INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy, occurring predominantly in a cirrhotic liver with estimated 5 years incidence of 25%[1-5], which can vary depending on the etiology of cirrhosisThe early diagnosis is very important, when curative treatments such as resection, transplantation, or local ablation therapy are possible[6]. All patients who are at high risk for HCC development should undergo active ultrasound surveillance every 6 mo, in order to detect small HCC lesions, as was suggested by the American Association for the Study of Liver Diseases (AASLD)[7,8]. If abnormal nodules larger than 1 cm are detected, further examination with computed tomography (CT), or magnetic resonance imaging (MRI) is necessary. According to AASLD and European Association for the Study of Liver Diseases guidelines, in cases where typical enhancement pattern, consisting of late arterial hyperenhancement followed by washout in the portal venous or delayed phases is present, the diagnosis of HCC can be made without further confirmation with tissue biopsy[7,9]. For nodules smaller than 1 cm, regular 3-mo follow-up ultrasound examination is recommended[7]. One of the shortcomings of both CT and MRI is the use of intravenous contrast agents, which can be problematic in patients with renal insufficiency[10]. In such cases, contrast enhanced ultrasound (CEUS) could be used for focal liver lesions characterization in cirrhotic liver[11]. CEUS is a safe imaging modality, which enables real-time depiction of the typical contrast-enhancement pattern of HCC, while CT and MRI may fail to show enhancement because of inappropriate arterial-phase timing[12,13].
Nevertheless, not all HCC lesions display typical enhancement pattern, which leads to many false negative findings on CT and MRI[14]. The difficulties in non-invasive HCC diagnosis could arise not only due to its atypical enhancement pattern, but also due to a variety of morphological growth patterns, different histological subtypes, and intralesional complications, such as haemorrhage, necrosis, and cystic degeneration. Therefore, the aim of this study was to present an overview of MRI features of atypical and rare HCC types, with emphasis on differential diagnosis.
PATHOLOGICAL FEATURES OF HCC
According to the size of the lesion, HCC can be divided into: (1) Small HCCs, including lesions with diameter up to 2 cm; and (2) Advanced HCCs, which consist of lesions larger than 2 cm[15,16]. Small HCC can be further divided into two types: A distinctly nodular type, and an indistinctly nodular type[17]. These two types differ not only in morphology, but also in their biological behavior. The distinctly nodular type is seen as a clear nodule with a fibrous capsule and/or fibrous septations, representing 80% of moderately-differentiated lesions. The indistinctly nodular type, on the other hand, is mostly an ill-defined, well-differentiated lesion which lacks hypervascularity in arterial phase imaging[17]. Namely, they receive portal blood supply in addition to an arterial blood supply, because of the presence of considerable number of portal tracts within the tumor with insufficient development of unpaired arteries[18]. Therefore, they are often presented as hypovascular nodules on contrast enhanced MRI with frequent intralesional fatty changes. In addition, invasion into portal vein branches and intrahepatic metastasis are not observed in the indistinctly nodular type, while they can be seen in up to 22% of cases in distinctly nodular type[17]. For this reason, indistinctly nodular small HCC is thought to represent carcinoma in situ, and is considered as early HCC.
Using Eggel’s classification, advanced HCC can be classified according to the gross morphology into three types: Nodular type, massive type, and diffuse type[19]. The nodular type consists of single or multiple nodular well demarcated tumors. The massive type represents a large ill-defined lesion occupying almost the entire right or left liver lobe, whereas the diffuse type consists of numerous small tumor nodules scattered throughout whole liver. As this classification is not always applicable to many surgically resected HCCs, further subclassification of the nodular type was introduced dividing this type into: (1) Simple nodular type; (2) Simple nodular type with extranodular growth; and (3) Confluent multinodular type. Simple nodular type is a well demarcated lesion, encapsulated by a fibrous capsule, while simple nodular type with extranodular growth is associated with multiple satellite lesions around the dominant tumor[20]. Confluent multinodular type consists of the confluence of multiple variably sized tumor nodules[20].
TYPICAL MRI FEATURES OF HCC
Typical HCCs are nodular lesions, hypointense on T1-weighted image, moderately hyperintense on T2-weighted image, and exhibit intense arterial phase contrast enhancement with rapid washout in portal-venous and delayed phase[9] (Figure 1). Hypervascularity in arterial phase is considered to be the main imaging finding of HCC, and when followed by washout in portal-venous phase, represents the typical vascular profile of HCC[21]. In cases where nodules have high signal intensity on precontrast MRI, the subtraction technique is useful for determining contrast enhancement in arterial phase[9].
Figure 1 Typical hepatocellular carcinoma in 68-year old man with cirrhosis.
A: On axial T2-weighted image a nodular heterogeneously hyperintense tumor (arrow) is seen; B-D: The lesion (arrow) is hypointense on T1-weighted image (B), hypervascular on arterial phase (C) with washout in portal venous phase (D).
All contrast agents used for HCC diagnosis can be divided into gadolinium-based chelated agents, which include extracellular and hepatobiliary (HB) agents, and non-gadolinium based agents[22]. Extracellular contrast agents whose pharmacokinetics is similar to iodinated contrast agents for CT, are most commonly used in everyday practice[22]. Namely, these contrast agents circulate in intravascular compartment, and then freely distribute in the extracellular compartment[22]. While these contrast agents provide only information about vascularity of the lesion, HB agents display also HB phase[23]. These dual-acting contrast agents are initially distributed in the extracellular fluid compartment, and thereafter taken up to varying degrees by functioning hepatocytes[23]. HB agents are taken up from sinusoids into hepatocytes by organic anion–transporting polypeptides (OATP), such as OATP1B1 (synonymous with OATP8) and OATP1B3[23]. On the other hand, multiple resistance-associated protein 2 (MRP2) located in the canalicular membrane of hepatocytes or tumour cells is responsible for excretion of HB agents into bile ducts, while MRP3 or MRP4 located in the sinusoidal membrane, return the contrast agents back to sinusoids[23]. The most commonly used HB agents are gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid (Gd-EOB-DTPA), and gadobenate dimeglumine (Gd-BOPTA)[23]. In HB phase, most HCCs are hypointense in comparison to surrounding liver parenchyma, indicating that these lesions do not contain functional hepatocytes[24].
In order to standardize terminology and provide uniform radiological reports for evaluation of the lesions identified in patients with chronic liver diseases, American College of Radiology developed the Liver Imaging-Reporting and Data System (LI-RADS)[21,25]. This system provides characterization of full spectrum of lesions and pseudolesions encountered in patients at risk. Moreover, imaging criteria for hypovascular HCC, and various benign and malignant hepatic tumors, including non-HCC malignancies like cholangiocarcinoma (CCC) are discussed. In addition, LI-RADS can also be applied when MRI studies are obtained with gadoxetic acid, and in these cases hypointensity of the lesion in HB phase is considered to be an ancillary finding of HCC. Hepatic lesions are categorized from LR1 to LR5, depending on their imaging features. LR1 correspond to definitely benign lesions, LR2 are lesions considered probably benign, LR3 are lesions with intermediate risk of being HCC, LR4 include lesions that are probably HCC, and LR5 are definitely HCC[21,25].
HCC WITH ATYPICAL ENHANCEMENT PATTERN
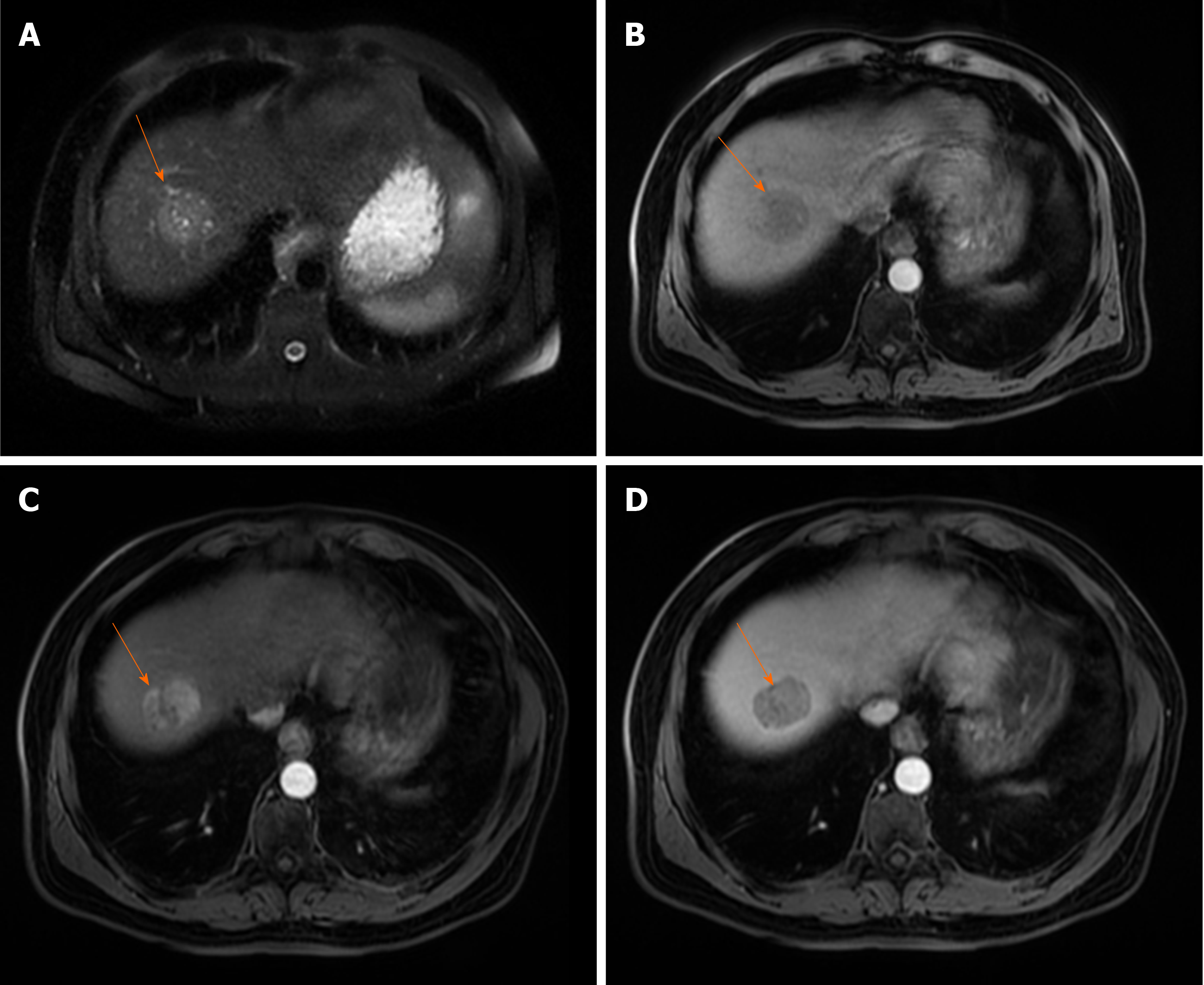
According to current guidelines, only the typical vascular profile, characterized by late arterial hyperenhancement followed by washout in lesions larger than 1 cm, can be recognized as a radiological hallmark for HCC in the setting of cirrhotic liver[21]. However, in the study by Piana et al[26], MRI had a sensitivity of only 37.1% in the diagnosis of small HCCs, and 78.8% in HCCs larger than 3 cm, using this typical vascular criteria. A slightly higher sensitivity for the detection of small HCC was reported in the study by Forner et al[14] (61.7%), since these authors used not only the vascular profile as diagnostic criteria, but also T2-weighted hyperintensity. Therefore, the use of validated diagnostic criteria still results in the need for biopsy in up to 67% of patients with HCC that are 2 cm or smaller[14]. This low sensitivity for the detection of small HCC could be explained by the multistep process of carcinogenesis, with the first step being loss of portal tracts before neovascularization occurs[27]. Thus, early HCC may be seen as an isointense nodule on late arterial phase and hypointense lesion on portal-venous phase. Reportedly, 17% of HCCs with a diameter 1-2 cm will exhibit these characteristics[28,29] (Figure 2). Further characterization of such nodules may be possible using more advanced MRI tools, such as hepatospecific contrast agents like gadoxetic acid, and diffusion weighted imaging (DWI)[30-33]. The additional value of these methods could be explained by the complexity of hepatocarcinogenesis which includes not only changes in vascularity, but also in architecture, cellular density, hepatocyte function, and Kupfer cell number[34]. Recent reports have shown that hyperintensity on high b-value DWI, and hypointensity on HB phase is strongly associated with progression to hypervascular HCC[29,35,36]. The usefulness of DWI was also shown by Le Moigne et al[37] who found that adding DWI to conventional sequences increased sensitivity in detecting small HCC from 75.7% to 87.8%.
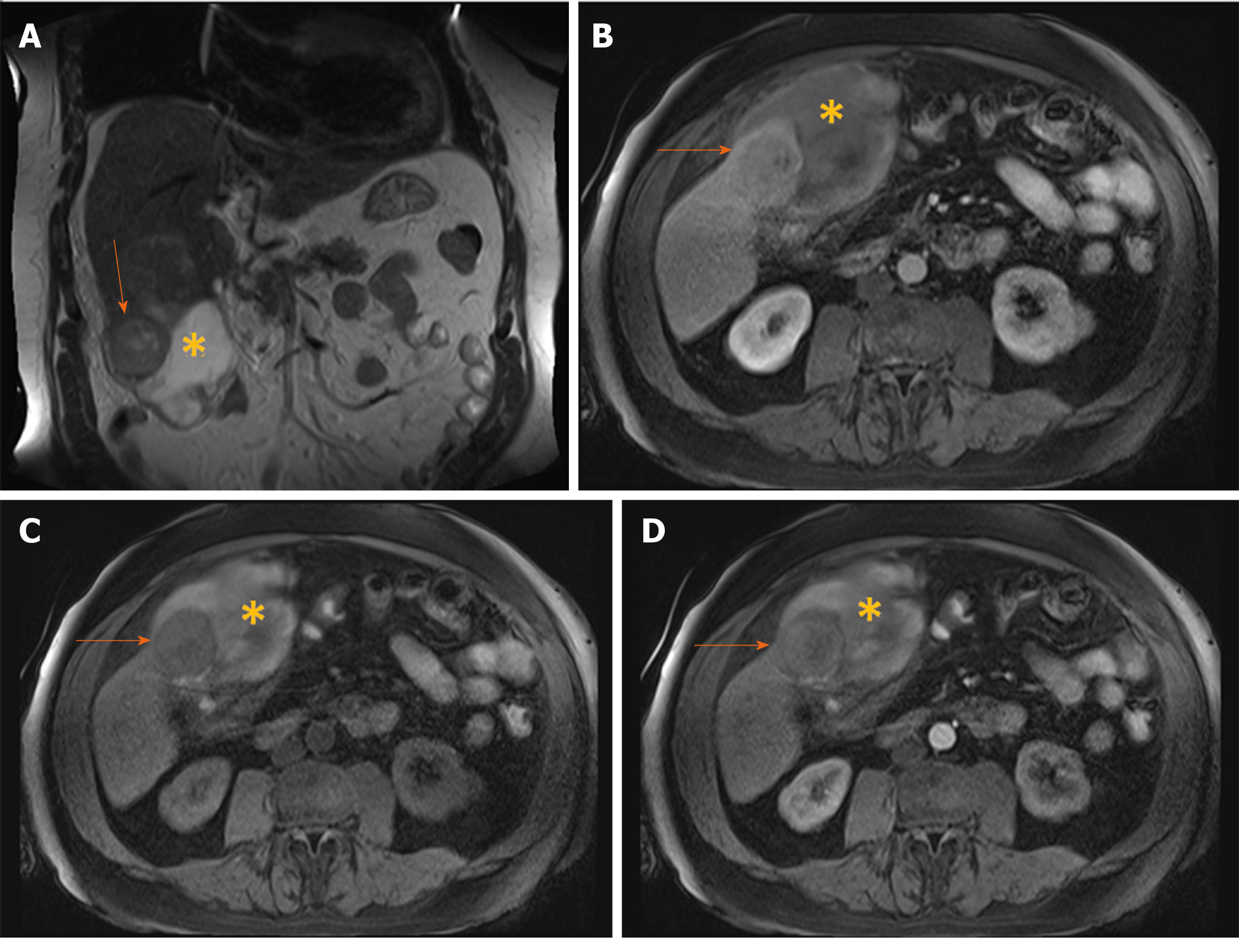
Figure 2 Hypovascular hepatocellular carcinoma in 58-year old woman with cirrhosis.
A: On axial in-phase image tumor (arrow) is isointense with surrounding liver parenchyma; B: On opposed-phase image there is a partial drop of signal intensity in the lesion corresponding to the fatty component; C-E: The lesion (arrow) is slightly hyperintense on T2-weighted FS image (C), hyperintense on diffusion weighted imaging (D), and hypointense on T1-weighted FS image (E); F and G: On arterial phase (F) the lesion is isointense with washout in portal venous phase (G); H: On hepatobiliary phase after administration of gadoxetic acid the tumor (arrow) is hypointense.
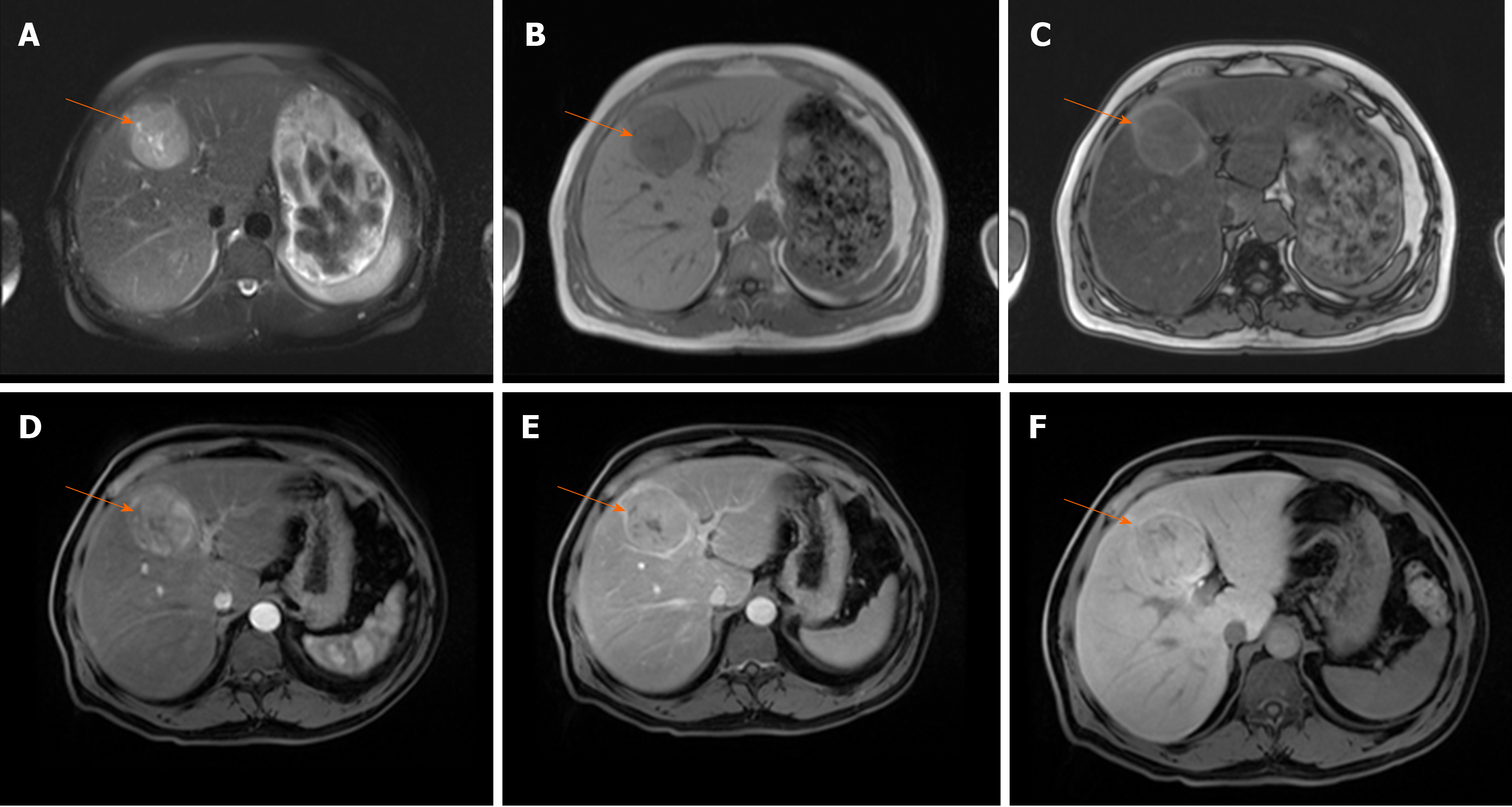
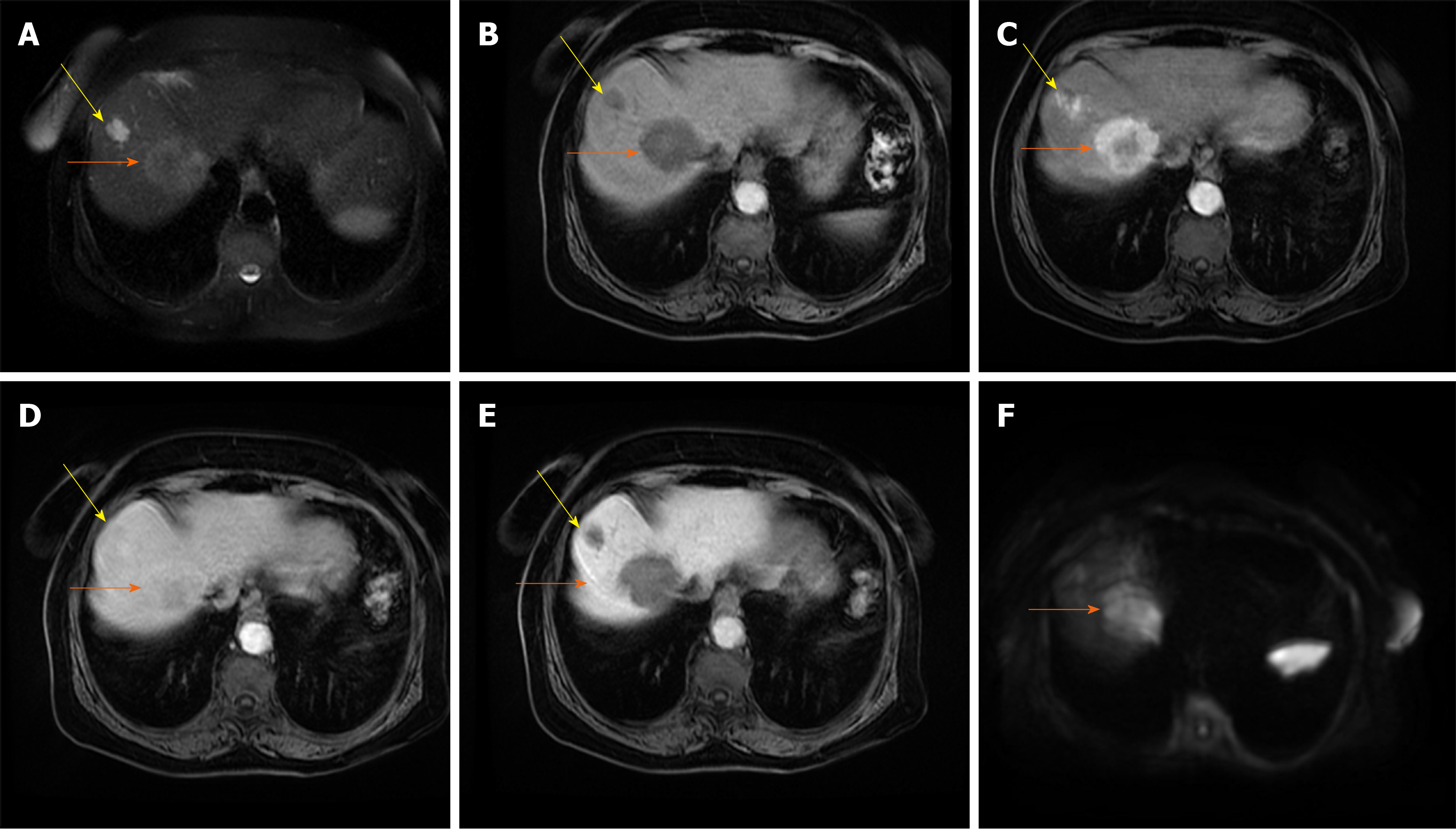
As the number of unpaired arteries increases during the hepatocarcinogenesis, the lesions become hypervascular on arterial phase imaging[27]. Depending on the number of portal tracts, the appearance of the lesion on portal venous phase can vary from isointensity to hypointensity[38]. The low sensitivity of typical vascular profile for detection of small HCC is namely due to hypervascular HCCs which do not washout in the portal or equilibrium phase, since truly hypovascular HCCs are very rare[39]. Kim et al[40] in their series on 131 HCCs have shown that 39% of lesions did not show washout. Since there are other benign lesions behaving as hypervascular nodules without washout, it is obvious that there is a need for additional imaging criteria for accurate diagnosis of such atypical HCC. In this regard, hyperintensity on DWI and/or hypointensity on hepatobilliary phase have been proposed as additional diagnostic criteria[34]. Thus, in case of hypervascular nodule in cirrhosis which does not have washout on delayed phase, the presence of either DWI hyperintensity, or HB phase hypointensity should raise suspicion of HCC[41] (Figure 3). Although this atypical enhancement pattern is mostly reported for small HCCs in the cirrhotic liver, it can rarely be seen even in large lesions (Figure 4). In such cases, differential diagnosis with other hypervascular lesions, especially with nonsteatotic adenoma is very difficult and usually made on pathology.
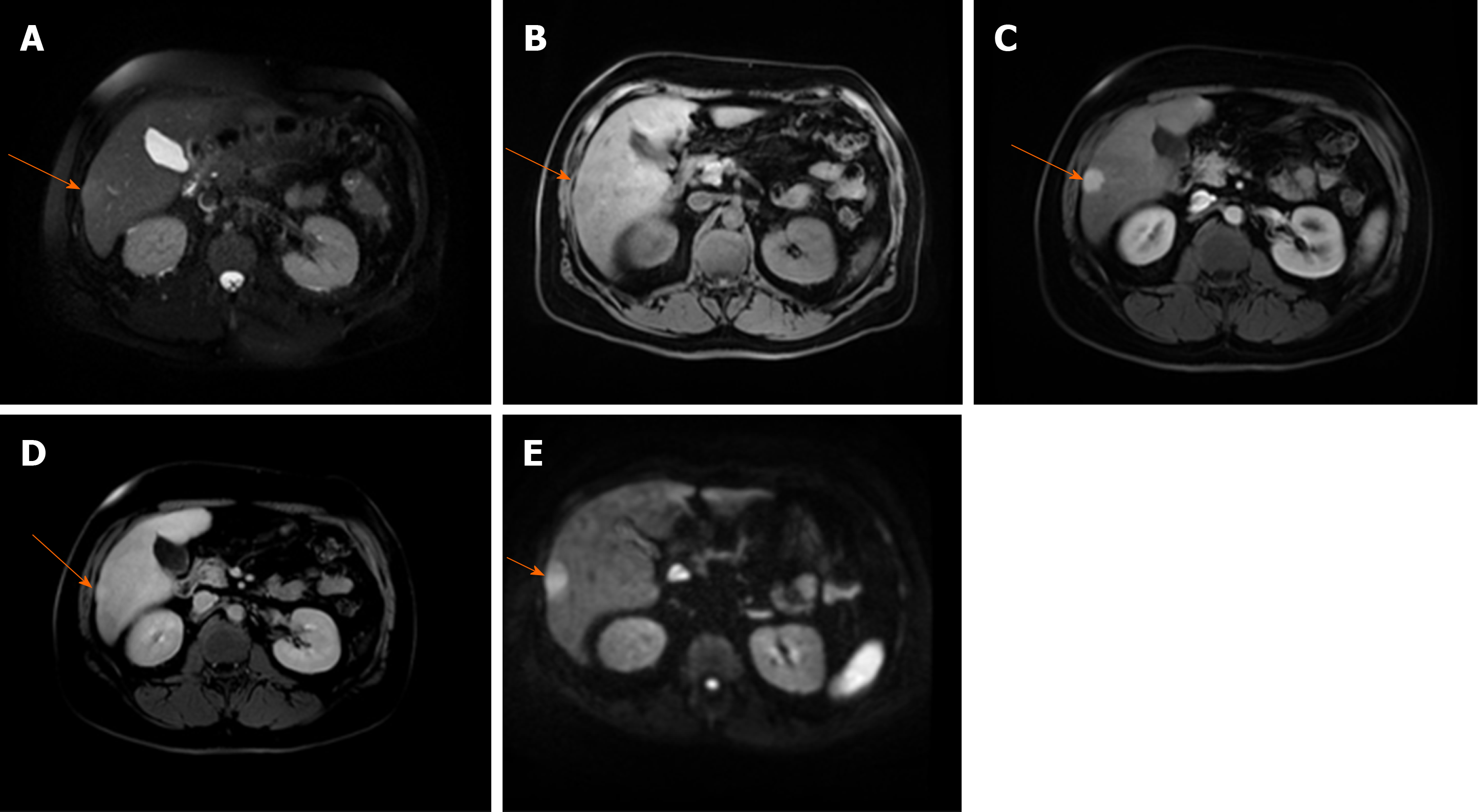
Figure 3 Hepatocellular carcinoma in 64-year old man with cirrhosis.
A: Axial T2-weighted FS image shows slightly hyperintense nodular lesion (arrow) located in segment VI; B-D: The lesion is hypointense on T1-weighted fat-saturated image (B), hypervascular on arterial phase (C) without washout on portal-venous phase (D); E: The tumor (arrow) is hyperintense on diffusion weighted imaging.
Figure 4 Hepatocellular carcinoma in 73-year old man with alcoholic cirrhosis.
A: Axial T2-weighted fat-saturated image shows slightly hyperintense well-defined nodular lesion (arrow) in segment Iva; B-D: The lesion (arrow) is hypointense on T1-weighted FS image (B), hypevascular on arterial phase (C) without washout on portal-venous phase (D); E and F: On hepatobiliary phase the tumor is strongly hyperintense (E) with diffusion restriction on diffusion-weighted image (F).
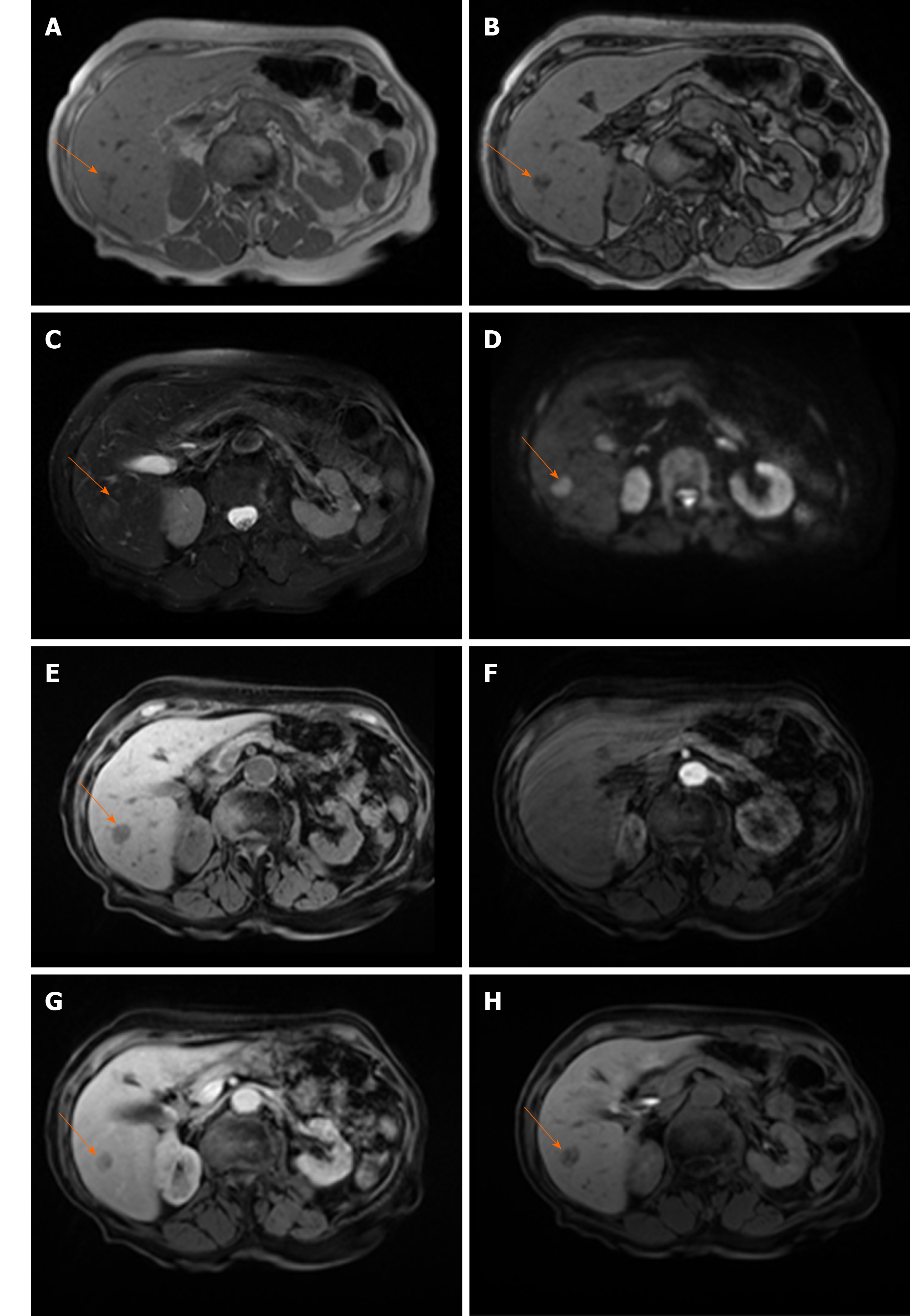
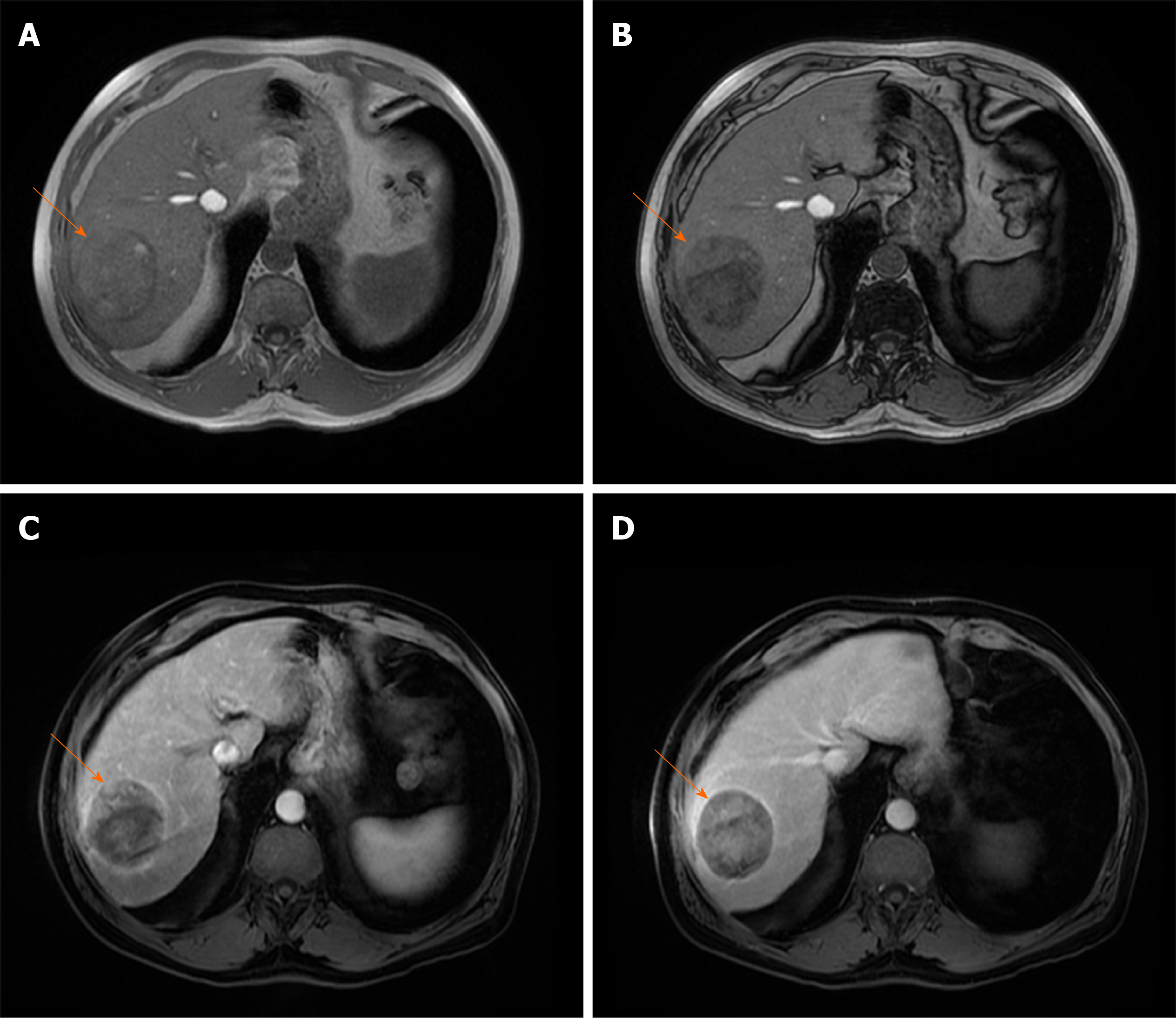
HCCs are generally hypointense on the HB phase due to decreased or absent retention of gadoxetic acid[42]. Nevertheless, 10%-25% of HCCs may be seen as isointense or even hyperintense lesions on the HB phase[43-46] (Figure 5). This could be explained by overexpression of OATP which is responsible for uptake of gadoxetic acid in hepatocytes[42]. Moreover, previous studies have shown that HCCs with gadoxetic acid uptake are specific genetic subtype with less aggressive behavior and better prognosis[43,47]. The possible explanation for such behavior is lower rate of microvascular invasion in HCCs hyperintense in HB phase, as was shown in study by Kim et al[48].
Figure 5 Hepatocellular carcinoma in 54-year old man with non-alcoholic fatty liver disease.
A: Axial T2-weighted FS image shows hyperintense lesion (arrow) in segment Ivb; B and C: Dual-echo images show that tumor (arrow) is hypointense on in-phase image (B) without signal drop on opposed-phase image, while background liver parenchyma shows diffuse signal drop as a consequenence of fatty liver disease (C); D and E: The lesion (arrow) is hypervascular on arterial phase (D) without washout on portal-venous phase (E); F: On hepatobiliary phase the nodule (arrow) is isointense with surrounding liver parenchyma.
UNUSUAL MORPHOLOGICAL TYPES OF HCC
Diffuse HCC
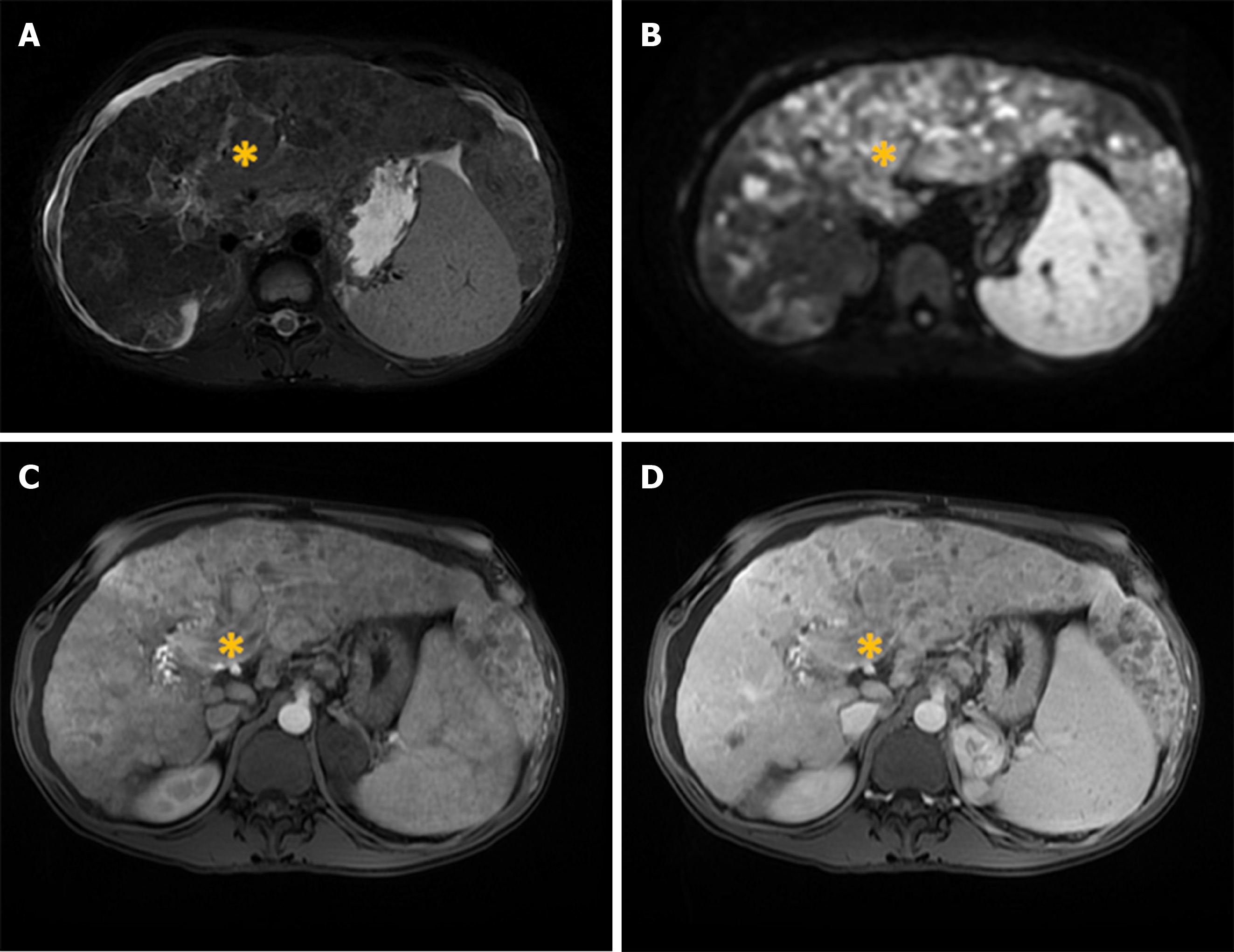
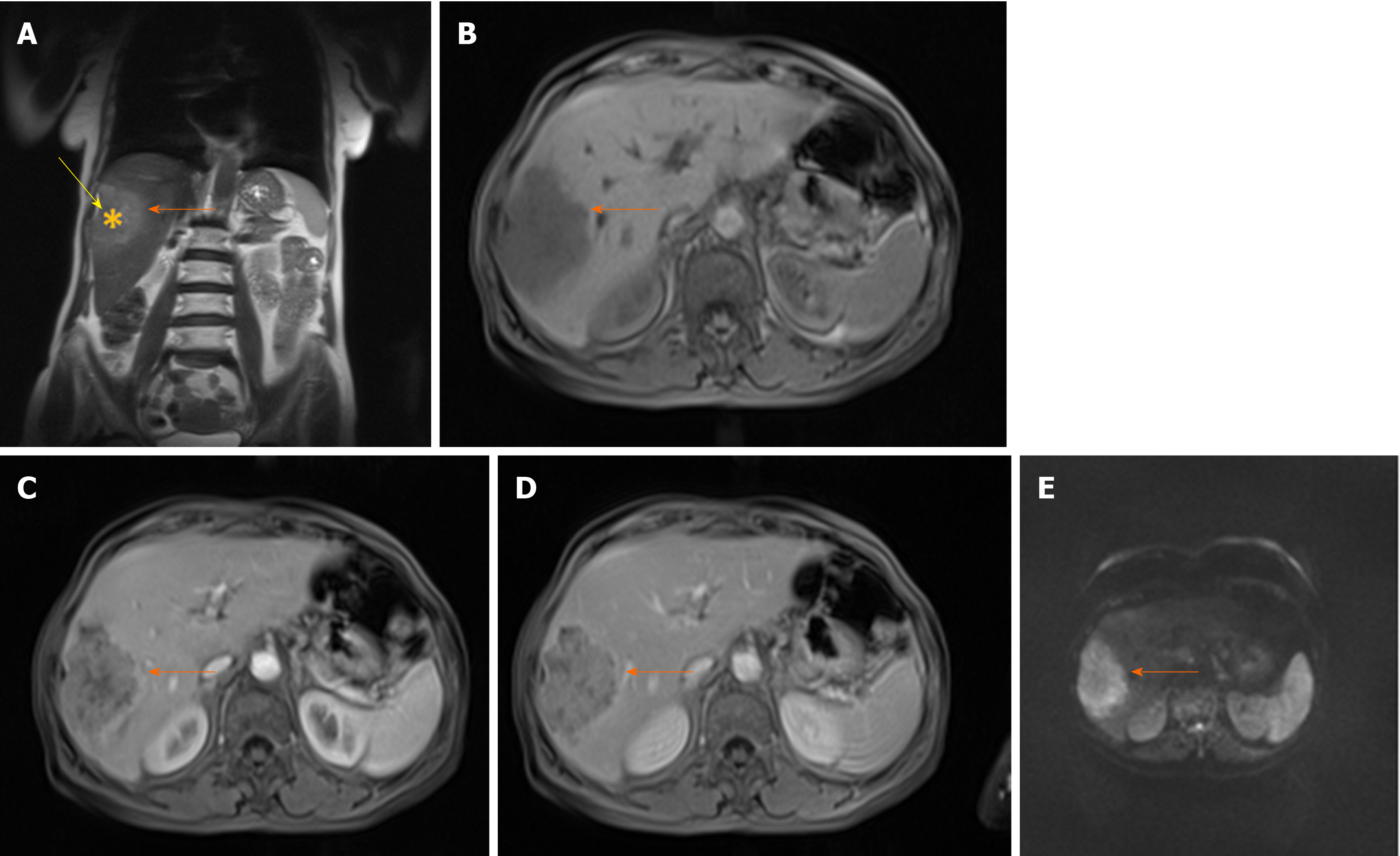
Diffuse or cirrhosis-like HCC is a rare type of HCC presenting as tumor that spreads throughout entire liver, with multiple uniformly sized nodules resembling cirrhotic nodules[49]. Macroscopically, in this type of tumor the liver contains multiple (more than 20) small subcentimeter nodules scattered among cirrhotic nodules[50]. It is still not known whether diffuse HCC develops as intrahepatic metastases from a single primary tumor occurring in a short period, or it is a multiclonal disease with multiple independent tumors[50,51]. The patients with this type of tumor usually have fast deterioration of their general condition with rapid liver enlargement leading to hepatic failure. It is often associated with portal vein thrombosis and high levels of alpha-fetoprotein (AFP)[50]. Moreover, because of subtle enhancement of the tumor in arterial phase, portal vein thrombosis may be the leading sign of diffuse HCC[49]. In this regard, it is of great clinical importance to differentiate bland tumor thrombus which occurs commonly in cirrhosis, and tumor thrombus[52]. Distension of portal vein lumen, and neovascularity of thrombus are shown to be highly sensitive signs for malignant thrombus[52].
There are only a few reports in the literature describing MRI features of diffuse HCC[53]. In the study by Kanematsu et al[53], patchy or miliary enhancement on arterial phase was found to be relatively specific for this type of HCC. Differential diagnosis of diffuse HCC and transitory hepatic intensity difference seen in the setting of portal-venous obstruction can be difficult. However, heterogeneous washout of the tumor nodules on portal-venous phase is characteristic for malignant lesions[53]. In addition, diffuse HCC mostly appears as irregular hypointense lesion on HB phase[53]. Due to reduced conspicuity of the diffuse HCC on postcontrast images, the tumor may be more visible on T1-, T2-weighted and DWI images[53]. These lesions are typically T1-weighted hypointense, heterogeneously hyperintense on T2-weighted images, and hyperintense on DWI[54] (Figure 6). Although very high levels of AFP have been observed in majority of patients with diffuse HCC, normal AFP values can be found[55]. As diffuse HCC displays typical morphological appearance, it can hardly be mistaken for other malignant lesions, specifically CCC and metastatic disease. Namely, both these entities have a more focal pattern of involvement, and are not associated with portal vein thrombosis although compression can be seen[54].
Figure 6 Diffuse hepatocellular carcinoma in 38-year-old man with long-standing Wilson disease.
Axial T2-weighted FS image shows multiple moderately hyperintense nodules scattered throughout liver parenchyma. A: Note also portal vein thrombosis with signal intensity of the thrombus similar to tumor nodules in the liver; B: On diffusion weighted image diffuse hyperintensity is seen corresponding to multiple tumor nodules; C and D: On arterial phase patchy enhancement can be seen including portal vein thrombus (C), with heterogeneous washout in portal vein phase (D).
Infiltrative HCC
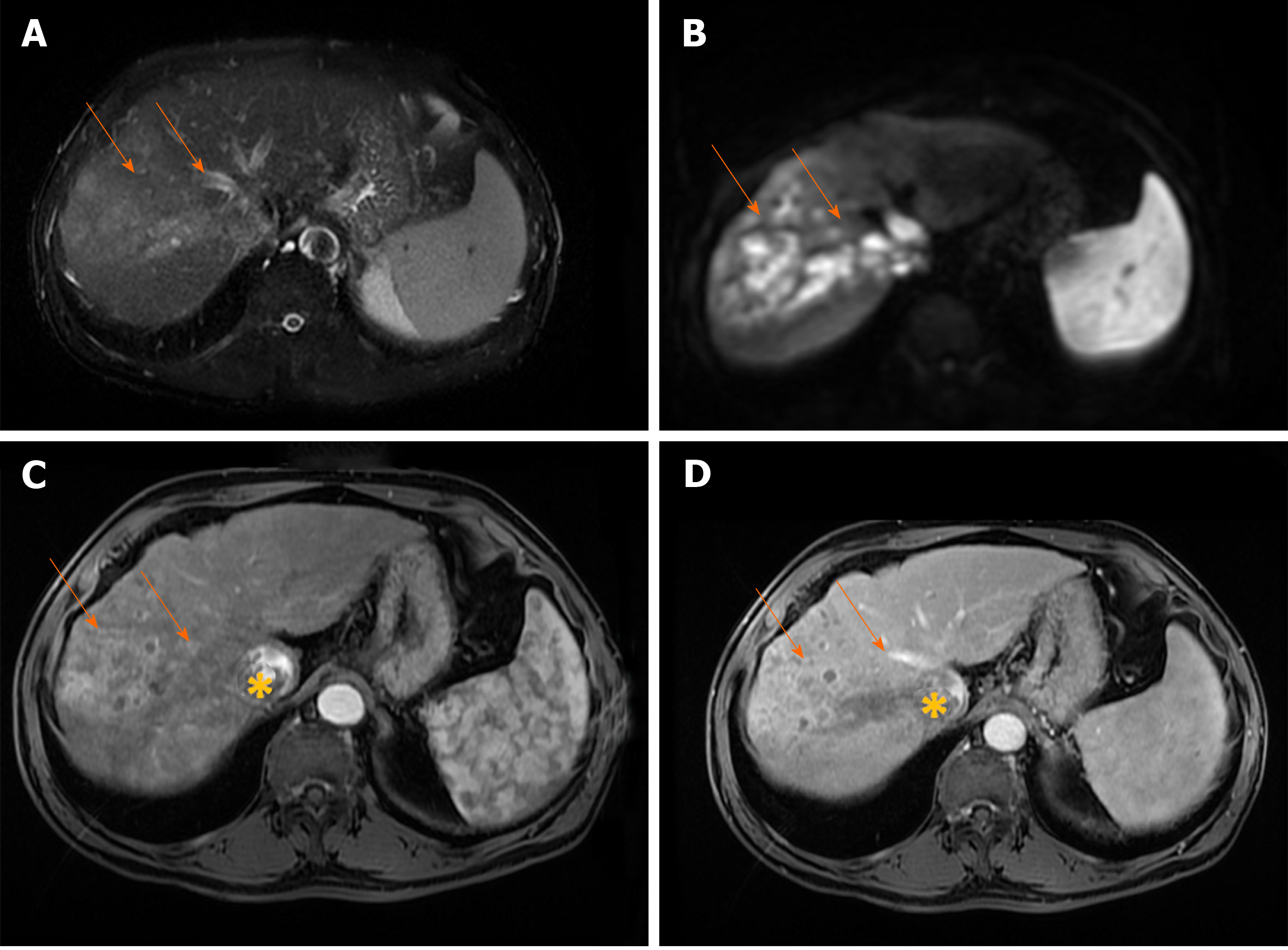
Infiltrative HCC is an ill-defined, non-encapsulated mass with irregular borders, seen in up to 8-18% of HCCs[56,57]. As a consequence of specific imaging features, infiltrative HCC is often not detected until it has progressed to an advanced stage[58]. Although there are many reports where infiltrative HCC is considered as synonym for diffuse HCC, these two terms should be distinguished[59,60]. While diffuse HCC presents with diffusely nodular liver without dominant mass, infiltrative HCC presents as a mass with irregular borders and frequently satellite nodules in the surrounding parenchyma, corresponding to the massive type in Eggel’s classification[60] (Figure 7). In the differential diagnosis of infiltrative HCC, focal confluent fibrosis in cirrhotic patients should be considered. The presence of lobular configuration, contour bulging, arterial enhancement, delayed washout, associated satellite nodules and portal vein thrombosis were proposed as highly suggestive MRI findings for differentiating infiltrative HCC from confluent fibrosis in liver cirrhosis[61-63].
Figure 7 Infiltrative hepatocellular carcinoma in 71-year old man with cirrhosis.
A: Axial T2-weighted FS image shows ill-defined mass (arrows) in segments VII and VIII; B: On diffusion weighted image the lesion is hyperintense; C and D: The tumor is heterogeneously hyperintense on arterial phase (C), with washout in portal vein phase (D). Note also right hepatic vein thrombosis with propagation of the tumor thrombus in vena cava inferior (asterix).
UNCOMMON HISTOLOGICAL VARIANTS OF HCC
Classical HCC is composed of liver-cell trabecular structure, and a stroma formed of sinusoid-like blood spaces[17]. According to cell differentiation, HCC can be divided into well, moderately-, and poorly-differentiated lesions[17]. However, besides typical histological organization, there are several variants including sarcomatous, scirrhous, fibrolamellar HCC, clear cell HCC, steatotic HCC, and HCC with lymphoid stroma[17].
Fibrolamellar carcinoma
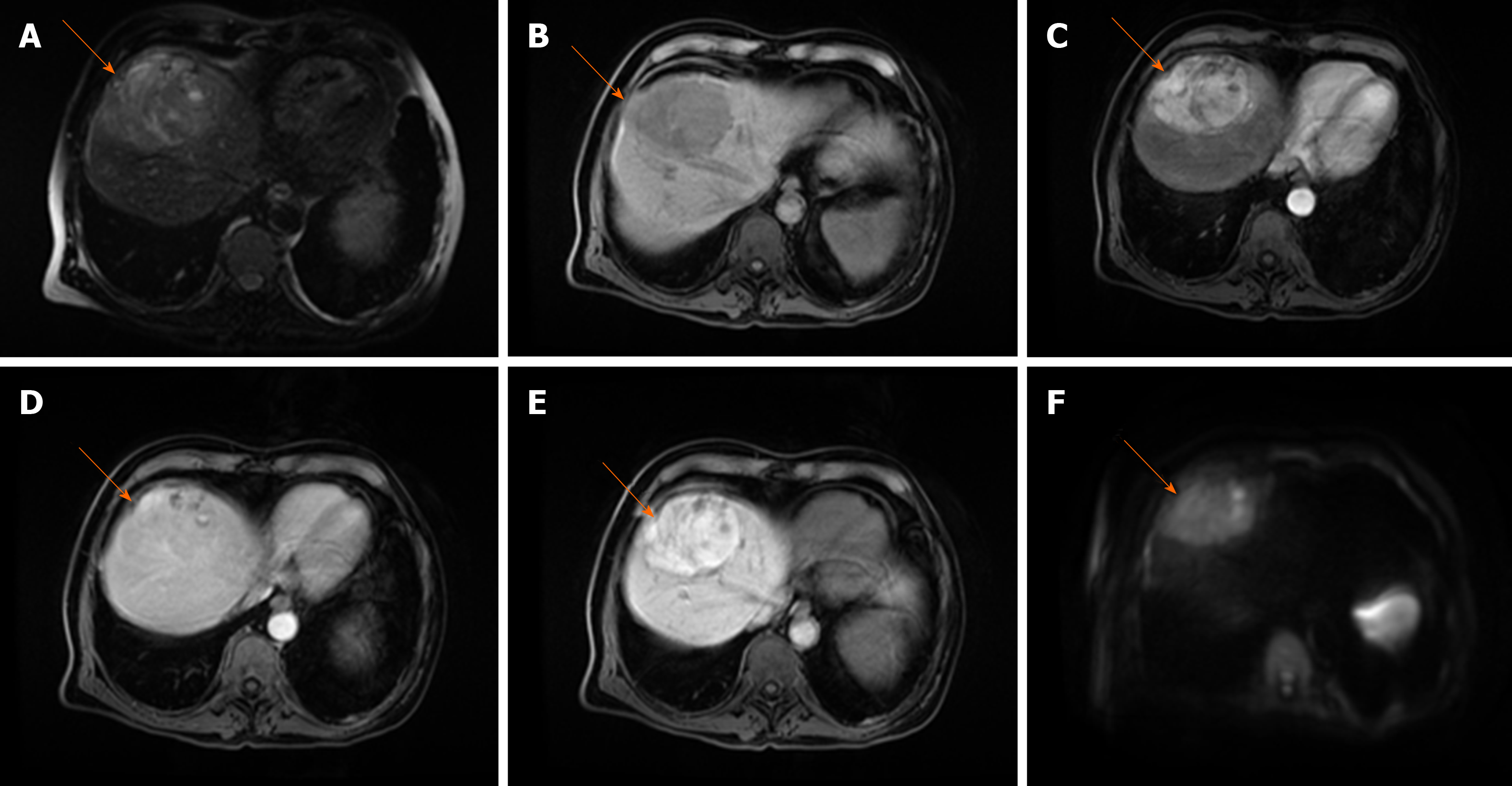
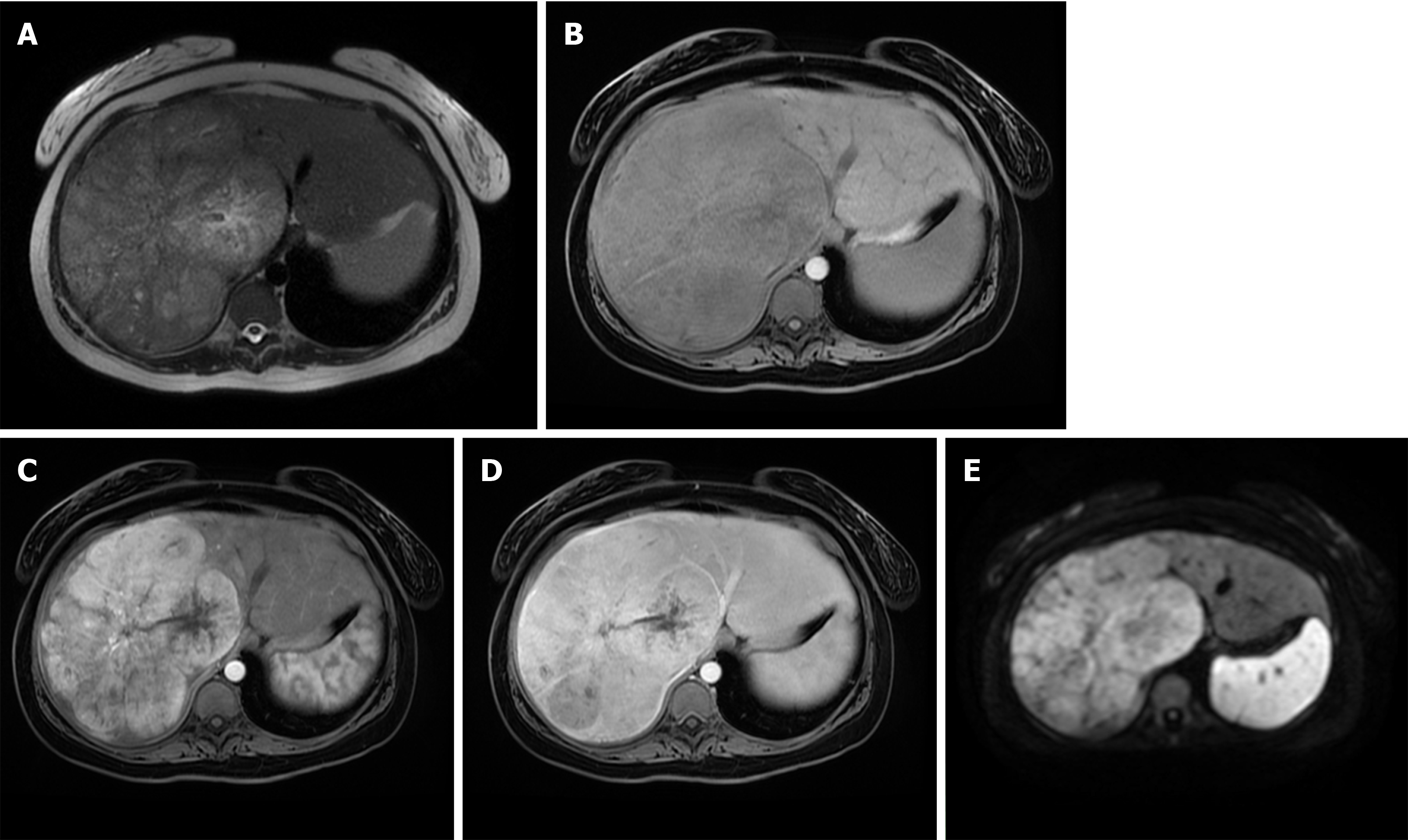
Fibrolamellar carcinoma (FLC) is a rare hepatic tumor, accounting for less than 1% of HCCs[64]. In contrast to classical HCC, FLC occurs in non-cirrhotic livers, mostly in young adults with more favorable prognosis than that of classical HCC[65]. Approximately 65%-85% of fibrolamellar HCCs occur in patients under 40 years old, while only 2%-4% of classic HCCs are found in this age group[66]. In addition, chronic liver disease and cirrhosis are not recognized as a risk factors for FLC[64,65]. Histologically, it has been characterized by lamellar pattern of fibrosis separating sheets of tumor cells, and a distinct cytology with abundant eosinophilic cytoplasm[67]. On MRI, FLC is usually hypointense on T1-weighted images, and moderately hyperintense on T2-weighted images[68]. Characteristic central stellate scar, present in 65%-70% of FLC, is mostly hypointense on T2-weighted images, in contrast to focal nodular hyperplasia whose central scar is hyperintense[68]. The presence of calcification within the central scar is characteristic for FLC, but is hardly detectable on MRI[69]. After intravenous administration of contrast material, most FLCs show marked heterogeneous enhancement on arterial phase, becoming isointense or hypointense on portal venous phase[68,69] (Figure 8). The variable appearance of FLC on portal venous phase could raise difficulties in differentiation from focal nodular hyperplasia. It has been reported that FLC does not uptake hepatospecific contrast agents in contrast to focal nodular hyperplasia, allowing differential diagnosis among these entities[70]. Moreover, the central scar in focal nodular hyperplasia enhances in delayed phases, while this feature is absent in most FLC[69]. Lymphadenopathy at hepatic hilum and hepatoduodenal ligament is a frequent finding in FLC, seen in up to 65% of cases, and is associated with poor prognosis[71].
Figure 8 Fibrolamellar hepatocellular carcinoma in 23-year old woman without chronic liver disease.
A: Axial T2-weighted image shows large heterogeneous mass occupying almost whole right liver lobe; B-E: The tumor is hypointense on T1-weghted FS image (B), hypervascular on arterial phase (C) with washout in parts of the lesion on portal venous phase (D) and diffusion restriction (E).
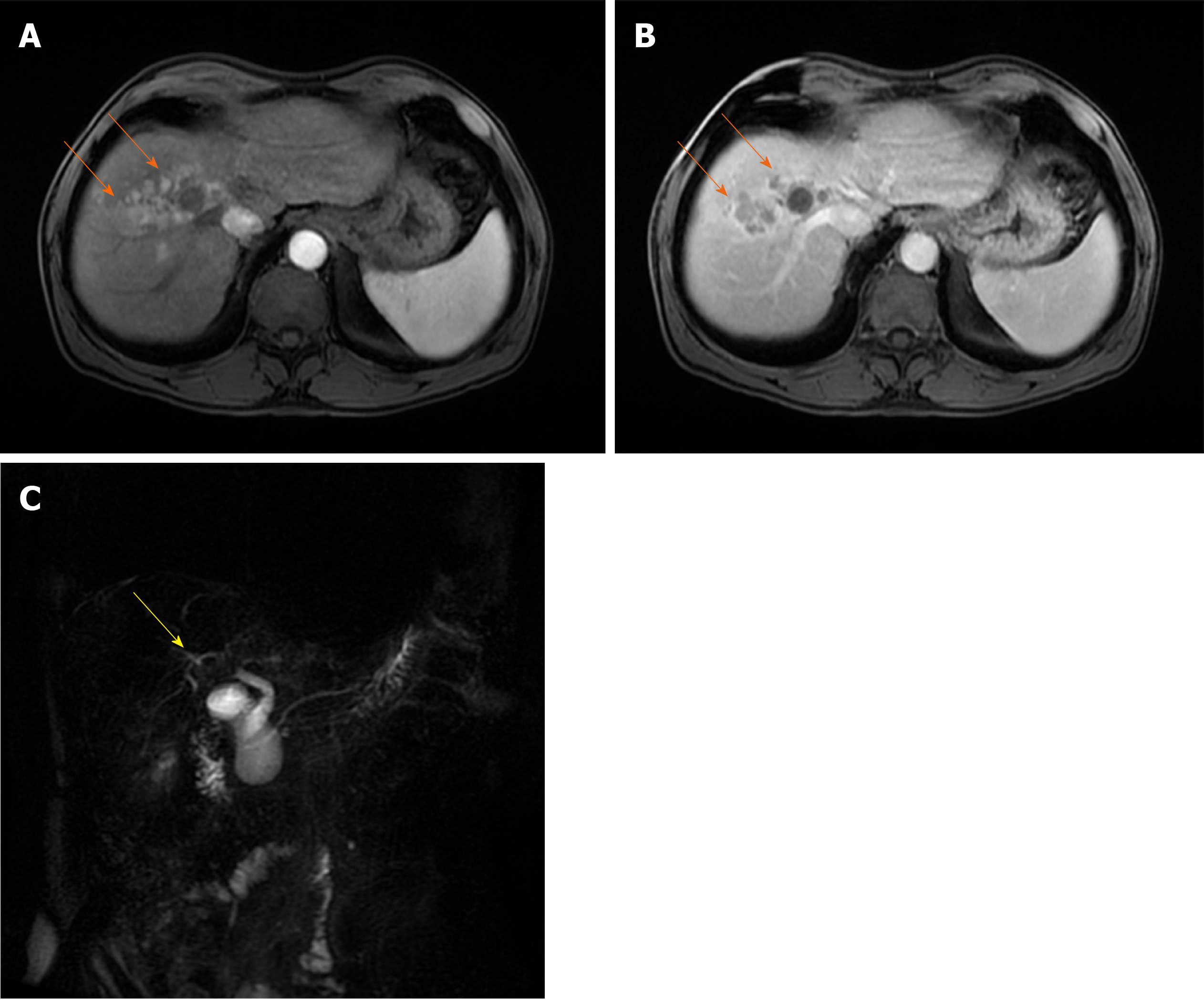
Combined HCC and CCC
Occasionally, other neoplastic tissues coexist with HCC, CCC being the most common[72,73]. These tumors named combined or byphenotipic HCC-CCC (cHC) occur in less than 1% of all liver carcinomas, with worse prognosis in comparison to pure HCC[74,75]. They can further be classified into three categories: (1) Double cancer representing tumors in which areas of HCC and CCC coexist separately; (2) Combined type where both components are present adjacent to each other, and mixed together; and (3) Mixed type in which both components are intimately mixed[76]. Nevertheless, this classification has its shortcomings as it is often very difficult to distinguish mixed and combined types. The pathogenesis of cHC has remained unclear for many years[77]. Recent advances in hepatic progenitor cell investigations have proposed the concept that cHC originates from these cells[73,78]. cHC may occur in cirrhotic liver, but also in patients with normal liver. The preoperative diagnosis of cHC is very difficult, and in most cases it is established on histopathology[79]. In the current literature there are only a few studies describing the radiological characteristics of cHC[80-83]. MRI features are very different, depending on the proportion of each component within the lesion[83,84]. The combined cHC is commonly solitary mass, heterogeneously hyperintense on T2-weighted image[84]. Nevertheless, the signal of the lesion may be high on T2-weighted images in contrast to pure HCC which is slightly T2-weighted hyperintense[83]. This feature could pose diagnostic dilemma with hemangioma. In the study by Campos et al[80] it was shown that early ring-enhancement with progressively enhancing central regions is the most common enhancement pattern in cHC, which is quite similar to metastases (Figure 9). The presence of washout in these lesions may suggest the diagnosis, but is rarely seen. Enhancement pattern may be geographic, with parts of the lesion exhibiting typical vascular profile of HCC, and other parts showing progressive enhancement[84]. Furthermore, lesions may show arterial hypervascularity typical for HCC without subsequent washout[85]. In such cases the use of hepatospecific contrast agents in conjunction with tumor characteristics on T1- and T2-weighted images allows differential diagnosis with focal nodular hyperplasia[85].
Figure 9 Combined hepatocellular carcinoma and cholangiocarcinoma in 65-year old woman without chronic liver disease.
A: Axial T2-weighted image shows lobulated heterogeneous mass (orange arrow) located centrally in segments VII and VIII; B-D: The tumor is hypointense on T1-weghted fat-saturated image (B), with thick rim of enhancement on arterial phase (C) and progressive enhancement on portal venous phase (D); E and F: The tumor (orange arrows) is hypointense on hepatobiliary phase (E) and hyperintense on diffusion-weighted image (F). Note also hemangioma peripherally in segment VIII (yellow arrow).
Steatotic HCC
Steatotic HCC is a distinct cytological type characterized by accumulation of the fat in hepatocytes, and is seen in 20% of HCCs[86]. It has been postulated that fatty change arises because of insufficient development of unpaired arteries and subsequent temporal hypoxic conditions[87]. This is considered to be the main reason for frequent fatty change in HCCs smaller than 2 cm, and is less common in larger tumors[87]. The occurrence of fatty change in well-differentiated larger tumors can be explained by overexpression of hypoxia-inducible proteins in addition to insufficient arterial network[88]. The microvesicular steatosis accounts for majority of steatotic HCCs, and can easily be detected using dual-echo imaging with low signal intensity on opposed phase images[89]. If macrovesicular steatosis is present, decrease of signal intensity can be seen also on fat-suppressed images[90]. Although fat containing HCC displays typical vascular profile, it must be considered that the attenuation effect of fat may overcome the effects of arterial phase enhancement[86] (Figure 10). Sometimes it may be very difficult to make differential diagnosis with other hypervascular fat containing liver lesions, such as angiomyolipoma and adenoma[91]. In such cases, clinical data should be considered, since hepatic adenoma is frequently encountered in young patients, especially after oral contraceptive or androgen usage.
Figure 10 Steatotic hepatocellular carcinoma in 67-year old man with cirrhosis.
A and B: T1-weighted in-phase image shows nodular tumor in segment VII (arrow) which is mostly isointense with surrounding liver parenchyma (A) with signal drop of posterior part of the lesion on opposed-phase image (B); C and D: On arterial phase image hypervascularization is clearly seen for nonstetaotic part of the tumor (C) with washout on portal-venous phase (D). Note relative lack of hypervascularity in steatotic part of the tumor, and extracapsular growth on the lateral border of the lesion.
Scirrhous HCC
Scirrhous HCC is a variant of HCC characterized by abundant intralesional fibrosis occurring in up to 4.6% of HCC cases[92]. Differentiation from intrahepatic CCC is usually difficult, since both lesions have rich fibrous stroma, and occur in cirrhotic livers[93]. Scirrhous HCCs usually present as lobulated tumors, hypointense on T1-weighted images, and heterogeneously hyperintense on T2-weighted images[93]. Concerning enhancement characteristics, in the study by Kim et al[94], the most frequent enhancement pattern was a peripheral rim-like enhancement on arterial and portal phase, seen in 62% of cases, followed by progressive enhancement of the central hypoattenuating area on the equilibrium phase. In the same study, washout was seen in only 19% of scirrhous HCCs, compared with 99.7% of typical HCCs[94]. The characteristic enhancement pattern of scirrhous HCC could be explained by histological features of the lesion, containing both carcinoma cells with rich vascularity on the periphery of the lesion, and fibrous stroma in the center of the lesion[94,95] (Figure 11). When these lesions are localized in subcapsular location, capsular retraction may also be seen, similarly to cholangiocellular carcinoma[95]. However, peripheral biliary dilatation is rarely present[95]. Choi et al[95] reported ancillary features for discriminating scirrhous HCC and cholangiocellular carcinoma including T2-weighted central darkness, and the presence of capsule, which were shown to be significant and independent MRI predictors for scirrhous HCC[95].
Figure 11 Scirrhous hepatocellular carcinoma in 68-year old woman with chronic hepatitis C infection.
A: Coronal T2-weighted image shows moderately hyperintense subcapsullary located lesion in segments VI and V (orange arrow); B-D: The tumor (orange arrows) is hypointense on axial T1-weighted FS image (B), hypervascular on arterial phase (C) with only small regions of washout in portal venous phase (D); E: On diffusion-weighted image the lesion is hyperintense. Note also capsular retraction on A (yellow arrow).
UNUSUAL HCC GROWTH PATTERNS
Bile duct tumor growth
Intra-bile duct growth of HCC is a very rare complication, with only a few cases described in the literature[96] (Figure 12). Direct tumor invasion into the bile ducts leads to CT and/or MRI detection of soft tissue mass within the lumen of the duct[96]. The bile duct tumor thrombus shows typical enhancement pattern as dominant tumor, with arterial hypervascularity, and portal venous washout[96]. Periductal infiltrative growth is not described in HCC lesions.
Figure 12 Infiltrative hepatocellular carcinoma in 63-year old man with intra-bile tumor growth.
A and B: On arterial phase image ill-defined hypervascular lesion (arrows) in segment VIII is seen (A) with washout in portal venous phase (B); C: Intra-bile tumor growth is better depicted on coronal magnetic resonance cholangiopancreatography image seen as defect in the lumen right hepatic duct and its posterior branch (yellow arrow).
Intra-atrial tumor growth
Rarely, large HCC with hepatic venous invasion can propagate in the lumen of vena cava up to left atrium (Figure 13). Sometimes, the detection of intra-atrial thrombus using ultrasonography could be the first sign of HCC[97].
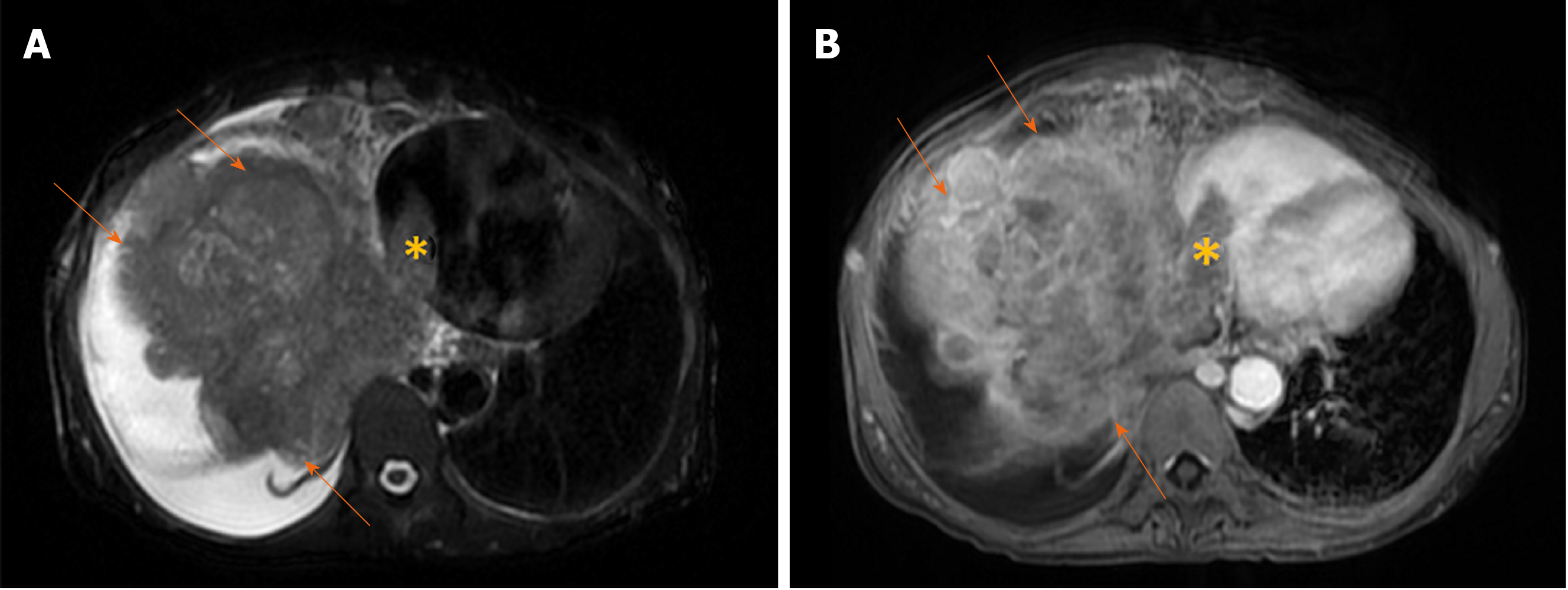
Figure 13 Massive hepatocellular carcinoma in 69-year old woman with cirrhosis.
A: Axial T2-weighted image shows a large mass (arrows) predominantly located in right liver lobe with infiltration of vena cava and tumor thrombus seen in left atrium (asterix); B: Axial image in portal venous phase shows washout in hepatocellular carcinoma (arrows) and thrombus (asterix) in left atrium.
HCC with rupture and intraabdominal bleeding
Ruptured HCC is seen in up to 15% of HCC patients[98]. Acute abdominal pain is usually the first manifestation of this complication. In this setting, CT is usually the primary diagnostic modality displaying hemoperitoneum, perihepatic hematoma, liver tumor associated with discontinuity of liver surface and enucleation sign[99] (Figure 14). Large HCC with liver contour bulging, and portal venous thrombosis is at risk for subsequent rupture[99]. Rarely, rupture of HCC can be the result of trauma or transcatheter arterial chemoembolization[100,101].
Figure 14 Hemorrhagic hepatocellular carcinoma in 70-year old man with cirrhosis presenting with acute abdominal pain.
A: Coronal T2-weighted image shows a nodular tumor (arrows) in the subcapsular location in segment V, and subhepatic hematoma (asterix); B: Axial T1-weighted FS image shows hypointense tumor (arrows) and hyperintense content of the hematoma (asterix); C and D: Arterial phase displays only subtle hypervascularity in the part of the tumor (arrows) depicted on this section (C) with washout in portal venous phase (D), and hematoma (asterix) located anteriorly.
CONCLUSION
HCC is the most common malignant liver tumor with high morbidity and high mortality, occuring usually in the setting of cirrhotic liver. When typical imaging features are present the diagnosis of HCC is straightforward. However, HCC can display wide variety of atypical forms, including uncommon growth or enhancement pattern, different histologic variants and morphological types. Although these atypical appearances of HCC are rare, it is important to recognize their imaging featues in order to privide patients appropriate treatment. MRI is the preferable diagnostic modality for diagnosis of atypical forms of HCC.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Suda T S-Editor: Wang YQ L-Editor: A E-Editor: Ma YJ