipitated a metabolic transition from carbohydrate fermentation dominance to proteolytic metabolism dominance, whereas the results of the GF mouse experiments confirmed that this transformation worsened HFD-induced adiposity in the context of dysbiosis microbiota transplantation[24].
The intestinal barrier is profoundly affected by HFD-induced microbial dysregulation. Through coordinated mecha
Dietary lipid composition critically influences microbial interactions and metabolic effects. Studies have shown that lard-based SFA diets promote pro-inflammatory Bilophila wadsworthia; in contrast, marine omega-3 polyunsaturated fatty acids enrich beneficial taxa such as Lactobacillus, Bacteroides, Akkermansia, and Coprococcus, while increasing fecal butyrate and isovalerate levels[27]. Modern dietary imbalances in omega-6/omega-3 ratios disrupt these protective mechanisms, although microbial biotransformation of polyunsaturated fatty acids offers compensatory pathways[22]. For example, GM-mediated conversion of linoleic acid to 10-hydroxy-cis-12-octadecenoic acid activates G protein-coupled receptor (GPR) 40/120 signaling, stimulating glucagon-like peptide-1 (GLP-1) secretion, enhancing gut motility and reducing lipid absorption, whose effects are transmissible via FMT[22].
Emerging nutritional interventions leverage lipid structure-specific microbial modulation. Camellia seed oil, which is rich in monounsaturated fatty acids, provides dual benefits by enriching beneficial GM taxa (Dubosiella, Lactobacillus, and Alistipes). It also improves lipid profiles by inhibiting mammalian target of rapamycin signaling, a key regulator of lipid metabolism through sterol regulatory element-binding protein 1 activation[28,29]. Similarly, sea buckthorn juice and algal-derived fibers ameliorate hyperlipidemia and adiposity by selectively promoting beneficial taxa and SCFA producers[24,30]. These findings collectively underscore the therapeutic potential of structure-guided dietary strategies targeting the GM-lipid metabolism axis.
Insights from Mediterranean and plant-based dietary patterns: The Mediterranean diet (MD) is characterized by high intake of plant-based foods (fruits, vegetables, nuts, and legumes), extra-virgin olive oil, and moderate wine consump
Plant-based dietary models, particularly strict vegan regimens, induce Prevotella-dominant enterotypes associated with leanness and enhanced SCFA production[35]. Fiber subtype specificity governs microbial modulation: Insoluble wheat bran selectively enriches Lactobacillus and Bifidobacterium, whereas resistant starch from whole grains promotes Eubacterium rectale and Roseburia while suppressing Clostridium and Enterococcus[36,37]. Paradoxically, excessive fructose intake in plant-heavy diets may counteract these benefits, inducing GM dysbiosis and impairing intestinal barrier function, ultimately triggering hepatic Kupffer cell activation and inflammation.
Central to these dietary patterns is microbiota-accessible carbohydrate (MAC) availability. Western diets with chronic MAC deprivation drive transgenerational loss of Bacteroidales and Clostridiales, creating ecological voids resistant to short-term fiber intervention[18]. Strikingly, combinatorial MAC restoration with targeted missing-taxa supplementation achieves certain degree of recovery of GM ecological balance compared to traditional diets, highlighting the therapeutic potential of precision microbial rehabilitation[18]. These findings position dietary fiber not merely as a microbial substrate but as an ecological engineering tool for GM reprogramming.
Host weight stability exerts profound regulatory effects on gut microbial ecosystems, with weight fluctuations demonstrating greater impacts on microbial composition than temporal variations alone[38]. In individuals with stable weights and lifestyles, the GM profiles show remarkable stability over time[38]. These microbial communities remain consistent for more than 3 months and potentially up to five years, indicating that the host metabolic state is a key determinant of microbial ecology[38]. This interdependence holds clinical significance in obesity, which disrupts lipid metabolism through multiple pathways. Key dysregulation features include hepatic very LDL (VLDL) overproduction, impaired lipoprotein lipase (LPL) activity, LDL receptor downregulation, reduced HDL-C levels, and dysfunctional lipid transport via cholesteryl ester transferase protein/phospholipid transfer protein axis alterations[39].
Obesity is closely linked to GM dysbiosis, which is characterized by reduced microbial diversity and taxonomic shifts, including depletion of beneficial genera (Bacteroides, Prevotella, Lactobacillus, Bifidobacterium, Akkermansia) and expansion of pro-inflammatory taxa (Clostridium, Staphylococcus, and Escherichia)[40-42]. A key feature of this dysbiosis is an elevated F/B ratio, which contributes to obesity progression by disrupting energy homeostasis, promoting fat storage, modulating appetite, and inducing chronic low-grade inflammation[43]. Cross-species evidence supports the F/B ratio as a conserved obesity signature, with metabolically compromised individuals (e.g., obese women) showing positive correlations between the F/B ratio and atherogenic lipids (TC, LDL-C), as well as inverse associations with fecal SCFA[41,44]. Fecal transplantation studies further confirmed the causal role of the GM in adiposity regulation, demonstrating that obesity-associated microbiota can transfer metabolic dysfunction, affecting GM harvest efficiency and fermentative capacity, as well as promoting chronic inflammation[41,44]. Weight loss interventions (e.g., caloric restriction and bariatric surgery) partially reverse obesity-associated dysbiosis, typically reducing Firmicutes while increasing Bacteroidetes[45]. However, the diagnostic utility of F/B ratio remains inconsistent due to heterogeneous study designs, population-specific variables, and clinical confounders (e.g., obesity severity and intervention duration). Despite these discrepancies, MetS is consistently associated with elevated F/B ratios across multiple studies[42,44], suggesting its potential as a context-dependent biomarker. Therapeutic strategies targeting GM modulation, such as probiotics, prebiotics, and FMT, may help restore a balanced F/B ratio and mitigate obesity-related metabolic dysfunction[43].
The GM actively modulates adipose biology through diet-microbe-host crosstalk, functioning as an environmental amplifier during the multifactorial pathogenesis of obesity. Current evidence reveals three key mechanisms, including LPS-mediated inflammation, SCFA signaling, and BA axis regulation. The development of targeted microbiota therapies for obesity-related dyslipidemia will require standardized multi-omics approaches to clarify these complex interactions.
Antibiotics as a double-edged sword: While indispensable for infection control, antibiotics induce profound GM perturbations through biodiversity loss and selection of resistant pathogens[46]. Early-life exposure to antibiotics exerts particularly durable effects, disrupting microbial colonization patterns and increasing lifelong risks of obesity and metabolic dysfunction, while these developmental programming effects are synergistically exacerbated by concurrent HFD co-exposure[47,48]. Cumulative antibiotic courses progressively deplete low-abundance taxa and impair microbial functions that are essential for lipid metabolism, with subtherapeutic antibiotic exposure recapitulating obesity-associated GM profiles characterized by Firmicutes enrichment and Bacteroidetes depletion[46]. These shifts correlate with enhanced energy harvest and adipogenesis, positioning antibiotics as inadvertent metabolic disruptors necessitating judicious clinical use[49].
Metformin as a microbiota-mediated metabolic regulator: The first-line antidiabetic agent metformin demonstrates GM-dependent lipid modulation through selective enrichment of beneficial Akkermansia and functional enhancement of butyrate synthesis and carbohydrate metabolism[50,51]. Clinical trials reveal metformin-herbal combinations increasing Blautia and Faecalibacterium abundances alongside lipid profile improvement in dyslipidemic diabetics[50], paralleled by rodent studies showing metformin counteracts HFD-induced dysbiosis[52]. FMT from metformin-treated donors improve glucose tolerance in GF mice, mechanistically linked to altered fatty acid metabolism and antimicrobial activity[53].
Statins lowering cholesterol with microbial implications: Statins, while primarily acting via HMG-CoA reductase inhibition to lower LDL-C, exhibit microbiota-mediated pleiotropic effects[16,54-56]: (1) Microbial secondary BAs modulate simvastatin bioavailability and LDL-C reduction efficacy; (2) Pregnane X receptor signaling mediates statin-induced restoration of microbial diversity and BA metabolic modulation; and (3) Bacteroidetes elevation and Faecalibacterium reduction correlate with statin-mediated metabolic improvements. Rodent models further demonstrate statins alter butyrate-producing taxa and inflammatory pathways, suggesting GM modulation contributes to both therapeutic and adverse effects[57].
Current evidence positions medications as potent GM modulators with cascading metabolic consequences. Critical knowledge gaps persist in understanding precise drug-microbiota interaction mechanisms, individual response heterogeneity, and direct microbial contributions to therapeutic outcomes. While microbial profiling holds promise for personalized pharmacotherapy, clinical translation requires mechanistic validation through standardized multi-omics approaches[39]. Prioritizing these investigations will enable harnessing GM’s therapeutic potential in dyslipidemia management.
Circadian rhythms are regulated by hypothalamic suprachiasmatic nucleus-driven transcriptional-translational feedback loops. These rhythms coordinate physiological processes from thermoregulation to lipid homeostasis through core clock genes, such as circadian locomotor output cycles kaput and brain and muscle-ARNT-like 1, which form heterodimers that activate period and cryptochrome[10,58]. These endogenous 24-hour oscillations synchronize peripheral tissues (e.g., liver, adipose, and gastrointestinal tracts) with environmental light-dark cycles, precisely regulating nutrient absorption dynamics and microbial metabolic activity[58,59]. Clinical studies have identified circadian misalignment (e.g., shift work) as an independent risk factor for dyslipidemia and obesity[11,12]. The risk associated with hypercaloric diets, while chronotherapies such as time-restricted feeding, shows metabolic benefits even under HFD conditions[11,12].
The GM engages in reciprocal regulation with the host circadian system through synchronized diurnal oscillations in composition and function[9]. Disruption of host rhythms via sleep deprivation, erratic feeding, or genetic clock disruption induces GM dysbiosis characterized by depletion of beneficial taxa and expansion of pro-inflammatory genera[10,60,61]. Conversely, over 20% of microbial genes show intrinsic rhythmicity, which is directly modulated by host-derived circadian signals[11,12,60]. Specific taxa (e.g., Lactobacillus and Klebsiella) program host metabolism through hepatic gene regulation and systemic metabolite fluxes (e.g., SCFAs and secondary BAs)[11,12,60].
Environmental perturbations exert profound effects on this circadian-microbiota axis: Chronic HFD exposure abolishes microbial rhythmicity, elevates F/B ratios, suppresses Verrucomicrobia and dysregulates hepatic clock gene expression, whereas antibiotic-induced eradication of oscillating microbiota exacerbates circadian disruption, manifesting as metabolic dysfunction (e.g., adiposity and glucose intolerance)[10,61]. Genetic evidence from brain and muscle-ARNT-like 1 knockout mice confirms the indispensability of the host circadian machinery, demonstrating the complete loss of microbial and metabolic rhythms irrespective of dietary input[11,59]. This bidirectional dysregulation establishes a self-reinforcing cycle: GM dysbiosis amplifies host metabolic disturbances (e.g., insulin resistance and dyslipidemia), whereas circadian dysfunction perpetuates microbial instability, clinically presenting as atherogenic lipid profiles, progressive weight gain and hepatic steatosis[11,59].
The evolutionary conservation of this axis underscores temporal synchrony as a prerequisite for metabolic health. Therapeutic strategies targeting circadian-microbiota crosstalk, including timed feeding protocols and microbial metabolite supplementation, have the potential to restore host-microbial rhythmicity, offering novel mechanistic avenues for metabolic disease management. The preservation of circadian integrity through chrononutrition and microbiota-directed interventions may thus constitute a critical frontier in combating global metabolic disorder epidemics[12].
The liver operates as the metabolic command center governing systemic lipid homeostasis through an intricate balance of de novo lipogenesis converting excess carbohydrates to fatty acids, VLDL assembly, TG transport, and mitochondrial β-oxidation regulating fatty acid catabolism (Figure 2)[62-64]. These coordinated pathways normally distribute fatty acids into structural components (phospholipids and cholesterol easters) and signaling molecules. Dyslipidemia develops when this balance is disrupted, manifesting in excessive fatty acid synthesis, diminished catabolic capacity, and impaired peripheral lipid clearance, ultimately resulting in the production of atherogenic lipoproteins[65,66].
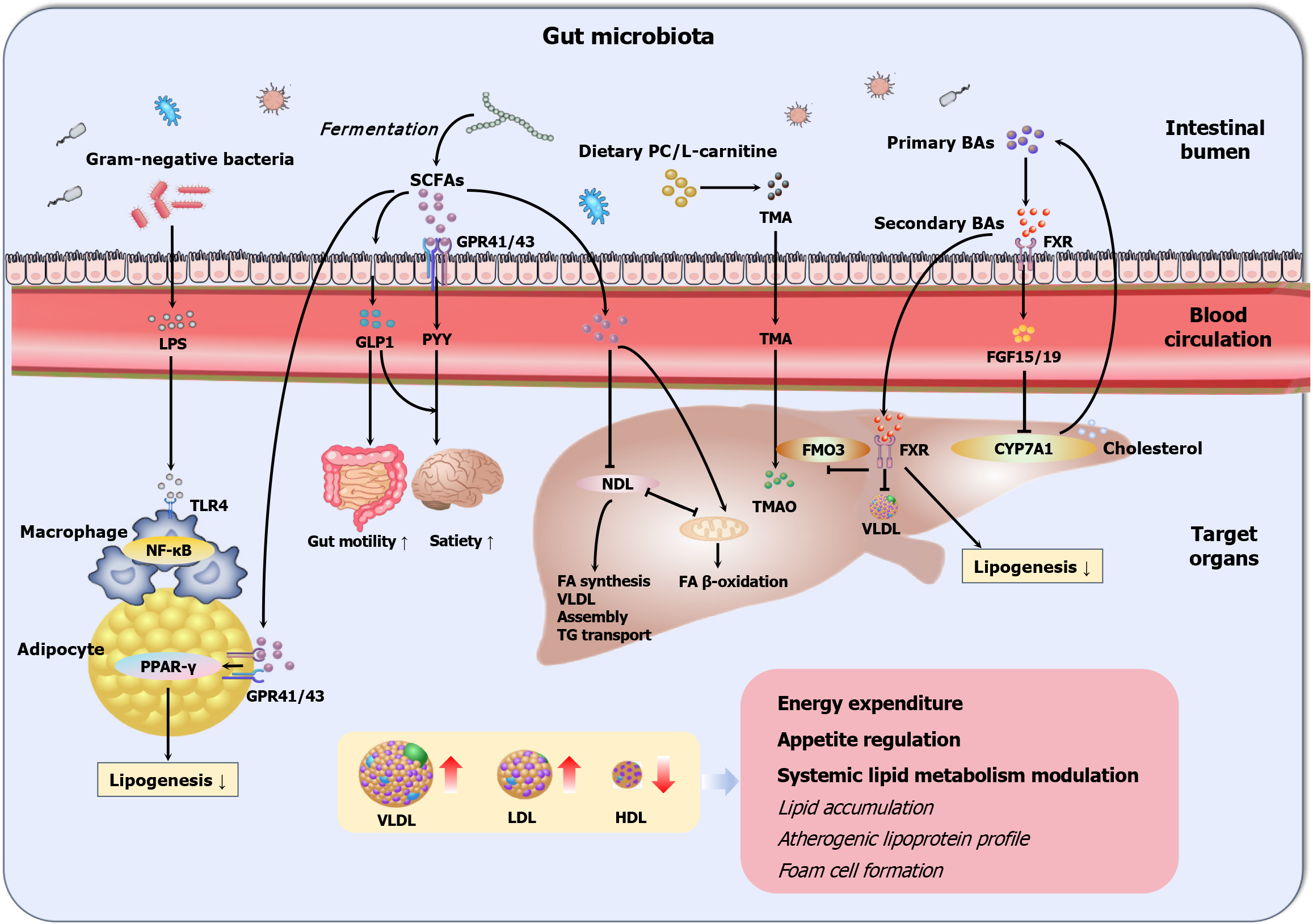
Parallel to hepatic regulation, the GM engages in systemic lipid modulation through bidirectional molecular dialogues. Microbial structural components such as LPS and extracellular polysaccharides translocate across the intestinal barrier, activating host pattern recognition receptors, including membrane-bound toll-like receptors (TLRs) (e.g., TLR2/4/5) on intestinal epithelial surfaces and endosomal TLR3/7/9 in immune cells[67]. These interactions trigger pro-inflammatory or immunomodulatory cascades that perturb hepatic LDL receptor recycling and HDL maturation. Concurrently, GM-derived metabolites exert multifaceted regulatory effects: SCFAs and BAs increase hepatic fatty acid oxidation, VLDL secretion, and cholesterol flux, whereas TMAO exacerbates atherosclerosis through foam cell formation[67]. A third layer of regulation emerges through microbial extracellular vesicles (EVs), which traverse the gut-liver axis via systemic circulation. These nanoscale carriers deliver bioactive cargo, such as microbial microRNAs (miRNAs) and proteins, to local intestinal cells and distant organs, including hepatocytes, where they epigenetically reprogram lipidogenic genes and post-translationally modify metabolic enzymes, thereby reshaping lipid flux[68,69]. This tripartite communication system, encompassing structural component signaling, metabolite-driven regulation, and EV-mediated genetic repro
Gram-negative opportunistic bacteria and their endotoxin byproducts constitute an important pathophysiological axis in metabolic dysregulation, with LPS emerging as a potent mediator linking GM dysbiosis to dyslipidemia. Under physiological conditions, LPS remains sequestered within the intestinal lumen by zonulin-1/occluding-mediated tight junction integrity[70]. HFDs, circadian disruption, or microbial imbalance impair gut barrier function, allowing the translocation of LPS into the circulation, a condition called metabolic endotoxemia[60]. This elevates plasma LPS levels several-fold, activating pattern recognition receptors (e.g., TLR-4, CD14, and nucleotide-binding oligomerization domain 1) on macrophages and dendritic cells[60]. This initiates a self-perpetuating inflammatory cascade: Nuclear factor-kappa B activation downregulates intestinal tight junction proteins while inducing fasting-induced adipose factor (FIAF), an endogenous LPL inhibitor that promotes ectopic lipid deposition in adipose and muscle tissues[71]. Preclinical models have demonstrated that LPS-mediated metabolic dysregulation occurs through coordinated pathways[72]. Chronic LPS administration in mice recapitulates the atherogenic lipid triad, specifically elevated TG levels, increased LDL-C levels, and reduced HDL-C levels, via TLR2/4-dependent mechanisms that impair hepatic VLDL clearance and suppress adipose LPL activity[73].
Clinical investigations corroborate these preclinical findings, revealing elevated abundances of endotoxin-producing bacteria and circulating LPS levels in obese individuals, which strongly correlate with atherogenic lipid profiles and chronic low-grade inflammation[74,75]. Fei and Zhao[76] reported that a traditional whole-grain Chinese diet with prebiotics reduced the abundance of Enterobacter (a potent endotoxin producer) from 35% to undetectable levels in an obese diabetic patient. This intervention resulted in dramatic metabolic improvements, including 51.4 kg weight loss (from 174.8 kg baseline), better lipid profiles, and reduced endotoxemia[76]. GF mice colonized with the patient’s isolated Enterobacter cloacae B29 strain developed HFD-dependent metabolic dysfunction characterized by elevated serum LPS, adipose inflammation, and dyslipidemia, effects that are absent in chow-fed counterparts[76].
These findings establish a mechanistic paradigm in which endotoxin-producing bacteria drive dyslipidemia through barrier disruption followed by metabolic endotoxemia, TLR-induced systemic inflammation, and FIAF-mediated lipid partitioning[76]. Future research must delineate strain-specific endotoxin production kinetics and develop targeted strategies to disrupt this pathophysiological axis, potentially through precision dietary interventions or anti-endotoxin therapies.
SCFAs, the major microbial fermentation products of non-digestible carbohydrates (e.g., pectin, hemicelluloses, and galactose-oligosaccharides), are predominantly acetate (60%), propionate (20%) and butyrate (20%)[77,78]. SCFA synthe
SCFAs regulate host metabolism through three interconnected mechanisms[88-90]. At the local colonic level, SCFAs serve as essential energy substrates for epithelial cells, while butyrate specifically enhances intestinal barrier integrity through the modulation of tight junctions and mucus production. Systemically, the portal circulation distributes SCFAs to peripheral tissues, where they profoundly influence hepatic TG metabolism, cholesterol metabolism, and adipocyte lipogenesis. Additionally, SCFAs function as endocrine signaling molecules, regulating appetite and energy expenditure through free fatty acid receptor (FFAR)-mediated pathways. The biological effects of SCFAs are mediated primarily through GPRs, particularly FFAR2 (GPR43) and FFAR3 (GPR41). These SCFA-sensing receptors are widely distributed across enteroendocrine cells, adipocytes, sympathetic ganglia, and immune cells, underscoring their systemic signaling potential[91].
Each major SCFA has distinct metabolic fates and physiological roles: Acetate, which constitutes the largest proportion of SCFAs, undergoes peripheral oxidation, participates in adipocyte lipogenesis, or may be converted to butyrate by the GM[92]; butyrate preferentially serves as the primary energy source for colonocytes while simultaneously enhancing barrier function and suppressing local inflammation[93]; and propionate uniquely stimulates intestinal gluconeogenesis and promotes the secretion of anorexigenic hormones [GLP-1/2, peptide YY (PYY), leptin][44,91,94], while also exerting anti-adipogenic effects through visceral fat reduction[5].
The regulatory effects of SCFAs on lipid metabolism are particularly noteworthy[95]. Acetate and propionate collectively suppress endogenous lipolytic activity, with propionate additionally modulating circulating lipid levels through increased LPL expression[96,97]. These coordinated actions contribute to reduced plasma lipid concentrations and body weight regulation. In addition to their effects on lipid mobilization, SCFAs significantly influence adipocyte development. Experimental studies have demonstrated that propionate and acetate stimulate preadipocyte differentiation through the upregulation of FFAR2 and proliferator-activated receptor-gamma (PPARγ) pathways via GPR41/43 activation[98-100]. Furthermore, SCFAs impact cholesterol homeostasis through multiple mechanisms, including the facilitation of hepatic cholesterol uptake (by acetate, propionate, and butyrate) and the direct suppression of cholesterol biosynthesis (specifically by propionate)[101-103], processes that involve BA synthesis and microbial coprostanol conversion[104]. Notably, the majority of current research has focused on butyrate, which is typically present at undetectable or very low concentrations in the body, unlike acetate and propionate[91].
The metabolic impacts of SCFAs exhibit context-dependent characteristics[1]. While collectively contributing more than 10% of daily caloric requirements and generally improving lipid profiles, excessive acetate production may paradoxically elevate plasma TG and promote obesity through ghrelin-mediated hyperphagia[105,106]. The F/B ratio, often proposed as a microbial metabolic indicator, demonstrates species-specific complexity[78]. Murine models (ob/ob and GF mice) associate an elevated F/B ratio with increased SCFA production and energy harvest[107], whereas human studies present conflicting associations, with some reporting no differences between obese and lean individuals[108] and others demon
As master regulators of intestinal integrity, neuroendocrine signaling, and systemic lipid homeostasis, SCFAs represent promising therapeutic targets. However, their dualistic effects require precise modulation of concentration gradients, compositional ratios, and tissue-specific receptor activation patterns[91]. Translating these insights requires elucidating temporal dynamics and individualized responses to SCFA-mediated metabolic regulation.
The GM-derived metabolite TMAO has emerged as a pivotal mediator of cardiovascular pathology, counterbalancing the cardioprotective effects of SCFAs through distinct pro-atherogenic mechanisms. This pathogenic cascade begins when the GM metabolizes dietary phosphatidylcholine and L-carnitine into trimethylamine, after which hepatic flavin monooxygenase 3 (FMO3) then oxidizes trimethylamine to form TMAO[6,7]. Isotopic tracing confirmed that most circulating TMAO is derived from this microbial pathway. At the molecular level, TMAO exacerbates atherosclerosis through coordinated disruption of cholesterol homeostasis[6,110]: (1) Suppression of RCT, impairing cholesterol efflux from peripheral tissues; (2) Inhibition of BA transporter expression, reducing cholesterol catabolism; and (3) Potentiation of foam cell formation through macrophage scavenger receptor (CD36, SR-A1)-dependent uptake of oxidized LDL.
Preclinical models conclusively establish the causal role of TMAO: Chronic administration in mice elevates circulating atherogenic lipids[111], whereas FMO3 silencing enhances RCT and cholesterol clearance[112]. Clinical cohort studies corroborate these findings, demonstrating that individuals with combined high plasma L-carnitine and elevated TMAO levels exhibit increased cardiovascular risk, surpassing traditional LDL-C risk stratification[6]. Dietary regulation through vegetarian regimens mitigates TMAO production from L-carnitine through microbial composition modulation, high
Despite mechanistic clarity, therapeutic translation faces substantial barriers. While GF- and antibiotic-treated models abolish trimethylamine production[6], clinical trials reveal paradoxical outcomes: Broad-spectrum antibiotics reduce TMAO levels but concurrently induce microbial diversity loss and enrich antibiotic resistance genes without improving cardiovascular endpoints[19,114]. Precision targeting of trimethylamine-producing taxa remains technically challenging owing to functional redundancy across microbial consortia[113]. These limitations underscore the urgent need for the development of non-antibiotic approaches, such as competitive inhibition of microbial trimethylamine lyases or pharmacological FMO3 antagonism, to disrupt the TMAO axis while preserving the commensal ecology.
BAs exemplify the GM’s capacity to generate metabolically beneficial compounds through structural modification of host-derived molecules[39]. Initiated by hepatic cytochrome P450-mediated conversion of cholesterol into primary BAs, cholic acid and chenodeoxycholic acid, this pathway serves dual roles in cholesterol catabolism and systemic metabolic regulation[115,116]. Following conjugation with glycine/taurine and biliary secretion, 90%-95% of primary BAs undergo efficient ileal reabsorption via apical sodium-dependent BA transporter-mediated enterohepatic circulation[117]. The residual 5% reaching the colon become substrates for microbial transformation through three sequential enzymatic processes, including bile salt hydrolase-catalyzed deconjugation, 7α-dehydroxylase-mediated generation of secondary BAs such as deoxycholic acid from cholic acid and lithocholic acid from chenodeoxycholic acid, and epimerization yielding tertiary BAs like ursodeoxycholic acid[115,117]. This microbial diversification creates a bioactive BA pool functioning as endocrine regulators through nuclear and membrane receptor networks, modulating energy expenditure, glucose homeostasis, and cholesterol balance[8,115].
The nuclear receptor (FXR, NR1H4) serves as the central coordinator of BA signaling, orchestrating tissue-specific metabolic responses. Hepatic FXR activation suppresses sterol regulatory element-binding protein 1c-driven lipogenesis and VLDL-C production, while increasing PPARα-mediated fatty acid oxidation and HDL-C synthesis[118]. Intestinal FXR stimulates fibroblast growth factor 15/19 secretion, which through hepatic fibroblast growth factor receptor 4/β-Klotho receptor complexes inhibits cholesterol 7α-hydroxylase, establishing a negative feedback loop regulating BA synthesis[119]. Notably, FXR exhibits pleiotropic microbial crosstalk, suppressing FMO3 to inhibit TMAO production while shaping the gut microbial composition[7]. The membrane G protein-coupled BA receptor 1 (TGR5) complements FXR signaling, with secondary BA-mediated TGR5 activation stimulating GLP-1 secretion and improving glucose tolerance[120]. Furthermore, BAs additionally engage diverse receptor systems including sphingosine-1-phosphate receptor 2, epidermal growth factor receptor, pregnane X receptor, and vitamin D receptor, enabling integrated metabolic and immune regulation[19,121].
This bidirectional BA-microbiota axis creates a self-regulating metabolic circuit[122]: Microbial BA transformation determines receptor activation states, while BAs reciprocally modulate microbial ecology through bacteriostatic effects[10,123,124]; besides, microbial BA transformations directly influence host lipid metabolism, as evidenced by FXR-mediated suppression of TMAO-producing taxa[7]. This circuit maintains metabolic homeostasis but presents therapeutic opportunities through FXR modulators (e.g., glycine-beta-muricholic acid)[125], TGR5 agonists, and probiotic interven
Emerging evidence has established the GM as a pivotal regulator of systemic fatty acid metabolism through the dynamic modulation of energy harvesting and oxidative pathways. SFAs, recognized drivers of adipogenesis and hepatic steatosis, exert their metabolic effects in part by reshaping the GM composition to favor lipogenic pathways. The metabolic impact of the GM is further evidenced by GF mouse models, which exhibit resistance to diet-induced obesity through synergistic mechanisms, including increased hepatic β-oxidation and suppressed lipid storage via reduced LPL activity[127]. This protective phenotype arises from the ability of the GM to suppress adenosine monophosphate-activated protein kinase (AMPK) signaling, a key regulator of mitochondrial biogenesis and fatty acid catabolism, in skeletal muscle and liver. Microbial suppression of AMPK phosphorylation inhibits fatty acid transport into mitochondria and promotes ectopic lipid accumulation in adipose and hepatic tissues[128]. Concurrently, GM-mediated downregulation of FIAF, an endogenous LPL inhibitor, enhances adipocyte LPL activity, driving TG hydrolysis and white adipose tissue expansion[127]. Compared with lean donor recipients, GF mice receiving microbiota from obese donors develop significantly greater adiposity, accompanied by significant reductions in skeletal muscle AMPK activity and hepatic β-oxidation capacity[71,107]. These findings collectively demonstrate the ability of the GM to reprogram host energy partitioning, optimizing caloric harvest through the suppression of fatty acid oxidation and the potentiation of lipid anabolism. The intricate interplay between microbial ecology and fatty acid metabolism underscores the role of the GM as a metabolic rheostat, balancing energy expenditure and storage through molecular crosstalk involving AMPK-FIAF-LPL axis modulation. Therapeutic targeting of these pathways may offer novel strategies for MetS management.
NcRNAs have emerged as pivotal regulators of metabolic homeostasis, orchestrating complex host-microbiota interac
As about 22 nucleotide post-transcriptional silencers, miRNAs occupy a central position in host-microbe communications[135]. Intestinal epithelial cells secrete miRNAs into the lumen, where they are internalized by microbial cells via endocytosis, selectively modulating the bacterial gene expression through targeted mRNA degradation[136,137]. Conversely, GM-derived EVs deliver microbial miRNAs and metabolites that reprogram host barrier integrity and cholesterol metabolism[136,138]. For example, fecal miR-10b, miR-26, miR-27a/b, miR-30c, miR-33a/b, miR-106b, and miR-144 target genes govern RCT[132,139], whereas miR-122 downregulation is correlated with reduced plasma cholesterol and TG levels[140]. Microbial metabolites further refine this axis: Indole-3-propionic acid attenuate atherosclerosis by upregulating ATP-binding cassette transporter A1 expression through miR-142-5p inhibition, thereby enhancing macrophage RCT[141]; whereas protocatechuic acid inhibits miR-10b to increase ATP-binding cassette transporter A1/G1 expression[142]. In obesity, GM-driven ethanolamine metabolism defects increase miR-101a-3p expression via ARID3a activation, destabilizing ZO-1 mRNA and impairing intestinal barrier function[143], a phenotype reversible by Lactobacillus rhamnosus HL-200 through ARID3a/miR-101a/ZO-1 axis normalization[143]. Probiotic interventions, such as Lactobacillus fermentum/salivarius, further demonstrate therapeutic potential by upregulating barrier-protective miRNAs (miR-143/miR-150/miR-155/223)[144].
LncRNAs (> 200 nt) extend this regulatory paradigm through chromatin remodeling, transcriptional interference, and miRNA sponging[145,146]. GM metabolites dynamically modulate lncRNA networks. For instance, deoxycholic acid upregulates macrophage lncRNA 57RIK via sphingosine-1-phosphate receptor 2, potentiating caspase-4/11-dependent pyroptosis upon LPS stimulation[147]. Conversely, Roseburia intestinalis flagellin induces intestinal lncRNA HIF1A-AS2, suppressing nuclear factor-kappa B/c-Jun N-terminal kinase signaling and reducing colonic inflammation[148]. Microbial suppression of intestinal lncRNA Snhg9 reprograms lipid metabolism via PPARγ activation, altering systemic lipid absorption and storage in mice[149-151]. Such findings position lncRNAs as versatile mediators of microbial influence on host metabolism. CircRNAs further diversify this network through covalently closed loop structures that sequester miRNAs or scaffold metabolic enzymes[152,153]. Notably, GM-derived EVs function as essential interkingdom signaling mediators by delivering functional nucleic acids to host cells, thereby post-transcriptionally modulating gene expression in recipient cells. This vesicle-mediated genetic regulation mechanism establish a novel paradigm whereby commensal microorganisms directly orchestrate host physiological homeostasis at the molecular level[154,155].
The ncRNA-microbiota axis presents novel therapeutic opportunities. MiRNA-based strategies, such as mimics or anti-miRNA oligonucleotides, could correct dysregulated pathways in metabolic diseases, while lncRNA/circRNA modulation offers novel avenues for precision intervention. Moreover, fecal ncRNA profiling show promise as non-invasive biomarkers for dysbiosis-associated metabolic disorders[134]. However, interspecies differences in ncRNA conservation and tissue-specific expression patterns complicate this therapeutic translation. Future research must delineate spatiotemporal dynamics of microbial RNA transfer mechanisms and develop organoid-based models to accelerate clinical translation of ncRNA-targeted metabolic therapies.
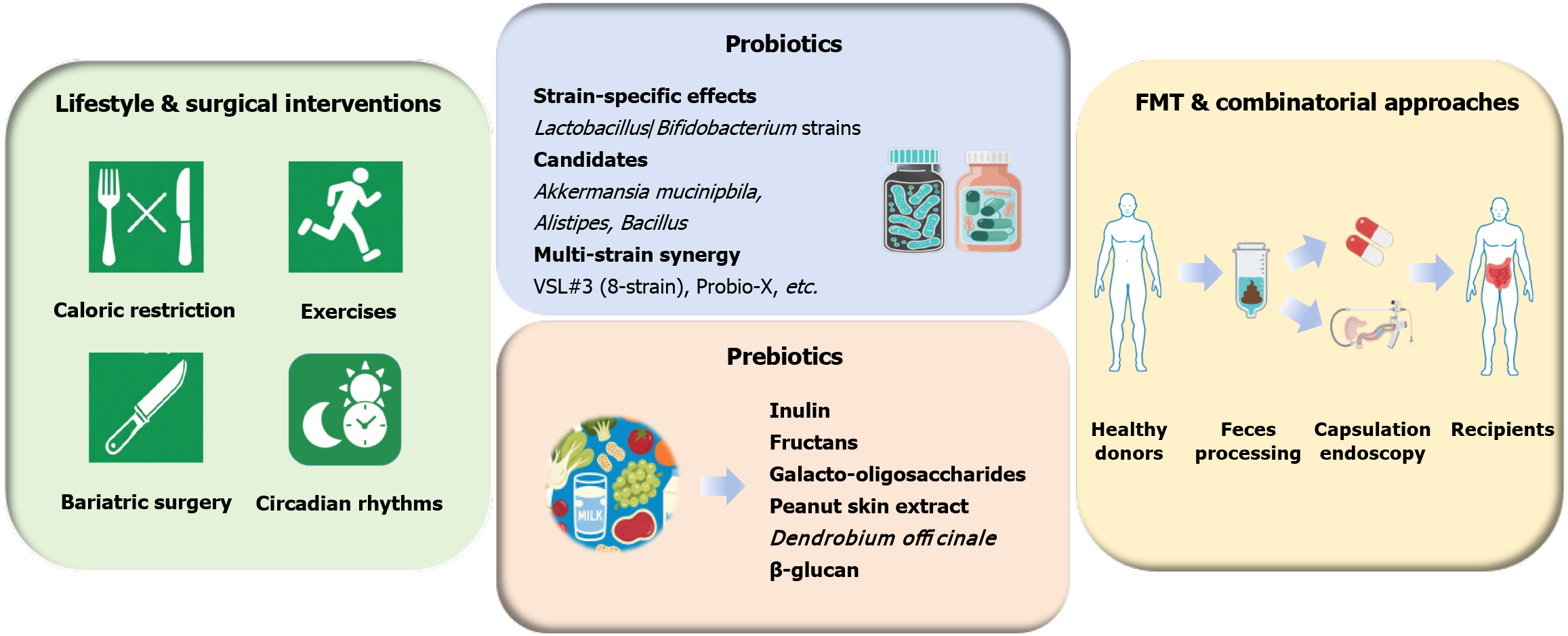
The GM has emerged as both a pathogenic mediator and therapeutic target in dyslipidemia management, with conventional interventions exerting profound yet bidirectional effects on microbial ecology (Figure 3). Lifestyle modifications, including caloric restriction and aerobic exercise, induce consistent shifts towards Bacteroides-Prevotella-dominant enterotypes while suppressing sulfate-reducing Enterobacteriaceae and Clostridium histolyticum/lituseburense populations, which are strongly correlated with restored microbial gene richness and improved lipid profiles[25]. In refractory obesity, bariatric surgery, such as Roux-en-Y gastric by-pass, drives dramatic microbial restructuring, expanding the anti-inflammatory Faecalibacterium prausnitzii and carbohydrate-fermenting Gammaproteobacteria while suppressing energy-harvesting methanogens[156]. These changes increase satiety hormone secretion (PYY, GLP-1) through microbial γ-aminobutyric acid/SCFA-mediated enteroendocrine activation[157]. Notably, the therapeutic potential of GM modu
Probiotics, defined by the Food and Agricultural Organization/World Health Organization as live microorganisms conferring health benefits when administered in adequate amounts, exert targeted effects on the gut microbial ecology and host metabolism through strain-specific mechanisms[160-163]. Clinical and preclinical studies have validated their therapeutic potential in dyslipidemia, with Lactobacillus and Bifidobacterium species demonstrating multifaceted benefits: Restoring intestinal barrier integrity, reducing adiposity, and attenuating systemic inflammation[164]. Meta-analyses further confirmed its lipid-modulating efficacy, showing that probiotic supplementation significantly lowered TC, LDL-C, and TG levels, particularly in younger individuals with elevated baseline BMIs[165].
Therapeutic outcomes potentially depend on strain specificity. Among the Lactobacillus strains, Lactobacillus rhamnosus CGMCC1.3724 induces sex-dependent weight loss in obese females[166], whereas GG improves dyslipidemia via GM restoration in murine models[167]. Cholesterol-lowering effects are pronounced in Lactobacillus plantarum strains: CECT7527-CECT7529 reduce TC and LDL-C levels in hypercholesterolemic humans[168], and CAI6/SC4 decreases serum TC levels in dyslipidemic mice[169], whereas their combination with Lactobacillus curvatus further reduces both serum and hepatic lipid accumulation in HFD-fed mice through conjugated linoleic acid production, leptin/fatty acid synthetase regulation, enhanced fatty acid oxidation, and angiopoietin like-4-mediated LPL inhibition[169,170]. Akkermansia muciniphila, which constitutes 1%-4% of the healthy colonic microbiota, represents a paradigm-shifting therapeutic candidate[171,172]. Its oral administration enhances intestinal barrier integrity, improves insulin sensitivity, and reduces visceral adiposity in both rodent models and human trials[55,173,174], with Akkermansia muciniphila-derived EVs recapitulating these benefits by reversing diet-induced cholesterol dysregulation and adipose inflammation[175,176]. Novel candidates, such as Alistipes (SCFA producers)[177] and Bacillus (cholesterol lowering via microbial richness modulation)[178], further expand the probiotic therapeutic options.
Polybiotic formulations demonstrate enhanced efficacy through synergistic interactions[165]. The 8-strain VSL#3 consortium (Streptococcus thermophilus, multiple Bifidobacterium and Lactobacillus strains) improved BMI and adiposity metrics[179], whereas Probio-X, enriched which Bifidobacterium animalis and Lactobacillus plantarum, effectively improved the serum lipid profile[180]. Similarly, Lactobacillus curvatus HY7601/Lactobacillus plantarum KY1032 combinations specifically lower serum TG in hypertriglyceridemic subjects[181], and Lactobacillus plantarum 9–41-A plus Lactobacillus fermentum M1-16 preferentially reduces TC levels in hypercholesterolemic models[182]. Compared with single-strain interventions, these formulations enhance the production of beneficial metabolites, such as SCFAs, and promote GLP-1 secretion, amplifying lipid-lowering effects[179,180].
Despite promising results, therapeutic variability persists due to confounding factors, including host genetics, baseline microbiota composition, and treatment duration. While probiotics modulate lipid metabolism, current evidence precludes their use as monotherapy for metabolic disorders, and only considers them as adjunctive therapies, leveraging GM modulation to support metabolic health without displacing pharmacological interventions[66,165]. Key challenges require solution include standardization of strain-specific dosing regimens, mechanistic dissection of host-microbe molecular crosstalk, and development of phenotype-guided formulations using metagenomics-metabolomics integration. Addressing these barriers will enable transition from empirical supplementation to precision probiotics targeting dyslipidemia pathophysiology through defined microbial effector networks.
Prebiotics, defined as non-digestible substrates selectively utilized by host microorganisms to confer health benefits, reshape gut microbial ecology through targeted nutritional supplementation. This class of compounds, including inulin, fructans, and galacto-oligosaccharides, serves as fermentable substrates for commensal bacteria, with inulin/fructans, naturally abundant in wheat, fruits, and vegetables, promoting beneficial taxa enrichment, and galacto-oligosaccharides exhibiting potent bifidogenic and butyrogenic properties[183]. In addition to taxonomic modulation, prebiotics amplify microbial metabolite production, strengthen intestinal barrier integrity, and regulate gut motility, positioning them as multifaceted tools for metabolic intervention[183]. Specifically, the therapeutic efficacy of prebiotics stems from their capacity to reconfigure microbial networks, reducing Firmicutes abundance while enriching Bacteroides, Bifidobacteria, Bifidobacterium, Lactobacillus, Prevotella, and Roseburia, a profile associated with improved insulin sensitivity and lipid metabolism[183-185]. At the neuroendocrine level, prebiotics stimulate anorexigenic hormone secretion (PYY and GLP-1) while suppressing ghrelin, collectively strengthening intestinal barrier integrity, regulating energy balance, reducing visceral adiposity, and attenuating chronic low-grade inflammation[184,185].
Emerging plant-derived prebiotics demonstrate targeted lipid modulation. Peanut skin extract exerts dose-dependent anti-dyslipidemic and anti-inflammatory effects in atherosclerotic mouse models, by enriching beneficial Roseburia, Rothia, Parabacteroides, and Akkermansia, and suppressing pro-inflammatory Bilophila and Alistipes[186]. Similarly, Dendrobium officinale elevate Akkermansia and Bifidobacterium, increase acetate/taurine production, lowering LDL-C and VLDL-C levels in HFD-fed mice, effects partially transmissible via FMT[187]. Moreover, barley-derived β-glucan demonstrates lipid-lowering efficacy correlating with increased Faecalibacterium, Bifidobacterium, Ruminococcaceae, and Lachnospira abundances[188]. Clinical meta-analyses confirm prebiotic/synbiotic interventions reduce TC and LDL-C in overweight dyslipidemic individuals, though TG modulation remains inconsistent[189,190]. Notably, the purified polysaccharide from alkali extraction and molecular sieve purification (PNS2A) from Phyllostachys nigra roots demon
Current limitations include non-specific microbial modulation and interindividual variability in baseline microbiota and metabolic status[113]. Future formulations may combine prebiotics with targeted probiotics (e.g., Akkermansia muciniphila) to amplify strain-specific effects. While prebiotics demonstrate robust lipid-modulating capacity, clinical translation require resolving several critical challenges, including dose optimization across ethnicities, biomarker-driven algorithms matching prebiotic types to microbial signatures, and synergistic design integrating prebiotics with probiotics/pharmacotherapies. Advancements in multi-omics-guided intervention will enable transition from empirical to precision microbial therapeutics for metabolic disorders.
FMT has revolutionized the treatment of recurrent or refractory Clostridioides difficile infection, achieving > 90% efficacy regardless of the administration modality (colonoscopic, nasogastric, or oral capsules), by reconstituting donor-like microbial communities with ecological stability[192-195]. Encapsulated formulations such as SER-109, a purified preparation of Firmicutes spores, have demonstrated non-inferior efficacy compared with traditional invasive methods while increasing patient compliance[196,197]. Since SER-109 exemplifies the promise of microbiome therapeutics, SER-109 might regulate host metabolism by regulating the GM composition; however, its metabolic applications require rigorous validation, and future studies should prioritize this topic[193]. In addition to refractory Clostridioides difficile infection, FMT shows exploratory promise in inflammatory bowel disease and metabolic disorders, although broader application necessitates standardized donor screening protocols, optimized preparation methods, and rigorous long-term safety monitoring for antibiotic resistance gene transfer risks[198].
Preclinical models illuminate the capacity of FMT to transfer metabolic phenotypes[1]: GF mice receiving conventional microbiota exhibit 60% increased adiposity and elevated hepatic de novo lipogenesis despite reduced food intake[71]. However, human trials have revealed only transient effects; lean donor FMT transiently increases butyrate-producing taxa in obese MetS recipients, but microbial profiles and metabolic parameters revert to baseline within 18 weeks[199]. Notably, multiple clinical trials reported no significant effects on body weight, resting energy expenditure, or enteroendocrine hormone levels, despite the observed increases in beneficial taxa such as Bifidobacterium pseudolongum and acetate production[199-201]. This dichotomy underscores the resilience of recipient microbial ecosystems and the need for adjunctive strategies. Combining FMT with lifestyle interventions (e.g., dietary modifications and physical activity) improves microbial engraftment, increasing beneficial taxa (e.g., Lactobacillus and Bifidobacterium) and enhancing lipid metabolism (e.g., lower TC and LDL-C)[202,203]. These benefits correlate with PPARα activation (α-hydroxy-3-methylvalerate), which reduces inflammation in both intermuscular and subcutaneous adipose tissue[202,203]. For example, GM modulation through dietary intervention and FMT significantly improved metabolic parameters, including blood glucose levels, lipid profiles, and blood pressure, in type 2 diabetic patients, with FMT inducing faster microbial shifts than diet alone. The abundance of beneficial bacteria (e.g., Bifidobacterium) increased and correlated negatively with glycemic/Lipid indices, whereas the abundance of sulfate-reducing bacteria (Bilophila, Desulfovibrio) decreased and was positively associated with hyperglycemia, suggesting that GM restructuring is a key mediator of metabolic improvement. The combined approach (diet plus FMT) accelerated weight loss and glycemic control, with a notable transition from Bacteroides- to Prevotella-dominant microbiota, suggesting a promising therapeutic strategy for type 2 diabetic and comorbid metabolic disorders[204]. These findings position FMT as both a therapeutic modality and a discovery platform for host-microbe metabolic crosstalk.
While current clinical trials have not consistently demonstrated the efficacy of FMT in individuals with obesity or MetS, baseline GM diversity may predict treatment responsiveness[199]. Successful microbial engraftment requires the resolution of three critical factors: (1) Engraftment variability: For example, conspecific strains exhibit superior coloniza
Decades of multidisciplinary research have unequivocally established the GM as a potent regulator of lipid metabolism, operating at the nexus of genetic predisposition, dietary patterns, and environmental exposures[1]. While multi-omics studies delineate characteristic dysbiotic signatures in dyslipidemia, critical knowledge gaps persist in distinguishing causal microbial taxa from epiphenomenal bystanders[25,39]. Current GM-targeted interventions, although mechanistically promising, face translational roadblocks: Probiotics/prebiotics transiently enrich beneficial taxa such as Bifidobacterium and Lactobacillus but fail to durably remodel microbial networks, whereas FMT results in variable engraftment and a lack of metabolic endpoint consistency[208,209].
Three paradigm-shifting opportunities have emerged: (1) Precision microbial consortia: Engineered assemblages combining several beneficial taxa to target atherogenic pathways; (2) Chronotherapeutic delivery: Time-restricted administration synchronized to host circadian metabolite oscillations; and (3) Artificial intelligence-driven personalization: Machine learning integration of metagenomic, lipidomic, and clinical data to predict individualized microbial therapy responses. Notably, personalized GM modulation must account for host genetic polymorphisms, baseline microbial ecology, and geographical variations while addressing ethical challenges in donor screening, long-term safety monitoring, and equitable access to microbiota-based therapies[210]. Realizing these potential demands requires coordinated efforts across microbiology, bioengineering, and computational biology to overcome ecological complexity and host-microbe reciprocity. Next-generation microbial therapeutics may ultimately bridge the gap between mechanistic promise and clinical reality in dyslipidemia management.
| 1. | Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129:4050-4057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 450] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 2. | Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017;66:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 388] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 3. | Guo L, Wang YY, Wang JH, Zhao HP, Yu Y, Wang GD, Dai K, Yan YZ, Yang YJ, Lv J. Associations of gut microbiota with dyslipidemia based on sex differences in subjects from Northwestern China. World J Gastroenterol. 2022;28:3455-3475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 4. | Stojanov S, Berlec A, Štrukelj B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms. 2020;8:1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 1070] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 5. | De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1618] [Article Influence: 147.1] [Reference Citation Analysis (0)] |
| 6. | Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2783] [Cited by in RCA: 3165] [Article Influence: 263.8] [Reference Citation Analysis (0)] |
| 7. | Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 789] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 8. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1684] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 9. | Bishehsari F, Voigt RM, Keshavarzian A. Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat Rev Endocrinol. 2020;16:731-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 10. | Cui Y, Li S, Yin Y, Li X, Li X. Daytime restricted feeding promotes circadian desynchrony and metabolic disruption with changes in bile acids profiles and gut microbiota in C57BL/6 Male Mice. J Nutr Biochem. 2022;109:109121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 11. | Altaha B, Heddes M, Pilorz V, Niu Y, Gorbunova E, Gigl M, Kleigrewe K, Oster H, Haller D, Kiessling S. Genetic and environmental circadian disruption induce weight gain through changes in the gut microbiome. Mol Metab. 2022;66:101628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 12. | Qiu Y, Wu L, Zhou W, Wang F, Li N, Wang H, He R, Tian Y, Liu Z. Day and Night Reversed Feeding Aggravates High-Fat Diet-Induced Abnormalities in Intestinal Flora and Lipid Metabolism in Adipose Tissue of Mice. J Nutr. 2024;154:2772-2783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Le Roy T, Lécuyer E, Chassaing B, Rhimi M, Lhomme M, Boudebbouze S, Ichou F, Haro Barceló J, Huby T, Guerin M, Giral P, Maguin E, Kapel N, Gérard P, Clément K, Lesnik P. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019;17:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 14. | Flaig B, Garza R, Singh B, Hamamah S, Covasa M. Treatment of Dyslipidemia through Targeted Therapy of Gut Microbiota. Nutrients. 2023;15:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 15. | Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, Gonzalez A, Kosciolek T, McCall LI, McDonald D, Melnik AV, Morton JT, Navas J, Quinn RA, Sanders JG, Swafford AD, Thompson LR, Tripathi A, Xu ZZ, Zaneveld JR, Zhu Q, Caporaso JG, Dorrestein PC. Best practices for analysing microbiomes. Nat Rev Microbiol. 2018;16:410-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 1011] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 16. | Fromentin S, Forslund SK, Chechi K, Aron-Wisnewsky J, Chakaroun R, Nielsen T, Tremaroli V, Ji B, Prifti E, Myridakis A, Chilloux J, Andrikopoulos P, Fan Y, Olanipekun MT, Alves R, Adiouch S, Bar N, Talmor-Barkan Y, Belda E, Caesar R, Coelho LP, Falony G, Fellahi S, Galan P, Galleron N, Helft G, Hoyles L, Isnard R, Le Chatelier E, Julienne H, Olsson L, Pedersen HK, Pons N, Quinquis B, Rouault C, Roume H, Salem JE, Schmidt TSB, Vieira-Silva S, Li P, Zimmermann-Kogadeeva M, Lewinter C, Søndertoft NB, Hansen TH, Gauguier D, Gøtze JP, Køber L, Kornowski R, Vestergaard H, Hansen T, Zucker JD, Hercberg S, Letunic I, Bäckhed F, Oppert JM, Nielsen J, Raes J, Bork P, Stumvoll M, Segal E, Clément K, Dumas ME, Ehrlich SD, Pedersen O. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat Med. 2022;28:303-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 148] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 17. | Wang B, Yao M, Lv L, Ling Z, Li L. The Human Microbiota in Health and Disease. Engineering. 2017;3:71-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 499] [Article Influence: 62.4] [Reference Citation Analysis (1)] |
| 18. | Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 965] [Cited by in RCA: 1171] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 19. | Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4098] [Cited by in RCA: 4544] [Article Influence: 324.6] [Reference Citation Analysis (1)] |
| 20. | David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5625] [Cited by in RCA: 6835] [Article Influence: 569.6] [Reference Citation Analysis (0)] |
| 21. | Wang DD, Nguyen LH, Li Y, Yan Y, Ma W, Rinott E, Ivey KL, Shai I, Willett WC, Hu FB, Rimm EB, Stampfer MJ, Chan AT, Huttenhower C. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat Med. 2021;27:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 237] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 22. | Miyamoto J, Igarashi M, Watanabe K, Karaki SI, Mukouyama H, Kishino S, Li X, Ichimura A, Irie J, Sugimoto Y, Mizutani T, Sugawara T, Miki T, Ogawa J, Drucker DJ, Arita M, Itoh H, Kimura I. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat Commun. 2019;10:4007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 277] [Article Influence: 46.2] [Reference Citation Analysis (2)] |
| 23. | Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med. 2021;218:e20201606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 580] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 24. | Lan Y, Sun Q, Ma Z, Peng J, Zhang M, Wang C, Zhang X, Yan X, Chang L, Hou X, Qiao R, Mulati A, Zhou Y, Zhang Q, Liu Z, Liu X. Seabuckthorn polysaccharide ameliorates high-fat diet-induced obesity by gut microbiota-SCFAs-liver axis. Food Funct. 2022;13:2925-2937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 25. | Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol. 2014;20:16079-16094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 342] [Cited by in RCA: 363] [Article Influence: 33.0] [Reference Citation Analysis (6)] |
| 26. | Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, Storlien LH. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 469] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 27. | Vijay A, Astbury S, Le Roy C, Spector TD, Valdes AM. The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial. Gut Microbes. 2021;13:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 28. | Gao J, Ma L, Yin J, Liu G, Ma J, Xia S, Gong S, Han Q, Li T, Chen Y, Yin Y. Camellia (Camellia oleifera bel.) seed oil reprograms gut microbiota and alleviates lipid accumulation in high fat-fed mice through the mTOR pathway. Food Funct. 2022;13:4977-4992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Sanches-Silva A, Testai L, Nabavi SF, Battino M, Pandima Devi K, Tejada S, Sureda A, Xu S, Yousefi B, Majidinia M, Russo GL, Efferth T, Nabavi SM, Farzaei MH. Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway. Pharmacol Res. 2020;152:104626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 30. | Shen F, Zhuang J, Wang Q, Zhang J, Huang Y, Mo Q, Zhao M, Wang J, Zhong H, Feng F. Enhancement in the metabolic profile of sea buckthorn juice via fermentation for its better efficacy on attenuating diet-induced metabolic syndrome by targeting gut microbiota. Food Res Int. 2022;162:111948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 31. | American Diabetes Association. 4. Lifestyle Management: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S38-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 422] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 32. | Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Fitó M, Gea A, Hernán MA, Martínez-González MA; PREDIMED Study Investigators. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018;378:e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2350] [Cited by in RCA: 2063] [Article Influence: 294.7] [Reference Citation Analysis (1)] |
| 33. | Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, Giampieri E, Jennings A, Candela M, Turroni S, Zoetendal EG, Hermes GDA, Elodie C, Meunier N, Brugere CM, Pujos-Guillot E, Berendsen AM, De Groot LCPGM, Feskins EJM, Kaluza J, Pietruszka B, Bielak MJ, Comte B, Maijo-Ferre M, Nicoletti C, De Vos WM, Fairweather-Tait S, Cassidy A, Brigidi P, Franceschi C, O'Toole PW. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69:1218-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 568] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 34. | Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, Giacco R, Mennella I, Ferracane R, Pons N, Pasolli E, Rivellese A, Dragsted LO, Vitaglione P, Ehrlich SD, Ercolini D. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69:1258-1268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 352] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 35. | Hjorth MF, Blædel T, Bendtsen LQ, Lorenzen JK, Holm JB, Kiilerich P, Roager HM, Kristiansen K, Larsen LH, Astrup A. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int J Obes (Lond). 2019;43:149-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 36. | Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, Kahleova H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front Nutr. 2019;6:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 402] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 37. | McBurney MI, Davis C, Fraser CM, Schneeman BO, Huttenhower C, Verbeke K, Walter J, Latulippe ME. Establishing What Constitutes a Healthy Human Gut Microbiome: State of the Science, Regulatory Considerations, and Future Directions. J Nutr. 2019;149:1882-1895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 38. | Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1329] [Cited by in RCA: 1454] [Article Influence: 121.2] [Reference Citation Analysis (0)] |
| 39. | Zwartjes MSZ, Gerdes VEA, Nieuwdorp M. The Role of Gut Microbiota and Its Produced Metabolites in Obesity, Dyslipidemia, Adipocyte Dysfunction, and Its Interventions. Metabolites. 2021;11:531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Cândido FG, Valente FX, Grześkowiak ŁM, Moreira APB, Rocha DMUP, Alfenas RCG. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: mechanisms and clinical implications on obesity. Int J Food Sci Nutr. 2018;69:125-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 41. | Napolitano M, Covasa M. Microbiota Transplant in the Treatment of Obesity and Diabetes: Current and Future Perspectives. Front Microbiol. 2020;11:590370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 42. | Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 1316] [Article Influence: 263.2] [Reference Citation Analysis (0)] |
| 43. | Cheng Z, Zhang L, Yang L, Chu H. The critical role of gut microbiota in obesity. Front Endocrinol (Lausanne). 2022;13:1025706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 44. | Hassan NE, El Shebini SM, El-Masry SA, Ahmed NH, Kamal AN, Ismail AS, Alian KM, Mostafa MI, Selim M, Afify MAS. Brief overview of dietary intake, some types of gut microbiota, metabolic markers and research opportunities in sample of Egyptian women. Sci Rep. 2022;12:17291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 45. | Zeng Q, Li D, He Y, Li Y, Yang Z, Zhao X, Liu Y, Wang Y, Sun J, Feng X, Wang F, Chen J, Zheng Y, Yang Y, Sun X, Xu X, Wang D, Kenney T, Jiang Y, Gu H, Li Y, Zhou K, Li S, Dai W. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci Rep. 2019;9:13424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 269] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 46. | Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 563] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 47. | Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T; CHILD Study Investigators, Mohn WW, Turvey SE, Finlay BB. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 1211] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 48. | Shelton CD, Sing E, Mo J, Shealy NG, Yoo W, Thomas J, Fitz GN, Castro PR, Hickman TT, Torres TP, Foegeding NJ, Zieba JK, Calcutt MW, Codreanu SG, Sherrod SD, McLean JA, Peck SH, Yang F, Markham NO, Liu M, Byndloss MX. An early-life microbiota metabolite protects against obesity by regulating intestinal lipid metabolism. Cell Host Microbe. 2023;31:1604-1619.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 49. | Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science. 2016;352:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 278] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 50. | Tong X, Xu J, Lian F, Yu X, Zhao Y, Xu L, Zhang M, Zhao X, Shen J, Wu S, Pang X, Tian J, Zhang C, Zhou Q, Wang L, Pang B, Chen F, Peng Z, Wang J, Zhen Z, Fang C, Li M, Chen L, Zhao L. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: a Multicenter, Randomized, Open Label Clinical Trial. mBio. 2018;9:e02392-e02317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 51. | Vich Vila A, Collij V, Sanna S, Sinha T, Imhann F, Bourgonje AR, Mujagic Z, Jonkers DMAE, Masclee AAM, Fu J, Kurilshikov A, Wijmenga C, Zhernakova A, Weersma RK. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020;11:362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 477] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 52. | Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X, Zhang X, Zhao L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 475] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 53. | Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 1137] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 54. | Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, Chakaroun R, Forslund SK, Assmann K, Valles-Colomer M, Nguyen TTD, Proost S, Prifti E, Tremaroli V, Pons N, Le Chatelier E, Andreelli F, Bastard JP, Coelho LP, Galleron N, Hansen TH, Hulot JS, Lewinter C, Pedersen HK, Quinquis B, Rouault C, Roume H, Salem JE, Søndertoft NB, Touch S; MetaCardis Consortium, Dumas ME, Ehrlich SD, Galan P, Gøtze JP, Hansen T, Holst JJ, Køber L, Letunic I, Nielsen J, Oppert JM, Stumvoll M, Vestergaard H, Zucker JD, Bork P, Pedersen O, Bäckhed F, Clément K, Raes J. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 55. | Khan TJ, Ahmed YM, Zamzami MA, Siddiqui AM, Khan I, Baothman OAS, Mehanna MG, Kuerban A, Kaleemuddin M, Yasir M. Atorvastatin Treatment Modulates the Gut Microbiota of the Hypercholesterolemic Patients. OMICS. 2018;22:154-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 56. | Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 686] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 57. | Caparrós-Martín JA, Lareu RR, Ramsay JP, Peplies J, Reen FJ, Headlam HA, Ward NC, Croft KD, Newsholme P, Hughes JD, O'Gara F. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome. 2017;5:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 58. | Fowler S, Hoedt EC, Talley NJ, Keely S, Burns GL. Circadian Rhythms and Melatonin Metabolism in Patients With Disorders of Gut-Brain Interactions. Front Neurosci. 2022;16:825246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 59. | Zhen Y, Wang Y, He F, Chen Y, Hu L, Ge L, Wang Y, Wei W, Rahmat A, Loor JJ, Wang M. Homeostatic crosstalk among gut microbiome, hypothalamic and hepatic circadian clock oscillations, immunity and metabolism in response to different light-dark cycles: A multiomics study. J Pineal Res. 2023;75:e12892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 60. | Yang DF, Huang WC, Wu CW, Huang CY, Yang YSH, Tung YT. Acute sleep deprivation exacerbates systemic inflammation and psychiatry disorders through gut microbiota dysbiosis and disruption of circadian rhythms. Microbiol Res. 2023;268:127292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 61. | Song D, Yang CS, Zhang X, Wang Y. The relationship between host circadian rhythms and intestinal microbiota: A new cue to improve health by tea polyphenols. Crit Rev Food Sci Nutr. 2021;61:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 62. | Wang Y, Ai Z, Xing X, Fan Y, Zhang Y, Nan B, Li X, Wang Y, Liu J. The ameliorative effect of probiotics on diet-induced lipid metabolism disorders: A review. Crit Rev Food Sci Nutr. 2024;64:3556-3572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 63. | Badmus OO, Hillhouse SA, Anderson CD, Hinds TD, Stec DE. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways. Clin Sci (Lond). 2022;136:1347-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 177] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 64. | Ngo J, Choi DW, Stanley IA, Stiles L, Molina AJA, Chen PH, Lako A, Sung ICH, Goswami R, Kim MY, Miller N, Baghdasarian S, Kim-Vasquez D, Jones AE, Roach B, Gutierrez V, Erion K, Divakaruni AS, Liesa M, Danial NN, Shirihai OS. Mitochondrial morphology controls fatty acid utilization by changing CPT1 sensitivity to malonyl-CoA. EMBO J. 2023;42:e111901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 65. | Kopecka J, Trouillas P, Gašparović AČ, Gazzano E, Assaraf YG, Riganti C. Phospholipids and cholesterol: Inducers of cancer multidrug resistance and therapeutic targets. Drug Resist Updat. 2020;49:100670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 66. | You M, Zhou L, Wu F, Zhang L, Zhu SX, Zhang HX. Probiotics for the treatment of hyperlipidemia: Focus on gut-liver axis and lipid metabolism. Pharmacol Res. 2025;214:107694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 67. | Jia X, Xu W, Zhang L, Li X, Wang R, Wu S. Impact of Gut Microbiota and Microbiota-Related Metabolites on Hyperlipidemia. Front Cell Infect Microbiol. 2021;11:634780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 68. | Díaz-Garrido N, Badia J, Baldomà L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles. 2021;10:e12161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 69. | Bittel M, Reichert P, Sarfati I, Dressel A, Leikam S, Uderhardt S, Stolzer I, Phu TA, Ng M, Vu NK, Tenzer S, Distler U, Wirtz S, Rothhammer V, Neurath MF, Raffai RL, Günther C, Momma S. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe-host-communication in vivo. J Extracell Vesicles. 2021;10:e12159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 70. | Moszak M, Szulińska M, Bogdański P. You Are What You Eat—The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients. 2020;12:1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 71. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718-15723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4530] [Cited by in RCA: 4417] [Article Influence: 210.3] [Reference Citation Analysis (4)] |
| 72. | Zhang C, Zhang M, Pang X, Zhao Y, Wang L, Zhao L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012;6:1848-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 383] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 73. | Xiao HB, Sun ZL, Zhang HB, Zhang DS. Berberine inhibits dyslipidemia in C57BL/6 mice with lipopolysaccharide induced inflammation. Pharmacol Rep. 2012;64:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Lassenius MI, Pietiläinen KH, Kaartinen K, Pussinen PJ, Syrjänen J, Forsblom C, Pörsti I, Rissanen A, Kaprio J, Mustonen J, Groop PH, Lehto M; FinnDiane Study Group. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34:1809-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 293] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 75. | Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, Salvador J, Ricart W, Frühbeck G, Burcelin R, Fernández-Real JM. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond). 2012;36:1442-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 76. | Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 542] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 77. | Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1162] [Cited by in RCA: 1493] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 78. | Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 647] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 79. | McBurney MI, Thompson LU, Cuff DJ, Jenkins DJ. Comparison of ileal effluents, dietary fibers, and whole foods in predicting the physiological importance of colonic fermentation. Am J Gastroenterol. 1988;83:536-540. [PubMed] |
| 80. | Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1559] [Cited by in RCA: 1679] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 81. | Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 239] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 82. | Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1896] [Cited by in RCA: 2193] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 83. | Bloemen JG, Venema K, van de Poll MC, Olde Damink SW, Buurman WA, Dejong CH. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr. 2009;28:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 84. | Bloemen JG, Olde Damink SW, Venema K, Buurman WA, Jalan R, Dejong CH. Short chain fatty acids exchange: Is the cirrhotic, dysfunctional liver still able to clear them? Clin Nutr. 2010;29:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 86. | Feng W, Ao H, Peng C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front Pharmacol. 2018;9:1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 248] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 87. | Ma J, Li H. The Role of Gut Microbiota in Atherosclerosis and Hypertension. Front Pharmacol. 2018;9:1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 88. | Overby HB, Ferguson JF. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota:Host Cross talk and Modulate Obesity and Hypertension. Curr Hypertens Rep. 2021;23:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 89. | Barrea L, Muscogiuri G, Annunziata G, Laudisio D, Pugliese G, Salzano C, Colao A, Savastano S. From gut microbiota dysfunction to obesity: could short-chain fatty acids stop this dangerous course? Hormones (Athens). 2019;18:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 90. | Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J, van Tol R, Vaughan EE, Verbeke K. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11:411-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 91. | van der Hee B, Wells JM. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021;29:700-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 564] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 92. | Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt-Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S, Frost G. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744-1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 940] [Cited by in RCA: 956] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 93. | Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, Hanash A, Toubai T, Oravecz-Wilson K, Wu SR, Sun Y, Rossi C, Fujiwara H, Byun J, Shono Y, Lindemans C, Calafiore M, Schmidt TM, Honda K, Young VB, Pennathur S, van den Brink M, Reddy P. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17:505-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 505] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 94. | Drucker DJ. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metab. 2022;57:101351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 228] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 95. | Nogal A, Valdes AM, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 449] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 96. | Lee SH, Hossner KL. Coordinate regulation of ovine adipose tissue gene expression by propionate. J Anim Sci. 2002;80:2840-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Al-Lahham S, Roelofsen H, Rezaee F, Weening D, Hoek A, Vonk R, Venema K. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur J Clin Invest. 2012;42:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 98. | Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 833] [Cited by in RCA: 1084] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 99. | Li G, Yao W, Jiang H. Short-chain fatty acids enhance adipocyte differentiation in the stromal vascular fraction of porcine adipose tissue. J Nutr. 2014;144:1887-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 100. | Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092-5099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 476] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 101. | Zhao Y, Liu J, Hao W, Zhu H, Liang N, He Z, Ma KY, Chen ZY. Structure-Specific Effects of Short-Chain Fatty Acids on Plasma Cholesterol Concentration in Male Syrian Hamsters. J Agric Food Chem. 2017;65:10984-10992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 102. | Nguyen TD, Prykhodko O, Hållenius FF, Nyman M. Monobutyrin Reduces Liver Cholesterol and Improves Intestinal Barrier Function in Rats Fed High-Fat Diets. Nutrients. 2019;11:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 103. | Demigné C, Morand C, Levrat MA, Besson C, Moundras C, Rémésy C. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br J Nutr. 1995;74:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 217] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 104. | Matysik S, Krautbauer S, Liebisch G, Schött HF, Kjølbaek L, Astrup A, Blachier F, Beaumont M, Nieuwdorp M, Hartstra A, Rampelli S, Pagotto U, Iozzo P. Short-chain fatty acids and bile acids in human faeces are associated with the intestinal cholesterol conversion status. Br J Pharmacol. 2021;178:3342-3353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Goffredo M, Mass K, Parks EJ, Wagner DA, McClure EA, Graf J, Savoye M, Pierpont B, Cline G, Santoro N. Role of Gut Microbiota and Short Chain Fatty Acids in Modulating Energy Harvest and Fat Partitioning in Youth. J Clin Endocrinol Metab. 2016;101:4367-4376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 106. | Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 951] [Article Influence: 105.7] [Reference Citation Analysis (1)] |
| 107. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9796] [Cited by in RCA: 8763] [Article Influence: 461.2] [Reference Citation Analysis (1)] |
| 108. | Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond). 2008;32:1720-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 866] [Article Influence: 50.9] [Reference Citation Analysis (1)] |
| 109. | Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 1787] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 110. | Zhu Y, Li Q, Jiang H. Gut microbiota in atherosclerosis: focus on trimethylamine N-oxide. APMIS. 2020;128:353-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 111. | Ding L, Chang M, Guo Y, Zhang L, Xue C, Yanagita T, Zhang T, Wang Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018;17:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 112. | Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015;10:326-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 305] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 113. | Brown JM, Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med. 2015;66:343-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 330] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 114. | Schloissnig S, Arumugam M, Sunagawa S, Mitreva M, Tap J, Zhu A, Waller A, Mende DR, Kultima JR, Martin J, Kota K, Sunyaev SR, Weinstock GM, Bork P. Genomic variation landscape of the human gut microbiome. Nature. 2013;493:45-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 648] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 115. | Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55:1553-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 116. | Lucas LN, Barrett K, Kerby RL, Zhang Q, Cattaneo LE, Stevenson D, Rey FE, Amador-Noguez D. Dominant Bacterial Phyla from the Human Gut Show Widespread Ability To Transform and Conjugate Bile Acids. mSystems. 2021;e0080521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 117. | Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1852] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 118. | Colin S, Briand O, Touche V, Wouters K, Baron M, Pattou F, Hanf R, Tailleux A, Chinetti G, Staels B, Lestavel S. Activation of intestinal peroxisome proliferator-activated receptor-α increases high-density lipoprotein production. Eur Heart J. 2013;34:2566-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Guthrie G, Vonderohe C, Burrin D. Fibroblast growth factor 15/19 expression, regulation, and function: An overview. Mol Cell Endocrinol. 2022;548:111617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 120. | Chiang JY. Recent advances in understanding bile acid homeostasis. F1000Res. 2017;6:2029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 121. | Fiorucci S, Distrutti E, Carino A, Zampella A, Biagioli M. Bile acids and their receptors in metabolic disorders. Prog Lipid Res. 2021;82:101094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 122. | Vítek L, Haluzík M. The role of bile acids in metabolic regulation. J Endocrinol. 2016;228:R85-R96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 123. | Li T, Chiang JYL. Bile acids as metabolic regulators: an update. Curr Opin Gastroenterol. 2023;39:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 124. | Watanabe M, Fukiya S, Yokota A. Comprehensive evaluation of the bactericidal activities of free bile acids in the large intestine of humans and rodents. J Lipid Res. 2017;58:1143-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 125. | Zhang L, Xie C, Nichols RG, Chan SH, Jiang C, Hao R, Smith PB, Cai J, Simons MN, Hatzakis E, Maranas CD, Gonzalez FJ, Patterson AD. Farnesoid X Receptor Signaling Shapes the Gut Microbiota and Controls Hepatic Lipid Metabolism. mSystems. 2016;1:e00070-e00016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 126. | Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T; MetaHIT consortium, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2727] [Cited by in RCA: 3215] [Article Influence: 267.9] [Reference Citation Analysis (2)] |
| 127. | Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2086] [Cited by in RCA: 1877] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 128. | Rodriguez J, Delzenne NM. Modulation of the gut microbiota-adipose tissue-muscle interactions by prebiotics. J Endocrinol. 2021;249:R1-R23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 129. | Slack FJ, Chinnaiyan AM. The Role of Non-coding RNAs in Oncology. Cell. 2019;179:1033-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 1113] [Article Influence: 222.6] [Reference Citation Analysis (0)] |
| 130. | Chiba M, Uehara H, Kuwata H, Niiyama I. Extracellular miRNAs in the serum and feces of mice exposed to highdose radiation. Biomed Rep. 2024;20:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 131. | Wortelboer K, Bakker GJ, Winkelmeijer M, van Riel N, Levin E, Nieuwdorp M, Herrema H, Davids M. Fecal microbiota transplantation as tool to study the interrelation between microbiota composition and miRNA expression. Microbiol Res. 2022;257:126972. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 132. | Ionescu RF, Enache RM, Cretoiu SM, Cretoiu D. The Interplay Between Gut Microbiota and miRNAs in Cardiovascular Diseases. Front Cardiovasc Med. 2022;9:856901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 133. | Fardi F, Khasraghi LB, Shahbakhti N, Salami Naseriyan A, Najafi S, Sanaaee S, Alipourfard I, Zamany M, Karamipour S, Jahani M, Majidpoor J, Kalhor K, Talebi M, Aghaei-Zarch SM. An interplay between non-coding RNAs and gut microbiota in human health. Diabetes Res Clin Pract. 2023;201:110739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 134. | Huang Z, Yao Q, Ma S, Zhou J, Wang X, Meng Q, Liu Y, Yu Z, Chen X. The synergistic role of gut microbiota and RNA in metabolic diseases: mechanisms and therapeutic insights. Front Microbiol. 2025;16:1504395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 135. | Bahari Khasraghi L, Nouri M, Vazirzadeh M, Hashemipour N, Talebi M, Aghaei Zarch F, Majidpoor J, Kalhor K, Farnia P, Najafi S, Aghaei Zarch SM. MicroRNA-206 in human cancer: Mechanistic and clinical perspectives. Cell Signal. 2023;101:110525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 136. | Behrouzi A, Ashrafian F, Mazaheri H, Lari A, Nouri M, Riazi Rad F, Hoseini Tavassol Z, Siadat SD. The importance of interaction between MicroRNAs and gut microbiota in several pathways. Microb Pathog. 2020;144:104200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 137. | Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234:5451-5465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1431] [Cited by in RCA: 1335] [Article Influence: 222.5] [Reference Citation Analysis (0)] |
| 138. | Li M, Chen WD, Wang YD. The roles of the gut microbiota-miRNA interaction in the host pathophysiology. Mol Med. 2020;26:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 139. | Mistry RH, Verkade HJ, Tietge UJ. Reverse Cholesterol Transport Is Increased in Germ-Free Mice-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 140. | Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 1062] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 141. | Xue H, Chen X, Yu C, Deng Y, Zhang Y, Chen S, Chen X, Chen K, Yang Y, Ling W. Gut Microbially Produced Indole-3-Propionic Acid Inhibits Atherosclerosis by Promoting Reverse Cholesterol Transport and Its Deficiency Is Causally Related to Atherosclerotic Cardiovascular Disease. Circ Res. 2022;131:404-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 124] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 142. | Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012;111:967-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 143. | Mishra SP, Wang B, Jain S, Ding J, Rejeski J, Furdui CM, Kitzman DW, Taraphder S, Brechot C, Kumar A, Yadav H. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut. 2023;72:1848-1865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 121] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 144. | Rodríguez-Nogales A, Algieri F, Garrido-Mesa J, Vezza T, Utrilla MP, Chueca N, Garcia F, Olivares M, Rodríguez-Cabezas ME, Gálvez J. Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: impact on microRNAs expression and microbiota composition. Mol Nutr Food Res. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 145. | Najafi S, Tan SC, Raee P, Rahmati Y, Asemani Y, Lee EHC, Hushmandi K, Zarrabi A, Aref AR, Ashrafizadeh M, Kumar AP, Ertas YN, Ghani S, Aghamiri S. Gene regulation by antisense transcription: A focus on neurological and cancer diseases. Biomed Pharmacother. 2022;145:112265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 146. | Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3257] [Cited by in RCA: 3039] [Article Influence: 759.8] [Reference Citation Analysis (0)] |
| 147. | Gao Y, Yue J, Ha F, Wang Y, Wang R, Yang X, Zhang J, Liu X, Zhang Y, Han T, Yang R. Bile acid derivatives from gut microbiota promote GBPs-mediated activation of caspase-4/11 by LPS through lncRNA57RIK. Int J Biol Sci. 2024;20:5831-5849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 148. | Quan Y, Song K, Zhang Y, Zhu C, Shen Z, Wu S, Luo W, Tan B, Yang Z, Wang X. Roseburia intestinalis-derived flagellin is a negative regulator of intestinal inflammation. Biochem Biophys Res Commun. 2018;501:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 149. | Ghafouri-Fard S, Taheri M. The expression profile and role of non-coding RNAs in obesity. Eur J Pharmacol. 2021;892:173809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 150. | Chen J, Chen X, Gao J. The gut-Snhg9 interplay as a new path to metabolic health. Trends Endocrinol Metab. 2024;35:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 151. | Wang Y, Wang M, Chen J, Li Y, Kuang Z, Dende C, Raj P, Quinn G, Hu Z, Srinivasan T, Hassell B, Ruhn KA, Behrendt CL, Liang T, Dou X, Song Z, Hooper LV. The gut microbiota reprograms intestinal lipid metabolism through long noncoding RNA Snhg9. Science. 2023;381:851-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 82] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 152. | Najafi S. The emerging roles and potential applications of circular RNAs in ovarian cancer: a comprehensive review. J Cancer Res Clin Oncol. 2023;149:2211-2234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 153. | Najafi S, Aghaei Zarch SM, Majidpoor J, Pordel S, Aghamiri S, Fatih Rasul M, Asemani Y, Vakili O, Mohammadi V, Movahedpour A, Arghiani N. Recent insights into the roles of circular RNAs in human brain development and neurologic diseases. Int J Biol Macromol. 2023;225:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 154. | Gilmore WJ, Johnston EL, Zavan L, Bitto NJ, Kaparakis-Liaskos M. Immunomodulatory roles and novel applications of bacterial membrane vesicles. Mol Immunol. 2021;134:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 155. | Dauros-Singorenko P, Blenkiron C, Phillips A, Swift S. The functional RNA cargo of bacterial membrane vesicles. FEMS Microbiol Lett. 2018;365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 156. | Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, Rizkalla S, Clément K. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049-3057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 898] [Cited by in RCA: 891] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 157. | Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, Bloom SR, Darzi A, Athanasiou T, Marchesi JR, Nicholson JK, Holmes E. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 324] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 158. | Hild B, Dreier MS, Oh JH, McCulloch JA, Badger JH, Guo J, Thefaine CE, Umarova R, Hall KD, Gavrilova O, Rosshart SP, Trinchieri G, Rehermann B. Neonatal exposure to a wild-derived microbiome protects mice against diet-induced obesity. Nat Metab. 2021;3:1042-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 159. | Du Y, Li X, Su C, Xi M, Zhang X, Jiang Z, Wang L, Hong B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br J Pharmacol. 2020;177:1754-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 160. | Hu H, Lin A, Kong M, Yao X, Yin M, Xia H, Ma J, Liu H. Intestinal microbiome and NAFLD: molecular insights and therapeutic perspectives. J Gastroenterol. 2020;55:142-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 161. | Kim J, Yun JM, Kim MK, Kwon O, Cho B. Lactobacillus gasseri BNR17 Supplementation Reduces the Visceral Fat Accumulation and Waist Circumference in Obese Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J Med Food. 2018;21:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 162. | Minami J, Iwabuchi N, Tanaka M, Yamauchi K, Xiao JZ, Abe F, Sakane N. Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: a randomized, double-blind, placebo-controlled trial. Biosci Microbiota Food Health. 2018;37:67-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 163. | Pedret A, Valls RM, Calderón-Pérez L, Llauradó E, Companys J, Pla-Pagà L, Moragas A, Martín-Luján F, Ortega Y, Giralt M, Caimari A, Chenoll E, Genovés S, Martorell P, Codoñer FM, Ramón D, Arola L, Solà R. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int J Obes (Lond). 2019;43:1863-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 164. | Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity (Silver Spring). 2013;21:2310-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 165. | Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: A systematic review and meta-analysis. Clin Nutr. 2018;37:532-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 166. | Sanchez M, Darimont C, Panahi S, Drapeau V, Marette A, Taylor VH, Doré J, Tremblay A. Effects of a Diet-Based Weight-Reducing Program with Probiotic Supplementation on Satiety Efficiency, Eating Behaviour Traits, and Psychosocial Behaviours in Obese Individuals. Nutrients. 2017;9:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 167. | Kim B, Park KY, Ji Y, Park S, Holzapfel W, Hyun CK. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem Biophys Res Commun. 2016;473:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 168. | Guerrero-Bonmatty R, Gil-Fernández G, Rodríguez-Velasco FJ, Espadaler-Mazo J. A Combination of Lactoplantibacillus plantarum Strains CECT7527, CECT7528, and CECT7529 Plus Monacolin K Reduces Blood Cholesterol: Results from a Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2021;13:1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 169. | Wang LX, Liu K, Gao DW, Hao JK. Protective effects of two Lactobacillus plantarum strains in hyperlipidemic mice. World J Gastroenterol. 2013;19:3150-3156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 170. | Tomaro-Duchesneau C, Saha S, Malhotra M, Jones ML, Labbé A, Rodes L, Kahouli I, Prakash S. Effect of orally administered L. fermentum NCIMB 5221 on markers of metabolic syndrome: an in vivo analysis using ZDF rats. Appl Microbiol Biotechnol. 2014;98:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 171. | Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066-9071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2639] [Cited by in RCA: 3316] [Article Influence: 276.3] [Reference Citation Analysis (0)] |
| 172. | Belzer C, de Vos WM. Microbes inside--from diversity to function: the case of Akkermansia. ISME J. 2012;6:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 519] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 173. | Cani PD, de Vos WM. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 698] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 174. | Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 774] [Cited by in RCA: 1429] [Article Influence: 238.2] [Reference Citation Analysis (0)] |
| 175. | Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, Park HS, Kim YK, Ryu SH. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 541] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 176. | Ashrafian F, Behrouzi A, Shahriary A, Ahmadi Badi S, Davari M, Khatami S, Rahimi Jamnani F, Fateh A, Vaziri F, Siadat SD. Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction. Gastroenterol Hepatol Bed Bench. 2019;12:163-168. [PubMed] |
| 177. | Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front Immunol. 2020;11:906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 1084] [Article Influence: 216.8] [Reference Citation Analysis (0)] |
| 178. | Huang J, Xiao N, Sun Y, Wu S, Tian W, Lai Y, Li P, Du B. Supplementation of Bacillus sp. DU-106 reduces hypercholesterolemia and ameliorates gut dysbiosis in high-fat diet rats. Appl Microbiol Biotechnol. 2021;105:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 179. | Jones RB, Alderete TL, Martin AA, Geary BA, Hwang DH, Palmer SL, Goran MI. Probiotic supplementation increases obesity with no detectable effects on liver fat or gut microbiota in obese Hispanic adolescents: a 16-week, randomized, placebo-controlled trial. Pediatr Obes. 2018;13:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 180. | Nuankham K, Sitdhipol J, Chonpathompikunlert P, Khongrum J, Kittichaiworakul R, Noisagul P, Thongkumkoon P, Kampoun T, Dissook S. Impact of Lactocaseibacillus (Lactobacillus) paracasei sup. paracasei TISTR 2593 Probiotic Supplementation on the Gut Microbiome of Hypercholesterolemia Patients: A Randomized Controlled Trial. Nutrients. 2024;16:2916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 181. | Ahn HY, Kim M, Chae JS, Ahn YT, Sim JH, Choi ID, Lee SH, Lee JH. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduces fasting triglycerides and enhances apolipoprotein A-V levels in non-diabetic subjects with hypertriglyceridemia. Atherosclerosis. 2015;241:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 182. | Xie N, Cui Y, Yin YN, Zhao X, Yang JW, Wang ZG, Fu N, Tang Y, Wang XH, Liu XW, Wang CL, Lu FG. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement Altern Med. 2011;11:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 183. | Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 557] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 184. | Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr. 2012;107:601-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 185. | Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One. 2011;6:e20944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 353] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 186. | Xu M, Lv C, Wang H, Lu Q, Ye M, Zhu X, Liu R. Peanut skin extract ameliorates high-fat diet-induced atherosclerosis by regulating lipid metabolism, inflammation reaction and gut microbiota in ApoE(-/-) mice. Food Res Int. 2022;154:111014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 187. | Zheng H, Ji H, Fan K, Xu H, Huang Y, Zheng Y, Xu Q, Li C, Zhao L, Li Y, Gao H. Targeting Gut Microbiota and Host Metabolism with Dendrobium officinale Dietary Fiber to Prevent Obesity and Improve Glucose Homeostasis in Diet-Induced Obese Mice. Mol Nutr Food Res. 2022;66:e2100772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 188. | Maruyama S, Matsuoka T, Hosomi K, Park J, Nishimura M, Murakami H, Konishi K, Miyachi M, Kawashima H, Mizuguchi K, Kobayashi T, Ooka T, Yamagata Z, Kunisawa J. Classification of the Occurrence of Dyslipidemia Based on Gut Bacteria Related to Barley Intake. Front Nutr. 2022;9:812469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 189. | Kellow NJ, Coughlan MT, Reid CM. Metabolic benefits of dietary prebiotics in human subjects: a systematic review of randomised controlled trials. Br J Nutr. 2014;111:1147-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 190. | Beserra BT, Fernandes R, do Rosario VA, Mocellin MC, Kuntz MG, Trindade EB. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin Nutr. 2015;34:845-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 191. | Zhao K, Wu X, Han G, Sun L, Zheng C, Hou H, Xu BB, El-Bahy ZM, Qian C, Kallel M, Algadi H, Guo Z, Shi Z. Phyllostachys nigra (Lodd. ex Lindl.) derived polysaccharide with enhanced glycolipid metabolism regulation and mice gut microbiome. Int J Biol Macromol. 2024;257:128588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 192. | Ramai D, Zakhia K, Fields PJ, Ofosu A, Patel G, Shahnazarian V, Lai JK, Dhaliwal A, Reddy M, Chang S. Fecal Microbiota Transplantation (FMT) with Colonoscopy Is Superior to Enema and Nasogastric Tube While Comparable to Capsule for the Treatment of Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2021;66:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 193. | Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, Sims M, Kraft CS, Cohen SH, Berenson CS, Korman LY, Ford CB, Litcofsky KD, Lombardo MJ, Wortman JR, Wu H, Auniņš JG, McChalicher CWJ, Winkler JA, McGovern BH, Trucksis M, Henn MR, von Moltke L. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N Engl J Med. 2022;386:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 326] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 194. | Halaweish HF, Boatman S, Staley C. Encapsulated Fecal Microbiota Transplantation: Development, Efficacy, and Clinical Application. Front Cell Infect Microbiol. 2022;12:826114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 195. | Wilson BC, Vatanen T, Jayasinghe TN, Leong KSW, Derraik JGB, Albert BB, Chiavaroli V, Svirskis DM, Beck KL, Conlon CA, Jiang Y, Schierding W, Holland DJ, Cutfield WS, O'Sullivan JM. Strain engraftment competition and functional augmentation in a multi-donor fecal microbiota transplantation trial for obesity. Microbiome. 2021;9:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 196. | van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2678] [Article Influence: 223.2] [Reference Citation Analysis (0)] |
| 197. | Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, Chang HJ, Coward S, Goodman KJ, Xu H, Madsen K, Mason A, Wong GK, Jovel J, Patterson J, Louie T. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 2017;318:1985-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 448] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 198. | Allegretti JR, Mullish BH, Kelly C, Fischer M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet. 2019;394:420-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 199. | Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, Knop FK, Blaak EE, Zhao J, Smidt H, Harms AC, Hankemeijer T, Bergman JJGHM, Romijn HA, Schaap FG, Olde Damink SWM, Ackermans MT, Dallinga-Thie GM, Zoetendal E, de Vos WM, Serlie MJ, Stroes ESG, Groen AK, Nieuwdorp M. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26:611-619.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 681] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 200. | Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, Marchesi JR, McDonald JAK, Pechlivanis A, Barker GF, Miguéns Blanco J, Garcia-Perez I, Wong WF, Gerardin Y, Silverstein M, Kennedy K, Thompson C. Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin Gastroenterol Hepatol. 2020;18:855-863.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 201. | Yu EW, Gao L, Stastka P, Cheney MC, Mahabamunuge J, Torres Soto M, Ford CB, Bryant JA, Henn MR, Hohmann EL. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020;17:e1003051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 202. | Ng SC, Xu Z, Mak JWY, Yang K, Liu Q, Zuo T, Tang W, Lau L, Lui RN, Wong SH, Tse YK, Li AYL, Cheung K, Ching JYL, Wong VWS, Kong APS, Ma RCW, Chow EYK, Wong SKH, Ho ICH, Chan PKS, Chan FKL. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut. 2022;71:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 203. | van der Vossen EWJ, Bastos D, Stols-Gonçalves D, de Goffau MC, Davids M, Pereira JPB, Li Yim AYF, Henneman P, Netea MG, de Vos WM, de Jonge W, Groen AK, Nieuwdorp M, Levin E. Effects of fecal microbiota transplant on DNA methylation in subjects with metabolic syndrome. Gut Microbes. 2021;13:1993513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 204. | Su L, Hong Z, Zhou T, Jian Y, Xu M, Zhang X, Zhu X, Wang J. Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci Rep. 2022;12:1152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 205. | Bibbò S, Settanni CR, Porcari S, Bocchino E, Ianiro G, Cammarota G, Gasbarrini A. Fecal Microbiota Transplantation: Screening and Selection to Choose the Optimal Donor. J Clin Med. 2020;9:1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 206. | Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, Putignani L, Fischer M, Keller JJ, Costello SP, Sokol H, Kump P, Satokari R, Kahn SA, Kao D, Arkkila P, Kuijper EJ, Vehreschild MJG, Pintus C, Lopetuso L, Masucci L, Scaldaferri F, Terveer EM, Nieuwdorp M, López-Sanromán A, Kupcinskas J, Hart A, Tilg H, Gasbarrini A. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68:2111-2121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 207. | DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, Hohmann EL. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. 2019;381:2043-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 834] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 208. | Tözün N, Vardareli E. Gut Microbiome and Gastrointestinal Cancer: Les liaisons Dangereuses. J Clin Gastroenterol. 2016;50 Suppl 2, Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13-15, 2015:S191-S196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 209. | Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology. 2017;152:1671-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 210. | Shariff S, Kwan Su Huey A, Parag Soni N, Yahia A, Hammoud D, Nazir A, Uwishema O, Wojtara M. Unlocking the gut-heart axis: exploring the role of gut microbiota in cardiovascular health and disease. Ann Med Surg (Lond). 2024;86:2752-2758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |