Published online May 28, 2020. doi: 10.3748/wjg.v26.i20.2570
Peer-review started: January 1, 2020
First decision: January 19, 2020
Revised: March 2, 2020
Accepted: May 13, 2020
Article in press: May 13, 2020
Published online: May 28, 2020
Processing time: 148 Days and 11 Hours
Circulating microRNAs (miRNAs) are potential biomarkers for many diseases. However, they can originate from non-disease specific sources, such as blood cells, and compromise the investigations for miRNA biomarkers. While small extracellular vesicles (sEVs) have been suggested to provide a purer source of circulating miRNAs for biomarkers discovery, the most suitable blood sample for sEV miRNA biomarker studies has not been defined.
To compare the miRNA profiles between matched serum and plasma sEV preparations to determine their suitability for biomarker studies.
Matched serum and plasma samples were obtained from 10 healthy controls and 10 patients with esophageal adenocarcinoma. sEV isolates were prepared from serum and plasma using ExoQuickTM and quantified using NanoSight. RNA was extracted from sEV preparations with the miRNeasy Serum/Plasma kit and profiled using the Taqman Openarray qPCR. The overall miRNA content and the expression of specific miRNAs of reported vesicular and non-vesicular origins were compared between serum and plasma sEV preparations. The diagnostic performance of a previously identified multi-miRNA biomarker panel for esophageal adenocarcinoma was also compared.
The overall miRNA content was higher in plasma sEV preparations (480 miRNAs) and contained 97.5% of the miRNAs found in the serum sEV preparations (412 miRNAs).The expression of commonly expressed miRNAs was highly correlated (Spearman’s R = 0.87, P < 0.0001) between the plasma and serum sEV preparations, but was consistently higher in the plasma sEV preparations. Specific blood-cell miRNAs (hsa-miR-223-3p, hsa-miR-451a, miR-19b-3p, hsa-miR-17-5p, hsa-miR-30b-5p, hsa-miR-106a-5p, hsa-miR-150-5p and hsa-miR-92a-3p) were expressed at 2.7 to 9.6 fold higher levels in the plasma sEV preparations compared to serum sEV preparations (P < 0.05). In plasma sEV preparations, the percentage of protein-associated miRNAs expressed at relatively higher levels (Ct 20-25) was greater than serum sEV preparations (50% vs 31%). While the percentage of vesicle-associated miRNAs expressed at relatively higher levels was greater in the serum sEV preparations than plasma sEV preparations (70% vs 44%). A 5-miRNA biomarker panel produced a higher cross validated accuracy for discriminating patients with esophageal adenocarcinoma from healthy controls using serum sEV preparations compared with plasma sEV preparations (AUROC 0.80 vs 0.54, P < 0.05).
Although plasma sEV preparations contained more miRNAs than serum sEV preparations, they also contained more miRNAs from non-vesicle origins. Serum appears to be more suitable than plasma for sEV miRNAs biomarkers studies.
Core tip: Current evidence suggests that circulating small extracellular vesicles (sEVs) function as delivery cargo shuttles for various molecules. MicroRNAs are small non-coding RNAs with important roles in the regulation of gene expression, are often dysregulated in diseases, and are relatively stable in the circulation. MicroRNAs circulating in sEVs are consequently considered as highly suitable candidates for use as non-invasive biomarkers. Extracellular vesicle preparations derived from serum and plasma are recognised to be enriched in sEVs, but not purely comprised of them. Most circulating sEV microRNA biomarker studies have used plasma, but here we show that sEVs isolated from serum are less contaminated with blood cell and protein-associated microRNAs.
- Citation: Chiam K, Mayne GC, Wang T, Watson DI, Irvine TS, Bright T, Smith LT, Ball IA, Bowen JM, Keefe DM, Thompson SK, Hussey DJ. Serum outperforms plasma in small extracellular vesicle microRNA biomarker studies of adenocarcinoma of the esophagus. World J Gastroenterol 2020; 26(20): 2570-2583
- URL: https://www.wjgnet.com/1007-9327/full/v26/i20/2570.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i20.2570
MicroRNAs (miRNAs) are small non-coding RNA molecules (21-23 nucleotides) that can regulate gene expression via various mechanisms including repression of messenger RNA translation. miRNAs are important regulators because a single miRNA can target multiple genes. Furthermore, specific miRNA expression signatures have been shown to be tissue-specific[1], and disease-specific[2-4]. miRNAs are found in a range of body fluids such as serum, plasma, whole blood, urine and saliva. These circulating miRNAs are highly stable in different conditions (e.g., temperature, pH and storage period) and can be easily measured. For these reasons, circulating miRNAs have garnered significant research interest as potential biomarkers for diagnostic, prognostic and treatment prediction purposes.
However, there are many challenges in the process of biomarker discovery to clinical practice for circulating miRNAs. Various factors can influence the quality and outcome of biomarker studies, which include the choice of sample, processing conditions, biomarker detection and analysis methods[5,6]. There is also increasing awareness about the multiple origins of specific circulating miRNAs and the implications this has on how we should evaluate and interpret miRNAs biomarkers studies[7-10]. A study by Pritchard et al[8] highlighted that a large proportion of circulating miRNA cancer biomarkers identified in the literature overlapped with those that have been reported to be highly expressed in blood cells. This has raised concerns on factors such as hemolysis and whether different types of blood samples used in miRNA studies may vary in their content of miRNAs originating from blood-cells.
Small extracellular microvesicles (sEVs), are considered to be a more stable and disease-specific source of circulating miRNAs for biomarker development[11]. In cancers, circulating miRNAs encapsulated in sEVs have been shown to have critical functional roles such as regulating disease progression, metastasis and sensitivity to specific drugs[12]. Circulating miRNAs can also be found complexed with the Argonaute2 (Ago2) protein, which functions to protect the miRNAs against RNases and enhance their stability in the circulation[13,14]. Although these protein-associated circulating miRNAs have been found to be present in larger quantities than sEV miRNAs, their functional roles in disease pathogenesis and potential utility as biomarkers have not been investigated. Thus far, the focus remains on circulating sEV miRNAs as preferred candidates for biomarkers development and protocol-related studies[11,15-17].
A crucial step in the development of robust circulating miRNAs biomarkers is to determine which blood sample is optimum for the study. This is a challenging question to address due to the multiple origins of circulating miRNAs and experimental factors that can influence miRNA levels. Previous studies have endeavoured to address this question by comparing circulating miRNA profiles, mostly of cell-free miRNAs across different blood samples, and have reported inconsistent results[10,11,16,18,19]. There are only limited studies that have comprehensively investigated and reported sEV miRNAs profiles between different blood samples[11,13]. In this study, we compared miRNA profiles between matched serum and plasma sEV preparations, collected from healthy controls and patients with esophageal adenocarcinoma, for the presence of reported specific vesicular and non-vesicular miRNAs. We also compared the performance of a previously identified multi-biomarker panel (comprising of 5 sEV miRNA ratios)[20], between serum and plasma sEV preparations, to discriminate patients with esophageal adenocarcinoma from the healthy individuals.
Individuals visiting Flinders Medical Centre (Adelaide, South Australia) and the Royal Adelaide Hospital (Adelaide, South Australia) for endoscopy procedures and management of esophageal cancer were recruited for a biomarker research study. Ethical approval was obtained from the Southern Adelaide Clinical Human Research Ethics Committee and the Royal Adelaide Hospital Research Committee. All individuals provided written informed consent for blood and personal data collection for research purposes. The study was conducted in accordance with the Declaration of Helsinki’s (2008) statement for the ethical principles for medical research involving human subjects.
Blood samples from 10 healthy controls (median age 56.5 ± 10) and 10 patients with locally advanced esophageal adenocarcinoma (median age 59.5 ± 7) were used. The individuals were previously part of a larger biomarker study for esophageal adenocarcinoma[20]. The “healthy controls” all underwent endoscopy with biopsies and were not identified as having Barrett’s esophagus, gastroesophageal reflux disease, or cancer. Only individuals with no endoscopic or histological abnormality were included in the control group. Matched serum and plasma samples from each individual was collected at the same time prior to their endoscopy procedure. Blood was collected from the patients with cancer prior to any treatment. Collection was performed with 8 mL Z Serum Separator Clot Activator tubes Vacuette® (cat# 455078) and 9 mL K3E K3EDTA tubes Vacuette® (cat# 455036) respectively.
All blood samples were left at room temperature for a period of 16-24 h before processing with a standardised protocol established in our laboratory. Serum was collected via centrifugation of blood at 650 g for 15 min and stored as 1 mL aliquots at -80 °C for later use. Plasma was collected via centrifugation at 650 g for 15 min to separate the plasma supernatant from the red blood cells and buffy coat containing white blood cells. The top clear layer of plasma supernatant was transferred to a fresh 10 mL tube (Techno-Plas Pty Ltd., Australia; cat# S9716-V06) for a second centrifugation at 650 g for 15 min and the supernatant was stored as 1 mL aliquots at -80 °C for later use.
For extracellular vesicle isolation, aliquots (1 mL) of the matched serum and plasma from the 10 healthy controls and 10 patients with esophageal adenocarcinoma were retrieved from -80 °C and quick thawed. The aliquots were centrifuged at 16000 g at 4 °C for 30 min to exclude large microparticles. Two hundred and fifty microliter supernatants from each sample was processed with the ExoQuickTM kit (System Biosciences, CA, United States; EXOQ20A-1) according to the manufacturer’s protocol. All samples were incubated with ExoQuickTM at 4 °C for 16 h. The extracellular vesicle pellet isolated from each sample was resuspended with 50 µL phosphate buffered saline.
The size and concentration of extracellular vesicles isolated from each sample was measured using a NanoSight LM10 Nanoparticle Analysis System and Nanoparticle Tracking Analysis Software (NanoSight Ltd., Malvern, United Kingdom). One microliter of vesicle suspension was serially diluted in pre-filtered phosphate buffered saline to a dilution factor of 1:3200 for the NanoSight measurement. This dilution factor was determined in the laboratory to achieve an average particle concentration range of 108-109/mL for our samples, which is the optimal measurement range recommended by the manufacturer’s protocol. The diluted sample was injected into the NanoSight instrument sample inlet port and a 60 s video were captured for measurement. The measurements were performed in triplicate for each diluted sample by re-injecting the same sample into the sample inlet port. Average particle size and concentration for each sample was evaluated using the batch-processing settings within the NTA software.
The miRNeasy Serum/Plasma kit (QIAGEN, #217184) was used according to the manufacturer’s protocol. After the addition of 500 µL QIAzol Lysis reagent to each vesicle pellet, 5 µL (0.1 picomole) of each of the synthetic RNA molecules ath-miR-159a and cel-miR-54 were added (Shanghai Genepharma Co. Ltd.). The final RNA elution from each sample was performed with 24 µL of RNase-free ultrapure water.
The Taqman® OpenArray® Human microRNA panel (Life technologies, #4461104) was used to profile the expression of 758 miRNAs. The detailed steps for the miRNA profiling were as previously described[20]. The profiling was performed using the Biotrove OpenArray NT cycler at the Flinders Genomics Facility (Flinders University, South Australia). The Realtime PCR Statminer® software (v4.5, Integromics) was used to assess the miRNA expression as cycle threshold (Ct) value per assay. The relative miRNA expression was calculated as 2(40-Ct). The data has been submitted to the Gene Expression Omnibus website (GSE142855).
Wilcoxon signed-rank test was used to investigate the pairwise differences between the matched serum and plasma samples of individual. This included comparisons on the particle concentrations, number of miRNAs detected and relative expression of specific miRNA. Correlation was assessed using the Spearman’s rank correlation coefficient. The diagnostic accuracy of a previously identified 5-miRNA ratio panel[20] was determined using leave-one-out cross-validation and receiver-operating characteristics (ROC) curve analysis. Statistical significance was defined by a P value < 0.05. Statistical analyses were performed using Stata software version 13.1 (StataCorp, College station, TX, United States) and IBM® SPSS® Statistics software version 25.
The Nanosight system was used to compare the profiles of particles isolated from the matched serum and plasma of healthy individuals. The main population of particles isolated from serum and plasma were similar in size, at 97.7 ± 3.3 nm and 93.1 ± 3.1 nm respectively (Figure 1A). The range of particle sizes detected in the samples, including those from the cancer patients (Supplementary Figure 1A), were consistent with the reported sizes of exosomes (30-150 nm)[16,21,22]. To be consistent with the Minimal Information for Studies of Extracellular Vesicles 2018 guidelines, we refer here to the majority particle population in the preparations as “small extracellular vesicles (sEVs)” , while noting that a minor population of “medium-large extracellular vesicles (m/lEVs)” were also detected[23]. The average concentration of particles from healthy controls was 1.2-fold higher in the serum sEV preparations compared to the matched plasma sEV preparations (Wilcoxon signed-rank test, P = 0.047) (Figure 1B). However, there was no statistical difference in the yield of particles in the matched serum sEV preparations and plasma sEV preparations from the cancer patients (Wilcoxon signed-rank test, P = 0.56) (Supplementary Figure 1B).
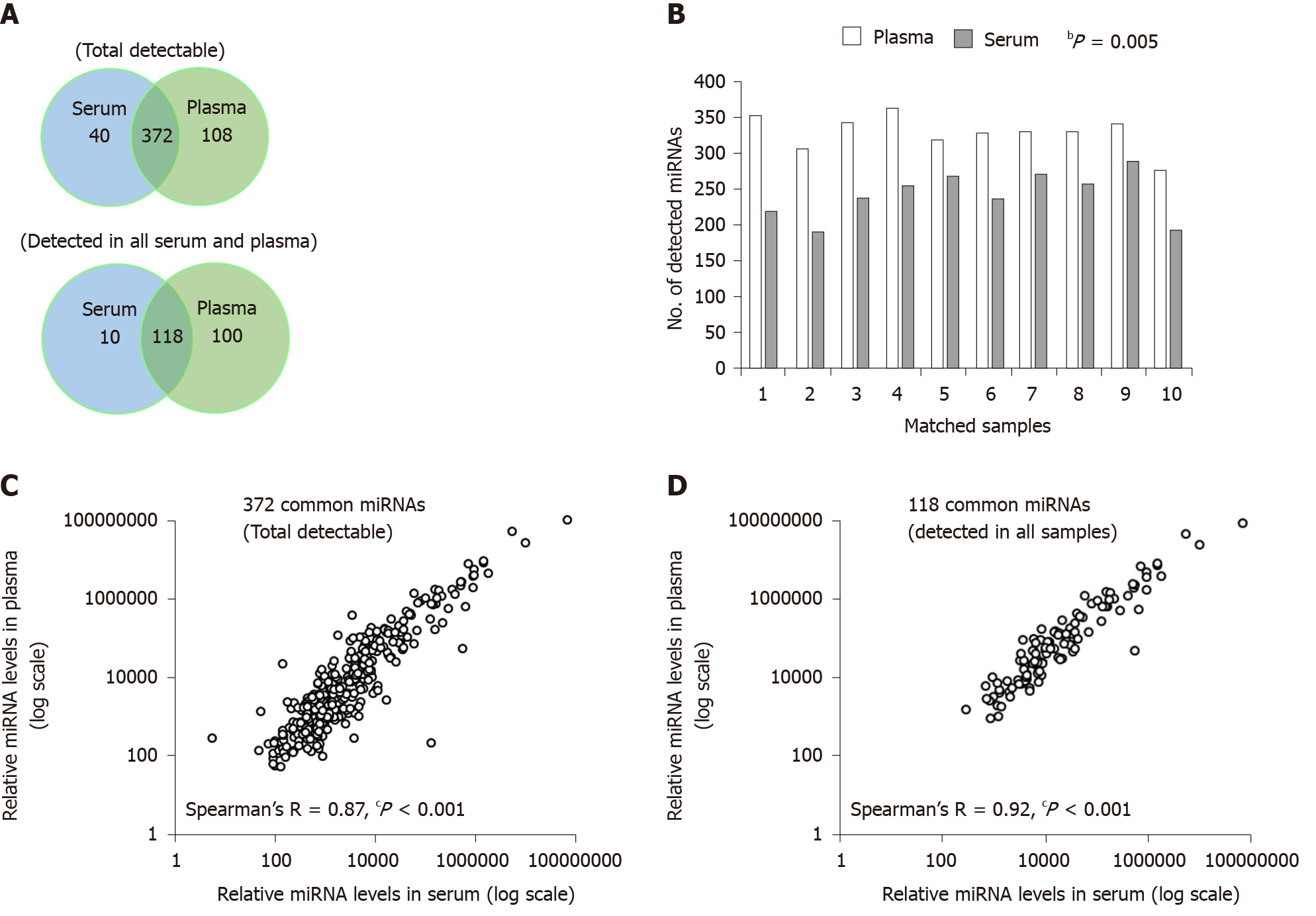
The number of miRNAs detected was greater in plasma sEV preparations than serum sEV preparations, either for those detected in each sample (total detectable), or for those detected in all samples (Figure 2A). In plasma sEV preparations, 480 miRNAs were detected, and 45.4% (218 miRNAs) of these were robustly expressed in all samples. In serum sEV preparations, 412 miRNAs were detected and 31.1% (128 miRNAs) of these were robustly expressed in all samples. Pairwise comparison of the number of total miRNAs that were detectable was consistently higher in the plasma sEV preparations (Wilcoxon signed-rank test, P = 0.005) (Figure 2B). The number of miRNAs unique to plasma sEV preparations was also greater than the number of miRNAs unique to serum sEV preparations (108 vs 40). Furthermore, a large proportion of the miRNAs unique to plasma sEV preparations were expressed in at least 50% of the cohort (at least 5 out of 10 samples) (Supplementary Table 1). While for miRNAs unique to serum sEV preparations, only 1 miRNA (hsa-miR-1233) was expressed in at least 50% of the cohort.
| Rank | Plasma miRNA | Relative levels ± SD (× 105) | Serum miRNA | Relative levels ± SD (× 105) |
| 1 | hsa-miR-223-3p1 | 521.3 ± 310.6 | hsa-miR-92a-3p1 | 100.2 ± 178.0 |
| 2 | hsa-miR-92a-3p1 | 268.7 ± 188.5 | hsa-miR-223-3p1 | 53.8 ± 24.8 |
| 3 | hsa-miR-20a-5p1 | 89.8 ± 38.3 | hsa-miR-451a1 | 17.6 ± 22.1 |
| 4 | hsa-miR-19b-3p1 | 83.2 ± 40.1 | hsa-miR-19b-3p1 | 14.7 ± 15.8 |
| 5 | hsa-miR-24-3p1 | 79.0 ± 44.7 | hsa-miR-20a-5p1 | 14.6 ± 14.3 |
| 6 | hsa-miR-30a-5p1 | 54.8 ± 31.1 | hsa-miR-30a-5p1 | 9.4 ± 11.0 |
| 7 | hsa-miR-451a1 | 43.3 ± 22.0 | hsa-miR-320a-3p1 | 9.3 ± 13.5 |
| 8 | hsa-miR-320a-3p1 | 40.1 ± 17.5 | hsa-miR-328-3p1 | 9.1 ± 3.7 |
| 9 | hsa-miR-484 | 36.7 ± 23.4 | hsa-miR-24-3p1 | 7.2 ± 3.2 |
| 10 | hsa-miR-17-5p1 | 27.2 ± 12.6 | hsa-miR-1274b | 6.4 ± 3.9 |
| 11 | hsa-miR-106a-5p1 | 26.0 ± 11.7 | RNU6-1 | 5.5 ± 4.0 |
| 12 | hsa-miR-16-5p1 | 21.2 ± 20.8 | hsa-miR-106a-5p1 | 5.4 ± 4.2 |
| 13 | hsa-miR-328-3p1 | 19.2 ± 5.4 | hsa-miR-16-5p1 | 5.1 ± 8.8 |
| 14 | hsa-miR-130a-3p | 17.5 ± 7.2 | hsa-miR-17-5p1 | 5.0 ± 3.9 |
| 15 | hsa-miR-30c-5p | 17.1 ± 11.6 | hsa-miR-150-5p1 | 4.0 ± 2.1 |
| 16 | hsa-miR-146a-5p1 | 16.2 ± 12.0 | hsa-miR-517a-3p | 2.8 ± 2.4 |
| 17 | hsa-miR-221-3p | 13.4 ± 7.2 | hsa-miR-30b-5p1 | 2.1 ± 1.2 |
| 18 | hsa-miR-150-5p1 | 13.3 ± 8.5 | hsa-miR-146a-5p1 | 1.8 ± 1.1 |
| 19 | has-hsa-miR-30b-5p1 | 11.5 ± 6.6 | hsa-miR-191-5p1 | 1.7 ± 0.9 |
| 20 | hsa-miR-191-5p1 | 10.6 ± 5.9 | hsa-miR-25-3p | 1.6 ± 2.3 |
The majority of the miRNAs detected in serum sEV preparations were also detected in plasma sEV preparations. 372 miRNAs were commonly expressed between serum and plasma sEV preparations, which represented 90.3% of the total miRNA content in serum sEV preparations. Of these, 118 miRNAs were commonly expressed in all serum and plasma sEV preparations. The relative expression of the 372 commonly expressed miRNAs was significantly correlated (Spearman’s R = 0.87, P < 0.0001) (Figure 2C). There was a stronger correlation among the common 118 miRNAs expressed in all serum and plasma sEV preparations (Spearman’s R = 0.92, P < 0.0001) (Figure 2D). Similar observations of the overall miRNA content were found in the matched serum and plasma sEV preparations from the patients with esophageal adenocarcinoma (Supplementary Figure 2), although the overall number of miRNAs were higher in the sEV preparations from the cancer patients compared to healthy individuals.
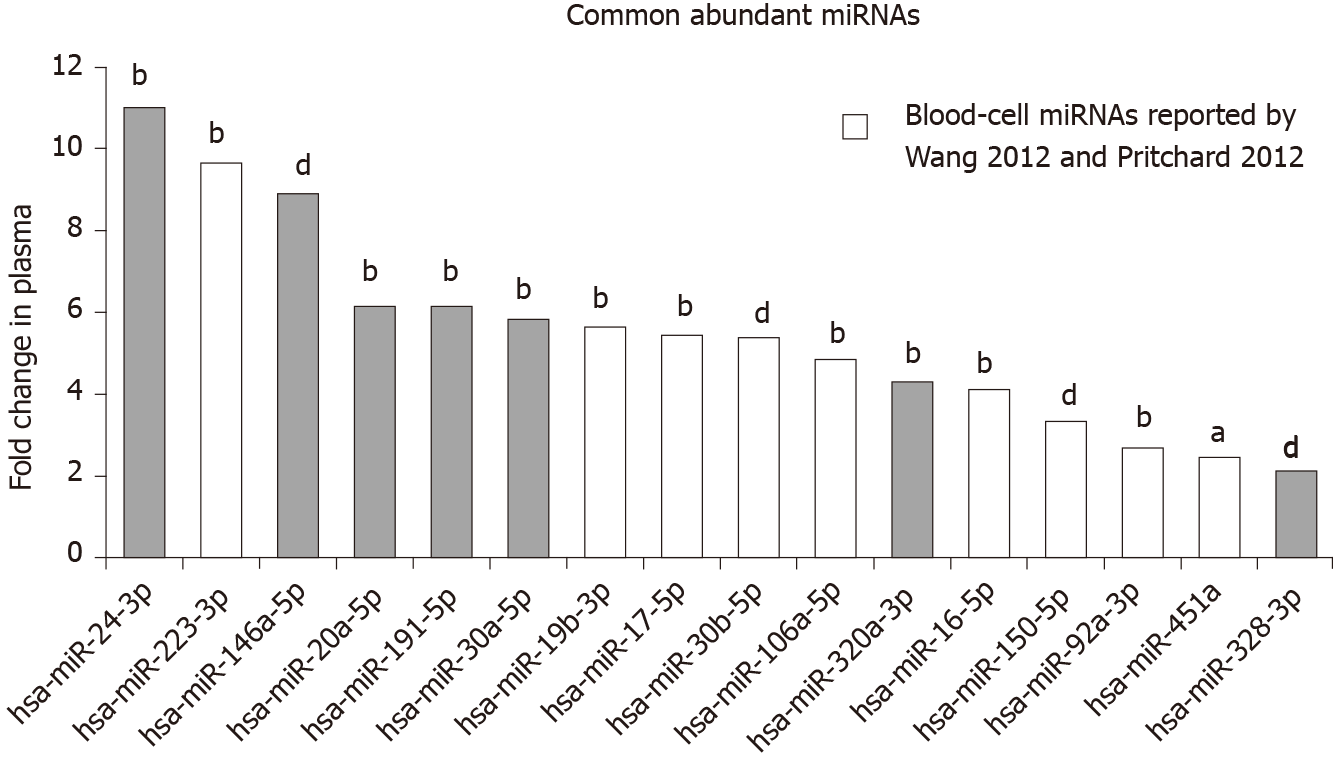
The top 20 most abundant miRNAs expressed in the serum and plasma sEV preparations were compared (Table 1). 16 out of 20 of the most abundant miRNAs were common between serum and plasma sEV preparations. However, the expression levels of the 16 common miRNAs were 2 to 11-fold higher in the plasma sEV preparations compared to serum sEV preparations (Wilcoxon signed-rank test, P < 0.05) (Figure 3). Of the 20 most highly expressed miRNAs in plasma sEV preparations, hsa-miR-484, hsa-miR-130a-3p, hsa-miR-30c-5p and hsa-miR-221-3p were not detected in serum sEV preparations. Of the 20 miRNAs that were highly expressed in serum sEV preparations, hsa-miR-1274b, RNU6-1, hsa-miR-517a-3p and hsa-miR-25-3p were not detected in plasma sEV preparations.
The presence of miRNAs reported by Wang et al[10], 2012, and Pritchard et al[8], 2012, to be highly expressed or uniquely expressed in blood cells was examined in our serum and plasma derived sEV preparations. Both Wang et al[10] and Pritchard et al[8] identified hsa-miR-223-3p and hsa-miR-451a as highly abundant in blood cells. Wang et al[10] reported 27 miRNAs that were uniquely expressed in blood cells. Pritchard et al[8] 2012 reported 44 additional miRNAs that were highly expressed in blood cells.
Both hsa-miR-223-3p and hsa-miR-451a were found to be among the top 20 most highly expressed miRNAs in our serum and plasma sEV preparations (Figure 3). Compared to serum sEV preparations, hsa-miR-223-3p was expressed at 9.6-fold higher in plasma sEV preparations (Wilcoxon signed-rank test, P = 0.0051), while hsa-miR-451a was expressed at 2.5-fold higher in plasma sEV preparations (Wilcoxon signed-rank test, P = 0.01). An additional 6 blood-cell miRNAs were identified in the top 20 most highly expressed miRNAs as consistently expressed at higher levels in plasma sEV preparations compared to serum sEV preparations (2.7 to 5.6 fold; hsa-miR-19b-3p, hsa-miR-17-5p, hsa-miR-30b-5p, hsa-miR-106a-5p, hsa-miR-150-5p and hsa-miR-92a-3p; Figure 3). In addition, we identified 4 blood-cell miRNAs (hsa-miR-98-5p, hsa-miR-30d-3p, hsa-miR-146b-3p and hsa-miR-19b-1-5p) that were robustly expressed in at least 50% of the plasma sEV preparations, that were not expressed in the serum sEV preparations (Supplementary Table 1).
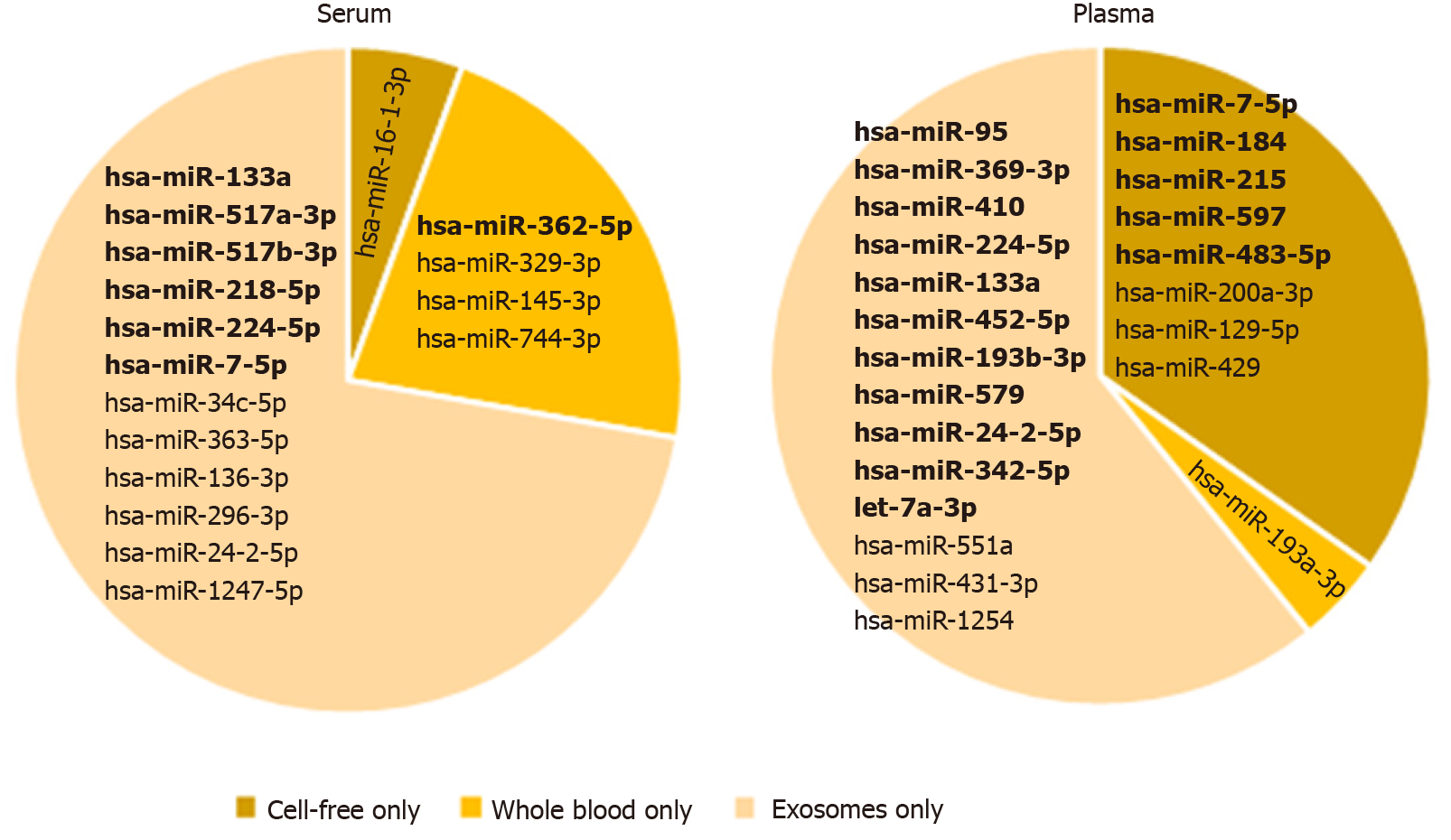
The presence of unique vesicular miRNAs, whole blood miRNAs (blood-cell miRNAs) and cell-free miRNAs (protein-associated miRNAs) reported by Cheng et al[11] were compared in our plasma and serum sEV preparations (Figure 4). Overall, we detected 12 of Cheng et al[11]’s unique vesicular miRNAs in our serum sEV preparations, and 14 of Cheng et al[11]’s unique vesicular miRNAs in our plasma sEV preparations (Figure 4). Smaller numbers of Cheng et al[11]’s unique whole blood miRNAs and cell-free miRNAs were detected in our plasma and serum sEV preparations (Figure 4). Several of these unique miRNAs were detected in only a small number of serum or plasma sEV preparations. We therefore identified those that were more reliably and robustly expressed in at least 50% of samples. In serum derived preparations, 6 unique vesicular miRNAs and only 1 unique whole blood miRNA were robustly expressed. In comparison, there were more unique vesicular miRNAs (11 miRNAs) and cell-free miRNAs (5 miRNAs) robustly expressed in plasma derived preparations. These observations were consistent in the matched samples from patients with esophageal adenocarcinoma (Supplementary Table 2).
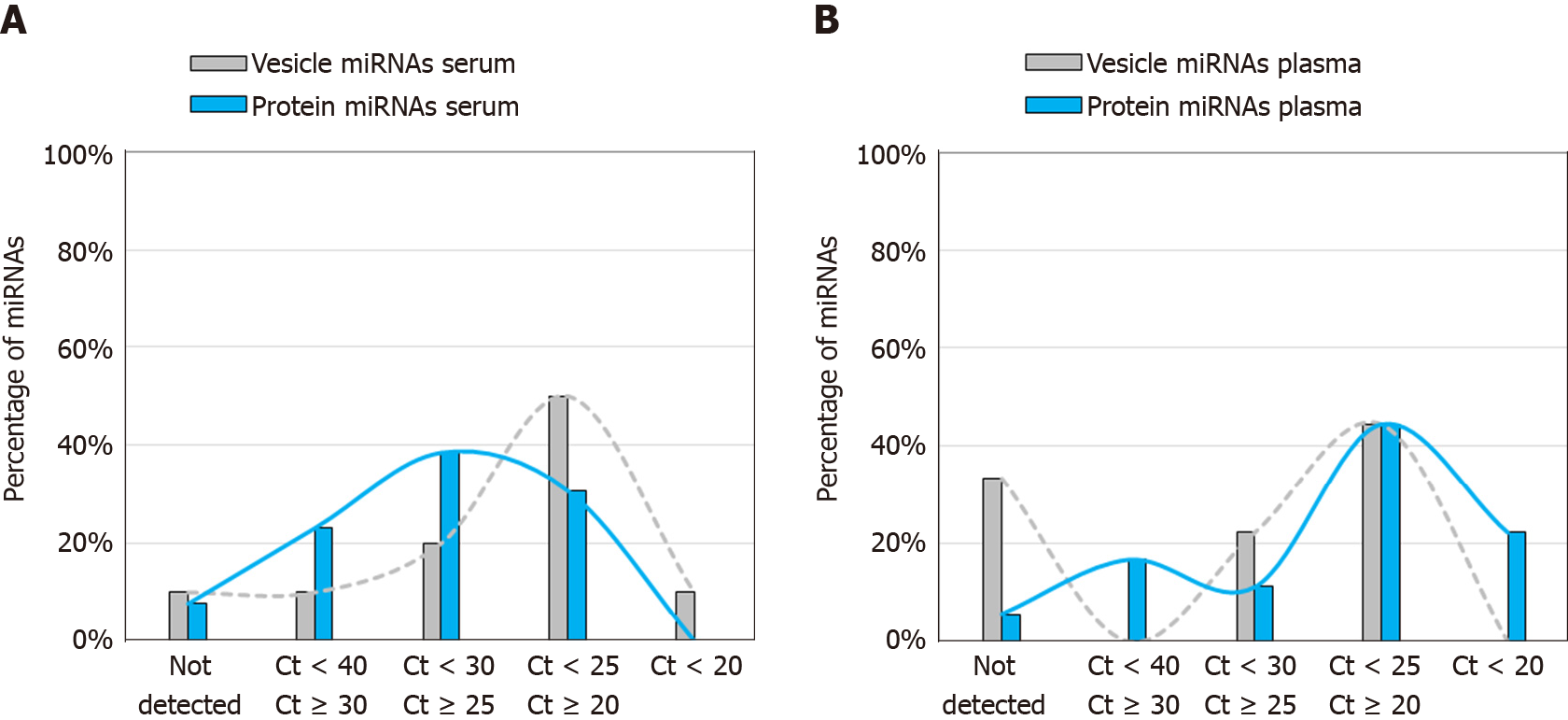
We next evaluated for the presence of vesicle-associated miRNAs and protein-associated miRNAs reported by Arroyo et al[13] (Figure 5). The list of miRNAs that were assessable on the TaqMan OpenArray platform are provided in Supplementary Tables 3 and 4. To investigate the relative expression levels of these miRNAs in serum and plasma sEV preparations, we partitioned them into the following 5 bins using their qPCR Ct’s: bin-1, not detected; bin-2, 40 > Ct ≥ 30; bin-3, 30 > Ct ≥ 25; bin-4, 25 > Ct ≥ 20; bin-5, Ct < 20. The percentage of the total number of vesicle-associated miRNAs, and protein-associated miRNAs, was then determined for each bin, for serum sEV preparations and for plasma sEV preparations.
We found that serum sEV preparations had a greater percentage of vesicle-associated miRNAs expressed at relatively high levels (Ct’s < 25) than plasma sEV preparations (60% vs 44%), whereas plasma sEV preparations had a greater percentage of protein-associated miRNAs expressed at relatively high levels than serum sEV preparations (62% vs 31%; Figure 5). Plasma sEV preparations also had a greater percentage, than serum sEV preparations, of protein-associated miRNAs expressed at very high levels (Ct’s < 20; 22% vs 0%), and plasma sEV preparations had a higher percentage of undetected vesicle-associated miRNAs than serum sEV preparations (33% vs 10%). We observed similar distributions of vesicle-associated and protein-associated miRNAs in serum and plasma sEV preparations from patients with esophageal adenocarcinoma (Supplementary Figure 3). Overall these results indicated that serum sEV preparations contained higher levels of vesicle associated miRNAs, and lower levels of protein associated miRNAs, compared with plasma sEV preparations.
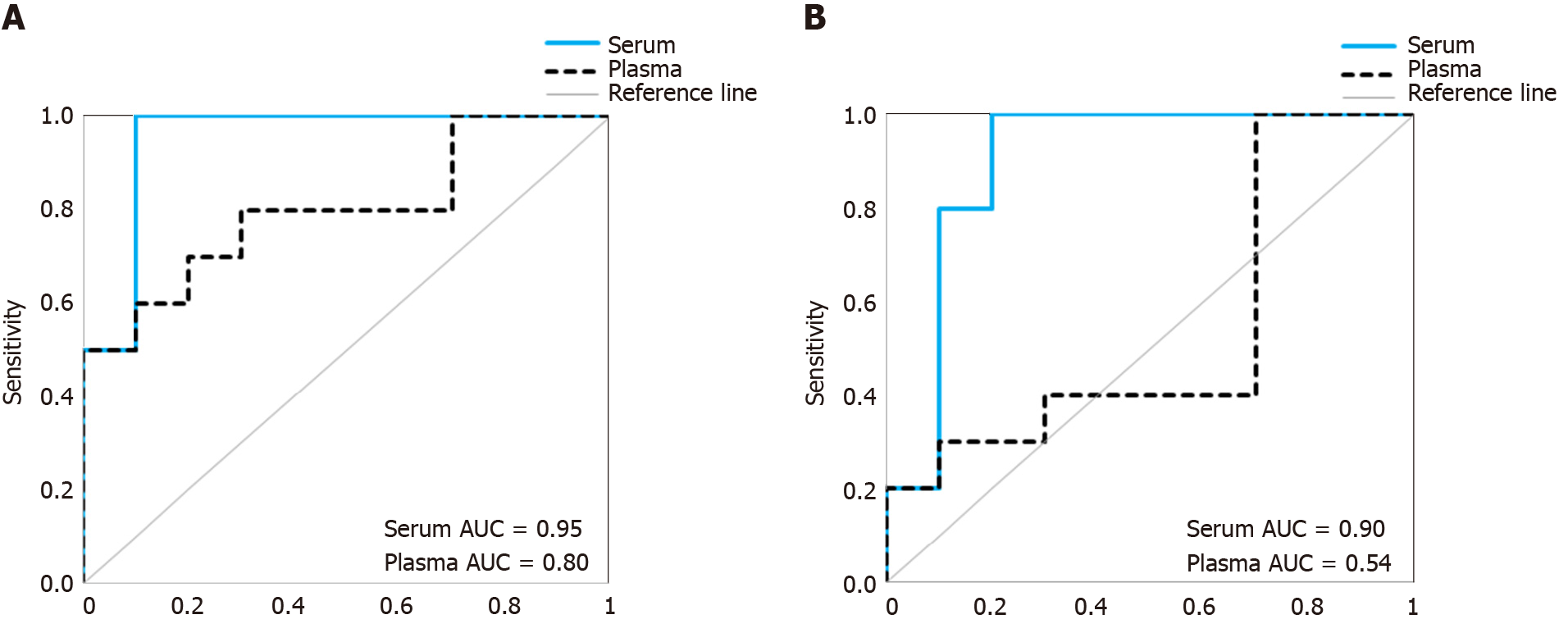
To investigate whether the above observed differences in proportions of non-vesicular to vesicular miRNAs in serum and plasma sEV preparations may influence outcomes of biomarker studies, we compared the diagnostic performance of a previously identified multi-biomarker panel[20] in the matched sEV preparations from serum and plasma samples (Figure 6). The multi-biomarker panel consisted of 5 specific miRNA ratios (RNU6-1/hsa-miR-16-5p, hsa-miR-25-3p/hsa-miR-320a, hsa-let-7e-5p/hsa-miR-15b-5p, hsa-miR-30a-5p/hsa-miR-324-5p, hsa-miR-17-5p/hsa-miR-194-5p) that discriminated esophageal adenocarcinoma patients from healthy controls and non-dysplastic Barrett’s esophagus[20]. When assessed in the serum sEV preparations, the multi-biomarker panel achieved a good prediction accuracy (AUROC = 0.95) and remained robust in leave-one-out cross validation (AUROC = 0.90). When assessed in the matched plasma sEV preparations, the multi-biomarker panel was less accurate in predicting which patients had esophageal adenocarcinoma (AUROC = 0.80) and performed considerably worse in leave-one-out cross validation (AUROC = 0.54).
To date, there have only been limited studies investigating sEV miRNA profiles concurrently in serum and plasma sEV preparations to determine their suitability for biomarker studies[11,16]. Based on our overall study findings, we observed significant differences in the proportion of reported sEV associated miRNAs between serum and plasma sEV preparations. Our results suggest that there is a greater concern for potential contamination of non-vesicular miRNAs in the plasma sEV preparations, and that this may influence biomarker studies. Therefore, we propose serum to be the preferred choice over plasma for future sEV miRNA biomarkers studies.
Under our specific study conditions, we isolated similar sEV yields yet overall higher miRNA content in plasma compared to serum sEV preparations. These findings are in contrast with previous studies that reported an overall higher miRNA content in serum sEV preparations compared to plasma sEV preparations[11,16]. In Cheng et al[11], next generation sequencing was used to profile a larger number of miRNAs than our TaqMan OpenArray platform. However, the study only utilised matched samples from 3 healthy individuals and used different methods than us for sEV isolation. The number of miRNAs detected was also marginally higher in the serum sEV preparations compared to plasma sEV preparations (412 vs 386 miRNAs). Although Ding et al[16] used the same sEV isolation and quantification techniques as us, the blood processing and miRNA detection methods were different to our study. The authors reported higher sEV yield, higher albumin contamination, larger microvesicles and higher number of miRNAs detected in the serum compared to plasma sEV preparations[16]. However, blood samples used for the sEV quantification and sEV miRNA profiles were derived from different individuals (5 and 20 healthy individuals respectively). Despite the disparities among these studies, the evidence that miRNA profiles differ between matched serum and plasma sEV preparations is consistent.
Possible explanations for the different miRNA profiles of sEV preparations from serum and plasma might include factors that impact upon the amount of protein-bound (non-vesicular) miRNAs present, and/or upon the sEVs produced from blood cells. These factors could include the different tubes with different additives that are used for producing plasma compared to serum, and that the production of serum involves blood clotting while the production of plasma specifically avoids this by using anti-coagulants. Blood clot formation involves trapping an array of proteins into the clot mesh, and this results in a significantly lower protein content in serum than in plasma[24], which may directly contribute towards an overall depletion of protein-bound miRNAs in serum compared to plasma. Besides Ago2 protein, high density lipoprotein (HDL) is another type of protein based vehicle for peripherally circulating miRNAs in plasma[25]. Of particular interest, it has been reported that HDL is trapped in the mesh that forms during clotting[26]. Therefore, it is possible that HDL-bound miRNAs are trapped in the clot, thereby resulting in lower numbers of HDL-bound miRNAs in serum than plasma. The method that we used for preparing sEVs involves the precipitation of membrane particles, and methods based upon this technique are known to result in co-precipitation of lipoproteins[27]. Taken together, it is possible that our serum sEV preparations contain less HDL-bound miRNAs than our plasma sEV preparations, and this may explain the lower overall miRNA abundance in our serum sEV preparations, as well as the tendency for our serum sEV preparations to contain a higher proportion of sEV associated miRNAs than our plasma sEV preparations. Different blood collection tube components have been shown to interfere with clinical chemistry assays in different ways[24]. The blood collection tubes used for plasma preparation in our study contained EDTA as the anti-coagulant. It has been reported that EDTA results in platelet activation, which increases the release of microvesicles, including sEVs, from platelets[28,29]. This might suggest that our plasma preparations would be more biased, than our serum sEV preparations, towards containing platelet derived / activated platelet sEV derived miRNAs. Consistent with this possibility, the top two miRNAs that were most heavily biased towards our plasma derived preparations were hsa-miR-223-3p, which is the most abundant miRNA in platelets, and hsa-miR-24-3p, which is a biomarker for platelet activation[30]. Another possibility is that the different blood tube components in the tubes used for preparing serum and plasma have different impacts upon the number of sEVs produced from blood cells, and / or the miRNA expression in blood cells, which translates into differences in the miRNA composition of sEVs derived from them[31]. It is also possible that the differences in blood tube components might impact upon the specific blood cell miRNAs that are sorted into sEVs[31].
One of the most significant differences between the miRNA profiles of our serum and plasma sEV preparations was the higher level of expression of reported blood cell miRNAs in plasma sEV preparations. In previous studies, circulating cell-free hsa-miR-451a, hsa-miR-16-5p and hsa-miR-223-3p are among the most common miRNAs assessed as indicators of haemolysis or blood cells contamination[10,18,32-35]. Although we found several reported blood cell miRNAs, including hsa-miR-451a, hsa-miR-16-5p and hsa-miR-223-3p, to be abundant in both plasma and serum sEV preparations, they were all more highly expressed in plasma sEV preparations. The presence of higher blood cell contamination in plasma sEV preparations was further supported by the observation that several reported blood-cell miRNAs were uniquely expressed only in our plasma derived samples, and a greater number of unique cell-free miRNAs, reported by Cheng 2014, were detected in the plasma derived samples.
Although the overall miRNA content was higher in our plasma sEV preparations, the concern was the high abundance of miRNAs in plasma sEV preparations that were reported to be from non-vesicular origins. Contrary to the consistently high miRNA content in plasma compared to serum derived sEV preparations, we observed a larger percentage of highly expressed vesicle-associated miRNAs in the serum sEV preparations, but a larger percentage of highly expressed protein-associated miRNAs in the matched plasma sEV preparations. We acknowledge that this conclusion is reliant on the findings of a single study (Arroyo 2011) and will require further validation. However, unlike blood-cell miRNAs, specific miRNAs that are highly expressed or uniquely expressed in sEVs or in protein-complexes with Ago2 are not as well-established. We identified only two studies, Cheng 2014[11], and Arroyo 2011[13], comprehensively reporting specific sEV miRNA profiles and protein-associated miRNA profiles in serum and plasma. Interestingly, RNU6-1, which has been reported to be enriched in sEVs, was also found to be abundant in our serum sEV preparations (top 20 most highly expressed miRNAs) but not in our plasma sEV preparations[36-39]. Altogether, these findings suggest that although a large proportion of miRNAs were consistently more highly expressed in plasma sEV preparations compared to serum sEV preparations, we identified a subset of miRNA candidates reported to be of sEV origin to be more highly expressed in serum sEV preparations.
Taking all these findings together, serum appears preferable to plasma for sEV miRNA biomarkers studies. As a proof-of-concept, we evaluated the diagnostic performance of a multi-biomarker panel to discriminate esophageal adenocarcinoma patients from the healthy controls in this study cohort when assessed in the serum sEV preparations compared to plasma sEV preparations. The diagnostic accuracy of the biomarker panel had higher cross validated prediction accuracy when assessed in the serum sEV preparations than in plasma sEV preparations. However, we recognise that our study findings are based on a small sample size and are specific to our study conditions, and further work is necessary to validate these findings. In particular, there is currently limited understanding on circulating miRNAs in protein-complexes, and a need to consider their role in future sEV miRNAs studies.
Small extracellular vesicles (sEVs), including exosomes, are shed from tumors into the blood circulation. These circulating sEVs are an excellent source of microRNA (miRNA) biomarkers for cancer research. Blood serum and blood plasma both contain sEVs, however at present there is no consensus on which of these two blood sample types is most useful for biomarker analysis.
Extracellular vesicle preparations derived from serum and plasma are known to be enriched in sEVs, but they also contain significant amounts of non-vesicle associated miRNAs derived from sources such as blood cells and protein-bound miRNAs. These non-vesicles associated miRNAs could interfere with cancer biomarker discovery. Our study was motivated by the need to determine which blood sample contains the least amount of non-vesicle associated miRNAs. This knowledge has the potential to improve cancer biomarker discovery and translation.
We sought to compare the miRNA profiles between serum and plasma sEV preparations to determine their suitability for biomarker studies. We also sought to compare the diagnostic performance of these two sample types using a previously established multi-miRNA biomarker panel for esophageal adenocarcinoma.
Matched serum and plasma samples from 10 healthy controls and 10 patients with esophageal adenocarcinoma were used for this study. sEVs were isolated with using ExoquickTM. RNA extracted from the vesicles was profiled using the Taqman Openarray qPCR.
The overall miRNA content was higher in plasma sEV preparations (480 miRNAs) and contained 97.5% of the miRNAs found in the serum sEV preparations (412 miRNAs). The expression of commonly expressed miRNAs was highly correlated (Spearman’s R = 0.87, P < 0.0001) between the plasma and serum sEV preparations but was consistently higher in the plasma sEV preparations. Specific blood-cell miRNAs (hsa-miR-223-3p, hsa-miR-451a, miR-19b-3p, hsa-miR-17-5p, hsa-miR-30b-5p, hsa-miR-106a-5p, hsa-miR-150-5p and hsa-miR-92a-3p) were expressed at 2.7 to 9.6 fold higher levels in the plasma sEV preparations compared to serum sEV preparations (P < 0.05). In plasma sEV preparations, the percentage of protein-associated miRNAs expressed at relatively higher levels (cycle threshold 20-25) was greater than serum sEV preparations (50% vs 31%). While the percentage of vesicle-associated miRNAs expressed at relatively higher levels was greater in the serum sEV preparations than plasma sEV preparations (70% vs 44%). A 5-miRNA biomarker panel produced a higher cross validated accuracy for discriminating patients with esophageal adenocarcinoma from healthy controls using serum sEV preparations compared with plasma sEV preparations (AUROC 0.80 vs 0.54, P < 0.05).
Although plasma sEV preparations contained more miRNAs than serum sEV preparations, they also contained more miRNAs from non-vesicle origins.
Serum appears to be more suitable than plasma for sEV miRNAs biomarkers studies. Future studies on sEV associated cancer biomarkers may benefit from using serum as the sample type for analysis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li YH, Zhang XM S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ
| 1. | Sood P, Krek A, Zavolan M, Macino G, Rajewsky N. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc Natl Acad Sci USA. 2006;103:2746-2751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 500] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 2. | Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5705] [Cited by in RCA: 6032] [Article Influence: 317.5] [Reference Citation Analysis (0)] |
| 3. | Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE, Green AR, Ellis IO, Tavaré S, Caldas C, Miska EA. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 706] [Cited by in RCA: 739] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 4. | Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, Calabrò E, Croce CM, Pastorino U, Sozzi G. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci USA. 2011;108:3713-3718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 570] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 5. | Tiberio P, Callari M, Angeloni V, Daidone MG, Appierto V. Challenges in using circulating miRNAs as cancer biomarkers. Biomed Res Int. 2015;2015:731479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 6. | Cheng HH, Yi HS, Kim Y, Kroh EM, Chien JW, Eaton KD, Goodman MT, Tait JF, Tewari M, Pritchard CC. Plasma processing conditions substantially influence circulating microRNA biomarker levels. PLoS One. 2013;8:e64795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 7. | Ma R, Jiang T, Kang X. Circulating microRNAs in cancer: origin, function and application. J Exp Clin Cancer Res. 2012;31:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, Tait JF, Tewari M. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila). 2012;5:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 723] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 9. | Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci. 2012;37:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 416] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 10. | Wang K, Yuan Y, Cho JH, McClarty S, Baxter D, Galas DJ. Comparing the MicroRNA spectrum between serum and plasma. PLoS One. 2012;7:e41561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 517] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 11. | Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 630] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 12. | Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 605] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 13. | Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003-5008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2345] [Cited by in RCA: 2643] [Article Influence: 188.8] [Reference Citation Analysis (0)] |
| 14. | Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223-7233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1356] [Cited by in RCA: 1525] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 15. | Bhome R, Del Vecchio F, Lee GH, Bullock MD, Primrose JN, Sayan AE, Mirnezami AH. Exosomal microRNAs (exomiRs): Small molecules with a big role in cancer. Cancer Lett. 2018;420:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 16. | Ding M, Wang C, Lu X, Zhang C, Zhou Z, Chen X, Zhang CY, Zen K, Zhang C. Comparison of commercial exosome isolation kits for circulating exosomal microRNA profiling. Anal Bioanal Chem. 2018;410:3805-3814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 17. | Salehi M, Sharifi M. Exosomal miRNAs as novel cancer biomarkers: Challenges and opportunities. J Cell Physiol. 2018;233:6370-6380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 18. | Foye C, Yan IK, David W, Shukla N, Habboush Y, Chase L, Ryland K, Kesari V, Patel T. Comparison of miRNA quantitation by Nanostring in serum and plasma samples. PLoS One. 2017;12:e0189165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | El-Mogy M, Lam B, Haj-Ahmad TA, McGowan S, Yu D, Nosal L, Rghei N, Roberts P, Haj-Ahmad Y. Diversity and signature of small RNA in different bodily fluids using next generation sequencing. BMC Genomics. 2018;19:408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Chiam K, Wang T, Watson DI, Mayne GC, Irvine TS, Bright T, Smith L, White IA, Bowen JM, Keefe D, Thompson SK, Jones ME, Hussey DJ. Circulating Serum Exosomal miRNAs As Potential Biomarkers for Esophageal Adenocarcinoma. J Gastrointest Surg. 2015;19:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 21. | Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, Liu Y. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS One. 2017;12:e0170628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 476] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 22. | Tang YT, Huang YY, Zheng L, Qin SH, Xu XP, An TX, Xu Y, Wu YS, Hu XM, Ping BH, Wang Q. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40:834-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 341] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 23. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7702] [Article Influence: 1100.3] [Reference Citation Analysis (1)] |
| 24. | Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb). 2014;24:31-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 25. | Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2292] [Cited by in RCA: 2188] [Article Influence: 156.3] [Reference Citation Analysis (0)] |
| 26. | Talens S, Leebeek FW, Demmers JA, Rijken DC. Identification of fibrin clot-bound plasma proteins. PLoS One. 2012;7:e41966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Karttunen J, Heiskanen M, Navarro-Ferrandis V, Das Gupta S, Lipponen A, Puhakka N, Rilla K, Koistinen A, Pitkänen A. Precipitation-based extracellular vesicle isolation from rat plasma co-precipitate vesicle-free microRNAs. J Extracell Vesicles. 2019;8:1555410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791-3799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1078] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 29. | Taus F, Meneguzzi A, Castelli M, Minuz P. Platelet-Derived Extracellular Vesicles as Target of Antiplatelet Agents. What Is the Evidence? Front Pharmacol. 2019;10:1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Maués JHDS, Aquino Moreira-Nunes CF, Rodriguez Burbano RM. MicroRNAs as a Potential Quality Measurement Tool of Platelet Concentrate Stored in Blood Banks-A Review. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Bæk R, Søndergaard EK, Varming K, Jørgensen MM. The impact of various preanalytical treatments on the phenotype of small extracellular vesicles in blood analyzed by protein microarray. J Immunol Methods. 2016;438:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Kirschner MB, Edelman JJ, Kao SC, Vallely MP, van Zandwijk N, Reid G. The Impact of Hemolysis on Cell-Free microRNA Biomarkers. Front Genet. 2013;4:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 33. | Pizzamiglio S, Zanutto S, Ciniselli CM, Belfiore A, Bottelli S, Gariboldi M, Verderio P. A methodological procedure for evaluating the impact of hemolysis on circulating microRNAs. Oncol Lett. 2017;13:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Blondal T, Jensby Nielsen S, Baker A, Andreasen D, Mouritzen P, Wrang Teilum M, Dahlsveen IK. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2013;59:S1-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 546] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 35. | Kirschner MB, Kao SC, Edelman JJ, Armstrong NJ, Vallely MP, van Zandwijk N, Reid G. Haemolysis during sample preparation alters microRNA content of plasma. PLoS One. 2011;6:e24145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 439] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 36. | Lopatina T, Favaro E, Grange C, Cedrino M, Ranghino A, Occhipinti S, Fallo S, Buffolo F, Gaykalova DA, Zanone MM, Romagnoli R, Camussi G. PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia. Sci Rep. 2018;8:17458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, Miyado K, Higashi Y, Ochi M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl Med. 2016;5:1620-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 341] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 38. | Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012;40:10937-10949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 354] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 39. | Jenjaroenpun P, Kremenska Y, Nair VM, Kremenskoy M, Joseph B, Kurochkin IV. Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. PeerJ. 2013;1:e201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |