Published online Sep 14, 2021. doi: 10.3748/wjg.v27.i34.5630
Peer-review started: January 27, 2021
First decision: May 13, 2021
Revised: May 19, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: September 14, 2021
Processing time: 225 Days and 3.2 Hours
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respi
Core Tip: The Coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), was declared a pandemic by the World Health Organization in 2020. It has since affected millions of persons wor
- Citation: Vargas-Mendoza N, García-Machorro J, Angeles-Valencia M, Martínez-Archundia M, Madrigal-Santillán EO, Morales-González Á, Anguiano-Robledo L, Morales-González JA. Liver disorders in COVID-19, nutritional approaches and the use of phytochemicals. World J Gastroenterol 2021; 27(34): 5630-5665
- URL: https://www.wjgnet.com/1007-9327/full/v27/i34/5630.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i34.5630
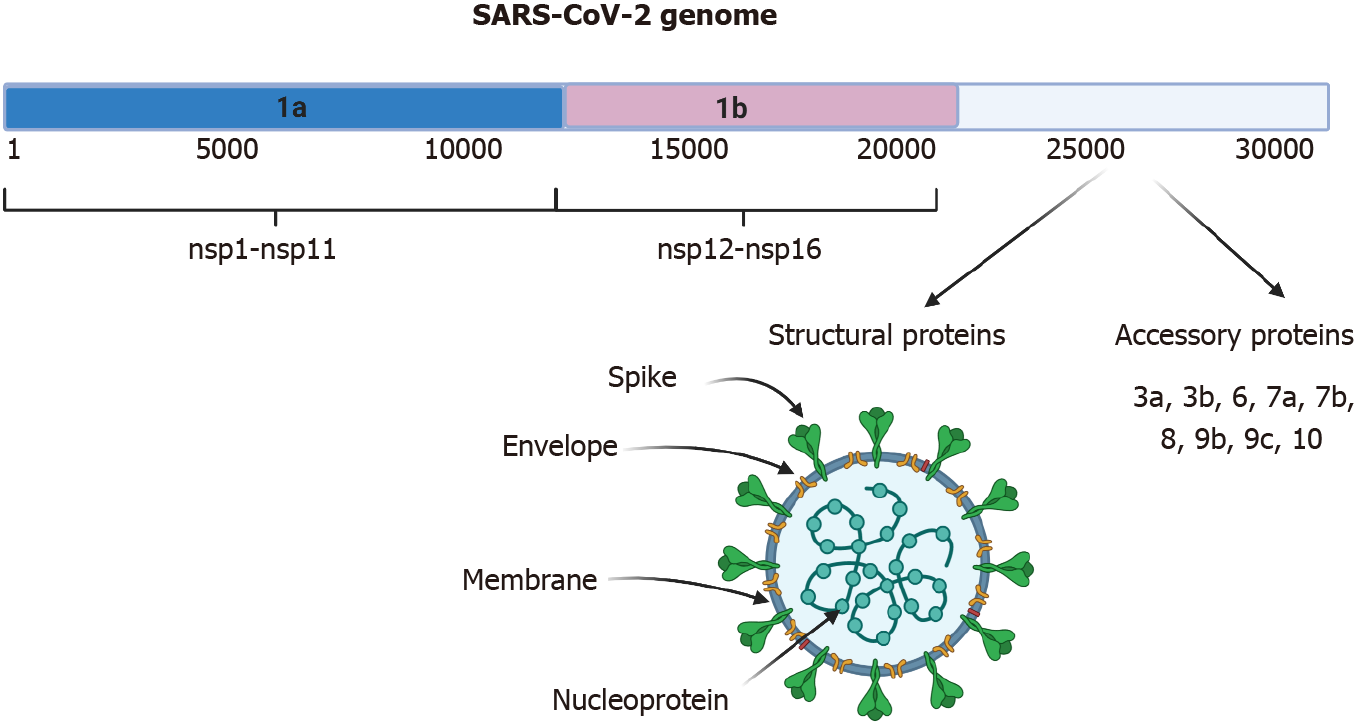
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) belongs to the family of coronaviruses; the genome is +ssRNA, which is nonsegmented and has a size of 30 kb[1,2]. In the cytoplasm of the host cell, it can be translated immediately into protein, as occurs with messenger (m)RNA. The genome encodes 16 nonstructural proteins (nsps) that are not part of the virion and have various functions in genome replication, protein processing, viral assembly, the exit of viral progeny, and others[3]. It also codes for four structural proteins, including spike (S), nucleocapsid (N), membrane (M), and envelope (E) that are required to constitute the complete virus particle (Figure 1)[2,4]. The origin of the disease may be from BatCov RaTG13 (GenBank: MN996532), a relatively close virus and one that was isolated from horseshoe bats[5]. SARS-CoV-2 causes the disease known as coronavirus disease 2019 (COVID-19). Since March 2020, a pandemic has been declared, and up to December 31, 2020, 81475053 cases were confirmed and 1798050 deaths had been reported. The global lethality rate is 2.22%[6].
SARS-CoV-2 can affect various organs that express the entry receptor. The main receptor described is angiotensin-converting enzyme 2 (ACE2)[1]. Distribution of the ACE2 protein was investigated by immunohistochemistry, and the protein was found present in endothelial cells from small and large arteries and veins, arterial smooth muscle cells, myofibroblasts, the membrane of fat cells in various organs, and in the basal layer of nonkeratinizing squamous epithelium. Importantly, ACE2 is expressed in nasal and oral mucosae and the nasopharynx in type I and type II alveolar epithelial cells in normal lungs, which explains the respiratory tract infection. Gastrointestinal manifestations can be explained by the presence of the receptor in smooth muscle cells and in the endothelium of vessels in the stomach, small intestine, and colon. In addition, the receptor can be found in the brush border of enterocytes in the small intestine, including the duodenum, jejunum, and ileum, but not in enterocytes of the colon. In the kidney, weak glomerular visceral ACE2 staining was observed, whereas parietal epithelial cells were moderately positive. In the skin, ACE2 was present in the basal cell layer of the epidermis extending to the basal cell layer of hair follicles. Smooth muscle cells surrounding the sebaceous glands were also positive for ACE2. In the brain, ACE2 receptors were found in only endothelial and smooth muscle cells[7]. Some of the extra respiratory manifestations can be explained by the previous fin
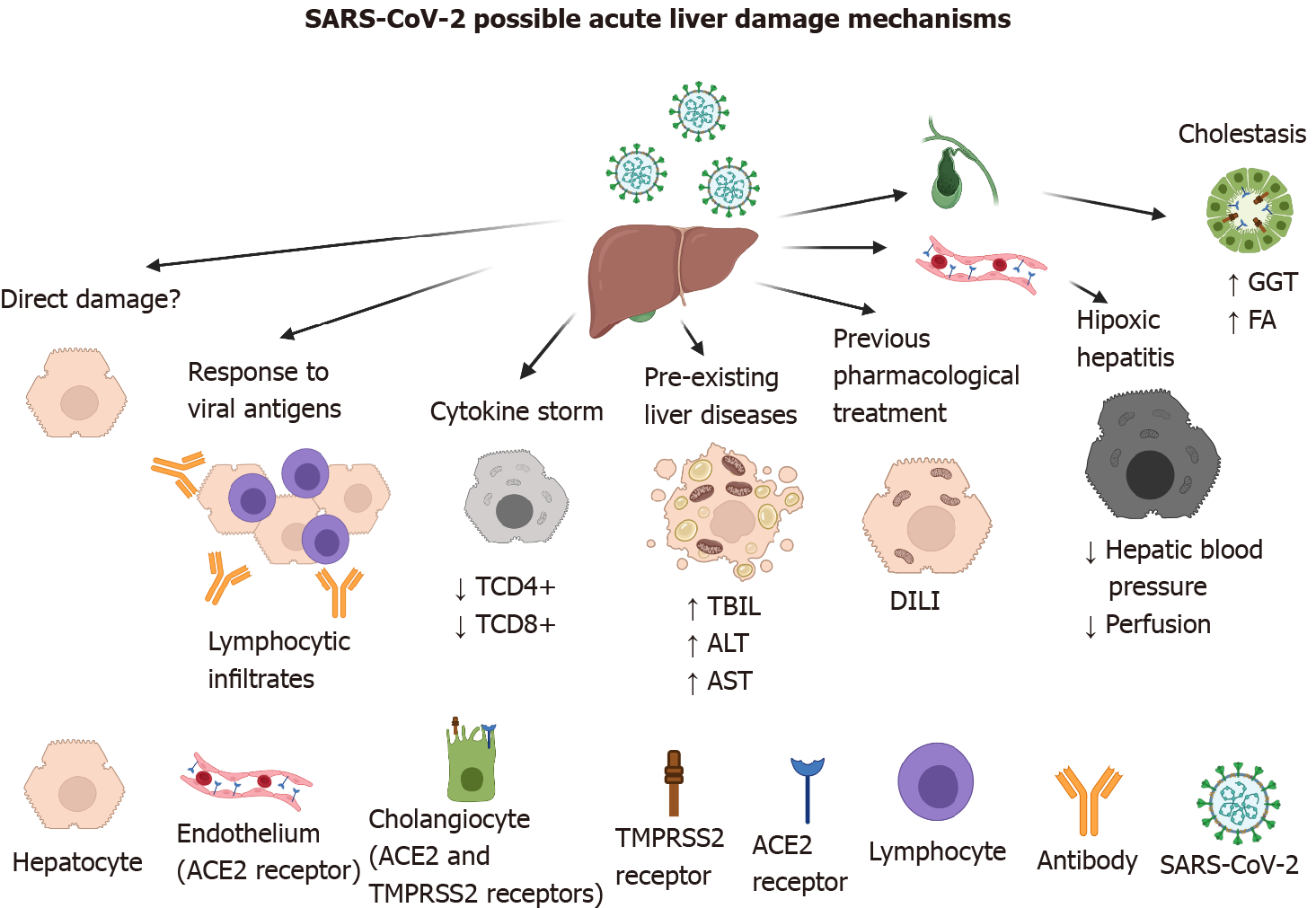
The ACE2 receptor has been described as the main entry receptor for the SARS-CoV and SARS-CoV-2 viruses, and it is abundantly expressed in the cells of the biliary system (i.e. cholangiocytes)[7]. Thus, it has been implicated in elevated levels of alkaline phosphatase and gamma-glutamyl transferase (GGT). However, those changes have been found in a small number of patients[8], compared with elevated levels of aminotransferases directly related to functions of hepatocytes that do not express the ACE2 receptor (Figure 2)[9].
Direct damage of liver cells by SARS-CoV-2 has been reported, based on ultra
In addition to the above observations, we would ask how the virus enters cells that do not have the receptor? In the case of cells with high phagocytic capacity that could swallow the virus, viral replication capacity would have to be investigated by the detection of nonstructural proteins. In the case of suspected viral reservoir cells, detecting at least some of the viral structural proteins would require staining, as has been reported for other viruses[12].
On the other hand, SARS-CoV-2 can use transmembrane protease serine 2 (TMPRSS2), which has not been detected in the liver, to enter cells. However, other transmembrane serine proteases have been detected, in particular furin and hepsin (in Huh7-25-CD81 cells)[13]. In three-dimensional (3D) culture systems, liver bile duct-derived progenitor cells form “liver ductal organoids” that retain their tissue-of-origin commitment and genetic stability. In a SARS-CoV-2 infection model with human liver ductal organoids, cholangiocytes expressing ACE2 and TMPRSS2 were preserved ex vivo in long-term culture. In addition, the expression of TMPRSS2 mRNA was found in a subset of hepatocytes and cholangiocytes[14]. In other experiments, human liver ductal organoids showed increased expression of viral mRNA 24 h after being infected with SARS-CoV-2[15]. The experimental evidence suggests that viral receptors are not static and that they can be regulated by mechanisms that involve the presence of the virus and have not yet been described.
Damage to the liver is known to be caused by hepatotropic viruses that replicate in the liver (e.g., hepatitis A, B, C, and E viruses). However, some viruses that attack the respiratory tract can cause liver damage, from small alterations in transaminases to fulminant liver failure, for example, the influenza virus[16,17], parvovirus[18], and respiratory syncytial virus bronchiolitis[19]. In general, hepatitis is thought to be a consequence of an immune response to viral antigens and to the loss of regulation of the inflammatory response. SARS-CoV-2 infection has been associated with hepatitis characterized by focal lobular lymphocytic infiltrates[20]. SARS-CoV-2 viral antigens were recently detected in liver as nucleocapsids and spike proteins[21].
The first line of antiviral defense is the innate immune response, initiated by the production of interferon (IFN) type 1 or IFN-α/β[22,23]. IFN-α/β binds to its cellular receptor and initiates autocrine and paracrine signaling to stimulate the expression of genes involved in the antiviral response (interferon-stimulated genes, ISG), in order to build resistance to infection and limit the spread of the virus. However, poor inter
Correlations between the severity of COVID-19 and chronic diseases such as diabetes mellitus, arterial hypertension, obesity, renal alterations, and cardiovascular disease have been clearly described[32-34]. Regarding the impact of liver diseases on the progression of COVID-19, ALT and AST activity, and the concentration of C-reactive protein (CRP) have been related to the prediction of disease severity. The prediction of mortality was correlated with liver failure, total bilirubin, platelet count, and serum albumin concentration. Chronic hepatitis B and chronic liver disease were not related to disease severity, the requirement for treatment in the intensive care unit (ICU), or mortality[35]. In another study, 17 inactive hepatitis B virus carriers with SARS-CoV-2 co-infection were found to have abnormal liver function tests (total bilirubin, ALT, and AST)[36]. Thus, it is not clear whether pre-existing liver diseases, such as viral hepa
The liver contributes mainly to the elimination of lipophilic drugs by increasing their solubility, therefore facilitating their elimination through the cytochrome P450 isoenzyme complex. Therefore, it is important to inquire whether patients diagnosed with COVID-19 are using medications for the treatment of chronic diseases or even anti-influenza or antipyretic drugs[38]. Drugs that are especially potentially hepato
Ischemic hepatitis, also known as hypoxic hepatitis or liver shock, is defined as extensive and potentially severe predominantly centrilobular hepatocellular necrosis resulting from a significant decrease in hepatic perfusion. In patients with severe COVID-19, mechanical ventilation, or hemodynamic disturbance and the occurrence of a sudden drop in systemic blood pressure, can lead to a reduction in hepatic blood pressure, decreased perfusion, and hepatocellular hypoxia. The pathogenesis involves hepatic ischemia and hepatic venous congestion because of elevated central venous pressure, which can predispose the hepatocytes to irreversible hypoxic injury[39,40]. Additionally, the hypoxia can directly affect the tissue–oxygen demand[41].
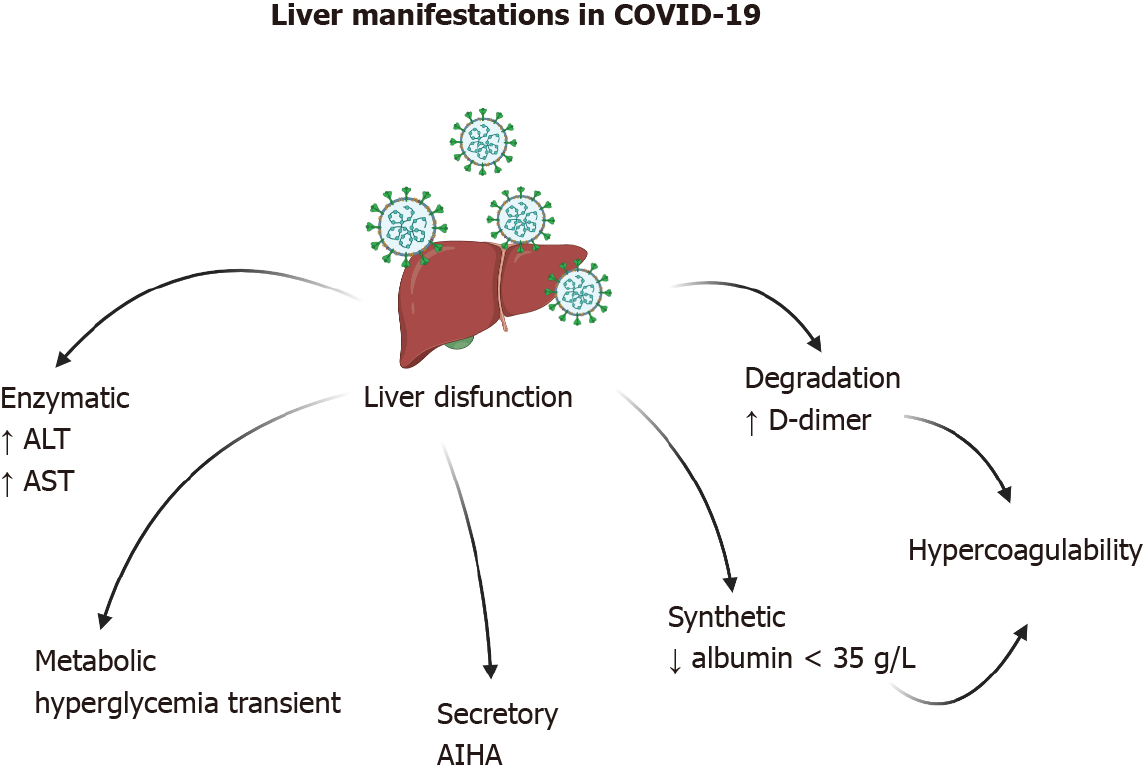
Various hepatic manifestations associated with COVID-19 have been documented, ranging from mild enzyme disruption to acute hepatic injury (Figure 3). The manifestations can be grouped into enzymatic (ALT and AST), metabolic (hypoglycemia and hyperammonemia), secretory (hyperbilirubinemia), synthetic (hypoalbuminemia and prothrombin time), and degradation (D-dimer) dysfunctions. Cholestasis with impai
Liver enzyme abnormalities in patients with COVID-19 are usually transient and are associated with disease severity. Alanine and aspartate aminotransferases (ALT and AST) are generally found to be elevated in the serum and are more often observed in hospitalized patients[33,42-44] than in patients with subclinical disease. Acute hepatic injury can be diagnosed by an ALT at 10 times the upper reference limit and by AST at three times the appropriate upper reference limit[45].
Hypoglycemia and hyperammonemia suggest liver failure and have not been found in COVID-19 patients, but there is a hypothesis that coronaviruses can cause a transient dysfunction of pancreatic beta cells[46], leading to acute hyperglycemia and relative insulin deficiency. The hypothesis is supported by a previous study including 39 patients with SARS and without a history of diabetes. Twenty of the patients deve
Regarding bilirubin, no relationship has been directly associated with SARS-CoV-2. However, there are reports of autoimmune hemolytic anemia (AIHA) in patients with symptomatic COVID-19[48,49]. Additionally, there is a report of a patient without clear evidence of SARS-CoV-2 infection for 2 wk and a subsequent positive PCR test. The final diagnosis was AIHA secondary to COVID-19)[50].
Albumin is a protein synthesized in the liver and has a serum half-life of approximately 21 d[51]. In the majority of cases, a decrease in serum albumin can be ex
D-dimer is the end-product of fibrin degradation, and it serves as a serological indicator of the activation of coagulation and of the fibrinolytic system. D-dimer levels have been reported as elevated in patients with COVID-19 and have been used as a tool for the prediction of disease severity in 178 studies[54]. In one study, it was determined that elevated D-dimer levels predicted in-hospital mortality in patients with COVID-19[55]. However, the usefulness of D-dimer is diminished by poor reporting of the values. The majority of publications did not identify either the assay manufacturer or the D-dimer product used for the determination. The majority of the authors did not identify whether D-dimer values were reported as D-dimer units or fibrinogen equivalent units (FEU), which differ by approximately ×2. The studies did not report normal cutoff values, and the units of comparison were not the same[56].
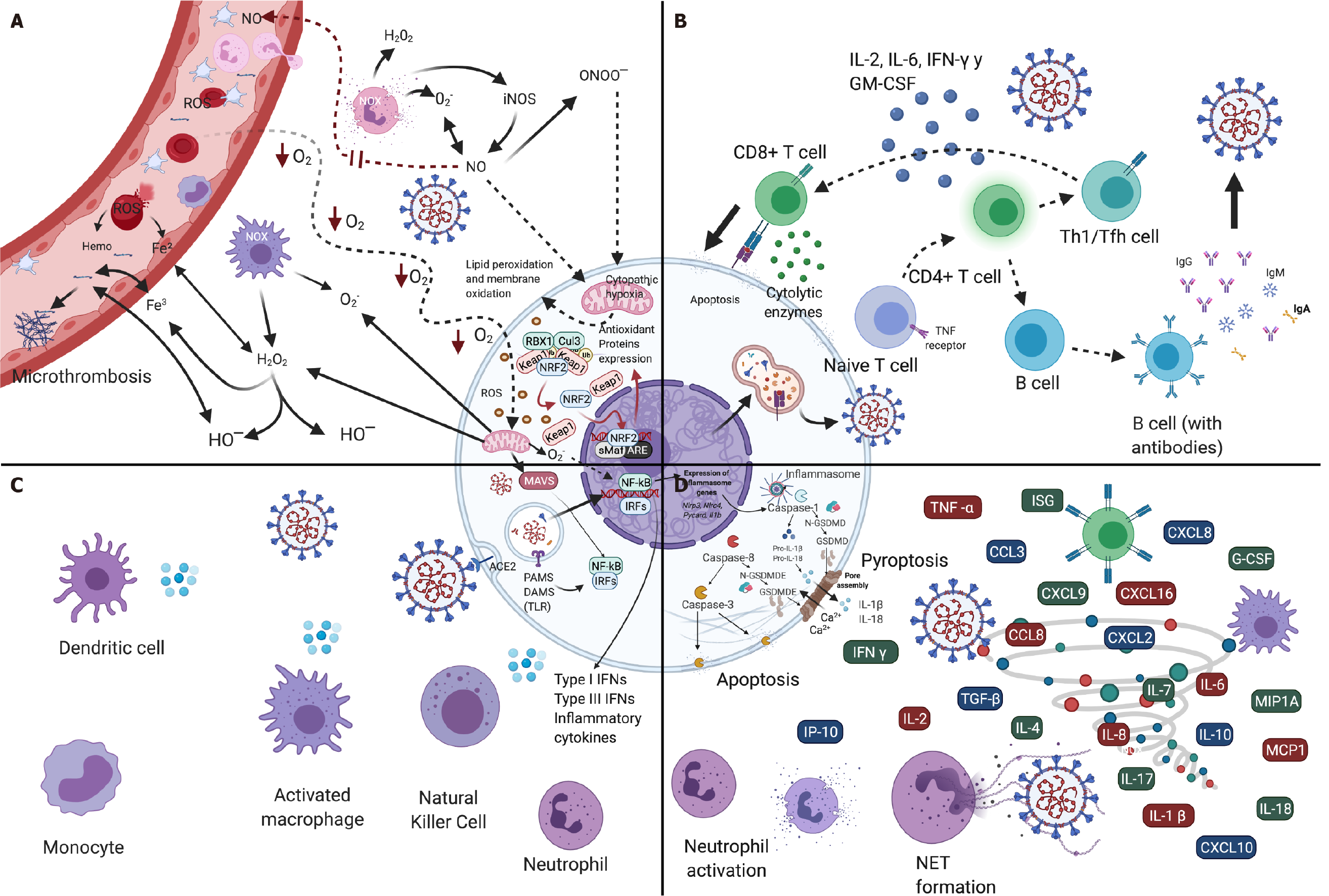
The innate immunological system functions as the first line of defense of the host against infection by SARS-CoV-2; it is crucial for identifying and eliminating the infected cells, and at the same time, for coordinating an adaptive immunity response[57]. The immune response of the host in cases of COVID-19 can be described as an early local immune response (antiviral defense) and a later local/systemic response phase, followed by uncontrolled inflammatory responses and cytokine storm syn
If the immunological system of the individual does not counteract the virus during the initial phase of exposure through a rapid early response, the virus advances to the lower respiratory tract (LRT)[62,63]. Once the virus reaches terminal respiratory pa
In response to viral invasion, the innate immunological system recognizes viral nucleic acids by host recognition-pattern (HRP) receptors, which are expressed in innate immune cells (e.g., neutrophils, dendritic cells, epithelial cells, and macro
Once the virus was detected by an HRP receptor, intracellular signaling pathways are triggered that activate interferon regulatory factor 3 (IRF3) and the nuclear factor kappa beta (NF-κB) signaling cascade[58]. The initial phase of the production of interferon (IFN) mediated by IRF3 performs the initial phase of the innate immune response to detect and brake viral replication[69]. Viral detection stimulates the pro
HRP receptors, particularly the nucleotide-binding oligomerization domain-like (NOD)-like receptor (NLR) family, subsequent to the SARS-CoV-2 infection, assemble a multiprotein complex called inflammasome NLRP3, which results in the activation of caspase-1. Activated caspase-1 splits from the pro-interleukin IL-1β, pro-IL-18, and gasdermin D (GSDMD) and releases the GSDMD N-terminal fragment that can be oligomerized within membranes to form membrane pores and “pyroptosis”[57]. The excision of caspase-1-dependent GSDMD leads to the release of IL-1β and IL-18[72], which are key mediators of the inflammatory response, and their increase in the plasma have been correlated with COVID-19 mortality or severity[73]. Apoptosis, another type of cell death, also occurs during SARS-CoV-2 infection and is driven by the excision of the caspase-8, -9, -10 initiators of the executioner caspase-3 and -7. Apoptotic caspase-3 activates gasdermin E (GSDME) to induce the lytic form of cell death; the protein ORF3a of the SARS-CoV-2 virus also induces the excision of caspase-8 and -9 and causes apoptosis[57]. The activation associated with the death pathways of the inflammatory cells can give rise to critical tissue damage, severe inflammation, and lactate dehydrogenase (LDH), which is a marker of cell death that has high concentrations in COVID-19 patients. The LDH concentration is considered a predictive factor for the early recognition of pulmonary lesions in severe cases[74].
Macrophages are a key respiratory system. They produce chemokines and IFN-β. Infected dendritic cells (DCs) produce antiviral cytokines, like IFN-α and IFN-β; the proinflammatory cytokine TNF, IL-6, and high levels of the inflammatory chemokines CCL3, CCL5, CCL2, and CXCL10. The cytokines/chemokines are key factors for the chemotaxis of neutrophils, monocytes, and activated T cells[75]. Activated neutrophils, whose main function is the elimination of pathogens and dendrites by means of phagocytosis[67], release leukotrienes and reactive oxygen species (ROS) that induce local damage to pneumocytes and the endothelium, which in turn leads directly to acute lung lesions[68].
Neutrophils can also develop DNA networks called neutrophilic extracellular traps (NETs)[68] through the process of NETosis, or the release of nucleic acids enveloped in histones, which retain viral particles and promote the inactivation of the viral infection and cytokine production to restrict replication of the virus[67]. NETosis is conditioned to the production of ROS by means of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. In addition to the physical containment promoted by NETosis, NETs contain proteases and cytotoxic enzymes that permit the neutrophils to cen
The inflammatory responses in the respiratory system intended to eliminate SARS-CoV-2 result in the generation of metabolic-acid waste material that, together with an increase in respiratory muscular work, lead to the development of metabolic acidosis. Metabolic acidosis compromises adaptive cellular immunity and the efficient era
Infection by SARS-CoV-2 promotes mechanisms that antagonize proinflammatory signals, particularly the signaling of IFN-I and IFN-III, but increases the expression of chemokines and proinflammatory cytokines in order to counteract the host’s innate immune response[57,58,63]. Thus, the expansion and early differentiation of T cells depend on the direct action of IFN-I[82]. The descending production of interferons promotes intracellular antiviral defenses in neighboring epithelial cells that can limit viral dissemination, while the release of IL-6 and IL-1β from other immune cells promotes neutrophil recruitment and immune cell activation[68].
The three most critical components of the adaptive immune responses are viral protein-specific CD4+ T cells, CD8+ T cells, and nAbs. The nAbs produced by B cells can bind to and neutralize the extracellular SARS-CoV-2 proteins. If the Abs cannot prevent the virus from entering cells, cytotoxic CD8+ T cells are called upon to destroy the cells directly infected with their granules[83,84]. Pulmonary cytotoxic CD8+ T cells recognize and induce apoptosis in cells infected through direct mechanisms (i.e. cell–cell contact) and indirect mechanisms with the participation of the perforin and granzyme-secreted cytolytic enzymes, as well as with the cytokines IFN-γ and TNF-α[85]. However, cytotoxic cells by nature do not prevent the infection, they destroy already infected cells, thus reducing propagation of the infection (Figure 4B)[59]. Transitory increases of the CD8 effector T and memory T cells constitute an effective and efficient response during early viral infection[81]. High counts of CD8+ T cells in the lungs are correlated with better control of SARS-CoV-2[80,85].
CD4+ T cells, the third arm, are auxiliaries and coordinators of the production of Abs and of the activation of the cytotoxic CD8+ T cells[83,84]. After being infected with SARS-CoV-2, CD4+ T cells are activated and differentiate to Th1 cells or cir
Lymphocytes gradually decrease as the disease advances, which results in immu
The host capacity to generate efficient T cell responses after infection by SARS-CoV-2 probably depends on the directed epitopes, the presence or absence of pre-existing cross-reactive T cells, and genetic factors such as the human lymphocyte antigen (HLA) type, and the repertory of T cell receptors (TCRs)[85]. Given that activated T cells in elderly persons and in those with chronic disease present reduced responses to IFN-I, a longer time is needed to generate effective adaptive immune responses because of the deterioration of the immune functions such as the production of virgin T cells and memory T cells, which diminish with aging[84], and present asynchronous immune responses with high Ab levels and weak T cell responses[83]. Delayed activation of SARS-CoV-2-specific T cells and a reduction of the clarification of the virus increase the risk of cytokine storm, the earlier appearance of severe disease, and increased mortality[68].
In contrast with innate immune responses, which are produced before the infection and participate fully in the elimination of the virus, the adaptive immune responses begin 4-7 d after infection. If the body does not generate effective adaptive antiviral responses in time to eliminate the virus, the innate immune responses will be main
The inflammatory cytokine storm, also known as the cytokine release syndrome, is a severe excessive immune response caused by positive biofeedback circuit damage of immune cells by the cytokines[67,75,87]. The formation of a cytokine storm leads to a “suicide attack” that not only limits additional propagation of the virus, but also induces secondary tissue damage[68]. The marked release of proinflammatory cyto
Oxidative stress is the result of disequilibrium between the oxidant system, which consists principally of free radicals, ROS), and reactive nitrogen species (RNS), and the antioxidant systems that neutralize the free radicals[90]. Reactive oxygen and nitrogen species (RONS) are characterized by unpaired valance electrons, obliging them to react with diverse biological molecules[90,91]. ROS comprise the hydroxyl (OH) radicals, superoxide anion (O2−), singlet oxygen (¹O2), hydrogen peroxide (H2O2), and ozone
In the pathology of COVID-19, the cytokine storm is an important source of endogenous oxidative stress, and excessive production of ROS that in turn stimulates the increased release of cytokines, causing an exaggeration of the already initiated inflammatory responses (Figure 4A)[93-97]. The interaction of ROS and cytokines ge
The cytokine storm with hyperinflammation accompanied by cytopenia and hy
SARS-CoV-2 activates oxidant-sensitive pathways through inflammatory responses following activation of the NF-κΒ pathway[93]. The reduction of oxygen saturation leads to the generation of superoxide radicals and H2O2 by the mitochondria. Hy
At the same time, the IFN-γ pathways are activated by oxidative stress induced by the inflammation intended to combat the infection by the virus[33]. Circulation of the inflammatory cytokines and ROS damage erythrocytes, leading to the generation of heme and free iron and diminish the circulating nitric oxide (NO), which worsens the existing ischemia of the organs. Deterioration of the mitochondria leads to cytopathic hypoxia, which results in a partial reduction of oxygen with the generation of ROS and the reduced energy production[98]. In addition, macrophages and activated neutro
Poorly coordinated iron, especially in the presence of high concentrations of oxygen and reducers have the potential to generate hydrogen peroxide, superoxide, and hydroxyl radicals in the lung[92]. The radical superoxide anion reduces Fe (III) to Fe (II) that, in the presence of H2O2, produces hydroxyl radicals (•OH), which are extremely toxic and promote the formation of lipid peroxidases in the cell membrane and the oxidation of proteins, causing cell death by apoptosis[94]. Hydroxyl radicals plus free iron convert soluble plasma fibrinogen into abnormal fibrin clots in the form of enzymatic degradation-resistant dense and entangled deposits, leading to micro
Oxidative damage resulting from SARS-CoV-2 infection can produce viral mu
Oxidative stress is already increased in the elderly and people with diabetes and chronic cardiovascular diseases[90,91]. Increase in the stress level in response to viral infection affords a possible explanation of the severity of COVID-19 in such patients. In addition, elderly individuals may particularly vulnerable to infection by SARS-CoV-2 because the level and the activity of Nrf2 diminish with age[98]. Therefore, aging is not only associated with alterations in the response to adaptive immunity, but also to a proinflammatory state in the host[97].
SARS-COV-2 infection and the resulting COVID-19 may cause multiorgan failure in addition to respiratory symptoms, including gastrointestinal (GI) and liver dysfun
Based on the previous statement, malnutrition should be identified as an early step in the assessment of patients with SARS-CoV-2 infection, especially in patients with a high risk of mortality or of poor outcomes, such as older adults and polymorbid individuals. The identification of malnutrition is crucial for establishing effective nutritional support in order to improve food consumption, nutritional status, the patient’s health prognosis, and even to prevent the occurrence of COVID-19 in the future. Particularly in the COVID-19 crisis, food and nutrient absorption is often impaired by nausea, vomiting, and diarrhea, the main GI symptoms, which lead to enhanced malnutrition. The international meta-analysis of McClave et al[106] of 47 studies in 10890 patients who were analyzed to determine the prevalence of liver and GI manifestations resulting from COVID-19 established consultative management of patients. Nausea/vomiting was a very frequent GI manifestation with a pooled prevalence of 7.8% (95%CI, 7.1%-8-5%) followed by diarrhea in 7.7% (95%CI, 7.2%-8.2%) and abdominal pain in 2.7% (95%CI, 2.0-3.4%). Moreover, ALT had a pooled prevalence of 15% (95%CI, 13.6%-16.5%) and AST had a prevalence of 15% (95%CI, 13.6%-15.4%) as manifestations of liver disorders. A cohort study of patients with COVID-19 in Hong Kong revealed that GI symptoms were present in 17.6% of patients and that diarrhea appeared to be the most common symptom. In the meta-analysis, the RNA virus was detected in 48.1% (95%CI, 38.3%-57-9%) of stool samples, and 70.3% (95%CI, 49.6%-85.1%) of the samples were collected after respiratory samples were found to be negative. Therefore, good management must be considered as a low risk of infection through endoscopic procedures or saving stool samples[107]. Thus, it is suggested that, prior to the administration of any treatment, it is necessary to evaluate the nutritional status of every infected patient.
In clinical practice, malnutrition is assessed with various tools (Table 1). The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends using the Malnutrition Universal Screening Tool (MUST) for the identification of malnu
| Tool | Target patients | Criteria |
| MUST | Low weight | Objective criteria: |
| Objective measures: weight and height to obtain BMI | ||
| Other measures (optional): ulna length and mid upper arm circumference | ||
| Weight loss in las 3-6 mo | ||
| Obese patients | Subjective criteria: | |
| Reduced food intake in last 5 d: clinical management, psychological factors | ||
| Weight loss appearance (clothes, jewelry) | ||
| NRS-2002 | Hospitalized individuals | BMI |
| Weight loss within 3 mo | ||
| Reduced dietary intake in last week | ||
| NUTRIC score | Hospitalized patients at ICU | Age |
| Days hospitalized or in the ICU | ||
| Number of comorbidities | ||
| IL-6 levels (optional) | ||
| APACHE II score | ||
| SOFA score | ||
| APACHE II score | Patients at ICU (predicting mortality) | Age |
| Temperature | ||
| Mean arterial pressure | ||
| pH | ||
| Heart rate/pulse | ||
| Respiratory rate | ||
| Sodium, potassium levels | ||
| Creatinine | ||
| Acute renal failure | ||
| SOFA score | Patients at ICU (estimation of mortality) | PaO2 |
| FiO2 | ||
| Medical ventilation | ||
| Platelets level | ||
| Glasgow Coma Scale | ||
| Bilirubin levels | ||
| Mean arterial pressure or administration of vasoactive agents required | ||
| Creatinine levels | ||
| Is a COVID-19 patient? | ||
| GLIM | Individuals at risk in general | Phenotypic criteria: |
| Weight loss | ||
| Low BMI | ||
| Loss of muscle mass | ||
| Etiologic criteria: | ||
| Reduced food intake or assimilation | ||
| Presence of disease or inflammation | ||
| NRF-NPT | Detection of malnutrition in liver patients disease | Unplanned weight loss in las 3-6 mo |
| BMI | ||
| Reduced dietary intake and uncompleted meals |
Several tools developed for assessment of hospitalized patients have been applied in COVID-19. Nutrition Risk Screening-2002 (NRS-2002) criteria, also recommended by ESPEN, predict malnutrition in hospitalized individuals and are recommended by the American College of Gastroenterology (ACG) guidelines for nutrition therapy[106]. NRS-2002 takes into account BMI; weight loss within the past 3 mo; reduced dietary intake in the previous week, and an ICU stay. In addition, the Nutrition Risk in the Critically ill (NUTRIC) score[108-111] is designed for patients, particularly for those in the ICU, and for those who can benefit from nutritional therapy considering their age, days hospitalized or time in the ICU, number of comorbidities, IL-6 Levels (optional). The Acute Physiology And Health Evaluation II (APACHE II) score[112,113], which is the most widely used tool for predicting mortality in the ICU, and the Sequential Organ Failure Assessment (SOFA) score[114,115], which estimates ICU mortality based on clinical data and laboratory results have both been used in COVID-19 clinical trials[116].
Recently, the Global Leadership Initiative on Malnutrition (GLIM), which includes the leading clinical nutrition societies worldwide, achieved a consensus with the purpose of establishing global criteria for the diagnosis of malnutrition in clinical practice. Such a consensus is a two-step approach. The first consists of the identification of “at risk” status by validated screening tools, and the second is the asse
In patients with liver damage caused by COVID-19, the risk of malnutrition may be increased by the presence of GI-associated abnormalities. In severe cases of COVID-19 infection, liver injury has most commonly been observed. At the same time, patients with previous liver damage represent more critical cases of COVID-19[118]. The incidence of liver injury ranges from 14%-53%[8]. Approximately one-third of the infected population reported altered aminotransferase levels. Liver damage may affect glucose, amino acid, and lipid metabolism, and result in poor clearance of lactate and protein catabolism as a consequence of hyperaminoacidemia, and hyperammonemia, which contributes to malnutrition. On the other hand, it is crucial to determine whether the liver injury is a consequence of pre-existing liver injury (cirrhosis, viral hepatitis, NASH, and ASH) or the result of COVID-19 infection and/or the reported drug-induced liver injury. In conjunction with the MUST and NRS-2002 tools for malnutrition assessment, ESPEN recommends the Royal Free Hospital-Nutritional Prioritizing tool (RFH-NPT), which has been developed for the detection of mal
In COVID-19, disease complications are basically the result of a hyperinflammatory response mediated by cytokine storm and several immune-related stimuli mentioned above. Thus, nutrition treatment should be designed to strengthen the immune system during the COVID-19 crisis, providing nutrients that relieve inflammation and oxidative stress. Whether liver injury emerges as a consequence of COVID-19 or pre-existing before infection, nutrition strategies should also correct liver and GI manifestations as related symptoms to prevent or treat the associated malnutrition.
Liver injury associated with COVID-19, as in any disease, could enhance inflammation, which alters the metabolic rate. In critically ill patients, it is recommended to estimate energy needs by means of indirect calorimetry considering all of the conditions for sterility during the measurement procedure. Significant increases in resting energy expenditure (REE) in patients with acute liver failure (ALF, 18%-30%)[121], alcoholic hepatitis (55%)[122] and alcoholic cirrhosis (26%)[123] have been reported. Hypermetabolism reported in patients with liver cirrhosis (> 30%)[124] could be related to effect of delay in the improvement of body composition on clinical outcomes. Hence, because of the individual variability in liver damage, REE should be measured by indirect calorimetry. Recently, the estimation of REE with a less-expensive handheld calorimeter method based on a respiratory quotient of 0.85, and which is very accurate for REE has been proposed[125]. However, when accessibility to calorimetric equipment is limited, prediction equations for the estimation of energy expenditure could be employed. A summary of caloric and nutrient recommendations is shown in Table 2. Caloric intake should be 1500-2000 kcal/d for normal maintenance by oral diets, with an increase of 400-500 kcals under conditions of stress or in an infection crisis[126]. ESPEN guidelines[100] suggest 27 kcal/kg body weight (Bw)/day in polymorbid patients and in patients > 65 years of age. For low-weight or older patients, it is suggested to achieve 30 kcal/kg Bw/day. In malnourished cirrhotic patients with muscle depletion, the energy supply must provide 30-35 kcal/kg Bw/day. Contrariwise, in overweight or obese patients with liver disease, the prognosis may be worse. In such cases, obesity has been associated with portal hypertension[127]. For that reason, an increased energy intake is not recommended. Nonetheless, all energy recommendations must be adjusted individually, taking into account disease severity, mobility, physical activity, and tolerance. In severely underweight patients, the energy supply must be carefully administered in order to prevent the refeeding syndrome, which is very common in such patients. Whenever possible, oral feeding should be the first energy and nutrient-supply option. When oral feeding is not feasible, support nutrition therapy by nasogastric tube through enteral nutrition (EN) or parenteral nutrition (PN) should be available as the next step.
| Energy/Nutrient | Criteria | Recommendation |
| Estimation of REE | All individuals with COVID-19 | Estimation by indirect calorimetry |
| Prediction equations | ||
| Calories | Normal oral diets | 1500-2000 kcals/d |
| Increase 400-500 kcals in stress or infection crisis | ||
| Polymorbid, old patients > 65 yr | 27 kcals/kg Bw/day | |
| Low weight, older patients | 30 kcals/kg Bw/day | |
| Malnourish chronic patients and muscle depletion | 30-35 kcals/kg Bw/day | |
| -Patients with COVID-19 outside ICU | ONS with low oral intolerance: | |
| 150-400 kcals/service | ||
| 70-100 g protein/service | ||
| Carbohydrates, fiber, PUFAs, vitamins, minerals, probiotics | ||
| Consuming for a month | ||
| Protein | Normal individuals (prevent loss and muscle mass) | 1 g/kg Bw/day |
| 70-100 g/d | ||
| Form animal (milk, yogurt, meat, fish, chicken, cheese) and vegetable sources (beans, soy, nuts, peas) | ||
| Patients with liver cirrhosis sarcopenic | 1.2-1.5 g/kg Bw/day | |
| Obese sarcopenic | Oral supplementation of BCAA 0.20-0.25 g/kg Bw/day or 30 g/d | |
| Glutamine and arginine supplementation | ||
| Carbohydrates/fat | Patients with COVID-19 without respiratory impairment | Ratio 70:30 carbohydrates/fat |
| Medium and low glycemic | ||
| Fiber 25-30 g/d | ||
| PUFAs: DHA, EPA, ALA | ||
| Patients with ventilator support | Ratio 50:50 carbohydrates/fat | |
| Vitamins | All individuals with COVID-19 | A, C, D, E, folate, B6 and B12 (monitoring in patients with liver abnormalities) |
| Minerals | All individuals with COVID-19 | Zinc, copper, selenium (monitoring in patients with liver abnormalities) |
| Critically ill patients | EN after 24-36 h. after ICU admission | |
| Initiate with trophic low-dose (10-20 mL/h.) | ||
| Polymeric formula: 15-20 kcals/kg Bw and 1.2-2.0 g/kg Bw/day of protein vitamins, minerals, fiber, probiotics | ||
| Provide 70%-80% needs in over 1 wk | ||
| Sever obese patients BMI > 50 | Energy 22-25 kcals/kg IBW | |
| Protein 2 g/kg per day (Class I, II) or 2.5 g/kg IBW/day of (Class III) | ||
| Vitamins, minerals, fiber, probiotics | ||
| Gastric intolerance individuals | Use prokinetics | |
| Post-pyloric feeding in persistence intolerance or at high risk of aspiration | ||
| Patients with no GI feasible | PN recommended | |
| Poor nutrition status | Limit the use of omega-6 soy-based ILE during first week | |
| Prolonged stay at ICU | Mixture of lipids such as olive oil based ILE or SMOF (soy, medium chain triglycerides, olive oil, fish oil) |
Proteins in the diet are known to be crucial nutrients for gut-associated lymphoid tissue, as are active immunoglobulins, which act against infection in the gut mucosa[128]. The consumption of high-value proteins that contain essential amino acids is associated with immune responses, for example, adequate production of antibodies; activation of T and B lymphocytes, macrophages, and NK cells; and the production of cytokines and other immune elements that prevent infectious diseases[129]. In addition, in order to prevent body weight loss and muscle mass, > 1 g/kg Bw/day of protein intake is recommended for patients with anorexia or reduced food intake. In general, 1 g/kg Bw/day is sufficient to meet the requirement, but protein intake should be adapted to individual needs according to disease status, physical activity, and tolerance. Overall, 70-100 g/d of protein are acceptable in the oral diet, preferably consumed from animal (milk, yogurt, meat, fish, chicken, and cheese) and vegetable sources (beans, soy, nuts, and peas)[126]. Oral nutrition supplements (ONS) are a fairly good option when the goals of oral feeding are not fully achieved. In the latter cases, ONS are suggested to supply 150-400 kcals and 15-30 g of protein and should be consumed for at least 1 mo[100,126].
In liver injury, nutritional approaches must be attended to according to the type and grade of the lesion(s). In patients with acute liver failure, nutrition therapy must be centered on providing sufficient energy in the form of glucose and fatty acids along with vitamins and mineral elements, preventing hypoglycemia and hypertriglyceridemia, and protein and amino acid sources adequate to avoid catabolism and to promote protein synthesis. Patients with liver cirrhosis are very likely to experience malnutrition and muscle wasting because of a decrease in protein synthesis and increased total protein breakdown. The recommendation is 1.2-1.5 g/kg Bw/day of protein to ameliorate protein synthesis in patients with sarcopenia, including those with obese sarcopenia, preferably with high-quality proteins from animal and ve
During liver injury, the metabolism of glucose is altered, the glucose oxidation rate is reduced, and the glucose production rate in the liver is low because of the depletion of glycogen stores despite increased gluconeogenesis. Glucose deposition in skeletal muscle and liver as glycogen is impaired. At the same time, glucose uptake in tissues is reduced because of insulin resistance in response to high secretion and reduced degradation[138,139]. Lipid metabolism reflects an augmented rate of lipid oxidation parallel to insulin resistance. Additionally, the plasma levels of essential and polyunsaturated fatty acids are reduced in relation to the nutritional status and to the severity of the injury[140]. In patients with COVID-19, the ratio of carbohydrates to fat should fall within the range of 70:30 for patients without respiratory impairment and within 50:50 in patients on ventilatory support[141]. Nonetheless, it is noteworthy to take into consideration the previously mentioned changes in glucose and lipid metabolism in patients with liver disorders. In oral diets, low glycemic index foods, such as ve
For patients outside the ICU, nutrition treatment should begin early, within 24-48 h of hospitalization, orally and mainly in older and polymorbid patients, whose nutritional situation may be compromised. The use of ONS could be fully applicable in patients with COVID-19 and liver injury in order to prevent or treat malnutrition and reestablish liver function. ONS are recommended to meet energy, macronutrient (carbohydrate, protein, and lipids) and micronutrient (vitamins and mineral elements) needs. Fiber and probiotics are suggested to promote optimal intestinal function. The increase of dietary fiber from whole grains benefits gut microbiome composition and is also correlated with reducing both systemic and gut inflammation by reducing IL-6, TNF-α, and hs-CRP, and by increasing short chain fatty acids (SCFAs)[74]. SCFA are produced by gut microbiota as a result of dietary fermentation. They are potential activators of anti-inflammatory signaling cascades and inhibitors of proinflammatory cytokines, as well as the reduced expression of NF-kB[72]. Furthermore, dietary fiber has been reported to promote healthy gut microbiota, which is related to inhibition of systemic inflammation and enhanced mucosal thickness, protecting the gut barrier from the infiltration of pathogens[77]. Dietary fiber is also thought to influence the gut microbiome and respiratory function, and it is noteworthy that, in some cases, the macrophage response to respiratory viruses is linked to the composition of the gut microbiome[148].
Some vitamins and mineral elements have attracted special attention because of the potential benefit that they may have during the COVID-19 crisis. In general, treatment with vitamins A, C, D, and E, folate, vitamin B6 and B12, and minerals including zinc, selenium, copper, iron, and calcium is recommended as ONS as well as by consum
Regarding vitamin D, recent investigations have pointed out its role in preventing infections, including COVID-19. Vitamin D has been described as possessing anti-inflammatory and immunomodulatory effects and may interfere with viral replication, probably by its effects on innate and adaptive responses by the modulation of defensins and cathelicidins and reduction of the Th1 helper cell response[152]. Fur
It appears that the role of vitamin A in preventing the effects of COVID-19 is not fully understood, but it is known that retinoic acid, the most active retinoid form of vitamin A, has an impact on the production of IL-1β and IL-1 receptor antagonists by means of alveolar macrophages and the consequent infiltration of neutrophils into the lungs during the course of ARDS. Retinoic acid contributes to surfactant production, which may be another way to protect against ARDS. Meanwhile, carotenes attenuate ROS production, diminishing the level of oxidative stress in the lungs. Under con
The most relevant activity of vitamin E is it antioxidant activity, which protects against free radicals like superoxides. In addition, the vitamin E effect is associated with the immune response of lymphocytes, NK cells, and neutrophils, which decline in the elderly[157]. Vitamin E supplementation in older adults was shown to enhance immune cell functions including neutrophil chemotaxis and phagocytosis, NK cell activity, and mitogen-induced lymphocyte proliferation[158].
With regard to the vitamin B group, vitamins B6, B9 (folate), and B12 are considered relevant to immune function in the context of the COVID-19 pandemic framework[159]. Low concentrations of B6 influence on lymphocyte maturation, antibody res
The trace minerals zinc, copper, and selenium are intricately involved in immune function. Zinc is highlighted because of its immunomodulatory effects on NK cell activity and macrophage and neutrophil function, complementary activity, and T cell-mediated function. Antioxidant activity has been attributed to trace minerals because they are cofactors of antioxidant metalloenzymes such as SOD. Zinc deficiency is implicated in the systemic activation of NF-κΒ[162]. Zinc might reduce viral repli
Selenium is considered another important trace element with antioxidant and anti-inflammatory effects. Selenium, together with vitamin D deficiency, probably impairs immune responses to COVID-19 and increases the severity of the disease[167]. In contrast, other clinical studies reveal that the recovery rate in surviving patients was related to increased selenium levels in patients with COVID-19[168,169]. Selenium is intricately involved in a wide range of immune activities, such as T cell activation, maturation, differentiation, and proliferation. It exerts antiviral activity by regulating the TCD4+ response, modulating TCD8+ and NK cells with cytotoxic activity, and it has a crucial role in the production of antibodies[170]. It is thought that a selenium deficiency has an influence on the high incidence of thromboembolism in patients critically ill with COVID-19 in the ICU[171]. A possible explanation is that low selenium levels are correlated with an abnormal thromboxane A2-to-prostacyclin I2 ratio, which is implicated in vasoconstriction and coagulation[172]. However, further studies are needed for clarification[170].
Copper protects DNA integrity by reducing oxidative damage, and its deficiency alters the immune response and promotes infectious diseases. Copper appears to participate in immune cell differentiation and to inhibit viral replication. It exerts an influence on the optimal functioning of NK cells, lymphocytes, neutrophils, ma
The hyperinflammatory response is one of the main characteristics of patients critically ill with COVID-19. Accordingly, the GI tract could play a central role in modulation of the inflammatory response, as it is the largest immune organ in the body, with the greatest microbiome. Nutritional therapy through EN strengthens gut barrier defenses and the microbial burden by providing luminal nutrients that impair dysbiosis and stimulate the anti-inflammatory response[176]. EN nutrition should start early, within 24-36 h after admission to the ICU or within 12 h of mechanical ventilation. To avoid refeeding syndrome, EN should be initiated with low trophic doses (10-20 mL/h) of a standard isosmotic polymeric formula that supplies 15-20 kcal/kg Bw and 1.2-2.0 g/kg Bw/day of protein, considering the actual body weight. The goal is to provide 70-80% of energy needs during 1 wk. In obese individuals with a BMI of 30-50, EN provides 11-14 kcals/kg Bw/day; for patients with severe obesity with a BMI >50 energy 22-25 kcal/kg Bw and 2 g/kg/d (classes I and II) or 2.5 g/kg/d (class III), taking into account the ideal body weight (IBW)[102]. Various complications may occur in patients with EN, such as GI disorders (e.g., diarrhea, vomiting, and nausea) and mechanical disorders related to intubation and metabolic disorders (e.g., hyper
For patients in whom GI feeding is not feasible, PN should be considered. Patients with a poor nutrition status, significant intolerance to EN, or those with a prolonged length-of-stay in the ICU, are potential candidates for PN. To avoid proinflammatory infusion, it is suggested to limit the use of omega-6 soy-based intravenous lipid emulsion (ILE) in week 1 or to change to a mixture of lipids, such as olive oil-based ILE or to soy, medium chain triglycerides, olive oil, and fish oil (SMOF)[102]. PN complications include catheter-related bloodstream infections and PN liver-associated damage[99]. At present, there are no reported cases associated with these complications. Nevertheless, the background must be taken into account for patients with sustained PN[126].
Certain other aspects must be taken into consideration when determining nu
The outbreak of COVID-19 has revolutionized medical science, and has been accom
From structural studies of SARS-CoV-2, it has been possible to determine the major drug targets, which include 3-chymotrypsin-like protease (3CLpro), papain-like pro
Previous studies of SARS-CoV and Middle East Respiratory Syndrome provided relevant information for understanding the behavior of SARS-CoV-2 and the effectiveness of natural compounds that inhibit viral activity. Among the compounds that appear to possess effective antiviral activity against coronaviruses silvestrol, tryp
A few computer modeling studies have been performed to screen the targeted biding sites of phytochemicals with SARS-CoV-2. Recently, Kurman et al[76] used molecular docking or molecular dynamics (MD) software to predict phytochemical interactions with Nps15, a nidoviral RNA uridylate-specific endoribonuclease (NendoU) protein commonly present in coronaviruses, and responsible for interfering with the innate immune response. It was found that 50 phytochemicals had the potential to bond with Nsp15 and obstruct viral replication. The phytochemicals with the effective bonding potential were sarsasapogenin, ursonic acid, curcumin, aj
In addition, phytochemicals can also induce protection against SARS-CoV-2 by activating cytoprotective systems. A variety of these compounds have been shown to trigger signaling pathways related to endogenous defenses such as Nrf2/Keap1/ARE. Nuclear factor erythroid 2-related factor 1 (Nrf2) is a nuclear transcription factor coded by the NFE2L2 gene. It is known as the master regulator of cytoprotection and antioxidant responses[188]. Nrf2 impacts approximately 250 genes involved in an
To our knowledge, silymarin (SM) could be one of the most remarkable phyto
Moreover, recent investigations have identified SM antiviral activity against he
Curcumin is another phytochemical with potential for treating SARS-CoV-2 and liver damage. Curcumin is the main polyphenol in turmeric, present the rhizome of Curcuma longa and other species. In traditional medicine, it is used as an anti-inflammatory, antiseptic, analgesic, antimalarial, and as an insect repellent[205]. Current evidence supports the ability of curcumin to attenuate oxidative stress produced by ROS and RNS. Curcumin induces the antioxidant enzymes GST and γ-Glutamyl cysteine ligase (γ-GCL) and, at the same time, inhibits ROS-producing enzymes (oxidases, lipooxygenase, xanthine hydrogenase, and cyclooxygenase) and removes peroxide radicals, hydrogen peroxide, hydroxyl radicals, oxygen, and nitric oxide. The activities are associated with regulation of the Nrf2/Keap1/ARE pathway in addition to the modulation of other transcription factors, such as NF-kB and AP1[206]. In rat hepatic stellate-T6 (HSC-T6) cells, curcumin reduced the levels of malonaldehyde (MDA), and ROS treated with glucose oxidase, upregulated the nuclear translocation of Nrf2, augmenting glutathione levels, and reduced the expression of smooth muscle α-actin and extracellular matrix molecules[207]. In a rat model of arthritis, curcumin reduced inflammation by increasing methionine sulfoxide reductase A and the antioxidant enzymes CAT, SOD, and GPx[208]. In macrophages and RAW264.7 cells, curcumin protected cells from stress by inducing H2O2, triggering the Nrf2/Keap1/ ARE pathway and the expression of antioxidant enzymes, thus promoting cell survival[209]. Additional evidence that supports the effect of curcumin on Nrf2 activation, curcumin is also described as an antiviral. Curcumin interacts with viral proteins on the cell surface, impairing entry of the virus. It also interacts with RNA polymerase and other proteins to hamper replication and may also interact with protease Mpro[210,211]. In the previously mentioned MD study, curcumin was an effective inhibitor of the SARS-CoV-2 Nps15 protein. The hyperinflammatory response initiated in the “cytokine storm” is also weakened by curcumin, which interferes in the NF-κΒ and MAPK inflammatory pathways to reduce the expression of the proinflammatory cytokines IFN-γ, monocyte chemoattractant protein 1 (MCP)-1, IL-6, IL-10, and TNF-α[212]. Curcumin appears to prevent apoptosis and lung fibrosis by inhibiting the TGF-β pathway[213]. The envelope protein of SARS-CoV-2 is the smallest of the four structural proteins of the virus and it is known to participate in the viral entry process. We have performed some in-silico studies to investigate that protein.
The three-dimensional model of the SARS-CoV-2 envelope protein of was built using Modeller 9.23 Software and considering the crystal structure of the transmembrane region of the envelope protein of SARS-CoV (PDB: 5X29). The resulting model re
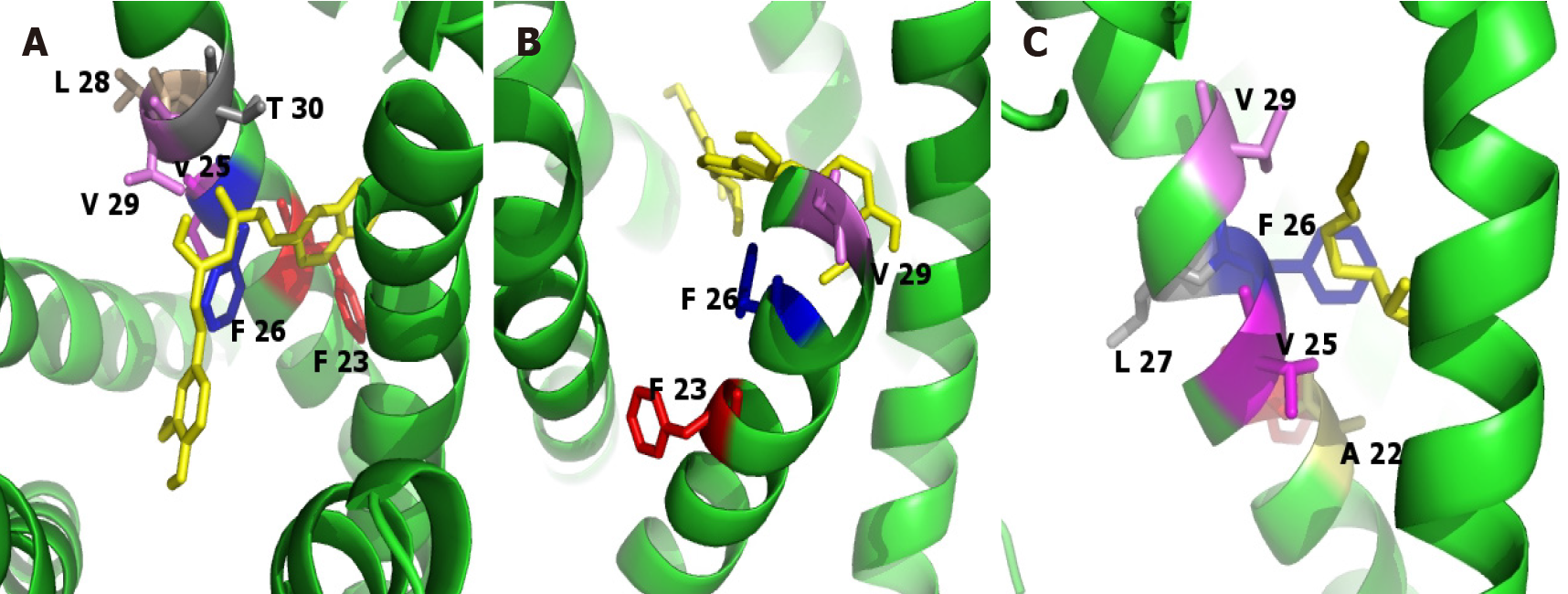
Docking studies were used to predict the binding affinity of the compounds of interest and the SARS-CoV-2 envelope protein. Results of the analysis of silibylin, curcumina, and sulfuraphane were performed with Autodock 4.2 (Table 3 and Figure 5). The input files were prepared with the AutoDock Tools 1.5.2 graphic interface[214]. A grid box of 120 Å × 120 Å × 120 Å and a grid spacing of 0.375 Å was used for the docking studies. The Lamarckian genetic algorithm was selected for the sampling score, considering a randomized initial population of 100 and a maximum number of energy evaluations of 107.
| Ligand | Binding energy (kcal/mol) | kI µmol/L | Residue interactions |
| Curcumine | -4.6 | 428.13 | Phe 23, Val 25, Leu 28, Phe 26, Thr 30, Val 26 |
| Silybin | -5.73 | 62.57 | Phe 23, Phe 26, Val 29 |
| Sulforaphane | -3.75 | 1.79 | Val 29, Phe 26, Val 25, Ala 22, Phe 23, Leu 27 |
The current pandemic situation caused by COVID-19 infection has generated global changes in not only human health, but in all economic, political, and social levels. The lack of an effective vaccine that prevents COVID-19 infections and the absence of an effective treatment that reduces multiorgan consequences, only allows us to establish guidelines and recommendations based on direct experience with patients and ob
Nancy Vargas-Mendoza and Marcelo Angeles-Valencia are scholarship holders for their postgraduate studies by the Consejo Nacional de Ciencia y Tecnología (CONACyT) and the BEIFI program, Instituto Politécnico Nacional, Mexico.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tumminello G S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Mittal A, Manjunath K, Ranjan RK, Kaushik S, Kumar S, Verma V. COVID-19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 2020;16:e1008762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 2. | Palacios Cruz M, Santos E, Velázquez Cervantes MA, León Juárez M. COVID-19, a worldwide public health emergency. Rev Clin Esp. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Yoshimoto FK. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. Protein J. 2020;39:198-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 360] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 4. | Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1889] [Cited by in RCA: 2096] [Article Influence: 209.6] [Reference Citation Analysis (0)] |
| 5. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14131] [Article Influence: 2826.2] [Reference Citation Analysis (1)] |
| 6. | WHO. Coronavirus disease (COVID-19) pandemic. 2020. [cited 31 December 2020]. Available from: https://www.who.int/es/emergencies/diseases/novel-coronavirus-2019. |
| 7. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 8. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 9. | Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 949] [Article Influence: 189.8] [Reference Citation Analysis (1)] |
| 10. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 11. | Philips CA, Ahamed R, Augustine P. SARS-CoV-2 related liver impairment - perception may not be the reality. J Hepatol. 2020;73:991-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Pedro KD, Henderson AJ, Agosto LM. Mechanisms of HIV-1 cell-to-cell transmission and the establishment of the latent reservoir. Virus Res. 2019;265:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Esumi M, Ishibashi M, Yamaguchi H, Nakajima S, Tai Y, Kikuta S, Sugitani M, Takayama T, Tahara M, Takeda M, Wakita T. Transmembrane serine protease TMPRSS2 activates hepatitis C virus infection. Hepatology. 2015;61:437-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 15. | Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 16. | Polakos NK, Cornejo JC, Murray DA, Wright KO, Treanor JJ, Crispe IN, Topham DJ, Pierce RH. Kupffer cell-dependent hepatitis occurs during influenza infection. Am J Pathol. 2006;168:1169-1178; quiz 1404-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Morton A. Presumed acute fatty liver of pregnancy following influenza A hepatitis. Obstet Med. 2017;10:186-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Pardi DS, Romero Y, Mertz LE, Douglas DD. Hepatitis-associated aplastic anemia and acute parvovirus B19 infection: a report of two cases and a review of the literature. Am J Gastroenterol. 1998;93:468-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Eisenhut M, Thorburn K, Ahmed T. Transaminase levels in ventilated children with respiratory syncytial virus bronchiolitis. Intensive Care Med. 2004;30:931-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 305] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 21. | Cheung CCL, Goh D, Lim X, Tien TZ, Lim JCT, Nerurkar SN, Shihleone L, Cheow PC, Chan CY, Koh YX, Tan TT, Kalimuddin S, David Tai WM, Ng JL, Hong Low JG, Yeong J, Hon Lim TK. Residual SARS-CoV-2 viral antigens detected in gastrointestinal and hepatic tissues from two recovered COVID-19 patients. 2020 Preprint. Available from: medRxiv:2020.10.28.20219014. [DOI] [Full Text] |
| 22. | Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19:181-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1056] [Cited by in RCA: 1165] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 23. | Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1858] [Cited by in RCA: 1960] [Article Influence: 392.0] [Reference Citation Analysis (0)] |
| 24. | Bouayad A. Innate immune evasion by SARS-CoV-2: Comparison with SARS-CoV. Rev Med Virol. 2020;30:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 853] [Article Influence: 170.6] [Reference Citation Analysis (0)] |
| 26. | Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 27. | McGonagle D, Sharif K, O'Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19:102537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1194] [Cited by in RCA: 1186] [Article Influence: 237.2] [Reference Citation Analysis (0)] |
| 28. | Li Y, Xiao SY. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92:1491-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 29. | de Lucena TMC, da Silva Santos AF, de Lima BR, de Albuquerque Borborema ME, de Azevêdo Silva J. Mechanism of inflammatory response in associated comorbidities in COVID-19. Diabetes Metab Syndr. 2020;14:597-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 30. | Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 581] [Article Influence: 116.2] [Reference Citation Analysis (0)] |
| 31. | Edeas M, Saleh J, Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020;97:303-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 32. | Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, Tang C, Sang L, Liu J, Ni Z, Hu Y, Liu L, Shan H, Lei C, Peng Y, Wei L, Liu Y, Peng P, Wang J, Chen Z, Li G, Zheng Z, Qiu S, Luo J, Ye C, Zhu S, Liu X, Cheng L, Ye F, Zheng J, Zhang N, Li Y, He J, Li S, Zhong N; Medical Treatment Expert Group for COVID-19. Risk Factors of Fatal Outcome in Hospitalized Subjects With Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest. 2020;158:97-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 410] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 33. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18877] [Article Influence: 3775.4] [Reference Citation Analysis (7)] |
| 34. | Zádori N, Váncsa S, Farkas N, Hegyi P, Erőss B; KETLAK Study Group. The negative impact of comorbidities on the disease course of COVID-19. Intensive Care Med. 2020;46:1784-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Váncsa S, Hegyi PJ, Zádori N, Szakó L, Vörhendi N, Ocskay K, Földi M, Dembrovszky F, Dömötör ZR, Jánosi K, Rakonczay Z Jr, Hartmann P, Horváth T, Erőss B, Kiss S, Szakács Z, Németh D, Hegyi P, Pár G. Pre-existing Liver Diseases and On-Admission Liver-Related Laboratory Tests in COVID-19: A Prognostic Accuracy Meta-Analysis With Systematic Review. Front Med (Lausanne). 2020;7:572115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Lin Y, Yuan J, Long Q, Hu J, Deng H, Zhao Z, Chen J, Lu M, Huang A. Patients with SARS-CoV-2 and HBV co-infection are at risk of greater liver injury. Genes Dis. 2021;8:484-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 38. | Ferron PJ, Gicquel T, Mégarbane B, Clément B, Fromenty B. Treatments in Covid-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury? Biochimie. 2020;179:266-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 39. | Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 40. | Kavoliuniene A, Vaitiekiene A, Cesnaite G. Congestive hepatopathy and hypoxic hepatitis in heart failure: a cardiologist's point of view. Int J Cardiol. 2013;166:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Salehi B, Calina D, Docea AO, Koirala N, Aryal S, Lombardo D, Pasqua L, Taheri Y, Marina Salgado Castillo C, Martorell M, Martins N, Iriti M, Suleria HAR, Sharifi-Rad J. Curcumin's Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 42. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30122] [Article Influence: 6024.4] [Reference Citation Analysis (3)] |
| 43. | Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 44. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (2)] |
| 45. | Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46:2050-2068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 233] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 46. | Ilias I, Zabuliene L. Hyperglycemia and the novel Covid-19 infection: Possible pathophysiologic mechanisms. Med Hypotheses. 2020;139:109699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 781] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 48. | Lopez C, Kim J, Pandey A, Huang T, DeLoughery TG. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br J Haematol. 2020;190:31-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 49. | Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, Merabet F, Mekinian A, Braun T, Damaj G, Delmer A, Cymbalista F. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190:29-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 50. | Jawed M, Hart E, Saeed M. Haemolytic anaemia: a consequence of COVID-19. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 51. | Rothschild MA, Oratz M, Schreiber SS. Serum albumin. Hepatology. 1988;8:385-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 317] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | Violi F, Ceccarelli G, Cangemi R, Alessandri F, D'Ettorre G, Oliva A, Pastori D, Loffredo L, Pignatelli P, Ruberto F, Venditti M, Pugliese F, Mastroianni CM. Hypoalbuminemia, Coagulopathy, and Vascular Disease in COVID-19. Circ Res. 2020;127:400-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 53. | Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, Lin S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92:2152-2158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 54. | U.S. NIH National Library of Medicine. COVID-19 Information. 2020. [cited 31 December 2020]. Available from: https://www.clinicaltrials.gov/. |
| 55. | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2337] [Cited by in RCA: 2488] [Article Influence: 497.6] [Reference Citation Analysis (0)] |
| 56. | Favaloro EJ, Thachil J. Reporting of D-dimer data in COVID-19: some confusion and potential for misinformation. Clin Chem Lab Med. 2020;58:1191-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 57. | Lee S, Channappanavar R, Kanneganti TD. Coronaviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Trends Immunol. 2020;41:1083-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 58. | Liu BM, Hill HR. Role of Host Immune and Inflammatory Responses in COVID-19 Cases with Underlying Primary Immunodeficiency: A Review. J Interferon Cytokine Res. 2020;40:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 59. | Russell MW, Moldoveanu Z, Ogra PL, Mestecky J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front Immunol. 2020;11:611337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (1)] |
| 60. | Isho B, Abe KT, Zuo M, Jamal AJ, Rathod B, Wang JH, Li Z, Chao G, Rojas OL, Bang YM, Pu A, Christie-Holmes N, Gervais C, Ceccarelli D, Samavarchi-Tehrani P, Guvenc F, Budylowski P, Li A, Paterson A, Yue FY, Marin LM, Caldwell L, Wrana JL, Colwill K, Sicheri F, Mubareka S, Gray-Owen SD, Drews SJ, Siqueira WL, Barrios-Rodiles M, Ostrowski M, Rini JM, Durocher Y, McGeer AJ, Gommerman JL, Gingras AC. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Cervia C, Nilsson J, Zurbuchen Y, Valaperti A, Schreiner J, Wolfensberger A, Raeber ME, Adamo S, Weigang S, Emmenegger M, Hasler S, Bosshard PP, De Cecco E, Bächli E, Rudiger A, Stüssi-Helbling M, Huber LC, Zinkernagel AS, Schaer DJ, Aguzzi A, Kochs G, Held U, Probst-Müller E, Rampini SK, Boyman O. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild vs severe COVID-19. J Allergy Clin Immunol. 2021;147:545-557.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 279] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 62. | Chakraborty J, Banerjee I, Vaishya R, Ghosh S. Bioengineered in Vitro Tissue Models to Study SARS-CoV-2 Pathogenesis and Therapeutic Validation. ACS Biomater Sci Eng. 2020;6:6540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | Karlsson AC, Humbert M, Buggert M. The known unknowns of T cell immunity to COVID-19. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 64. | Ren L, Zhang L, Chang, Wang J, Hu Y, Chen H, Guo L, Wu C, Wang C, Wang Y, Wang G, Yang S, Dela Cruz CS, Sharma L, Wang L, Zhang D. The kinetics of humoral response and its relationship with the disease severity in COVID-19. Commun Biol. 2020;3:780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 65. | Hashem AM, Algaissi A, Almahboub SA, Alfaleh MA, Abujamel TS, Alamri SS, Alluhaybi KA, Hobani HI, AlHarbi RH, Alsulaiman RM, ElAssouli MZ, Hala S, Alharbi NK, Alhabbab RY, AlSaieedi AA, Abdulaal WH, Bukhari A, Al-Somali AA, Alofi FS, Khogeer AA, Pain A, Alkayyal AA, Almontashiri NAM, Ahmad BM, Li X. Early Humoral Response Correlates with Disease Severity and Outcomes in COVID-19 Patients. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Lenti MV, Aronico N, Pellegrino I, Boveri E, Giuffrida P, Borrelli de Andreis F, Morbini P, Vanelli L, Pasini A, Ubezio C, Melazzini F, Rascaroli A, Antoci V, Merli S, Di Terlizzi F, Sabatini U, Cambiè G, Tenore A, Picone C, Vanoli A, Arcaini L, Baldanti F, Paulli M, Corazza GR, Di Sabatino A. Depletion of circulating IgM memory B cells predicts unfavourable outcome in COVID-19. Sci Rep. 2020;10:20836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Borges L, Pithon-Curi TC, Curi R, Hatanaka E. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Mediators Inflamm. 2020;2020:8829674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 68. | Tang L, Yin Z, Hu Y, Mei H. Controlling Cytokine Storm Is Vital in COVID-19. Front Immunol. 2020;11:570993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 69. | Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 873] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 70. | Desai N, Neyaz A, Szabolcs A, Shih AR, Chen JH, Thapar V, Nieman LT, Solovyov A, Mehta A, Lieb DJ, Kulkarni AS, Jaicks C, Xu KH, Raabe MJ, Pinto CJ, Juric D, Chebib I, Colvin RB, Kim AY, Monroe R, Warren SE, Danaher P, Reeves JW, Gong J, Rueckert EH, Greenbaum BD, Hacohen N, Lagana SM, Rivera MN, Sholl LM, Stone JR, Ting DT, Deshpande V. Temporal and spatial heterogeneity of host response to SARS-CoV-2 pulmonary infection. Nat Commun. 2020;11:6319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 71. | Blot M, Bour JB, Quenot JP, Bourredjem A, Nguyen M, Guy J, Monier S, Georges M, Large A, Dargent A, Guilhem A, Mouries-Martin S, Barben J, Bouhemad B, Charles PE, Chavanet P, Binquet C, Piroth L; LYMPHONIE study group. The dysregulated innate immune response in severe COVID-19 pneumonia that could drive poorer outcome. J Transl Med. 2020;18:457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 72. | Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1616] [Article Influence: 146.9] [Reference Citation Analysis (0)] |
| 73. | Rodrigues TS, Sa KS, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Goncalves AV, Perucello DB, Andrade WA, Castro R, Veras FP, Toller-Kawahisa JE, Nascimento DC, de Lima MH, Silva CM, Caetite DB, Martins RB, Castro IA, Pontelli MC, de Barros FC, do Amaral NB, Giannini MC, Bonjorno LP, Lopes MIF, Benatti MN, Santana RC, Vilar FC, Auxiliadora-Martins M, Luppino-Assad R, de Almeida SC, de Oliveira FR, Batah SS, Siyuan L, Cunha TM, Alves-Filho JC, Cunha FQ, Cunha LD, Frantz FG, Kohlsdorf T, Fabro AT, Arruda E, de Oliveira RD, Louzada-Junior P, Zamboni DS. Inflammasome activation in COVID-19 patients. 2020 Preprint. [DOI] [Full Text] |
| 74. | Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2013;98:872-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 75. | Zhang Y, Chen Y, Meng Z. Immunomodulation for Severe COVID-19 Pneumonia: The State of the Art. Front Immunol. 2020;11:577442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 76. | Kumar S, Kashyap P, Chowdhury S, Kumar S, Panwar A, Kumar A. Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine. 2021;85:153317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 77. | Myhrstad MCW, Tunsjø H, Charnock C, Telle-Hansen VH. Dietary Fiber, Gut Microbiota, and Metabolic Regulation-Current Status in Human Randomized Trials. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 78. | Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, Scherer C, Rudelius M, Zoller M, Höchter D, Keppler O, Teupser D, Zwißler B, von Bergwelt-Baildon M, Kääb S, Massberg S, Pekayvaz K, Stark K. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation. 2020;142:1176-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 79. | Sun S, Cai X, Wang H, He G, Lin Y, Lu B, Chen C, Pan Y, Hu X. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. 2020;507:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 80. | Gutiérrez-Bautista JF, Rodriguez-Nicolas A, Rosales-Castillo A, Jiménez P, Garrido F, Anderson P, Ruiz-Cabello F, López-Ruz MÁ. Negative Clinical Evolution in COVID-19 Patients Is Frequently Accompanied With an Increased Proportion of Undifferentiated Th Cells and a Strong Underrepresentation of the Th1 Subset. Front Immunol. 2020;11:596553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 81. | Liao WH, Yang GG, Henneberg M. The renin-angiotensin-aldosterone system inhibitors in COVID-19: from acidosis to ventilation and immunity. Swiss Med Wkly. 2020;150:w20444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Chen Y, Luo G, Yuan J, Wang Y, Yang X, Wang X, Li G, Liu Z, Zhong N. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014;2014:426740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 83. | Shi Y, Zhou G, Li Q. Asynchronous actions of immune responses in COVID-19 patients. Signal Transduct Target Ther. 2020;5:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Kalpakci Y, Hacibekiroglu T, Trak G, Karacaer C, Demirci T, Kocayigit H, Sunu C, Varim C, Falay M. Comparative evaluation of memory T cells in COVID-19 patients and the predictive role of CD4+CD8+ double positive T lymphocytes as a new marker. Rev Assoc Med Bras (1992). 2020;66:1666-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Shomuradova AS, Vagida MS, Sheetikov SA, Zornikova KV, Kiryukhin D, Titov A, Peshkova IO, Khmelevskaya A, Dianov DV, Malasheva M, Shmelev A, Serdyuk Y, Bagaev DV, Pivnyuk A, Shcherbinin DS, Maleeva AV, Shakirova NT, Pilunov A, Malko DB, Khamaganova EG, Biderman B, Ivanov A, Shugay M, Efimov GA. SARS-CoV-2 Epitopes Are Recognized by a Public and Diverse Repertoire of Human T Cell Receptors. Immunity. 2020;53:1245-1257.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 86. | Saeed F, Nadeem M, Ahmed RS, Tahir Nadeem M, Arshad MS, Ullah A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds – a review. Food Agric Immunol. 2016;27:205. [RCA] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 87. | Bacher P, Rosati E, Esser D, Martini GR, Saggau C, Schiminsky E, Dargvainiene J, Schröder I, Wieters I, Khodamoradi Y, Eberhardt F, Vehreschild MJGT, Neb H, Sonntagbauer M, Conrad C, Tran F, Rosenstiel P, Markewitz R, Wandinger KP, Augustin M, Rybniker J, Kochanek M, Leypoldt F, Cornely OA, Koehler P, Franke A, Scheffold A. Low-Avidity CD4+ T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity. 2020;53:1258-1271.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 243] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 88. | Singh Y, Gupta G, Kazmi I, Al-Abbasi FA, Negi P, Chellappan DK, Dua K. SARS CoV-2 aggravates cellular metabolism mediated complications in COVID-19 infection. Dermatol Ther. 2020;33:e13871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Bulló M, Casas R, Portillo MP, Basora J, Estruch R, García-Arellano A, Lasa A, Juanola-Falgarona M, Arós F, Salas-Salvadó J. Dietary glycemic index/load and peripheral adipokines and inflammatory markers in elderly subjects at high cardiovascular risk. Nutr Metab Cardiovasc Dis. 2013;23:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 90. | Ntyonga-Pono MP. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan Afr Med J. 2020;35:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 91. | Suhail S, Zajac J, Fossum C, Lowater H, McCracken C, Severson N, Laatsch B, Narkiewicz-Jodko A, Johnson B, Liebau J, Bhattacharyya S, Hati S. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020;39:644-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 92. | Muhoberac BB. What Can Cellular Redox, Iron, and Reactive Oxygen Species Suggest About the Mechanisms and Potential Therapy of COVID-19? Front Cell Infect Microbiol. 2020;10:569709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 93. | Dutta S, Sengupta P. SARS-CoV-2 infection, oxidative stress and male reproductive hormones: can testicular-adrenal crosstalk be ruled-out? J Basic Clin Physiol Pharmacol. 2020;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Bakadia BM, Boni BOO, Ahmed AAQ, Yang G. The impact of oxidative stress damage induced by the environmental stressors on COVID-19. Life Sci. 2021;264:118653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 95. | Zinovkin RA, Grebenchikov OA. Transcription Factor Nrf2 as a Potential Therapeutic Target for Prevention of Cytokine Storm in COVID-19 Patients. Biochemistry (Mosc). 2020;85:833-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 96. | Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 97. | Delgado-Roche L, Mesta F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch Med Res. 2020;51:384-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 407] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 98. | Wu J. Tackle the free radicals damage in COVID-19. Nitric Oxide. 2020;102:39-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 99. | Xu ZW, Li YS. Pathogenesis and treatment of parenteral nutrition-associated liver disease. Hepatobiliary Pancreat Dis Int. 2012;11:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 100. | Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, Pirlich M, Singer P; endorsed by the ESPEN Council. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 510] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 101. | Federico A, Dallio M, Loguercio C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 290] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 102. | Patel JJ, Martindale RG, McClave SA. Relevant Nutrition Therapy in COVID-19 and the Constraints on Its Delivery by a Unique Disease Process. Nutr Clin Pract. 2020;35:792-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 103. | Violi F, Oliva A, Cangemi R, Ceccarelli G, Pignatelli P, Carnevale R, Cammisotto V, Lichtner M, Alessandri F, De Angelis M, Miele MC, D'Ettorre G, Ruberto F, Venditti M, Pugliese F, Mastroianni CM. Nox2 activation in Covid-19. Redox Biol. 2020;36:101655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 104. | Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1419] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 105. | Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MA, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 1481] [Article Influence: 164.6] [Reference Citation Analysis (0)] |
| 106. | McClave SA, DiBaise JK, Mullin GE, Martindale RG. ACG Clinical Guideline: Nutrition Therapy in the Adult Hospitalized Patient. Am J Gastroenterol. 2016;111:315-334; quiz 335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 107. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1133] [Article Influence: 226.6] [Reference Citation Analysis (1)] |
| 108. | Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15:R268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 498] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 109. | Kalaiselvan MS, Renuka MK, Arunkumar AS. Use of Nutrition Risk in Critically ill (NUTRIC) Score to Assess Nutritional Risk in Mechanically Ventilated Patients: A Prospective Observational Study. Indian J Crit Care Med. 2017;21:253-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 110. | Rosa M, Heyland DK, Fernandes D, Rabito EI, Oliveira ML, Marcadenti A. Translation and adaptation of the NUTRIC Score to identify critically ill patients who benefit the most from nutrition therapy. Clin Nutr ESPEN. 2016;14:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 111. | Compher C, Chittams J, Sammarco T, Nicolo M, Heyland DK. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit Care Med. 2017;45:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 112. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10902] [Cited by in RCA: 11139] [Article Influence: 278.5] [Reference Citation Analysis (0)] |
| 113. | Capuzzo M, Valpondi V, Sgarbi A, Bortolazzi S, Pavoni V, Gilli G, Candini G, Gritti G, Alvisi R. Validation of severity scoring systems SAPS II and APACHE II in a single-center population. Intensive Care Med. 2000;26:1779-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 114. | Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6591] [Cited by in RCA: 7774] [Article Influence: 268.1] [Reference Citation Analysis (1)] |
| 115. | Cárdenas-Turanzas M, Ensor J, Wakefield C, Zhang K, Wallace SK, Price KJ, Nates JL. Cross-validation of a Sequential Organ Failure Assessment score-based model to predict mortality in patients with cancer admitted to the intensive care unit. J Crit Care. 2012;27:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 116. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18201] [Article Influence: 3640.2] [Reference Citation Analysis (0)] |
| 117. | Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats AJS, Crivelli AN, Evans DC, Gramlich L, Fuchs-Tarlovsky V, Keller H, Llido L, Malone A, Mogensen KM, Morley JE, Muscaritoli M, Nyulasi I, Pirlich M, Pisprasert V, de van der Schueren MAE, Siltharm S, Singer P, Tappenden K, Velasco N, Waitzberg D, Yamwong P, Yu J, Van Gossum A, Compher C; GLIM Core Leadership Committee, GLIM Working Group. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10:207-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 632] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 118. | Aguila EJT, Cua IHY, Dumagpi JEL, Francisco CPD, Raymundo NTV, Sy-Janairo MLL, Cabral-Prodigalidad PAI, Lontok MAD. COVID-19 and its effects on the digestive system and endoscopy practice. JGH Open. 2020;4:324-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 119. | Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 405] [Article Influence: 67.5] [Reference Citation Analysis (3)] |
| 120. | Arora S, Mattina C, McAnenny C, O'Sullivan N, McGeeney L, Calder N, Gatiss G, Davidson B, Morgan MY. The development and validation of a nutritional prioritising tool for use in patients with chronic liver disease. J Hepatol. 2012;56:S241. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 121. | Schneeweiss B, Pammer J, Ratheiser K, Schneider B, Madl C, Kramer L, Kranz A, Ferenci P, Druml W, Grimm G. Energy metabolism in acute hepatic failure. Gastroenterology. 1993;105:1515-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 122. | John WJ, Phillips R, Ott L, Adams LJ, McClain CJ. Resting energy expenditure in patients with alcoholic hepatitis. JPEN J Parenter Enteral Nutr. 1989;13:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 123. | Levine JA, Harris MM, Morgan MY. Energy expenditure in chronic alcohol abuse. Eur J Clin Invest. 2000;30:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Müller MJ, Böttcher J, Selberg O, Weselmann S, Böker KH, Schwarze M, von zur Mühlen A, Manns MP. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. 1999;69:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 155] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 125. | Glass C, Hipskind P, Cole D, Lopez R, Dasarathy S. Handheld calorimeter is a valid instrument to quantify resting energy expenditure in hospitalized cirrhotic patients: a prospective study. Nutr Clin Pract. 2012;27:677-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 126. | Aguila EJT, Cua IHY, Fontanilla JAC, Yabut VLM, Causing MFP. Gastrointestinal Manifestations of COVID-19: Impact on Nutrition Practices. Nutr Clin Pract. 2020;35:800-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 127. | Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, Calleja JL, Bañares R, García-Pagán JC, Mesonero F, Bosch J; Ciberehd SportDiet Collaborative Group. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65:1293-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 128. | Amaral JF, Foschetti DA, Assis FA, Menezes JS, Vaz NM, Faria AM. Immunoglobulin production is impaired in protein-deprived mice and can be restored by dietary protein supplementation. Braz J Med Biol Res. 2006;39:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 129. | Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98:237-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 985] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 130. | Swart GR, Zillikens MC, van Vuure JK, van den Berg JW. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. BMJ. 1989;299:1202-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 131. | Zillikens MC, van den Berg JW, Wattimena JL, Rietveld T, Swart GR. Nocturnal oral glucose supplementation. The effects on protein metabolism in cirrhotic patients and in healthy controls. J Hepatol. 1993;17:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 132. | Les I, Doval E, García-Martínez R, Planas M, Cárdenas G, Gómez P, Flavià M, Jacas C, Mínguez B, Vergara M, Soriano G, Vila C, Esteban R, Córdoba J. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011;106:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 133. | Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 134. | Ren M, Zhang SH, Zeng XF, Liu H, Qiao SY. Branched-chain Amino Acids are Beneficial to Maintain Growth Performance and Intestinal Immune-related Function in Weaned Piglets Fed Protein Restricted Diet. Asian-Australas J Anim Sci. 2015;28:1742-1750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 135. | Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 136. | Shah AM, Wang Z, Ma J. Glutamine Metabolism and Its Role in Immunity, a Comprehensive Review. Animals (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 137. | Kim SH, Roszik J, Grimm EA, Ekmekcioglu S. Impact of l-Arginine Metabolism on Immune Response and Anticancer Immunotherapy. Front Oncol. 2018;8:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 138. | Selberg O, Burchert W, vd Hoff J, Meyer GJ, Hundeshagen H, Radoch E, Balks HJ, Müller MJ. Insulin resistance in liver cirrhosis. Positron-emission tomography scan analysis of skeletal muscle glucose metabolism. J Clin Invest. 1993;91:1897-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 139. | Müller MJ, Willmann O, Rieger A, Fenk A, Selberg O, Lautz HU, Bürger M, Balks HJ, von zur Mühlen A, Schmidt FW. Mechanism of insulin resistance associated with liver cirrhosis. Gastroenterology. 1992;102:2033-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 140. | Croci I, Byrne NM, Choquette S, Hills AP, Chachay VS, Clouston AD, O'Moore-Sullivan TM, Macdonald GA, Prins JB, Hickman IJ. Whole-body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut. 2013;62:1625-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 141. | Gomes F, Schuetz P, Bounoure L, Austin P, Ballesteros-Pomar M, Cederholm T, Fletcher J, Laviano A, Norman K, Poulia KA, Ravasco P, Schneider SM, Stanga Z, Weekes CE, Bischoff SC. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37:336-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 142. | Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang CY, Noar K, Song X, Lampe JW. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 143. | Radzikowska U, Rinaldi AO, Çelebi Sözener Z, Karaguzel D, Wojcik M, Cypryk K, Akdis M, Akdis CA, Sokolowska M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 144. | Innis SM. Dietary lipids in early development: relevance to obesity, immune and inflammatory disorders. Curr Opin Endocrinol Diabetes Obes. 2007;14:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 145. | Siegers JY, Novakovic B, Hulme KD, Marshall RJ, Bloxham CJ, Thomas WG, Reichelt ME, Leijten L, van Run P, Knox K, Sokolowski KA, Tse BWC, Chew KY, Christ AN, Howe G, Bruxner TJC, Karolyi M, Pawelka E, Koch RM, Bellmann-Weiler R, Burkert F, Weiss G, Samanta RJ, Openshaw PJM, Bielefeldt-Ohmann H, van Riel D, Short KR. A High-Fat Diet Increases Influenza A Virus-Associated Cardiovascular Damage. J Infect Dis. 2020;222:820-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 146. | Singer P, Shapiro H. Enteral omega-3 in acute respiratory distress syndrome. Curr Opin Clin Nutr Metab Care. 2009;12:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 147. | Dushianthan A, Cusack R, Burgess VA, Grocott MP, Calder PC. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst Rev. 2019;1:CD012041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 148. | Schenck LP, Surette MG, Bowdish DM. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016;590:3705-3720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 149. | Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 322] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 150. | Hemilä H. Vitamin C and Infections. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 268] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 151. | Erol N, Saglam L, Saglam YS, Erol HS, Altun S, Aktas MS, Halici MB. The Protection Potential of Antioxidant Vitamins Against Acute Respiratory Distress Syndrome: a Rat Trial. Inflammation. 2019;42:1585-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 152. | Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 153. | Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458-5467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 575] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 154. | Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 851] [Cited by in RCA: 743] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 155. | Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 450] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 156. | Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, Holford P, Thornton CA, Whitaker IS. Could Vitamins Help in the Fight Against COVID-19? Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 157. | Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 158. | Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 493] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 159. | Galmés S, Serra F, Palou A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 160. | Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, Potter JD, Ulrich CM. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 308] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 161. | Sheybani Z, Dokoohaki MH, Negahdaripour M, Dehdashti M, Zolghadr AR. The Role of Folic Acid in the Management of Respiratory Disease Caused by COVID-19. ChemRexiv. 2020;. [DOI] [Full Text] |
| 162. | Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 449] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 163. | te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 576] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 164. | Doboszewska U, Wlaź P, Nowak G, Młyniec K. Targeting zinc metalloenzymes in coronavirus disease 2019. Br J Pharmacol. 2020;177:4887-4898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 165. | Razzaque MS. COVID-19 Pandemic: Can Maintaining Optimal Zinc Balance Enhance Host Resistance? Tohoku J Exp Med. 2020;251:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 166. | Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int J Infect Dis. 2020;99:307-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 167. | Im JH, Je YS, Baek J, Chung MH, Kwon HY, Lee JS. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 168. | Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111:1297-1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 169. | Moghaddam A, Heller RA, Sun Q, Seelig J, Cherkezov A, Seibert L, Hackler J, Seemann P, Diegmann J, Pilz M, Bachmann M, Minich WB, Schomburg L. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 170. | Bae M, Kim H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 171. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3488] [Cited by in RCA: 3408] [Article Influence: 681.6] [Reference Citation Analysis (0)] |
| 172. | Haberland A, Neubert K, Kruse I, Behne D, Schimk I. Consequences of long-term selenium-deficient diet on the prostacyclin and thromboxane release from rat aorta. Biol Trace Elem Res. 2001;81:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 173. | Turnlund JR, Jacob RA, Keen CL, Strain JJ, Kelley DS, Domek JM, Keyes WR, Ensunsa JL, Lykkesfeldt J, Coulter J. Long-term high copper intake: effects on indexes of copper status, antioxidant status, and immune function in young men. Am J Clin Nutr. 2004;79:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 174. | Andreou A, Trantza S, Filippou D, Sipsas N, Tsiodras S. COVID-19: The Potential Role of Copper and N-acetylcysteine (NAC) in a Combination of Candidate Antiviral Treatments Against SARS-CoV-2. In Vivo. 2020;34:1567-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 175. | Raha S, Mallick R, Basak S, Duttaroy AK. Is copper beneficial for COVID-19 patients? Med Hypotheses. 2020;142:109814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 176. | Martindale R, Patel JJ, Taylor B, Arabi YM, Warren M, McClave SA. Nutrition Therapy in Critically Ill Patients With Coronavirus Disease 2019. JPEN J Parenter Enteral Nutr. 2020;44:1174-1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 177. | Bodoky G, Kent-Smith L. Basics in clinical nutrition: Complications of enteral nutrition. E Spen Eur E J Clin Nutr Metab. 2009;4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 178. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 179. | Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World J Gastroenterol. 2020;26:2286-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 180. | López-Romero D, Izquierdo-Vega JA, Morales-González JA, Madrigal-Bujaidar E, Chamorro-Cevallos G, Sánchez-Gutiérrez M, Betanzos-Cabrera G, Alvarez-Gonzalez I, Morales-González Á, Madrigal-Santillán E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 2: Plants, Vegetables, and Natural Resin. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 181. | Izquierdo-Vega JA, Morales-González JA, SánchezGutiérrez M, Betanzos-Cabrera G, Sosa-Delgado SM, Sumaya-Martínez MT, Morales-González Á, Paniagua-Pérez R, Madrigal-Bujaidar E, Madrigal-Santillán E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 1: Fruits and Polysaccharides. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 182. | Ben-Shabat S, Yarmolinsky L, Porat D, Dahan A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv Transl Res. 2020;10:354-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 183. | Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, Wang Q, Xu Y, Li M, Li X, Zheng M, Chen L, Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1221] [Cited by in RCA: 1415] [Article Influence: 283.0] [Reference Citation Analysis (0)] |
| 184. | Mani JS, Johnson JB, Steel JC, Broszczak DA, Neilsen PM, Walsh KB, Naiker M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020;284:197989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 185. | Park JY, Yuk HJ, Ryu HW, Lim SH, Kim KS, Park KH, Ryu YB, Lee WS. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhib Med Chem. 2017;32:504-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 186. | Shree P, Mishra P, Selvaraj C, Singh SK, Chaube R, Garg N, Tripathi YB. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants - Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi) - a molecular docking study. J Biomol Struct Dyn. 2020;1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 187. | Swargiary A, Mahmud S, Saleh MA. Screening of phytochemicals as potent inhibitor of 3-chymotrypsin and papain-like proteases of SARS-CoV2: an in silico approach to combat COVID-19. J Biomol Struct Dyn. 2020;1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 188. | Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926-9930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1274] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 189. | Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 813] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 190. | Tu W, Wang H, Li S, Liu Q, Sha H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019;10:637-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 465] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 191. | Houghton CA, Fassett RG, Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev. 2013;71:709-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 192. | Houghton CA, Fassett RG, Coombes JS. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician's Expectation Be Matched by the Reality? Oxid Med Cell Longev. 2016;2016:7857186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 193. | de Figueiredo SM, Binda NS, Nogueira-Machado JA, Vieira-Filho SA, Caligiorne RB. The antioxidant properties of organosulfur compounds (sulforaphane). Recent Pat Endocr Metab Immune Drug Discov. 2015;9:24-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 194. | Kamal MM, Akter S, Lin CN, Nazzal S. Sulforaphane as an anticancer molecule: mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch Pharm Res. 2020;43:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 195. | Liu Y, Yu Q, Chen Y. Effect of silibinin on CFLAR-JNK pathway in oleic acid-treated HepG2 cells. Biomed Pharmacother. 2018;108:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 196. | Ou Q, Weng Y, Wang S, Zhao Y, Zhang F, Zhou J, Wu X. Silybin Alleviates Hepatic Steatosis and Fibrosis in NASH Mice by Inhibiting Oxidative Stress and Involvement with the Nf-κB Pathway. Dig Dis Sci. 2018;63:3398-3408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 197. | Zhao F, Shi D, Li T, Li L, Zhao M. Silymarin attenuates paraquat-induced lung injury via Nrf2-mediated pathway in vivo and in vitro. Clin Exp Pharmacol Physiol. 2015;42:988-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 198. | Zhou J, Chao G, Li Y, Wu M, Zhong S, Feng Z. Activation of NRF2/ARE by isosilybin alleviates Aβ25-35-induced oxidative stress injury in HT-22 cells. Neurosci Lett. 2016;632:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 199. | Abdelsalam HM, Samak MA, Alsemeh AE. Synergistic therapeutic effects of Vitis vinifera extract and Silymarin on experimentally induced cardiorenal injury: The pertinent role of Nrf2. Biomed Pharmacother. 2019;110:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 200. | Arafa Keshk W, Zahran SM, Katary MA, Abd-Elaziz Ali D. Modulatory effect of silymarin on nuclear factor-erythroid-2-related factor 2 regulated redox status, nuclear factor-κB mediated inflammation and apoptosis in experimental gastric ulcer. Chem Biol Interact. 2017;273:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 201. | Li L, Sun HY, Liu W, Zhao HY, Shao ML. Silymarin protects against acrylamide-induced neurotoxicity via Nrf2 signalling in PC12 cells. Food Chem Toxicol. 2017;102:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 202. | Vargas-Mendoza N, Morales-González Á, Morales-Martínez M, Soriano-Ursúa MA, Delgado-Olivares L, Sandoval-Gallegos EM, Madrigal-Bujaidar E, Álvarez-González I, Madrigal-Santillán E, Morales-Gonzalez JA. Flavolignans from Silymarin as Nrf2 Bioactivators and Their Therapeutic Applications. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 203. | Liu CH, Jassey A, Hsu HY, Lin LT. Antiviral Activities of Silymarin and Derivatives. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 204. | Lalani SS, Anasir MI, Poh CL. Antiviral activity of silymarin in comparison with baicalein against EV-A71. BMC Complement Med Ther. 2020;20:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 205. | Lestari ML, Indrayanto G. Curcumin. Profiles Drug Subst Excip Relat Methodol. 2014;39:113-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 206. | Asouri M, Ataee R, Ahmadi AA, Amini A, Moshaei MR. Antioxidant and free radical scavenging activities of curcumin. Asian J Chem. 2013;25:7593. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 207. | Liu Z, Dou W, Zheng Y, Wen Q, Qin M, Wang X, Tang H, Zhang R, Lv D, Wang J, Zhao S. Curcumin upregulates Nrf2 nuclear translocation and protects rat hepatic stellate cells against oxidative stress. Mol Med Rep. 2016;13:1717-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 208. | Meshkibaf MH, Maleknia M, Noroozi S. Effect of curcumin on gene expression and protein level of methionine sulfoxide reductase A (MSRA), SOD, CAT and GPx in Freund's adjuvant inflammation-induced male rats. J Inflamm Res. 2019;12:241-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 209. | Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H, Huang X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. 2019;14:e0216711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 210. | Zahedipour F, Hosseini SA, Sathyapalan T, Majeed M, Jamialahmadi T, Al-Rasadi K, Banach M, Sahebkar A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;34:2911-2920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 211. | Mathew D, Hsu WL. Antiviral potential of curcumin. J Functional Foods. 2018;40:692. [RCA] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 212. | Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A. Immune modulation by curcumin: The role of interleukin-10. Crit Rev Food Sci Nutr. 2019;59:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 213. | Avasarala S, Zhang F, Liu G, Wang R, London SD, London L. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PLoS One. 2013;8:e57285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 214. | Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785-2791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19127] [Cited by in RCA: 16296] [Article Influence: 1018.5] [Reference Citation Analysis (0)] |