Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3666
Peer-review started: September 28, 2021
First decision: November 18, 2021
Revised: November 21, 2021
Accepted: June 30, 2022
Article in press: June 30, 2022
Published online: July 28, 2022
Processing time: 301 Days and 17.3 Hours
In Korea, infliximab was approved for use in children with ulcerative colitis (UC) in October 2012.
To compare the clinical course of UC before and after the introduction of biolo
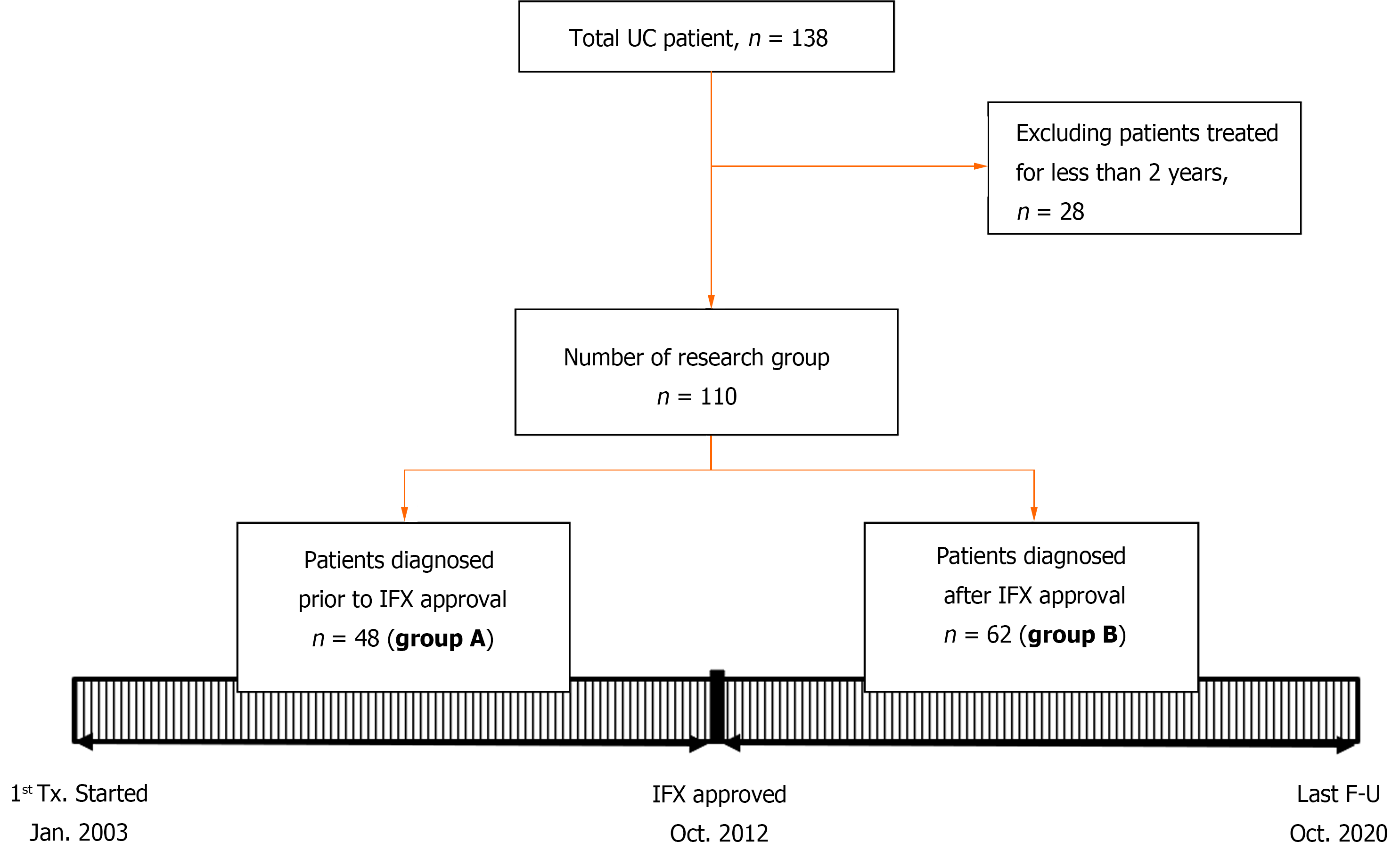
Patients under 18 years of age, who were diagnosed with UC and followed from January 2003 to October 2020, were included in the study. Group A (n = 48) was followed for at least 2 years between January 2003 and October 2012, and Group B (n = 62) was followed for at least 2 years between November 2012 and October 2020. We compared endoscopic remission, drug composition, relapse rate, steroid-free period, and the quality of life of each group. We plotted the clinical course of the included patients using the pediatric UC activity index score, and compared our patients with those in the IBSEN study.
After 2 years of treatment, colonoscopy evaluation revealed different outcomes in the two treatment groups. Remission was confirmed in 14 patients (29.2%) of Group A, and in 31 patients (50.0%) of Group B (P < 0.012). The median cumu
The incidence of relapse has decreased and the steroid-free period has increased after the introduction of the biological agent. The clinical course also showed a different pattern from that of IBSEN study. The active use of biological agents may change the long-term disease course in moderate to severe pediatric UC.
Core Tip: This was a retrospective study that assessed how the introduction of biological agents has altered the disease course over time in pediatric ulcerative colitis (UC). Endoscopic remission, relapse rate, steroid-free period, and the quality of life of each group were evaluated as outcomes. Clinical course was plotted with the pediatric UC activity index score, and compared to that of the IBSEN study. The incidence of relapse has decreased and the steroid-free period has increased after the introduction of biological agents. The clinical course also showed a different pattern from the IBSEN study.
- Citation: Kwon Y, Kim ES, Choe YH, Kim MJ. How has the disease course of pediatric ulcerative colitis changed throughout the biologics era? A comparison with the IBSEN study. World J Gastroenterol 2022; 28(28): 3666-3681
- URL: https://www.wjgnet.com/1007-9327/full/v28/i28/3666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i28.3666
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) that was first described in 1875 by two English physicians. Although it has been the subject of many studies over the years, the etiology remains unknown[1]. The potential causes explained in the literature include immune system dysfunction, genetics, changes in normal intestinal bacteria, environmental factors, and a combination of any or all of these variables[2]. The incidence of UC is higher in developed countries than in less developed ones, which is thought to be the result of reduced exposure to intestinal infections and a Western-style diet and lifestyle[3]. As people in Asia adopt more Westernized dietary habits, the incidence rate is gradually increasing in that part of the world[4].
The age of onset of UC can be anywhere from 15 to 30 to > 60 years, and the incidence of UC is lower than that of Crohn’s disease in children[5]. For this reason, the number of large-scale studies and long-term follow-up studies for pediatric patients with UC is insufficient, and more studies are needed to evaluate the clinical course of UC in such patients. The IBSEN study evaluated the long-term clinical course and outcomes of 423 adult patients with UC during the first 10 years. Mortality risk and cumulative colorectal resection were evaluated as clinical outcomes. The proportion of patients who relapsed and who remain in remission was evaluated as a clinical course. In addition, the authors divided the disease course of UC into four types and showed them in graphs, which became a representative figure of this paper[6]. However, this study was done before any biological agents were approved as treatments. No studies have compared the clinical course of UC before and after the introduction of biological agents in a single cohort.
Infliximab was first introduced in Europe in 1999 for the treatment of Crohn’s disease[7]. Since the introduction of infliximab, several studies have reported improvements in clinical outcomes with infliximab treatment[8-11]. Nevertheless, relapse has been observed in patients treated with infliximab, and many studies have been conducted on factors that can predict relapse in these patients[12-14]. Therefore, we assessed what changes have occurred in the long-term clinical course of UC since the introduction of biological agents as treatment.
In Korea, infliximab was approved for use in adults with UC in May 2007, and in children in October 2012. Since long-term follow up is possible due to the characteristics of pediatric patients, the aim of this study was to compare the clinical course of UC before and after the introduction of biological agents. Another goal of this study was to compare the clinical course of our patients to that in the IBSEN study.
Patients under 18 years of age, who were diagnosed with UC and had been followed from January 2003 to October 2020, were included in this study. Initially, the total number of patients was 138, but patients who had been observed for less than 2 years were eliminated. Finally, 110 patients were selected as the study group (Figure 1). All patients were children and adolescents under 18 years of age at the time of diagnosis, but some patients became adults during the follow-up period. UC was diagnosed in accordance with the guidelines of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (the Porto criteria)[15]. This study was approved by the institutional review board of Samsung Medical Center (IRB File No. SMC 2020-12-005).
This was a retrospective study. The first goal was to evaluate how the active use of biological agents causes a change in the clinical course of UC. This was done using data from patients who were treated after the approval of infliximab in October 2012. With the cut-off date of October 2012, the patients were divided into two groups, A and B. Group A had been followed for at least 2 years between January 2003 and October 2012, and Group B had been followed for at least 2 years between November 2012 and October 2020. Since the two groups were classified according to a time difference, we first checked whether there was any difference in the baseline characteristics of the patients, and then conducted a comparative analysis.
Both patient groups were evaluated by laboratory tests (including hemoglobin, albumin, erythrocyte sedimentation rate [ESR], and C-reactive protein [CRP]), colonoscopy, abdominal computed tomography (CT), and pediatric UC activity index (PUCAI) score at the time of diagnosis. Laboratory tests were performed and a PUCAI score questionnaire was submitted at each outpatient follow-up visit, which took place at intervals of 1-3 mo. We confirmed that there were no clinical differences between the two groups in terms of demographic characteristics, the extent and severity of disease according to the laboratory test and colonoscopy results, or the PUCAI score at the time of diagnosis. The fecal calprotectin test has only been available in Korea since 2017 and thus could not be included as a comparative test. Based on the colonoscopy findings, disease extent was classified into E1 (proctitis), E2 (left colitis), E3 (right colitis), and E4 (pancolitis) according to the Paris classification[16], and severity was classified from 0 to 3 using the Mayo endoscopic subscore (MES)[17].
Colonoscopy was performed at the time of initial diagnosis and at intervals of 1 to 2 years during the follow-up period. The colonoscopy was performed with a CF scope after sedation with midazolam and pethidine. All procedures reached the cecum and the entire colon was examined. Characteristic endoscopic findings of UC such as peri-appendiceal patches, demarcation line, mucosal edema, decreased vascularity of loss of vascularity, and superficial tiny ulcers were observed and described. We reviewed the colonoscopy findings at 2 and 5 years after the start of treatment. Remission was defined as endoscopic mucosal healing, which means that no lesion was observed and the MES was 0 with histological healing (Geboes grade 0-1). The reason for excluding MES 1 is that the authors aimed at deep remission because they experienced cases of MES 1 that relapsed easily. In addition, we investigated the drugs prescribed to both groups of patients during the follow-up period.
To compare the clinical course between the groups, the number of relapses that occurred during the follow-up period was investigated, along with the time interval at which the first relapse occurred (from the time of diagnosis) and the time interval of each relapse when multiple relapses occurred in the same patient. Since the follow-up period was different for each patient, the relapse rate was finally evaluated between the two groups. Clinical relapse was defined as a PUCAI score > 10 with modification of treatment (addition of steroids or biologic agents). To evaluate the clinical risk factors for relapse, age, PUCAI score, disease extent, MES, and initial use of corticosteroids were statistically analyzed. Situations in which symptoms temporarily worsened due to infections such as gastroenteritis were excluded.
In addition, the corticosteroid-free period, cumulative colectomy, and changes in PUCAI score during the follow-up period were evaluated as clinical outcomes of both groups. As another quality of life-related factor, patients who did not experience relapse but were hospitalized or admitted to the emergency room for antibiotic use or blood transfusions were also investigated and the rates of such patients were compared between the groups.
The second goal of this study was to actually draw a clinical course using PUCAI score in these two patient groups and compare these distributions to the four predefined curves, depicting different clinical courses of UC, as published in the IBSEN study[6].
The demographic and clinical characteristics of the study groups at initial diagnosis and the clinical outcomes of the study groups were compared using the χ2 test and Mann-Whitney test. Steroid-free survival curves were presented for the whole cohort using the Kaplan-Meier method. Differences in survival curves between the two groups were evaluated using the log-rank test. Repeated event data for relapses were exhibited by estimating mean cumulative function (MCF), which is the average number of cumulative events experienced by an individual in the study at each point in time since the start of follow-up[18,19]. Univariable/multivariable analyses of the associations between relapse rate and other factors were analyzed by Cox’s proportional hazard regression using count process[20] because of multiple events for relapse. Multivariable analysis was performed by selecting variables with P < 0.1 in univariate analysis. The 95% confidence intervals (CIs) of the hazard ratio (HR) using robust sandwich covariance estimate to account the within subject correlation[21] were estimated. All of the above statistical analyses were conducted using SAS version 9.4. MCF was analyzed using reReg 1.4.0 package in R 4.0.4 (Vienna, Austria; http://www.R-project.org/. P < 0.05 was considered statistically significant.
Table 1 shows the patient characteristics and disease severity information of Group A (n = 48) and Group B (n = 62) at the time of diagnosis, as well as information on the medications that were used for treatment. The median age at the time of diagnosis of UC was 14.4 years for Group A and 15.8 years for Group B. There was no significant difference between the two groups. The median value of the total follow-up period was 3.3 years in Group A and 4.5 years in Group B, and there was no significant difference between the two groups. Serum hemoglobin, albumin, ESR, and CRP levels confirmed by laboratory tests at the time of diagnosis also did not show any significant difference between the two groups.
| Group A (n = 48) | Group B (n = 62) | P value | |
| Age at diagnosis, yr | 14.4 (12.2-17.1) | 15.8 (13.1-16.5) | 0.574c |
| Total duration of follow up with treatment, yr | 4.0 (4.0-5.0) | 5.5 (3.0-6.9) | 0.073c |
| PUCAIa at diagnosis | 35 (30-65) | 45 (35-55) | 0.969c |
| Hemoglobin at diagnosis, g/dL | 12.3 (10.5-14.1) | 12.1 (9.6-13.5) | 0.245c |
| Albumin at diagnosis, g/dL | 4.3 (4.0-4.6) | 4.2 (3.9-4.6) | 0.702c |
| ESR at diagnosis, mm/h | 14.5 (7-31.3) | 20 (7.5-39) | 0.249c |
| CRP at diagnosis, mg/dL | 0.04 (0.03-0.13) | 0.12 (0.03-0.56) | 0.791c |
| Disease extent of Paris classification at diagnosis | 0.018d | ||
| E1 proctitis | 18 (37.5) | 13 (21) | |
| E2 left colitis | 8 (16.7) | 8 (12.9) | |
| E3 right colitis | 6 (12.5) | 7 (11.3) | |
| E4 pancolitis | 16 (33.3) | 34 (54.8) | |
| Mayo endoscopic subscore at diagnosisb | 0.310d | ||
| 0 normal or inactive | 0 | 0 | |
| 1 mild | 12 (25.0) | 7 (11.3) | |
| 2 moderate | 26 (54.2) | 43 (69.4) | |
| 3 severe | 10 (20.8) | 12 (19.4) | |
| Corticosteroid use at baseline | 21 (43.8) | 30 (48.4) | 0.630d |
| Corticosteroid-dependent | 8/21 (38.1) | 9/30 (30.0) | 0.550d |
| Corticosteroid-refractory | 1/21 (4.8) | 1/30 (3.3) | 0.360d |
| Cumulative number receiving medication by | 0.211d | ||
| 3 mo after diagnosis | 0.453d | ||
| 5-aminosalicylate | 48 (100) | 60 (96.8) | |
| Azathioprine | 42 (87.5) | 51 (82.3) | |
| Methotrexate | 0 (0) | 1 (1.6) | |
| Cyclosporine | 1 (2.1) | 0 (0) |
Based on the results of endoscopy performed at the time of diagnosis, the disease extents of the two groups were classified according to the Paris classification, and severity was evaluated using the endoscopy subscore. There was no difference in disease severity between the two groups, but regarding disease extent, Group B patients had a higher rate of pancolitis and lower rate of proctitis. In Group A, 18 patients (37.5%) had proctitis and 16 (33.3%) had pancolitis, but in Group B, 13 (21%) had proctitis and 34 (54.8%) had pancolitis.
Within 3 mo of diagnosis, most patients in both groups were treated with 5-aminosalicylate and immunosuppressants, primarily azathioprine. In both groups, about 50% of patients used corticosteroids to reduce disease activity at the time of diagnosis. Among the 21 patients who used corticosteroids in Group A, 8 (38.1%) showed dependence and 1 (4.8%) showed refractory findings. In Group B, 9/30 (30.0%) of patients were corticosteroid-dependent and 1/30 (3.3%) was corticosteroid-refractory. There was no statistical difference between the two groups.
When the drugs that were used for treatment were investigated at the 2-year follow-up visits, 5 patients in Group B did not use any drugs, but no patient in Group A terminated drug treatment (Supplem
After 5 years, drug composition was re-evaluated in patients who were treated for more than 5 years (Group A = 24 patients, Group B = 31 patients). The same 5 patients in Group B continued to not use any drugs, and 2 patients in Group A also terminated drug treatment. In Group A, mesalazine (87.5%) and azathioprine (58.3%) use had been tapered and discontinued in many patients. Nevertheless, the proportion of patients using oral drugs was still high compared to Group B (mesalazine: 48.4%, azathioprine: 45.2%). In Group B, the percentage of patients taking infliximab and other biological agents increased (67.7%) after 5 years. In terms of drug composition, the rate of use of mesalazine and infliximab was significantly different, with P values of 0.003 and 0.001, respectively (Supplem
The patients underwent colonoscopy to re-evaluate disease extent and severity after 2 years of treatment (Table 2). In Group A, remission was confirmed in 14 patients and the proportion of each category of disease extent decreased slightly in comparison to the time of diagnosis, but the differences were small and pancolitis patients still accounted for 27.1% of the total. On the other hand, although the incidence of pancolitis was significantly higher in Group B at the time of diagnosis, 50% of the patients reached remission, and the number of pancolitis patients was significantly reduced to 4 (6.5%). In addition, severity according to the MES also showed a significant difference between the two groups (P = 0.037). A large percentage of patients in both groups had moderately severe disease at the time of diagnosis, but after 2 years of treatment, more had mildly severe disease; this was especially true in Group B, in which the percentage of patients with subscores of 0 or 1 was high. In Group B, 53.2% of cases was evaluated as normal or inactive.
| Group A (n = 48) | Group B (n = 62) | P value | |
| Maintenance treatment 2 year after diagnosis | |||
| None | 0 | 5 (8.1) | 0.045 |
| 5-Aminosalicylate | 47 (97.9) | 46 (74.2) | 0.001 |
| Azathioprine | 43 (89.6) | 36 (58.1) | 0.001 |
| Infliximab | 0 | 34 (54.8) | < 0.001 |
| Adalimumab | 0 | 0 | |
| Vedolizumab | 0 | 0 | |
| Ustekinumab | 0 | 0 | |
| Tofacitinib | 0 | 0 | |
| Disease extent of Paris classification 2 years after diagnosis | 0.012b | ||
| Remission | 14 (29.2) | 31 (50.0) | |
| E1 proctitis | 13 (27.1) | 11 (17.7) | |
| E2 left colitis | 2 (4.2) | 8 (12.9) | |
| E3 right colitis | 6 (12.5) | 8 (12.9) | |
| E4 pancolitis | 13 (27.1) | 4 (6.5) | |
| Mayo endoscopic subscore 2 years after diagnosisa | 0.037b | ||
| 0 normal or inactive | 15 (24.2) | 33 (53.2) | |
| 1 mild | 24 (50.0) | 21 (33.9) | |
| 2 moderate | 8 (16.7) | 8 (12.9) | |
| 3 severe | 1 (2.1) | 0 | |
| Group A (n = 24) | Group B (n = 31) | P value | |
| Maintenance treatment 5 year after diagnosis | |||
| None | 2 (8.3) | 5 (16.1) | 0.394 |
| 5-aminosalicylate | 21 (87.5) | 15 (48.4) | 0.003 |
| Azathioprine | 14 (58.3) | 14 (45.2) | 0.337 |
| Infliximab | 0 | 18 (58.1) | < 0.001 |
| Adalimumab | 0 | 2 (6.5) | |
| Vedolizumab | 0 | 1 (3.2) | |
| Ustekinumab | 0 | 0 | |
| Tofacitinib | 0 | 0 | |
| Disease extent of Paris classification 5 years after diagnosis | 0.016b | ||
| Remission | 3 (12.5) | 13 (41.9) | |
| E1 proctitis | 9 (37.5) | 9 (29.0) | |
| E2 left colitis | 3 (12.5) | 4 (12.9) | |
| E3 right colitis | 2 (8.3) | 2 (6.5) | |
| E4 pancolitis | 7 (29.2) | 3 (9.7) | |
| Mayo endoscopic subscore 5 years after diagnosisa | < 0.001b | ||
| 0 normal or inactive | 3 (12.5) | 13 (41.9) | |
| 1 mild | 6 (25.0) | 16 (51.6) | |
| 2 moderate | 14 (58.3) | 2 (6.5) | |
| 3 severe | 1 (4.2) | 0 |
Colonoscopy findings were analyzed again after 5 years of treatment (Table 2). As with year 2, the proportion of patients who reached remission at 5 years was greater in Group B (41.9%) than in Group A (12.5%). Overall, a higher proportion of cases in Group B was evaluated as E1 or E2, so disease severity in Group B was still milder than that in Group A (P = 0.016). Regarding MES, 41.9% of patients in Group B maintained an inactive disease state, whereas 12.5% of patients in Group B remained low. In Group A, 58.3% of cases was evaluated as moderate, and evidence of the disease was often difficult to see as it was well controlled. In Group B, 51.6% of patients had mild disease. The difference in disease severity between the two groups was statistically significant (P < 0.001).
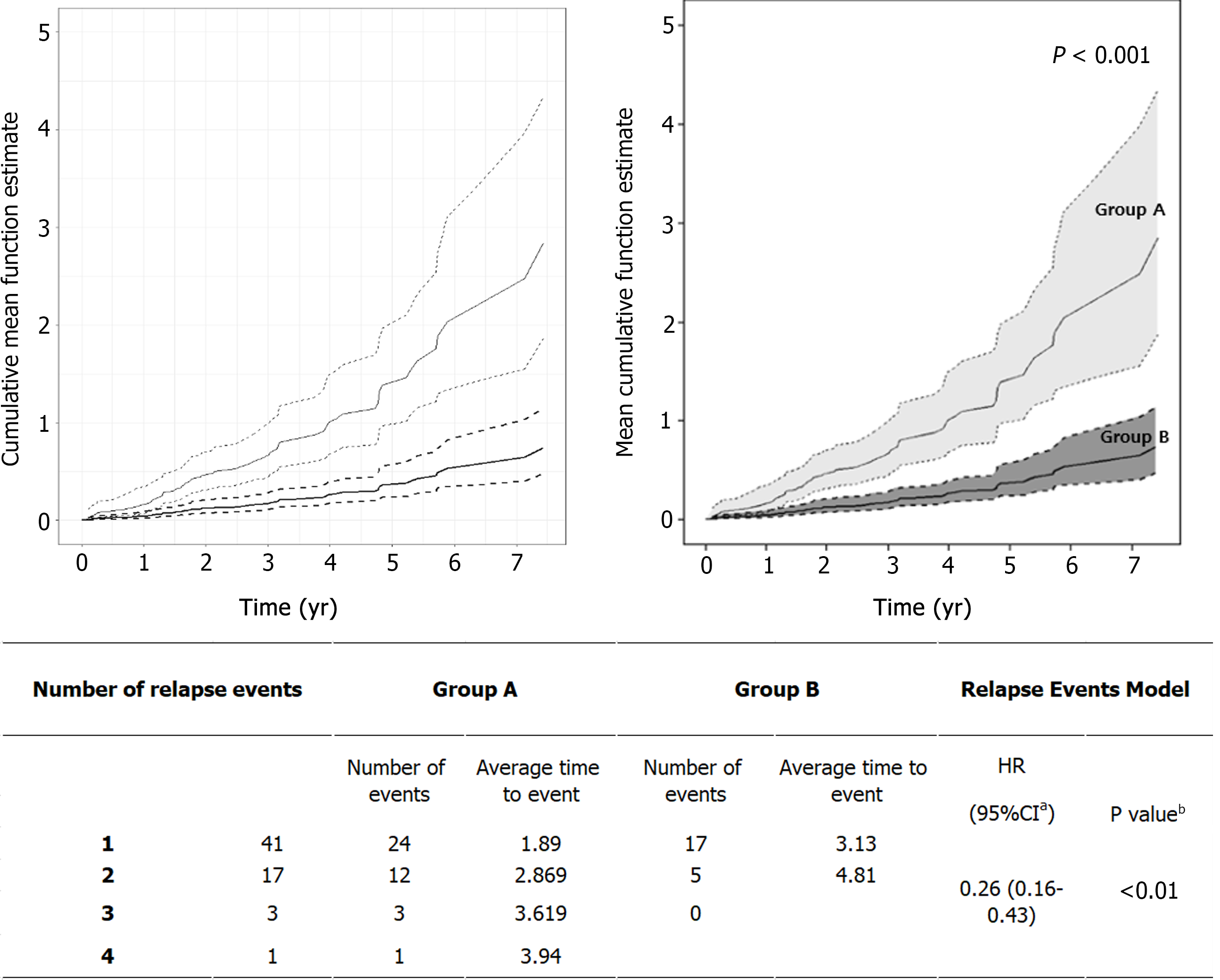
Disease relapse occurred in 23 patients (47.9%) in Group A and 16 (25.8%) in Group B, which was a significant difference (P = 0.027) (Table 3). The total cumulative number of relapses was also significantly higher in Group A (40 in Group A vs 22 in Group B; P = 0.006). In addition, although the difference was not significant, the number of relapses that occurred per person per year was also higher in Group A, with a ratio of 0.44 (Group A) vs 0.25 (Group B). The first relapse occurred at a median of 1.2 years after diagnosis in Group A, but in Group B, relapse occurred after a median interval of 1.95 years; thus, first relapse seemed to be delayed in Group B. The time interval between each relapse was investigated in patients who experienced multiple relapses. In Group A, relapse occurred approximately every 1.3 years, whereas relapse took about 1.7 years in Group B, showing that the interval between relapses was relatively wide in Group B. Since each patient's follow-up period was different, we also evaluated the relapse rate by comparing the cumulative hazard rate up to a specific point in time. There were statistically significant differences (P < 0.001) between the two groups, as shown in Figure 2.
| Group A (n = 48) | Group B (n = 62) | P value | |
| Number of relapsed patients | 23 (47.9) | 16 (25.8) | 0.027b |
| Cumulative total relapses | 40 | 22 | 0.006b |
| Number of relapses per person | 1.74 | 1.38 | |
| Number of relapses per person per year | 0.44 | 0.25 | |
| First relapse interval from diagnosis, yr | 1.20 (0.60-2.50) | 1.95 (1.35-3.93) | 0.194b |
| Each relapse interval, yr | 1.30 (0.60-3.55) | 1.70 (1.00-4.20) | 0.943b |
| Initial disease extent of relapsed patients | 0.080c | ||
| E1 proctitis | 7 (30.4) | 0 | |
| E2 left colitis | 2 (8.7) | 0 | |
| E3 right colitis | 3 (13.0) | 2 (12.5) | |
| E4 pancolitis | 11 (47.8) | 14 (87.5) | |
| Number of hospitalizations per person | 0.13 | 0.18 | 0.964b |
| Median PUCAIa during treatment period | 10 (5-20) | 5 (3.75-15) | < 0.001b |
| Median PUCAIa at the time of relapse | 65 (52.5-75) | 45 (45-55) | < 0.001b |
| Median cumulative corticosteroid free period | 3.0 (2.6-3.7) | 4.4 (3.1-6.0) | < 0.001b |
| Number of cumulative colectomies1 | 1 | 0 |
The PUCAI scores at each outpatient visit during maintenance treatment (excluding times at which the patients had relapsed) were significantly lower in Group B than in Group A (P < 0.001) (Table 3). The PUCAI score at the time of relapse was also compared, which showed that the PUCAI score was significantly lower in Group B than in Group A (P < 0.001).
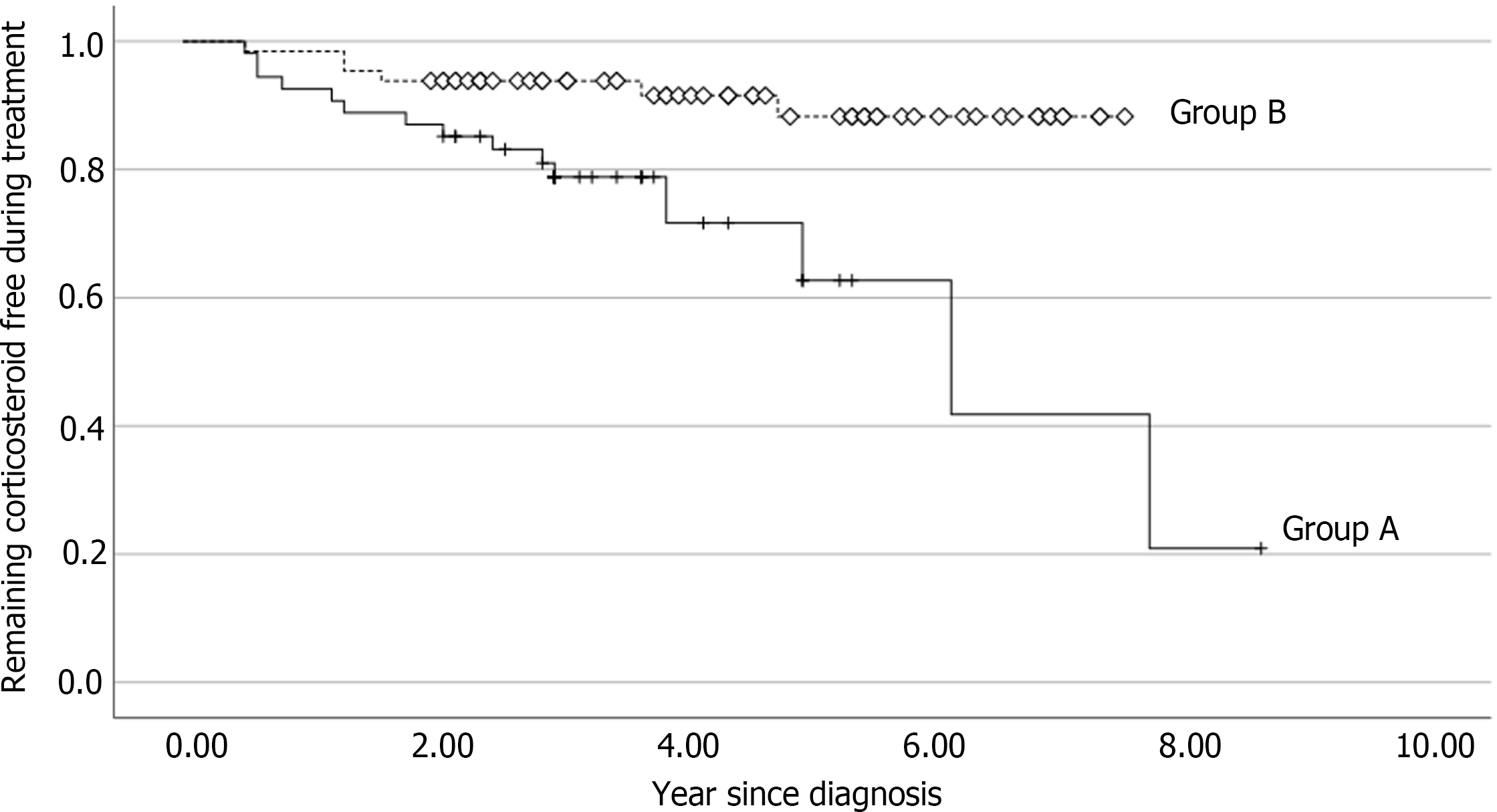
Because Group B patients were treated with infliximab, the frequency of steroid use was lower in that group (Table 3). The corticosteroid-free period was compared between the two groups. The median value of the cumulative corticosteroid-free period was 3.0 years in Group A and 4.4 years in Group B. The steroid-free period of Group B was significantly longer than that of Group A (P < 0.001). Figure 3 shows the analysis of corticosteroid-free survival curve after initiation of treatment using the Kaplan-Meier method. Over the course of 2 years, the likelihood of remaining corticosteroid-free in Group A was 21%, while it was 88% in Group B (P = 0.003).
Only 1 patient underwent colectomy in Group A. At the time of colectomy, the patient used mesalazine, azathioprine, methylprednisolone, and antibiotics. The patient finally underwent total proctocolectomy due to persistent uncontrolled hematochezia at 3 years after diagnosis.
Since relapse was often observed in both Group A and Group B, the risk factors associated with an increase in relapse rate were investigated in all patients who experienced relapse using Cox’s proportional hazard regression for counting processes (Table 4). Since all patients were not followed up for the same period of time, the relapse rate was evaluated but not simply the number of relapses. All factors that were analyzed reflected the status at the time of diagnosis. Young age (> 10 years), PUCAI score over 45, disease extent according to the Paris classification, MES, and initial corticosteroid use were investigated as potential risk factors. In univariable analysis, MES, and initial corticosteroid use were identified as clinical risk factors with statistical significance (P = 0.010 and < 0.001) In multivariable analysis, only severe status remained an independent risk factor with statistical significance (P = 0.015).
| Parameter | Univariable analysisb | Multivariable analysisb | |||||||
| Pr > ChiSq | HR | 95%CIc of HR | Pr > ChiSq | HR | 95%CIc of HR | ||||
| Age at diagnosis, yr (< 10 yr) | 0.063 | 0.434 | 0.642 | 3.714 | |||||
| Disease extent at diagnosis | 0.317 | ||||||||
| E2 | 1 | 0.786 | 0.194 | 3.185a | |||||
| E3 | 1 | 1.097 | 0.555 | 2.166a | |||||
| E4 | 0.374 | 1.758 | 1.058 | 2.921a | |||||
| Corticosteroid uses at baseline (Yes) | 0.010 | 2.111 | 1.201 | 3.712 | 0.109 | 1.682 | 0.890 | 3.176 | |
| Mayo endoscopic subscore at diagnosis (> 2 Moderate) | < 0.001 | 2.496 | 1.478 | 4.217 | 0.015 | 2.108 | 1.157 | 3.843 | |
| PUCAId at diagnosis (> 45) | 0.558 | 1.202 | 0.650 | 2.222 | |||||
Supplementary Figure 2 shows a comparison of the timing of the start of infliximab treatment after diagnosis among non-relapsed and relapsed patients who used infliximab. In the group of 29 non-relapsed patients, the majority (22, 75.9%) of them began infliximab treatment within 3 mo. On the other hand, in the group that experienced relapse, there were many patients who first used oral drugs or steroids and then began to use infliximab after 6 mo as the disease progressed.
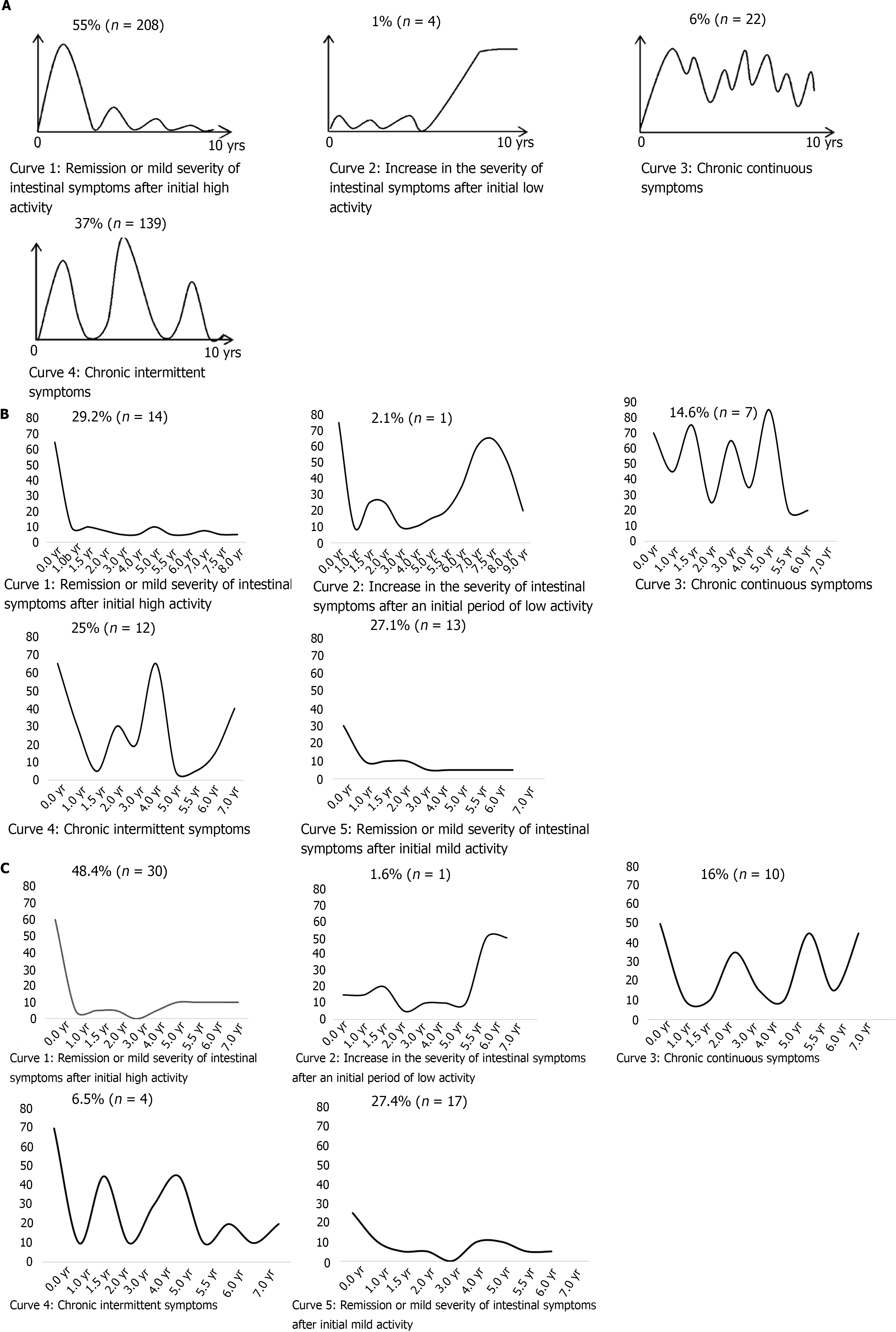
In the IBSEN study, patients were asked to choose which of four predefined patterns best reflected their clinical course (Figure 4A). The predefined patterns were as follows: remission or mild severity of intestinal symptoms after initial high activity, increase in the severity of intestinal symptoms after an initial period of low activity, chronic continuous symptoms, or chronic intermittent symptoms[6]. These curves do not include the curve of the patients whose disease is consistently well controlled after showing mild disease activity at the time of diagnosis; they were probably included in curve 1 in the IBSEN study. In the current study, we plotted the clinical courses of all patients in both groups using the PUCAI score at each outpatient visit and the PUCAI score at relapse. We separated the patients with mild initial disease activity (PUCAI < 35) from the group with good disease control after treatment and drew a new curve (curve 5). Therefore, the proportion of patients corresponding to curve 1 was different from the IBSEN study. In this study, 27.1% of patients in Group A were included in curve 5, and 29.2% of patients showed moderate to severe initial disease activity and maintained well after treatment; they were included in curve 1 (Figure 4B). When these two were combined, it was 56%, which was similar to the ratio of curve 1 in the IBSEN study. In the case of Group B, 27.4% of patients were included in curve 5 and the proportion was similar to Group A (Figure 4C). The patients of curve 1 in Group B were 48.4%, showing an increase in the proportion of patients compared to Group A. When combined with patients with mild disease initial activity, it was 76%, which was higher than that of curve 1 patients in the IBSEN study.
The other clinical courses were also classified among patients with similar types and compared with the predefined curves of the IBSEN study. Patients in Group A were distributed among the four types in similar proportions as patients in the IBSEN study (Figure 4B). However, in Group B, the percentage of patients corresponding to curve 4 was relatively small (16%) (Figure 4C).
Many previous papers have cited young age at diagnosis as a risk factor for poor clinical outcomes such as relapse and colectomy in pediatric UC patients[22-25]. Thus, research on children with UC is vital. This study evaluated the ways in which the clinical course of children and adolescents with UC changed when the treatment options were diversified after the introduction of biological agents. Infliximab was approved in Korea in 2012 and is now widely used in patients with moderate to severe UC, but many patients who exhibit a good response to oral medication continue to be treated with oral medication only. Here, we compared the clinical course of patients before and after approval of the biological agent according to the passage of time, rather than directly comparing patients who used infliximab to those who did not, as was done in previous studies[8,26,27].
Table 1 shows the characteristics of the included patients. It is evident that the two groups had similar characteristics and differed only in terms of the initial disease extent. The difference in initial disease extent is thought to be due to the rise in incidence of IBD over time as dietary habits become more Westernized; accordingly, the severity of the disease is increasing in newly diagnosed patients[28-30]. Since all patients in this study were followed for at least 2 years, drug and endoscopic evaluation were performed in every patient at 2 years post-diagnosis; it was thus possible to assess changes in the disease extent after 2 years to follow up on this difference in initial disease extent. Only half of all included patients were followed for more than 5 years, but the two groups still exhibited a statistically significant difference in disease extent and severity at 5 years. Despite the high proportion of pancolitis at initial diagnosis, many patients in Group B reached remission. This finding indicates that, in patients with broad disease extent and severity, there is an active and ongoing need for biological agents such as infliximab.
The number of patients who relapsed, the total number of relapses, and the relapse rate were significantly lower in Group B than in Group A. Though both measures significantly differed between the two groups, because every patient had a different follow-up period, we assume that the relapse rate is a more relevant outcome than the total number of relapses (Figure 2). We believe that the higher relapse rate in Group A is attributable to the differences in treatment. Most of the patients with relapse in Group B initially showed pancolitis. Many previous studies have identified disease extent as a risk factor for relapse[23,31,32]. Our risk factor analysis also demonstrated that the initial disease severity (MES) was a significant risk factor for relapse, with P values of 0.006 in the univariate analysis and 0.0149 in the multivariate analysis (Table 4). The worse the initial disease state, the worse the prognosis; this relationship may be taken for granted, but it is important information to keep in mind when establishing a treatment strategy. We thought it was strange that disease extent was not significantly associated with relapse, so we delved a bit deeper into the issue. As a result, pancolitis at initial diagnosis was identified as a risk factor in the univariate analysis in Group A. In Group B, the relapse rate was relatively low, so statistical evaluation was not possible. In our opinion, disease extent can indicate how long a patient has been suffering from the disease without appropriate treatment. Therefore, it is less likely to be related to relapse rate than disease severity.
As with the endoscopic findings, a high PUCAI score and the use of corticosteroids, which indirectly indicate greater initial disease severity, were also identified as risk factors for relapse. We wondered whether the time at which the patients started infliximab treatment would change the clinical course in relapsed patients even in Group B. Rapid use of infliximab can be expected to reduce the risk of relapse in patients with severe disease, as shown in Supplementary Figure 2. In the non-relapsed group, there were 16 patients with pancolitis at the time of diagnosis, but no relapse occurred among those who received infliximab within 3 mo of diagnosis (Supplementary Figure 2). Since all of our patients were treated with mesalazine and azathioprine for severe disease before receiving additional treatments such as corticosteroids and infliximab, they received similar medications at similar times with the exception of infliximab. Therefore, we propose that the active use of infliximab for initial treatment of moderate to severe UC is important.
As the field of IBD research has expanded and more treatments have been developed, there have been many studies on the quality of life of IBD patients[33-35]. The average PUCAI score during maintenance treatment can be considered an important factor in terms of improving quality of life and preventing relapse. The PUCAI scores during maintenance treatment and at the time of relapse were significantly lower in Group B, indicating that the daily lives of patients in Group B may have been more enriching.
Perhaps the most important factors in evaluating the clinical outcome of treatment are how long the steroid-free period was maintained and whether colectomy was performed[31]. As children and adolescents are not yet finished growing, using steroids more sparingly can prevent osteoporosis and slow growth, which are the typical side effects of steroids[36]. It can also prevent hirsutism, moon face, and buffalo hump in adolescent patients, who tend to be sensitive to appearance. We confirmed that steroid dependency could be avoided through the use of biological agents in patients with high-severity UC (Figure 3). Since colectomy, like the length of the steroid-free period, is an important factor when evaluating long-term outcomes, other studies have also reported on colectomy rates according to treatment[37-39]. In this study, only 1 patient in Group A underwent colectomy due to uncontrolled hematochezia, so it was not possible to compare colectomy as a long-term outcome.
The IBSEN study has been cited in several papers because it is a representative paper that evaluated the clinical course of UC on a large scale[40,41]. The IBSEN study developed predefined curves to represent the clinical course of the disease (Figure 4A). Each patient was asked to choose which predefined graph was most similar to their clinical course. We wondered whether the actual clinical course is represented by these curves and whether the clinical course differs between adults and children. We also wondered whether the clinical course changed after the introduction of biological agents. Therefore, we plotted the clinical course using PUCAI scores. The PUCAI score was chosen because laboratory results such as ESR and CRP are only weakly correlated with symptoms in patients with UC. The shapes of our graphs are similar to the four curves of the IBSEN study. However, there are some differences. The biggest difference is that we separated the patients whose initial disease activity was mild and disease was well controlled from curve 1, which was not shown in the IBSEN study. We believe that patients with such clinical features were also included in curve 1 in the IBSEN study. What was newly confirmed in the process of dividing into curves 1 and 5 is that the proportion of patients in curve 5 was similar in Group A and Group B. It is an epidemiologically convincing finding that the proportion of mild cases is similar over time.
In Group A, if the patients in curve 1 and those in curve 5 were combined, the ratio was similar to that of curve 1 in the IBSEN study. Since the patients of Group A received treatments that were similar to those given in the IBSEN study, the proportion of patients corresponding to each curve was similar. In the case of curve 2, the relapse that occurred in the second half did not persist as relapses tended to do in the IBSEN study, and instead showed a trend towards improvement with treatment. In addition, curve 3 differed in that it showed a lower baseline than the IBSEN curve when disease was partially controlled.
When comparing the graphs of the two groups, fewer patients in Group B than Group A corresponded to curves 3 and 4, and more corresponded to curve 1, indicating that the disease course of patients in Group B was better controlled. Therefore, the proportion of Group B patients corresponding to each curve was also different from the IBSEN study. As mentioned above, the relapse rate dropped after the introduction of infliximab and patients showed improved clinical outcomes. Similarly, the disease course appeared to be changing due to the change in treatment. Taken together, this study suggests that the course of chronic diseases may change over time due to the expanded availability of different types of therapeutic drugs.
The main limitation of this study is that the time difference between the two groups may have introduced some degree of bias. The diagnostic environment and degree of training of the clinicians may have improved over time. To minimize this bias, we evaluated the patients’ baseline characteristics using objective indicators and found that there were no significant differences between the two groups. Also, when assessing disease extent and Mayo subscore from the colonoscopy findings, the same experts evaluated all patients using the same criteria. Another limitation is that it was conducted at a single center. However, this hospital is one of the biggest pediatric IBD centers in Korea and has the advantage of having a steady patient cohort through long-term care.
As patients with moderate and severe UC have been treated with infliximab since its introduction almost a decade ago, the rate of relapse has decreased and the steroid-free period has increased. In addition, based on the PUCAI score and objective colonoscopy results, the disease is better controlled and patients’ quality of life has improved. Before the introduction of the biological agent, the clinical course of pediatric patients showed patterns that resembled those in the IBSEN study, but since its introduction, the proportion of patients who have reached remission has significantly increased. The current goal of treatment for IBD is to change the disease course for a better quality of life, and appropriate treatment with biologic agents may be an option for achieving that goal.
There are many articles comparing the clinical outcomes of patients treated with biological agents vs those who did not use biological agents, but no studies have compared the disease course of UC by era before and after the introduction of biological agents. The authors assessed how introduction of new treatment altered disease course over time.
The number of large-scale and long-term follow-up studies for pediatric patients with ulcerative colitis (UC) is insufficient. A representative paper dealing with the clinical course of adult UC is the IBSEN study. This paper dealt with the clinical course before the introduction of biological agents. If the use of biological agents in pediatric UC changed the clinical course of UC, it would be helpful in future treatment decisions.
The aim of this study was to compare the clinical course of pediatric UC by era before and after the introduction of biological agents, and to compare them with clinical course curve of the IBSEN study.
Infliximab was approved for use in children in October 2012 in Korea. Group A (n = 48) was followed between January 2003 and October 2012, and Group B (n = 62) was followed between November 2012 and October 2020. Endoscopic remission, drug composition, relapse rate, steroid-free period, and the quality of life of the groups were evaluated as outcomes. Clinical course was plotted with the pediatric UC activity index score, and compared to the curve of the IBSEN study.
Despite a higher rate of pancolitis, patients in Group B had a higher rate of achieving endoscopic remission, longer steroid-free periods and reduced relapse rate. Unlike the clinical course curve of the IBSEN study, we drew one more independent curve (curve 5), and the proportion of the patients in Group B corresponding to curve 1 (remission or mild severity after initial high activity) was higher. In terms of quality of life, the number of hospitalizations and emergency room visits have improved after the introduction of biological agents. Comparison of treatment costs is also an important issue that needs future research.
The active use of biological agents may change the long-term disease course in moderate to severe pediatric UC. Growth can also be achieved by reducing the use of steroids.
Because biological agents are an expensive treatment option, whether there is a difference in economic quality of life caused by treatment with biological agents is also an important topic for future research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Iizuka M, Japan; Nakaji K, Japan S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Cai YX
| 1. | Wilks S. Morbid appearances in the intestine of Miss Bankes. Med Times Gazette. 1859;2:264-265. |
| 2. | Akiho H, Yokoyama A, Abe S, Nakazono Y, Murakami M, Otsuka Y, Fukawa K, Esaki M, Niina Y, Ogino H. Promising biological therapies for ulcerative colitis: A review of the literature. World J Gastrointest Pathophysiol. 2015;6:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, Bartkowiak-Wieczorek J, Mądry E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 407] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 4. | Rizzello F, Spisni E, Giovanardi E, Imbesi V, Salice M, Alvisi P, Valerii MC, Gionchetti P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 5. | Kugathasan S, Cohen S. Searching for new clues in inflammatory bowel disease: tell tales from pediatric IBD natural history studies. Gastroenterology. 2008;135:1038-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, Bernklev T, Henriksen M, Sauar J, Vatn MH, Moum B; IBSEN Study Group. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 535] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 7. | Cornillie F. Ten years of infliximab (remicade) in clinical practice: the story from bench to bedside. Eur J Pharmacol. 2009;623 Suppl 1:S1-S4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG, Hanauer SB, Lichtenstein GR, Present D, Sands BE, Sandborn WJ. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 728] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 9. | Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Stephens M, Evans J, Otley A, Carvalho R, Mack D, Bousvaros A, Rosh J, Grossman A, Tomer G, Kay M, Crandall W, Oliva-Hemker M, Keljo D, LeLeiko N, Markowitz J; Pediatric Inflammatory Bowel Disease Collaborative Research Group. Outcome following infliximab therapy in children with ulcerative colitis. Am J Gastroenterol. 2010;105:1430-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Ferrante M, Vermeire S, Fidder H, Schnitzler F, Noman M, Van Assche G, De Hertogh G, Hoffman I, D'Hoore A, Van Steen K, Geboes K, Penninckx F, Rutgeerts P. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis. 2008;2:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Oussalah A, Evesque L, Laharie D, Roblin X, Boschetti G, Nancey S, Filippi J, Flourié B, Hebuterne X, Bigard MA, Peyrin-Biroulet L. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response, optimization, colectomy, and hospitalization. Am J Gastroenterol. 2010;105:2617-2625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Farkas K, Lakatos PL, Nagy F, Szepes Z, Miheller P, Papp M, Palatka K, Bálint A, Bor R, Wittmann T, Molnár T. Predictors of relapse in patients with ulcerative colitis in remission after one-year of infliximab therapy. Scand J Gastroenterol. 2013;48:1394-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Fiorino G, Cortes PN, Ellul P, Felice C, Karatzas P, Silva M, Lakatos PL, Bossa F, Ungar B, Sebastian S, Furfaro F, Karmiris K, Katsanos KH, Muscat M, Christodoulou DK, Maconi G, Kopylov U, Magro F, Mantzaris GJ, Armuzzi A, Boscà-Watts MM, Ben-Horin S, Bonovas S, Danese S. Discontinuation of Infliximab in Patients With Ulcerative Colitis Is Associated With Increased Risk of Relapse: A Multinational Retrospective Cohort Study. Clin Gastroenterol Hepatol. 2016;14:1426-1432.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | De Vos M, Louis EJ, Jahnsen J, Vandervoort JG, Noman M, Dewit O, Dʼhaens GR, Franchimont D, Baert FJ, Torp RA, Henriksen M, Potvin PM, Van Hootegem PP, Hindryckx PM, Moreels TG, Collard A, Karlsen LN, Kittang E, Lambrecht G, Grimstad T, Koch J, Lygren I, Coche JC, Mana F, Van Gossum A, Belaiche J, Cool MR, Fontaine F, Maisin JM, Muls V, Neuville B, Staessen DA, Van Assche GA, de Lange T, Solberg IC, Vander Cruyssen BJ, Vermeire SA. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. 2013;19:2111-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 15. | IBD Working Group of the European Society for Paediatric Gastroenterology Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis--the Porto criteria. J Pediatr Gastroenterol Nutr. 2005;41:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 498] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 16. | Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, Fell J, Ruemmele FM, Walters T, Sherlock M, Dubinsky M, Hyams JS. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1144] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 17. | Mazzuoli S, Guglielmi FW, Antonelli E, Salemme M, Bassotti G, Villanacci V. Definition and evaluation of mucosal healing in clinical practice. Dig Liver Dis. 2013;45:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 18. | Nelson WB. Recurrent events data analysis for product repairs, disease recurrences, and other applications: SIAM, 2003. [DOI] [Full Text] |
| 19. | Lawless JF, Nadeau C. Some simple robust methods for the analysis of recurrent events. Technometrics. 1995;37:158-168. [RCA] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 209] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Andersen PK, Borgan O, Gill RD, Keiding N. Statistical models based on counting processes: Springer Science & Business Media, 2012. |
| 21. | Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074-1078. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1451] [Cited by in RCA: 1414] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 22. | Jang ES, Lee DH, Kim J, Yang HJ, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Kim N, Jung HC, Song IS. Age as a clinical predictor of relapse after induction therapy in ulcerative colitis. Hepatogastroenterology. 2009;56:1304-1309. |
| 23. | Bitton A, Peppercorn MA, Antonioli DA, Niles JL, Shah S, Bousvaros A, Ransil B, Wild G, Cohen A, Edwardes MD, Stevens AC. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Lee JH, Cheon JH, Moon CM, Park JJ, Hong SP, Kim TI, Kim WH. Do patients with ulcerative colitis diagnosed at a young age have more severe disease activity than patients diagnosed when older? Digestion. 2010;81:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Leo S, Leandro G, Di Matteo G, Caruso ML, Lorusso D. Ulcerative colitis in remission: it is possible to predict the risk of relapse? Digestion. 1989;44:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2885] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 27. | Guo C, Wu K, Liang X, Liang Y, Li R. Infliximab clinically treating ulcerative colitis: A systematic review and meta-analysis. Pharmacol Res. 2019;148:104455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Kim BJ, Song SM, Kim KM, Lee YJ, Rhee KW, Jang JY, Park SJ, Yoon CH. Characteristics and trends in the incidence of inflammatory bowel disease in Korean children: a single-center experience. Dig Dis Sci. 2010;55:1989-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Jung YS, Han M, Kim WH, Park S, Cheon JH. Incidence and Clinical Outcomes of Inflammatory Bowel Disease in South Korea, 2011-2014: A Nationwide Population-Based Study. Dig Dis Sci. 2017;62:2102-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Kim HJ, Hann HJ, Hong SN, Kim KH, Ahn IM, Song JY, Lee SH, Ahn HS. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015;21:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 31. | Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 235] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Qiu Y, Chen B, Li Y, Xiong S, Zhang S, He Y, Zeng Z, Ben-Horin S, Chen M, Mao R. Risk factors and long-term outcome of disease extent progression in Asian patients with ulcerative colitis: a retrospective cohort study. BMC Gastroenterol. 2019;19:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Guthrie E, Jackson J, Shaffer J, Thompson D, Tomenson B, Creed F. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn's disease. Am J Gastroenterol. 2002;97:1994-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Carcamo L, Miranda P, Zúñiga A, Alexander E, Molina ME, Urrejola G, Larach T, Miguieles R, Bellolio F. Ileal pouch-anal anastomosis in ulcerative colitis: outcomes, functional results, and quality of life in patients with more than 10-year follow-up. Int J Colorectal Dis. 2020;35:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Hagelund LM, Elkjær Stallknecht S, Jensen HH. Quality of life and patient preferences among Danish patients with ulcerative colitis - results from a survey study. Curr Med Res Opin. 2020;36:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med. 2009;122:599-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 37. | Abou Khalil M, Boutros M, Nedjar H, Morin N, Ghitulescu G, Vasilevsky CA, Gordon P, Rahme E. Incidence Rates and Predictors of Colectomy for Ulcerative Colitis in the Era of Biologics: Results from a Provincial Database. J Gastrointest Surg. 2018;22:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Aratari A, Papi C, Clemente V, Moretti A, Luchetti R, Koch M, Capurso L, Caprilli R. Colectomy rate in acute severe ulcerative colitis in the infliximab era. Dig Liver Dis. 2008;40:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Worley G, Almoudaris A, Bassett P, Segal J, Akbar A, Ghosh S, Aylin P, Faiz O. Colectomy rates for ulcerative colitis in England 2003-2016. Aliment Pharmacol Ther. 2021;53:484-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Ossum AM, Palm Ø, Lunder AK, Cvancarova M, Banitalebi H, Negård A, Høie O, Henriksen M, Moum BA, Høivik ML; IBSEN Study Group. Ankylosing Spondylitis and Axial Spondyloarthritis in Patients With Long-term Inflammatory Bowel Disease: Results From 20 Years of Follow-up in the IBSEN Study. J Crohns Colitis. 2018;12:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 41. | Monstad IL, Solberg IC, Cvancarova M, Hovde O, Henriksen M, Huppertz-Hauss G, Gunther E, Moum BA, Stray N, Vatn M, Hoie O, Jahnsen J. Outcome of Ulcerative Colitis 20 Years after Diagnosis in a Prospective Population-based Inception Cohort from South-Eastern Norway, the IBSEN Study. J Crohns Colitis. 2021;15:969-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |