Published online Jan 14, 2023. doi: 10.3748/wjg.v29.i2.332
Peer-review started: August 10, 2022
First decision: October 20, 2022
Revised: October 25, 2022
Accepted: November 18, 2022
Article in press: November 18, 2022
Published online: January 14, 2023
Processing time: 145 Days and 3.5 Hours
Magnesium (Mg2+) has an important role in numerous biological functions, and Mg2+ deficiency is associated with several diseases. Therefore, adequate intestinal absorption of Mg2+ is vital for health. The small intestine was previously thought to absorb digested Mg2+ exclusively through an unregulated paracellular mechanism, which is responsible for approximately 90% of total Mg2+ absorption. Recent studies, however, have revealed that the duodenum, jejunum, and ileum absorb Mg2+ through both transcellular and paracellular routes. Several regulatory factors of small intestinal Mg2+ uptake also have been explored, e.g., parathyroid hormone, fibroblast growth factor-23, apical acidity, proton pump inhibitor, and pH-sensing channel and receptors. The mechanistic factors underlying proton pump inhibitor suppression of small intestinal Mg2+, such as magnesiotropic protein dysfunction, higher mucosal bicarbonate secretion, Paneth cell dysfunction, and intestinal inflammation, are currently being explored. The potential role of small intestinal microbiomes in Mg2+ absorption has also been proposed. In this article, we reviewed the current knowledge on the mechanisms and regulatory factors of small intestinal Mg2+ absorption.
Core Tip: Small intestinal epithelium absorbs digested magnesium (Mg2+) through both transcellular active and paracellular passive mechanisms. Several regulatory factors of small intestinal Mg2+ uptake have been reported. Parathyroid hormone and fibroblast growth factor-23 directly inhibit transcellular Mg2+ absorption in the duodenum, jejunum, and ileum. The apical proton triggers acid-sensing ion-channel 1a and purinergic P2Y2 receptor activities, which stimulates mucosal bicarbonate secretion and induces MgCO3 precipitation to suppress absorption. Omeprazole suppresses Mg2+ absorption in the duodenum, jejunum, and ileum.
- Citation: Chamniansawat S, Suksridechacin N, Thongon N. Current opinion on the regulation of small intestinal magnesium absorption. World J Gastroenterol 2023; 29(2): 332-342
- URL: https://www.wjgnet.com/1007-9327/full/v29/i2/332.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i2.332
Magnesium (Mg2+) has an essential role in numerous cellular biochemical functions ranging from DNA structure stability and repairing, cell proliferation, neuronal excitability, bronchodilatation, vasodilatation, muscle contraction, myocardial excitability, bone hydroxyapatite formation, and anti-inflammatory function to exocrine and endocrine function of the pancreas[1]. Mg2+ deficiency has been implicated in several diseases, such as Alzheimer’s disease[2], osteoporosis[3], hypertension[4], diabetes mellitus[5], and cancer[6]. Therefore, its plasma level is tightly regulated within a narrow range (0.7–1.1 mmol/L) by the collaborative actions of intestinally digested Mg2+ absorption, bone and muscle Mg2+ storage, and excess renal Mg2+ excretion[1]. The mechanism underlying regulation of transepithelial Mg2+ transport has been extensively explored in the renal tubular epithelium[1]. However, few research articles on the mechanism and regulatory factors of intestinal Mg2+ absorption have been published.
Since dietary intake is the sole source of Mg2+ in humans, adequate intestinal absorption of Mg2+ is vital for normal Mg2+ balance. It was previously hypothesized that bulk Mg2+ uptake occurs in the small intestine through an unregulated paracellular pathway, whereas fine-tuning of colonic Mg2+ absorption occurs through a regulated transcellular mechanism[1,7,8]. Colonic Mg2+ absorption can be modulated by dietary Mg2+ content and inulin fibers[7,9] but not by hormones[1,7]. In contrast, recent studies have provided new insights into the mechanisms and modulatory factors of small intestinal Mg2+ uptake. The aim of this article was to review the current knowledge of the mechanisms and regulatory factors of small intestinal Mg2+ absorption.
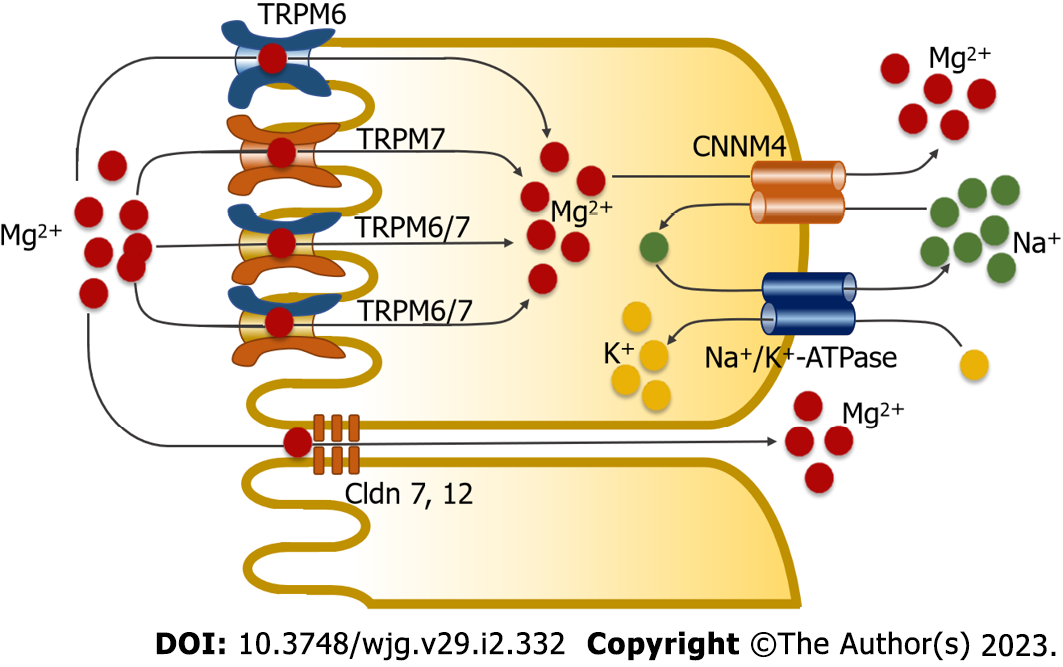
The mechanism of small intestinal Mg2+ absorption is currently under debate. One research group has proposed that transient receptor potential melastatin 6 homodimer channel (TRPM6) mRNA expression and transcellular Mg2+ absorption were not present in the small intestine[1,7,8]. However, a study from the same group showed positive immunofluorescence staining of TRPM6 protein in the absorptive cells along the brush border membrane of the villi in the duodenum[10]. Another group has proposed that the small intestinal epithelium absorbs Mg2+ through transcellular active and paracellular passive transport mechanisms[11-13]. In an Ussing chamber study, transport of transcellular and paracellular Mg2+ was detected in the duodenum, jejunum, and ileum[11-13]. The proposed mechanism of small intestinal Mg2+ absorption is shown in Figure 1.
Transcellular Mg2+ absorption occurs through mucosal Mg2+ uptake by TRPM6 and TRPM7 homodimer channel, both of which were markedly detected in the small intestinal epithelium of human and murine cells[10-14]. In addition, recent mass spectrometric peptide sequence analysis confirmed the expression of TRPM6 and TRPM7 in the duodenum and jejunum[15]. The channel activities of both homodimers of TRPM6 and of TRPM7 are negatively regulated by physiological Mg·ATP and Mg2+ levels[10,16-19]. A recent study reported the expression of a heterodimer TRPM6/7 channel in the plasma membrane of duodenal and jejunal epithelium[15]; therefore, Mg2+ enters the small intestinal epithelial cells through TRPM6/7, TRMP6, and TRPM7. However, the heterodimer TRPM6/7 channels do not respond to physiological intracellular Mg2+ and Mg·ATP[17,19]; thus, continuous epithelial Mg2+ absorption can occur through the TRPM6/7 channel, regardless of intracellular Mg2+ and concentrations. Basolateral Mg2+ extrusion from the small intestinal epithelium occurs through cystathionine β-synthase domain divalent metal cation transport mediator 4[11-13,20] by means of a sodium (Na+) gradient-dependent secondary active transport[20]. However, mutation of cystathionine β-synthase domain divalent metal cation transport mediator 4 does not affect the plasma concentration in humans[21,22], suggesting that other Mg2+ extrusion mechanisms probably occur.
It has been suggested that paracellular Mg2+ absorption is responsible for 90% of total intestinal Mg2+ uptake[23]. Paracellular permeability is regulated by the paracellular claudin (Cldn) channel of the tight junction[24]. In 1999, the first discovery of a paracellular channel at the tight junction was Cldn-19 or paracellin-1, which form a paracellular Mg2+ channel[25]. It is thought that paracellular Mg2+ channels in epithelial tissues are formed by Cldn-16 and -19[25-27]; mutations in these genes lead to severe hypomagnesemia. The small intestinal epithelium expresses Cldn-1–5, -7, -8, -12, and -15 but not -16 and -19[28,29]. A previous study proposed that Cldn-7 and -12 modulated intestinal paracellular Mg2+ absorption[30]. However, the processes involving Cldn-regulated paracellular Mg2+ absorption in the small intestine still must be elucidated.
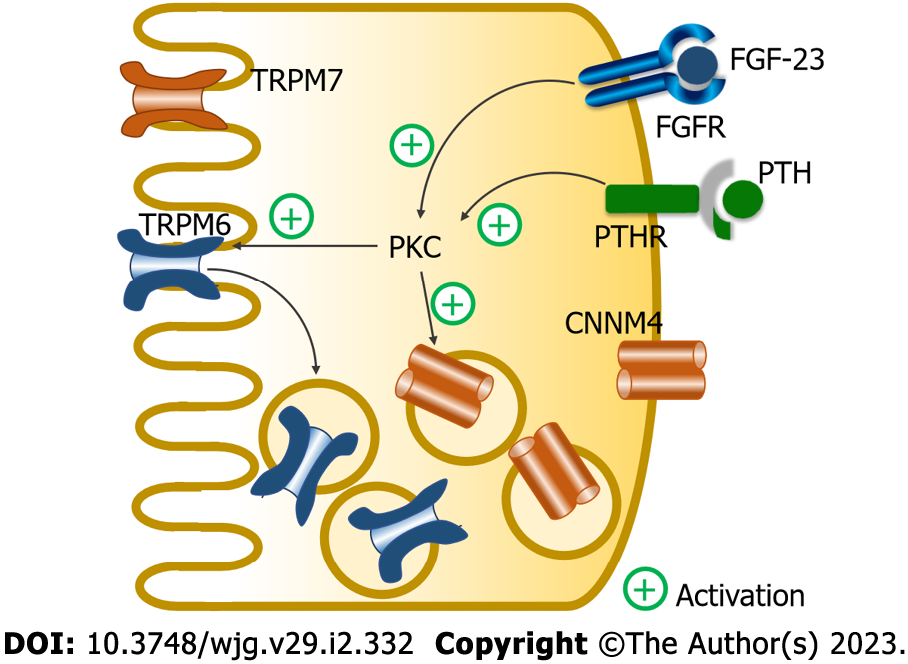
In general, hormones mainly modulate the transcellular electrolyte transport to regulate epithelial electrolyte absorption or secretion. Hormonal regulation of small intestinal Mg2+ absorption also modulates transcellular Mg2+ absorption. A recent study reported that parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF-23) systemically and directly inhibited transcellular, but not paracellular, Mg2+ absorption in the duodenum, jejunum, and ileum[13]. There was no additional effect of PTH and FGF-23, suggesting that they acted through the same intracellular signaling molecule. Both PTH and FGF-23 activate their corresponding receptors that further stimulate the same protein kinase C pathway to suppress plasma membrane-associated TRPM6 expression (Figure 2). Since native TRPM6 primarily functions as a subunit of heteromeric TRPM6/7 channels[31], the suppression of plasma membrane TRPM6 probably suppresses plasma TRPM6/7 heterodimer expression. The suppression of plasma TRPM6 and TRPM6/7 activity leads to diminution of transcellular Mg2+ absorption[13]. The inhibitory effect of PTH and FGF-23 could be nullified by Gö 6850[13], which inhibits the conventional (α, β1, β2, and γ) and novel (δ and ε) protein kinase C isoforms. However, the exact signaling pathway of PTH and FGF-23 inhibition of small intestinal transcellular Mg2+ absorption requires further study.
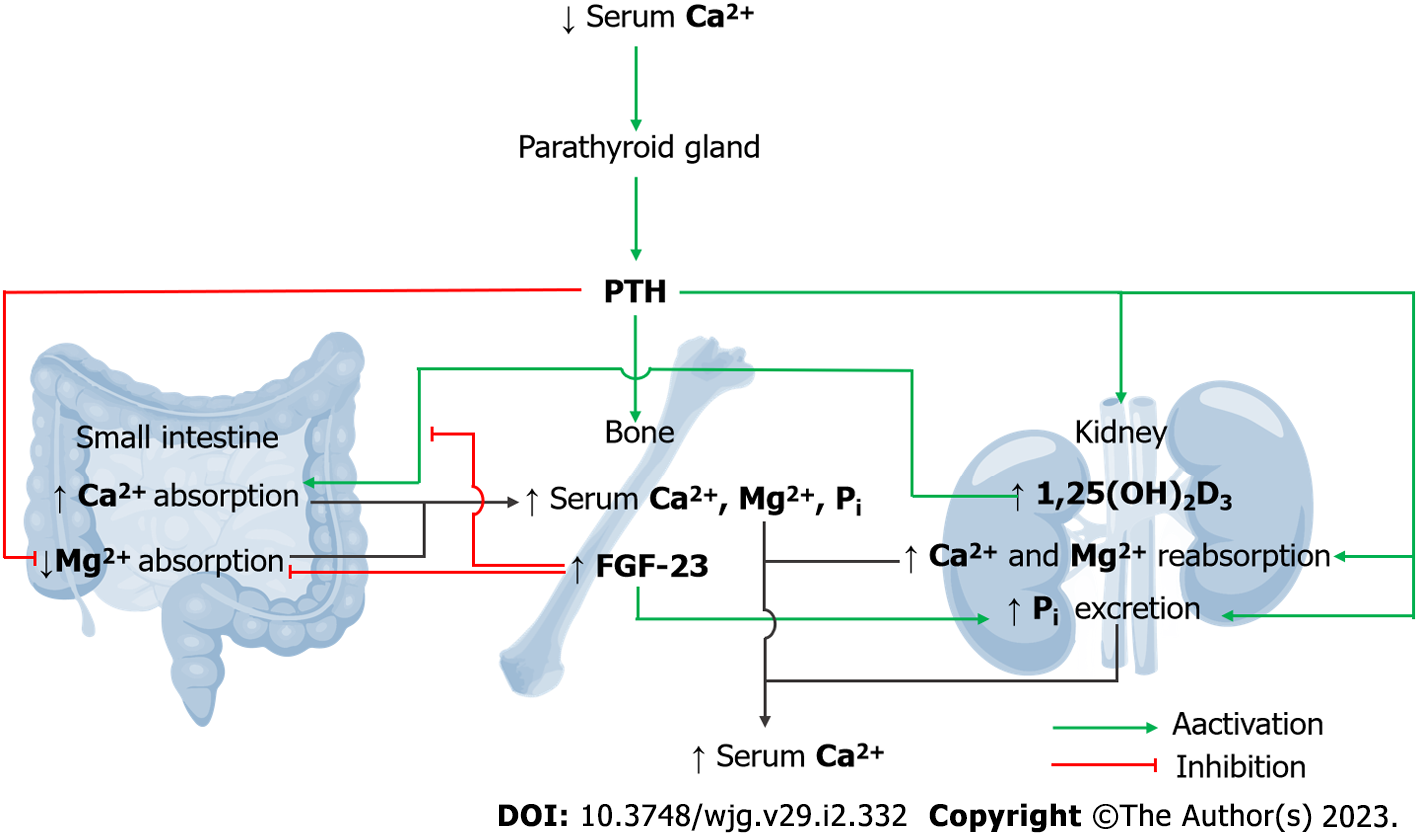
The proposed physiologically relevant magnesiotropic actions of PTH and FGF-23 are shown in Figure 3. During hypocalcemia, the parathyroid gland actively secretes PTH into the blood stream. PTH stimulates the bone resorption process, which increases plasma calcium (Ca2+), inorganic phosphate (Pi), and Mg2+ levels[32,33]. PTH stimulates renal 1,25-dihydroxy vitamin D3 [1,25(OH)2D3] production, which subsequently induces small intestinal Ca2+ absorption[34]. PTH also activates renal tubular Ca2+ and Mg2+ reabsorption[32]. Plasma Pi and PTH trigger bone-derived FGF-23 release, which acts as a negative feedback regulator to abolish 1,25(OH)2D3-induced intestinal Ca2+ absorption[33]. PTH and FGF-23 synergistically suppress the small intestinal absorption of dietary Mg2+[13] to prevent hypermagnesemia. PTH and FGF-23 downregulate the Na2+-dependent Pi cotransporters, (NaPi)-IIa and NaPi-IIc, and increase urinary Pi excretion[32] to prevent hyperphosphatemia. Therefore, PTH and FGF-23 exert their calcemic effect by preventing hyperphosphatemia and hypermagnesemia.
The hypothesis that apical acidity and mucosal bicarbonate secretion (MBS) affect luminal Mg2+ solubility and intestinal Mg2+ absorption was previously proposed in 2014[11,35], which was confirmed in a recent review article[36]. The luminal acidity along the entire human and rodent small bowel varies from pH 5.0–7.3[12,37]. The luminal protons provide an appropriate environment for mineral absorption by stabilizing their ionized forms[38]. The elevation of luminal pH led to a lower soluble Mg2+, which decreased from 79.61% of total luminal Mg content at pH 5.15% to 8.71% of total luminal Mg at pH 7.8[39]. Therefore, luminal acidity enhances Mg2+ absorption in the human small intestine[40] and epithelial-like Caco-2 monolayers[30,35]. The MBS and luminal pH elevation diminished duodenal, jejunal, and ileal Mg2+ absorption[11,12].
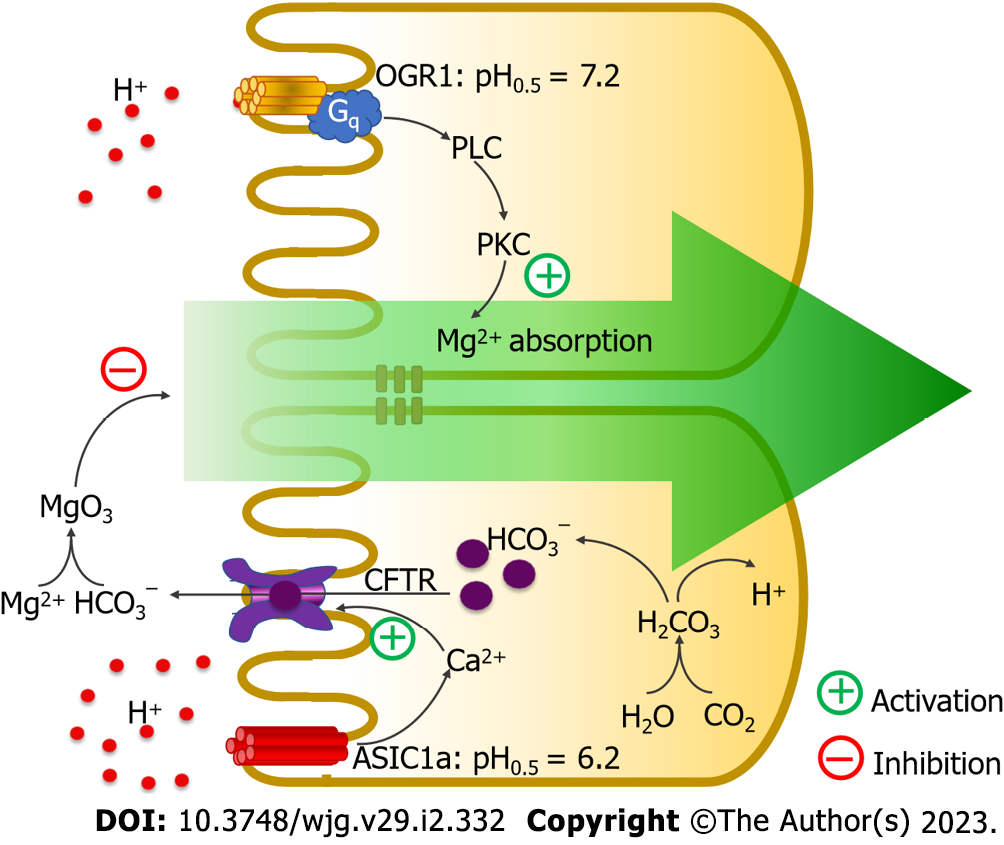
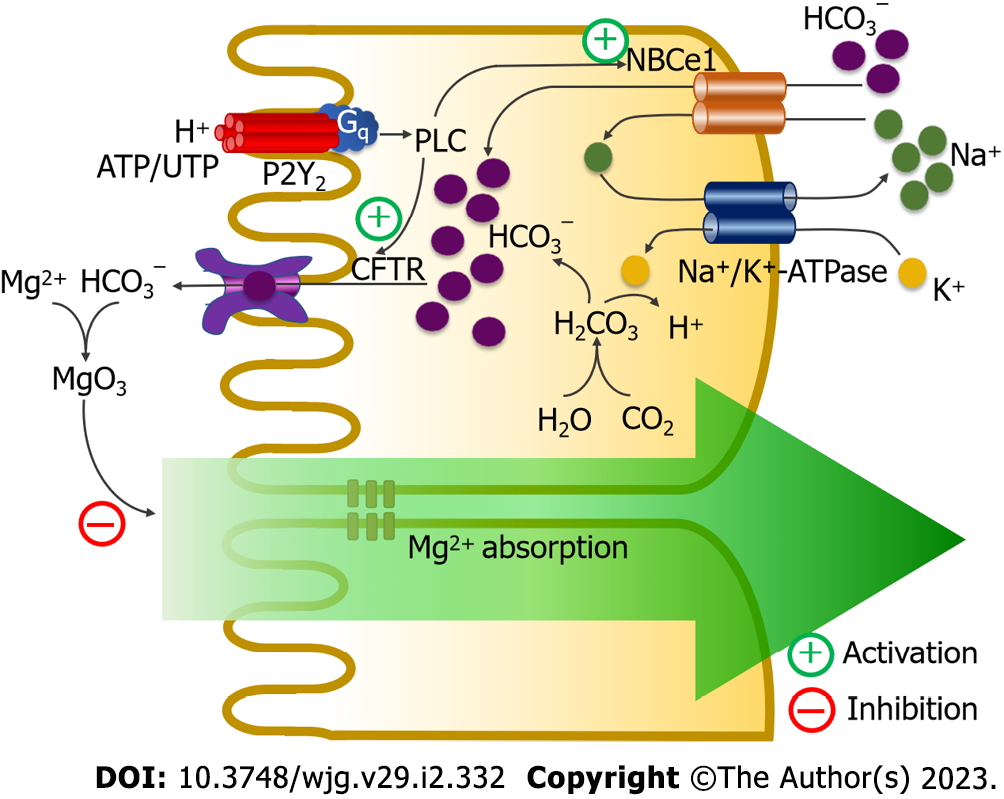
Small intestinal enterocytes are regularly exposed to strong gastric acid. When luminal protons are present in the duodenal lumen, the intestinal epithelium cells can directly detect and modulate their cellular response through the proton-sensing channels, e.g., the acid-sensing ion-channel 1a (ASIC1a) or proton-sensing receptors, such as ovarian cancer G protein-coupled receptor 1 (OGR1) and P2Y2 purinoceptor[41-44].
OGR1, also known as GPR68, is expressed in the human small intestine, spleen, testes, brain, lungs, placenta, heart, and kidneys but not in the colon[44]. OGR1 is a proton-sensitive receptor with pH values at half activation (pH0.5) and full activation of 7.2 and 6.8, respectively[45-47]. When the luminal pH decreases to 6.5, OGR1 activity is inactivated[45]. Activation of OGR1 triggers the phospholipase C–protein kinase C signaling pathway to activate intestinal Mg2+ absorption[35] (Figure 4).
ASIC1a is a proton-sensitive Ca2+ channel with a pH0.5 of 6.2[41,43]. Activation of ASIC1a activates intracellular Ca2+ signaling and subsequently induces MBS. In the intestinal epithelium, luminal proton stimulates ASIC1a activity that further activates MBS in a Ca2+ signaling-cystic fibrosis transmembrane conductance regulator-dependent mechanism[35] (Figure 4). Secreted bicarbonate has previously been found to reduce luminal protons[48] and induce precipitation of luminal free Mg2+[49], thus reducing free soluble Mg2+ and suppressing intestinal Mg2+ absorption.
Purinergic regulation of luminal pH and electrolyte transport in the small intestine have been described[50-52]. Duodenocytes regularly secrete ATP into its lumen. If luminal pH is low, luminal alkaline phosphatase activity is diminished and luminal ATP increases, which subsequently activates P2Y2 purinoceptor. Simultaneously, P2Y2 is a proton-sensitive receptor that is activated by luminal protons[42]. Active P2Y2 purinoceptors further activate MBS to increase luminal pH. A previous study showed that P2Y2 activation induced MBS through a cystic fibrosis transmembrane conductance regulator- and Na+-HCO3− cotransporter-1-dependent mechanism, which subsequently suppressed intestinal Mg2+ absorption[53] (Figure 5).
Proton pump inhibitor (PPI)-induced hypomagnesemia (PPIH) and hypomagnesuria in humans have been reported since 2006[54-57]. Intravenous Mg2+ supplementation or withdrawal of the PPI was able to rapidly normalize plasma and urinary Mg2+ levels in PPIH patients, though oral Mg2+ supplementation could not. Clinical assessments have reported that PPIH patients had normal renal Mg2+ handling[54,56,57]. These findings suggest that PPIs could suppress intestinal Mg2+ absorption. Our group has extensively studied the underlying mechanisms of PPI-suppressed intestinal Mg2+ absorption for a decade[11,12,15,30,35,53,58,59]. Our results suggest that PPIs mainly suppressed small intestinal Mg2+ absorption.
Omeprazole, the first introduced PPI, significantly suppressed total, transcellular, and paracellular Mg2+ absorption in the duodenum, jejunum, ilium, and colon of PPIH rats[11,12]. Regarding the percent suppression of total Mg2+ absorption in the duodenum (81.86%), jejunum (70.59%), ileum (69.45%), and colon (39.25%), the small intestine is the segment most adversely affected by prolonged PPI administration. However, previous articles have proposed that PPIs mainly inhibit colonic Mg2+ absorption[36,60,61], but those study results remain controversial[60,61]. They also proposed that colonic fermentation of dietary fibers probably increased serum Mg2+ and cured patients with PPIH[36]. A previous study clearly showed that dietary inulin fibers significantly induced cecal and colonic fermentation, but not plasma Mg2+ levels, in control and PPIH mice[61]. In contrast, dietary inulin fibers significantly induced renal Mg2+ excretion in PPIH mice[61], which should aggravate hypomagnesemia in PPIH. Therefore, the large intestine may not be a suitable intestinal segment that should be modulated to counteract PPIH.
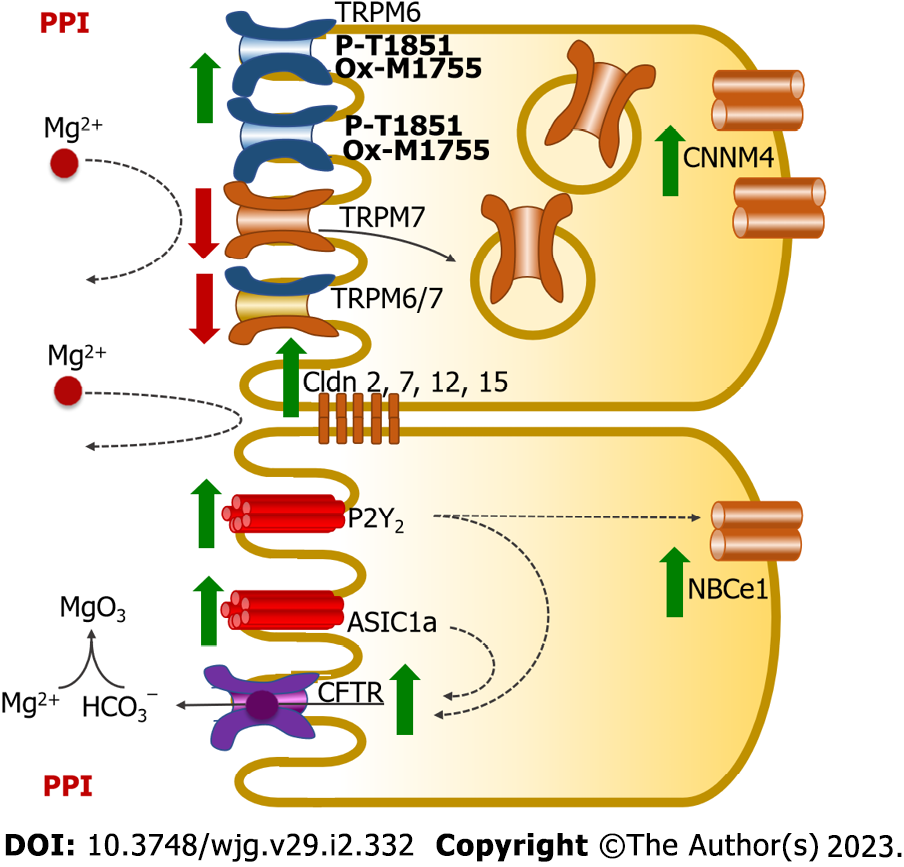
The proposed mechanism of PPI-suppression of small intestinal Mg2+ absorption is shown in Figure 6. PPIs markedly suppress membranous TRPM7 and TRPM6/7[15]. Membranous TRPM6-channel activity is suppressed by hyperphosphorylation at the T1851 residue and hyperoxidation at the M1755 residue[15]. Phosphorylation of the T1851 residue of the TRPM6 protein induces TRPM6-channel suppression by intracellular free Mg2+ and activated 5 C-kinase 1[62]. Oxidation of the M1755 residue in the TRPM6 protein also suppresses its channel permeability[63]. Suppression of membranous TRPM6, TRPM7, and TRPM6/7 disrupts mucosal Mg2+ entry into the small intestinal epithelium and then inhibits transcellular Mg2+ absorption[11,12]. Plasma FGF-23 was markedly increased in PPIH rats[12]. The mechanism by which FGF-23 inhibits transcellular small intestinal Mg2+ absorption is described in the above section[13]. Therefore, PPI-suppressed transcellular Mg2+ absorption is due, at least in part, to FGF-23.
PPIs suppress paracellular Mg2+ absorption (Figure 6). The small intestinal epithelium only expresses Cldn-1, -2, -3, -4, -5, -7, -8, -12, and -15[28,29]. Overexpression of Cldn proteins and higher paracellular resistance have been demonstrated in the small intestines of PPIH rats[11,12]. Paracellular tight junction width was significantly decreased in the small intestine of PPIH rats[58]. PPIs also suppress epithelial paracellular Mg2+ permeability and cation selectivity[30,59]. These results shed light on the mechanism of PPI-suppressed paracellular Mg2+ absorption in the small intestine.
PPI-induced small intestinal MBS (Figure 6) has been reported in humans[64], PPIH rats[11], and PPI-treated Caco-2 monolayers[35,53]. PPIs have also been shown to significantly increase ASIC1a and P2Y2 expression in PPI-treated epithelium[35,53]. Active ASIC1a and P2Y2 trigger MBS. Higher secreted bicarbonate in PPIH small intestines reduces free soluble Mg2+, which disrupts Mg2+ absorption (Figure 6). Inhibition of MBS significantly increases duodenal Mg2+ absorption in PPIH rats[11].
In addition to the change in magnesiotropic protein expression and function and MBS, PPIs have been shown to induce structural change in the absorptive epithelium of the small intestine[58]. Prolonged PPI administration markedly decreased the villous length and absorptive area in the duodenal, jejunal, and ilial epithelium of PPIH rats. The underlying mechanism involves Paneth cell dysfunction in the small intestine[58]. Paneth cells have an important role in host-microorganism homeostasis in the small intestine by providing antimicrobial α-defensin peptides[65,66]. Disruption of the secretory function of Paneth cells leads to infection and chronic inflammation of the small intestine[65,66]. In PPIH rats, a reduction in secretory granules and metaplasia of Paneth cells occurs in the duodenum, jejunum, and ileum, suggesting Paneth cell secretory dysfunction[58]. Chronic inflammation in the small intestinal epithelium leads to villous atrophy and reduction of the absorptive area in the small intestine of PPIH rats[58].
The potential role of gut microbiota in colonic Mg2+ absorption has previously been proposed[36]. However, it is currently unknown how the small intestinal microbiome affects small intestinal Mg2+ absorption. Previous studies have shown that the small intestine is colonized by a complex gut microbiota community and is less numerous and diverse (approximately 103–107 microbial cells/gram) than in the colon (approximately 1012 microbial cells/gram)[67]. The dominant bacterial phyla in the small intestine are Streptococcus sp., Lactobacillaceae, and Enterobacteriaceae, whereas in the colon, the dominant phyla are Bacteroidaceae, Prevotellaceae, Rikenellaceae, Lachnospiraceae, and Ruminococcaceae[68,69]. Prolonged PPI treatment can lead to gut microbiota dysbiosis, such as the reduction of Actinobacteria and Bifidobacteria spp., which are responsible for maintaining the mucosal barrier function[68].
Furthermore, long-term treatment with PPIs causes small intestinal bacterial overgrowth because of the loss of the gastric acid defensive barrier[70]. The jejunal samples of small intestinal bacterial overgrowth patients regularly showed increased production of toxic agents, such as serum endotoxin and bacterial compounds that stimulate the secretion of proinflammatory cytokines[71]. Apart from these findings, our previous study showed Paneth dysfunction and chronic inflammation in the small intestine of PPIH rats[58]. From the perspective of relevant gut microbiota, Paneth cell defects have been found to be associated with increased Bacteroidetes and Enterococcus and decreased Bifidobacterium[72], whereas Bifidobacterium longum has been found to promote cell proliferation and expression of Lgr5 and Wnt3a in intestinal organoids and alleviate microbiota dysbiosis by regulating the functions of Paneth cells[73]. It is also possible that the synthesis of gut microbiota metabolites could lead to changes in the absorptive surface in the gut and/or stimulate gene expression[74].
In the colon, bifidobacterial fermentation leads to acidification of the colon, which shows beneficial absorption of Mg2+[9,61,75]. In humans, small intestinal microbiota can also ferment the available carbohydrates and induce intestinal acidification[76]. In the human small intestine, a dominant bacterial phylum is Streptococcus sp.[77,78], which is an anaerobe that can ferment relatively simple carbohydrates at a high rate[79]. According to the above, luminal acidity markedly induces small intestinal Mg2+ absorption. Therefore, small intestinal fermentation should induce small intestinal Mg2+ absorption.
Bulk absorption of digested Mg2+ occurs in the small intestine through transcellular active and paracellular passive mechanisms. PTH, FGF-23, luminal protons, ASIC1a, OGR1, P2Y2, PPIs, and the microbiome have recently been proposed as regulatory factors of small intestinal Mg2+ uptake. However, the regulatory mechanism of small intestinal Mg2+ requires additional extensive studies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: The Physiological Society of Thailand, No. 376.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tuo BG, China; Wu LH, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 1011] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 2. | Yamanaka R, Shindo Y, Oka K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 3. | Rondanelli M, Faliva MA, Tartara A, Gasparri C, Perna S, Infantino V, Riva A, Petrangolini G, Peroni G. An update on magnesium and bone health. Biometals. 2021;34:715-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 4. | Dominguez L, Veronese N, Barbagallo M. Magnesium and Hypertension in Old Age. Nutrients. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | Pelczyńska M, Moszak M, Bogdański P. The Role of Magnesium in the Pathogenesis of Metabolic Disorders. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 6. | Trapani V, Wolf FI. Dysregulation of Mg2+ homeostasis contributes to acquisition of cancer hallmarks. Cell Calcium. 2019;83:102078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Groenestege WM, Hoenderop JG, van den Heuvel L, Knoers N, Bindels RJ. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J Am Soc Nephrol. 2006;17:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Lameris AL, Nevalainen PI, Reijnen D, Simons E, Eygensteyn J, Monnens L, Bindels RJ, Hoenderop JG. Segmental transport of Ca²⁺ and Mg²⁺ along the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2015;308:G206-G216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Coudray C, Demigné C, Rayssiguier Y. Effects of dietary fibers on magnesium absorption in animals and humans. J Nutr. 2003;133:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 428] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 11. | Thongon N, Penguy J, Kulwong S, Khongmueang K, Thongma M. Omeprazole suppressed plasma magnesium level and duodenal magnesium absorption in male Sprague-Dawley rats. Pflugers Arch. 2016;468:1809-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Suksridechacin N, Kulwong P, Chamniansawat S, Thongon N. Effect of prolonged omeprazole administration on segmental intestinal Mg2+ absorption in male Sprague-Dawley rats. World J Gastroenterol. 2020;26:1142-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Suksridechacin N, Thongon N. Fibroblast growth factor-23 and parathyroid hormone suppress small intestinal magnesium absorption. Physiol Rep. 2022;10:e15247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 520] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 15. | Kampuang N, Thongon N. Mass spectrometric analysis of TRPM6 and TRPM7 from small intestine of omeprazole-induced hypomagnesemic rats. Front Oncol. 2022;12:947899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 16. | Chubanov V, Gudermann T. TRPM6. Handb Exp Pharmacol. 2014;222:503-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Zhang Z, Yu H, Huang J, Faouzi M, Schmitz C, Penner R, Fleig A. The TRPM6 kinase domain determines the Mg·ATP sensitivity of TRPM7/M6 heteromeric ion channels. J Biol Chem. 2014;289:5217-5227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Fleig A, Chubanov V. TRPM7. Handb Exp Pharmacol. 2014;222:521-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Penner R, Fleig A. The Mg2+ and Mg(2+)-nucleotide-regulated channel-kinase TRPM7. Handb Exp Pharmacol. 2007;313-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Yamazaki D, Funato Y, Miura J, Sato S, Toyosawa S, Furutani K, Kurachi Y, Omori Y, Furukawa T, Tsuda T, Kuwabata S, Mizukami S, Kikuchi K, Miki H. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet. 2013;9:e1003983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Parry DA, Mighell AJ, El-Sayed W, Shore RC, Jalili IK, Dollfus H, Bloch-Zupan A, Carlos R, Carr IM, Downey LM, Blain KM, Mansfield DC, Shahrabi M, Heidari M, Aref P, Abbasi M, Michaelides M, Moore AT, Kirkham J, Inglehearn CF. Mutations in CNNM4 cause Jalili syndrome, consisting of autosomal-recessive cone-rod dystrophy and amelogenesis imperfecta. Am J Hum Genet. 2009;84:266-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Polok B, Escher P, Ambresin A, Chouery E, Bolay S, Meunier I, Nan F, Hamel C, Munier FL, Thilo B, Mégarbané A, Schorderet DF. Mutations in CNNM4 cause recessive cone-rod dystrophy with amelogenesis imperfecta. Am J Hum Genet. 2009;84:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Quamme GA. Recent developments in intestinal magnesium absorption. Curr Opin Gastroenterol. 2008;24:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Meoli L, Günzel D. Channel functions of claudins in the organization of biological systems. Biochim Biophys Acta Biomembr. 2020;1862:183344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 744] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 26. | Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 354] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 27. | Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A. 2009;106:15350-15355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, Kojima T, Yamashita T, Sawada N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J Histochem Cytochem. 2006;54:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017;1397:66-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 30. | Thongon N, Krishnamra N. Apical acidity decreases inhibitory effect of omeprazole on Mg(2+) absorption and claudin-7 and -12 expression in Caco-2 monolayers. Exp Mol Med. 2012;44:684-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Chubanov V, Ferioli S, Wisnowsky A, Simmons DG, Leitzinger C, Einer C, Jonas W, Shymkiv Y, Bartsch H, Braun A, Akdogan B, Mittermeier L, Sytik L, Torben F, Jurinovic V, van der Vorst EP, Weber C, Yildirim ÖA, Sotlar K, Schürmann A, Zierler S, Zischka H, Ryazanov AG, Gudermann T. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Leaf DE, Christov M. Dysregulated Mineral Metabolism in AKI. Semin Nephrol. 2019;39:41-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Zofková I, Kancheva RL. The relationship between magnesium and calciotropic hormones. Magnes Res. 1995;8:77-84. [PubMed] |
| 34. | Khuituan P, Teerapornpuntakit J, Wongdee K, Suntornsaratoon P, Konthapakdee N, Sangsaksri J, Sripong C, Krishnamra N, Charoenphandhu N. Fibroblast growth factor-23 abolishes 1,25-dihydroxyvitamin D₃-enhanced duodenal calcium transport in male mice. Am J Physiol Endocrinol Metab. 2012;302:E903-E913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Thongon N, Ketkeaw P, Nuekchob C. The roles of acid-sensing ion channel 1a and ovarian cancer G protein-coupled receptor 1 on passive Mg2+ transport across intestinal epithelium-like Caco-2 monolayers. J Physiol Sci. 2014;64:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Gommers LMM, Hoenderop JGJ, de Baaij JHF. Mechanisms of proton pump inhibitor-induced hypomagnesemia. Acta Physiol (Oxf). 2022;235:e13846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 37. | Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 485] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 38. | Evenepoel P. Alteration in digestion and absorption of nutrients during profound acid suppression. Best Pract Res Clin Gastroenterol. 2001;15:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Ben-Ghedalia D, Tagari H, Zamwel S, Bondi A. Solubility and net exchange of calcium, magnesium and phosphorus in digesta flowing along the gut of the sheep. Br J Nutr. 1975;33:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Heijnen AM, Brink EJ, Lemmens AG, Beynen AC. Ileal pH and apparent absorption of magnesium in rats fed on diets containing either lactose or lactulose. Br J Nutr. 1993;70:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Dong X, Ko KH, Chow J, Tuo B, Barrett KE, Dong H. Expression of acid-sensing ion channels in intestinal epithelial cells and their role in the regulation of duodenal mucosal bicarbonate secretion. Acta Physiol (Oxf). 2011;201:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Holzer P. Taste receptors in the gastrointestinal tract. V. Acid sensing in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2007;292:G699-G705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol. 2009;283-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 44. | Xu Y, Casey G. Identification of human OGR1, a novel G protein-coupled receptor that maps to chromosome 14. Genomics. 1996;35:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 564] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 46. | Seuwen K, Ludwig MG, Wolf RM. Receptors for protons or lipid messengers or both? J Recept Signal Transduct Res. 2006;26:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Mohebbi N, Benabbas C, Vidal S, Daryadel A, Bourgeois S, Velic A, Ludwig MG, Seuwen K, Wagner CA. The proton-activated G protein coupled receptor OGR1 acutely regulates the activity of epithelial proton transport proteins. Cell Physiol Biochem. 2012;29:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Allen A, Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:C1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 403] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 49. | Kurita Y, Nakada T, Kato A, Doi H, Mistry AC, Chang MH, Romero MF, Hirose S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1402-R1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 50. | Akiba Y, Mizumori M, Guth PH, Engel E, Kaunitz JD. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1223-G1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Dong X, Smoll EJ, Ko KH, Lee J, Chow JY, Kim HD, Insel PA, Dong H. P2Y receptors mediate Ca2+ signaling in duodenocytes and contribute to duodenal mucosal bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2009;296:G424-G432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Kaunitz JD, Akiba Y. Purinergic regulation of duodenal surface pH and ATP concentration: implications for mucosal defence, lipid uptake and cystic fibrosis. Acta Physiol (Oxf). 2011;201:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Thongon N, Chamniansawat S. The inhibitory role of purinergic P2Y receptor on Mg2+ transport across intestinal epithelium-like Caco-2 monolayer. J Physiol Sci. 2019;69:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Epstein M, McGrath S, Law F. Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med. 2006;355:1834-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 55. | Broeren MA, Geerdink EA, Vader HL, van den Wall Bake AW. Hypomagnesemia induced by several proton-pump inhibitors. Ann Intern Med. 2009;151:755-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Cundy T, Dissanayake A. Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clin Endocrinol (Oxf). 2008;69:338-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 57. | Shabajee N, Lamb EJ, Sturgess I, Sumathipala RW. Omeprazole and refractory hypomagnesaemia. BMJ. 2008;337:a425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Chamniansawat S, Kampuang N, Suksridechacin N, Thongon N. Ultrastructural intestinal mucosa change after prolonged inhibition of gastric acid secretion by omeprazole in male rats. Anat Sci Int. 2021;96:142-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Thongon N, Krishnamra N. Omeprazole decreases magnesium transport across Caco-2 monolayers. World J Gastroenterol. 2011;17:1574-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (6)] |
| 60. | Lameris AL, Hess MW, van Kruijsbergen I, Hoenderop JG, Bindels RJ. Omeprazole enhances the colonic expression of the Mg(2+) transporter TRPM6. Pflugers Arch. 2013;465:1613-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Hess MW, de Baaij JH, Gommers LM, Hoenderop JG, Bindels RJ. Dietary Inulin Fibers Prevent Proton-Pump Inhibitor (PPI)-Induced Hypocalcemia in Mice. PLoS One. 2015;10:e0138881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Cao G, Thébault S, van der Wijst J, van der Kemp A, Lasonder E, Bindels RJ, Hoenderop JG. RACK1 inhibits TRPM6 activity via phosphorylation of the fused alpha-kinase domain. Curr Biol. 2008;18:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Cao G, Lee KP, van der Wijst J, de Graaf M, van der Kemp A, Bindels RJ, Hoenderop JG. Methionine sulfoxide reductase B1 (MsrB1) recovers TRPM6 channel activity during oxidative stress. J Biol Chem. 2010;285:26081-26087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Mertz-Nielsen A, Hillingsø J, Bukhave K, Rask-Madsen J. Omeprazole promotes proximal duodenal mucosal bicarbonate secretion in humans. Gut. 1996;38:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 875] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 66. | Lueschow SR, McElroy SJ. The Paneth Cell: The Curator and Defender of the Immature Small Intestine. Front Immunol. 2020;11:587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 67. | El Aidy S, Merrifield CA, Derrien M, van Baarlen P, Hooiveld G, Levenez F, Doré J, Dekker J, Holmes E, Claus SP, Reijngoud DJ, Kleerebezem M. The gut microbiota elicits a profound metabolic reorientation in the mouse jejunal mucosa during conventionalisation. Gut. 2013;62:1306-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 68. | Bruno G, Zaccari P, Rocco G, Scalese G, Panetta C, Porowska B, Pontone S, Severi C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J Gastroenterol. 2019;25:2706-2719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 157] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (7)] |
| 69. | Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14:20-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1265] [Cited by in RCA: 1607] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 70. | Fujimori S. What are the effects of proton pump inhibitors on the small intestine? World J Gastroenterol. 2015;21:6817-6819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 383] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (4)] |
| 72. | Riba A, Olier M, Lacroix-Lamandé S, Lencina C, Bacquié V, Harkat C, Gillet M, Baron M, Sommer C, Mallet V, Salvador-Cartier C, Laurent F, Théodorou V, Ménard S. Paneth Cell Defects Induce Microbiota Dysbiosis in Mice and Promote Visceral Hypersensitivity. Gastroenterology. 2017;153:1594-1606.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 73. | Zhou C, Fang X, Xu J, Gao J, Zhang L, Zhao J, Meng Y, Zhou W, Han X, Bai Y, Li Z, Zou D. Bifidobacterium longum alleviates irritable bowel syndrome-related visceral hypersensitivity and microbiota dysbiosis via Paneth cell regulation. Gut Microbes. 2020;12:1782156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Cashman K. Prebiotics and calcium bioavailability. Curr Issues Intest Microbiol. 2003;4:21-32. [PubMed] |
| 75. | Schuchardt JP, Hahn A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium-An Update. Curr Nutr Food Sci. 2017;13:260-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 76. | Zoetendal EG, Raes J, van den Bogert B, Arumugam M, Booijink CC, Troost FJ, Bork P, Wels M, de Vos WM, Kleerebezem M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012;6:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 511] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 77. | Booijink CC, El-Aidy S, Rajilić-Stojanović M, Heilig HG, Troost FJ, Smidt H, Kleerebezem M, De Vos WM, Zoetendal EG. High temporal and inter-individual variation detected in the human ileal microbiota. Environ Microbiol. 2010;12:3213-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 78. | Hayashi H, Takahashi R, Nishi T, Sakamoto M, Benno Y. Molecular analysis of jejunal, ileal, caecal and recto-sigmoidal human colonic microbiota using 16S rRNA gene libraries and terminal restriction fragment length polymorphism. J Med Microbiol. 2005;54:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 239] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 79. | Holt JG. Genus Streptococcus In: Bergy DH, Holt JG, Krieg NR, Sneath PH (eds). Bergey’s Manual of Determinative Bacteriology. Lippincott Williams & Wilkins: Baltimore, MD, 1994: 523. |