Published online Oct 28, 2020. doi: 10.3748/wjg.v26.i40.6224
Peer-review started: June 23, 2020
First decision: July 28, 2020
Revised: August 8, 2020
Accepted: September 11, 2020
Article in press: September 11, 2020
Published online: October 28, 2020
Processing time: 126 Days and 15.2 Hours
Intestinal dysbiosis has been shown to be associated with the pathogenesis of alcoholic liver disease (ALD), which includes changes in the microbiota composition and bacterial overgrowth, but an effective microbe-based therapy is lacking. Pediococcus pentosaceus (P. pentosaceus) CGMCC 7049 is a newly isolated strain of probiotic that has been shown to be resistant to ethanol and bile salts. However, further studies are needed to determine whether P. pentosaceus exerts a protective effect on ALD and to elucidate the potential mechanism.
To evaluate the protective effect of the probiotic P. pentosaceus on ethanol-induced liver injury in mice.
A new ethanol-resistant strain of P. pentosaceus CGMCC 7049 was isolated from healthy adults in our laboratory. The chronic plus binge model of experimental ALD was established to evaluate the protective effects. Twenty-eight C57BL/6 mice were randomly divided into three groups: The control group received a pair-fed control diet and oral gavage with sterile phosphate buffered saline, the EtOH group received a ten-day Lieber-DeCarli diet containing 5% ethanol and oral gavage with phosphate buffered saline, and the P. pentosaceus group received a 5% ethanol Lieber-DeCarli diet but was treated with P. pentosaceus. One dose of isocaloric maltose dextrin or ethanol was administered by oral gavage on day 11, and the mice were sacrificed nine hours later. Blood and tissue samples (liver and gut) were harvested to evaluate gut barrier function and liver injury-related parameters. Fresh cecal contents were collected, gas chromatography–mass spectrometry was used to measure short-chain fatty acid (SCFA) concentrations, and the microbiota composition was analyzed using 16S rRNA gene sequencing.
The P. pentosaceus treatment improved ethanol-induced liver injury, with lower alanine aminotransferase, aspartate transaminase and triglyceride levels and decreased neutrophil infiltration. These changes were accompanied by decreased levels of endotoxin and inflammatory cytokines, including interleukin-5, tumor necrosis factor-α, granulocyte colony-stimulating factor, keratinocyte-derived protein chemokine, macrophage inflammatory protein-1α and monocyte chemoattractant protein-1. Ethanol feeding resulted in intestinal dysbiosis and gut barrier disruption, increased relative abundance of potentially pathogenic Escherichia and Staphylococcus, and the depletion of SCFA-producing bacteria, such as Prevotella, Faecalibacterium, and Clostridium. In contrast, P. pentosaceus administration increased the microbial diversity, restored the relative abundance of Lactobacillus, Pediococcus, Prevotella, Clostridium and Akkermansia and increased propionic acid and butyric acid production by modifying SCFA-producing bacteria. Furthermore, the levels of the tight junction protein ZO-1, mucin proteins (mucin [MUC]-1, MUC-2 and MUC-4) and the antimicrobial peptide Reg3β were increased after probiotic supplementation.
Based on these results, the new strain of P. pentosaceus alleviated ethanol-induced liver injury by reversing gut microbiota dysbiosis, regulating intestinal SCFA metabolism, improving intestinal barrier function, and reducing circulating levels of endotoxin and proinflammatory cytokines and chemokines. Thus, this strain is a potential probiotic treatment for ALD.
Core Tip: Gut microbiota dysbiosis plays an important role in the progression of ethanol-induced liver injury, but an effective microbe-based therapy is lacking. Our study screened an ethanol-resistant strain of Pediococcus pentosaceus (P. pentosaceus) as a potential probiotic. Preliminary results indicated that the new strain of P. pentosaceus modulated the composition of the gut microbiota and short-chain fatty acid metabolism to protect against ethanol-induced liver injury in a mouse model.
- Citation: Jiang XW, Li YT, Ye JZ, Lv LX, Yang LY, Bian XY, Wu WR, Wu JJ, Shi D, Wang Q, Fang DQ, Wang KC, Wang QQ, Lu YM, Xie JJ, Li LJ. New strain of Pediococcus pentosaceus alleviates ethanol-induced liver injury by modulating the gut microbiota and short-chain fatty acid metabolism. World J Gastroenterol 2020; 26(40): 6224-6240
- URL: https://www.wjgnet.com/1007-9327/full/v26/i40/6224.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i40.6224
Alcoholic liver disease (ALD), which includes a spectrum of conditions, remains one of the most common causes of liver cirrhosis and liver failure worldwide, and it is responsible for 1.2% and 0.7% of all global deaths in men and women, respectively[1]. Most heavy drinkers exhibit liver steatosis, but only 10%–35% of them progress to alcoholic hepatitis, and even fewer ultimately develop cirrhosis, indicating that multiple factors, such as sex, genetics, obesity, drinking patterns and comorbidity, are involved in the progression of ALD[2-4]. Furthermore, recent studies have also suggested a close association between intestinal dysbiosis and the progression of ethanol-induced liver injury[4].
Intestinal dysbiosis has been shown to be associated with the pathogenesis of ALD, which includes changes in the microbiota composition and bacterial overgrowth. Several studies have reported alterations in the microbiota composition in patients with ALD, with a decreasing proportion of Bacteroidetes and increasing proportions of Firmicutes and Actinobacteria; a lower abundance of lactic acid-producing bacteria, such as Lactobacillus and Pediococcus, was also closely associated with ALD[5-7]. In addition, intestinal bacterial overgrowth is very common in patients with ALD and in animal models involving ethanol feeding[8,9]. Ethanol-induced intestinal bacterial overgrowth contributes to bacterial translocation that is associated with the expression of the antimicrobial peptides Reg3β and Reg3γ, which inhibit gram-positive bacteria and protect against bacterial overgrowth, and Reg3β- and Reg3γ-deficient mice exhibit mucosal bacterial overgrowth and increased translocation of bacteria to the liver[10]. Fecal microbiota transplantation from patients with ALD to germ-free mice increased the susceptibility to ethanol-induced liver injury and revealed a causal relationship between intestinal dysbiosis and the progression of ALD[4]. Moreover, alcohol and the metabolite acetaldehyde disrupt the gut mucosal barrier and increase gut permeability, promoting the translocation of pathological bacteria and microbiota-associated products such as lipopolysaccharide from the gut to the liver, which promotes immune cell activation by Toll-like receptors (TLRs) and the release of cytokines and chemokines to induce liver inflammation and fibrosis[11,12]. Thus, manipulation of the microbiota with prebiotics, probiotics and fecal microbiota transplantation to restore gut homeostasis represent new therapeutic strategies for ALD[13].
Pediococcus pentosaceus (P. pentosaceus) belongs to the Lactobacillaceae family and is widely used as a probiotic. Several strains of P. pentosaceus have been proven to exert anti-inflammatory effects on fatty liver and intestinal inflammation. Our laboratory has focused on the isolation of potential probiotics from the gut microbiota of healthy volunteers. P. pentosaceus CGMCC 7049 is a newly isolated strain of bacteria that has been shown to be acid-tolerant and resistant to bile salts, with a high tolerance to 5% ethanol[14]. As shown in our previous studies, the administration of P. pentosaceus effectively inhibits pathogenic bacteria, such as Staphylococcus aureus and Clostridium difficile[14,15]. The relative abundance of Pediococcus is also significantly reduced in patients with ALD and animal models involving ethanol feeding[9,16]. Based on findings from previous studies, we screened the ethanol-resistant strain of P. pentosaceus CGMCC 7049 to further evaluate its potential protective effect on the chronic plus binge NIAAA animal model of ALD[17,18]. The short-chain fatty acid (SCFA) concentrations and composition of the gut microbiota were analyzed to explore the potential mechanisms.
P. pentosaceus CGMCC 7049 was cultured in MRS broth in an anaerobic chamber. Anaerobic workstations (Electrotek, England) filled with 10% H2, 10% CO2, and 80% N2 were used to maintain anaerobic conditions. The cultured bacteria were centrifuged and washed twice with sterile phosphate buffered saline (PBS). P. pentosaceus was resuspended in PBS and adjusted to a dose of 2 × 109 colony forming units (CFUs) for animal experiments.
Eight- to ten-week-old C57BL/6 mice were fed a liquid diet for one week to adapt to alcohol feeding. The mice were then randomly divided into three groups: the mice in the control group received a pair-fed control diet and oral gavage with sterile PBS (n = 8), mice in the EtOH group received a ten-day Lieber-DeCarli diet containing 5% ethanol and oral gavage with PBS (n = 10), and the mice in the P. pentosaceus group received a 5% ethanol Lieber-DeCarli diet but were treated with P. pentosaceus (n = 10). The mice in the P. pentosaceus group were administered 2 × 109 CFUs of P. pentosaceus suspended in PBS, and an equal volume of PBS was administered by oral gavage to the control group and EtOH group once daily. Mice in the EtOH group and P. pentosaceus group received oral gavage with one dose of ethanol (5 g/kg) on day 11, and the control group received the same volume of isocaloric maltose dextrin solution as the control reagent instead of ethanol by oral gavage; the mice were sacrificed nine hours later. Blood and tissue samples (liver and gut) were harvested to evaluate gut barrier function and liver injury-related parameters. Fresh cecal contents were collected after the mice were sacrificed on day 11 and immediately stored at -80°C until 16S rRNA gene sequencing and SCFA analyses.
A dry chemistry analyzer was used to measure the concentrations of aspartate transaminase (AST) and alanine aminotransferase (ALT). The liver tissue samples were homogenized, the supernatant was collected, and hepatic triglyceride (TG) concentrations were measured with a triglyceride assay kit (Applygen Technologies Inc., China). Endotoxin was quantified indirectly with lipopolysaccharide-binding protein (LBP) due to the inaccurate results of direct measurements, and serum LBP concentrations were determined using an LBP ELISA Kit (Guduo, Shanghai, China) according to the manufacturer’s protocols.
Serum cytokine concentrations were measured using a 23-Plex Panel kit with the MAGPIX system according to the manufacturer’s protocols. The following cytokines and chemokines were detected using this multiplex assay: Interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12 (p70), IL-13, IL-17A, IL-18, interferon-γ, tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-3α, C-C chemokine ligand 5, vascular endothelial growth factor, granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor, and keratinocyte-derived protein chemokine (KC).
The liver and gut tissues were fixed with 4% paraformaldehyde overnight and then embedded in paraffin. Hematoxylin and eosin (HE) staining of liver and intestinal sections was performed, and the sections were then analyzed with a digital pathology system. For the analysis of hepatic lipid accumulation, liver samples were frozen in optimal cutting temperature compound and cryosectioned. After air-drying, the sections were fixed and stained with oil red O in propylene glycol and counterstained with hematoxylin.
Immunohistochemical staining was performed on paraffin sections to detect neutrophil (myeloperoxidase [MPO], Abcam, 1:200 dilution) infiltration. Briefly, liver sections were deparaffinized with xylene and dehydrated with a gradient of ethanol solutions. Rabbit MPO antibodies and secondary anti-rabbit antibodies were applied. Images were obtained with the NanoZoomer Digital Pathology system. Immunofluorescence staining was performed in colon sections to detect a tight junction protein (ZO-1). The cells were incubated with a ZO-1 antibody overnight, followed by an incubation with secondary goat anti-rabbit antibodies and DAPI staining for 45 min. Images were captured with a 340 confocal microscope (Zeiss, Oberkochen, Germany).
RNA was purified from the liver and gut with the RNeasy Plus kit. An Applied Biosystems system was used for quantitative PCR (qPCR). The mRNA expression of the following genes was assessed using qPCR: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), TNF-α, IL-6, IL-10, MCP-1, ZO-1, TLR2, TLR4, Reg3β, Reg3γ, MUC-1, MUC-2, and MUC-4.
Gas chromatography–mass spectrometry (GC-MS) was performed to measure the levels of SCFAs, including acetic acid, propionic acid, butyric acid, isobutyric acid, 2-methylbutyric acid and valeric acid, in the cecal contents. SCFA concentrations in cecal contents are reported in milligrams per gram (mg/g) of feces.
Total bacterial DNA was obtained with a QIAamp Fast DNA Stool Mini Kit (Qiagen, CA, United States). DNA extraction was verified by standard agarose gel electrophoresis and quantified with a Nanodrop 2000 spectrophotometer. Library preparations and sequencing were performed at BGI (Shenzhen, China). PCR was performed to prepare amplicons from the V3-V4 region using the 341F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) primers. Libraries were finally submitted for sequencing on the Illumina MiSeq platform. Paired-end raw reads from the Illumina platform were overlapped and merged using FLASH with standard parameters. Quality control of the merged reads was performed using the QIIME platform. Finally, the reads were taxonomically assigned by mapping them to the Greengenes reference database based on 97% sequence similarity using closed-reference operational taxonomic unit (OTU) mapping in QIIME. Calculations of strain composition, rarefied alpha diversity indices and beta diversity indices were performed using QIIME. The similarity of microbiota communities among groups was determined by performing a principal coordinate analysis (PCoA). The differentially abundant biomarkers among groups were further investigated with linear discriminant analysis effect size (LEfSe) analysis. The raw sequencing data were deposited in the GenBank Sequence Read Archive (Accession number: PRJNA555778).
The results are presented as means ± SE. The normality of the data was evaluated with the Kolmogorov–Smirnov test. One-way ANOVA followed by Tukey’s multiple comparison tests were used to evaluate the statistical significance of differences among groups. Spearman’s rank correlation coefficients were calculated to establish the correlations between gut bacterial genera and injury-related indices. P < 0.05 was considered statistically significant. Statistical analyses were performed and graphs were constructed using GraphPad Prism version 7.0 (GraphPad Software Inc., La Jolla, CA, United States) and SPSS 20.0 (SPSS, Inc., Chicago, IL, United States).
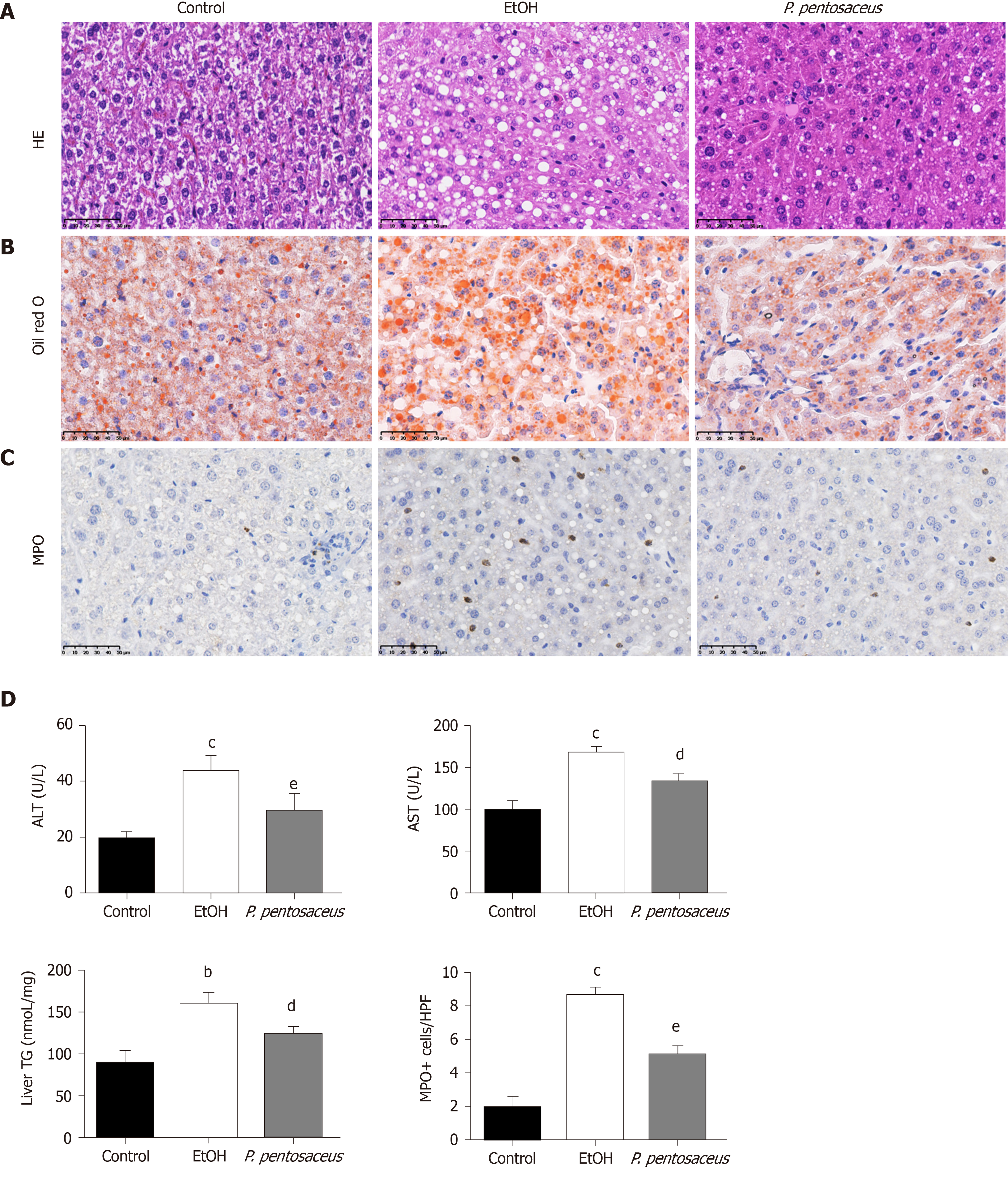
We established the chronic plus binge NIAAA animal model of experimental ALD to evaluate the therapeutic properties of P. pentosaceus. Ethanol-fed mice exhibited a significant induction of liver steatosis (Figure 1A), which was confirmed by the measurement of hepatic TG concentrations and oil red O staining of hepatic neutral lipids (Figure 1B and D). In addition, P. pentosaceus supplementation attenuated liver steatosis, with lower hepatic TG levels and less fat accumulation in hepatocytes in the histological images. Moreover, compared to the pair-fed control mice, ethanol-fed mice exhibited signs of liver injury and inflammation, as indicated by increases in serum ALT and AST levels (Figure 1D) and neutrophil infiltration (Figure 1C). However, P. pentosaceus administration protected against liver injury by reducing ALT and AST levels and neutrophil infiltration, which was also confirmed by the quantification of MPO-positive cells after immunohistochemical staining for MPO in the liver (Figure 1C and D).
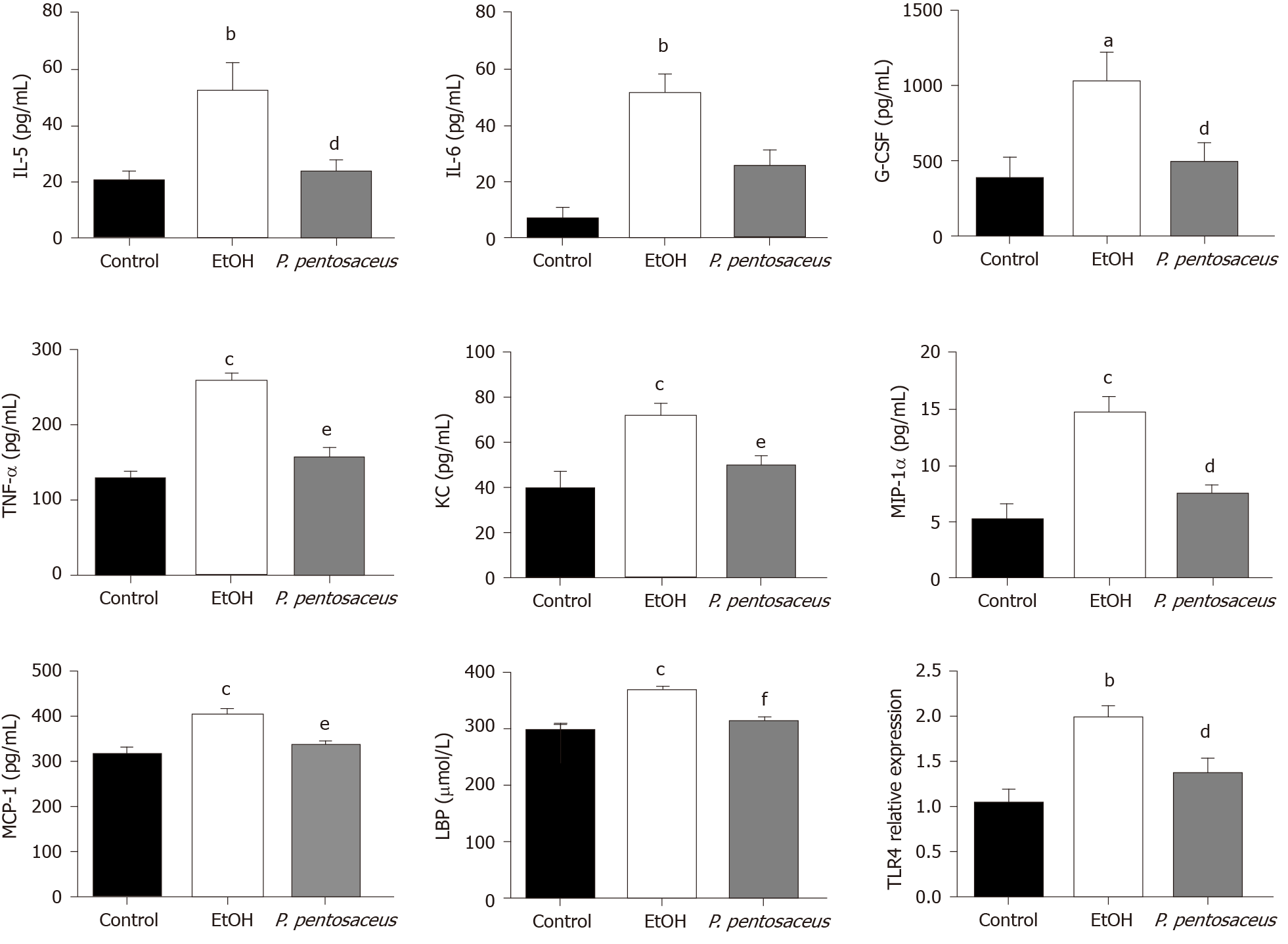
The serum LBP and cytokine levels were subsequently assessed. The serum level of LBP was increased in ethanol-fed mice, but the mice treated with P. pentosaceus showed lower levels of LBP than mice in the EtOH group (Figure 2). Additionally, the expression of TLR4 mRNA was also upregulated in the liver of ethanol-fed mice compared with control mice, while TLR4 expression was downregulated in the P. pentosaceus-treated group (Figure 2). The serum cytokine and chemokine levels were also measured, and the levels of proinflammatory cytokines, including IL-5, IL-6, TNF-α, G-CSF, KC, MIP-1α and MCP-1, were markedly increased in ethanol-fed mice. As expected, P. pentosaceus treatment significantly reduced the levels of the proinflammatory cytokines IL-5, TNF-α, G-CSF, KC, MIP-1α and MCP-1 (Figure 2).
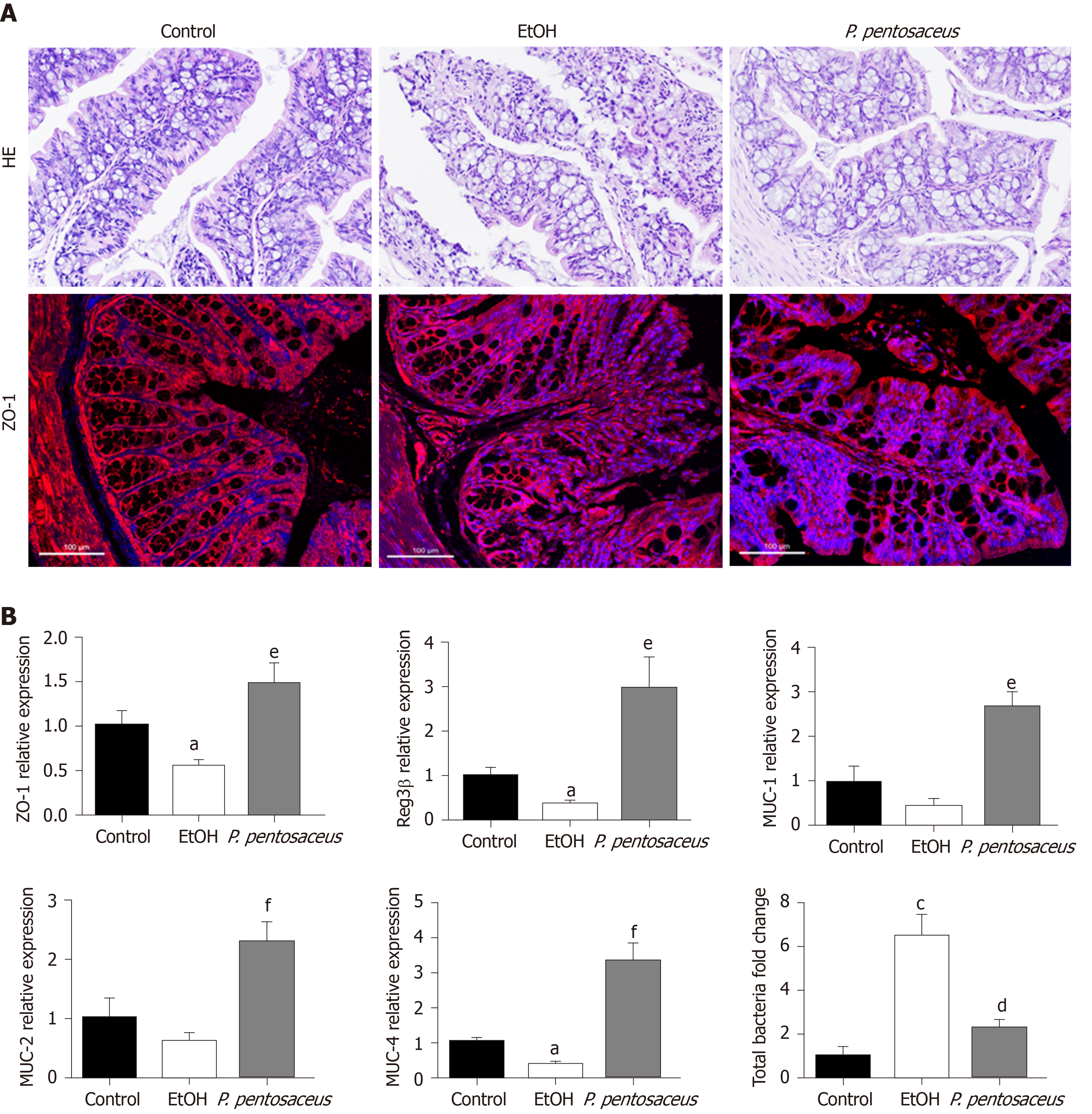
According to HE staining of colon tissues, alcohol exposure significantly increased tissue inflammation in the absence of a loss of villi or intestinal crypts. P. pentosaceus supplementation significantly ameliorated the histological abnormalities associated with ethanol-induced intestinal epithelial injury (Figure 3A). Tight junction proteins are essential to maintain intestinal barrier function, and thus we next evaluated the expression of ZO-1 in the colon. Alcohol feeding significantly reduced the expression of ZO-1 mRNA, whereas the expression of ZO-1 mRNA was upregulated following P. pentosaceus supplementation. The results of immunofluorescence staining were also consistent with the gene expression results, as the fluorescence intensity of ZO-1 decreased during alcohol feeding and P. pentosaceus supplementation stabilized the expression of this tight junction protein and improved intestinal integrity (Figure 3A and B). Moreover, we also investigated the expression of mucin mRNAs that protect against pathogen penetration of the mucus layer after alcohol administration. Although the expression of intestinal mucin mRNAs, including MUC-1 and MUC-2, was not significantly different between the EtOH group and the control group, significantly higher mucin mRNA expression (MUC-1, MUC-2 and MUC-4) was observed in mice treated with P. pentosaceus (Figure 3B). In addition, we further evaluated the overall bacterial load and the expression of the antibacterial peptides Reg3β and Reg3γ. The total bacterial load was significantly higher in ethanol-fed animals than in the control animals, but P. pentosaceus supplementation significantly ameliorated intestinal bacterial overgrowth during alcohol feeding (Figure 3B). The expression of Reg3β mRNA was significantly inhibited in alcohol-fed mice compared with control mice. Conversely, much higher levels of Reg3β mRNA were observed in mice that received the P. pentosaceus supplement (Figure 3B).
Bacterial DNA was extracted and the gut microbiota composition was analyzed using 16S rRNA sequencing. The resulting paired reads were assembled and filtered to generate 1077504 valid tags (44896 tags per sample) for subsequent analysis. The nonchimeric sequences were then clustered into 398 OTUs based on 97.0% sequence similarity.
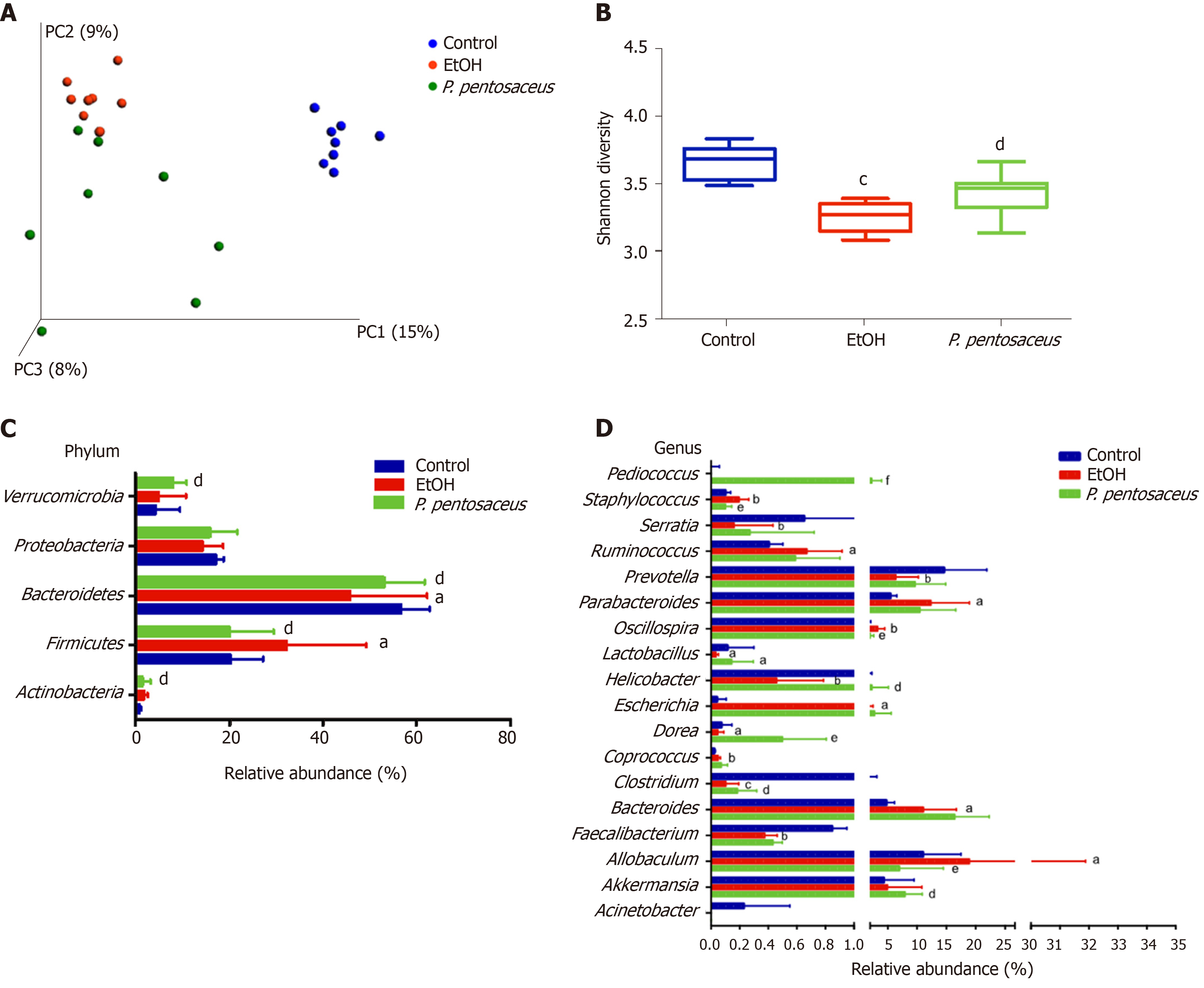
The microbial alpha diversity was evaluated with the Chao, Shannon and Simpson indices. The Chao richness index and Shannon and Simpson diversity indices were significantly reduced after alcohol consumption, but the changes in microbial diversity were partially reversed by P. pentosaceus treatment (Figure 4B and Supplementary Figure 1). Furthermore, we constructed a PCoA plot with unweighted UniFrac distances to assess microbial beta diversity among the three groups (Figure 4A). The microbial communities of the three groups were distinctly separated, and the microbial communities in the alcohol-fed group were clustered and significantly different from the microbial communities in the control group (ANOSIM, P = 0.002, r = 0.71). Likewise, the microbial composition also differed between the P. pentosaceus group and the EtOH group (ANOSIM, P = 0.003, r = 0.23). Based on these results, P. pentosaceus supplementation altered the bacterial community and increased the microbial diversity.
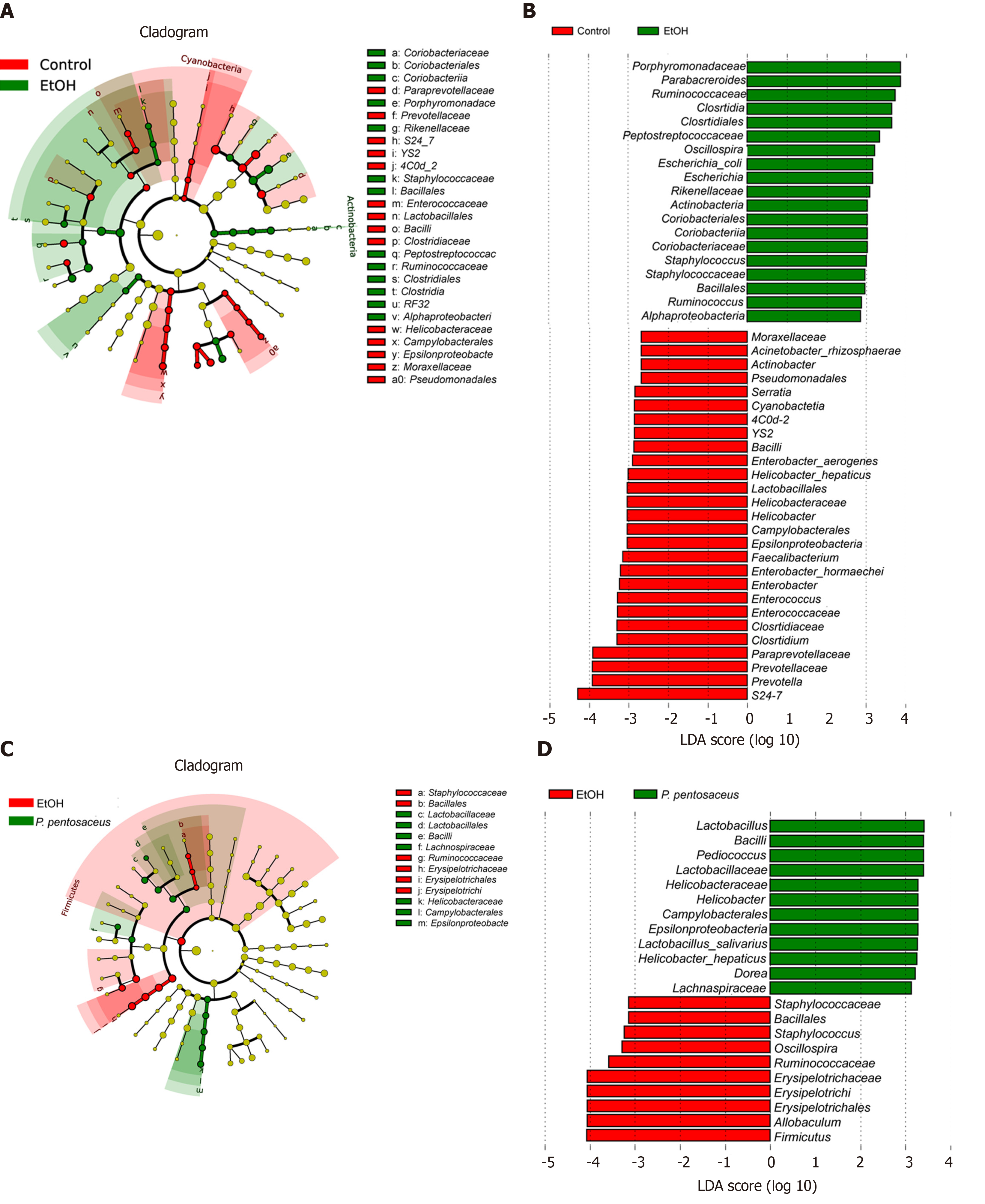
We further investigated the changes in the microbial composition at the phylum and genus levels. At the phylum level, alcohol feeding resulted in an increase in the abundance of Actinobacteria and Firmicutes, but lower proportions of Bacteroidetes compared to the control group (Figure 4C). However, P. pentosaceus supplementation restored the abundance of Bacteroidetes and Verrucomicrobia and prevented the expansion of Firmicutes (Figure 4C). At the genus level, significant reductions in Prevotella, Helicobacter, Lactobacillus, Faecalibacterium, and Clostridium and increases in Escherichia, Staphylococcus, and Parabacteroides were observed in mice after alcohol consumption (Figure 4D). In contrast, P. pentosaceus administration restored the relative abundance of Lactobacillus, Pediococcus, Clostridium and Akkermansia and decreased the abundance of Staphylococcus, Oscillospira and Allobaculum (Figure 4D). We performed a LEfSe analysis to investigate the microbial markers between the groups. Similarly, Prevotella, Helicobacter, Lactobacillus, Faecalibacterium, Clostridium and Serratia were more frequently represented in the control group, whereas Escherichia, Staphylococcus, Parabacteroides, Oscillospira and Allobaculum were the predominant microbiota in the EtOH group (Figure 5A). Remarkably, the family Lactobacillaceae and the genera Lactobacillus and Pediococcus were depleted after alcohol feeding and were significantly overrepresented after P. pentosaceus supplementation (Figure 5B).
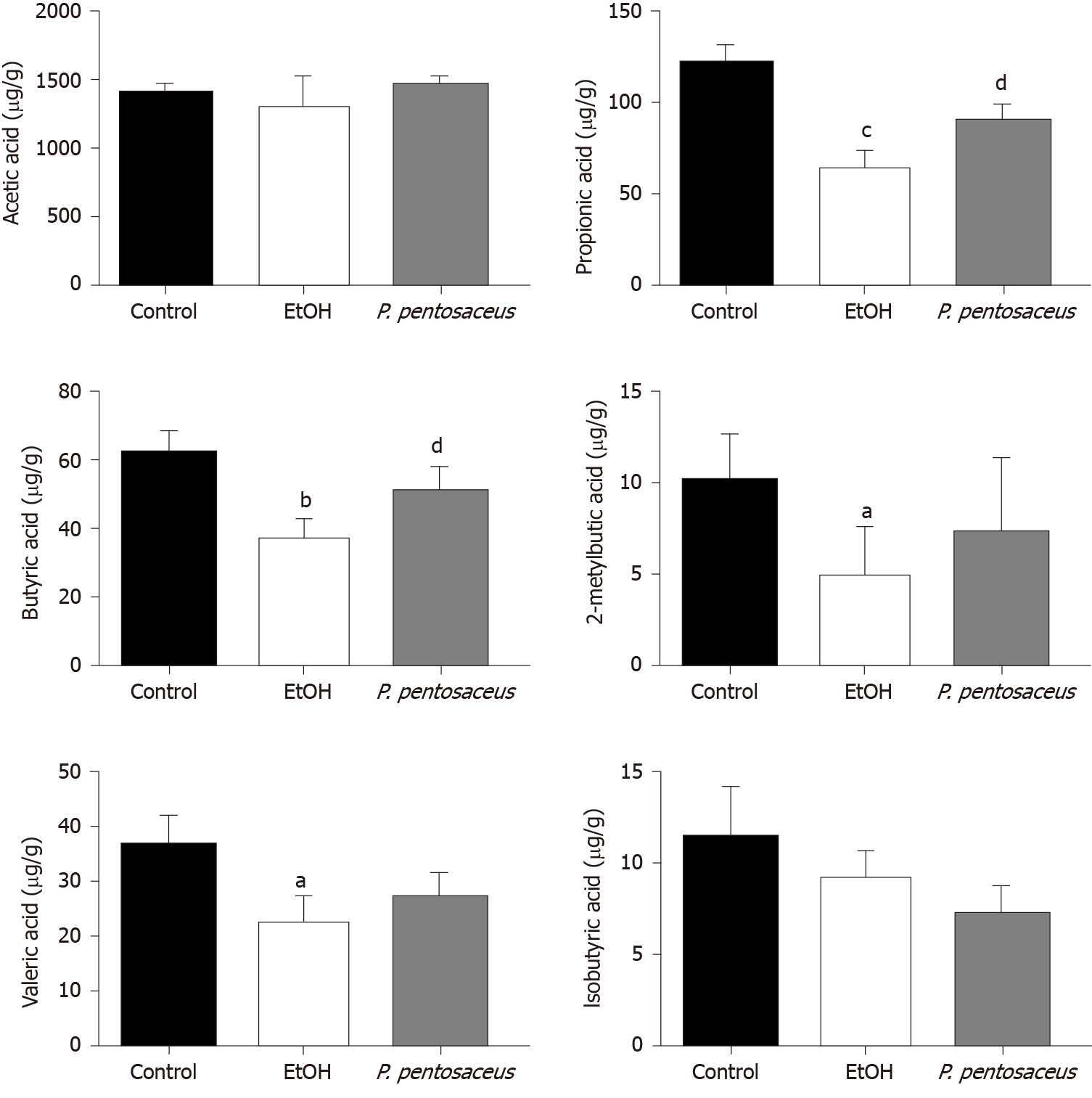
GC-MS was used to measure the concentrations of SCFAs, including acetic acid, propionic acid, butyric acid, isobutyric acid, 2-methylbutyric acid and valeric acid, in mouse feces. Ethanol feeding resulted in marked reductions in propionic acid, butyric acid, valeric acid, and 2-methylbutyric acid concentrations (Figure 6). Conversely, P. pentosaceus administration increased the production of propionic acid and butyric acid compared to the EtOH group (Figure 6). A decreasing trend in the levels of acetic acid and isobutyric acid was observed in mice after ethanol consumption, but this trend was not statistically significant among the three groups (Figure 6). Thus, alcohol feeding significantly decreased the production of SCFAs, but P. pentosaceus supplementation partially reversed this trend.
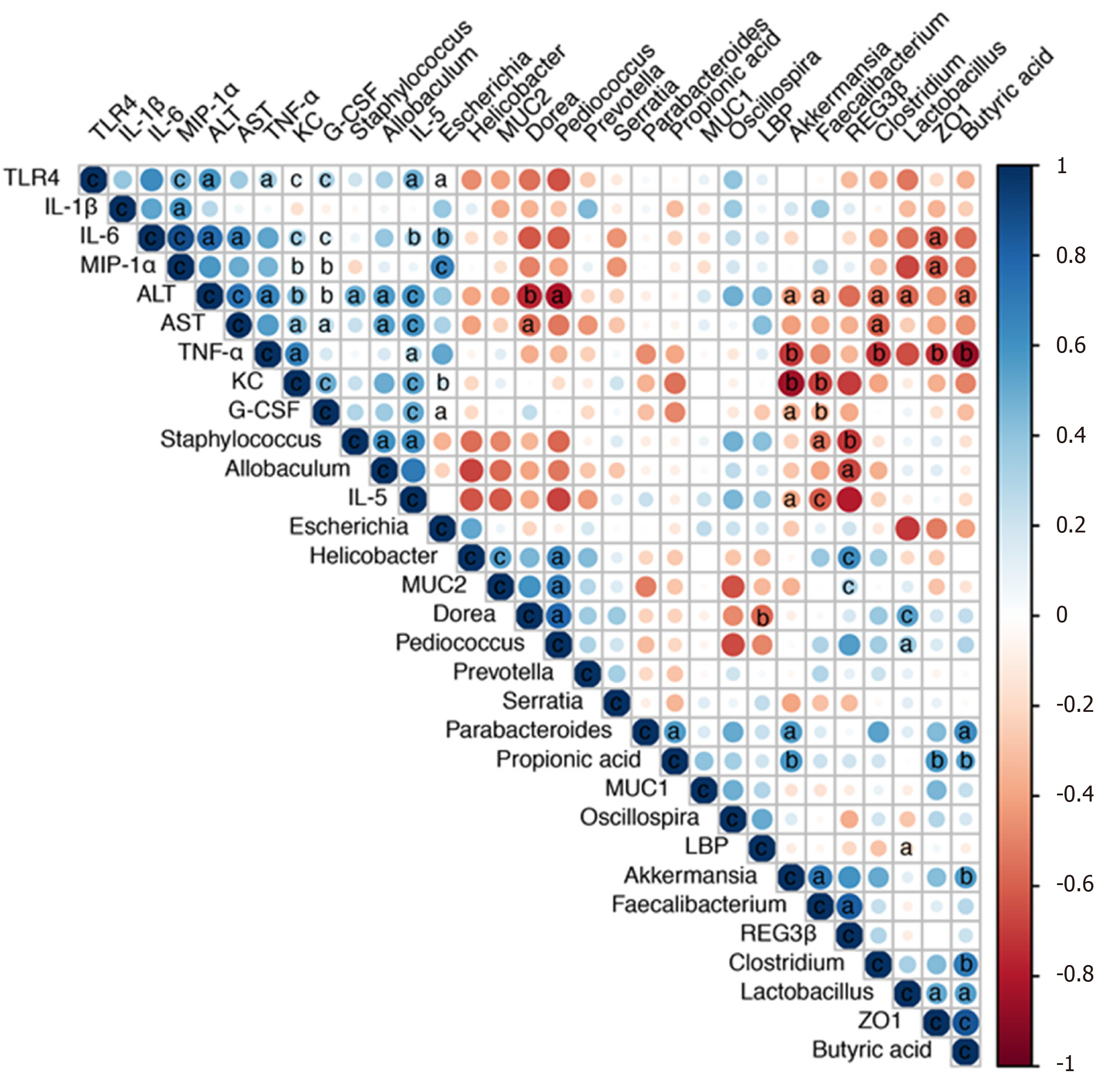
We next performed a correlation analysis of altered bacterial genera, gut barrier markers, inflammatory cytokines and liver injury-related indices (Figure 7). The concentrations of proinflammatory cytokines (IL-5, IL-6, TNF-α, G-CSF, KC, and MIP-1α) were positively correlated with liver injury-related indices (ALT and AST). TLR4 mRNA expression was also positively correlated with the levels of IL-6, TNF-α, G-CSF, KC, MIP-1α and ALT, indicating that TLR activation and inflammatory cytokine release contributed to liver injury. Additionally, the altered microbiota showed positive or negative correlations with gut barrier markers and liver injury-related parameters. The relative abundance of potentially pathogenic Escherichia was enriched after alcohol feeding and showed positive correlations with the levels of proinflammatory cytokines (IL-5, IL-6, KC, and MIP-1α). Pediococcus and Lactobacillus were enriched in the P. pentosaceus group and exhibited positive correlations with gut barrier markers (ZO-1 and MUC-2) and negative correlations with ALT levels. The relative abundance of Akkermansia and Faecalibacterium was significantly higher after P. pentosaceus supplementation and exhibited positive correlations with SCFA concentrations (propionic acid and butyric acid) and gut barrier markers (Reg3β), but negative correlations with the levels of proinflammatory cytokines (IL-5, G-CSF, TNF-α and KC) and liver injury parameters (ALT). Similarly, the proportion of Clostridium was negatively correlated with ALT, AST and TNF-α levels and positively correlated with the butyric acid concentration. The gut barrier marker ZO-1 was positively correlated with SCFA concentrations (propionic acid and butyric acid) but negatively correlated with proinflammatory cytokine levels (IL-6, MIP-1α, and TNF-α). Therefore, the correlation network analysis indicated that the modified gut microbiota, SCFAs and the gut barrier contributed to alleviating systemic inflammation and liver injury.
Based on the results of the present study, P. pentosaceus supplementation is effective at alleviating ethanol-induced liver inflammation and steatosis. The potential mechanisms were further investigated. Ethanol feeding results in intestinal dysbiosis and gut barrier dysregulation, while P. pentosaceus supplementation modulates the microbiota diversity and composition, improves gut barrier function and SCFA metabolism, and reduces endotoxin and proinflammatory cytokine levels, which might be associated with the protective effect on ethanol-induced liver injury.
Regulation of the intestinal microbiota with probiotics, prebiotics and fecal microbiota transplantation is another promising treatment option for ALD[19,20]. P. pentosaceus is widely used as a probiotic and is considered beneficial for health. In our study, P. pentosaceus CGMCC 7049 is a newly isolated strain of bacteria that was shown to be resistant to acid and bile salts, with a high tolerance to ethanol[21]. However, we had not yet determined whether P. pentosaceus exerts a protective effect on ALD and the potential mechanism. Therefore, this potential protective effect was further evaluated using chronic plus binge animal models[17].
Intestinal dysbiosis and gut barrier disruption result in the translocation of PAMPs, such as endotoxins, from the gut to the liver[22]. An increase in endotoxin levels in the systemic circulation activates the TLR pathway in Kupffer cells, and increased production of proinflammatory cytokines and chemokines result in liver inflammation[18,23]. In the present study, P. pentosaceus supplementation ameliorated liver inflammation and steatosis, decreased ALT, AST and TG levels, and reduced neutrophil infiltration. Alcohol feeding induced gut leakiness, increased the endotoxin level and upregulated the expression of the TLR4 mRNA in the liver. Consistent with these results, the levels of proinflammatory cytokines, including IL-5, IL-6, TNF-α, G-CSF, KC, MIP-1α and MCP-1, were significantly increased. Increased levels of cytokines, such as IL-6 and TNF-α, induce liver inflammation and fibrosis, and increased levels of chemokines recruit neutrophils to aggravate liver inflammation in patients with ALD[21]. Based on our findings, P. pentosaceus supplementation decreased endotoxin levels and downregulated TLR4 expression. In addition, the levels of proinflammatory cytokines, including IL-5, TNF-α, G-CSF, KC, MIP-1α and MCP-1, were significantly decreased. Chemokines such as MCP-1 and MIP-1α, which are upregulated in patients with alcoholic hepatitis[21], are responsible for the recruitment and activation of macrophages and monocytes and modulate the production of the proinflammatory cytokines TNF-α, IL-1β and IL-6[24]. Therefore, P. pentosaceus supplementation reduced the circulating endotoxin level and subsequently decreased the levels of proinflammatory cytokines and chemokines, which might explain the protective effect on liver inflammation.
The gut barrier plays a crucial role in preventing endotoxin translocation from the gut to the liver, and alcohol consumption disrupts the epithelial barrier and gut-vascular barrier and results in increased intestinal permeability in animal models and patients with ALD[22,25]. In our study, the mRNA expression of the tight junction protein ZO-1 was decreased after alcohol consumption, but the probiotic P. pentosaceus treatment increased the level of the ZO-1 protein. On the other hand, the intestinal mucus layer, which consists of mucins that are synthesized and secreted by goblet cells, is critical for protecting the epithelial barrier against colonization by pathogenic bacteria[25-27]. In our study, the mRNA expression of intestinal mucins, including MUC-1, MUC-2 and MUC-4, was significantly increased after P. pentosaceus supplementation. Antimicrobial peptides such as Reg3β and Reg3γ are secreted by epithelial cells and Paneth cells to inhibit bacterial overgrowth, and Reg3β peptides exert bacteriostatic effects on gram-negative bacteria and protect against pathogenic bacterial colonization[10,28,29]. Reg3β-deficient mice exhibit restricted bacterial overgrowth and translocation to prevent ethanol-induced liver injury[21]. Treatment with the probiotic P. pentosaceus significantly increased the expression of Reg3β mRNA in the present study, which may explain the inhibitory effect of this probiotic on intestinal bacterial overgrowth. Thus, the levels of tight junction proteins, mucin proteins and antimicrobial peptides were upregulated by probiotic supplementation to improve gut barrier function.
Excessive alcohol consumption induces changes in the gut microbiota composition and bacterial overgrowth and increases intestinal permeability and bacterial translocation from the gut to the liver to promote alcoholic steatohepatitis[22,23]. In the present study, the microbiota diversity and richness were reduced, and the microbial composition was also altered after alcohol consumption; the change in microbial diversity was partially reversed by P. pentosaceus treatment. The reduction in bacterial diversity was consistent with the finding that patients with alcoholic hepatitis have lower Shannon diversity and Chao richness indices[30]. In the present study, alcohol feeding increased the Firmicutes-to-Bacteroidetes ratio (F/B ratio), and P. pentosaceus supplementation restored the F/B ratio to a value similar to that of the control group. These results are consistent with the finding that Firmicutes and Bacteroidetes are the predominant phyla detected in patients with alcoholic hepatitis, and the F/B ratio is 127% higher than in healthy controls[30]. At the genus level, the abundance of Escherichia and Staphylococcus increased with alcohol consumption, which was related to intestinal inflammation. Escherichia are also enriched in patients with nonalcoholic steatohepatitis, liver cirrhosis and hepatocellular carcinoma[31-33]. Escherichia increase the production of endotoxins, secondary bile acids and endogenous ethanol to promote liver inflammation[34], and one possible explanation for the increase of Escherichia in patients with ALD might be its tolerance to ethanol[35]. Staphylococcus is an important pathogen that causes infection in patients with alcoholic liver cirrhosis and increases the risk of mortality[36]. According to a previous study, antimicrobial substances from P. pentosaceus are effective at inhibiting pathogenic Staphylococcus[37]. Consistent with this finding, the relative abundance of Staphylococcus was significantly decreased after P. pentosaceus supplementation in the present study. On the other hand, the relative abundance of Lactobacillus, Prevotella, Faecalibacterium, and Clostridium decreased with alcohol feeding. Supplementation with Lactobacillus rhamnosus GG promotes Lactobacillus expansion and is a beneficial treatment for alcohol-induced liver injury[38-41]. Likewise, P. pentosaceus administration restored the relative abundance of Lactobacillus and Pediococcus in the present study. In addition, Prevotella, Faecalibacterium and Clostridium are considered SCFA-producing bacteria, and their levels are also decreased in patients with alcoholic liver cirrhosis[42]. In the present study, P. pentosaceus supplementation partially restored the abundance of Prevotella and Clostridium. Akkermansia has been reported to be depleted and negatively correlated with the severity of ALD, and an Akkermansia treatment increases the number of goblet cells and the expression of mucin proteins to alleviate ethanol-induced liver injury[43]. An increasing trend in Akkermansia abundance was observed after P. pentosaceus supplementation, which exhibited a positive correlation with SCFA concentrations and gut barrier markers. Thus, probiotic supplementation may attenuate intestinal dysbiosis.
SCFAs, which are derived from dietary fiber fermentation by the commensal gut microbiota, provide an energy source for intestinal epithelial cells and regulate hepatic glucose and energy metabolism[43-45]. Butyrate-producing probiotic or butyrate supplementation improve intestinal permeability and inhibit HDAC1 expression to alleviate hepatic steatosis and injury[46-49]. In human studies, a reduction in SCFA concentrations, including acetic acid, propionic acid and butyric acid, was observed in patients with severe alcoholic hepatitis[43]. Similarly, the levels of SCFAs, including propionic acid, butyric acid, valeric acid, and 2-methylbutyric acid, were markedly decreased in the present study, accompanied by a reduction in the abundance of SCFA-producing bacteria, including Prevotella, Faecalibacterium and Clostridium, after alcohol feeding. However, P. pentosaceus administration increased the production of propionic acid and butyric acid by modifying the abundance of SCFA-producing bacteria, including Prevotella, Clostridium and Akkermansia, and the higher abundance of Clostridium and Akkermansia was also positively correlated with the SCFA concentrations. Based on these results, the increase in the abundance of SCFA-producing bacteria might increase the production of propionic acid and butyric acid, which are associated with an improvement in gut barrier function.
Nevertheless, our study had some potential limitations. Although the chronic plus binge ethanol feeding animal model mimics ethanol-induced liver inflammation and steatosis, liver fibrosis was not induced in this model[17]. The results of animal experiments have shown the protective effect of probiotic supplementation, but further human clinical studies are needed to confirm its safety and efficacy. Differentially abundant bacteria were identified at the genus level, but the exploration of taxa at the species level was not possible due to the depth of 16S rRNA sequencing[50]. Although P. pentosaceus supplementation modified the gut microbiota composition and SCFA metabolism, the causal relationship between the modified microbiota and the alleviation of liver injury was unclear, and the host microbiota and metabolism interaction requires further investigation[51].
In conclusion, P. pentosaceus supplementation was effective at alleviating ethanol-induced liver inflammation and steatosis by reversing gut microbiota dysbiosis, regulating SCFA metabolism, improving intestinal barrier function, and reducing circulating endotoxin and proinflammatory cytokine and chemokine levels. These results provide a theoretical basis for the use of P. pentosaceus CGMCC 7049 as a prospective probiotic treatment option for patients with ALD.
Gut microbiota dysbiosis plays an important role in the progression of ethanol-induced liver injury, and microbe-based therapy including probiotics, prebiotics and fecal microbiota transplantation, has emerged as a prospective treatment option for patients with alcoholic liver disease (ALD).
Pediococcus pentosaceus (P. pentosaceus) belongs to the Lactobacillaceae family and is widely used as a probiotic. P. pentosaceus CGMCC 7049 is a newly isolated strain of bacteria that has been shown to be resistant to acid and bile salts, with a high tolerance to ethanol. Moreover, further studies are needed to determine the effects of P. pentosaceus supplementation on ethanol-induced liver injury.
The aim of our study was to evaluate the protective effect of the probiotic P. pentosaceus on an experimental ALD model and to investigate the potential mechanisms.
P. pentosaceus CGMCC 7049 was isolated from healthy adults in our laboratory. The chronic plus binge NIAAA model was used to evaluate the protective effects. Mice were randomly divided into three groups: the control group received a pair-fed control diet and oral gavage with sterile phosphate buffer saline (PBS), the EtOH group received 5% ethanol Lieber-DeCarli diet and oral gavage with PBS, and the P. pentosaceus group received a 5% ethanol Lieber-DeCarli diet and P. pentosaceus treatment. Gut and liver tissue samples were harvested to assess the gut barrier function and liver injury-related parameters. Fresh cecal contents were collected for the 16S rRNA gene sequencing and short-chain fatty acid (SCFA) analyses.
The P. pentosaceus treatment improved ethanol-induced liver injury by reducing alanine aminotransferase, aspartate transaminase and triglyceride levels, and neutrophil infiltration, which was accompanied by decreased levels of endotoxin and inflammatory cytokines. In addition, P. pentosaceus administration increased the expression of a tight junction protein, mucin proteins and antibacterial peptides to improve the gut barrier function. Ethanol administration induced intestinal dysbiosis and increased the relative abundance of pathogenic Escherichia and Staphylococcus but depleted SCFA-producing bacteria. In contrast, P. pentosaceus treatment increased the microbial diversity and restored the relative abundance of SCFA-producing bacteria, such as Prevotella, Clostridium and Akkermansia, and increased the production of propionic acid and butyric acid.
Based on the results of the present study, the newly isolated strain of P. pentosaceus was an effective treatment that protected against ethanol-induced liver injury by modulating the gut microbiota and improving SCFA metabolism and gut barrier function.
An ethanol-resistant strain of probiotic P. pentosaceus alleviated ethanol-induced liver injury in a chronic plus binge animal model, which might represent a promising microbe-based therapy for patients with ALD.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Miyoshi E, Yi SQ S-Editor: Gao CC L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 538] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 2. | Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 320] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1496] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 4. | Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, Puchois V, Martin JC, Lepage P, Le Roy T, Lefèvre L, Langelier B, Cailleux F, González-Castro AM, Rabot S, Gaudin F, Agostini H, Prévot S, Berrebi D, Ciocan D, Jousse C, Naveau S, Gérard P, Perlemuter G. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 424] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 5. | Ciocan D, Voican CS, Wrzosek L, Hugot C, Rainteau D, Humbert L, Cassard AM, Perlemuter G. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment Pharmacol Ther. 2018;48:961-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS, Alexeev DG, Taraskina AY, Nasyrova RF, Krupitsky EM, Shalikiani NV, Bakulin IG, Shcherbakov PL, Skorodumova LO, Larin AK, Kostryukova ES, Abdulkhakov RA, Abdulkhakov SR, Malanin SY, Ismagilova RK, Grigoryeva TV, Ilina EN, Govorun VM. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 7. | Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966-G978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 595] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 8. | Casafont Morencos F, de las Heras Castaño G, Martín Ramos L, López Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1996;41:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 638] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 10. | Wang L, Fouts DE, Stärkel P, Hartmann P, Chen P, Llorente C, DePew J, Moncera K, Ho SB, Brenner DA, Hooper LV, Schnabl B. Intestinal REG3 Lectins Protect against Alcoholic Steatohepatitis by Reducing Mucosa-Associated Microbiota and Preventing Bacterial Translocation. Cell Host Microbe. 2016;19:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 293] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 11. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 395] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 12. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 13. | Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol. 2019;70:260-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 14. | Yao MF, Li B, Ye HW, Huang WH, Luo QX, Xiao H, McClements DJ, Li LJ. Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles. Food Hydrocolloid. 2018;83:246-252. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Xu Q, Gu S, Chen Y, Quan J, Lv L, Chen D, Zheng B, Xu L, Li L. Protective Effect of Pediococcus pentosaceus LI05 Against Clostridium difficile Infection in a Mouse Model. Front Microbiol. 2018;9:2396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Wu ZW, Lu HF, Wu J, Zuo J, Chen P, Sheng JF, Zheng SS, Li LJ. Assessment of the fecal lactobacilli population in patients with hepatitis B virus-related decompensated cirrhosis and hepatitis B cirrhosis treated with liver transplant. Microb Ecol. 2012;63:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc. 2013;8:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 903] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 18. | Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 746] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 19. | Ferrere G, Wrzosek L, Cailleux F, Turpin W, Puchois V, Spatz M, Ciocan D, Rainteau D, Humbert L, Hugot C, Gaudin F, Noordine ML, Robert V, Berrebi D, Thomas M, Naveau S, Perlemuter G, Cassard AM. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2017;66:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 20. | Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, Kumar G, Sharma MK, Maiwall R, Jindal A, Choudhary A, Hussain MS, Sharma S, Sarin SK. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017;15:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 21. | Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Tuomisto S, Pessi T, Collin P, Vuento R, Aittoniemi J, Karhunen PJ. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol. 2014;14:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 469] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 24. | Esche C, Stellato C, Beck LA. Chemokines: key players in innate and adaptive immunity. J Invest Dermatol. 2005;125:615-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 25. | Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, Stärkel P, Belzer C, Hellerbrand C, Tsukamoto H, Ho SB, Schnabl B. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 26. | Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064-15069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1534] [Article Influence: 90.2] [Reference Citation Analysis (1)] |
| 27. | Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1222] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 28. | Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1080] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 29. | Miki T, Holst O, Hardt WD. The bactericidal activity of the C-type lectin RegIIIβ against Gram-negative bacteria involves binding to lipid A. J Biol Chem. 2012;287:34844-34855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Smirnova E, Puri P, Muthiah MD, Daitya K, Brown R, Chalasani N, Liangpunsakul S, Shah VH, Gelow K, Siddiqui MS, Boyett S, Mirshahi F, Sikaroodi M, Gillevet P, Sanyal AJ. Fecal Microbiome Distinguishes Alcohol Consumption From Alcoholic Hepatitis But Does Not Discriminate Disease Severity. Hepatology. 2020;72:271-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 31. | Fukui H. Improve gut microbiome: a new horizon of cancer therapy. Hepatobiliary Surg Nutr. 2017;6:424-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1283] [Article Influence: 106.9] [Reference Citation Analysis (1)] |
| 33. | Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 454] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 34. | Fukui H. Role of Gut Dysbiosis in Liver Diseases: What Have We Learned So Far? Diseases. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 35. | Ingram LO, Vreeland NS, Eaton LC. Alcohol tolerance in Escherichia coli. Pharmacol Biochem Behav. 1980;13 Suppl 1:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Kang CI, Song JH, Ko KS, Chung DR, Peck KR; Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group. Clinical significance of Staphylococcus aureus infection in patients with chronic liver diseases. Liver Int. 2010;30:1333-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Jang S, Lee D, Jang IS, Choi HS, Suh HJ. The Culture of Pediococcus pentosaceus T1 Inhibits 
Listeria Proliferation in Salmon Fillets and Controls Maturation of Kimchi. Food Technol Biotechnol. 2015;53:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Chen P, Torralba M, Tan J, Embree M, Zengler K, Stärkel P, van Pijkeren JP, DePew J, Loomba R, Ho SB, Bajaj JS, Mutlu EA, Keshavarzian A, Tsukamoto H, Nelson KE, Fouts DE, Schnabl B. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015;148:203-214.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 39. | Wang YH, Kirpich I, Liu YL, McClain CJ, Feng WK. Lactobacillus GG Treatment increases Intestinal Barrier Protecting Factors and Ameliorates Alcohol-Induced Intestinal Dysfunction and Alcoholic Hepatosteatosis. Gastroenterology. 2011;140:S982-S983. [DOI] [Full Text] |
| 40. | Wang Y, Kirpich I, Liu Y, Ma Z, Barve S, McClain CJ, Feng W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol. 2011;179:2866-2875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 41. | Zhao H, Zhao C, Dong Y, Zhang M, Wang Y, Li F, Li X, McClain C, Yang S, Feng W. Inhibition of miR122a by Lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicol Lett. 2015;234:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker BR. Fiber-utilizing capacity varies in Prevotella- vs Bacteroides-dominated gut microbiota. Sci Rep. 2017;7:2594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 379] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 43. | Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, Enrich B, Ciocan D, Macheiner S, Mayr L, Drach M, Moser P, Moschen AR, Perlemuter G, Szabo G, Cassard AM, Tilg H. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 457] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 44. | Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P, Geier A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United European Gastroenterol J. 2018;6:1496-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 45. | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1566] [Cited by in RCA: 2373] [Article Influence: 263.7] [Reference Citation Analysis (0)] |
| 46. | Cresci GA, Glueck B, McMullen MR, Xin W, Allende D, Nagy LE. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J Gastroenterol Hepatol. 2017;32:1587-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Donde H, Ghare S, Joshi-Barve S, Zhang J, Vadhanam MV, Gobejishvili L, Lorkiewicz P, Srivastava S, McClain CJ, Barve S. Tributyrin Inhibits Ethanol-Induced Epigenetic Repression of CPT-1A and Attenuates Hepatic Steatosis and Injury. Cell Mol Gastroenterol Hepatol. 2020;9:569-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Seo B, Jeon K, Moon S, Lee K, Kim WK, Jeong H, Cha KH, Lim MY, Kang W, Kweon MN, Sung J, Kim W, Park JH, Ko G. Roseburia spp. Abundance Associates with Alcohol Consumption in Humans and Its Administration Ameliorates Alcoholic Fatty Liver in Mice. Cell Host Microbe. 2020;27:25-40.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 49. | Han Y, Glueck B, Shapiro D, Miller A, Roychowdhury S, Cresci GAM. Dietary Synbiotic Supplementation Protects Barrier Integrity of Hepatocytes and Liver Sinusoidal Endothelium in a Mouse Model of Chronic-Binge Ethanol Exposure. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Poretsky R, Rodriguez-R LM, Luo C, Tsementzi D, Konstantinidis KT. Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS One. 2014;9:e93827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (1)] |
| 51. | Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3236] [Article Influence: 248.9] [Reference Citation Analysis (0)] |