Published online Jul 28, 2025. doi: 10.3748/wjg.v31.i28.109078
Revised: May 24, 2025
Accepted: July 4, 2025
Published online: July 28, 2025
Processing time: 86 Days and 17.9 Hours
Hepatic hemangiomas represent the most prevalent benign liver tumors. Surgical management of large symptomatic hepatic hemangiomas remains controversial and there is an increasing interest in minimally invasive techniques, such as trans
To evaluate the efficacy and safety of TACE combined with MWA for large hepa
This retrospective cohort study was conducted at Peking Union Medical College Hospital between January 2015 and January 2024. Eighty-two patients with he
At baseline, the median tumor diameter was 8.3 (range: 5.0-19.2) cm in the obser
Combination treatment enhances tumor shrinkage, promotes long-term tumor control, and reduces the compli
Core Tip: This study compares two treatment options for large liver hemangiomas, a type of benign tumor. We found that combining transcatheter arterial chemoembolization and microwave ablation was more effective at shrinking tumors and preventing recurrence compared to transcatheter arterial chemoembolization alone. Patients with large liver hemangiomas experienced fewer complications on the combined treatment which may be a safer alternative to surgery.
- Citation: Sun JP, Zhou K, Pan J, Yang N, Sun XN, Zhao HT, Yang XB. Efficacy and safety of transcatheter arterial chemoembolization combined with microwave ablation for hepatic hemangiomas (> 5 cm). World J Gastroenterol 2025; 31(28): 109078
- URL: https://www.wjgnet.com/1007-9327/full/v31/i28/109078.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i28.109078
Hepatic hemangiomas are the most prevalent benign liver tumors, with an incidence rate of approximately 2.5% to 5.0%, and are more frequently observed in women aged 30-50 years[1,2]. Although most asymptomatic small hemangiomas (diameter < 5 cm) do not require intervention, larger tumors (diameter > 5 cm) or those presenting with clinical symp
According to the 2016 European Association for the Study of the Liver (EASL) guidelines[1], surgical resection is the preferred treatment for symptomatic and large hemangiomas. However, as outlined in the EASL and Associazione Italiana Studio del Fegato guidelines[7], surgery is applicable only to a subset of patients. The surgical resection of large symptomatic hepatic hemangiomas remains controversial due to the associated invasive risks[1,7]. This has led to in
TACE induces tumor ischemia and shrinkage by occluding the supplying arteries, while MWA generates heat through high-frequency electromagnetic waves, resulting in thermal coagulative necrosis of the tumor. While effective, TACE may be limited by its reduced efficacy in poorly vascularized tumors and less significant tumor shrinkage, whereas MWA carries a risk of hemolysis-related complications[9,11-13]. A combined TACE and MWA strategy has been proposed to mitigate these respective limitations, potentially enhancing therapeutic outcomes while reducing MWA-related compli
This retrospective cohort study was conducted at Peking Union Medical College Hospital between January 2015 and January 2024 and included 82 patients with hepatic hemangiomas. The patients were divided into two groups based on the treatment modality: The observation group (n = 50), which received a combination of TACE and MWA, and the control group (n = 32), which received TACE alone. The study adhered to strict inclusion and exclusion criteria to ensure both scientific rigor and reproducibility of the results.
All enrolled patients underwent a comprehensive pretreatment evaluation conducted by a multidisciplinary team (MDT) that included hepatobiliary surgeons, interventional radiologists, and anesthesiologists. During this evaluation, surgical candidacy was assessed for each patient, and surgical resection was considered in all cases. However, for most patients, surgical resection was either declined due to personal preferences or was deemed contraindicated due to medical comorbidities. Given the symptomatic burden and ineligibility or reluctance to undergo surgical resection, the MDT collectively determined that a minimally invasive interventional approach consisting of TACE combined with MWA was a clinically appropriate and justifiable alternative. This decision was based on the expected safety profile and efficacy of the combined treatment, which was tailored to address both the tumor characteristics and the patient’s overall clinical condition. The MDT’s decision-making process was guided by a thorough consideration of individual patient factors, including the risk-benefit profile of surgical resection vs the proposed interventional treatment.
The inclusion criteria were as follows: (1) Tumor diameter: The longest diameter of the hepatic hemangioma was > 5 cm, confirmed by contrast-enhanced magnetic resonance imaging (MRI) or computed tomography (CT); (2) Clinical symp
The exclusion criteria were as follows: (1) Comorbid malignant tumors: Presence of other primary or metastatic malignant tumors; (2) Severe systemic diseases: Patients with severe dysfunction of the liver, kidneys, heart, or lungs; (3) Coagula
The diagnosis of hepatic hemangioma was primarily confirmed through contrast-enhanced CT and/or MRI and was characterized by peripheral nodular enhancement in the arterial phase and centripetal filling in the venous phase. In cases with atypical tumor morphology, a pathological biopsy was performed to confirm the diagnosis of hepatic heman
All patients were treated by four interventional radiologists, each with more than 15 years of experience, using stan
The control group underwent TACE using the ionic contrast agent, diatrizoate meglumine and diatrizoate sodium (Urografin® 76%, 370 mgI/mL; Bayer AG, Berlin, Germany, H10970164), guided by a Philips Allura DSA system (Allura Xper FD20). The Seldinger technique was employed to puncture the right femoral artery, followed by selective catheterization of the common hepatic artery, during which hepatic arteriography was performed. Upon confirming the location of the lesion, the catheter was advanced into the blood vessel of the tumor, and the embolizing agent was slowly admi
In the observation group, MWA (water-cooled MWA system; Yigao Medical, China) was performed following TACE. The ablation protocol was standardized across all patients, with careful consideration of the tumor characteristics and patient safety. The patients received intravenous anesthesia, followed by the insertion of an endotracheal tube or laryngeal mask airway, with mechanical ventilation employed to control respiration, thereby ensuring patient stability during the ablation process. CT guidance was used to identify the precise location of the lesion, followed by meticulous planning of the puncture path to minimize damage to surrounding vital organs. To mitigate the risk of bleeding from the hemangioma, the ablation probe was typically directed through the normal liver tissue to reach the target lesion. The output power of MWA was generally set between 30 W and 80 W, with the ablation time determined according to the tumor volume. Ablation sites were carefully planned to optimize tumor necrosis. At a typical output power of 50 W, an ablation needle was inserted to deliver energy, and the ablation zone was designed to adequately cover the tumor. For hemangiomas with tumor diameter > 5 cm, a single point of ablation was focused on, with a target range of approximately 3.0-3.5 cm along the anteroposterior axis and a diameter of approximately 2 cm from the needle tip. The ablation zone was determined based on the manufacturer’s technical specifications and the clinical experience of the interventional radiologist. For giant (> 10 cm) or strategically located tumors, a two-circle overlapping ablation technique was used to ensure complete tumor coverage within the ablation zone. Throughout the procedure, the microwave probe was maintained at least 1 cm away from critical structures such as the bile ducts and inferior vena cava. Thermal shielding technology was utilized as necessary to protect these vital structures. MWA commenced at the edge of the tumor where the thermal sink effect was relatively minimal, thereby facilitating better bleeding control. Finally, needle tract ablation was performed by withdrawing the ablation electrode to further decrease the risk of postoperative bleeding. Following general anesthesia for ablation, the patients underwent 12 hours of electrocardiogram monitoring, 6 hours of fasting, and hydration with 1000-2000 mL of normal saline, adjusted according to the patient’s specific condition.
In this study, dynamic monitoring of the liver and kidney function was conducted before treatment, 1-week post-treatment, and 1-month post-treatment. We focused on key biochemical markers, including alanine aminotransferase (ALT), aspartate aminotransferase, total bilirubin (TBIL), direct bilirubin (DBIL), albumin (Alb), and creatinine. Postope
During imaging follow-up, enhanced MRI scans were performed at 1 month, 6 months, and 12 months after treatment to assess the longest tumor diameter and shrinkage of hepatic hemangiomas. Currently, the Response Evaluation Criteria in Solid Tumors (RECIST)[17] are not commonly used to assess hepatic hemangioma. This study employed RECIST to analyze the imaging results, thereby ensuring the objectivity and accuracy of treatment evaluations. For patients pre
The primary endpoints of this study were safety, assessed using the Clavien-Dindo classification[21] of ablation-related complications, and imaging, evaluated using the RECIST. Secondary endpoints included the clinical response, which encompassed the evaluation of postoperative symptom improvement, such as pain relief, restoration of digestive func
The sample size for this retrospective cohort study was determined by the complete enumeration of all eligible patients with hepatic hemangiomas (diameter > 5 cm) who met the predefined inclusion and exclusion criteria and underwent either TACE combined with MWA (observation group, n = 50) or TACE alone (control group, n = 32) at Peking the Union Medical College Hospital between January 2015 and January 2024, and for whom comprehensive clinical outcome data were available. All data were analyzed using SPSS software (version 26.0; IBM Corp., Armonk, NY, United States). The statistical review of the study was performed by a biomedical statistician. Appropriate statistical methods were chosen based on the type and distribution of the data, with statistical significance set at P < 0.05. For some of the key metrics, the risk ratios and their 95% confidence intervals (CIs) were calculated and reported to assess the effect sizes and clinical relevance of the differences between groups.
To evaluate the statistical power of the current study to detect differences in the primary efficacy endpoint, post-hoc power analysis was performed for the 12-month objective response rate (ORR). This analysis, based on the observed ORRs of 93.94% in the observation group (n = 50) and 33.33% in the control group (n = 32), was conducted using G*Power software (version 3.1.9.7; Faul, Erdfelder, Lang, and Buchner, 2007; Heinrich Heine University Düsseldorf, Düsseldorf, Germany). The analysis was set for a two-sided test with a significance level (α) of 0.05. The resulting statistical power (1-β) was 0.9999995, indicating that the study was amply powered to detect the observed between-group difference.
This study was reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital (approval No. 1-24PJ1959). All study participants, or their legal guardians, provided informed written consent prior to study enrollment. Data collection and analysis strictly adhered to the ethical guidelines for medical research involving humans, as outlined in the Declaration of Helsinki, ensuring full protection of patient privacy.
This study included 82 patients who were categorized into an observation (n = 50) or control (n = 32) group according to the type of intervention. No statistically significant differences were observed between the groups in terms of age, sex, baseline tumor diameter, disease duration, lesion distribution, and count, comorbidities, preoperative anxiety status, or other critical demographic and clinical indicators (all P > 0.05). This indicated that the baseline characteristics of the participants were comparable (Table 1).
| Characteristics1 | All (n = 82) | Control group (n = 32) | Observation group (n = 50) | P value2 |
| Age (years) | 46 (40-54) | 47 (41-55) | 44 (39-52) | 0.122 |
| Longest tumor diameter (cm) | 8.4 (5-20) | 8.5 (5-20) | 8.3 (5-19.2) | 0.860 |
| Disease duration (years) | 6 (1-13) | 7 (2-12) | 5 (1-14) | 0.668 |
| Sex | 0.527 | |||
| Female | 65 (79.27) | 27 (84.38) | 38 (76.00) | |
| Male | 17 (20.73) | 5 (15.62) | 12 (24.00) | |
| Measurable lesions3 | 0.993 | |||
| Single | 73 (89.02) | 29 (90.62) | 44 (88.00) | |
| Multiple | 9 (10.98) | 3 (9.38) | 6 (12.00) | |
| Lesion distribution | 0.909 | |||
| Right lobe | 59 (71.95) | 24 (75.00) | 35 (70.00) | |
| Left lobe | 10 (12.20) | 3 (9.38) | 7 (14.00) | |
| Junction of the right and left lobes | 11 (13.41) | 4 (12.50) | 7 (14.00) | |
| Caudate lobe | 2 (2.44) | 1 (3.12) | 1 (2.00) | |
| Lesion classification4 | 0.624 | |||
| Huge | 63 (76.83) | 26 (81.25) | 37 (74.00) | |
| Giant | 19 (23.17) | 6 (18.75) | 13 (26.00) | |
| Symptoms | 0.099 | |||
| Asymptomatic | 52 (63.41) | 16 (50.00) | 36 (72.00) | |
| Abdominal discomfort | 27 (32.93) | 15 (46.88) | 12 (24.00) | |
| Gastrointestinal symptoms | 3 (3.66) | 1 (3.12) | 2 (4.00) | |
| Comorbidities | 0.473 | |||
| No comorbidities | 67 (81.71) | 25 (78.12) | 42 (84.00) | |
| Hypertension | 10 (12.20) | 5 (15.62) | 5 (10.00) | |
| Hyperlipidemia | 2 (2.44) | 0 (0.00) | 2 (4.00) | |
| Diabetes mellitus | 2 (2.44) | 1 (3.12) | 1 (2.00) | |
| SLE | 1 (1.22) | 1 (3.12) | 0 (0.00) | |
| Anxiety status | 1.0 | |||
| No anxiety | 75 (91.46) | 29 (90.62) | 46 (92.00) | |
| Mild anxiety | 7 (8.54) | 3 (9.38) | 4 (8.00) | |
| Reason for treatment | 0.128 | |||
| Enlargement of hemangioma | 52 (63.41) | 19 (59.38) | 33 (66.00) | |
| Symptomatic hemangioma | 18 (21.95) | 6 (18.75) | 12 (24.00) | |
| Anxiety | 2 (2.44) | 0 (0.00) | 2 (4.00) | |
| ≥ 2 reasons | 10 (12.20) | 7 (21.88) | 3 (6.00) | |
As shown in Table 2, most patients in the observation group (n = 50) underwent a single ablation (47/50, 94.0%), while three patients (6.0%) required two ablation sessions due to the tumor diameter or clinical strategy. On average, surgeries in 66% of the patients required one probe, while 34% used two probes per ablation, with an average of 5.78 ± 3.00 ablation sites. Regarding the energy parameters, 50 W was the most common average output power (34/50, 68.0%) used in the cases, followed by 15 cases (30.0%) using 60 W, and only one case (2.0%) employing 40 W. The average duration of the ablation procedure was 27.88 ± 14.11 minutes, suggesting that the procedure duration may vary based on the tumor diameter and tissue characteristics. Furthermore, based on postoperative responses and clinical observations, the embolization to ablation interval was categorized into three groups: < 1 week (32.0%), 1-3 weeks (20.0%), and > 3 weeks (48.0%). In the observation group, ablation was generally performed within the appropriate time window following the initial embolization to enhance local control in lesions with residual or potential recurrence risk.
| Ablation parameters1 | Observation group (n = 50) |
| Number of ablation procedures | |
| 1 | 47 (94.00) |
| 2 | 3 (6.00) |
| Number of ablation probes | |
| 1 | 33 (66.00) |
| 2 | 17 (34.00) |
| Average ablation sites | 5.78 ± 3.00 |
| Ablation power (W)2 | |
| 40 | 1 (2.00) |
| 50 | 34 (68.00) |
| 60 | 15 (30.00) |
| Ablation duration (minute), mean ± SD | 27.88 ± 14.11 |
| Embolization to ablation interval3 | |
| < 1 week | 16 (32.00) |
| 1-3 weeks | 10 (20.00) |
| > 3 weeks | 24 (48.00) |
Perioperative adverse events were classified according to the Clavien-Dindo grading system, ranging from mild (grade I) to severe complications (grade IV), as detailed in Table 3. There were no significant differences in the occurrence of common adverse events, including postprocedural pain, fever, nausea, and vomiting, between the control and obser
| Adverse events1 | Clavien-Dindo grade | All (n = 82) | Control group (n = 32) | Observation group (n = 50) | P value2 |
| Postprocedural pain | I | 20 (24.39) | 8 (25.00) | 12 (24.00) | 1.0 |
| Fever | I | 5 (6.10) | 1 (3.12) | 4 (8.00) | 0.669 |
| Nausea and vomiting | I | 17 (20.73) | 6 (18.75) | 11 (22.00) | 0.94 |
| Mild perihepatic hemorrhage | I | 10 (12.20) | 0 (0.00) | 10 (20.00) | 0.019a |
| Anemia3 | I | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.0 |
| Neutropenia3 | I | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.0 |
| Thrombocytopenia3 | I | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.0 |
| Hemoglobinuria | I | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.0 |
| Hepatic injury | II | 20 (24.39) | 2 (6.25) | 18 (36.00) | 0.005a |
| Jaundice | II | 7 (8.54) | 0 (0.00) | 7 (14.00) | 0.071 |
| Biliary fistula | II | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.0 |
| Liver abscess | II | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.0 |
| Acute kidney injury | IV | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.0 |
| Characteristics | All (n = 82) | Control group (n = 32) | Observation group (n = 50) | P value1 |
| ALT (U/L) | ||||
| Pre-treatment | 25.34 ± 22.85 | 16.25 ± 11.10 | 31.16 ± 26.39 | < 0.001 |
| Postoperative within 1 week | 74.55 ± 122.15 | 30.62 ± 38.36 | 102.66 ± 147.19 | < 0.001 |
| Postoperative at 1 month | 23.39 ± 26.06 | 18.84 ± 7.13 | 26.30 ± 32.68 | 0.227 |
| TBIL (μmol/L) | ||||
| Pre-treatment | 12.81 ± 5.43 | 11.20 ± 4.53 | 13.85 ± 5.74 | 0.034 |
| Postoperative within 1 week | 18.83 ± 10.52 | 14.71 ± 7.53 | 21.46 ± 11.35 | < 0.001 |
| Postoperative at 1 month | 11.86 ± 4.05 | 11.20 ± 3.78 | 12.28 ± 4.20 | 0.248 |
| DBIL (μmol/L) | ||||
| Pre-treatment | 3.88 ± 1.68 | 3.44 ± 1.65 | 4.16 ± 1.66 | 0.038 |
| Postoperative within 1 week | 4.66 ± 2.36 | 3.59 ± 1.21 | 5.35 ± 2.65 | < 0.001 |
| Postoperative at 1 month | 3.65 ± 1.48 | 3.38 ± 1.46 | 3.83 ± 1.47 | 0.166 |
| Albumin (g/L) | ||||
| Pre-treatment | 43.85 ± 3.94 | 44.06 ± 5.10 | 43.72 ± 3.02 | 0.33 |
| Postoperative within 1 week | 40.95 ± 3.08 | 41.72 ± 3.31 | 40.46 ± 2.84 | 0.09 |
| Postoperative at 1 month | 43.98 ± 2.57 | 43.81 ± 3.34 | 44.08 ± 1.96 | 0.759 |
| WBC (× 109/L) | ||||
| Pre-treatment | 5.81 ± 1.52 | 5.77 ± 1.48 | 5.83 ± 1.56 | 0.844 |
| Postoperative within 1 week | 8.47 ± 3.08 | 8.86 ± 3.77 | 8.21 ± 2.56 | 0.794 |
| Postoperative at 1 month | 5.69 ± 1.11 | 5.69 ± 0.95 | 5.69 ± 1.21 | 0.555 |
| Neutrophils (× 109/L) | ||||
| Pre-treatment | 3.78 ± 1.38 | 3.53 ± 1.07 | 3.94 ± 1.53 | 0.274 |
| Postoperative within 1 week | 7.02 ± 2.98 | 7.34 ± 3.67 | 6.81 ± 2.46 | 0.761 |
| Postoperative at 1 month | 3.59 ± 1.04 | 3.54 ± 0.83 | 3.63 ± 1.16 | 0.775 |
| Platelets (× 109/L) | ||||
| Pre-treatment | 218.45 ± 56.88 | 220.47 ± 65.79 | 217.16 ± 51.05 | 0.799 |
| Postoperative within 1 week | 198.87 ± 60.03 | 201.38 ± 80.27 | 197.26 ± 43.29 | 0.868 |
| Postoperative at 1 month | 225.18 ± 53.08 | 228.53 ± 63.39 | 223.04 ± 45.87 | 0.816 |
| Hemoglobin (g/L) | ||||
| Pre-treatment | 134.45 ± 17.30 | 134.97 ± 16.50 | 134.12 ± 17.96 | 0.83 |
| Postoperative within 1 week | 128.63 ± 20.91 | 130.38 ± 15.33 | 127.51 ± 23.90 | 0.936 |
| Postoperative at 1 month | 132.59 ± 13.43 | 132.88 ± 14.73 | 132.40 ± 12.67 | 0.659 |
| Creatinine (μmol/L) | ||||
| Pre-treatment | 63.68 ± 13.16 | 62.09 ± 13.94 | 64.70 ± 12.67 | 0.232 |
| Postoperative within 1 week | 59.72 ± 12.02 | 59.36 ± 11.12 | 59.95 ± 12.68 | 0.985 |
| Postoperative at 1 month | 63.57 ± 9.05 | 62.25 ± 9.02 | 64.42 ± 9.06 | 0.175 |
Table 4 presents the dynamic monitoring results of key laboratory parameters, including the levels of ALT, TBIL, DBIL, Alb, WBCs, neutrophils, platelets, hemoglobin, and creatinine, for both groups at multiple time points (pre-treatment, 1-week post-treatment, and 1-month post-treatment). Overall, ALT and TBIL levels were significantly higher in the observation group at certain time points (including 1-week post-treatment) than in the control group (P < 0.001). This finding suggests that the combined intervention had a more pronounced short-term effect on liver cell injury. Follow-up assessments 1 month post-treatment indicated that the differences in ALT and TBIL between the two groups had gradually diminished and were no longer significantly different (P > 0.05). Additionally, no significant differences were observed in the Alb, WBC, platelet, or hemoglobin levels at any of the time points (all P > 0.05).
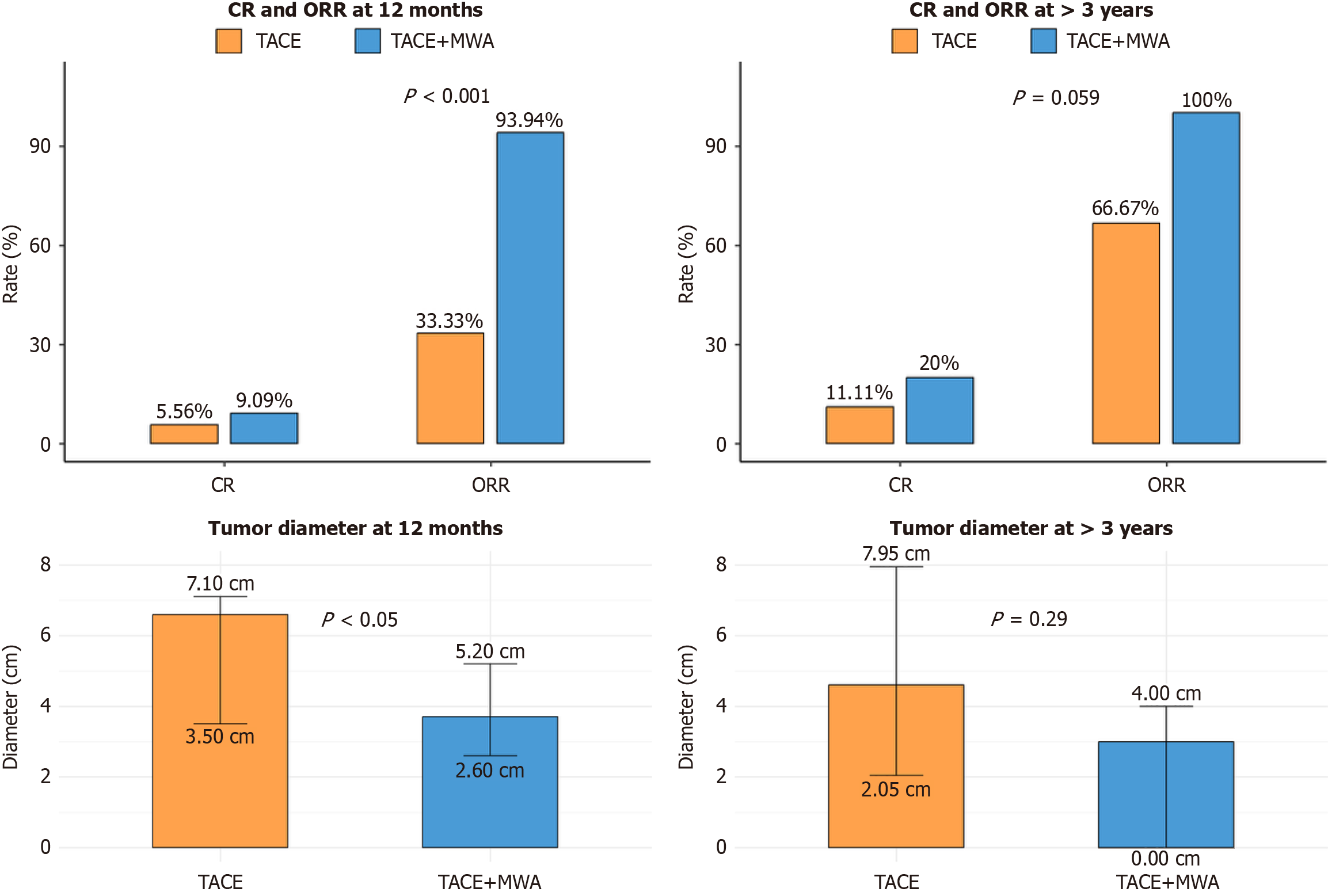
The median follow-up time for this study was 44.6 months (95%CI: 36.7-52.5 months), with a quartile range spanning from 21.3 months to 82.5 months. As indicated in Table 5, no statistically significant difference was observed in the longest tumor diameter between the two groups at the 1-month follow-up (P = 0.70). However, at the 6- and 12-month follow-ups, the observation group exhibited a significantly smaller tumor diameter than the control group (P < 0.05). The tumor reduction ratio at 12 months postoperatively was significantly greater in the observation group (50.98%, interquartile range: 37.5-61.8) than in the control group (23.28%, interquartile range: 17.5-47.27); P < 0.001), as illustrated in Figure 1. RECIST evaluation revealed a higher ORR in the observation group than in the control group (93.94% vs 33.33%). In the long-term follow-up period of 1-3 years, and in some cases extending beyond 3 years, the observation group consistently demonstrated a superior complete response and ORR. These outcomes are summarized in Figure 1. Notably, two patients in the control group experienced tumor recurrence during the extended follow-up, whereas no recurrence was observed in the observation group.
| Variable | All (n = 82) | Control group (n = 32) | Observation group (n = 50) | P value1 |
| Longest tumor diameter at 1 month | 7.0 (5.2-8.2) | 6.2 (5.9-9.0) | 7.0 (4.95-7.95) | 0.70 |
| Longest tumor diameter at 6 months | 4.4 (3.2-7.0) | 7.5 (4.6-8.9) | 4.1 (3.1-6.0) | < 0.05a |
| Longest tumor diameter at 12 months | 4.05 (2.8-6.6) | 6.6 (3.5-7.1) | 3.7 (2.6-5.2) | < 0.05a |
| Tumor reduction ratio (%) | 46.16 (26.48-58.73) | 23.28 (17.5-47.27) | 50.98 (37.5-61.8) | < 0.001c |
| RECIST | < 0.001c | |||
| CR | 4/51 (7.84%) | 1/18 (5.56%) | 3/33 (9.09%) | |
| PR | 33/51 (64.71%) | 5/18 (27.78%) | 28/33 (84.85%) | |
| SD | 14/51 (27.45%) | 12/18 (66.67%) | 2/33 (6.06%) | |
| PD | 0 | 0 | 0 | |
| ORR2 | 37/51 (72.55%) | 6/18 (33.33%) | 31/33 (93.94%) | |
| Longest tumor diameter at 1-3 years (n = 38)3 | 3.2 (0.5-5.8) | 4.5 (3.5-7.9) | 2.8 (0-4) | < 0.05a |
| RECIST | < 0.01b | |||
| CR | 7/38 (18.42%) | 1/12 (8.33%) | 6/26 (23.08%) | |
| PR | 24/38 (63.16%) | 5/12 (41.67%) | 19/26 (73.08%) | |
| SD | 6/38 (15.79%) | 5/12 (41.67%) | 1/26 (3.85%) | |
| PD | 1/38 (2.63%) | 1/12 (8.33%) | 0 | |
| ORR2 | 31/38 (81.58%) | 6/12 (50%) | 25/26 (96.15%) | |
| Longest tumor diameter at > 3 years (n = 29)3 | 3.6 (2-5.5) | 4.6 (2.05-7.95) | 3 (0-4) | 0.29 |
| RECIST | 0.059 | |||
| CR | 5/29 (17.24%) | 1/9 (11.11%) | 4/20 (20.00%) | |
| PR | 21/29 (72.41%) | 5/9 (55.56%) | 16/20 (80.00%) | |
| SD | 2/29 (6.90%) | 2/9 (22.22%) | 0 | |
| PD | 1/29 (3.45%) | 1/9 (11.11%) | 0 | |
| ORR2 | 26/29 (89.66%) | 6/9 (66.67%) | 20/20 (100.00%) | |
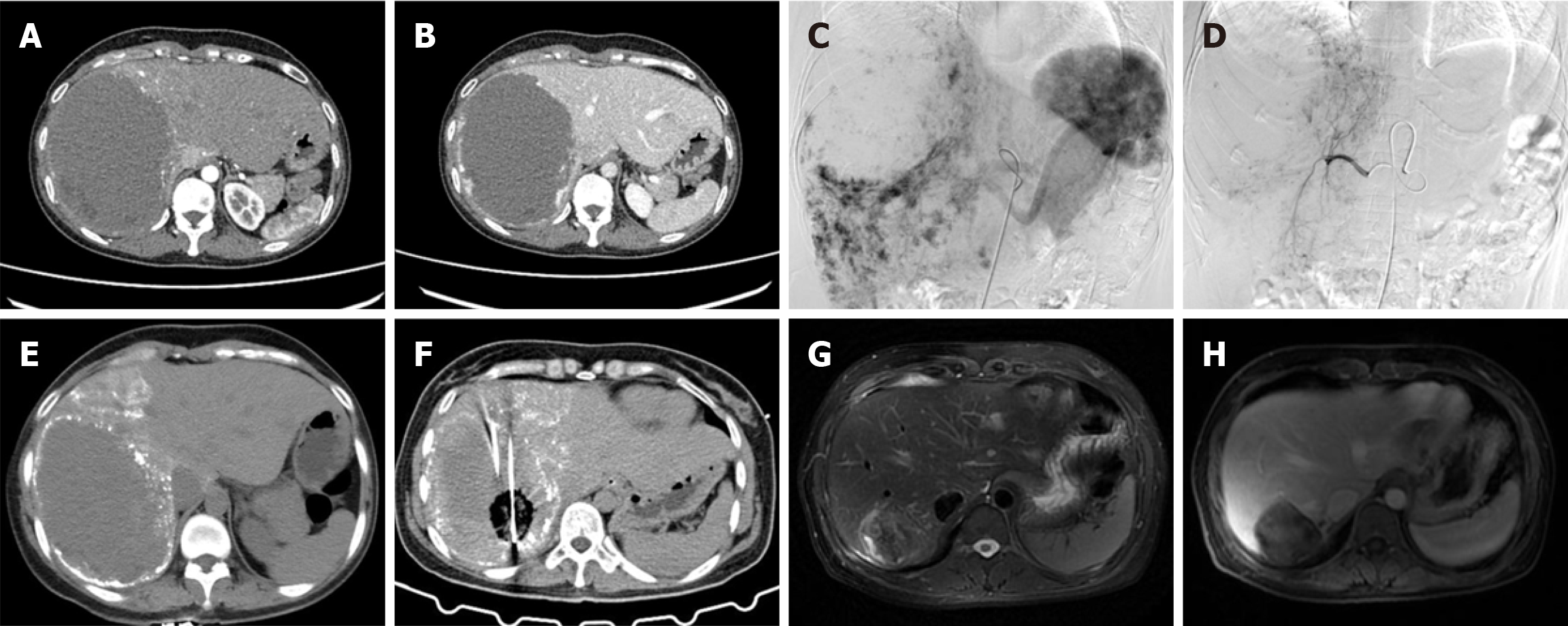
Figure 2 depicts the imaging results of a 48-year-old female with a 14-cm hepatic hemangioma, showing the tumor’s appearance on pre-treatment CT (Figure 2A and B), intraoperative angiographic embolization (Figure 2C and D) and post-embolization CT (Figure 2E), application of MWA (Figure 2F), and marked tumor reduction on the 40-month follow-up MRI (Figure 2G and H), consistent with a complete response.
Table 6 presents the activities of daily living scores of both groups at admission and discharge. At admission, the mean ± SD activities of daily living scores were 81.65 ± 21.36 overall, 82.50 ± 20.52 in the control group, and 81.00 ± 20.52 in the observation group (P = 0.849). At discharge, the corresponding scores were 96.71 ± 6.99, 96.41 ± 7.32, and 96.90 ± 6.84, respectively (P = 0.975). No statistically significant differences were observed between the two groups at any time point (all P > 0.05).
In recent years, the continuous advancement of interventional therapy technologies has led to minimally invasive treatments for hepatic hemangiomas, which have gradually emerged as viable alternatives to traditional surgical procedures[1,16]. Transcatheter arterial embolization and TACE have been employed to shrink tumors and alleviate symptoms by obstructing the blood supply. However, these treatments often require repetition because of the potential for vascular recanalization, which carries the risk of recurrence[10,14]. In the field of thermal ablation therapies, radiofrequency ablation (RFA) and MWA are widely used for the treatment of hepatic hemangiomas. According to Chinese experts, including Gao et al[4], RFA induces tumor necrosis through thermal coagulative necrosis. Although RFA is minimally invasive and facilitates rapid recovery, it is susceptible to thermal sink effects in highly vascular lesions, which can result in severe hemolysis and associated complications. A multicenter retrospective analysis by Wu et al[22] reported an 85.91% incidence of hemoglobinuria following RFA. In contrast, MWA employs dielectric heating principles, enabling it to achieve higher temperatures in a shorter timeframe, thus creating a larger and more uniform necrotic area while being less influenced by the cooling effects of surrounding blood flow[23-25]. However, due to the abundant blood supply and complex vascular structure of hepatic hemangiomas, MWA still encounters thermal sink effects caused by blood flow, leading to hemoglobinuria, hemolytic jaundice, and AKI, with incidence rates ranging from 3.8% to 48.6%[9,11-13]. Consequently, identifying both safe and effective minimally invasive treatment methods has become a pivotal research focus.
In this study, no instances of hemoglobinuria or AKI were observed in the patients who underwent TACE combined with MWA. This result notably diverges from those of previous studies in which hemolysis-related complications with RFA or MWA were reported. These hemolytic complications primarily stem from extensive erythrocyte destruction induced by thermal ablation in highly vascularized hepatic lesions, causing the release of hemoglobin and other cellular contents into the circulation, and ultimately impairing renal function through tubular injury and inflammation-driven processes[9,26]. The absence of such complications in our study is primarily attributable to the pre-ablation embolization strategy. Initial TACE markedly reduced the tumor blood volume and limited erythrocyte exposure and fragmentation during subsequent MWA. By decreasing intratumorally blood flow, TACE enhanced heat delivery efficiency and minimized hemolysis, aligning with the strategic embolization approach reported by Kacała et al[14] and Cai et al[11]. Furthermore, Fei and Hongsong[6] conducted a meta-analysis, revealing that shorter ablation durations were associated with reduced hemolysis rates, supporting our approach of targeted, abbreviated MWA sessions enabled by pre-ablation embolization. Our finding suggests that the “embolization followed by ablation” strategy not only enhances the efficacy of ablation but also mitigates the risk of hemolysis, a common complication associated with a rich blood supply[1,4,14,25]. These results underscore the superior safety profile of the TACE + MWA combination therapy compared to MWA alone.
The median follow-up time for this study was 44.6 months (95%CI: 36.7-52.5 months), with a quartile range spanning from 21.3-82.5 months, ensuring the long-term effectiveness of the treatment and the significance of the follow-up data. This comprehensive analysis better reflects the evolving trends in treatment effectiveness and recurrence risk, particularly in the management of giant hemangiomas (> 10 cm), enabling a more thorough evaluation of treatment comprehensiveness and durability. The study data indicated that at 12 months post-treatment, the median longest tumor diameter in the observation group decreased to 3.7 cm (interquartile range: 2.6-5.2 cm), which was significantly lower than the median of the control group at 6.6 cm (interquartile range: 3.5-7.1 cm; P < 0.05). The ORR in the observation group was 93.94% compared to 33.33% in the control group. During the long-term follow-up (> 3 years), the complete response and ORR in the observation group increased to 20.00% and 100.00%, respectively, both of which were significantly higher than those in the control group (11.11% and 66.67%, respectively; P = 0.059). As illustrated in Figure 1, the combined therapy achieved markedly better outcomes. These results demonstrate that the combined treatment strategy offers distinct advantages in terms of inhibiting tumor growth and reducing recurrence risk, effectively addressing the limitations of single treatments in managing hemolytic complications and the necessity for repeated procedures in large/giant hemangiomas. Guided by MDT evaluation, sequential TACE + MWA offered a key minimally invasive alternative for patients unsuitable for or declining surgery. This combined approach is particularly advantageous for those with giant hemangiomas (> 10 cm), pronounced symptoms, high expectations for cytoreduction, or lesions challenging for standalone MWA due to size or critical location (e.g., major vascular proximity, high heat-sink risk), where TACE may enhance subsequent MWA efficacy and safety[6,9,12,14].
All ablation treatments were conducted under general anesthesia to ensure a painless experience and minimize physiological stress responses. General anesthesia enhances safety and operability when addressing larger or more complex lesions, thereby optimizing treatment outcomes[4,6,27]. Furthermore, the combined approach underscores the importance of a well-planned needle insertion strategy. As indicated in Table 2, the observation group required an average of 5.78 ± 3.00 ablation sites, with ablation primarily performed at a power setting of 50-60 W. This not only reduced the ablation time but also ensured a more extensive necrotic area. The ischemia and inflammatory edema induced by TACE augmented conduction[4,14]. Although the observation group experienced a higher incidence of mild perihepatic hemorrhage (20.00%) and hepatic injury (36.00%) at 1-week post-treatment than the control group (0.00% and 6.25%, respectively), these adverse events were classified as Clavien-Dindo grade I or II and resolved rapidly with conservative management and short-term symptomatic treatment, with the majority improving within 1 week postoperatively. These findings underscore the overall safety and controllability of the combined therapeutic approach and reinforce its clinical viability without the need for further invasive interventions. One month post-treatment, ALT, TBIL, DBIL, and other indicators returned to levels comparable to those in the control group (P > 0.05), suggesting that liver function impairment was largely self-limiting. Stringent monitoring and timely interventions can effectively mitigate the risk of further deterioration[22,23,28]. Beyond mitigating MWA-induced hemolysis, a comprehensive safety assessment of the TACE approach also considers the systemic toxicities of bleomycin. While bleomycin’s primary dose-limiting concern is pulmonary toxicity (not observed in our cohort, likely due to patient selection and localized intra-arterial delivery), its potential for bone marrow suppression is generally characterized as mild and infrequent with regional administration at modest doses[14,15]. Our study’s findings strongly corroborate this favorable hematologic safety profile. Comprehensive hematologic follow-up (Table 4) demonstrated no clinically significant deviations in the white blood cell counts, neutro
In terms of improving clinical symptoms, interventional therapy has obvious advantages in the treatment of hepatic hemangiomas. Preoperative data indicated that 32.93% (27/82) of patients experienced abdominal discomfort, with 46.88% (17/32) in the control group and 24.00% (12/50) in the observation group. Gastrointestinal symptoms occurred in 3.66% of the patients, with one case (3.12%) in the control group and two cases (4.00%) in the observation group. This distribution aligns with prior studies, such as the study by Kong et al[9], in which the proportion of patients treated with RFA or MWA seeking treatment for abdominal pain or discomfort ranged from 7.56% to 11.63%. After treatment, 97.56% (80/82) of patients were asymptomatic, with 96.88% (31/32) in the control group and 98.00% (49/50) in the observation group. Only two patients (2.44%) reported persistent symptoms. The overall symptom improvement rate was approximately 93%; the control group improved from 50.00% to 3.12% (93.76% improvement), and the observation group improved from 28.00% to 2.00% (92.86% improvement). Shi et al[12] demonstrated that the complete symptom relief rate after MWA was 88.6%, compared to 69.2% in the transcatheter arterial embolization group. Consequently, interventional therapy for hepatic hemangiomas not only effectively alleviates symptoms but may also mitigate patients’ anxiety. This study evaluated changes in the psychological state and clinical symptoms of patients with hepatic hemangiomas before and after treatment, using widely validated scales, including the generalized anxiety disorder-7 and 36-Item Short Form Health Survey, to quantify changes in anxiety, depression, stress, and overall quality of life, with particular emphasis on anxiety relief and clinical symptom improvement. Among the 82 patients, nine (11.0%) exhibited anxiety prior to treatment. Following intervention, improvement was observed in six of these patients (66.7%), and only three (33.3%) continued to display anxiety. This finding is consistent with the treatment strategies outlined in the EASL guidelines[1] and corroborates the results of a multicenter study conducted by Tang et al[5] on the clinical symptoms and psychological status of patients with hepatic hemangiomas.
Short-term follow-up (within 12 months) revealed no recurrence in either group. However, during the 1- to 3-year follow-up period, one case of recurrence was observed in the control group, whereas no recurrence was observed in the observation group. The long-term follow-up imaging (Figure 2) confirms the durability of tumor control achieved with combined therapy. This trend indicates that embolization followed by ablation may help reduce the risk of long-term recurrence, particularly in patients with giant hemangiomas. Gao et al[4] highlighted that TACE for hepatic hemangiomas > 10 cm carries a risk of recurrence, while combined ablation reduces postoperative residue and enhances long-term tumor control. Some patients were evaluated as having a partial response or stable disease after treatment, as their tumors remained active. Additionally, some patients did not receive secondary treatment because of the decision to continue observation. Follow-up remains a reasonable strategy[9,14,29].
Although originally developed for malignancies, the RECIST standard provides valuable utility in assessing the treatment response of benign hepatic hemangiomas, particularly after interventional therapies[17]. RECIST measures changes in tumor diameter, specifically the reduction in the longest diameter, offering a standardized, reproducible method for evaluating therapeutic outcomes. However, RECIST has limitations in the context of benign tumors, such as hepatic hemangiomas, where treatment goals often focus on symptom relief and tumor growth inhibition rather than complete tumor eradication. Beyond RECIST measurements, volumetric assessment offers a potentially more precise method for quantifying the changes in the tumor burden, particularly for hepatic hemangiomas with irregular morpho
This study has several important limitations. First, this was a single-center retrospective cohort study with a limited sample size (n = 82). Although post-hoc power analysis for the primary efficacy endpoint (12-month ORR) demonstrated exceptionally high statistical power (> 0.999) for detecting the observed significant differences, the sample size might still pose limitations for the statistical power of analyses concerning certain secondary endpoints or within specific subgroups. Second, the efficacy assessment of the multiple hepatic hemangiomas focused primarily on the response of the index lesion. This may have incompletely captured precise changes in all secondary lesions and is an inherent challenge when retrospectively analyzing multiple lesions. Future studies may consider more detailed individualized follow-up of all treated lesions in patients with multiple lesions. Third, loss to follow-up was observed, particularly in patients with follow-up periods > 3 years. In addition, considerations of economic impact and healthcare accessibility are also para
In conclusion, TACE combined with MWA for the treatment of hepatic hemangiomas > 5 cm demonstrates superior efficacy compared to TACE alone, as evidenced by a higher tumor reduction ratio, complete response, and long-term local control. This combination therapy also effectively mitigates the risk of hemolytic complications potentially associated with MWA monotherapy, exhibiting an excellent safety and efficacy profile that offers an improved long-term prognosis for patients. Nevertheless, prospective, multicenter randomized controlled trials are warranted to definitively confirm these encouraging outcomes and refine patient selection criteria for this combined therapeutic strategy.
| 1. | European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol. 2016;65:386-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (2)] |
| 2. | Maruyama H, Tobari M, Nagamatsu H, Yamaguchi T, Shiina S. Ablation for Benign Liver Tumors: Current Concepts and Limitations. J Clin Transl Hepatol. 2023;11:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Jinhuan Y, Gang D, Binyao S, Huan M, Bin J. Is laparoscopic hepatectomy suitable for giant hepatic hemangioma larger than 10 cm in diameter? Surg Endosc. 2020;34:1224-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Gao J, Fan RF, Yang JY, Cui Y, Ji JS, Ma KS, Li XL, Zhang L, Xu CL, Kong XL, Ke S, Ding XM, Wang SH, Yang MM, Song JJ, Zhai B, Nin CM, Guo SG, Xin ZH, Lu J, Dong YH, Zhu HQ, Sun WB. Radiofrequency ablation for hepatic hemangiomas: A consensus from a Chinese panel of experts. World J Gastroenterol. 2017;23:7077-7086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Tang T, Wang X, Mao Y, Li J, Wen T, Jia W, Chen Y, Peng T, Liu L, Fan R, Ma K, Xia F. Real-world data on the clinicopathological traits and outcomes of hospitalized liver hemangioma patients: a multicenter study. Ann Transl Med. 2021;9:1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Fei L, Hongsong X. Effectiveness of microwave ablation for the treatment of hepatic hemangioma - meta-analysis and meta-regression. Int J Hyperthermia. 2023;40:2146214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Pompili M, Ardito F, Brunetti E, Cabibbo G, Calliada F, Cillo U, de Sio I, Golfieri R, Grova M, Gruttadauria S, Guido M, Iavarone M, Manciulli T, Pagano D, Pettinari I, Santopaolo F, Soresi M, Colli A. Benign liver lesions 2022: Guideline for clinical practice of Associazione Italiana Studio del Fegato (AISF), Società Italiana di Radiologia Medica e Interventistica (SIRM), Società Italiana di Chirurgia (SIC), Società Italiana di Ultrasonologia in Medicina e Biologia (SIUMB), Associazione Italiana di Chirurgia Epatobilio-Pancreatica (AICEP), Società Italiana Trapianti d'Organo (SITO), Società Italiana di Anatomia Patologica e Citologia Diagnostica (SIAPEC-IAP) - Part II - Solid lesions. Dig Liver Dis. 2022;54:1614-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Tang X, Ding M, Lu B, Chi J, Wang T, Shi Y, Wang Z, Cui D, Li P, Zhai B. Outcomes of ultrasound-guided percutaneous microwave ablation versus surgical resection for symptomatic large hepatic hemangiomas. Int J Hyperthermia. 2019;36:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Kong J, Gao R, Wu S, Shi Y, Yin T, Guo S, Xin Z, Li A, Kong X, Ma D, Zhai B, Sun W, Gao J. Safety and efficacy of microwave versus radiofrequency ablation for large hepatic hemangioma: a multicenter retrospective study with propensity score matching. Eur Radiol. 2022;32:3309-3318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Torkian P, Li J, Kaufman JA, Jahangiri Y. Effectiveness of Transarterial Embolization in Treatment of Symptomatic Hepatic Hemangiomas: Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol. 2021;44:80-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Cai Q, Qian TG, Zhao QY, Feng SY, Yang Q, Luo YC, Dai YQ, Liang P, Yu XL, Liu FY, Han ZY, Du QW, Li X, Yu J. Percutaneous microwave ablation versus sclerotherapy for large hepatic hemangioma: a multi-center cohort study. Int J Hyperthermia. 2024;41:2285705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Shi Y, Song J, Ding M, Tang X, Wang Z, Chi J, Wang T, Ji J, Zhai B. Microwave ablation versus transcatheter arterial embolization for large hepatic hemangiomas: clinical outcomes. Int J Hyperthermia. 2020;37:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Furumaya A, van Rosmalen BV, Takkenberg RB, van Delden OM, Dejong CHC, Verheij J, van Gulik TM. Transarterial (Chemo-)Embolization and Lipiodolization for Hepatic Haemangioma. Cardiovasc Intervent Radiol. 2019;42:800-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Kacała A, Dorochowicz M, Korbecki A, Sobański M, Puła M, Patrzałek D, Janczak D, Guziński M. Transarterial Bleomycin-Lipiodol Chemoembolization for the Treatment of Giant Hepatic Hemangiomas: An Assessment of Effectiveness. Cancers (Basel). 2024;16:380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Brandt JP, Gerriets V. Bleomycin. 2023 Aug 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 16. | Ayoobi Yazdi N, Mehrabinejad MM, Dashti H, Pourghorban R, Nassiri Toosi M, Rokni Yazdi H. Percutaneous Sclerotherapy with Bleomycin and Ethiodized Oil: A Promising Treatment in Symptomatic Giant Liver Hemangioma. Radiology. 2021;301:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21529] [Article Influence: 1345.6] [Reference Citation Analysis (1)] |
| 18. | Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21545] [Cited by in RCA: 28664] [Article Influence: 1194.3] [Reference Citation Analysis (0)] |
| 19. | Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11947] [Cited by in RCA: 18674] [Article Influence: 982.8] [Reference Citation Analysis (0)] |
| 20. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23352] [Cited by in RCA: 23946] [Article Influence: 725.6] [Reference Citation Analysis (0)] |
| 21. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8559] [Article Influence: 534.9] [Reference Citation Analysis (0)] |
| 22. | Wu S, Gao R, Yin T, Zhu R, Guo S, Xin Z, Li A, Kong X, Gao J, Sun W. Complications of Radiofrequency Ablation for Hepatic Hemangioma: A Multicenter Retrospective Analysis on 291 Cases. Front Oncol. 2021;11:706619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Hui TC, Kwan J, Pua U. Advanced Techniques in the Percutaneous Ablation of Liver Tumours. Diagnostics (Basel). 2021;11:585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (1)] |
| 25. | Mathew RP, Sam M, Raubenheimer M, Patel V, Low G. Hepatic hemangiomas: the various imaging avatars and its mimickers. Radiol Med. 2020;125:801-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Wu XK, Yang LF, Chen YF, Chen ZW, Lu H, Shen XY, Chi MH, Wang L, Zhang H, Chen JF, Huang JY, Zeng YY, Yan ML, Zhang ZB. Transcatheter arterial chemoembolisation combined with lenvatinib plus camrelizumab as conversion therapy for unresectable hepatocellular carcinoma: a single-arm, multicentre, prospective study. EClinicalMedicine. 2024;67:102367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 27. | Dong W, Qiu B, Xu H, He L. Invasive management of symptomatic hepatic hemangioma. Eur J Gastroenterol Hepatol. 2019;31:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Li X, An C, Liu F, Cheng Z, Han Z, Yu X, Dong L, Yu J, Liang P. The value of 3D visualization operative planning system in ultrasound-guided percutaneous microwave ablation for large hepatic hemangiomas: a clinical comparative study. BMC Cancer. 2019;19:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Aziz H, Brown ZJ, Baghdadi A, Kamel IR, Pawlik TM. A Comprehensive Review of Hepatic Hemangioma Management. J Gastrointest Surg. 2022;26:1998-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3287] [Article Influence: 219.1] [Reference Citation Analysis (36)] |