Published online Mar 7, 2020. doi: 10.3748/wjg.v26.i9.973
Peer-review started: October 30, 2019
First decision: December 12, 2019
Revised: January 9, 2020
Accepted: January 19, 2020
Article in press: January 19, 2020
Published online: March 7, 2020
Processing time: 128 Days and 7 Hours
Peroral endoscopic myotomy (POEM) is a promising therapeutic modality for esophageal achalasia worldwide. However, clinical failure and adverse events of POEM have still been concerned.
To compare the efficacy and safety of a novel mark-guided POEM with standard POEM.
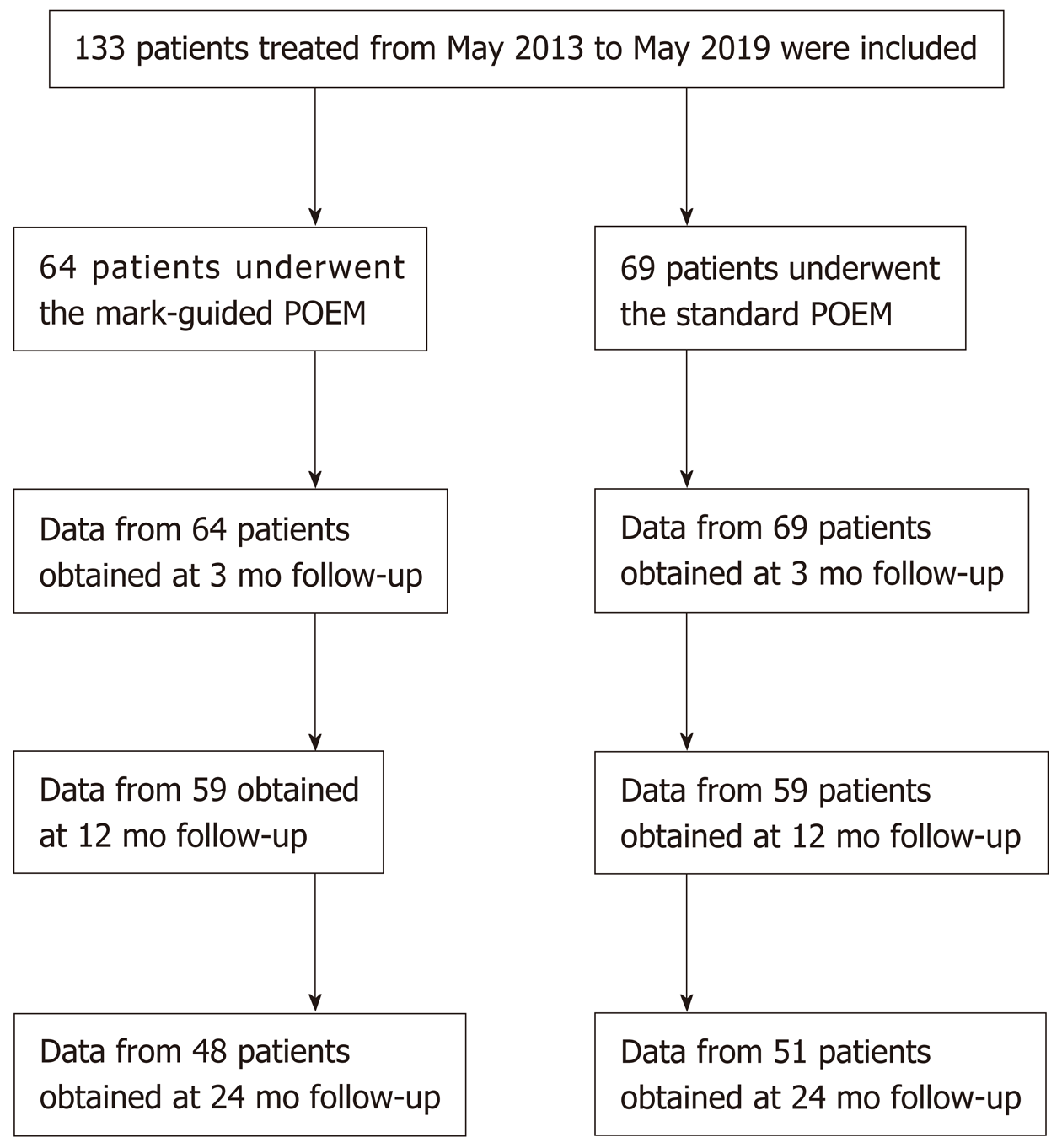
A total of 133 patients with esophageal achalasia who underwent POEM from May 2013 to May 2019 were enrolled in this retrospective study. Of the 133 patients, there were 64 patients in the mark-guided POEM group and 69 patients in the standard POEM group. The clinical success, procedural duration and adverse events were compared between the two groups at 3 mo, 12 mo and 24 mo postoperatively.
Characteristic baseline was similar in the mark-guided POEM group and standard POEM group. The clinical success was comparable between the two groups, ranging from 92% to 98%, at 3 mo, 12 mo and 24 mo postoperatively (all P > 0.5). Eckart score, Gastroesophageal Reflux Disease Questionnaire score and SF-36 score were not different between the two groups after treatment (all P > 0.05). No severe adverse events occurred in the two groups. However, mark-guided POEM required shorter procedural duration, and less use of proton pump inhibitors and lower incidence of reflux symptoms than the standard POEM (all P < 0.001).
Mark-guided POEM and standard POEM were both effective and safe for the treatment of esophageal achalasia. However, the mark-guided POEM was characterized by shorter procedural duration, less use of proton pump inhibitors and lower incidence of reflux symptoms.
Core tip: Mark-guided Peroral endoscopic myotomy (POEM) can create full and large separation through sufficient sub-mucosal injection, which can improve the operative filed, decrease the incidence of bleeding, perforation and intra-procedural mucosal injury, and enhance the clinical success. By mark-guided POEM, it was not necessary to repeatedly pull out the tunnel to check the direction, thus saving the procedural time. Moreover, mark-guided POEM required less use of proton pump inhibitors and showed a lower incidence of reflux symptoms after the procedure.
- Citation: Li DF, Xiong F, Yu ZC, Zhang HY, Liu TT, Tian YH, Shi RY, Lai MG, Song Y, Xu ZL, Zhang DG, Yao J, Wang LS. Effect and safety of mark-guided vs standard peroral endoscopic myotomy: A retrospective case control study. World J Gastroenterol 2020; 26(9): 973-983
- URL: https://www.wjgnet.com/1007-9327/full/v26/i9/973.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i9.973
As a rare esophageal motility disorder, esophageal achalasia is characterized by a failure of peristalsis in the esophageal body, leading to impaired lower esophageal sphincter relax and esophageal emptying[1,2]. The hampered passage of food from the esophagus to the stomach contributes to symptoms of dysphagia, regurgitation, chest pain and weight loss, as well as pulmonary complications[3,4].
Peroral endoscopic myotomy (POEM) was first described by Inoue et al[5] for achalasia treatment in 2010. Subsequently, it was demonstrated that POEM was effective and safe and has become the standard procedure for achalasia treatment worldwide[6-8]. Although several prospective studies have shown that POEM was superior in controlling symptoms of achalasia, POEM-associated clinical failure and adverse events have still been concerned[9-11]. The clinical success rate of POEM was reported to be more than 90%, however, reflux esophagitis which was the main adverse event developed in more than 40% of the patients after POEM treatment[12].
Several factors are associated with the efficacy and safety of POEM, such as mucosal injury, direction lossin the tunnel and oblique muscle damage[12,13]. Therefore, we here described a novel POEM procedure named mark-guided POEM, which may solve above-mentioned problems. We retrospectively compared the novel mark-guided POEM and standard POEM described by Inoue et al[5] in terms of clinical success, technical success and adverse events in our clinical center.
From May 2013 to May 2019, patients diagnosed with achalasia based on Eckardt score, barium esophagography and high-resolution manometry (HRM) were retrospectively collected at the Department of Gastroenterology of the Second Clinical Medicine College (Shenzhen People's Hospital) of Jinan University (Guangdong, China). The patients who were lost to follow-up were excluded. Demographic and clinical data included patient’s age, gender, disease duration, follow-up, procedural duration, clinical success, technical success, pre-operative and post-operative Eckardt score, post-operative length of stay, recurrence and adverse events (bleeding, perforation and reflux symptoms). A total of 133 patients who underwent POEM were included in this study. Of these patients, there were 64 patients in the mark-guided POEM group treated from September 2018 to May 2019 and 69 patients in the standard POEM group treated from May 2013 to September 2018. The initial follow-up barium esophagography was conducted at 3 mo post-operatively. Subsequently, Eckardt score, Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), reflux symptoms and proton pump inhibitor (PPI) use were assessed via telephone at 3, 12 and 24 mo post-operatively (Figure 1). The study protocol was approved by Shenzhen People's Hospital Ethics Committee.
Achalasia is divided into three distinct subtypes (type I, II and III) according to the pattern of esophageal contractility observed during high-resolution manometry (HRM) according to the Chicago Classification system[3]. Eckardt scores in 4-item questionnaire including dysphagia, regurgitation and chest pain ranging from 0 to 3 (0, none; 1, occasionally; 2, daily; 3, with every meal), and weight loss (0, no weight loss; 1, < 5 kg; 2, 5-10 kg; 3, > 10 kg) were used to evaluate the severity of achalasia, which were rated from the lowest severity (0 score) to the highest severity (12 scores)[14]. Clinical success was assessed using the Eckardt scores (≤ 3 scores), and failure of treatment was defined as Eckardt scores of more than 3 after treatment. The Gastroesophageal Reflux Disease Questionnaire (GERDQ) was used to assess reflux symptoms, including heartburn, regurgitation, epigastric pain, nausea, sleep disorder and use of over-the-counter drugs, and each of them was rated from 0 to 3 scores. Therefore, the total scores ranged from 0 to 18 points, and > 8 points was regarded as GERD[15]. SF-36 scoring system was composed of physical and mental components ranging from 0 to 100 scores, and higher scores indicated better quality of life[16]. Severe adverse events consisted of perforation and bleeding (defined as need of blood transfusion or endoscopy, radiologic and surgical intervention).
Patients were fasted for 24 h before the procedure. POEM was performed under general anesthesia with endotracheal intubation and CO2 insufflation. All participating endoscopists were experts, and standard POEM procedure in this study was in accordance with Inoue et al[5]. The steps of standard POEM were briefly described as follows. (1) At the middle of esophagus, a submucosal bleb was created by injecting saline containing 0.3 % indigo carmine. Subsequently, a 2-cm longitudinal mucosal incision was made by Dual Knife (Olympus, Japan) to create submucosal tunnel using Endocut mode (30 W, effect 3) (ERBE, Germany); (2) A tunnel passing gastroesophageal junction (GEJ) 2-3 cm into proximal stomach was created by Dual Knife on the plane of dissection of submucosal layer; (3) Circular muscle bundle dissection was extended from 3 cm below the mucosal entry onto the proximal gastric cardia using Triangle Knife; and (4) Clips were placed close to the mucosal entity site (Anrei, China) (Video 1 standard peroral endoscopic myotomy procedure) through endoscopy. In the first step of mark-guided POEM, the middle of esophagus to gastric cardia at esophageal mucosal surface was marked using Dual Knife. Then, submucosal injection was administered through the mark with saline containing 0.3 % indigo carmine. Next, submucosal layer dissection, circular muscle bundle dissection and closure of mucosal entity site were the same as standard POEM (Video 2 mark-guided peroral endoscopic myotomy procedure).
All patients were given antibiotics (Cefatriaxone and Metronidazole) and a double-dose PPI (Omeprazole) intravenously at the day of the procedure and kept nothing by mouth (NPO) at the night of the procedure. The next day, a gastrografin esophagram was performed to rule out leakage and perforation. All patients with no evidence of adverse events were discharged, and they were advised to take soft food for 2 wk and PPI (Omeprazole, 20 mg, once a day) was prescribed for 2 wk.
All patients were followed up with barium esophagography at 3 mo post-operatively, and Eckart score, GERDQ score, SF-36 score, reflux symptoms and PPI use were also assessed via telephone at 12 mo and 24 mo post-operatively.
The primary outcome was clinical success, and the second outcome included procedure duration, severe adverse events, Eckart score, GERDQ score, SF-36 score, reflux symptoms and PPI use.
All analyses were performed using the SPSS 23.0 software package (SPSS Company, Chicago, IL, United States). All categorical variables were expressed as the frequency with respective percentages. Continuous data were presented as mean ± SD or median (interquartile range) according to distribution. χ2 test or Fisher’s exact test was used to assess categorical variables, and unpaired t-test or Mann-Whitney test was used to assess continuous data. P values < 0.05 were considered statistically significant.
A total of 133 consecutive patients were included in this retrospective study. Of these patients, there were 64 patients in the mark-guided POEM group and 69 patients in the standard POEM group. There was no significant difference between the two groups in terms of sex, age, type of achalasia, disease duration, Eckardt score, esophageal height, esophageal diameter, HRM, GERDQ score and SF-36 score (Table 1).
| Characteristics | Mark-guided POEM (n = 64) | Standard POEM (n = 69) | P value |
| Sex | |||
| Male (n) | 33 (51.6%) | 36 (52.2%) | 0.94 |
| Female (n) | 31 (48.4%) | 33 (47.8%) | |
| Age (yr) | 33.5 (28-48.75) | 40 (30-47.75) | 0.22 |
| Achalasia Type | |||
| I | 21 (32.8%) | 23 (33.3%) | 0.98 |
| II | 36 (56.3%) | 38 (55.1%) | |
| III | 7 (10.9%) | 8 (11.6%) | |
| Disease duration (mo) | 32.5 (23-49.50) | 33 (22.5-49.50) | 0.95 |
| Eckardt score | 9.0 (8-9.75) | 8.0 (7-9) | 0.32 |
| Barium esophagraphy | |||
| Height (cm) | 8 (8-9) | 8 (7-9) | 0.55 |
| Diameter (cm) | 5 (4-6) | 5 (5-6) | 0.29 |
| HRM (mmHg) | 38 (28-41) | 38 (28-41.5) | 0.64 |
| GERDQ score | 7 (6-9) | 7 (6-8.5) | 0.74 |
| SF-36 score | 47.22 ± 7.25 | 46.81 ± 7.60 | 0.75 |
| Procedure duration (min) | 40 (38-43) | 49 (47-51) | < 0.001 |
| Technical success (n) | 64 (100%) | 69 (100%) | 1 |
| Postoperative stay (d) | 1 (1-2) | 1 (1-2) | 0.56 |
| Perforation (n) | 0 | 0 | 1 |
| Bleeding (n) | 0 | 0 | 1 |
Both groups successfully underwent POEM without any severe adverse events (perforation and bleeding) (P = 1). In addition, the hospital stay was not significantly different between the two groups (P = 0.56). However, the procedure duration was significantly shorter in the mark-guided POEM group compared with the standard POEM group (P < 0.001) (Table 1).
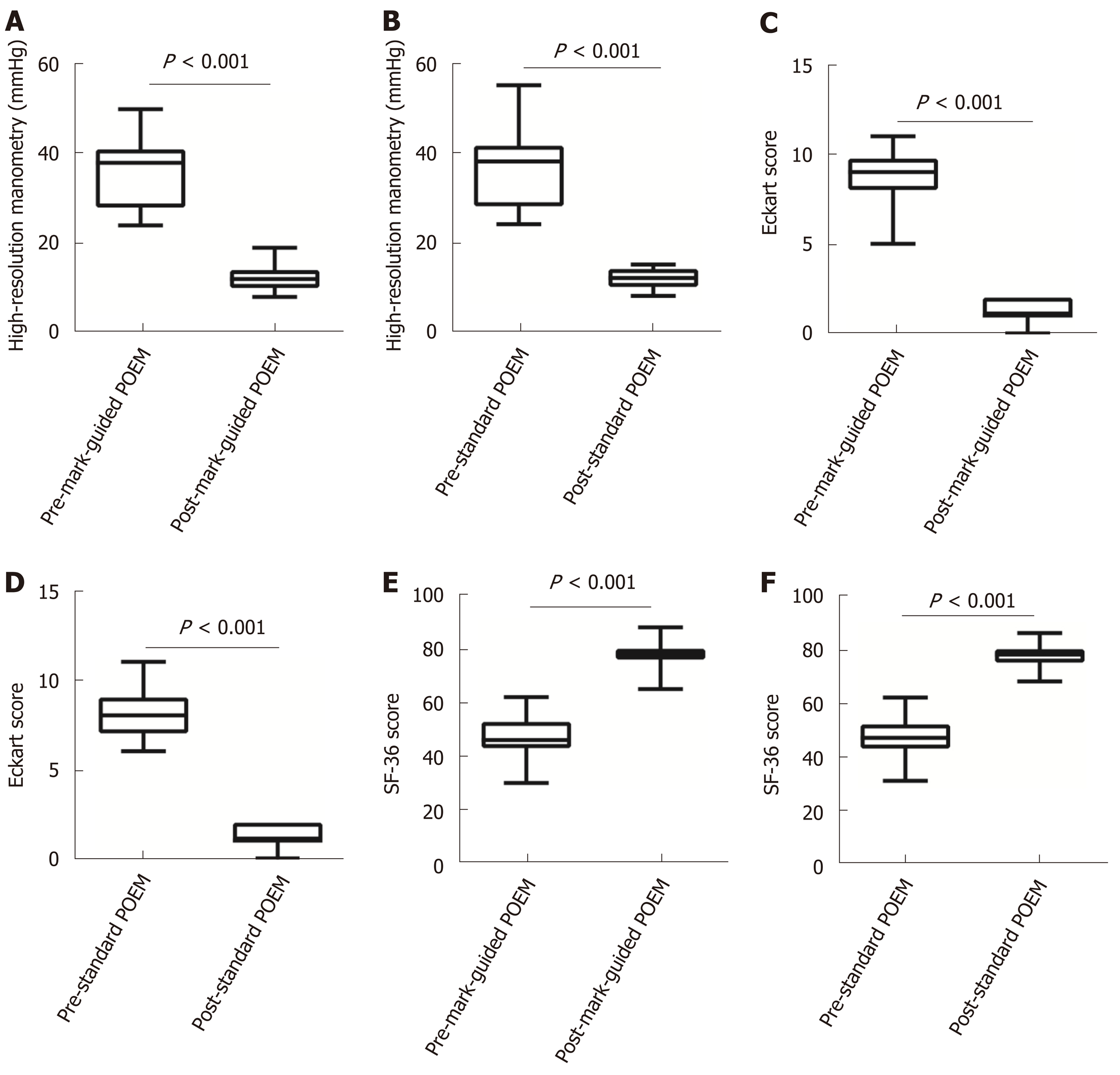
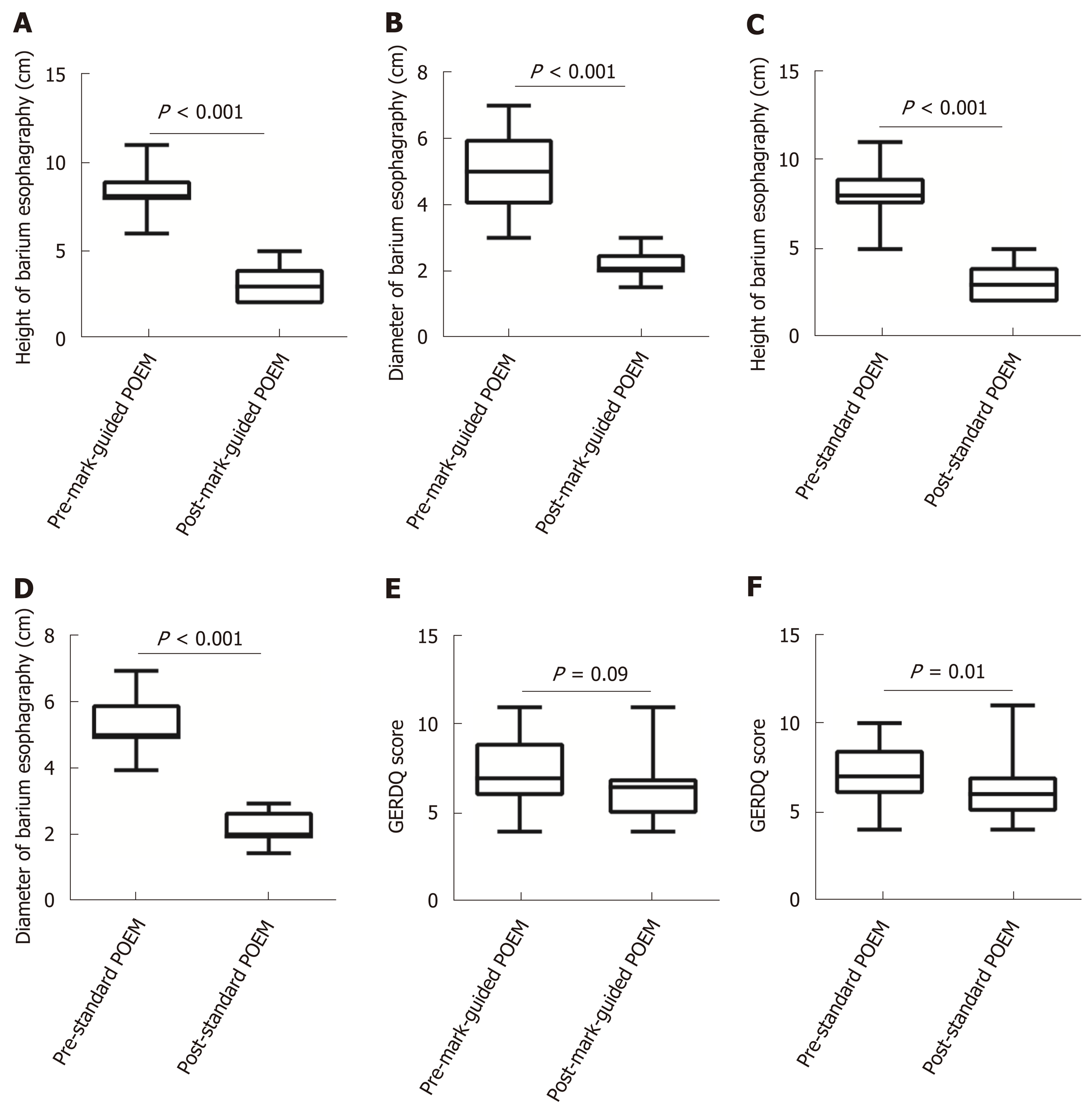
There were 64 and 69 patients in the mark-guided POEM group and standard POEM group at 3-mo follow-up, respectively. No significant difference was observed in the clinical success between the two groups (98.4% vs 98.6%, P = 0.3). Figure 2 shows that the pre-operative HRM and Eckart scores were significantly decreased compared with the post-operative values in both groups (Figure 2A-2D, all P < 0.001). Furthermore, the pre-operative SF-36 score was significantly improved compared with the postoperative value in both groups (Figure 2E, 2F, all P < 0.001). However, there was no significant difference between the two groups (Table 2). The post-operative height and diameter of barium esophagography were significantly decreased in both groups (Figure 3A-3D, all P < 0.001), whereas there was no significant difference between the two groups (Table 2). Moreover, the pre-operative GERDQ score was significantly decreased compared with its post-operative value in the standard POEM group (P = 0.01, Figure 3E), while such significant difference was not observed in the mark-guided POEM group (P = 0.09, Figure 3F). However, the incidence of reflux symptoms and PPI use were significantly different between mark-guided POEM and standard POEM groups (10.9% vs 24.6%, P = 0.04; and 12.7% vs 27.5%, P = 0.03, respectively) (Table 2).
| Mark-guided POEM (n = 64) | Standard POEM (n = 69) | P value | |
| Overall clinical success (n) | 63 (98.4%) | 68 (98.6%) | 0.3 |
| Eckart score | 1 (1-2) | 1 (1-2) | 0.78 |
| GERDQ score | 6 (5-9) | 6 (5-7) | 0.35 |
| SF-36 score | 78 (76-80) | 78 (75-80.5) | 0.87 |
| Barium esophagraphy | |||
| Height (cm) | 3 (2-4) | 3 (2-4) | 0.94 |
| Diameter (cm) | 2 (2-2.5) | 2 (2-2.75) | 0.86 |
| HRM (mmHg) | 12.2 ± 2.37 | 12.06 ± 1.93 | 0.7 |
| Reflux symptom (n) | |||
| Yes | 7 (10.9%) | 17 (24.6%) | 0.04 |
| No | 57 (89.1%) | 52(75.4%) | |
| PPI use (n) | |||
| Yes | 8 (12.7%) | 19 (27.5%) | 0.03 |
| No | 56 (87.3%) | 60 (72.5%) |
Table 3 shows that there were 59 patients in each group at 12-mo follow-up, and the clinical success was 93.5% (55/59) and 91.5% (54/59) in the mark-guided POEM group and standard POEM group, respectively, with no significant difference between the two groups (P = 0.73). Moreover, there was no significant difference between the two groups in terms of Eckart score, GERDQ score and SF-36 score (P = 0.9, P = 0.67 and P = 0.94, respectively). However, the incidence of reflux symptoms and PPI use was 16.9% and 18.6% in the mark-guided POEM group and 37.3% and 40.7% in the standard POEM group, respectively (P = 0.01 and P = 0.009).
| Mark-guided POEM (n = 59) | Standard POEM (n = 59) | P value | |
| Overall clinical success (n) | 55 (93.2%) | 54 (91.5%) | 0.73 |
| Eckart score | 1 (1-2) | 1 (1-2) | 0.9 |
| GERDQ score | 7 (6-9) | 6 (6-9) | 0.67 |
| SF-36 score | 75 (67-78) | 74 (70-78) | 0.94 |
| Reflux symptom (n) | |||
| Yes | 10 (16.9%) | 22 (37.3%) | 0.01 |
| No | 49 (83.1%) | 37 (62.7%) | |
| PPI use (n) | |||
| Yes | 11 (18.6%) | 24 (40.7%) | 0.009 |
| No | 48 (81.4%) | 35 (59.3%) |
There were 48 patients in the mark-guided POEM group and 51 patients in the standard POEM group at 24-mo follow-up. The results showed that there was no significant difference in clinical success between the mark-guided POEM group and standard POEM group (92.7% vs 92.2%, P = 0.93). Furthermore, there was no significant difference between the two groups in terms of Eckart score, GERDQ score and SF-36 score (P = 0.92, P = 0.74 and P = 0.73, respectively), whereas the incidence of reflux symptoms and PPI use were significantly lower in the mark-guided POEM group compared with the standard POEM group (27.1% vs 47.1%, P = 0.04 and 29.2% vs 51%, P = 0.02) (Table 4).
| Mark-guided POEM (n = 48) | Standard POEM (n = 51) | P value | |
| Overall clinical success (n) | 44 (92.7%) | 47 (92.2%) | 0.93 |
| Eckart score | 1 (1-2) | 1 (1-2) | 0.92 |
| GERDQ score | 7 (6-9) | 7 (6-9) | 0.74 |
| SF-36 score | 77 (71-80) | 76 (72-80) | 0.73 |
| Reflux symptom (n) | |||
| Yes | 13 (27.1%) | 24(47.1%) | 0.04 |
| No | 35(72.9%) | 27 (52.9%) | |
| PPI use (n) | |||
| Yes | 14 (29.2%) | 26 (51%) | 0.02 |
| No | 34 (70.8%) | 25 (49%) |
Nine and 10 patients with unsuccessful treatment in the mark-guided POEM group and standard POEM group, respectively, were all symptomatic (Eckart score > 3). Of the nine patients in the mark-guided POEM group, five patients required re-treatment and recovered uneventfully, whereas the other four patients refused additional treatments because of symptom improvement. Of the 10 patients in the standard POEM group, six patients successfully underwent re-treatment of POEM, while the other four patients refused additional re-treatment.
In this retrospective study, we compared the mark-guided POEM with standard POEM in terms of the clinical success, procedure duration, adverse events, reflux symptoms and PPI use at 3-mo, 12-mo and 24-mo follow-up. The results showed that the overall clinical success, hospital stay and severe adverse events were not significantly different between the two groups. However, the procedural duration, and incidence of reflux symptoms and PPI use were significantly lower in the mark-guided POEM group compared with the standard POEM group.
In the present study, we found that the clinical success ranged from 92.7% to 98.4% and 92.2% to 98.6% at 3-mo follow-up and 24-mo follow-up, respectively, in the mark-guided POEM group and standard POEM group, which was similar to a previous meta-analysis[17]. Moreover, there was no severe adverse event (perforation and bleeding) in the patients in this study. However, a previous study has shown that the overall rate of adverse events is 7.5%, and severe adverse events only occur in 90 cases of 1800 POEM procedures[18]. Our results indicated that the clinical success could be decreased with time in both groups, which was consistent with previous data that the recurrence rate after POEM can be increased with time[19,20]. However, POEM re-treatment was also effective for the recurrent patients, and some of them refused additional treatment because of symptom improvement. Therefore, both the mark-guided and standard POEM was effective for achalasia. Interestingly, we found that the mark-guided POEM showed a lower incidence of reflux symptoms and less PPI use compared with standard POEM, which wasmarkedly lower compared with the previous study as well[6]. Ponds et al[10] have demonstrated that the reflux esophagitis rate is 49%, and 8% are severe cases on endoscopy examination at 1-year follow-up after POEM treatment, which is markedly higher compared with the mark-guided POEM in the present study. Furthermore, Shiwaku et al[21] have found that the erosive esophagitis (Los Angeles grade A-D) and severe erosive esophagitis (Los Angeles grade C-D) account for 63% and 6.2%, respectively, whereas, symptomatic GERD is only observed in 14.8% of 1300 patients at 6-mo follow-up after POEM. Therefore, the erosive esophagitis might be more in this study. Fortunately, many studies including our current study have shown that reflux symptoms respond to treatment with a PPI[10,21].
To the best of our knowledge, we, for the first time, compared the mark-guided POEM with standard POEM. In addition to less procedure duration, and lower incidence of reflux symptoms and PPI use in the mark-guided POEM, there was no significant difference between the two groups. We considered that the mark-guided POEM had the following advantages: First, it could create full and large separation through sufficient sub-mucosal injection, which could improve operative filed, decrease the incidence of bleeding, perforation and intra-procedural mucosal injury, and increase the clinical success. Liu et al[22] have shown that intra-procedural mucosal injury is a risk factor for clinical failure. Second, it was not necessary to repeatedly pull out the tunnel to check the direction, thus saving much operating time.
There are several limitations in this study. First, this was a retrospective study from a single tertiary hospital, and the results need to be confirmed by multi-centers randomized controlled trials. Second, the Eckardt score was used to determine clinical success. However, its construct validity has recently been questioned[23]. Third, GERDQ has limitations to identify reflux symptoms or GERD after POEM. Fourth, patients were followed up at 3, 12 and 24 mo via telephone, and long-term conclusion is unavailable.
In summary, this retrospective study confirmed that the mark-guided POEM and standard POEM were both effective and safe for esophageal achalasia. However, the mark-guided POEM required less procedural duration and showed a lower incidence of reflux symptoms and PPI use compared with the standard POEM.
Peroral endoscopic myotomy (POEM) was first described by a study on achalasia treatment in 2010. Subsequently, it was demonstrated that POEM was effective and safe and has become the standard procedure for achalasia worldwide. However, clinical failure and adverse events of POEM have still been concerned. Indeed, POEM procedure can lead to a high incidence of reflux esophagitis.
Several factors are associated with the efficacy and safety of POEM, such as sufficient sub-mucosal injection, limiting mucosal injury and constructing sub-mucosal tunnel straightly. Therefore, we described a novel POEM procedure named mark-guided POEM, which may solve afore-mentioned problems.
This study aimed to compare the efficacy and safety of the novel mark-guided POEM with standard POEM in the improvement of efficacy and safety of achalasia treatment. This retrospective case control study will encourage us to explore the efficacy and safety of the mark-guided POEM for further research, such as multi-centers randomized controlled trials.
This retrospective case control study compared the efficacy and safety between the mark-guided POEM and standard POEM.
This study showed that mark-guided POEM and standard POEM were both effective and safe for achalasia treatment, however, the mark-guided POEM seemed to require less procedural duration and proton pump inhibitor (PPI) use and show a lower incidence of reflux symptoms. However, these results will be confirmed by randomized controlled trials.
POEM is a promising therapeutic procedure for esophageal achalasia worldwide. However, clinical failure and adverse events of POEM have still been concerned. In order to improve efficacy and safety of achalasia treatment, we described a novel POEM procedure named the mark-guided POEM. We retrospectively compared the efficacy and safety of the mark-guided POEM with standard POEM. The results showed that the clinical success was comparable between the two groups, ranging from 92% to 98%, at 3 mo, 12 mo and 24 mo postoperatively. However, the mark-guided POEM required less procedural duration, less use of PPI and lower incidence of reflux symptoms than the standard POEM. We will conduct multi-centers randomized controlled trial to confirm these results.
The mark-guided POEM may be superior to standard POEM for achalasia treatment; however, the findings need to be further confirmed using multi-centers randomized controlled trials.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdelfatah MM, Eleftheriadis N S-Editor: Dou Y L-Editor: MedE-Ma JY E-Editor: Zhang YL
| 1. | Richter JE. Achalasia - an update. J Neurogastroenterol Motil. 2010;16:232-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Sadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil. 2010;22:e256-e261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 3. | Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet. 2014;383:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 428] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 4. | Boeckxstaens GE. The lower oesophageal sphincter. Neurogastroenterol Motil. 2005;17 Suppl 1:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1235] [Article Influence: 82.3] [Reference Citation Analysis (1)] |
| 6. | Kahrilas PJ, Katzka D, Richter JE. Clinical Practice Update: The Use of Per-Oral Endoscopic Myotomy in Achalasia: Expert Review and Best Practice Advice From the AGA Institute. Gastroenterology. 2017;153:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Benias PC, Korrapati P, Raphael KL, D'Souza LS, Inamdar S, Trindade AJ, Lee C, Kumbhari V, Sejpal DV, Okolo P, Khashab MA, Miller L, Carr-Locke D. Safety and feasibility of performing peroral endoscopic myotomy as an outpatient procedure with same-day discharge. Gastrointest Endosc. 2019;90:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Liu Z, Wang Y, Fang Y, Huang Y, Yang H, Ren X, Xu M, Chen S, Chen W, Zhong Y, Zhang Y, Qin W, Hu J, Cai M, Yao L, Li Q, Zhou P. Short-term safety and efficacy of peroral endoscopic myotomy for the treatment of achalasia in children. J Gastroenterol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Werner YB, Hakanson B, Martinek J, Repici A, von Rahden BHA, Bredenoord AJ, Bisschops R, Messmann H, Vollberg MC, Noder T, Kersten JF, Mann O, Izbicki J, Pazdro A, Fumagalli U, Rosati R, Germer CT, Schijven MP, Emmermann A, von Renteln D, Fockens P, Boeckxstaens G, Rösch T. Endoscopic or Surgical Myotomy in Patients with Idiopathic Achalasia. N Engl J Med. 2019;381:2219-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 345] [Article Influence: 57.5] [Reference Citation Analysis (1)] |
| 10. | Ponds FA, Fockens P, Lei A, Neuhaus H, Beyna T, Kandler J, Frieling T, Chiu PWY, Wu JCY, Wong VWY, Costamagna G, Familiari P, Kahrilas PJ, Pandolfino JE, Smout AJPM, Bredenoord AJ. Effect of Peroral Endoscopic Myotomy vs Pneumatic Dilation on Symptom Severity and Treatment Outcomes Among Treatment-Naive Patients With Achalasia: A Randomized Clinical Trial. JAMA. 2019;322:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 11. | Shiwaku H, Inoue H, Sato H, Onimaru M, Minami H, Tanaka S, Sato C, Ogawa R, Okushima N, Yokomichi H. Peroral endoscopic myotomy for achalasia: a prospective multicenter study in Japan. Gastrointest Endosc. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Khashab MA, Vela MF, Thosani N, Agrawal D, Buxbaum JL, Abbas Fehmi SM, Fishman DS, Gurudu SR, Jamil LH, Jue TL, Bijun Sai Kannadath, Law JK, Lee JK, Naveed M, Qumseya BJ, Sawhney MS, Yang J, Wani S. ASGE guideline on the management of achalasia. Gastrointest Endosc. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 13. | Chiu PW, Inoue H, Rösch T. From POEM to POET: Applications and perspectives for submucosal tunnel endoscopy. Endoscopy. 2016;48:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Perbtani YB, Mramba LK, Yang D, Suarez J, Draganov PV. Life after per-oral endoscopic myotomy: long-term outcomes of quality of life and their association with Eckardt scores. Gastrointest Endosc. 2018;87:1415-1420.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Wang M, Zhang JZ, Kang XJ, Li L, Huang XL, Aihemaijiang K, Ayinuer A, Li YX, He XL, Gao F. Relevance between GerdQ score and the severity of reflux esophagitis in Uygur and Han Chinese. Oncotarget. 2017;8:74371-74377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Vigneswaran Y, Tanaka R, Gitelis M, Carbray J, Ujiki MB. Quality of life assessment after peroral endoscopic myotomy. Surg Endosc. 2015;29:1198-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Lee Y, Brar K, Doumouras AG, Hong D. Peroral endoscopic myotomy (POEM) for the treatment of pediatric achalasia: a systematic review and meta-analysis. Surg Endosc. 2019;33:1710-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Haito-Chavez Y, Inoue H, Beard KW, Draganov PV, Ujiki M, Rahden BHA, Desai PN, Pioche M, Hayee B, Haji A, Saxena P, Reavis K, Onimaru M, Balassone V, Nakamura J, Hata Y, Yang D, Pannu D, Abbas A, Perbtani YB, Patel LY, Filser J, Roman S, Rivory J, Mion F, Ponchon T, Perretta S, Wong V, Maselli R, Ngamruengphong S, Chen YI, Bukhari M, Hajiyeva G, Ismail A, Pieratti R, Kumbhari V, Galdos-Cardenas G, Repici A, Khashab MA. Comprehensive Analysis of Adverse Events Associated With Per Oral Endoscopic Myotomy in 1826 Patients: An International Multicenter Study. Am J Gastroenterol. 2017;112:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 19. | Von Renteln D, Fuchs KH, Fockens P, Bauerfeind P, Vassiliou MC, Werner YB, Fried G, Breithaupt W, Heinrich H, Bredenoord AJ, Kersten JF, Verlaan T, Trevisonno M, Rösch T. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology. 2013;145:309-11.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 20. | Werner YB, Costamagna G, Swanström LL, von Renteln D, Familiari P, Sharata AM, Noder T, Schachschal G, Kersten JF, Rösch T. Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years. Gut. 2016;65:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 21. | Shiwaku H, Inoue H, Onimaru M, Minami H, Sato H, Sato C, Tanaka S, Ogawa R, Okushima N. Multicenter collaborative retrospective evaluation of peroral endoscopic myotomy for esophageal achalasia: analysis of data from more than 1300 patients at eight facilities in Japan. Surg Endosc. 2020;34:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Liu XY, Cheng J, Chen WF, Liu ZQ, Wang Y, Xu MD, Chen SY, Zhong YS, Zhang YQ, Yao LQ, Zhou PH, Li QL. A risk-scoring system to predict clinical failure for patients with achalasia after peroral endoscopic myotomy. Gastrointest Endosc. 2020;91:33-40.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Taft TH, Carlson DA, Triggs J, Craft J, Starkey K, Yadlapati R, Gregory D, Pandolfino JE. Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenterol Motil. 2018;30:e13287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |