Published online Jun 7, 2025. doi: 10.3748/wjg.v31.i21.107395
Revised: April 11, 2025
Accepted: May 23, 2025
Published online: June 7, 2025
Processing time: 76 Days and 0.1 Hours
Diabetic gastroparesis (DGP), characterized by delayed gastric emptying and impaired motility, poses significant therapeutic challenges due to its complex neural and molecular pathophysiology. Emerging evidence suggests that electroa
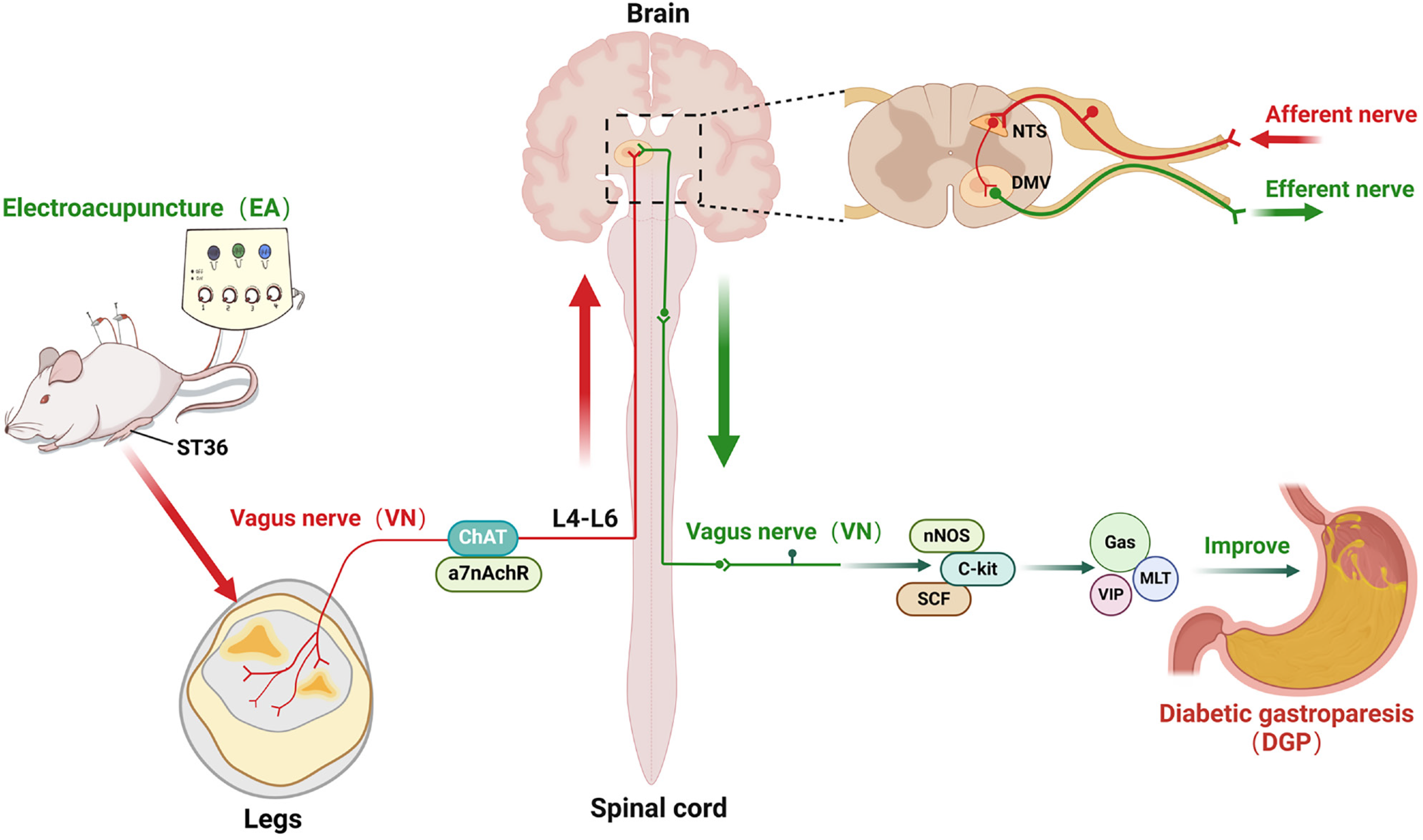
To elucidate the neural mechanisms underlying EA at ST36 improving DGP gastric motility through the nucleus tractus solitarius (NTS)-vagal axis.
The DGP model was established via a single high-dose intraperitoneal injection of 2% streptozotocin combined with an 8-week high-sugar/high-fat diet. Interven
The study found that EA significantly increased the rate of gastric emptying, restored the slow-wave rhythms of the stomach, and improved the architecture of the smooth muscles in the stomach. This was evidenced by a reduction in inflammatory infiltration and an increase in the expression of nNOS, C-kit, and SCF. Mechanistically, EA activated vagal targets (ChAT and α7nAChR) at ST36, transmitting signals via spinal segments L4-L6 to the NTS, subsequently regulating gastrointestinal peptides (Gas, MLT, VIP) and restoring interstitial cells of Cajal (ICCs) function via subdiaphragmatic vagal efferent pathways. It is crucial to note that subdiaphragmatic vagotomy led to the abrogation of EA-induced enhancements in gastric motility and ICC recovery, thereby confirming the indispensable role of vagal efferent signalling.
EA provides a novel molecular mechanism for improving gastrointestinal motility in DGP via a peripheral stimulation (ST36), spinal afferent (L4-L6), brainstem integration (NTS), vagal efferent (gastric) circuit.
Core Tip: Multimodal validation: Electroacupuncture (EA) significantly enhanced gastric emptying (validated by positron emission tomography-computed tomography), restored gastric slow-wave rhythms, and improved smooth muscle architecture via upregulation of neuronal nitric oxide synthase, cluster of differentiation 117, stem cell factor. Mechanistic insight: EA activates cholinergic targets (choline acetyltransferas/α7 nicotinic acetylcholine receptor) at ST36, transmits signals via spinal L4-L6 afferents to the nucleus tractus solitarius, and modulates gastrointestinal peptides (ghrelin, motilin, vasoactive intestinal peptide) through subdiaphragmatic vagal efferent, ultimately restoring interstitial cells of Cajal function. Translational relevance: Subdiaphragmatic vagotomy abolished EA’s therapeutic effects, unequivocally establishing vagal efferent signaling as indispensable.
- Citation: Zhang Y, Tang YW, Zhou J, Wei YR, Peng YT, Yan Z, Yue ZH. Electroacupuncture at ST36 ameliorates gastric dysmotility in rats with diabetic gastroparesis via the nucleus tractus solitarius-vagal axis. World J Gastroenterol 2025; 31(21): 107395
- URL: https://www.wjgnet.com/1007-9327/full/v31/i21/107395.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i21.107395
In 1958, Kassander[1] was the first to describe the association between diabetes and delayed gastric emptying, and he coined the term ‘diabetic gastroparesis’ (DGP). DGP has been observed to affect 50%-76% of individuals diagnosed with diabetes, with approximately 10% of these cases manifesting overt clinical symptoms, including nausea, vomiting, constipation, diarrhoea, early satiety and dyspepsia, often exacerbated by dietary triggers[2]. The multifactorial pathogenesis of DGP involves dysregulation of the autonomic and enteric nervous systems, smooth muscle dysfunction, abnormalities in interstitial cells of Cajal (ICCs), and gastrointestinal hormone imbalance, which collectively contribute to delayed gastric emptying[3]. Despite its clinical importance, the underlying pathophysiology remains incompletely understood and current therapeutic outcomes are suboptimal.
Electroacupuncture (EA), a modality integrating traditional acupuncture with electrical stimulation, has emerged as a promising intervention for DGP. The clinical guideline: Management of Gastroparesis by the American Gastroenterological Association emphasizes the efficacy of EA, as evidenced by numerous clinical studies[4,5]. Specifically, EA at the ST36 acupoint (Zusanli) has been shown to enhance gastric peristalsis frequency, modulate smooth muscle activity, and restore intrinsic circular currents function[6]. However, the precise mechanisms by which EA exerts its effect on the improvement of gastric motility remain to be elucidated.
Recent advances in the field have served to emphasize the critical role of the vagal pathways in the regulation of the gastrointestinal system. In mammals, the origin of vagal preganglionic neurons, characterized by choline acetyltransferase (ChAT) and α7 nicotinic acetylcholine receptor (α7nAChR) expression, located in the nucleus tractus solitarius (NTS)[7]. Vagal afferent signals activate NTS neurons prior to their synapsing with the dorsal motor nucleus of the vagus (DMV), which in turn relays information to target organs such as the stomach. Retrograde tracing studies demonstrated that gastric-projecting neurons predominantly localise to the DMV and NTS, with additional clusters in the raphe pallidus and lateral paragigantocellular nuclei[8]. Vagal modulation of gastric motility predominantly involves extrinsic autonomic pathways (e.g., vagovagal reflexes) and intrinsic enteric mechanisms. ChAT, the enzyme responsible for synthesising acetylcholine, is downregulated in DGP models, exacerbating disease progression through impaired cholinergic neurotransmission[9]. Building on these findings, mechanistic studies on EA at ST36 have identified neural regulation as a pivotal factor. Current research focuses on determining whether EA-mediated improvements in DGP involve the NTS-vagal axis, offering potential therapeutic targets for neuromodulation.
Seventy specific pathogen-free adult male Sprague-Dawley rats, with a weight range of 180-200 g, were supplied by the Animal Experiment Center of Hunan University of Chinese Medicine. The animals were housed under controlled conditions (temperature: 20-25 °C; humidity: 50%-70%) and fed a high-fat diet with free access to water. The bedding was changed on a daily basis, and the cages were disinfected twice a week. All experimental procedures were approved by the Animal Ethics Committee of Hunan University of Chinese Medicine (Approval No. HNUCM21-2311-08).
The DGP model was established as follows[10]: Rats were subjected to 12 hours of fasting, followed by intraperitoneal injection of 2% streptozotocin (STZ) solution (55 mg/kg). The rats were then maintained on a high-fat, high-sugar diet (composition: The composition of this diet was as follows: 58% basal feed, 15% lard, 20% sucrose, 5% milk powder, and 2% eggs). The rats were subjected to this diet for a period of 8 weeks. The DGP model criteria included: Firstly, non-fasting blood glucose levels of ≥ 16.7 mmol/L and secondly, reduced gastric motility and emptying rates in comparison to the Sham group. The rats in the control group were injected with an equivalent volume of sodium citrate buffer (0.1 mmol) and fed a standard diet. Following an eight-week modelling period, the EA group was administered EA at the ST36 acupoint once daily for a period of two weeks. The EA + antagonist alpha-NETA (AP) group received both EA and intra-acupoint injection of the ChAT antagonist AP (4 mg/kg/day, Abcam, United States) for a period of two weeks. The polygalacic acid (PA) group was injected with the ChAT agonist PA (4 mg/kg/day, MCE, United States) for a period of 2 weeks. The subdiaphragmatic vagotomy (SDV) group underwent SDV under isoflurane an aesthesia 8 weeks post-modeling. A 1 cm longitudinal incision was made approximately 0.2 cm to the left of the midline, below the diaphragm, to sever the anterior and posterior branches of the vagus nerve accompanying the esophagus. This was followed by continued high-fat diet feeding for a period of 2 weeks. The EA + SDV group received EA treatment for a period of two weeks following the same surgical procedure.
Bilateral ST36 acupoints (from the inferior border of the tibial tuberosity, measure 3 cun distally (where 1 cun is defined as the individual’s own thumb width at the interphalangeal joint) were punctured vertically to a depth of 5 mm using 25 mm acupuncture needles. Using an EA apparatus (Model SDZ-II; Hwato, China), a dense-disperse wave (20 Hz for disperse wave, 100 Hz for dense wave) was applied with a current intensity of 1 mA, causing slight skin vibration. The needles were left in place for 20 minutes once per day for a period of two weeks. Rats in other groups were anaesthetised and restrained without EA treatment.
Non-fasting blood glucose and body weight were recorded on a weekly basis. The rats were then restrained and their tails were disinfected with alcohol swabs. Following the evaporation of the alcohol, a lancet was used to puncture the tail tip and collect blood. The blood glucose levels were then measured using a blood glucose meter. The puncture site was then meticulously cleansed with a cotton ball to prevent infection. Body weight was measured using an electronic scale, and the values were recorded.
Following the conclusion of all treatment protocols, positron emission tomography (PET) scans were conducted utilizing an Inveon microPET scanner (PingSeng Scientific, China). The rats were fasted for over 12 hours and orally administered a contrast agent [68Ga] Ga-NOTA mixed with 0.2 g black sesame paste and 2 mL saline prior to scanning[11]. Dynamic images were continuously acquired from the time of administration up to 60 minutes post-administration. The dynamic data from each scan were divided into 60 frames (0 second × 10, 600 seconds × 10, 1200 seconds × 10, 1800 seconds × 10, 2400 seconds × 10, 3600 seconds × 10). To enhance the visualisation and anatomical alignment of PET images, micro-computed tomography (CT) scans were performed on the rats. CT scans were conducted from the nasal tip to the distal femur in order to capture the entire rat in the optimal field of view. A manual comparison of the acquired CT images was then performed to identify the frame that most closely matched the position and posture of the PET scan. Finally, the PET images were manually superimposed onto the selected CT images.
A quantitative analysis of PET images was performed using PMOD software (version 4.3) to accurately measure gastric content volume and total radioactivity. The pixel-based adaptive segmenter module was utilized to automatically extract three-dimensional volumes of interest (VOIs) for the stomach of each rat. Total radioactivity and volume were obtained from these VOIs. The radioactive concentration (RC) was then calculated as RC = total radioactivity/volume. The gastric emptying rate (GER) was subsequently calculated, where GER (t) denotes the percentage of gastric content emptied at a specific time point, using the following formula: GER (t) = [1 - RC (max)/RC (t)] × 100%, where RC (max) is the RC at the initial time point (e.g., 0 minutes).
Following a 12-hour fast, rats were anaesthetized with isoflurane and placed in a supine position. Abdominal hair was then shaved, and the skin was disinfected with iodine. A midline incision was then made below the xiphoid process in order to expose the gastric antrum. Needle electrodes were then inserted into the smooth muscle layer of the antrum, at a point approximately 0.5 cm from the pylorus, in a direction parallel to the longitudinal muscle axis, with an inter-electrode distance of 0.3 cm. The abdomen was then sutured. After a period of 10 minutes had elapsed, the electrode leads were connected to an MP160 multichannel electrophysiological recorder. The following parameters were set for the recording: Sensitivity at a gain of 1000, sampling frequency at 500 Hz, high-pass filter at DC, and low-pass filter at 1 Hz. Continuous slow-wave recordings were then performed for a duration of 30 minutes. Gastric slow wave discharges within 5-minute intervals were analyzed according to Edwards et al[12]. Five consecutive 5-minute segments of gastric myoelectric activity were randomly selected, and the number of slow wave discharges was calculated.
Following a 24-hour fast and a 2-hour water restriction, rats were administered 2 mL of a phenol red solution containing 50 mg/dL of the dye via oral gavage. Fifteen minutes later, an aesthesia was induced by intraperitoneal injection of 10% sodium pentobarbital (3 mL/kg). The abdominal cavity was then opened, and the entire stomach was excised and cut along the greater curvature. The stomach was then thoroughly rinsed with 20 mL of 0.9% sodium chloride solution, after which the contents were collected into a clean beaker. The contents were then mixed with 20 mL of 0.5 mol/L sodium hydroxide (NaOH) solution and left to stand for 1 hour. Thereafter, 5 mL of the resulting mixture was collected and mixed with 0.5 mL of a 20% trichloroacetic acid (TCA) solution. The mixture was then subjected to centrifugation at 3500 rpm for a period of 10 minutes. Thereafter, 2 mL of the resulting interface was analyzed for its optical density (OD) at 560 nm using a Ultraviolet spectrophotometer. A standard phenol red solution (2 mL phenol red solution, 18 mL distilled water, 20 mL 0.5 mol/L NaOH, and 4 mL 20% TCA) was prepared, and its OD value was measured using the same method. The GER was calculated as follows: GER (%) = (1 - sample OD value/standard phenol red OD value) × 100%[13].
The small intestine was meticulously dissected and positioned in a horizontal position on ice to ascertain its total length. The distal end, which had been stained with phenol red, was identified, and a small incision was made using ophthalmic scissors. A 0.5 mol/L NaOH solution was then added to the incision site, and a pinkish-purple colour change indicated the presence of phenol red. Additional drops of NaOH were applied proximal and distal to this point in order to determine the farthest point reached by phenol red. The intestinal propulsive rate (IPR) was calculated as follows: IPR (in percentage form) was thus calculated by the following method: IPR (in percentage form) = (farthest distance reached by phenol red/total length of the small intestine) × 100%[13].
Gastric tissue samples were fixed in 4% paraformaldehyde overnight, followed by paraffin embedding. Following fixation, the tissues were dehydrated, cleared, and embedded in paraffin. Serial sections of 4 μm-5 μm thickness were prepared using a microtome. The sections were then deparaffinized, rehydrated, and stained with hematoxylin and eosin (HE). Following this, the stained sections were dehydrated, cleared, and mounted with neutral balsam. Finally, images were captured under an optical microscope to evaluate morphological changes in the gastric tissues.
The tissues were minced into small fragments, and 20 mg of tissue was homogenised in 150-250 μL of lysis buffer until complete lysis was achieved. The lysate was then subjected to centrifugation at 12000 × g for 15 minutes at 4 °C. The resultant pellet was discarded, and the remaining fluid (supernatant) was collected for protein quantification. The samples were then stored at -80 °C. Polyacrylamide gels (10% or 12%) were prepared based on the molecular weight of target proteins. The gels were then cast into electrophoresis chambers, and an appropriate amount of running buffer was added. Following electrophoresis, proteins were transferred to membranes, which were blocked with 5% bovine serum albumin (BSA) (Elabscience) at 37 °C for 1 hour. Primary antibodies, including neuronal nitric oxide synthase (nNOS) (1:1500, 76067, Abcam), cluster of differentiation 117 (C-kit) (1:1000, 3074, Cell Signaling Technology), stem cell factor (SCF) (1:2500, 52603, Abcam), ChAT (1:1000, 178850, Abcam), α7nAChR (1:1000, 216485, Abcam), and β-actin (1:1000, 4970S, Cell Signaling Technology), were incubated overnight at 4 °C on a shaker. Following a thorough washing step with tris borate sodium tween-20, the membranes were subjected to an incubation with the relevant secondary antibodies (goat anti-rabbit, 1:2000, A-1003, Elabscience) at room temperature for a duration of one hour. Protein bands were detected using chemiluminescence, and band intensities were quantified using ImageJ software.
Tissues were fixed in 4% paraformaldehyde and then dehydrated through a graded ethanol series. Following this, they were cleared, embedded in paraffin, and sectioned. The paraffin sections were then deparaffinized, rehydrated, and subjected to antigen retrieval using ethylene diamine tetraacetic acid buffer. Following washing with phosphate buffered saline, autofluorescence quencher was applied, followed by blocking with 5% BSA. Primary antibodies, including rabbit anti-C-kit (1:200, 3074, Cell Signaling Technology), rabbit anti-c-FOS (1:200, 2250, Cell Signaling Technology), and mouse anti-ChAT (1:200, MAB5350, Sigma), were incubated overnight at 4 °C. Sections were then incubated with corresponding secondary antibodies (Alexa Fluor 488-conjugated goat anti-rabbit, 1:200, 11008, Invitrogen; Alexa Fluor 594-conjugated goat anti-mouse, 1:500, A21203, Invitrogen) at room temperature for 60 minutes in the dark. The nuclei were counterstained with 4’,6-diamidino-2-phenylindole for 10 minutes at room temperature in the dark. Following this, the sections were washed with phosphate buffered saline and mounted with antifade mounting medium. The samples were then observed under a fluorescence microscope. Images from three randomly selected fields per section were captured at 400 × magnification.
Levels of gastrointestinal peptides [i.e., gastrin (Gas), motilin (MLT) and vasoactive intestinal peptide (VIP)] were measured using enzyme-linked immunosorbent assay kits (purchased from LunChangShuoBiotech, China). Briefly, brain filtrates were subjected to a centrifugation process at 1000 × g for a duration of 10 minutes, with the objective of elimi
The analysis of the data was conducted utilising Prism 9 (GraphPad Software). Quantitative data are presented as mean ± SD. The differences between the groups were assessed using one-way analysis of variance, followed by Bonferroni’s post hoc test. For non-normally distributed data, Dunnett’s multiple comparison test was applied. A P value less than 0.05 was considered to be statistically significant.
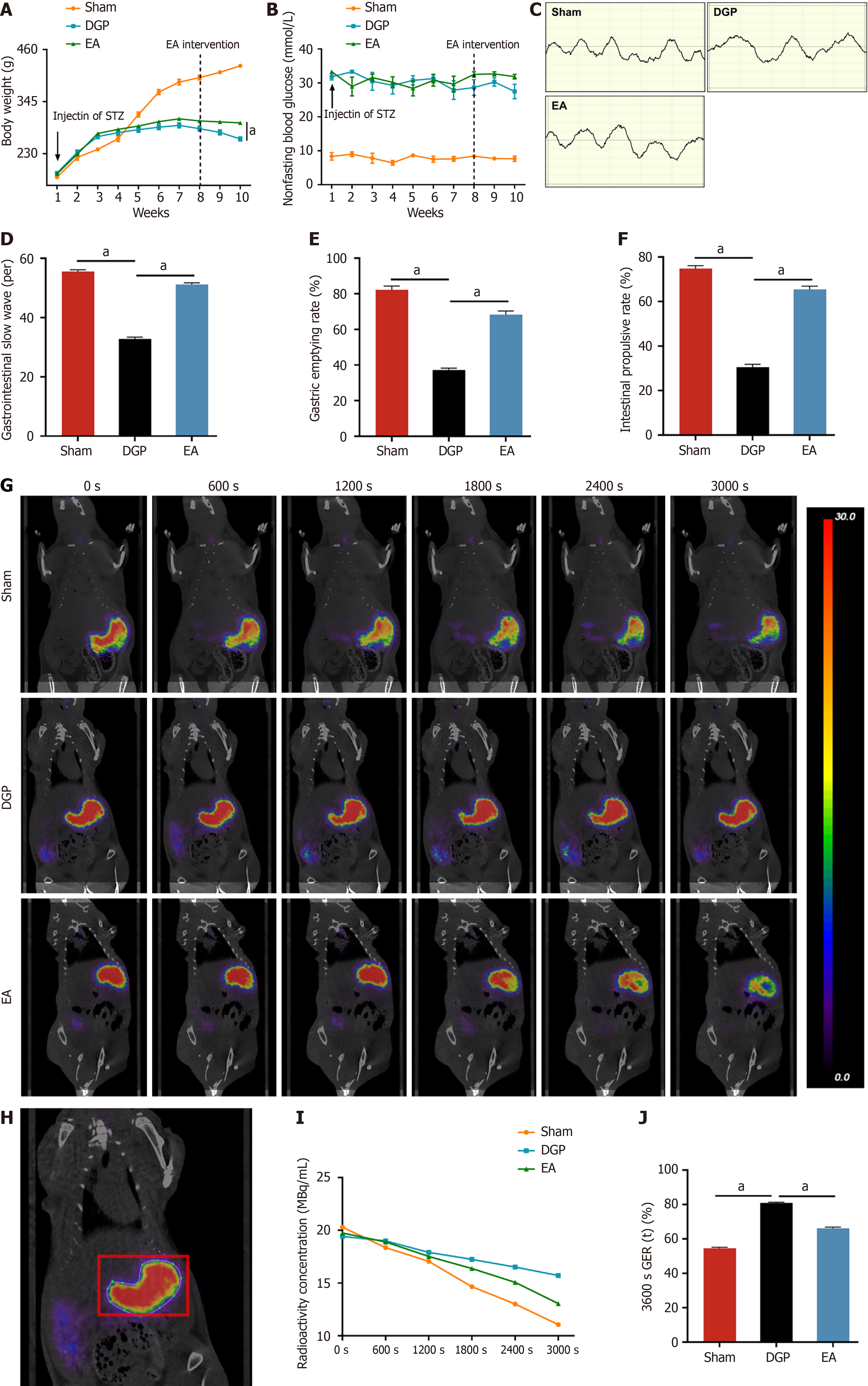
Longitudinal weight monitoring was conducted to evaluate diabetes-associated body weight dynamics (Figure 1A). The Sham group demonstrated progressive weight gain throughout the 10-week study period. In contrast, the STZ-induced diabetic rats (DGP and EA groups) demonstrated a less pronounced weight gain trajectory post-diabetes induction (week 1). The intervention of EA at week 8 led to a significant mitigation of diabetes-related weight loss, with the final body mass of EA-treated rats being 11.4% higher than that of the DGP controls at week 10 (P < 0.05). Metabolic profiling confirmed sustained hyperglycaemia (> 16.7 mmol/L, non-fasting) in the DGP and EA groups, with no significant glucose modulation by EA treatment (Figure 1B). Gastric motility was assessed via electrogastrography (Figure 1C and D). In comparison to the Sham group, DGP rats exhibited significantly reduced gastric slow wave discharges (P < 0.05), while EA intervention restored slow wave frequency to near-normal levels (P < 0.05). Further functional assessments revealed impaired gastric emptying and intestinal propulsion in DGP rats (P < 0.05), both of which were significantly improved by EA (P < 0.05) (Figure 1E and F). Furthermore, the gold standard for diagnosing DGP rats was employed to assess the efficacy of EA through the use of confirmatory PET-CT imaging with quantitative contrast tracking[11]. This revealed that EA-treated rats exhibited accelerated gastric emptying, with only 66.1% radioactivity remaining at 3600 seconds post-administration, in comparison to 80.9% in DGP and 54.5% in Sham controls (P < 0.05; Figure 1G-J). This multimodal validation, encompassing electrophysiological, functional, and imaging modalities, provides substantial evidence supporting the efficacy of EA as a robust intervention for restoring gastric motility in DGP.
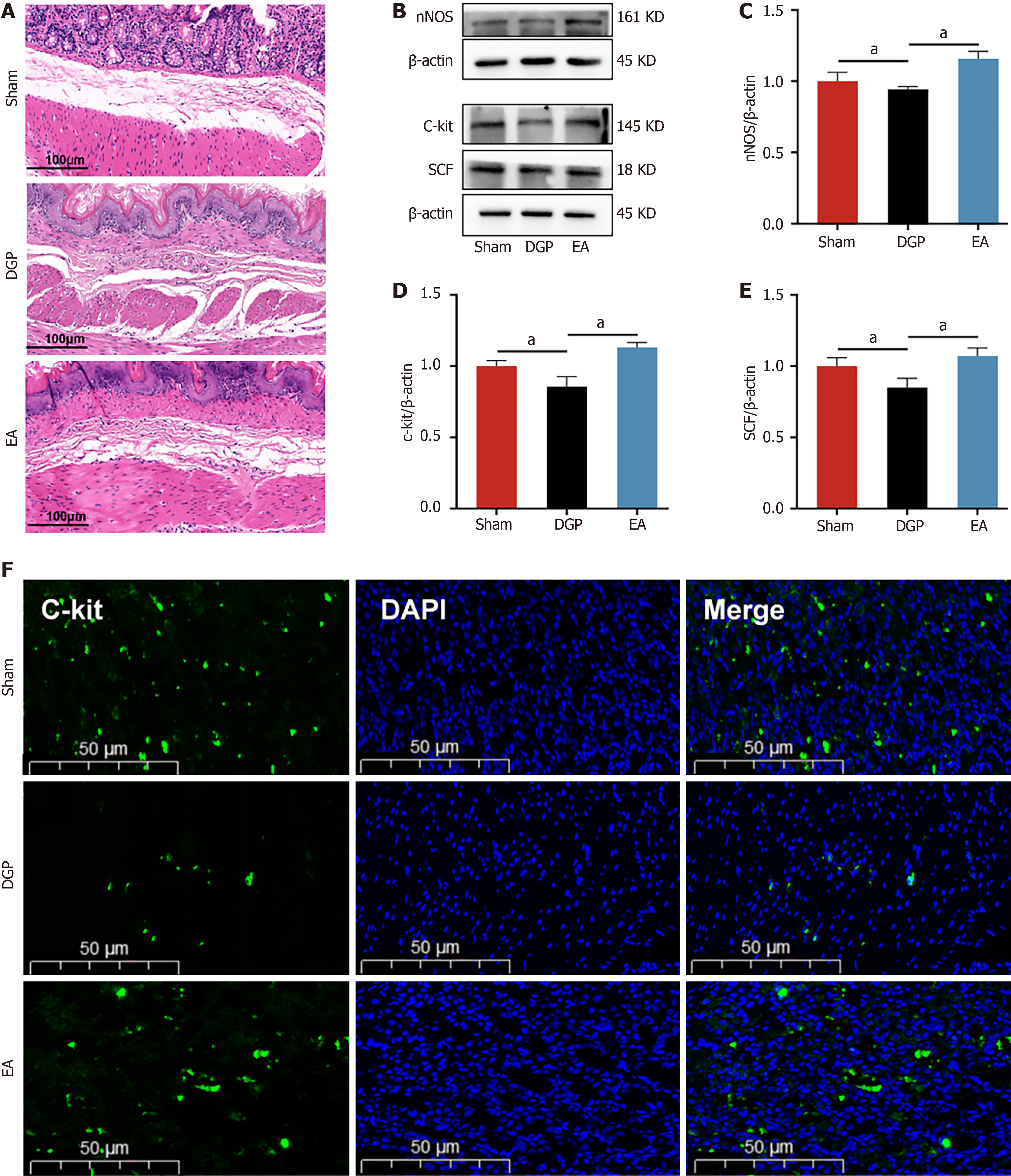
Having established the regulatory effects of EA on gastric emptying, the subsequent focus was on gastric smooth muscle dynamics, which are the primary driver of gastric contractility. Histopathological evaluation via HE staining (Figure 2A) revealed distinct morphological profiles across the experimental groups. Sham controls displayed preserved gastric architecture, with intact mucosal layers and orderly smooth muscle arrangement. In contrast, DGP rats exhibited marked mucosal degeneration, characterized by glandular atrophy, inflammatory infiltrates, submucosal fibrosis, and disrupted smooth muscle organization. The administration of EA significantly mitigated these pathological manifestations, as evidenced by the restoration of glandular density, the attenuation of inflammatory infiltration, and the enhancement of smooth muscle alignment. In order to investigate the molecular mechanisms underlying these morphological improvements, a study was conducted on the key regulators of smooth muscle function. Western blot analysis demon
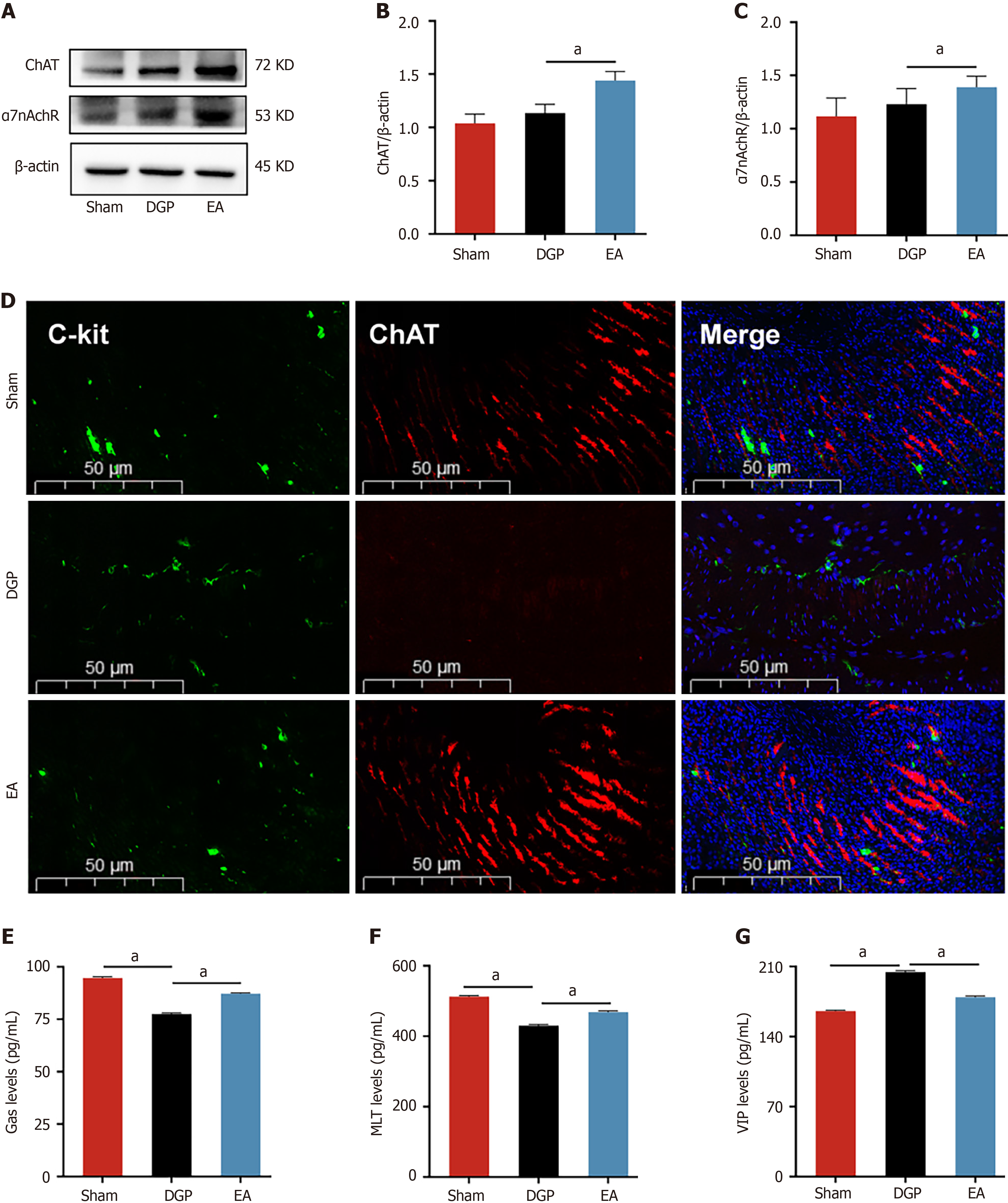
The vagus nerve is the principal neural regulator of gastrointestinal motility. In order to elucidate its role in EA-mediated improvements, a comprehensive analysis was conducted on key vagal targets in gastric tissues. The results of the immunoblot analysis revealed that EA induced upregulation of the cholinergic markers ChAT and α7nAchR in comparison with DGP controls (P < 0.05, Figure 3A-C). Furthermore, immunofluorescence co-localization analysis demonstrated parallel restoration of C-kit +/ChAT + expression patterns, with EA-treated rats showing distribution characteristics comparable to the Sham group (Figure 3D). This suggests that the vagus nerve target ChAT may be involved in the regulatory effect of EA on ICC. Assessment of gastrointestinal peptides further revealed characteristic alterations. Compared with the Sham group, DGP rats exhibited significant suppression of stimulatory mediators (Gas and MLT), accompanied by a concomitant paradoxical elevation in inhibitory VIP levels (P < 0.05). However, the EA intervention led to the normalization of these peptide profiles, with a significant increase in Gas and MLT levels (P < 0.05) and a reduction in VIP levels (P < 0.05) (Figure 3E-G). The EA intervention appears to have restored gastric motility in DGP rats by coordinating the activation of the vagal cholinergic pathway to regulate ICC cells and rebalance neuroendocrine signals.
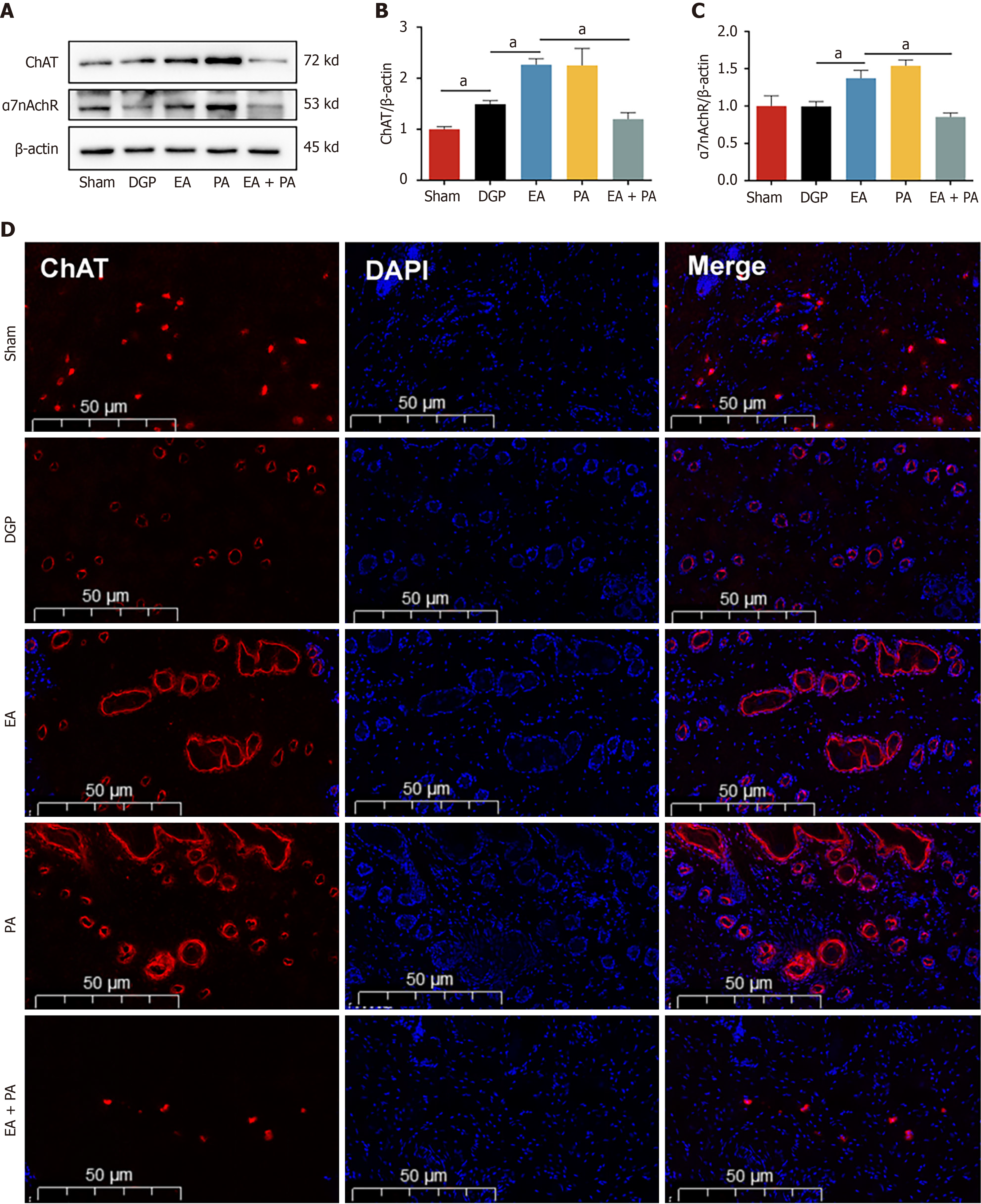
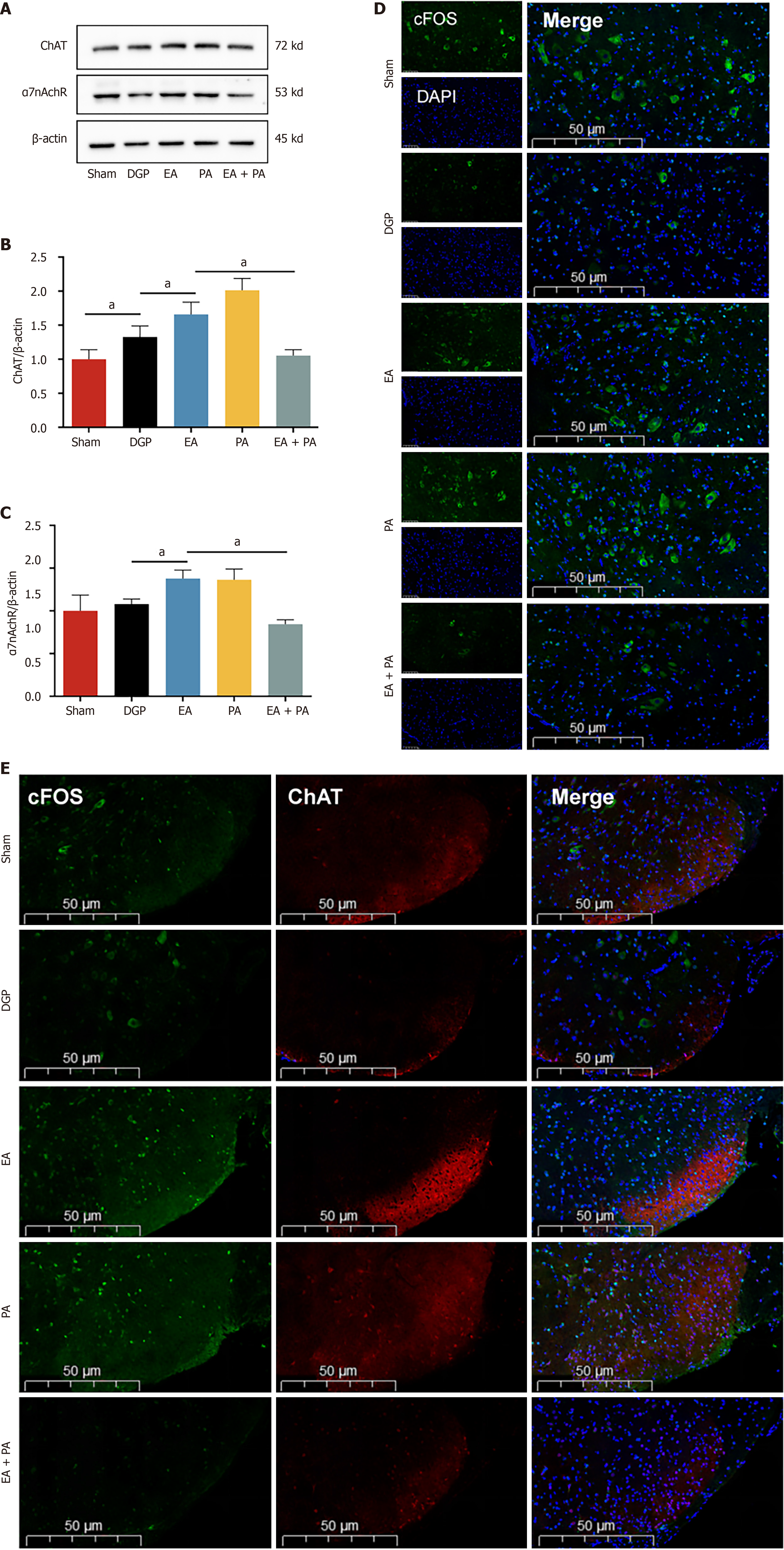
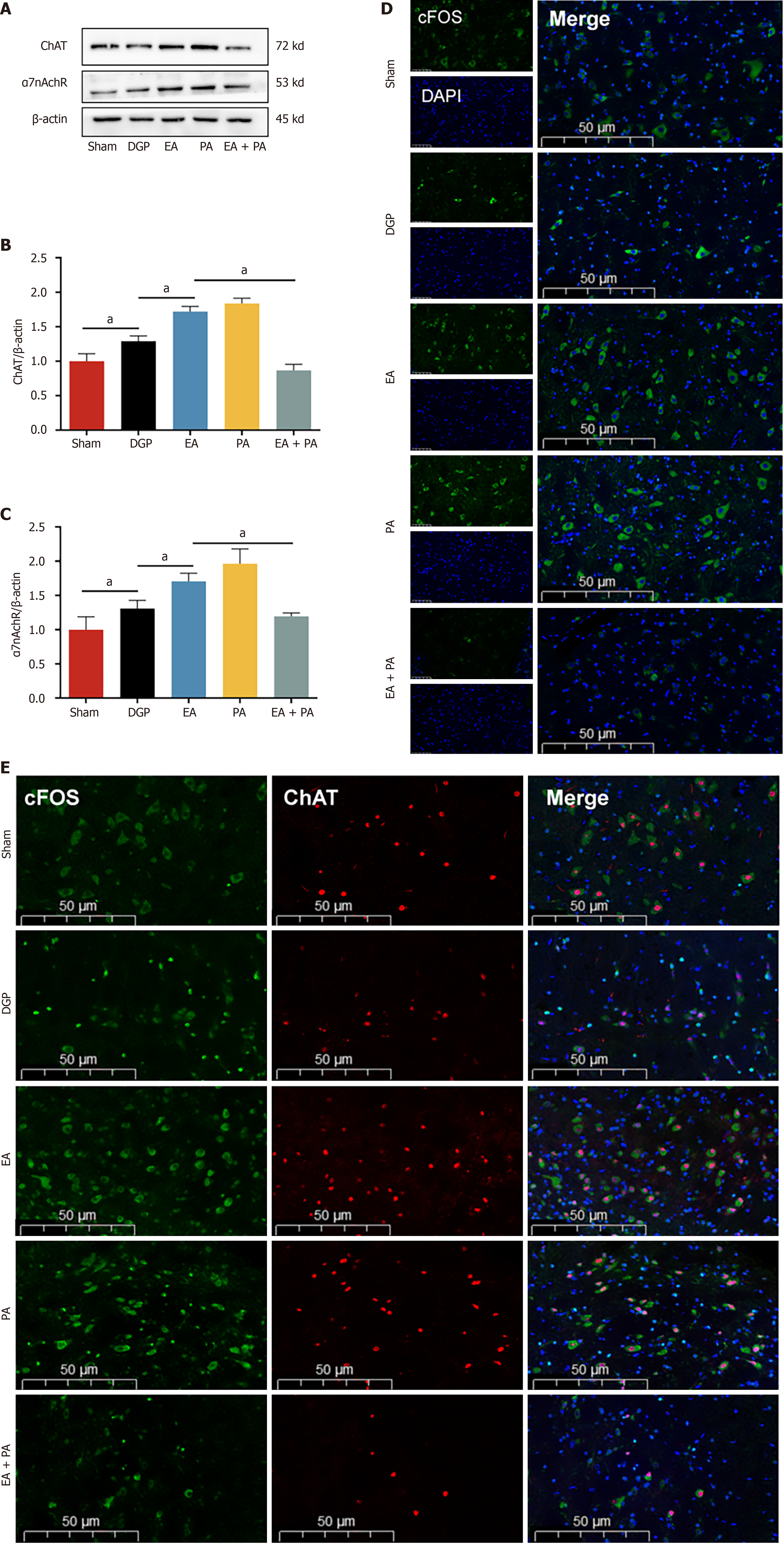
In order to elucidate the afferent neural pathways underlying EA-mediated gastric motility modulation in DGP rats, the ChAT inhibitor AP or the agonist PA was injected into the ST36 acupoint area and ChAT and α7nAChR protein expression was quantified across three key sites (Figures 4, 5 and 6): The ST36 acupoint skin, the spinal L4-L6 segments and the NTS (Figures 4A, 5A and 6A). Western blot analysis revealed significantly elevated ChAT levels in DGP rats compared to Sham controls across all sites (P < 0.05), with EA further amplifying ChAT expression (P < 0.05), mirroring trends observed in the PA group. However, the co-administration of AP with EA (EA + AP group) led to the abolition of this activation (P < 0.05; Figures 4B, 5B and 6B). In addition, EA induced an increase in α7nAChR expression in the ST36 skin, L4-L6 spinal region, and NTS (P < 0.05), an effect that was attenuated by AP co-treatment (P < 0.05; Figures 4C, 5C and 6C). Immunofluorescence analysis corroborated these observations. DGP rats exhibited reduced ChAT expression in ST36 skin, whereas EA and PA groups showed marked increases. AP administration nullified EA’s effects (Figure 4D). In the spinal L4-L6 segments, c-FOS immunostaining revealed robust neuronal activation in the EA and PA groups (P < 0.05), which was suppressed in the EA + AP rats (P < 0.05; Figure 5D). c-FOS +/ChAT + co-localization further confirmed that this activation was ChAT-dependent (Figure 5E). In the NTS, c-FOS expression was significantly elevated in the EA and PA groups (P < 0.05), with EA + AP rats showing reduced activation (P < 0.05; Figure 6D). c-FOS +/ChAT + co-expression mirrored these trends, demonstrating EA’s dual modulation of neuronal activity and cholinergic signalling (Figure 6E). The data taken together indicate that EA activates vagal ChAT/α7nAChR targets at ST36, transmitting signals via spinal L4-L6 afferents to NTS neurons, thereby restoring vagal tone in DGP.
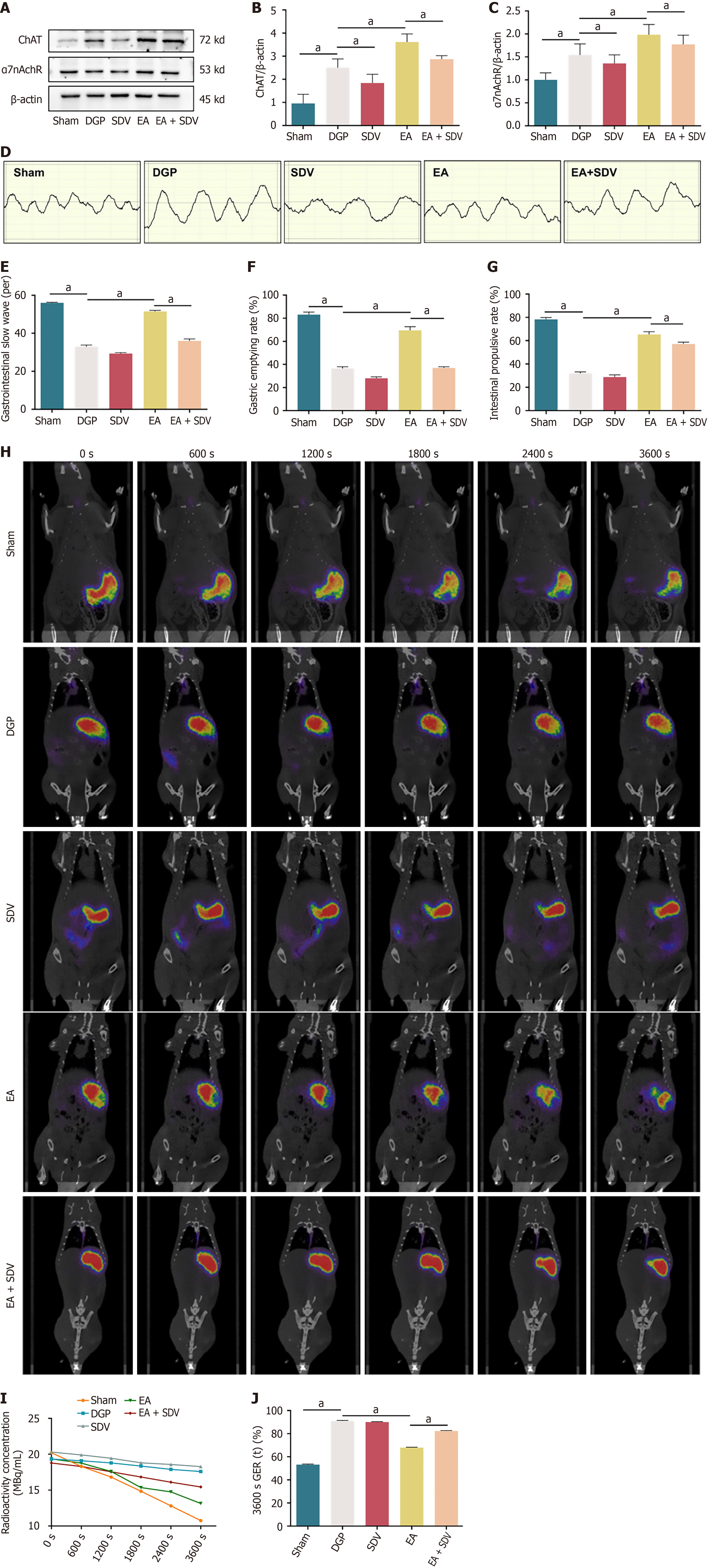
Utilizing the preceding evidence that EA activates the NTS through vagal afferent pathways, the present study investigated the role of vagal efferent signalling in EA-mediated gastric motility restoration by performing SDV in DGP rats. The results of the western blot analysis revealed a significant reduction in the expression of ChAT and α7nAChR in the stomach of the rats in the SDV group compared to the DGP group (P < 0.05). In contrast, EA significantly increased the expression of both proteins (P < 0.05). Crucially, SDV abolished EA-induced ChAT/α7nAChR activation (P < 0.05), confirming that EA’s therapeutic effects require intact vagal efferent signaling (Figure 7A-C). Electrogastrography demonstrated that EA restored gastric slow wave discharges in DGP rats (P < 0.05), an effect that was attenuated by SDV (P < 0.05; Figure 7D and E). The findings were corroborated by functional assessments: EA improved both gastric emptying and small intestinal propulsion rates (P < 0.05), whereas SDV negated these improvements (P < 0.05; Figure 7F and G). Dynamic PET-CT imaging further validated the prokinetic effects of EA, showing accelerated gastric radioactivity clearance in rats treated with EA (67.8% retention at 3600 seconds) compared to rats treated with DGP (90.9% retention; P < 0.05; Figure 7H and I). Post-spontaneous duodenal vagus stimulation (SDV), the efficacy of EA was significantly diminished, with residual gastric content increasing to 82.2% (P < 0.05; Figure 7J). The collective findings of this study demonstrate that EA enhances gastric motility via the subdiaphragmatic vagus nerve, thereby establishing it as a critical efferent pathway for neuromodulation in DGP.
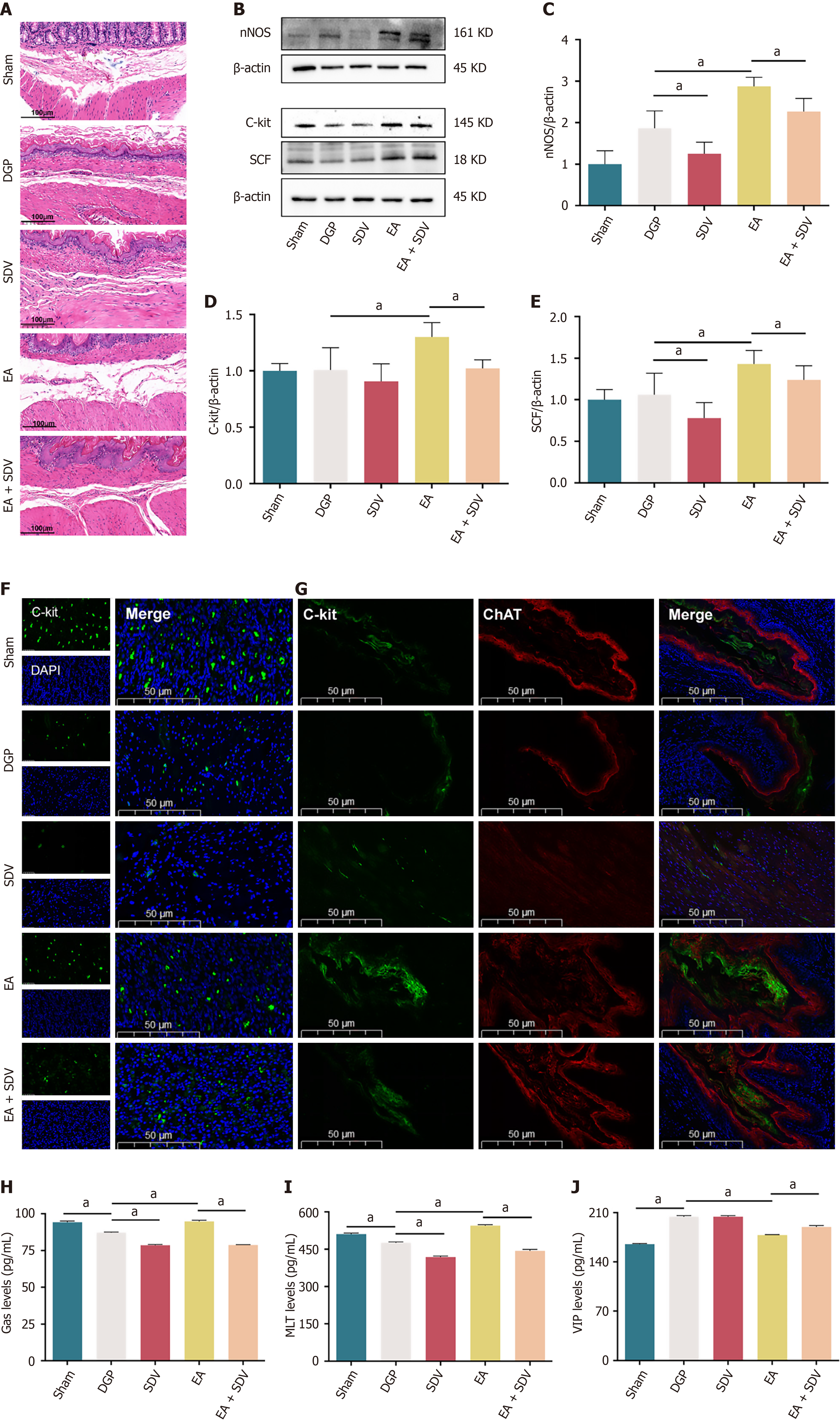
Histological analysis via HE staining revealed that EA ameliorated gastric pathology in DGP rats, characterized by reduced inflammatory infiltration and restored smooth muscle alignment. Conversely, SDV appeared to counteract these benefits, sustaining submucosal collagen deposition and mild smooth muscle disorganization (Figure 8A). In addition, the results of the western blotting analysis demonstrated that EA significantly increased the expression of nNOS, C-Kit, and SCF, while SDV significantly reduced the expression of these proteins in comparison with rats treated with EA (P < 0.05; Figure 8B-E). Immunofluorescence corroborated these findings: EA enhanced C-Kit expression, which was suppressed by SDV (P < 0.05; Figure 8F). Furthermore, the co-localization of C-Kit and ChAT further confirmed EA’s activation of vagal-ICC interactions, an effect that was attenuated by SDV (P < 0.05; Figure 8G). Furthermore, gastrointestinal peptide profiling revealed that EA-mediated upregulation of Gas and MLT was accompanied by suppression of VIP, with SDV reversing these trends (P < 0.05; Figure 8H-J). Collectively, these results establish that EA improves gastric dysmotility by activating subdiaphragmatic vagal efferent pathways, which regulate smooth muscle integrity, ICC function, and neuropeptide balance in DGP.
DGP is a chronic complication of diabetes characterized by impaired gastric motility, delayed gastric emptying and gastric dysrhythmia, which severely compromises patients’ quality of life[14]. Current pharmacological strategies targeting gastric prokinetic effects and glycemic control remain limited by adverse effects, drug resistance, and insufficient therapeutic efficacy[15]. EA, a traditional Chinese medical therapy, has demonstrated potential in improving gastrointestinal motility due to its high safety profile and cost-effectiveness, as evidenced by recent studies[16]. In this study, EA alleviated weight loss in DGP rats but did not significantly ameliorate hyperglycaemia. These findings suggest that a 14-day EA regimen may selectively stabilize body weight regulation, whereas chronic hyperglycemia-a hallmark of advanced diabetes-likely necessitates prolonged therapeutic interventions to achieve glycemic normalization, consistent with prior evidence on metabolic inertia in diabetic models[15]. The study found that EA significantly enhanced gastric emptying, as measured by the phenol red method, which is a diagnostic criterion for the DGP model. Electrogastrography revealed that EA markedly increased gastrointestinal slow wave activity, providing direct evidence of its prokinetic effects. Notably, a pioneering approach involved the use of PET-CT imaging, a gold-standard modality renowned for its superior quantitative accuracy and imaging resolution[17], to assess gastric emptying. The dynamic tracking based on PET-CT further validated the therapeutic potential of EA in DGP rats by demonstrating its robust promotion of gastric motility.
Mounting evidence indicates that smooth muscle pathology plays a pivotal role in the pathogenesis of DGP[18]. Of particular note is the finding that the downregulation of nNOS expression, a dimerized enzyme that is critical for inhibitory neurotransmission and gastric smooth muscle relaxation, is a hallmark of DGP. Its reduction has been shown to correlate closely with impaired nitrergic innervation and delayed intestinal transit[19,20]. In a seminal study, Han et al[16] demonstrated that EA mitigates nNOS loss in the enteric nervous system, thereby restoring neuro-mediated muscle responses and significantly accelerating gastric emptying. The present findings are in alignment with these observations: In DGP rats, EA resulted in the upregulation of gastric nNOS expression and a significant amelioration of gastric dysmotility. Beyond nNOS, ICCs dysfunction has been shown to be strongly associated with DGP. ICCs are essential for generating gastric slow waves and setting smooth muscle membrane potentials[21]. Clinical studies have revealed markedly reduced ICC density in gastric antral biopsies of DGP patients, with antral changes being more pronounced than those in the gastric body[22,23]. In addition, experimental studies on animals have corroborated these observations, demonstrating a decrease in the expression of the ICC markers C-kit and its receptor SCF in both the antrum and corpus of DGP rats[24]. This supports the hypothesis that ICC loss contributes to delayed gastric emptying. Lin et al[25] further validated this mechanism through EA intervention: EA-treated rats exhibited increased ICC populations, enhanced neuro-smooth muscle connectivity, and restored interactions between intrinsic nerves and ICCs compared to DGP models. Quantitative analysis confirmed that EA significantly increased C-kit and SCF expression, consistent with these morphological findings. Importantly, a positive correlation was observed between C-kit and ChAT expression, suggesting that vagal ChAT signalling may mediate EA’s regulatory effects on ICCs.
The present study investigates the association between vagal nerve dysfunction and DGP, given its status as the primary autonomic regulator of gastrointestinal motility. The role of the vagus nerve in modulating gastrointestinal smooth muscle contraction and glandular secretion is well-documented, and its contribution to enhancing gastroin
Hierarchical vagal regulatory pathways involve brainstem nuclei, particularly the DMV and the NTS, which serve as central hubs for integrating gastrointestinal signals (e.g., stretch reflexes and satiety) and coordinating gastrointestinal motility. EA at ST36 has been shown to modulate neuronal activity in these regions through vagal afferent pathways[27,30]. For instance, retrograde tracing using cholera toxin or pseudorabies virus injected at ST36 revealed abundant labeled neurons in the NTS and DMV, confirming direct neural connectivity between ST36 and these nuclei[31]. Fang et al[32] demonstrated that EA at ST36 activates NTS neurons, subsequently enhancing vagal efferent activity to promote gastrointestinal peristalsis. Consistent with this, our findings showed EA-induced activation of c-FOS-labeled neurons in the NTS accompanied by synchronized ChAT upregulation, further supporting the cascade activation of vagal central circuits. It is crucial to note that the efficacy of these EA-induced effects is contingent on the integrity of the vagus nerve. SDV abolished EA’s therapeutic benefits on gastric emptying and gastrointestinal peptide secretion[33]. In a similar manner, Song et al[34] reported that vagal denervation negated EA’s efficacy in ameliorating post-burn gastric dysfunction. The present study revealed that vagotomy not only blocked EA’s prokinetic effects but also attenuated its regulation of smooth muscle-related factors and gastrointestinal peptides. Most notably, vagal ablation completely abolished EA-induced ChAT upregulation, underscoring the indispensable role of vagal efferent pathways in EA’s anti-DGP mechanisms.
In summary, EA has been shown to ameliorate DGP through a multi-level neural pathway involving peripheral acupoint (ST36), spinal cord (L4-L6), brainstem nuclei (NTS), target organ (stomach), with therapeutic efficacy strictly dependent on intact vagal circuitry. These findings provide novel insights into the neural mechanisms underlying EA’s improvement of gastric dysmotility in DGP, advancing research directions for non-pharmacological interventions. However, this study has limitations. While the investigation focused on elucidating the role of the NTS-vagal axis in EA-mediated improvements, alternative regulatory pathways, such as sympathetic innervation and inflammatory cascades-were not systematically explored, which may overlook potential cross-talk mechanisms between neural and immune systems in DGP pathogenesis. Future studies will prioritize dissecting these interactions to comprehensively map the neuromodulatory network underlying EA therapy.
This study elucidates the mechanism by which EA improves gastric dysmotility in DGP rats through multi-tiered validation of vagal pathways. Firstly, EA at the ST36 acupoint activates peripheral vagal targets, specifically ChAT, transmitting signals via spinal L4-L6 afferents to the central nervous system. Subsequently, at the brainstem level, these signals enhance vagal central drive by activating c-FOS-expressing neurons in the NTS. Finally, EA upregulates smooth muscle-related factors (nNOS, C-kit, SCF) and promotes gastrointestinal peptide secretion, ultimately restoring gastric emptying (Figure 9). In this study, we systematically revealed for the first time the mechanism by which EA improves gastrointestinal motility in DGP through an integrated regulatory circuit of peripheral stimulation, spinal afferents, brainstem integration, vagal efferent, which provides a novel molecular mechanism for the treatment of DGP by EA.
| 1. | Kassander P. Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum). Ann Intern Med. 1958;48:797-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 221] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Ahmed MSO, Forde H, Smith D. Diabetic gastroparesis: clinical features, diagnosis and management. Ir J Med Sci. 2023;192:1687-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Bharucha AE, Kudva YC, Prichard DO. Diabetic Gastroparesis. Endocr Rev. 2019;40:1318-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (3)] |
| 4. | Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-37; quiz 38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 748] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 5. | Schol J, Huang IH, Carbone F, Fernandez LMB, Gourcerol G, Ho V, Kohn G, Lacy BE, Colombo AL, Miwa H, Moshiree B, Nguyen L, O'Grady G, Siah KTH, Stanghellini V, Tack J. Rome Foundation and international neurogastroenterology and motility societies' consensus on idiopathic gastroparesis. Lancet Gastroenterol Hepatol. 2025;10:68-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Yu B, Sun M, Wang Z, Zhu B, Xue J, Yang W, Gao X, Zhi M, Cao J, Zhao J, Zhao X, Liu W, Wang F, Li T. Effects of Stimulating Local and Distal Acupoints on Diabetic Gastroparesis: A New Insight in Revealing Acupuncture Therapeutics. Am J Chin Med. 2021;49:1151-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 7. | Zaman A, Özçelik H, Yücel E, Su Akkan S, Onsinejad T, Mert Yüksel S, Bülbül M. Effect of sex on chronic stress induced alterations in hindbrain catecholaminergic system and autonomic dysfunction resulting in gastrointestinal dysmotility. Brain Res. 2024;1842:149112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 8. | Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol. 2016;13:389-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 9. | Chen Y, Zhang S, Li Y, Yan H, Ba Y, Wang X, Shi N, Liu C. Gastric Electrical Stimulation Increases the Proliferation of Interstitial Cells of Cajal and Alters the Enteric Nervous System in Diabetic Rats. Neuromodulation. 2022;25:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Huang H, Peng Y, Xiao L, Wang J, Xin YH, Zhang TH, Li XY, Wei X. Electroacupuncture Promotes Gastric Motility by Suppressing Pyroptosis via NLRP3/Caspase-1/GSDMD Signaling Pathway in Diabetic Gastroparesis Rats. Chin J Integr Med. 2025;31:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Chen X, Liu Y, Pan D, Cao M, Wang X, Wang L, Xu Y, Wang Y, Yan J, Liu J, Yang M. (68)Ga-NOTA PET imaging for gastric emptying assessment in mice. BMC Gastroenterol. 2021;21:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Edwards FR, Hirst GD. An electrical analysis of slow wave propagation in the guinea-pig gastric antrum. J Physiol. 2006;571:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Wei X, Lin Y, Zhao D, Xiao X, Chen Q, Chen S, Peng Y. Electroacupuncture Relieves Suppression of Autophagy in Interstitial Cells of Cajal of Diabetic Gastroparesis Rats. Can J Gastroenterol Hepatol. 2020;2020:7920715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Jalleh RJ, Marathe CS, Jones KL, Horowitz M, Rayner CK. Digesting the pathogenesis of diabetic gastroparesis. J Diabetes Complications. 2021;35:107992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 15. | Camilleri M, Chedid V, Ford AC, Haruma K, Horowitz M, Jones KL, Low PA, Park SY, Parkman HP, Stanghellini V. Gastroparesis. Nat Rev Dis Primers. 2018;4:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 16. | Han X, Chen X, Wang X, Gong M, Lu M, Yu Z, Xu B, Yuan J. Electroacupuncture at ST36 Improve the Gastric Motility by Affecting Neurotransmitters in the Enteric Nervous System in Type 2 Diabetic Rats. Evid Based Complement Alternat Med. 2021;2021:6666323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Maalouf E, Khasawneh H, Karbhari A, AlAsfoor S, Breen-Lyles M, Bernard C, Rajan E, Farrugia G, Lowe V, Goenka A, Grover M. Preliminary study on the dynamic positron emission tomography imaging with (11)C-ER176 to delineate macrophage activation in diabetic gastroparesis. Neurogastroenterol Motil. 2024;36:e14762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Petri M, Singh I, Baker C, Underkofler C, Rasouli N. Diabetic gastroparesis: An overview of pathogenesis, clinical presentation and novel therapies, with a focus on ghrelin receptor agonists. J Diabetes Complications. 2021;35:107733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Edwards PT, Soni KG, Conner ME, Fowler SW, Foong JPP, Stavely R, Cheng LS, Preidis GA. Site-specific pathophysiology in a neonatal mouse model of gastroparesis. Neurogastroenterol Motil. 2023;35:e14676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Sprouse J, Sampath C, Gangula P. 17β-Estradiol Suppresses Gastric Inflammatory and Apoptotic Stress Responses and Restores nNOS-Mediated Gastric Emptying in Streptozotocin (STZ)-Induced Diabetic Female Mice. Antioxidants (Basel). 2023;12:758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Foong D, Zhou J, Zarrouk A, Ho V, O'Connor MD. Understanding the Biology of Human Interstitial Cells of Cajal in Gastrointestinal Motility. Int J Mol Sci. 2020;21:4540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 22. | Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, Ünalp-Arida A, Nguyen L, Koch KL, Calles J, Lee L, Tonascia J, Hamilton FA, Pasricha PJ; NIDDK Gastroparesis Clinical Research Consortium. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575-85.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 23. | Grover M, Bernard CE, Pasricha PJ, Parkman HP, Gibbons SJ, Tonascia J, Koch KL, McCallum RW, Sarosiek I, Hasler WL, Nguyen LAB, Abell TL, Snape WJ, Kendrick ML, Kellogg TA, McKenzie TJ, Hamilton FA, Farrugia G; NIDDK Gastroparesis Clinical Research Consortium (GpCRC). Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Li H, Cao W, Zhang XB, Zhang XX, Gu C, Gu LM, Pan CY, Tian YZ, Lu M. Atractylenolide-1 alleviates gastroparesis in diabetic rats by activating the stem cell factor/ckit signaling pathway. Mol Med Rep. 2021;24:691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Lin G, Zhang J, Li L, Zou Z, Chen C, Xue L, Zhao L. Effect of electroacupuncture on gastric interstitial cells of Cajal in a rat model of diabetic gastroparesis. Exp Ther Med. 2016;11:2489-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Yu Y, Hu L, Xu Y, Wu S, Chen Y, Zou W, Zhang M, Wang Y, Gu Y. Impact of blood glucose control on sympathetic and vagus nerve functional status in patients with type 2 diabetes mellitus. Acta Diabetol. 2020;57:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Jin Z, Shen Z, Yan S, Chen G, Yin Y, Zhang Y, Wu X. Electroacupuncture ameliorates gastrointestinal dysfunction by modulating DMV cholinergic efferent signals to drive the vagus nerve in p-MCAO rats. Heliyon. 2024;10:e29426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Liu S, Wang Z, Su Y, Qi L, Yang W, Fu M, Jing X, Wang Y, Ma Q. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 2021;598:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 391] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 29. | Jung SJ, Kook MG, Kim S, Kang KS, Soh KS. Homing of the Stem Cells from the Acupoint ST-36 to the Site of a Spinal Cord Injury: A Preliminary Study. J Acupunct Meridian Stud. 2018;11:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Lu MJ, Yu Z, He Y, Yin Y, Xu B. Electroacupuncture at ST36 modulates gastric motility via vagovagal and sympathetic reflexes in rats. World J Gastroenterol. 2019;25:2315-2326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 31. | Lee CH, Jung HS, Lee TY, Lee SR, Yuk SW, Lee KG, Lee BH. Studies of the central neural pathways to the stomach and Zusanli (ST36). Am J Chin Med. 2001;29:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Fang JF, Fang JQ, Shao XM, Du JY, Liang Y, Wang W, Liu Z. Electroacupuncture treatment partly promotes the recovery time of postoperative ileus by activating the vagus nerve but not regulating local inflammation. Sci Rep. 2017;7:39801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Zhang MT, Liang YF, Dai Q, Gao HR, Wang H, Chen L, Huang S, Wang XY, Shen GM. A spinal neural circuit for electroacupuncture that regulates gastric functional disorders. J Integr Med. 2025;23:56-65. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Song J, Yin J, Sallam HS, Bai T, Chen Y, Chen JD. Electroacupuncture improves burn-induced impairment in gastric motility mediated via the vagal mechanism in rats. Neurogastroenterol Motil. 2013;25:807-e635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |