Published online Jun 28, 2025. doi: 10.3748/wjg.v31.i24.106440
Revised: April 12, 2025
Accepted: June 5, 2025
Published online: June 28, 2025
Processing time: 120 Days and 11.3 Hours
Esophageal gastrointestinal stromal tumors (GISTs) are exceedingly rare, often detected incidentally due to their asymptomatic nature. Historically, esophagec
Core Tip: Endoscopic resection (ER) is emerging as a minimally invasive alternative to traditional surgery for esophageal gastrointestinal stromal tumors (GISTs), particularly for tumors ≤ 4 cm with low mitotic activity. The study by Xu et al provides compelling evidence supporting ER’s high en bloc resection rate and favorable long-term outcomes. However, critical questions remain regarding patient selection criteria, the oncologic safety of R1 resections, and the role of adjuvant therapy. Further prospective studies are needed to define ER’s place in the treatment algorithm and optimize management strategies for esophageal GISTs.
- Citation: Semash K, Dzhanbekov T. Redefining the treatment paradigm for esophageal gastrointestinal stromal tumors: The emerging role of endoscopic resection. World J Gastroenterol 2025; 31(24): 106440
- URL: https://www.wjgnet.com/1007-9327/full/v31/i24/106440.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i24.106440
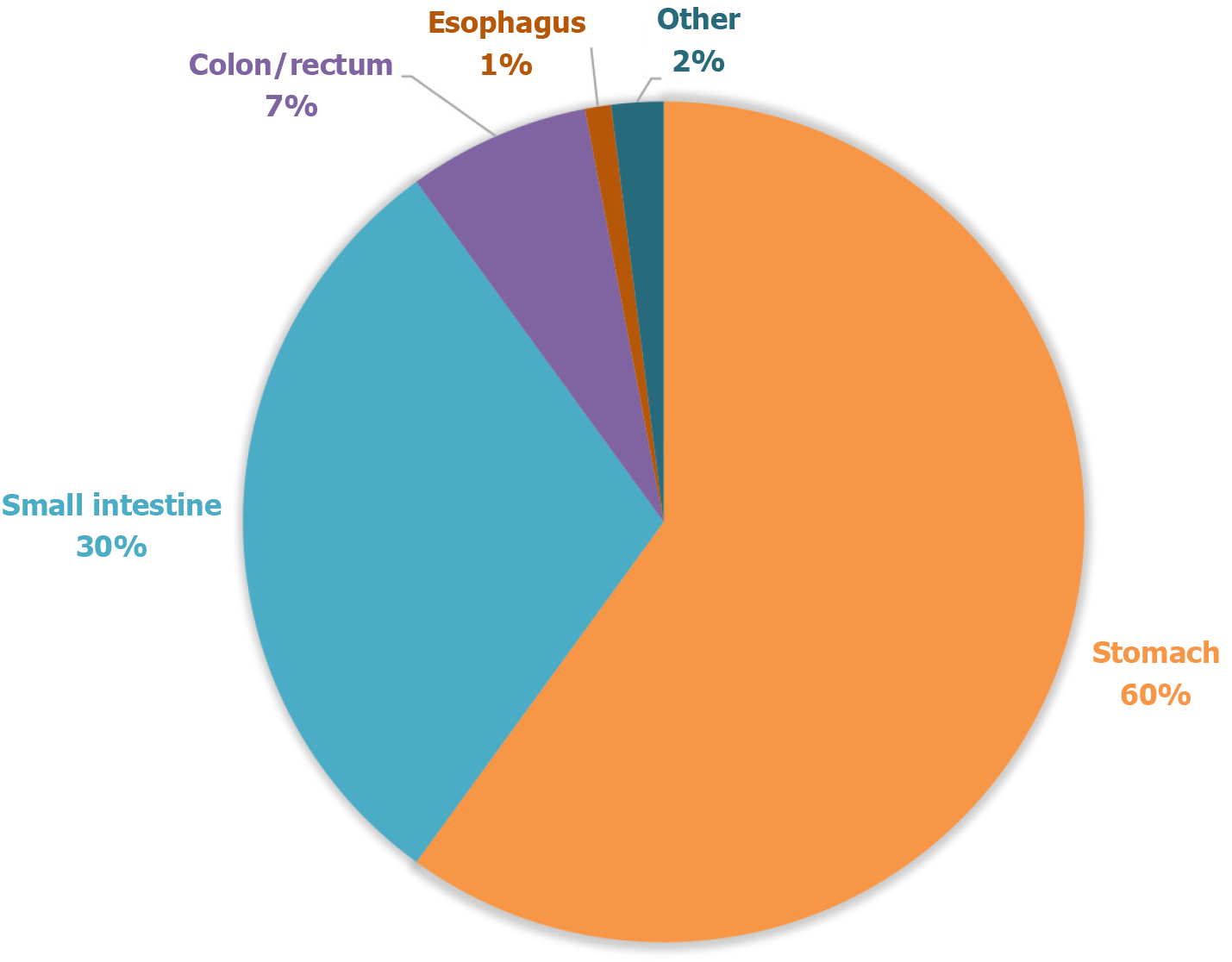
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal neoplasms, with an incidence of 10-15 cases per million population. The most common primary sites include the stomach (50%) and small intestine (30%), while esophageal GISTs remain extremely rare, comprising less than 1% of all cases[1] (Figure 1). The pathogenesis is predominantly driven by mutations in the c-KIT gene (80%) or platelet-derived growth factor receptor alpha (PDGFRA) (10%), with metastatic progression primarily affecting the liver and peritoneum[2].
Since 2020, esophageal GISTs have been staged according to the 8th edition of the tumor-node-metastasis (TNM) classification system. The primary prognostic factors include mitotic index, tumor size, and localization. According to the 2020 World Health Organization classification, all GISTs are considered potentially malignant mesenchymal tumors, with their biological behavior varying depending on location, size, and mitotic activity[3].
Histopathological features, particularly the mitotic index, remain critical in risk stratification. Tumors with a mitotic index of fewer than 5 mitoses per 50 high-power fields (HPFs) are considered low risk, whereas those exceeding this threshold are classified as high risk[1]. The molecular landscape of GISTs continues to evolve, with emerging evidence of succinate dehydrogenase-deficient subtypes exhibiting distinct biological behavior and therapeutic responses[1,3].
The primary diagnostic approach for GIST involves endoscopic examination. Tumor size serves as a key criterion for determining treatment strategy; thus, tumor diameter measurement is routinely performed[4]. Submucosal tumors smaller than 2 cm, exhibiting a hemispherical shape, smooth contours, and the absence of ulceration or depression, should be monitored once or twice per year. By contrast, lesions exceeding 2 cm in diameter and displaying irregular margins, ulceration, or depression require further evaluation. The second-line diagnostic approach consists of radiolo
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) provides sufficient tissue samples for diagnosis with minimal complications[6,7]. Thus, a definitive diagnosis of GIST can almost always be established through EUS-FNA combined with immunohistochemical staining. Immunohistochemical staining for cluster of differentiation 117 (KIT) and Discovered on GIST 1 (DOG1) remains the gold standard for diagnosis, with molecular genetic testing further aiding in cases of KIT and PDGFRA-negative tumors[1,8,9].
The primary treatment modality for localized and locally advanced forms of GISTs is surgical resection, whereas targeted therapy is the standard approach for recurrent or metastatic disease.
For small tumors (< 1-2 cm), active surveillance may be considered based on the clinical presentation. However, the potential risk of progression should be carefully evaluated, taking into account morphological findings and risk factors, particularly in cases where surgical intervention is not pursued. A study conducted by Polkowski et al[7] reported that subepithelial tumors exhibit a malignant potential of approximately 13%. For tumors larger than 2 cm, surgical resection is indicated. Limited (organ-preserving) resections with a 1-2 cm margin from the tumor edge are considered appropriate. Lymphadenectomy is only recommended in cases of succinate dehydrogenase-deficient GISTs; in other cases, lymph node dissection is generally not indicated[8]. For tumors located in the esophagus, gastroesophageal junction, duodenum, or rectum, preoperative therapy is recommended to reduce tumor size, thereby increasing the feasibility of organ-preserving surgery.
For tumors with uncertain resectability, neoadjuvant therapy with imatinib is recommended for 6-12 months. Prior to initiating treatment, molecular genetic testing should be performed to exclude the presence of the D842V mutation[9]. Adjuvant therapy with imatinib (400 mg/day for 3 years) is the standard treatment for patients at high risk of recurrence. In cases of KIT exon 9 mutations, higher-dose adjuvant imatinib (800 mg/day for 3 years) may be considered. Conversely, GISTs with the PDGFRA exon 18 D842V mutation should not be treated with adjuvant therapy[8,9]. Patients at very high risk of recurrence due to tumor rupture during surgery should be considered for adjuvant imatinib therapy[8].
Surgical resection remains the cornerstone of GIST treatment[10]. While traditional open surgery has historically been the standard, minimally invasive techniques, particularly endoscopic and thoracoscopic approaches, are increasingly favored due to their reduced morbidity and faster recovery times. Thoracoscopic and robotic-assisted resections are preferred for tumors larger than 5 cm or those located in challenging anatomical sites[8]. These approaches offer enhanced precision and reduced surgical trauma, making them viable alternatives to open surgery. Nevertheless, the thoracoscopic or robotic approach is not recommended for patients with large tumors due to the risk of tumor rupture, which is strongly associated with a significantly increased risk of recurrence[11]. Additionally, thoracoscopic surgery is not recommended for GISTs larger than 8 cm, as there is insufficient evidence demonstrating its superiority over open surgery for tumors of this size[10]. Nevertheless, there have been reports of successful treatment of large esophageal tumors using a thoracoscopic approach, followed by targeted therapy[12]. Hybrid techniques, such as laparoscopic-endoscopic cooperative surgery (LECS), further expand the feasibility of minimally invasive interventions by integrating the strengths of both modalities[13].
The treatment strategy for esophageal GISTs is determined on a case-by-case basis. Subtotal esophagectomy improves R0 resection rates; however, it involves major surgical intervention, posing risks to organ function and postoperative quality of life. In contrast, local resection is a minimally invasive approach, though concerns exist regarding potential tumor spread into the thoracic cavity[10,14].
Large tumors can cause significant defects in the muscular layer, leading to postoperative esophageal stenosis, dysfunction, and mucosal injury. Notably, successful enucleation using thoracoscopy or robotic-assisted techniques has been reported in cases of small tumors. However, endophytically growing tumors are often challenging to identify from the thoracic cavity and are not suitable for enucleation[15].
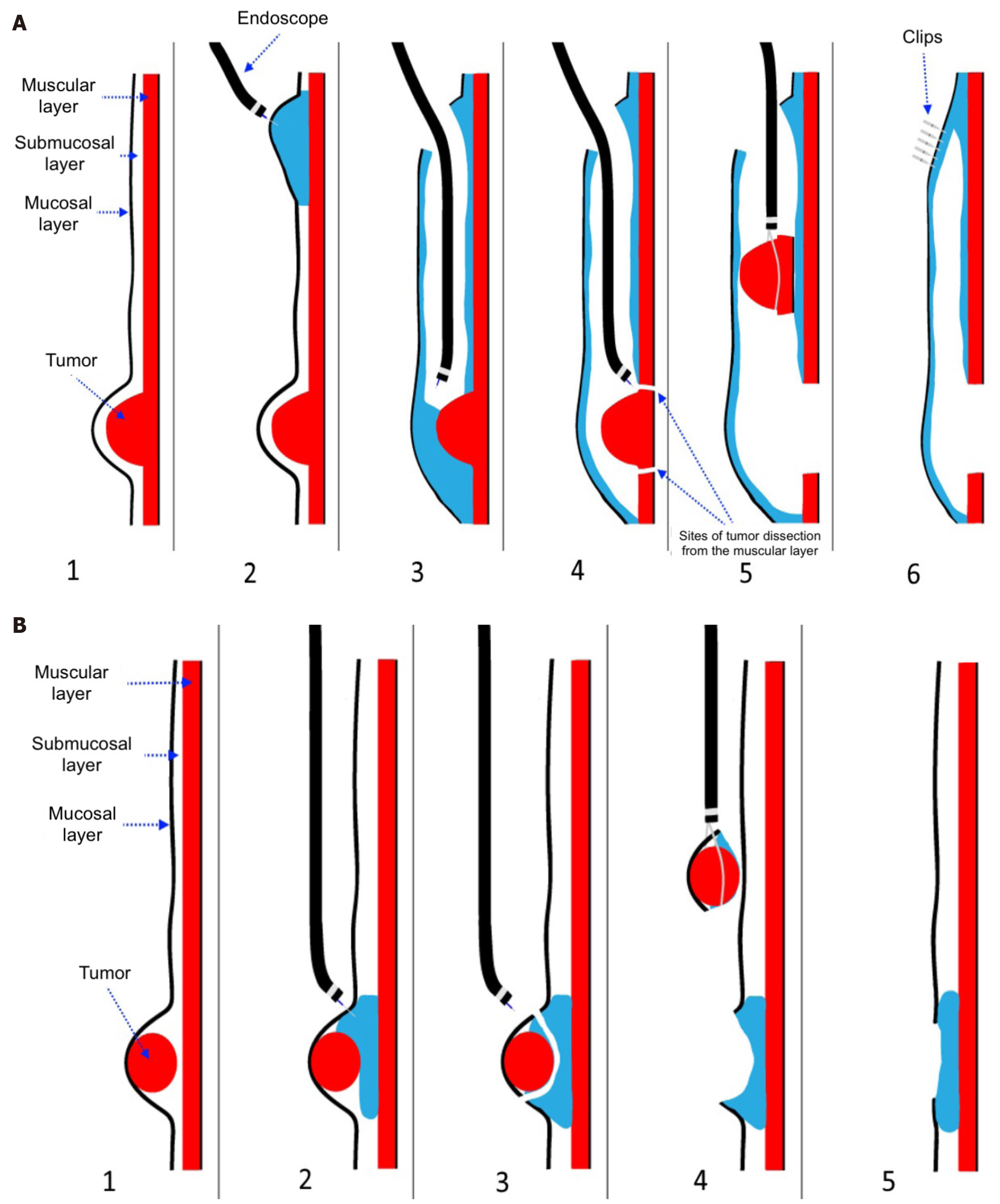
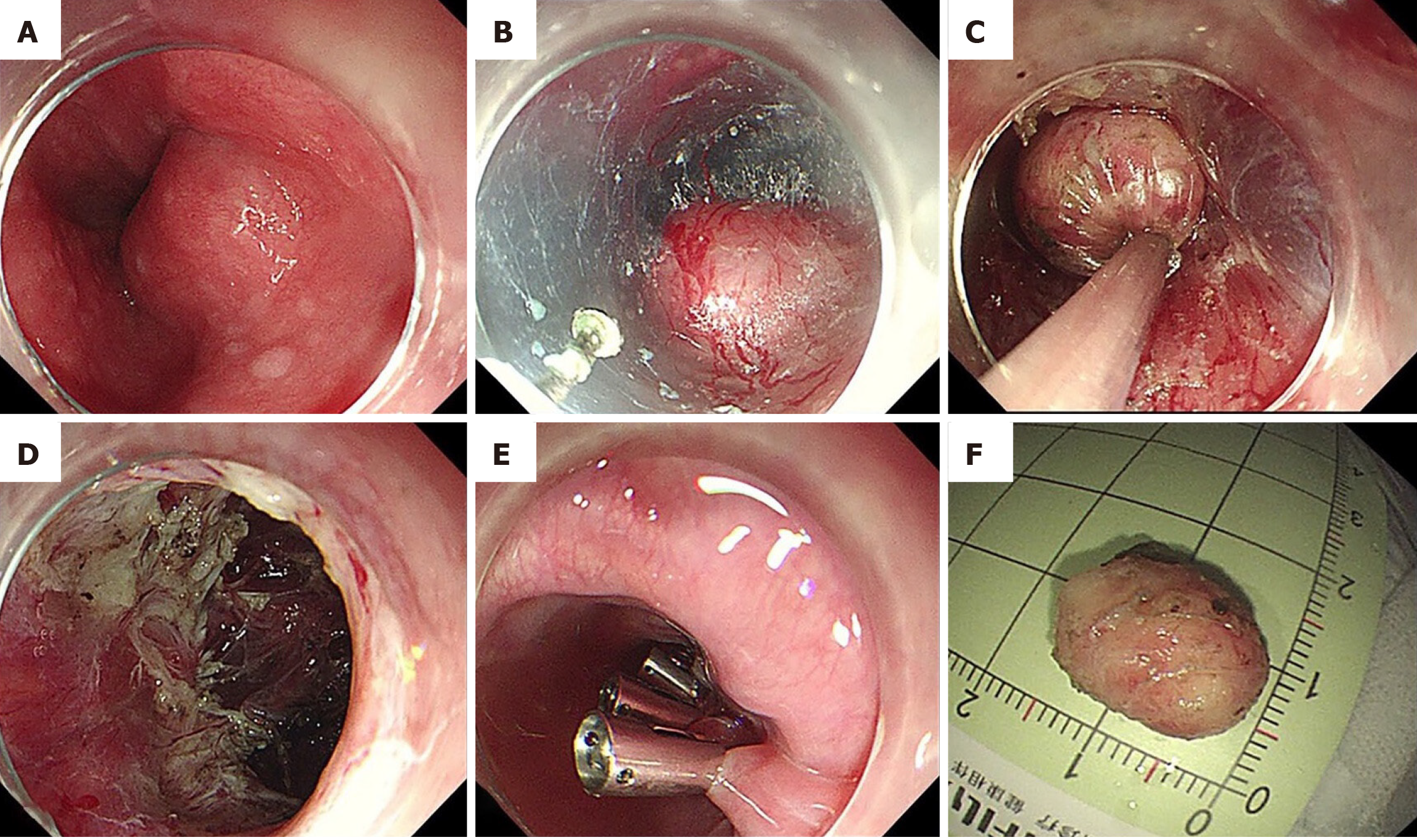
Recent studies have compared endoscopic and laparoscopic resections, highlighting the advantages of endoscopic techniques in select cases[16]. Endoscopic resection (ER) techniques, such as submucosal tunneling ER (STER) and endoscopic full-thickness resection (EFTR), have demonstrated promising results in the treatment of small GISTs, particularly in the upper gastrointestinal tract[10]. STER allows for en bloc resection of subepithelial lesions, minimizing the risk of tumor rupture and preserving organ function[17]. EFTR, which involves full-thickness resection with subsequent endoscopic closure, is particularly effective for GISTs confined to the muscularis propria. Both approaches provide oncologically sound resections with reduced postoperative complications compared to traditional surgery. The technical schemes of STER and EFTR are presented in Figure 2[18]. A comparative overview of surgical and ER approaches for esophageal GISTs is provided in Table 1, highlighting differences in invasiveness, organ preservation, complication risks, and clinical indications. Step-by-step execution of the STER technique, as applied in esophageal GIST resection, is illu
| Parameter | Surgical resection/esophagectomy | Endoscopic resection (STER/EFTR) |
| Invasiveness | High | Low |
| Organ preservation | Not always | Yes |
| Risk of perforation or rupture | Moderate to high | Low to moderate |
| R0 resection rate | High (especially with esophagectomy) | Moderate (especially in tumors > 4 cm) |
| Hospital stay | 7-14 days | 2-5 days |
| Tumor size indication | Often > 4 cm | Up to 4 cm (up to 5 cm in selected STER cases) |
| Possibility of surgical re-treatment | Limited | Feasible |
A study by Kahaleh et al[16] demonstrated comparable oncologic outcomes among STER, EFTR, and LECS, with reduced hospital stays and fewer complications in endoscopically treated patients. Similarly, a retrospective analysis by Lian et al[19] supported the use of STER for esophageal GISTs smaller than 4 cm, emphasizing its safety and efficacy[19]. Chen et al[20] compared ER with enucleation and concluded that STER is a less invasive approach, offering a shorter procedure time and reduced hospital stay compared to thoracic enucleation[20].
For metastatic or recurrent GIST, the National Comprehensive Cancer Network clinical guidelines recommend resection for gastric GISTs smaller than 2 cm if they exhibit high-risk features[20]. All clinical guidelines advocate for surgical intervention in non-gastric GISTs, even if their size is less than 2 cm[3,8,21-23]. Regarding the feasibility of laparoscopic surgery for tumors ≥ 5 cm, the authors provide a positive recommendation. Simple local resection is consistent with minimally invasive surgical principles, and careful handling to prevent tumor rupture is crucial for both open and laparoscopic approaches. However, there is no clear evidence defining a tumor size threshold for safe laparoscopic resection without compromising tumor integrity. Given the lack of evidence supporting systematic lymphadenectomy, local resection of the primary organ without lymph node dissection is the standard surgical approach for primary GISTs.
Highly invasive procedures, such as esophagectomy, may negatively impact postoperative quality of life and should be avoided whenever possible. The neoadjuvant therapy is recommended with imatinib for large GISTs (≥ 10 cm) and in cases where an incomplete resection is suspected. However, the long-term prognosis following adjuvant imatinib therapy in cases of tumor rupture remains unknown[23].
In 2025, Xu et al[24] published a study reporting the outcomes of treating esophageal GISTs using ER. ER techniques have demonstrated promising clinical results in selected patients. In the multicenter study by Xu et al[24], the en bloc resection rate was 96.9%, with an R0 resection rate of 75.0% and an R1 rate of 25.0%, none of which resulted in local recurrence over the follow-up period. The reported complication rate was 12.5%, including minor bleeding and transient perforation, all managed conservatively without conversion to surgery. The median hospital stay was 5.3 days, under
An essential component of esophageal GIST management is accurate risk stratification. The study utilized the modified National Institutes of Health criteria[25], categorizing patients into very low, low, intermediate, and high-risk groups. Notably, the majority of tumors in this cohort were classified as low or very low risk, reinforcing the viability of ER for carefully selected patients. The low recurrence rate observed suggests that complete resection, even with R1 margins in some cases, does not necessarily compromise long-term outcomes. This challenges conventional surgical dogma, which often prioritizes wide-margin resection.
Authors report that most tumors were detected incidentally (87.5%), indicating that early diagnosis plays a pivotal role in optimizing prognosis. Given that esophageal GISTs may remain asymptomatic until they reach a significant size, routine endoscopic surveillance in high-risk populations could facilitate early intervention. The potential for slow tumor progression and low mitotic activity in many esophageal GISTs suggests that timely ER may obviate the need for radical surgery in select patients[25].
Mai et al[26] reported the first successful case of curative cryoablation for an esophageal GIST, demonstrating the potential of this minimally invasive technique as an alternative to surgery. However, the conclusions were limited to a single observation, and further studies are required to assess its oncologic efficacy and safety.
Despite the promising results of ER for esophageal GISTs, several critical limitations must be addressed before this technique can be universally adopted as a standard treatment. The study by Xu et al[24] provides valuable insights into the feasibility and safety of ER; however, certain unresolved issues remain that warrant further investigation.
One of the primary limitations is the tumor size threshold for ER feasibility. The study suggests that tumors larger than 4 cm have a higher probability of incomplete (R1) resection and an increased recurrence rate. While ER has demonstrated high efficacy for tumors ≤ 4 cm[26,27], there is still insufficient evidence regarding its oncological safety in larger tumors. Future studies should focus on defining the upper size limit for ER and evaluating whether neoadjuvant therapy with tyrosine kinase inhibitors (TKIs) such as imatinib can reduce tumor size preoperatively, thereby facilitating R0 resection.
Although formal guidelines for selecting candidates for ER in esophageal GISTs are lacking, preliminary criteria can be inferred from published cohorts. Based on the study by Xu et al[24], ideal candidates for ER may include patients with tumors < 3 cm in diameter, a low mitotic index (< 5 mitoses per 50 HPF), and growth confined to the muscularis propria without evidence of ulceration or mucosal involvement[24]. Lesions located in the distal or middle third of the esopha
Neoadjuvant TKI therapy, particularly with imatinib, has been shown to reduce tumor size and facilitate organ-preserving surgery in large or borderline resectable GISTs, especially in the stomach and duodenum. Typical treatment duration ranges from 6 to 12 months, guided by radiologic and clinical response. Studies have demonstrated that neoadjuvant imatinib can improve R0 resection rates and reduce the need for extensive surgery without compromising oncologic outcomes[28]. While data on its use in esophageal GISTs are lacking, extrapolation from other locations suggests that preoperative TKI therapy may be a useful strategy in selected patients with large lesions considered borderline candidates for ER.
The long-term oncological outcomes of ER remain uncertain, given the relatively small sample size and the retrospective nature of available studies[29]. At the same time, numerous isolated reports of endoscopic treatment, including the use of adjuvant therapy, continue to appear in the literature[30]. While Xu et al[24] reported a 5-year disease-free survival rate of 90.6%, the follow-up period might not be sufficient to assess late recurrences, which are not uncommon in GISTs. Prospective, multicenter trials with extended follow-up are necessary to confirm whether ER can achieve recurrence-free survival comparable to surgical resection, particularly in intermediate- and high-risk patients.
The prognostic significance of R1 resection in ER is another unresolved issue. Unlike traditional surgical resection, where R1 status is associated with poor outcomes, the impact of microscopic residual tumor after ER remains unclear. Xu et al[24] reported an R0 resection rate of 75%, leaving 25% of patients with R1 resections. However, the recurrence rate in these cases was not significantly higher than in R0 resections. This raises the question of whether R1 margins in ER should be managed differently than in surgery. Further research should investigate whether adjuvant therapy is necessary for patients with R1 resections or whether close endoscopic surveillance alone is sufficient.
In addition to oncologic outcomes, functional results following ER are an important consideration. Several studies have reported favorable postoperative functional outcomes, including preserved esophageal motility, low rates of dysphagia, and better quality of life compared to surgical resection[19,20]. These aspects further support the use of ER in anatomically and biologically favorable cases.
In gastric GISTs, multiple studies have demonstrated that R1 margins do not significantly impact recurrence or survival, particularly when adjuvant therapy is administered in high-risk cases[31]. Similarly, data from rectal GISTs suggest that a microscopic margin involvement may be acceptable in the context of complete gross resection and favorable tumor biology. Whether these findings are applicable to esophageal tumors is not yet established, highlighting the need for site-specific data and long-term follow-up.
The role of adjuvant therapy after ER is yet to be determined. In the study by Xu et al[24], only two patients received adjuvant imatinib, making it difficult to assess its impact on recurrence prevention. While TKIs are widely used in adjuvant settings following surgical resection of high-risk GISTs[9], their utility after ER remains to be evaluated. Future research should identify specific risk factors that would indicate a benefit from adjuvant therapy in patients undergoing ER.
Technical advancements in ER methods require further exploration. The study highlights that STER is the preferred approach for small esophageal GISTs, as it minimizes mucosal injury and reduces complications such as strictures. However, for tumors originating from the deeper layers, endoscopic full-thickness resection may be a viable alternative[18]. Additional comparative studies are needed to determine which ER techniques yield the best oncological and functional outcomes.
Future research should focus on developing precise criteria for patient selection. While low- and very-low-risk GISTs appear to be ideal candidates for ER, the potential extension of indications to include intermediate-risk tumors remains an open question. Incorporating molecular profiling into the risk stratification of esophageal GISTs may further refine treatment algorithms and improve patient outcomes[1]. Also, future prospective, multicenter randomized controlled trials are warranted to evaluate the long-term oncologic efficacy and safety of ER techniques compared to surgical resection. Study designs comparing patient selection criteria or recurrence rates following R1 vs R0 resection would be particularly informative. In addition, advances in molecular oncology suggest that gene-based therapies targeting TP53 and CDH1 mutations, as explored in gastric cancer, may eventually offer novel therapeutic options for upper gas
In summary, while ER has emerged as a promising minimally invasive approach for esophageal GISTs, its limitations necessitate further research. Large-scale prospective studies, longer follow-up periods, and investigations into the role of neoadjuvant and adjuvant therapies will be crucial in establishing ER as a definitive treatment option for a broader range of patients.
ER has emerged as a viable, minimally invasive alternative to surgery for selected patients with esophageal GIST, particularly those ≤ 4 cm with low mitotic activity. It offers advantages such as organ preservation, reduced morbidity, and shorter recovery times. However, long-term oncologic outcomes, the significance of R1 resections, and the role of adjuvant therapy remain uncertain. Further prospective studies are needed to refine patient selection criteria, optimize treatment strategies, and establish ER as a definitive standard for managing esophageal GISTs.
| 1. | Kang G, Kang Y, Kim KH, Ha SY, Kim JY, Shim YM, Heinrich MC, Kim KM, Corless CL. Gastrointestinal stromal tumours of the oesophagus: a clinicopathological and molecular analysis of 27 cases. Histopathology. 2017;71:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Serrano C, Martín-Broto J, Asencio-Pascual JM, López-Guerrero JA, Rubió-Casadevall J, Bagué S, García-Del-Muro X, Fernández-Hernández JÁ, Herrero L, López-Pousa A, Poveda A, Martínez-Marín V. 2023 GEIS Guidelines for gastrointestinal stromal tumors. Ther Adv Med Oncol. 2023;15:17588359231192388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 3. | Hirota S, Tateishi U, Nakamoto Y, Yamamoto H, Sakurai S, Kikuchi H, Kanda T, Kurokawa Y, Cho H, Nishida T, Sawaki A, Ozaka M, Komatsu Y, Naito Y, Honma Y, Takahashi F, Hashimoto H, Udo M, Araki M, Nishidate S; Members of the Systematic Review Team of the Present Guidelines. English version of Japanese Clinical Practice Guidelines 2022 for gastrointestinal stromal tumor (GIST) issued by the Japan Society of Clinical Oncology. Int J Clin Oncol. 2024;29:647-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-41; quiz S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 5. | Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, Podoloff D. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17-21. [PubMed] |
| 6. | Hedenström P, Marschall HU, Nilsson B, Demir A, Lindkvist B, Nilsson O, Sadik R. High clinical impact and diagnostic accuracy of EUS-guided biopsy sampling of subepithelial lesions: a prospective, comparative study. Surg Endosc. 2018;32:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 7. | Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brodowicz T, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dufresne A, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Miah AB, Mir O, Montemurro M, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss SJ, Hall KS, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Gronchi A, Stacchiotti S; ESMO Guidelines Committee, EURACAN and GENTURIS. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 324] [Article Influence: 108.0] [Reference Citation Analysis (1)] |
| 9. | De Pasqual CA, Hetoja S, Gervasi MC, de Manzoni G. Esophageal gastrointestinal stromal tumors: a literature review. Gastrointest Stromal Tumor. 2023;6:7-7. [DOI] [Full Text] |
| 10. | Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 11. | Hiki N, Nunobe S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications. Ann Gastroenterol Surg. 2019;3:239-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 12. | Kanoda R, Kikuchi T, Utsumi A, Mochizuki S, Matsuishi A, Kaneta A, Nirei A, Hanayama H, Saze Z, Hikichi T, Hashimoto Y, Kono K. Thoracoscopic and endoscopic cooperative surgery for esophageal gastrointestinal stromal tumor: a case report. Surg Case Rep. 2024;10:237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Kurosaki T, Hoshino I, Kuwayama N, Suitou H, Kano M, Tonooka T, Chiba S, Soda H, Nabeya Y, Takayama W. Large esophageal gastrointestinal stromal tumors resected thoracoscopically after oral imatinib therapy: a report of two cases. Clin J Gastroenterol. 2023;16:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Mohammadi M, IJzerman NS, Hohenberger P, Rutkowski P, Jones RL, Martin-Broto J, Gronchi A, Schöffski P, Vassos N, Farag S, Baia M, Oosten AW, Steeghs N, Desar IME, Reyners AKL, van Sandick JW, Bastiaannet E, Gelderblom H, Schrage Y. Clinicopathological features and treatment outcome of oesophageal gastrointestinal stromal tumour (GIST): A large, retrospective multicenter European study. Eur J Surg Oncol. 2021;47:2173-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Andalib I, Yeoun D, Reddy R, Xie S, Iqbal S. Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: methods and feasibility data. Surg Endosc. 2018;32:1787-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Kahaleh M, Bhagat V, Dellatore P, Tyberg A, Sarkar A, Shahid HM, Andalib I, Alkhiari R, Gaidhane M, Kedia P, Nieto J, Kumta NA, Dixon RE, Salameh H, Mavrogenis G, Bassioukas S, Abe S, Arentes VN, Morita FH, Sakai P, de Moura EG. Subepithelial tumors: How does endoscopic full-thickness resection & submucosal tunneling with endoscopic resection compare with laparoscopic endoscopic cooperative surgery? Endosc Int Open. 2022;10:E1491-E1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Manta R, Zito FP, Pugliese F, Caruso A, Mangiafico S, D'Alessandro A, Castellani D, Germani U, Mutignani M, Conigliaro RL, Bonetti LR, Matsuda T, De Francesco V, Zullo A, Galloro G. Endoscopic Submucosal Dissection for Subepithelial Tumor Treatment in the Upper Digestive Tract: A Western, Multicenter Study. GE Port J Gastroenterol. 2023;30:115-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 18. | Smirnov AA, Burakov AN, Blinov EV, Saadulaeva MM, Semenikhin KD, Prudnikov AV, Dvoretskii SI, Kiriltseva MM, Bagnenko SV. The experience of endoscopic resection of benign tumors of the esophagus. Grekov's Bull Surg. 2018;177:40-44. [DOI] [Full Text] |
| 19. | Lian J, Ji Y, Chen T, Wang G, Wang M, Li S, Cao J, Shen L, Lu W, Xu M. Endoscopic resection for esophageal gastrointestinal stromal tumors: a multi-center feasibility study. Therap Adv Gastroenterol. 2024;17:17562848241255304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Chen T, Lin ZW, Zhang YQ, Chen WF, Zhong YS, Wang Q, Yao LQ, Zhou PH, Xu MD. Submucosal Tunneling Endoscopic Resection vs Thoracoscopic Enucleation for Large Submucosal Tumors in the Esophagus and the Esophagogastric Junction. J Am Coll Surg. 2017;225:806-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | von Mehren M, Kane JM, Riedel RF, Sicklick JK, Pollack SM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, Holder A, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Mesko NW, Meyer C, Pappo AS, Parkes AM, Petersen IA, Poppe M, Schuetze S, Shabason J, Spraker MB, Zimel M, Bergman MA, Sundar H, Hang LE. NCCN Guidelines® Insights: Gastrointestinal Stromal Tumors, Version 2.2022. J Natl Compr Canc Netw. 2022;20:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 22. | Khan J, Ullah A, Waheed A, Karki NR, Adhikari N, Vemavarapu L, Belakhlef S, Bendjemil SM, Mehdizadeh Seraj S, Sidhwa F, Ghleilib I, Foroutan S, Blakely AM, Del Rivero J, Karim NA, Vail E, Heneidi S, Mesa H. Gastrointestinal Stromal Tumors (GIST): A Population-Based Study Using the SEER Database, including Management and Recent Advances in Targeted Therapy. Cancers (Basel). 2022;14:3689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A, Hirota S, Zheng J, Zhang B, Tzen CY, Yeh CN, Nishida T, Shen L, Chen LT, Kang YK. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat. 2016;48:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 24. | Xu EP, Qi ZP, Zhang JW, Li B, Ren Z, Cai MY, Cai SL, Lv ZT, Chen ZH, Liu JY, Zhong YS, Zhou PH, Shi Q. Endoscopic treatment outcome of oesophageal gastrointestinal stromal tumours. World J Gastroenterol. 2025;31:102393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (4)] |
| 25. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 864] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 26. | Mai D, Hashimoto R, Yu A, Torralba EJ, Tran E, El-Hage Chehade N, Lee DP, Samarasena J. Successful Curative Cryoablation of an Esophageal Gastrointestinal Stromal Tumor. ACG Case Rep J. 2019;6:e00076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Yue L, Sun Y, Wang X, Hu W. Advances of endoscopic and surgical management in gastrointestinal stromal tumors. Front Surg. 2023;10:1092997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Kurokawa Y, Yang HK, Cho H, Ryu MH, Masuzawa T, Park SR, Matsumoto S, Lee HJ, Honda H, Kwon OK, Ishikawa T, Lee KH, Nabeshima K, Kong SH, Shimokawa T, Yook JH, Doki Y, Im SA, Hirota S, Hahn S, Nishida T, Kang YK. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer. 2017;117:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Pence K, Correa AM, Chan E, Khaitan P, Hofstetter W, Kim MP. Management of esophageal gastrointestinal stromal tumor: review of one hundred seven patients. Dis Esophagus. 2017;30:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Doan TT, Pham DT, Nguyen CV, Tran TT, Nguyen HV. Successfully treated esophageal gastrointestinal stromal tumor by minimally invasive esophagectomy followed by imatinib therapy: a case report. Ann Med Surg (Lond). 2025;87:939-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Judson I, Bulusu R, Seddon B, Dangoor A, Wong N, Mudan S. UK clinical practice guidelines for the management of gastrointestinal stromal tumours (GIST). Clin Sarcoma Res. 2017;7:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Cai HQ, Zhang LY, Fu LM, Xu B, Jiao Y. Mutational landscape of TP53 and CDH1 in gastric cancer. World J Gastrointest Surg. 2024;16:276-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |