Published online May 7, 2020. doi: 10.3748/wjg.v26.i17.2097
Peer-review started: February 11, 2020
First decision: February 27, 2020
Revised: March 26, 2020
Accepted: April 24, 2020
Article in press: April 24, 2020
Published online: May 7, 2020
Processing time: 86 Days and 1 Hours
Hemostasis of patients suffering from liver cirrhosis is challenging due to both, pro- and anticoagulatory disorders leading to hemostatic alterations with distinct abnormalities of coagulation. Pathological changes in conventional coagulation analysis and platelet count are common manifestations of decreased liver synthesis of coagulation factors and reduced platelet count in these patients. However, conventional coagulation analysis and platelet count do not reflect in-vivo coagulation status or platelet function. The purpose of this present observational study was therefore to assess the haemostatic profile including plasmatic coagulation using thrombelastometry and impedance aggregometry for platelet function in patients suffering from liver cirrhosis.
To assess the hemostatic profile of cirrhotic patients according to model for end-stage liver disease (MELD) score.
Our study included both in- and outpatients suffering from liver cirrhosis attending the out- and inpatient care of the department of hepatology. Demographic and biochemical data as well as medical history including cause of liver cirrhosis, end stage kidney failure and medication with anticoagulants were recorded. To assess the hemostatic profile, platelet function was analyzed by multiple electrode aggregometry (MEA) using Multiplate® (ADP-, ASPI- and TRAP-test) and thrombelastometry using ROTEM® (EXTEM, INTEM, FIBTEM). Data were compared using Mann-Whitney U- or χ2-test. Spearman correlation was performed to analyze the association between MELD Score and results of thrombelastometry and MEA.
A total of 68 patients attending the out- and inpatient care suffering from liver cirrhosis were screened. Of these, 50 patients were included and assigned to groups according to MELD score 6 to 11 (n = 25) or ≥ 17 (n = 25). Baseline patient characteristics revealed significant differences for MELD score (8 vs 22, P < 0.0001) and underlying laboratory parameters (international normalized ratio, bilirubine, creatinine) as well as fibrinogen level (275 mg/dL vs 209 mg/dL, P = 0.006) and aPTT (30 s vs 35 s, P = 0.047). MEA showed a moderately impaired platelet function (medians: AUCADP = 43U, AUCASPI = 71U, AUCTRAP = 92U) but no significant differences between both groups. Thrombelastometry using ROTEM® (EXTEM, INTEM, FIBTEM) revealed values within normal range in both groups. No significant correlation was observed between MELD score and results of MEA/thrombelastometry.
Our data demonstrate a partially impaired hemostatic profile in liver cirrhosis patients unrelated to MELD score. An individual assessment of a potential coagulopathy should therefore be considered.
Core tip: The results of our study show a moderately decreased platelet aggregation but no substantial impairment of the maximum clot firmness considering thrombelastometric results. Correlation analysis indicated that impedance aggregometric and thrombelastometric results did not correlate with model for end-stage liver disease score. Our data indicate that a potential coagulopathy in advanced liver cirrhosis may not be reflected by thrombelastometry or multiple electrode aggregometry as the underlying mechanisms may be beyond the platelet function of hemostasis. Rather, an individual assessment of a potential coagulopathy in patients with advanced disease is reasonable.
- Citation: Adam EH, Möhlmann M, Herrmann E, Schneider S, Zacharowski K, Zeuzem S, Weber CF, Weiler N. Assessment of hemostatic profile in patients with mild to advanced liver cirrhosis. World J Gastroenterol 2020; 26(17): 2097-2110
- URL: https://www.wjgnet.com/1007-9327/full/v26/i17/2097.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i17.2097
Liver cirrhosis is the end stage for a variety of hepatic diseases[1] and leads to hemostatic alterations with distinct abnormalities of coagulation[2]. Changes in both conventional coagulation analysis and platelet count are common manifestations of decreased synthesis of coagulation factors and reduced platelet count in these patients. Despite laboratory findings suggesting a coagulopathy, fewer bleeding events in cirrhosis patients occur not as often as expected[3]. In recent years, there is increasing evidence that the observed changes in both coagulation factors and platelet count in patients with liver cirrhosis cannot be interpreted as a reliable indicator of a diffuse bleeding risk[4,5]. The increasing knowledge on changes in hemostasis has meanwhile led to the concept of a rebalanced coagulation system[6]. However, a shift in hemostasis towards hypo- or hypercoagulation may occur in more advanced stages of liver failure. Routine diagnostic tests display relevant limitations, as they may not provide accurate information on the hemostatic status; they cannot predict bleeding risk and may therefore not provide clinically relevant information[7,8].
Viscoelastic tests are global tests of coagulation, that assess the viscoelastic properties of noncentrifuged whole blood. As standard coagulation tests cannot predict the risk of bleeding[9], viscoelastic tests are already widely used for haemostatic management during liver transplantation (LT)[10] resulting in a reduced need for transfusion[11,12]. Balancing the primary hemostasis is challenging and the underlying mechanisms that impair the primary hemostatic system are not completely understood[13].
A there is a lack of a universal platelet function assay in cirrhotic patients to assess bleeding risk or thrombotic events[14,15], Only limited and inconsistent data are available for the application of platelet function testing in patients suffering from liver cirrhosis[16-18]. Therefore, the aim of the present study was to assess the haemostatic profile of patients suffering from mild to advanced liver cirrhosis, including platelet function testing using multiple electrode aggregometry (MEA; Multiplate®) and viscoelastic testing using thrombelastometry (ROTEM®) and furthermore to correlate results from both tests with the severity of liver cirrhosis.
All patients attending the in- and outpatient care of the department of hepatology between December 2017 and May 2018 meeting the inclusion criteria were offered to participate in the present study. Inclusion criteria were liver cirrhosis and age of ≥ 18 years. Furthermore, a platelet count of at least 70 × 103/µL was mandatory to ensure adequate measurement of MEA using the Multiplate analyzer. Patients were excluded when given a platelet concentrate three weeks prior to inclusion or in case of pregnancy.
Written informed consent was obtained from all patients. The study was performed in accordance with the Declaration of Helsinki. Approval of the local ethics committee was obtained before study was performed (reference #195/17) and the study was registered to the Clinical Trials.gov Protocol Registration and Results System (NCT04265508). Patient care and study conduct complied with good clinical practice.
Demographic and biochemical data as well as medical history including cause of liver cirrhosis, end stage kidney failure, other diseases and medication with anticoagulants were recorded. Patients were included when having a model for end-stage liver disease (MELD) score of 6-11 or ≥ 17 and were stratified according to MELD score[19].
Venous blood was collected via a cannula inserted into a cubital vein. Collection tubes for conventional coagulation analysis were prefilled with sodium-citrate [S-Monovette® 1.8 mL, sodium-citrate 3.2% (1:10), Sarstedt AG, Nürnbrecht, Germany] and analyzed by ACL Top 700 CTS (Werfen GmbH, Barcelona, Spain). Hematological analyses were performed using collection tubes prefilled with ethylene-diaminetetraacetate (S-Monovette® 1.6 mL, K3 EDTA, Sarstedt AG Nürnbrecht, Germany) and analyzed by XN 9000 (Sysmex GmbH, Norderstedt, Germany). Platelet count was determined by fluorescence flow cytometry on XE 2100 (Sysmex GmbH, Norderstedt, Germany) and biochemical parameters were assessed using serum collection tubes (S-Monovette® 7.5 mL, Serum Gel with clotting activator, Sarstedt AG, Nürnbrecht, Germany) and analyzed by Cobas 8000 (Roche diagnostics, Mannheim, Germany).
For ROTEM analysis, blood was collected in collection tubes prefilled with sodium-citrate [S-Monovette® 1.8 mL, citrate 3.2% (1:10), Sarstedt AG, Nürnbrecht, Germany]. For MEA analysis, a heparinized blood gas analysis sample tube (safePICO, Radiometer, Krefeld, Germany) was used.
Platelet function was measured by MEA using the Multiplate analyzer 15min after blood draw and after activation with commercially available standard reagents (Roche, Basel, Swiss) as previously published[20]. Blood samples were analyzed at 37 °C. To test different ways of induction of aggregation, aggregation was stimulated (1) via adenosine diphosphate (ADP) receptors by ADP (ADP-test); (2) via arachidonic acid, the substrate of cyclooxygenase (COX), which subsequently forms the potent platelet activator thromboxane A2 (TXA2) (ASPI-test); and (3) by platelet receptor activating peptide 6 (TRAP-6) via the platelet surface platelet receptor (TRAP-test) as described in detail before[20]. To identify abnormal values in MEA assays, reference ranges were defined in accordance to the manufacturer’s recommendations for heparinized blood samples[21].
Thrombelastometric assays were performed 15 min after blood draw. Blood samples were analyzed at 37 °C using ROTEM delta analyzer. Detailed description of the ROTEM analysis was already illustrated earlier[22]. A runtime of 60 min was applied and regular quality control tests and ROTEM tests (Werfen GmbH, Barcelona, Spain) were run in accordance with the manufacturer’s instructions. For the present study, three tests were carried out using reagents provided by the manufacturer: Tissue factor triggered extrinsic pathway (EXTEM), which evaluates the extrinsic pathway; ellagic acid activated intrinsic pathway (INTEM), which evaluates the intrinsic pathway; cytochalasin D, which is an inhibitor of the rearrangement of microtubules in platelets and thus of platelet aggregation which evaluates the contribution of fibrinogen to clot formation (FIBTEM). To identify abnormal values in throm-belastometric assays, reference ranges were defined in accordance to previously published recommendations[23].
A sample size estimation expecting a median difference of AUCTRAP of ± 25% between the two groups was performed with α = 5% and power at 80% resulting in a computed sample size of n = 25 per group.
Data were tested for normality using Kolmogorov-Smirnov test. Data comparisons of patient characteristics and results of MEA and thrombelastometry were made using Mann-Whitney U- or Fisher’s exact-test, where applicable. To correlate parameters from MEA and thrombelastometry with other parameters, Spearman rank correlation analysis fitting a linear regression line were performed.
Results with P < 0.05 were considered to be statistically significant. All calculations/analyses were performed with SPSS (Version 25, Chicago, IL, United States) or GraphPad Prism (Version 8, San Diego, CA, United States).
The statistical methods of this study were reviewed by Prof. Eva Herrmann from the Department of Biostatistics and mathematical modeling of University Hospital Frankfurt.
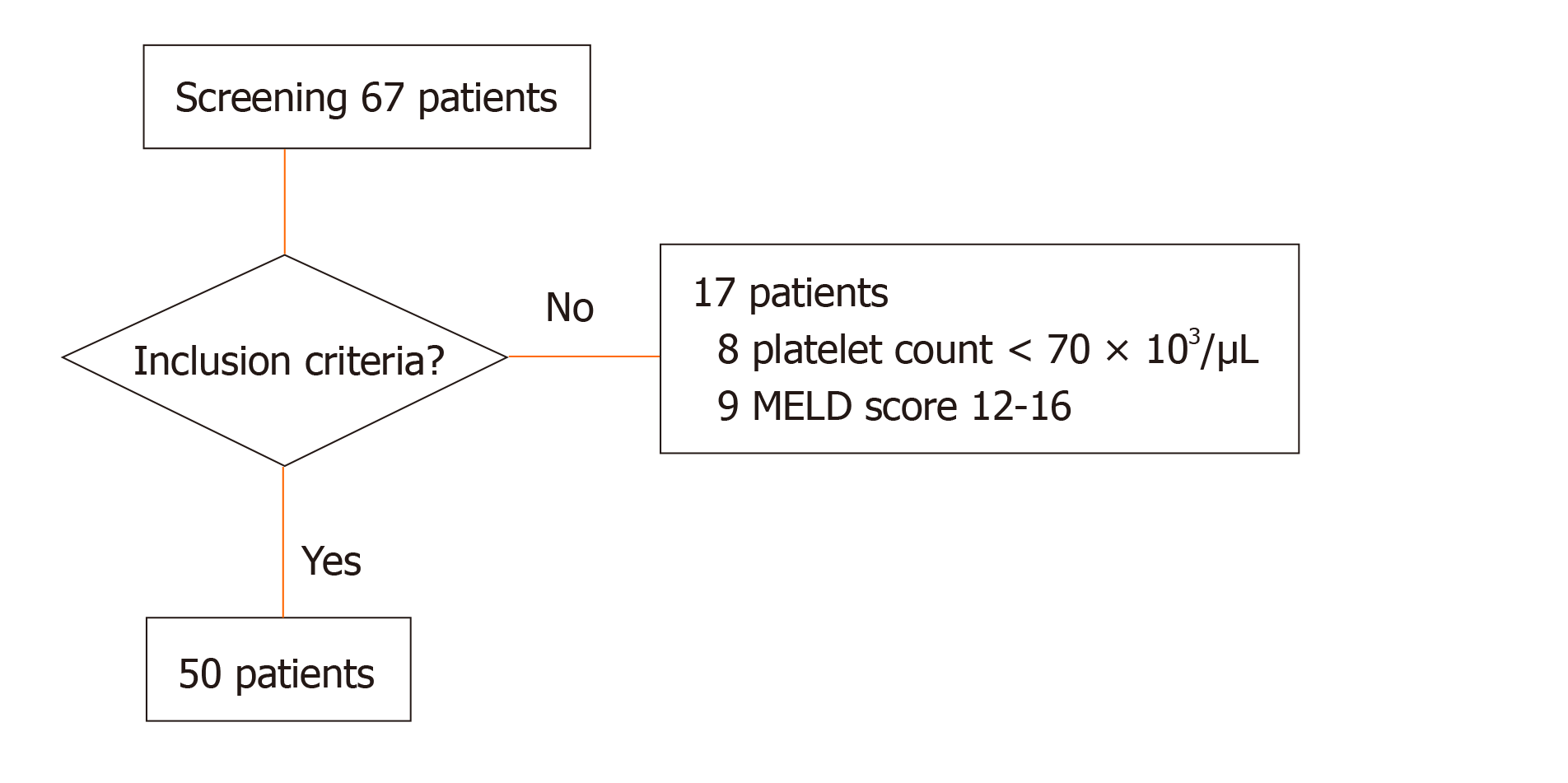
Between December 2017 and May 2018, a total of 67 patients suffering from liver cirrhosis attending the in- and out-patient care of the department of internal medicine were screened for inclusion into the study. Of these, 17 patients were not eligible to participate in the study: 8 patients presented with a platelet count lower than 70 × 103/µL at the day of inclusion; further, 9 patients, who had a calculated MELD score of 12-16 were excluded, in order to create more distinct subgroups according to previously published data and study protocol[19] (Figure 1). In total, 50 patients were included for analysis: Cirrhosis resulted from nutritive toxic origin in 24 patients (48%), from hepatitis C in 9 patients (18%), from non-alcoholic steatohepatitis (NASH) in 6 patients (12%), from hepatitis B in 4 patients (8%) and of other origin in 7 patients (14%). Of these, 7 patients (14%) were placed on the liver transplant waiting list (Table 1). Of the included patients, 25 suffered from mild liver cirrhosis according to a MELD score of 6 to 11, while the remaining 25 were included into advanced liver cirrhosis group presenting with a MELD score of 17 or above. Baseline patient characteristics revealed significant differences for MELD score and underlying laboratory parameters (international normalized ratio, bilirubin, creatinine) and for the level of fibrinogen an aPTT (Table 1).
| MELD 6-11 (n = 25) | MELD ≥ 17 (n = 25) | P value | |
| Age, yr (IQR) | 62 (56-69) | 58 (56-66) | 0.58 |
| Male sex, n (%) | 12 (48) | 16 (64) | 0.39 |
| BMI (SD) | 26 (6) | 27 (6) | 0.43 |
| MELD score (IQR) | 8 (7-10) | 22 (19-24) | < 0.0001 |
| Primary disease, n (%) | 0.20 | ||
| NASH | 2 (8) | 4 (16) | |
| Nutritive toxic | 15 (60) | 9 (36) | |
| Hepatitis B | 2 (8) | 2 (8) | |
| Hepatitis C | 5 (20) | 4 (16) | |
| Other | 1 (4) | 6 (24) | |
| Listed for LT, n (%) | 2 (8) | 5 (20) | 0.41 |
| Renal replacement therapy, n (%) | 0 (0) | 2 (8) | 0.49 |
| Anticoagulants, n (%) | 9 (36) | 11 (44) | 0.77 |
| P2Y12–receptor inhibitor | 1 (4) | 0 (0) | |
| ASA | 5 (20) | 8 (32) | |
| LMWH | 3 (12) | 3 (12) | |
| Laboratory parameters | |||
| Bilirubin, mg/dL (SD) | 0.9 (0.5) | 5.7 (4.4) | < 0.0001 |
| Creatinine, mg/dL (SD) | 0.8 (0.19) | 1.9 (1.5) | < 0.0001 |
| Urea, mg/dL (SD) | 28.7 (9.6) | 58.8 (41.6) | < 0.001 |
| INR | 1.1 (0.1) | 1.7 (0.6) | < 0.0001 |
| aPTT, s (SD) | 30 (3.7) | 35 (7.5) | 0.047 |
| Fibrinogen, mg/dL (SD) | 275 (51) | 209 (90) | 0.006 |
| Platelet count, 103/µL (SD) | 145 (51) | 138 (49) | 0.60 |
| Minimum, 103/µL | 80 | 72 | |
| Maximum, 103/µL | 275 | 274 | |
| Thrombocytopenia < 150 × 103/µL, n (%) | 16 (64) | 18 (72) | 1.00 |
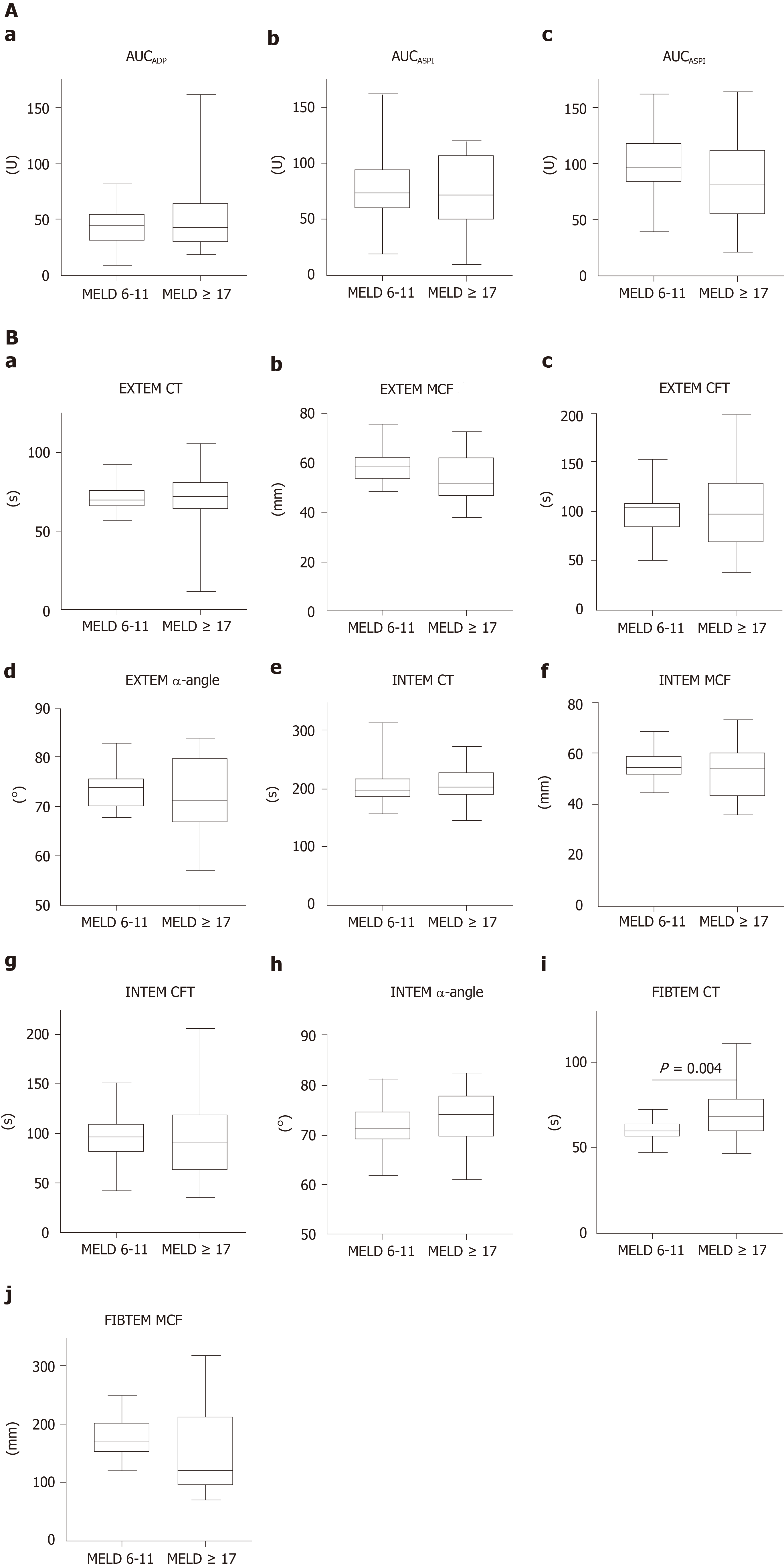
Comparing medians of the results from MEA, no significant differences were detected between the mild or advanced liver cirrhosis group (Table 2, Figure 2A). The overall median value of the ADP-test was 43 U (Table 2). One patient on clopidogrel therapy was excluded from analysis in the advanced liver cirrhosis group. No significant difference between mild and advanced liver cirrhosis patients (P = 0.80) was observed in regard to incidence of platelet dysfunction, as defined by AUCADP below 55 U (Figure 2A).
| All patients | MELD 6-11 | MELD ≥ 17 | P value | |
| Impedance aggregometry | ||||
| AUCADP1, U (IQR) | 43 (30-56) | 45 (30-56) | 42 (29-67) | 0.80 |
| AUCADP1< 55 U, n (%) | 34 (68) | 17 (68) | 17 (68) | 0.58 |
| AUC ASPI2, U (IQR) | 71 (57-99) | 72 (57-96) | 68 (43-104) | 0.53 |
| AUCASPI2 < 79 U, n (%) | 22 (44) | 12 (48) | 10 (40) | 0.94 |
| AUCTRAP, U (IQR) | 92 (61-106) | 94 (80-109) | 79 (60-110) | 0.26 |
| AUCTRAP < 92 U, n (%) | 24 (48) | 9 (36) | 15 (60) | 0.09 |
| Thrombelastometry | ||||
| EXTEM | ||||
| CT, s (IQR) | 71 (65-79) | 70 (65-77) | 73 (64-81) | 0.52 |
| CT < 42 s, n (%) | 1 (2) | 0 | 1 (4) | 0.10 |
| CT > 74 s, n (%) | 19 (38) | 8 (32) | 11 (44) | 0.38 |
| CFT, s (IQR) | 100 (75-115) | 103 (82-109) | 97 (67-132) | 0.96 |
| CFT < 46 s, n (%) | 1 (2) | 0 (0) | 1 (4) | 0.10 |
| CFT > 148 s, n (%) | 6 (12) | 1 (4) | 5 (20) | 0.18 |
| MCF, mm (IQR) | 57 (51-63) | 58 (53-63) | 52 (46-63) | 0.06 |
| MCF <49mm, n (%) | 10 (20) | 0 (0) | 10 (40) | < 0.001 |
| MCF >71mm, n (%) | 4 (8) | 2 (8) | 2 (8) | 1.00 |
| α-angle, ° (IQR) | 73 (69-77) | 74 (70-76) | 71 (66-80) | 0.31 |
| α-angle < 63°, n (%) | 3 (6) | 0 (0) | 3 (12) | 0.23 |
| α-angle > 81°, n (%) | 4 (8) | 1 (4) | 3 (12) | 0.60 |
| INTEM | ||||
| CT, s (IQR) | 197 (183-226) | 195 (181-217) | 202 (185-230) | 0.80 |
| CT < 137 s, n (%) | 0 (0) | 0 (0) | 0 (0) | a |
| CT > 246 s, n (%) | 9 (18) | 4 (16) | 4 (16) | 1.00 |
| CFT, s (IQR) | 92 (69-110) | 91 (80-109) | 91 (62-120) | 0.55 |
| CFT < 40 s, n (%) | 1 (2) | 0 (0) | 1 (4) | 1.00 |
| CFT > 100 s, n (%) | 22 (44) | 8 (32) | 11 (44) | 0.56 |
| MCF, mm (IQR) | 54 (48-59) | 55 (51-59) | 52 (42-59) | 0.16 |
| MCF < 52 mm, n (%) | 20 (40) | 8 (32) | 11 (44) | 0.38 |
| MCF > 72 mm, n (%) | 1 (2) | 0 (0) | 1 (4) | 1.00 |
| α-angle, °(IQR) | 73 (69-76) | 71 (67-75) | 74 (69-80) | 0.55 |
| α-angle < 71°, n (%) | 19 (38) | 10 (40) | 9 (36) | 0.77 |
| α-angle > 82°, n (%) | 0 (0) | 0 (0) | 0 (0) | a |
| FIBTEM | ||||
| CT, s (IQR) | 63 (56-71) | 59 (55-65) | 68 (59-79) | 0.004 |
| CT < 43 s, n (%) | 0 (0) | 0 (0) | 0 (0) | a |
| CT > 69 s, n (%) | 3 (6) | 4 (16) | 9 (36) | 0.19 |
| MCF, mm (IQR) | 17 (12-20) | 17 (14-20) | 12 (9-21) | 0.11 |
| MCF < 9 mm, n (%) | 4 (8) | 0 (0) | 4 (16) | 0.11 |
| MCF > 25 mm, n (%) | 3 (6) | 0 (0) | 3 (12) | 0.23 |
The overall median of the ASPI-test was 71 U (Table 2). To avoid a distortion of the results of the ASPI-test, 5 patients of the MELD 6-11 and 8 of the MELD ≥ 17 group with known acetylsalicylic acid intake were excluded from analysis. Comparing presence of platelet dysfunction, as defined by AUCASPI < 79 U, there was no significant difference between patients with mild or advanced liver cirrhosis (Table 2, Figure 2A).
The overall median of the TRAP-test was 92 U (Table 2). Platelet dysfunction defined as AUCTRAP below 92 U was detected in 9 (MELD 6-11) and 15 (MELD ≥ 17) patients, respectively, without any statistical significance between the groups (Figure 2A).
The overall median values of thrombelastometric analyses were within the normal reference ranges (Table 2). Comparing medians of clotting time (CT), clot formation time (CFT), maximum clot firmness (MCF) and α-angle of EXTEM assay, no statistical differences between mild and advanced liver cirrhosis group were detected (Figure 2B). Also, no statistically significant differences were observed regarding median values of CT, CFT, MCF and α-angle of INTEM assay (Figure 2B). Regarding FIBTEM assay, median CT differed significantly in comparison between mild and advanced liver cirrhosis group (59 vs 68, P = 0.004) (Table 2, Figure 2B). Comparing the frequency of pathological results of thrombelastometry, a significantly higher number of patients with reduced MCFEXTEM was observed in the advanced liver cirrhosis group (0 vs 10, P < 0.001) (Table 2).
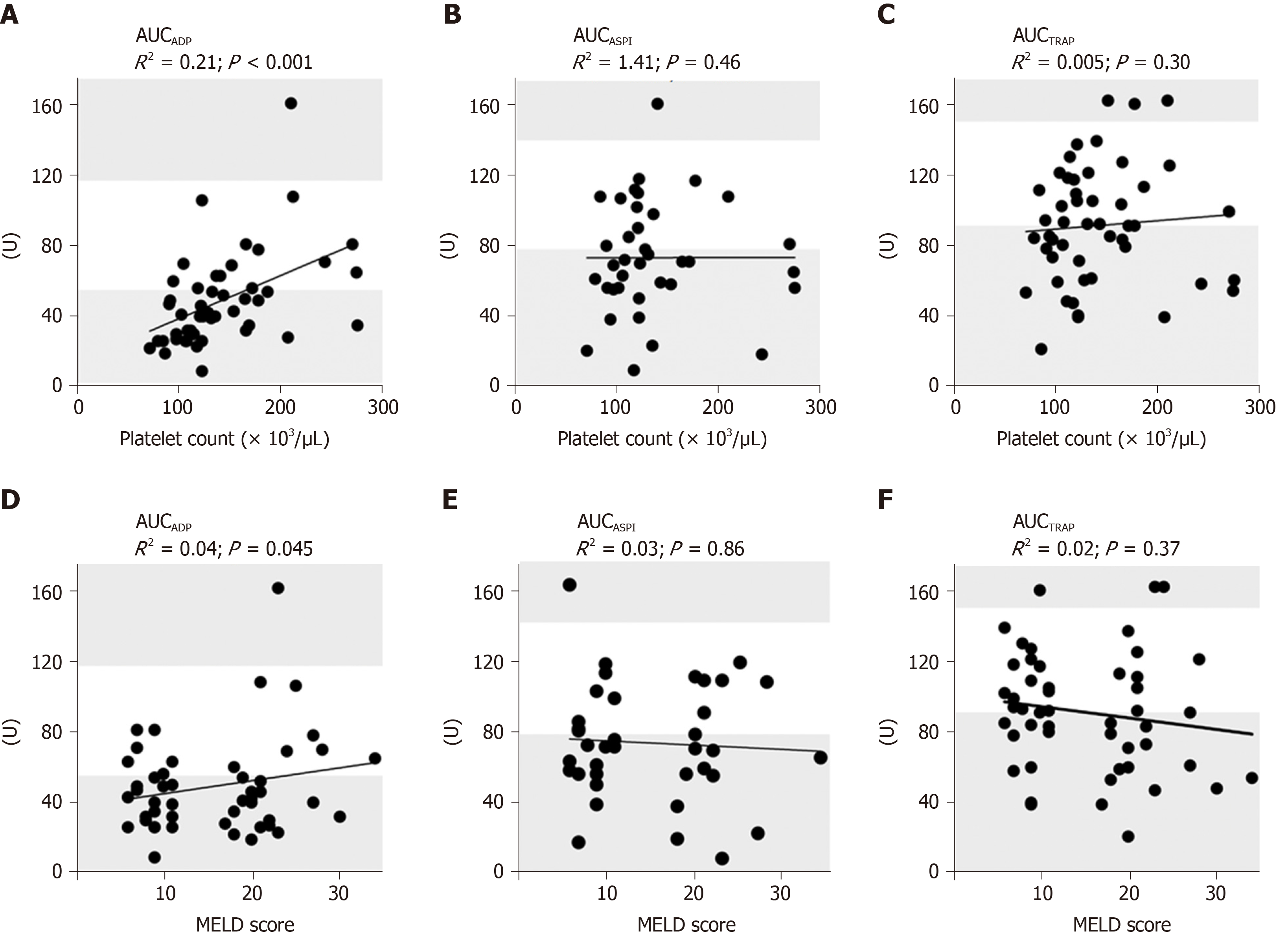
We performed a Spearman rank correlation analysis and fitted a linear regression line for platelet count and results of MEA. AUCTRAP (P = 0.30, R2 = 0.005) and AUCASPI (P = 0.46, R2 = 1.4e-006) revealed no significant correlation with platelet count. A significant correlation of AUCADP with platelet count was detected (P < 0.0001, R2 = 0.21, Figure 3A-C). Spearman rank correlation analysis and fitting a linear regression line were also performed for MELD score and results of MEA. AUCADP (P = 0.45, R2 = 0.04), AUCASPI, (P = 0.86, R2 = 0.003) and AUCTRAP (P = 0.37, R2 = 0.02) revealed no significant correlation with MELD score (Figure 3D-F).
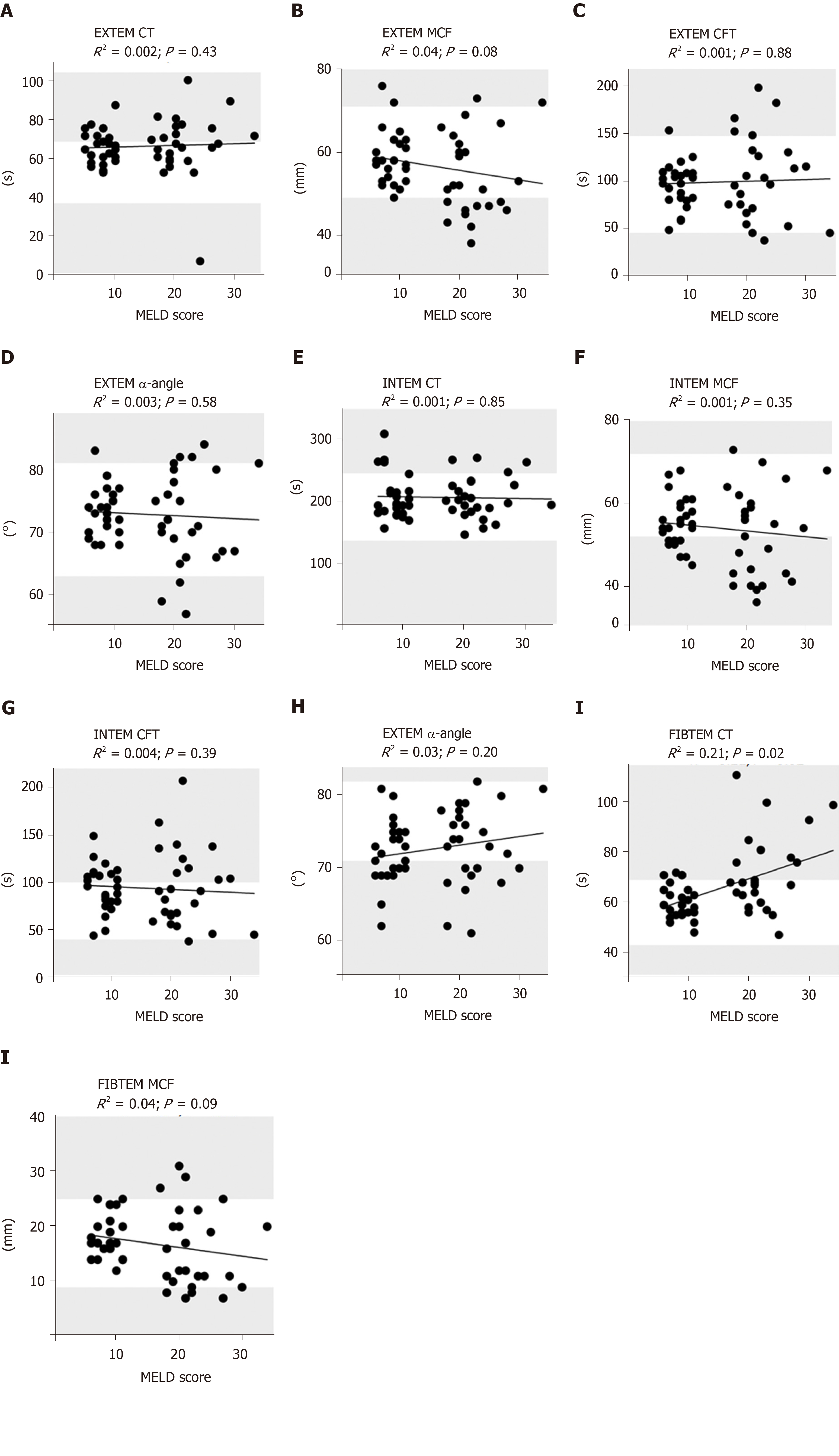
Spearman rank correlation analysis and fitting a linear regression line were also performed for MELD score and results of thrombelastometry. CT of EXTEM (P = 0.43, R2 = 0.002), INTEM (P = 0.85, R2 = 0.001) as well as CFT of EXTEM (P = 0.88, R2 = 0.001) and INTEM (P = 0.39, R2 = 0.004) revealed no significant correlation with MELD score (Figure 4). CT of FIBTEM (P = 0.02, R2 = 0.21) was significantly correlated to MELD score, demonstrating an increasing CTFIBTEM corresponding to an increasing MELD score (P = 0.02, R2 = 0.21, Figure 4).
In correlation analyses of MCF of EXTEM (P = 0.08, R2 = 0.04), INTEM (P = 0.38, R2 = 0.01) and FIBTEM (P = 0.09, R2 = 0.04) no significant correlations to MELD score were observed. α-angle of EXTEM (P = 0.58, R2 = 0.003) and INTEM (P = 0.20, R2 = 0.03) did not correlate significantly with MELD score (Figure 4).
Analysis of MCF of EXTEM (P < 0.0001, R2 = 0.45), INTEM (P < 0.0001, R2 = 0.44) and FIBTEM (P = 0.03, R2 = 0.09) showed significant correlations with platelet count (Supplementary Figure 1). Moreover, analysis of MCF of EXTEM (P < 0.0001, R2 = 0.35), INTEM (P < 0.0001, R2 = 0.31) and FIBTEM (P < 0.0001, R2 = 0.63) demonstrated significant correlations with fibrinogen level (Supplementary Figure 2).
Our prospective study in 50 patients with suffering from mild to advanced liver cirrhosis showed that the hemostatic profile assessed by MEA and thrombe-lastometric assays is not associated with the severity of liver cirrhosis according to MELD Score and ranged from normal to hypocoagulable state.
While most studies evaluating platelet function in liver cirrhosis are focused on LT, the aim of the present work was to conduct an analysis of platelet function in patients with liver cirrhosis in daily routine beyond surgery and to use MEA as the testing method. Our findings of MEA tests showed that platelet function was within or scarcely below the lower reference range for all performed tests. As a consequence, it appears to be inappropriate to universally label these patients as being pathologic concerning primary hemostasis.
In detail, comparing the observed MEA results to severity of liver cirrhosis, those patients who displayed abnormal results below the reference range showed mostly mild thrombopenia especially in the group of advanced liver cirrhosis. Thrombopenia in liver cirrhosis is well described and known to occur in affected patients[3,24]. However, a long-accepted association with thrombocytopathia in thrombopenic cirrhosis patients, has been increasingly questioned by various publications[6,15,17,25]. The mentioned publications provide evidence, that a consistent and predictable impairment in hemostasis may not be universal in patients with cirrhosis. Rather seems to be the coagulation status in a fragile but delicate balance between pro- and anticoagulation in cirrhosis patients[3,12]. Further supporting these findings, data from clinical trials showed a lack of association between thrombopenic cirrhotic patients and bleeding events[26,27].
Concerning platelet activation and aggregability, our findings show a slight to moderate impairment of platelet function in liver cirrhosis patients. Such decreased agonist-induced platelet aggregation has been demonstrated in previously published studies mainly by analyzing von-Willebrand factor (vWF) parameters[28,29], by biochemical investigations[30,31] or by using PFA-100[32,33]. Despite these various approaches, the causes for platelet dysfunction in liver cirrhosis are complex and not fully clarified yet. Earlier studies suggested hypoaggregability as a result of impaired platelet activation, but recent research identified an oversecretion of P-selectin by platelets resulting from increased cyclooxygenase 1 activation as a potential cause for hypoaggregability[34]. Similar to the extent of thrombocytopenia and stage of liver cirrhosis[35], an increased activation of platelets could be associated to progression of cirrhosis, suggesting diminution of platelet count as a non-desired, but protective effect against intensification of inflammatory processes[36,37]. Beyond that, an increased vWF level demonstrated for cirrhosis patients[38] might compensate for the quantitative platelet defect.
Considering all the above-mentioned alterations in platelet function of cirrhotic patients, a finely tuned balance seems to prevail, not only in plasmatic and cellular components, but also regarding for the platelet function.
However, a significant correlation of platelet dysfunction and platelet count was in contrast to results of non-cirrhotic patients[39] only detected for AUCADP, which reinforces previous published data[16,25] and highlights the alterations in primary hemostasis of these patients. This finding may be related to the higher sensitivity of the ADP-test in comparison to the thrombin-dependent tests as reported earlier in cardiac surgery patients[40].
Furthermore, ADP-, ASPI- and TRAP-tests were not significantly deteriorated in comparison of patients with mild and advanced liver cirrhosis, which may also be related to the mentioned compensating mechanisms of thrombocytopenia.
MEA records platelet aggregation in-vitro in response to given test reagents as mentioned above. The purpose of this method is to determine the platelet function in patients on antiaggregatory medication, as well as in the pre- and postoperative setting. Until today, only one other study showed results of measuring platelet function of liver cirrhotic patients used this approach[16]. It therefore remains unclear to what extent the alteration of e.g., hepatic TXA2 metabolism, over secretion of P-selectin, changes in thrombin generation and chronic inflammation might contribute to an impaired in-vitro platelet aggregation using MEA and further studies are required to assess MEA as an adequate tool in liver cirrhotic patients[15,41]. Additional studies addressing the impact of pathological coagulation as per MEA testing on the development of clinically relevant bleeding events are necessary.
In line with the literature our results of EXTEM, INTEM and FIBTEM assays varied from normal to hypocoagulable status[42] without significant differences according to MELD score. Our results reinforce the assumptions on alterations in plasmatic coagulation of cirrhotic patients[43] and follow the concept of a balanced hemostatic coagulation status[6]. These are important findings, as clinical practice of platelet transfusion is occasionally based on reduced MCFEXTEM. In line with previously published data suggesting platelet transfusion in cases of reduced maximum clot firmness[44,45], our findings again highlight the presumably specific hemostatic balance of these patients and support the current recommendations to avoid correction of pathological values as determined by conventional coagulation analysis. Since our results did not reveal correlation with the severity of liver cirrhosis - in contrast to previous published data[46] - the MELD score should not be used to assess hemostatic potential or to guide hemostatic therapy.
To ensure adequate performance of MEA, patients suffering from severe thrombocytopenia were not included into the study. Hence, findings in MEA or thrombelastometry may differ in those severely affected patients. Moreover, results of MEA provide only restricted information of platelet function, which might not be adequate to assess platelet function in liver cirrhosis. Measuring platelet function using other point-of-care tests with different approaches would be an interesting aim in future studies. Transfer of the presented findings to patients suffering from other diseases should be done with caution due to the distinct alterations in the hemostatic profile of patients with liver cirrhosis.
In conclusion, our data demonstrate a partially impaired hemostatic profile of both platelet function and plasmatic coagulation in cirrhotic patients, but no correlation of impairment with the severity of liver cirrhosis. Therefore, the hemostatic potential or a hemostatic therapy should not be judged by MELD score alone but rather by an individual and patient-specific assessment.
Liver cirrhosis leads to hemostatic alterations. Despite laboratory findings suggesting a coagulopathy, these do not reflect bleeding events in cirrhosis patients. Routine diagnostic tests display relevant limitations, as they may not provide accurate information on the hemostatic status; they cannot predict bleeding risk and may therefore not provide clinically relevant information.
Viscoelastic tests are global tests of coagulation, which assess the viscoelastic properties of blood. The aim of the present study was to assess the haemostatic profile of patients suffering from mild to advanced liver cirrhosis, including platelet function testing and viscoelastic testing. Results originating from these tests may close the gap in testing patients with liver cirrhosis for
The objective was to study the hemostatic profile of patients with liver cirrhosis and to correlate results from both tests with the severity of liver cirrhosis.
Platelet function was measured by multiple electrode aggregometry using the multiplate analyzer 15 min after blood draw and after activation with commercially available standard reagents. Blood samples were analyzed at 37 °C. Thrombelastometric assays were performed 15 min after blood draw. Blood samples were analyzed at 37 °C using ROTEM delta analyzer. A runtime of 60 min was applied and regular quality control tests and ROTEM tests were run in accordance with the manufacturer’s instructions. Data comparisons of patient characteristics and results of multiple electrode aggregometry (MEA) and thrombelastometry were made using Mann-Whitney U- or Fisher’s exact-test, where applicable. To correlate parameters from MEA and thrombelastometry with other parameters, Spearman rank correlation analysis fitting a linear regression line were performed.
Our prospective study in 50 patients with suffering from mild to advanced liver cirrhosis showed that the hemostatic profile assessed by viscoelastic tests is not associated with the severity of liver cirrhosis according to model for end-stage liver disease (MELD) score and ranged from normal to hypocoagulable state. In detail, comparing the observed MEA results to severity of liver cirrhosis, those patients who displayed abnormal results below the reference range showed mostly mild thrombopenia especially in the group of advanced liver cirrhosis.
Our data demonstrate a partially impaired hemostatic profile of both platelet function and plasmatic coagulation in cirrhotic patients, but no correlation of impairment with the severity of liver cirrhosis. Therefore, the hemostatic potential or a hemostatic therapy should not be judged by MELD score alone but rather by an individual and patient-specific assessment. Further, our data indicate that a potential coagulopathy in advanced liver cirrhosis may not be reflected by thrombelastometry or multiple electrode aggregometry as the underlying mechanisms may be beyond the platelet function of hemostasis. In clinical practice, viscoelastic testing may prove to be an asset in the assessment of hemostatic profiling of patients suffering from liver cirrhosis.
Ultimately, further tests need to be developed to further reflect/identify patients with an impaired hemostatic potential.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Goral V, Guo JS, Shimizu Y S-Editor: Wang YQ L-Editor: A E-Editor: Ma YJ
| 1. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2290] [Article Influence: 381.7] [Reference Citation Analysis (0)] |
| 2. | Bienholz A, Canbay A, Saner FH. [Coagulation management in patients with liver disease]. Med Klin Intensivmed Notfmed. 2016;111:224-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing Concepts of Cirrhotic Coagulopathy. Am J Gastroenterol. 2017;112:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | Harrison MF. The Misunderstood Coagulopathy of Liver Disease: A Review for the Acute Setting. West J Emerg Med. 2018;19:863-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Stravitz RT. Algorithms for managing coagulation disorders in liver disease. Hepatol Int. 2018;12:390-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost. 2017;1:150-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Segal JB, Dzik WH; Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 493] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Bonhomme F, Ajzenberg N, Schved JF, Molliex S, Samama CM; French Anaesthetic and Intensive Care Committee on Evaluation of Routine Preoperative Testing; French Society of Anaesthesia and Intensive Care. Pre-interventional haemostatic assessment: Guidelines from the French Society of Anaesthesia and Intensive Care. Eur J Anaesthesiol. 2013;30:142-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Haas T, Fries D, Tanaka KA, Asmis L, Curry NS, Schöchl H. Usefulness of standard plasma coagulation tests in the management of perioperative coagulopathic bleeding: is there any evidence? Br J Anaesth. 2015;114:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 10. | Smart L, Mumtaz K, Scharpf D, Gray NO, Traetow D, Black S, Michaels AJ, Elkhammas E, Kirkpatrick R, Hanje AJ. Rotational Thromboelastometry or Conventional Coagulation Tests in Liver Transplantation: Comparing Blood Loss, Transfusions, and Cost. Ann Hepatol. 2017;16:916-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Schumacher C, Eismann H, Sieg L, Friedrich L, Scheinichen D, Vondran FWR, Johanning K. Use of Rotational Thromboelastometry in Liver Transplantation Is Associated With Reduced Transfusion Requirements. Exp Clin Transplant. 2019;17:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Forkin KT, Colquhoun DA, Nemergut EC, Huffmyer JL. The Coagulation Profile of End-Stage Liver Disease and Considerations for Intraoperative Management. Anesth Analg. 2018;126:46-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 447] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 14. | Lisman T, Porte RJ. Platelet function in patients with cirrhosis. J Hepatol. 2012;56:993-4; author reply 994-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Violi F, Basili S, Raparelli V, Chowdary P, Gatt A, Burroughs AK. Patients with liver cirrhosis suffer from primary haemostatic defects? Fact or fiction? J Hepatol. 2011;55:1415-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Vinholt PJ, Alnor AB, Nybo M, Hvas AM. The primary haemostasis is more preserved in thrombocytopenic patients with liver cirrhosis than cancer. Blood Coagul Fibrinolysis. 2018;29:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Eyraud D, Suner L, Dupont A, Bachelot-Loza C, Smadja DM, Helley D, Bertil S, Gostian O, Szymezak J, Loncar Y, Puybasset L, Lebray P, Vezinet C, Vaillant JC, Granger B, Gaussem P. Evolution of platelet functions in cirrhotic patients undergoing liver transplantation: A prospective exploration over a month. PLoS One. 2018;13:e0200364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Alkozai EM, Porte RJ, Adelmeijer J, Zanetto A, Simioni P, Senzolo M, Lisman T. No evidence for increased platelet activation in patients with hepatitis B- or C-related cirrhosis and hepatocellular carcinoma. Thromb Res. 2015;135:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 617] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 20. | Adam EH, Baro D, Schmidt P, Mutlak H, Zacharowski K, Hanke AA, Weber CF. Aggregometric assessment of clonidine's impact on the efficacy of dual platelet inhibition. Clin Lab. 2014;60:1533-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Reference Ranges for Multiplate® analysis. Rotkreuz: Roche Diagnostics International Ltd., 2013. Available from: URL: https://www.roche.es/content/dam/rochexx/roche-es/roche_spain/es_ES/Home/Diagnostica/Multiplate_Reference_ranges.pdf. |
| 22. | Gronchi F, Perret A, Ferrari E, Marcucci CM, Flèche J, Crosset M, Schoettker P, Marcucci C. Validation of rotational thromboelastometry during cardiopulmonary bypass: A prospective, observational in-vivo study. Eur J Anaesthesiol. 2014;31:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, Lakner M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005;16:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 391] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 24. | Saner FH, Gieseler RK, Akız H, Canbay A, Görlinger K. Delicate balance of bleeding and thrombosis in end-stage liver disease and liver transplantation. Digestion. 2013;88:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Napolitano G, Iacobellis A, Merla A, Niro G, Valvano MR, Terracciano F, Siena D, Caruso M, Ippolito A, Mannuccio PM, Andriulli A. Bleeding after invasive procedures is rare and unpredicted by platelet counts in cirrhotic patients with thrombocytopenia. Eur J Intern Med. 2017;38:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 26. | Kalambokis GN, Oikonomou A, Christou L, Kolaitis NI, Tsianos EV, Christodoulou D, Baltayiannis G. von Willebrand factor and procoagulant imbalance predict outcome in patients with cirrhosis and thrombocytopenia. J Hepatol. 2016;65:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Basili S, Raparelli V, Napoleone L, Talerico G, Corazza GR, Perticone F, Sacerdoti D, Andriulli A, Licata A, Pietrangelo A, Picardi A, Raimondo G, Violi F; PRO-LIVER Collaborators. Platelet Count Does Not Predict Bleeding in Cirrhotic Patients: Results from the PRO-LIVER Study. Am J Gastroenterol. 2018;113:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 28. | Wannhoff A, Rauber C, Friedrich K, Rupp C, Stremmel W, Weiss KH, Schemmer P, Gotthardt DN. Von Willebrand factor and alkaline phosphatase predict re-transplantation-free survival after the first liver transplantation. United European Gastroenterol J. 2017;5:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Palyu E, Harsfalvi J, Tornai T, Papp M, Udvardy M, Szekeres-Csiki K, Pataki L, Vanhoorelbeke K, Feys HB, Deckmyn H, Tornai I. Major Changes of von Willebrand Factor Multimer Distribution in Cirrhotic Patients with Stable Disease or Acute Decompensation. Thromb Haemost. 2018;118:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Sayed D, Amin NF, Galal GM. Monocyte-platelet aggregates and platelet micro-particles in patients with post-hepatitic liver cirrhosis. Thromb Res. 2010;125:e228-e233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Ercin CN, Dogru T, Tapan S, Karslioglu Y, Haymana C, Kilic S, Sonmez A, Yesilova Z, Uygun A, Gulsen M, Bagci S, Kemal Erbil M. Levels of soluble CD40 ligand and P-Selectin in nonalcoholic fatty liver disease. Dig Dis Sci. 2010;55:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Pihusch R, Rank A, Göhring P, Pihusch M, Hiller E, Beuers U. Platelet function rather than plasmatic coagulation explains hypercoagulable state in cholestatic liver disease. J Hepatol. 2002;37:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Wannhoff A, Müller OJ, Friedrich K, Rupp C, Klöters-Plachky P, Leopold Y, Brune M, Senner M, Weiss KH, Stremmel W, Schemmer P, Katus HA, Gotthardt DN. Effects of increased von Willebrand factor levels on primary hemostasis in thrombocytopenic patients with liver cirrhosis. PLoS One. 2014;9:e112583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Vardareli E, Saricam T, Demirustu C, Gulbas Z. Soluble P selectin levels in chronic liver disease: relationship to disease severity. Hepatogastroenterology. 2007;54:466-469. [PubMed] |
| 35. | Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37:778-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 36. | Panasiuk A, Zak J, Kasprzycka E, Janicka K, Prokopowicz D. Blood platelet and monocyte activations and relation to stages of liver cirrhosis. World J Gastroenterol. 2005;11:2754-2758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Hartmann M, Szalai C, Saner FH. Hemostasis in liver transplantation: Pathophysiology, monitoring, and treatment. World J Gastroenterol. 2016;22:1541-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, Leebeek FW. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 419] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 39. | Hanke AA, Roberg K, Monaca E, Sellmann T, Weber CF, Rahe-Meyer N, Görlinger K. Impact of platelet count on results obtained from multiple electrode platelet aggregometry (Multiplate). Eur J Med Res. 2010;15:214-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 40. | Ranucci M, Pistuddi V, Di Dedda U, Menicanti L, De Vincentiis C, Baryshnikova E. Platelet function after cardiac surgery and its association with severe postoperative bleeding: the PLATFORM study. Platelets. 2019;30:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Blasi A. Coagulopathy in liver disease: Lack of an assessment tool. World J Gastroenterol. 2015;21:10062-10071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 42. | Lentschener C, Flaujac C, Ibrahim F, Gouin-Thibault I, Bazin M, Sogni P, Samama CM. Assessment of haemostasis in patients with cirrhosis: Relevance of the ROTEM tests?: A prospective, cross-sectional study. Eur J Anaesthesiol. 2016;33:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Saner FH, Abeysundara L, Hartmann M, Mallett SV. Rational approach to transfusion in liver transplantation. Minerva Anestesiol. 2018;84:378-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Görlinger K, Dirkmann D, Hanke AA. Potential value of transfusion protocols in cardiac surgery. Curr Opin Anaesthesiol. 2013;26:230-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Karkouti K, Callum J, Wijeysundera DN, Rao V, Crowther M, Grocott HP, Pinto R, Scales DC; TACS Investigators. Point-of-Care Hemostatic Testing in Cardiac Surgery: A Stepped-Wedge Clustered Randomized Controlled Trial. Circulation. 2016;134:1152-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 46. | Kohli R, Shingina A, New S, Chaturvedi S, Benson A, Biggins SW, Bambha K. Thromboelastography Parameters Are Associated with Cirrhosis Severity. Dig Dis Sci. 2019;64:2661-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |