INTRODUCTION
Parkinson's disease (PD)-a progressive neurodegenerative disorder-is characterized by motor symptoms, such as tremors, rigidity, and bradykinesia, and non-motor symptoms, such as gastrointestinal (GI) dysfunction[1-4]. The rising prevalence of PD has driven a global search for effective therapeutic strategies. The gut-brain axis plays a critical role in the development and progression of PD[5-10], and impaired GI function has been implicated in the early stages of PD, preceding the onset of motor symptoms. Pathological substances such as alpha-synuclein produced in the gut can be retrogradely transported to the brain via the vagus nerve, accelerating the pathological process of PD[8]. In addition, the gut-brain axis influences the onset and progression of PD through the interaction between the gut microbiota and the host nervous system[9]. Dysbiosis of the gut microbiota can trigger inflammatory responses and neurotransmitter metabolic disorder. Meanwhile, intestinal barrier dysfunction worsens neuroinflammation in PD by allowing inflammatory factors and toxins to enter the bloodstream[10].
Although the exact mechanism underlying GI dysfunction in PD is not fully understood, inflammation and oxidative stress may be involved in both the gut-brain axis and GI dysfunction[11,12]. Current treatments focus primarily on symptomatic relief rather than addressing the underlying pathophysiology, including inflammation. Therefore, novel interventions that can mitigate these pathological processes are crucial, as existing therapies often exhibit limited efficacy and involve significant side effects.
The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a well-established neurotoxin used to induce PD-like symptoms in animal models, mimicking the dopaminergic neuronal loss and motor deficits observed in human patients with PD[13-15]. MPTP exposure also results in significant GI dysfunction, including constipation and delayed gastric emptying, potentially mediated by inflammation[16,17]. Therefore, ameliorating inflammation in GI dysfunction could be a promising approach to ameliorate both motor and non-motor PD symptoms, providing new therapeutic avenues for the management of PD[18,19].
Rhapontin-a natural compound derived from Rheum palmatum-exhibits laxative effects and has been used in traditional Chinese medicine for centuries[20-23]. Rhapontin also exhibits potent antioxidant and anti-inflammatory properties[24]. Despite the promising pharmacological profile of rhapontin, a significant gap remains regarding its specific molecular mechanism of action in the context of PD. Rhapontin may reduce inflammation by activating the nuclear factor erythroid 2-related factor 2 (NRF2)[25,26]. NRF2 is a transcription factor that plays a pivotal role in regulating cellular responses to oxidative stress and inflammation[27,28]. The precise interactions between rhapontin and the NRF2 pathway in PD, as well as its effects on downstream signaling components, remain largely unexplored. This study aimed to bridge this gap by elucidating the mechanism through which rhapontin exerts its neuroprotective effects, particularly emphasizing its role in modulating oxidative stress, inflammation, and the gut-brain axis in MPTP-induced PD mouse models.
This study involved a multifaceted research approach, including bioinformatics analyses to identify potential rhapontin targets, construction of protein-protein interaction (PPI) networks to determine the signaling pathways involved, and behavioral assessments to assess the effects on motor and non-motor symptoms. By integrating network pharmacology and animal model experiments, this study achieves greater authenticity than pure bioinformatics analysis and higher credibility than approaches relying solely on animal models or molecular techniques. Additionally, we measured the levels of inflammatory markers and NRF2 expression and performed tyrosine hydroxylase-positive (TH+) staining. The specific objectives of this study were to identify the key molecular targets of rhapontin, evaluate its effect on motor and GI symptoms in PD, and investigate its anti-inflammatory effects mediated by NRF2. Our findings contribute valuable insights into the therapeutic viability of rhapontin as a candidate for PD treatment, paving the way for future research and potential clinical applications.
MATERIALS AND METHODS
Network pharmacology analysis
A network pharmacology analysis was conducted to explore the potential mechanisms of action of rhapontin in PD. The Online Mendelian Inheritance in Man (OMIM) and Gene Expression Omnibus (GEO) databases were searched using the term "Parkinson’s disease" to identify relevant genes. Subsequently, we also searched various databases, including the Traditional Chinese Medicine Systems Pharmacology Database (TCMSP; http://tcmspw.com/tcmsp.php), Search Tool for Interactions of Chemicals (STITCH; http://stitch.embl.de/), PharmMapper (http://pharmmapper.shsmu.edu.cn/), Evidence-Based TCM (ETCM; http://www.etcm.cn/), and PubChem (https://pubchem.ncbi.nlm.nih.gov/), to predict rhapontin targets. We used different keywords, such as rhapontin, rhaponitin, poniticin, and rhaponiticin, to enhance the accuracy of our target prediction. For gene selection, we set a threshold of P value < 0.05 and log2 (fold change) > 1 to ensure the identification of significantly associated genes. Only those consistently identified across multiple databases were included in our analysis. Venn diagrams were constructed to identify intersections between the compounds and disease targets. A PPI network was constructed using the STRING database. For PPI analysis, we used a confidence score threshold of 0.7 to filter high-confidence interactions. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed using the Metascape platform.
Animals and treatments
Forty male C57BL/6J mice, aged 8 weeks, were acquired from Hunan Slaughter Jingda Laboratory Animal Co., Ltd. The mice were acclimated to the laboratory conditions for 1 week, with a 12-hour light/dark cycle and ad libitum access to food and water, before the commencement of the experiment. All experimental procedures were approved by the Ethics Committee of Hainan Medical University and complied with the guidelines set forth by the National Institutes of Health for the humane treatment of laboratory animals. Carbon dioxide asphyxiation was used to euthanize all the mice. The mice were randomly divided into the MPTP (Sigma-Aldrich, United States) + rhapontin (Macklin, Shanghai, China) + ML385 (Targetmol, United States) group (n = 10), MPTP + rhapontin group (n = 10), MPTP group (n = 10), and control group (n = 10). Mice were intraperitoneally administered MPTP (20 mg/kg) daily for 4 consecutive days to induce PD, followed by a 3-day recovery period to allow for the manifestation of PD-like symptoms. Rhapontin (20 mg/kg/day) was gavaged to the mice starting 1 week prior to MPTP administration and continuing throughout the experimental period.
Behavioral tests
The open field test was conducted to assess general locomotor activity and exploratory behavior of the mice. Each mouse was placed in the center of a square arena (50 cm × 50 cm) with black walls, and the behavior was recorded for 10 min using a video-tracking system. After each trial, the apparatus was cleaned with 70% ethanol to eliminate olfactory cues. The total distance traveled was quantified to evaluate anxiety-like behaviors and motor function.
The pole-climbing test was used to assess forelimb asymmetry and motor coordination. The mouse was placed at the bottom, facing upward in a vertical wooden dowel (1 cm in diameter and 50 cm in height). The time required for the mouse to descend and reach the bottom was recorded. Each mouse was subjected to three trials, and the average time was calculated. This test is used to measure the asymmetry in forelimb use and the overall motor coordination ability of mice.
Fecal water content was used as an indicator of GI dysfunction. Fresh fecal pellets were collected from each mouse, weighed to obtain the wet weight, and then dry weight was obtained after drying them in an oven at 60 °C for 48 hours. The water content was calculated as the difference between the wet and dry weights and expressed as a percentage of the wet weight.
The number of fecal pellets produced within 15 minutes was used to assess GI motility. The mice were placed individually in clean cages with fresh bedding, and the number of fecal pellets excreted within a 15-minute period was counted. This test is used to quantitatively measure the bowel movement frequency, indicative of GI function.
All behavioral assays were conducted in a quiet, temperature-controlled room during the light phase of the circadian cycle to minimize external influences. The experimenters were blinded to the treatment groups. The data obtained from these tests were analyzed to evaluate the effects of MPTP-induced Parkinsonism and the potential ameliorative effects of rhapontin on motor and GI functions.
Immunofluorescence analysis
Brain sections containing the substantia nigra and striatum were subjected to immunofluorescence analysis to visualize TH+ dopaminergic neurons. Sections were permeabilized with 0.3% Triton X-100, blocked with 5% normal donkey serum, and incubated with a primary antibody against TH (1:500, Servicebio, Wuhan, China) overnight at 4 °C. After washing with phosphate-buffered saline (PBS), the sections were incubated with a secondary antibody conjugated to Alexa Fluor 594 (1:200, Servicebio) for 1 hour at room temperature. Nuclei were counterstained with DAPI, images were captured using a confocal microscope, and the intensity of TH immunoreactivity in the striatum and substantia nigra was quantified using image analysis software.
Enzyme-linked immunosorbent assay
Interleukin (IL)-10, IL-6, IL-1β, and tumor necrosis factor (TNF) levels in the substantia nigra, striatum, and colon tissues were quantified using enzyme-linked immunosorbent assay (ELISA) kits (Thermo Fisher Scientific) following the manufacturer's instructions. Tissues were homogenized in PBS containing a protease inhibitor cocktail, and supernatants were collected after centrifugation. ELISA plates were coated with specific antibodies, blocked, and incubated with the tissue supernatants and standards. After incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies and TMB substrate, absorbance was measured at 450 nm. The optical density values were plotted against the standard curve to determine cytokine concentrations in the samples, calculated in pg/mL and normalized to the total protein content of the tissue samples, which was determined using a BCA protein assay kit (Thermo Fisher Scientific).
Western blotting
NRF2 expression in the substantia nigra, striatum, and colon tissues was analyzed by western blotting. Briefly, tissues were homogenized in radioimmunoprecipitation assay buffer, and protein concentrations were measured using a BCA protein assay kit. Equal amounts of protein were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, transferred onto polyvinylidene fluoride membranes, and incubated with primary antibodies against NRF2 (1:1000, Servicebio) and β-actin (1:5000, Servicebio) overnight at 4 °C. Membranes were then incubated with HRP-conjugated secondary antibodies (1:2000, Servicebio), and bands were visualized using an enhanced chemiluminescence detection system.
Statistical analysis
Data are presented as the mean ± SE. Statistical comparisons between the groups were performed using t-tests (our data met the assumptions of normality and equal variances). Statistical significance was set at P < 0.05. All statistical analyses were conducted using GraphPad Prism 8.0 software.
RESULTS
PPI network and key targets of rhapontin in PD
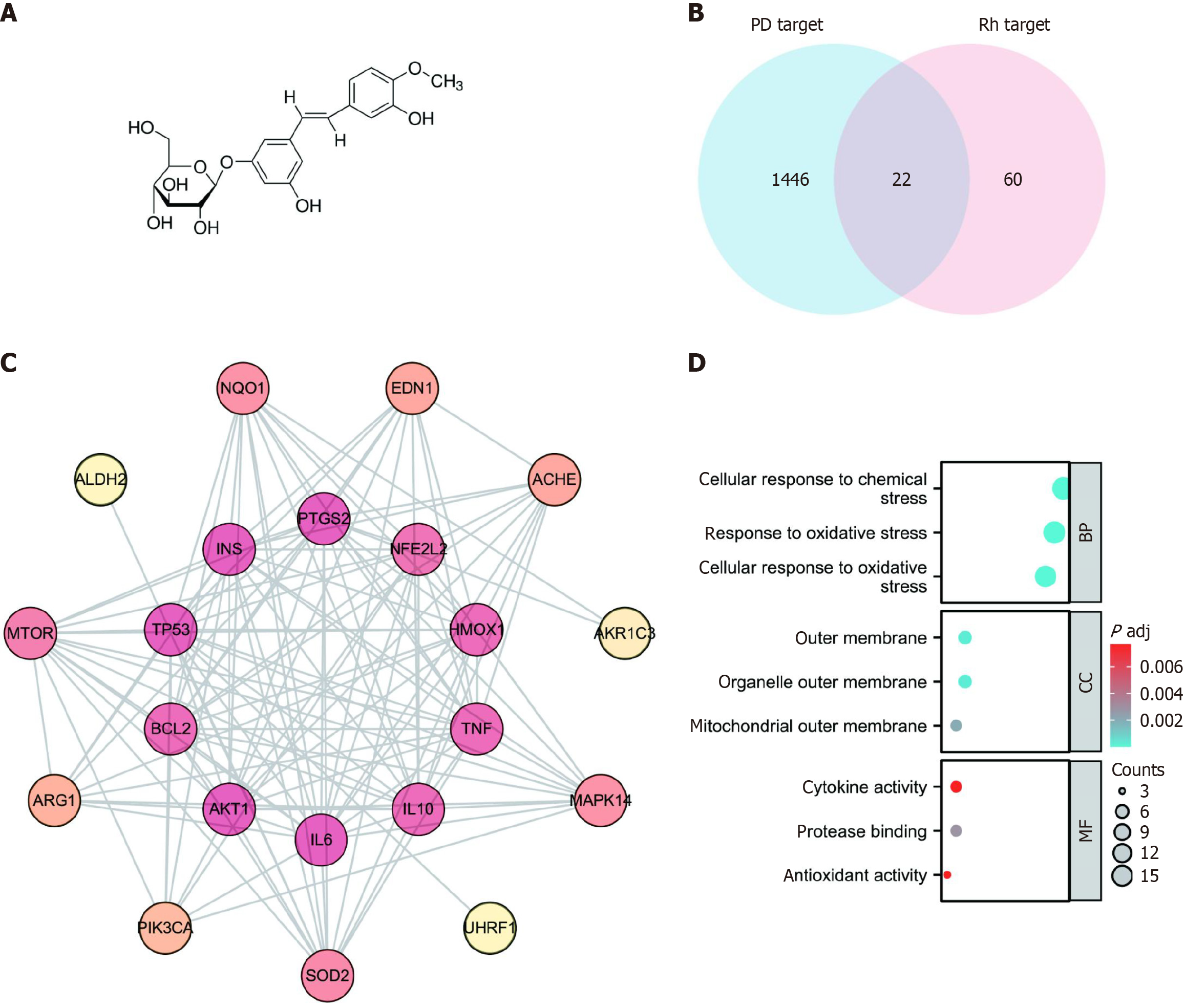
Figure 1A illustrates the chemical structure of rhapontin. We identified 83 genes from TCMSP, STITCH, PharmMapper, ETCM, and PubChem databases after integrating and deleting the reduplicated terms (Supplementary material). Furthermore, a total of 1468 genes were identified from OMIM and GEO databases after integrated deduplication (Supplementary material). The 22 overlapping genes from the two datasets were identified as potential rhapontin targets in PD (Figure 1B). Subsequently, the PPI network was constructed using the STRING database. The top 10 key targets were NFE2 L2, HMOX1, TNF, IL10, IL6, AKT1, BCL2, TP53, INS, and PTGS2 (Figure 1C). We then performed KEGG pathway enrichment analysis on potential therapeutic targets to gain insight into the key physiological mechanisms of rhapontin in PD. The response to oxidative stress and antioxidant activity were critical biological processes and molecular functions of rhapontin in the treatment of PD (Figure 1D).
Figure 1 The network pharmacology analysis of rhapontin treatment of Parkinson's disease.
A: The chemical structure of rhapontin; B: Venn diagram of overlapping targets associated with rhapontin in Parkinson's disease (PD); C: Protein-protein interaction of potential targets of rhapontin to treat PD. The deeper the color, the greater the degree. The line between two nodes represents the interaction; D: Gene ontology functional enrichment analysis. PD: Parkinson's disease; PPI: Protein-protein interaction; GO: Gene ontology; BP: Biological process; CC: Cell component; MF: Molecular function.
Motor symptoms in the MPTP-induced mouse model were alleviated by rhapontin by activating NRF2
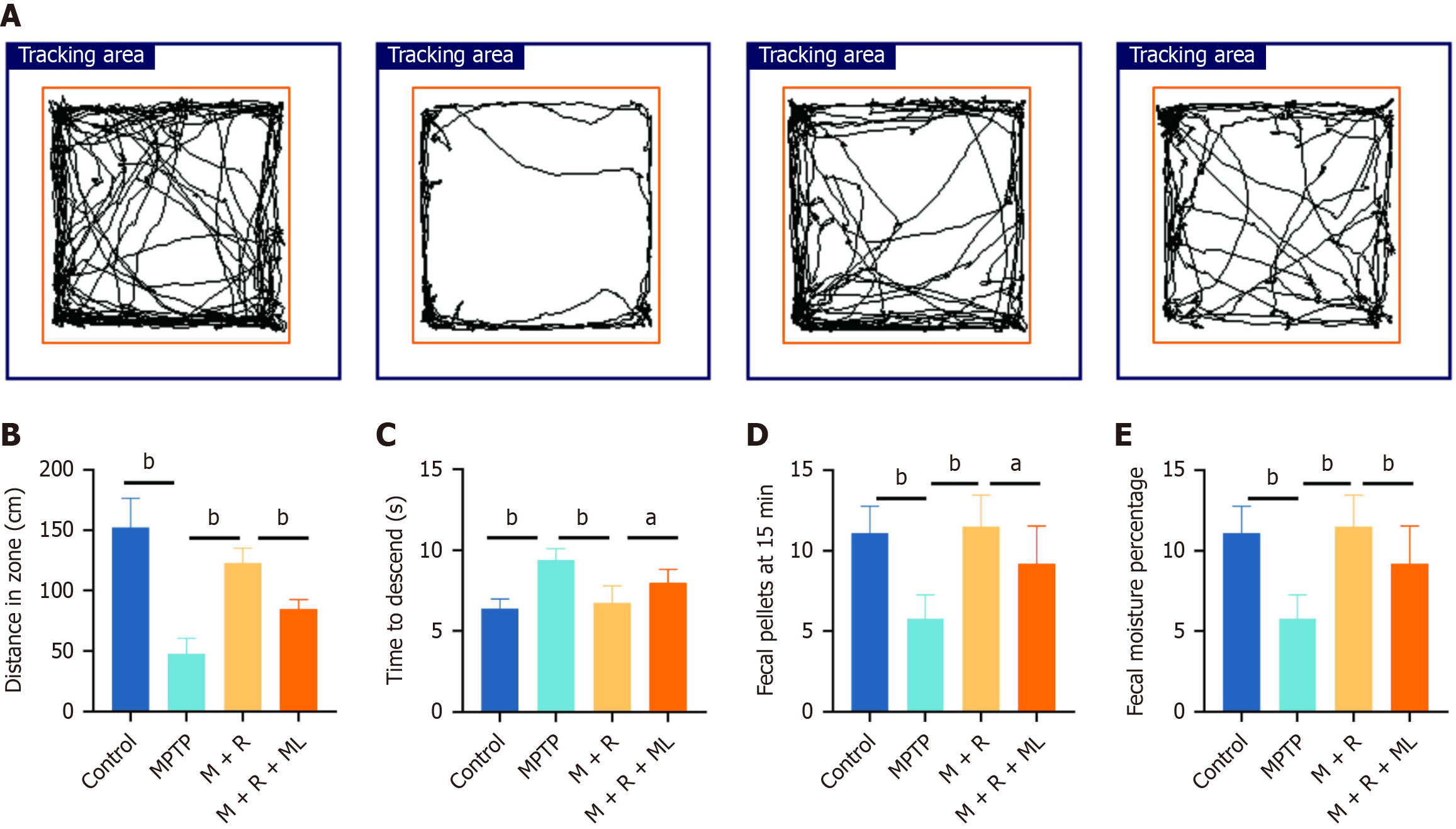
The above results suggested NRF2 as a key transcription factor underlying rhapontin mechanism of action in PD owing to its critical role in antioxidant and anti-inflammatory activities. Subsequently, we evaluated rhapontin effects on movement disorders and GI dysfunction in MPTP-induced mice using behavioral tests. Figure 2A presents the representative tracks from the open field test. Rhapontin restored the distance traveled by MPTP-induced mice (P < 0.01). The mice in the MPTP + rhapontin + ML385 group traveled shorter distances in the open field test than those in the MPTP + rhapontin group (P < 0.01; Figure 2B). Furthermore, MPTP-induced mice took more time to climb down the pole in the pole-climbing task than mice in the control (P < 0.01) and MPTP + rhapontin groups (P < 0.01). However, the NRF2 inhibitor, ML385, partly abolished the effect of rhapontin on the time taken to climb down the pole (P < 0.05) (Figure 2C).
Figure 2 Rhapontin ameliorates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced motor and gastrointestinal dysfunction of Parkinson's disease mice.
A: Representative traces of the open field test; B: Distance of the open field test; C: Time to descend of pole test; D: Fecal pellets of each group at 15 minutes; E: Fecal moisture percentage of each group; n = 10. aP < 0.05; bP < 0.01; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; M: MPTP; R: Rhapontin; ML: ML385.
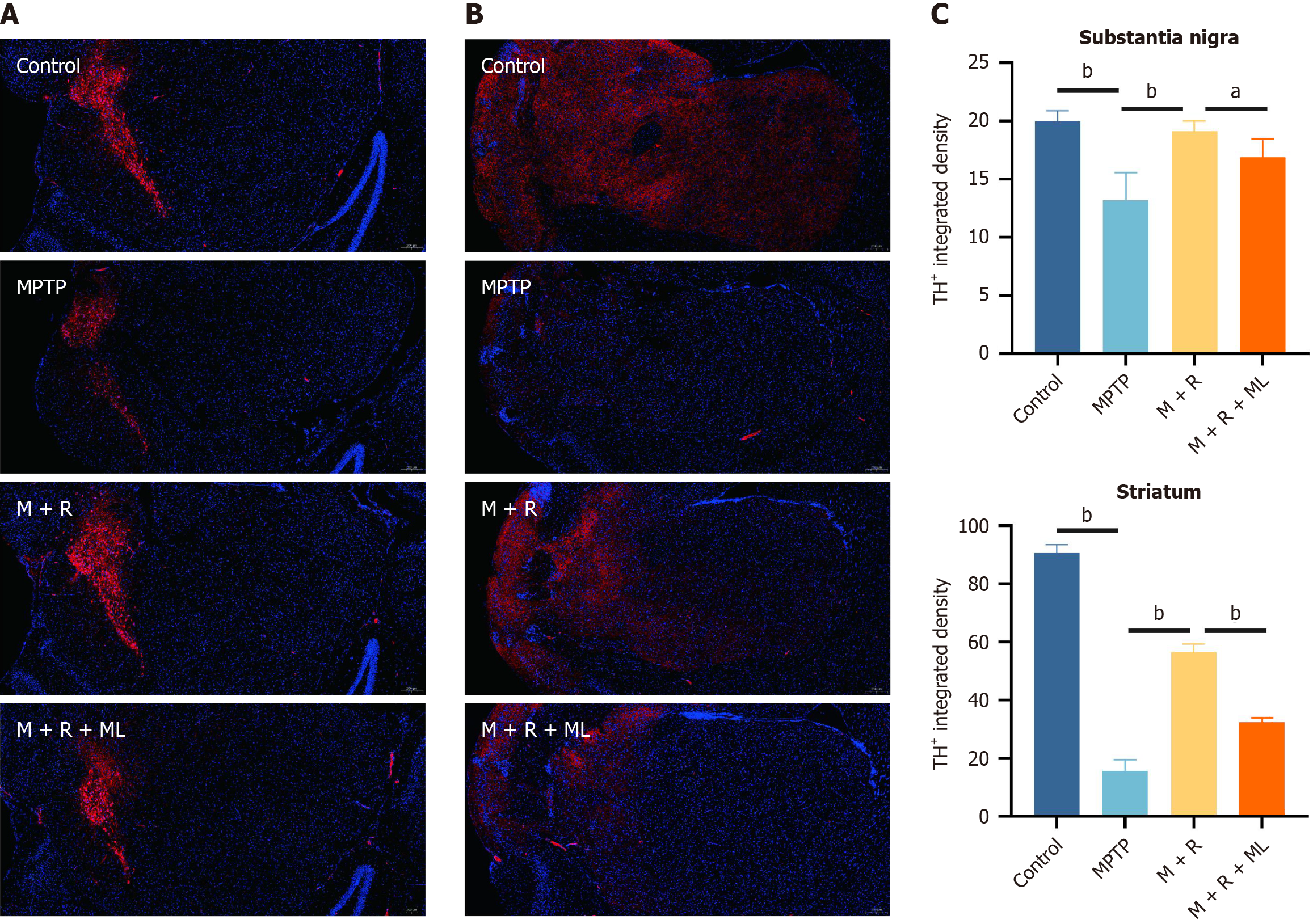
Figure 3A and B shows dopaminergic neurons in each group based on the TH staining. We further analyzed the pathological changes. Notably, we observed the enhanced fluorescence intensity of TH+ cells in the substantia nigra (P < 0.01) and striatum (P < 0.01) of the mice in the MPTP + rhapontin group compared with that in the mice in the MPTP group. However, ML385 treatment in the rhapontin + MPTP group decreased fluorescence intensity of TH+ cells in the substantia nigra (P < 0.05) and striatum (P < 0.01; Figure 3C).
Figure 3 Rhapontin ameliorates dopaminergic neuronal damage of Parkinson's disease mice.
A: Representative immunofluorescence images of TH-positive cells in the substantia nigra; B: Representative immunofluorescence images of TH-positive cells in the striatum; C: Quantification analysis of TH-positive cell number in Substantia nigra and Striatum. n = 10. aP < 0.05; bP < 0.01; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; M: MPTP; R: Rhapontin; ML: ML385.
GI dysfunction in the MPTP-induced mouse model was ameliorated by rhapontin by activating NRF2
A series of experiments focusing on fecal pellets and fecal moisture were conducted to investigate rhapontin effects on GI dysfunction in the MPTP-induced PD mice (Figure 2D and E). MPTP-treated mice exhibited a significant reduction in the number of fecal pellets produced within 15 minutes compared with those in the control group (P < 0.01). Rhapontin treatment significantly increased the number of fecal pellets in the MPTP-induced PD mice (P < 0.01), indicating an improvement in GI motility. Additionally, fecal water content was significantly lower in the mice in the MPTP group than in the control group (P < 0.01). Rhapontin administration to MPTP-induced PD mice considerably increased their fecal water content, further supporting its beneficial effects on GI function (P < 0.01). Next, we co-administered ML385 and rhapontin to explore the underlying mechanisms. ML385 partially inhibited the beneficial effects of rhapontin, indicating that the improvement in GI function [fecal pellets (P < 0.05) and fecal moisture (P < 0.01)] by rhapontin may be mediated through a pathway involving NRF2 to some extent.
Rhapontin exerts its anti-inflammatory effects by activating NRF2, particularly in the colon
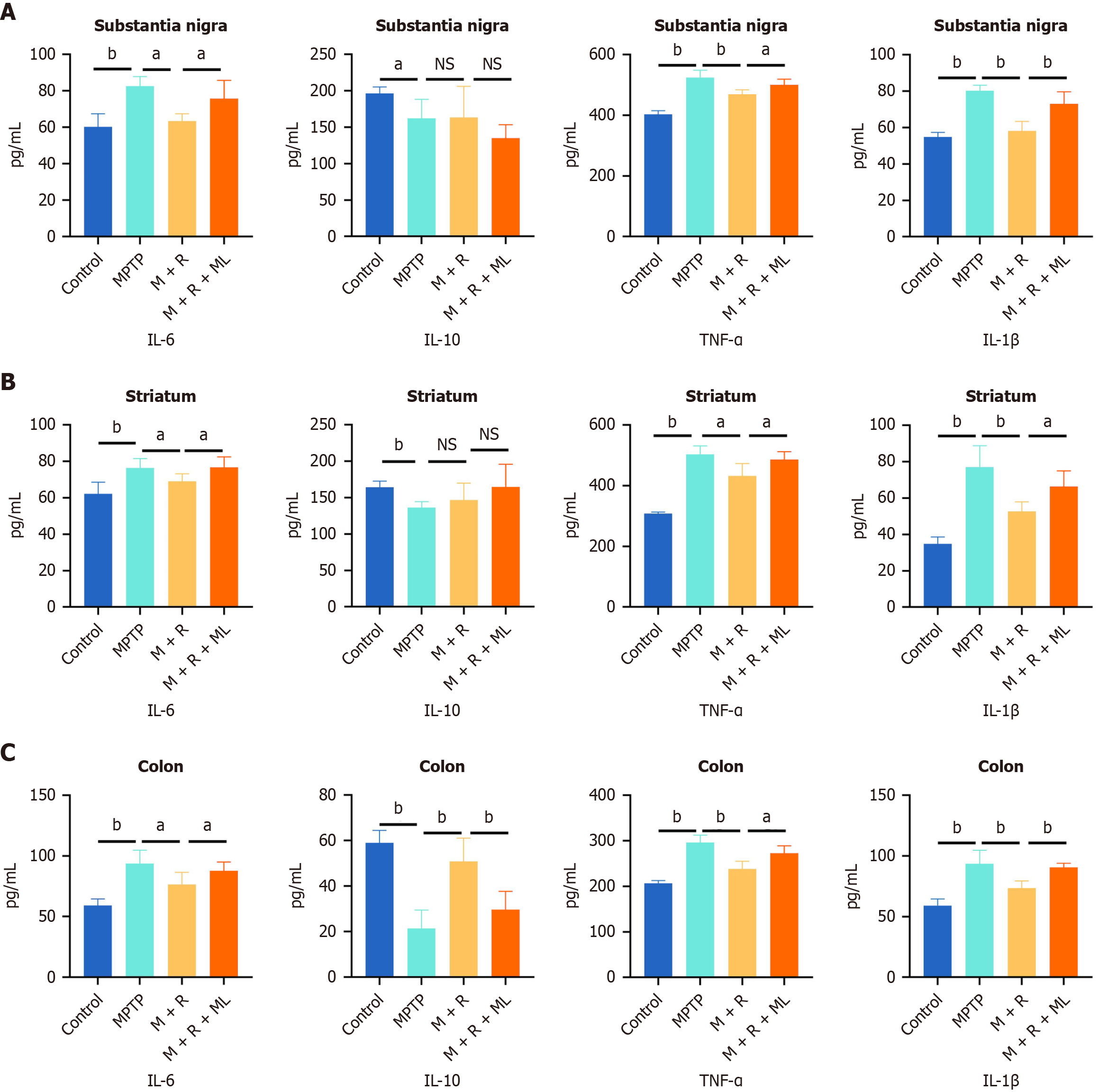
We quantified IL-6, IL-10, TNF-α, and IL-1β levels in the substantia nigra, striatum, and colon to investigate the anti-inflammatory effects of rhapontin in the MPTP-induced PD mouse model using ELISA. Additionally, we determined NRF2 expression in these regions using western blotting.
In the substantia nigra, MPTP treatment remarkably increased IL-6, TNF-α, and IL-1β levels compared with their levels in the substantia nigra of the mice in the control group (P < 0.01). However, rhapontin administration reduced the levels of these pro-inflammatory cytokines (Figure 4A), although the levels of IL-10, an anti-inflammatory cytokine, did not significantly change in the MPTP and MPTP + rhapontin groups (Figure 4A).
Figure 4 Rhapontin ameliorates inflammation of Parkinson's disease mice.
A: Quantification analysis of pro-inflammatory cytokines [Interleukin (IL)-6, tumor necrosis factor-α, and IL-1β] and anti-inflammatory cytokines (IL-10) in the substantia nigra; B: Quantification analysis of pro-inflammatory cytokines and anti-inflammatory cytokines in the striatum; C: Quantification analysis of pro-inflammatory cytokines and anti-inflammatory cytokines in the colon; n = 10. aP < 0.05; bP < 0.01; NS: Not significant; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; IL: Interleukin; TNF-α: Tumor necrosis factor-α; M: MPTP; R: Rhapontin; ML: ML385.
Similar trends were observed in the striatum. MPTP treatment significantly increased IL-6, TNF-α, and IL-1β levels (P < 0.01). However, rhapontin treatment attenuated these effects (Figure 3B), although IL-10 Levels did not significantly differ between the MPTP and MPTP + rhapontin groups (Figure 4B).
MPTP treatment increased IL-6, TNF-α, and IL-1β levels in the colon (P < 0.01). However, rhapontin treatment significantly reduced their levels compared with the MPTP group (Figure 4C). Unlike in the substantia nigra and striatum, IL-10 Levels were significantly elevated in the colon in the rhapontin-treated group compared with those in the MPTP group (P < 0.01; Figure 4C).
ML385 partially reversed the anti-inflammatory effects of rhapontin in all three regions, as indicated by increased IL-6, TNF-α, and IL-1β levels (Figure 4).
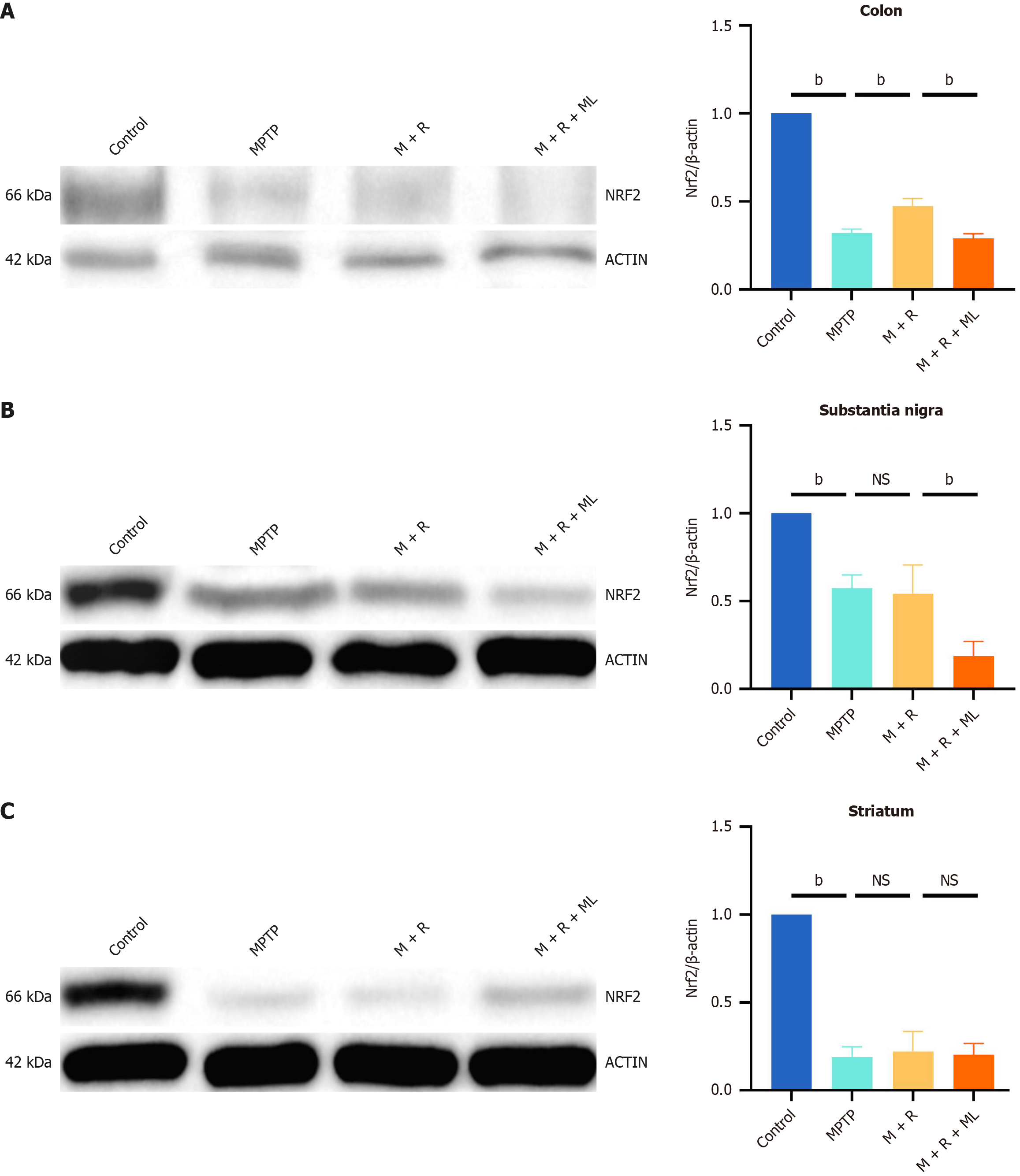
Western blotting revealed significantly increased NRF2 expression in the colon following rhapontin treatment (P < 0.01; Figure 5A). However, NRF2 expression in the substantia nigra and striatum did not significantly change in the MPTP and MPTP + rhapontin groups (Figure 5B and C).
Figure 5 Rhapontin activates nuclear factor erythroid 2-related factor 2 in the colon.
A: Western Blot image and analysis of nuclear factor erythroid 2-related factor 2 (NRF2) in colon; B: Western blot image and analysis of NRF2 in substantia nigra; C: Western blot image and analysis of NRF2 in striatum. n = 10. aP < 0.05; bP < 0.01; NS: Not significant; NRF2: Nuclear factor erythroid 2-related factor 2; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; M: MPTP; R: Rhapontin; ML: ML385.
These findings suggest that the gut-brain axis may play a crucial role in the protective effects of rhapontin against MPTP-induced inflammation in PD, and rhapontin exerts its anti-inflammatory effects by activating NRF2, particularly in the colon. We selected an appropriate sample size based on previous studies[29,30] and in accordance with the principle of minimizing the use of experimental animals. However, the modest sample size used in the experimental design may limit the statistical power and generalizability of the conclusions drawn. Future studies should address these limitations by incorporating larger cohorts and conducting clinical trials to verify the efficacy and safety of rhapontin in human populations, enhancing the robustness of our findings.
DISCUSSION
PD is a prevalent neurodegenerative disorder characterized by progressive loss of dopaminergic neurons in the substantia nigra, leading to debilitating motor and non-motor symptoms[31]. Clinical manifestations include tremors, rigidity, bradykinesia, and postural instability, which significantly affect the quality of life of affected individuals. Moreover, the increasing incidence of PD, particularly in the aging population, underscores the urgent need for effective therapeutic interventions to manage its symptoms and slow disease progression[32]. Current pharmacological strategies primarily focus on dopaminergic replacement therapy; however, they often fail to address the underlying pathophysiological processes, such as oxidative stress and neuroinflammation, which contribute to neuronal degeneration[32,33].
The earliest pathological changes in PD originate in the gut[34], as GI symptoms, such as constipation, often precede the onset of motor symptoms by several years[35]. The gut, which acts as a critical interface between the external environment and the internal milieu, is particularly susceptible to oxidative stress and inflammation, which are key contributors to PD pathogenesis[36]. Inflammation in the gut may trigger neuroinflammation in PD[37]. The activation of enteric glial cells and the subsequent release of pro-inflammatory cytokines can lead to a cascade of events, including increased intestinal permeability, oxidative stress, and the propagation of neuroinflammatory signals to the central nervous system (CNS)[38]. This gut-brain axis is further reinforced by the role of the gut microbiota, which can modulate immune responses and oxidative stress, thereby influencing PD progression[39]. Moreover, the functional impairment of the gut in PD is intricately linked to oxidative stress and inflammation[40]. In PD, oxidative stress can exacerbate intestinal inflammation, leading to a vicious cycle of tissue damage and further oxidative stress[41]. The resulting inflammation propagates to the CNS, contributing to the degeneration of dopaminergic neurons in the substantia nigra pars compacta[42]. Collectively, the interplay among gut inflammation, oxidative stress, and neuroinflammation provides a comprehensive framework for understanding the early stages and progression of PD.
Rhapontin is increasingly recognized for its potential therapeutic benefits in GI disorders[29]. In this study, rhapontin effectively alleviated constipation symptoms in PD by modulating intestinal motility and improving fecal water content. This effect is likely mediated by its ability to inhibit GI inflammation and improve the overall gut environment. Specifically, rhapontin activated the NRF2 pathway in the colon, thereby suppressing the intestinal inflammation and improving GI function in mice with PD. Notably, NRF2 activation in the colon did not extend to the substantia nigra or striatum, suggesting a specific and localized effect on the GI tract. Furthermore, rhapontin may act via the gut-brain axis by activating NRF2 in the colon and reducing gut inflammation, thereby attenuating the systemic inflammatory response and oxidative stress that contribute to neuronal damage in PD. These insights provide a compelling rationale for further exploration of rhapontin as a novel therapeutic option for PD, potentially addressing both symptom management and disease progression.
NRF2, a transcription factor known for its role in antioxidant defense, plays a key role in maintaining the cellular redox balance and protecting against oxidative stress-induced damage[43]. In our study, rhapontin significantly increased NRF2 expression in the colon of mice with PD, which reduced oxidative stress and inflammation by upregulating downstream antioxidant genes. The localized activation of NRF2 in the colon rather than in the brain suggests that rhapontin primarily targets the GI tract to exert anti-inflammatory effects. NRF2 activation by rhapontin in the colon reduced the expression of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, which contribute to the development and progression of inflammatory bowel disease and other GI disorders[44]. Furthermore, rhapontin effectively alleviated intestinal inflammation by suppressing pro-inflammatory cytokines, which is a common feature of patients with PD[41]. This finding is consistent with those of previous studies highlighting the importance of NRF2 in the protection against intestinal inflammation. Rhapontin also improved GI function in mice with PD, evident from the normalization of bowel movements and reduction in constipation symptoms. Improvement in GI function is likely mediated by the suppression of intestinal inflammation and NRF2 activation, which together help maintain the integrity of the intestinal barrier and promote normal bowel function.
Indeed, beyond the canonical antioxidant pathways, NRF2 activation influences several other molecular pathways that may contribute to the effects of rhapontin on PD. For example, NRF2 can inhibit the NF-κB signaling pathway, which is a pivotal mediator of inflammation[45]. By reducing the expression of pro-inflammatory cytokines and chemokines, NRF2 activation can shift the inflammatory response towards an anti-inflammatory phenotype, thereby mitigating inflammation. Additionally, NRF2 prevents the formation of the NLRP3 inflammasome, which is a key regulator of neuroinflammatory processes[46].
In addition to the molecular mechanisms, this study highlights the implications of rhapontin on the gut-brain axis. Unlike NRF2 activation in the colon, its activation was not observed in the substantia nigra and striatum following rhapontin treatment. However, rhapontin inhibited the expression of pro-inflammatory cytokines in these brain regions, indicating that its beneficial effects on the brain may be indirectly mediated by its action in the GI tract. The gut-brain axis-a bidirectional communication network between the gut and brain-plays a crucial role in the pathogenesis of PD[47]. By improving GI function and reducing intestinal inflammation, rhapontin may help reduce systemic inflammation, which contributes to PD progression.
The observation that IL-10 levels were significantly increased in the colon, but not in the brain, is intriguing. This localized effect in the colon may be attributed to the unique immunoregulatory environment of the gut, which is highly responsive to anti-inflammatory signaling. IL-10 is a key anti-inflammatory cytokine that limits immune cell activation and cytokine production in innate immune cell types[48]. The localized increase in IL-10 in the colon may contribute to the neuroprotective properties of rhapontin through several mechanisms. Its activation, specifically in the colon, is driven by the gut microbiota, which is known to modulate local immune responses through the production of short-chain fatty acids and other metabolites. These metabolites enhance IL-10 expression, thereby suppressing pro-inflammatory pathways and maintaining gut homeostasis[49]. In contrast, IL-10 can influence the gut microbiota composition, thus promoting a more anti-inflammatory microbial profile that further supports the overall immune balance.
Furthermore, IL-10 promotes microglial phagocytic and clearance activities[50]. IL-10 expression did not significantly increase in the substantia nigra or striatum in our study, suggesting that IL-10 activation was primarily localized in the colon. This localized effect of rhapontin on IL-10 expression was consistent with that on NRF2. The observed reduction in pro-inflammatory cytokines in the substantia nigra and striatum, along with the elevation of anti-inflammatory cytokines such as IL-10 only in the colon, indicates that rhapontin mitigates neuroinflammation by affecting GI health, which is often compromised in patients with PD. Therefore, rhapontin plays a dual role in modulating both central and peripheral inflammatory responses, thereby improving overall patient outcomes. Understanding the relationship between gut health and neuroinflammation further emphasizes the need for a holistic approach in the management of PD, wherein the gut-brain axis could be a significant target for therapeutic interventions.
Recent studies have highlighted the critical role of the gut-brain axis in PD pathogenesis[41]. Rhapontin’s effects on the colon, particularly its ability to reduce gut inflammation, may indirectly influence PD progression through this axis. Our study suggests that the anti-inflammatory properties of rhapontin in the gut can reduce inflammation in the CNS, which may be a key factor in delaying the progression of PD. Recent study indicate that α-synuclein pathology is induced by GI inflammation and then transfers to the brain by the gut-brain axis[51]. Rhapontin’s impact on gut inflammation may reduce the accumulation of α-synuclein in the GI tract, which is known to spread to the brain and induce PD pathology. The relationship between intestinal inflammation and gut microbiota was close in PD[52]. Rhapontin may inhibit intestinal inflammation by modulating the gut microbiota, similar to many traditional Chinese medicine compounds[53]. Meanwhile, the inhibition of intestinal inflammation by rhapontin may also affect the gut microbiota. This crosstalk is worth further exploration. To further explore we will investigate the direct impact of rhapontin on α-synuclein pathology in both gut and brain models. Examine the role of rhapontin in modulating the gut microbiota and its downstream effects on neuroinflammation. We believe that further exploration of these mechanisms will provide valuable insights.
This study also has some limitations. First, the absence of clinical validation restricts the translational potential of the results to humans. Second, the integration of multiple datasets introduces variability, which could potentially skew the results and complicate data interpretation. Finally, this study has yet to fully elucidate the specific molecular mechanisms underlying rhapontin-mediated upregulation of the NRF2 pathway. While we have explored the role of the NRF2/IL-10 in the gut-brain axis of PD, the precise mechanism by which rhapontin activates NRF2 remains an area for further investigation in future studies.
We can speculate that rhapontin activates NRF2 in the colon, which subsequently promotes the increase of IL-10 and suppresses intestinal inflammation. This effect may alleviate neuroinflammation through the gut-brain axis, rather than by directly activating NRF2 in the brain. However, these conclusions lack clinical data support. For future clinical trials on rhapontin, we recommend a sample size of at least 100 participants, selected based on age (18-65 years), absence of chronic diseases, and normal lifestyle habits. The intervention should include an 8-12-week rhapontin administration with a placebo-controlled design. Outcome measures should focus on changes in gut microbiota composition (via metagenomic sequencing) and key physiological markers (e.g., inflammatory and metabolic profiles). Long-term follow-up (1-2 years) is crucial for assessing safety and efficacy. Multi-center trials and individualized analyses are also advised to enhance study robustness and applicability. The detailed experimental design needs to be refined in future clinical studies. Furthermore, questions concerning the clinical dosage, bioavailability, and biosafety of rhapontin remain to be answered, and these explorations are vital for successful clinical translation.