Published online Feb 21, 2019. doi: 10.3748/wjg.v25.i7.789
Peer-review started: December 8, 2018
First decision: December 28, 2018
Revised: January 10, 2019
Accepted: January 14, 2019
Article in press: January 14, 2019
Published online: February 21, 2019
Processing time: 77 Days and 5.3 Hours
Systemic therapy for hepatocellular carcinoma (HCC) has markedly advanced since the survival benefit of a molecular targeted agent, sorafenib, were demonstrated in the SHARP and Asia Pacific trials in 2007. Treatment options for patients with advanced HCC increased by sorafenib, and long-term survival for patients with advanced stage HCC has become possible to some extent. However, development of a more potent first-line novel molecular targeted agent replacing sorafenib and a potent second-line agent after disease progression on or intolerant to sorafenib has been warranted because sorafenib lacks tumor shrinking/necrotizing effects and induces relatively severe adverse events such as hand foot skin reaction. Many agents in the 1st line and 2nd line setting were attempted to develop between 2007 and 2016, but all of these clinical trials failed. On the other hand, clinical trials of 4 agents (regorafenib, lenvatinib, cabozantinib, and ramucirumab) succeeded in succession in 2017 and 2018, and their use in clinical practice is possible (regorafenib and lenvatinib) or underway (cabozantinib and ramucirumab). Furthermore, all of 5 clinical trials of combination therapy with transcatheter chemoembolization (TACE) plus a molecular targeted agent failed to date, however, the combination of TACE and sorafenib (TACTICS trials) was reported to be successful and presented at ASCO in 2018. Phase 3 clinical trials of immune checkpoint inhibitors and a combination therapy of immune checkpoint inhibitors and molecular targeted agents are also ongoing, which suggests treatment paradigm of HCC in all stages from early, intermediate and advanced stage, is expected to be changed drastically in the very near future.
Core tip: Systemic therapy for hepatocellular carcinoma (HCC) has markedly advanced since sorafenib was approved in 2007. Since then, there was no active drug for 10 years that prolong overall survival, however, in 2017 and 2018, clinical trials of 4 more molecular targeted agents including lenvatinib as first line agent, regorafenib, cabozantinib and ramucirumab as second line agent have shown their survival benefit. In addition, immune check point inhibitors, nivolumab and pembrolizumab, were approved by Food and Drug Administration. Combination cancer immunotherapy, that combines immune checkpoint inhibitors and molecular targeted agents show great promise in the treatment of HCC.
- Citation: Kudo M. Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2019 and beyond. World J Gastroenterol 2019; 25(7): 789-807
- URL: https://www.wjgnet.com/1007-9327/full/v25/i7/789.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i7.789
Hepatocellular carcinoma (HCC) is treated by surgical resection, local ablation, transarterial chemoembolization (TACE), or intra-arterial infusion chemotherapy. Most practice guidelines relevant to HCC were revised in 2017 and 2018[1-5]. In this article, the current status and future perspective of systemic therapy for HCC, which has advanced markedly will be reviewed.
Since first appearance of the molecular targeted agent, sorafenib, in 2007, systemic therapy for HCC has changed markedly. Treatment options for extrahepatic spread and vascular invasion have increased, and relatively long-term survival has been achieved, even for patients with Barcelona Clinic Liver Cancer (BCLC) stage C advanced HCC. However, sorafenib does not shrink or induce necrosis in tumors and has relatively severe adverse events (AEs), including hand foot skin reaction. Thus, development of a novel molecular targeted agent that can replace sorafenib, along with a second-line agent that can prevent/slow disease progression while a patient is undergoing treatment with sorafenib is desirable. Between 2007 and 2016, many comparative trials of new agents with sorefenib have been conducted; however, eight clinical trials of first-line agents and eight of second-line agents failed (Table 1)[6-20]. In 2017 and 2018, clinical trials of three agents (regorafenib, lenvatinib, cabozantinib, and ramucirumab) reported successful outcomes; indeed, some of these drugs are now used in clinical practice (Table 1). All of three trials in adjuvant setting after curative treatments failed (Table 2)[21-24]. In addition, five trials of combination therapy with transcatheter chemoembolization (TACE) plus a molecular targeted agent have been conducted to date, but all of them failed to show its benefit[25-29] (Table 2). In 2018, the combination of TACE and sorafenib, the TCTICS trial, reported improved progression-free survival (PFS); the results were presented at the 2018 ASCO-GI and ASCO meetings in 2018[30]. Herein, recent advances and future prospects for molecular targeted therapy for HCC will be discussed.
| Target population | Design | Trial name | Result | Presentation | Publication | 1st author | |
| Advanced | First line | 1 Sorafenib vs Sunitinib | SUN1170 | Negative | ASCO 2011 | JCO 2013[6] | Cheng AL |
| 2 Sorafenib +/- Erlotinib | SEARCH | Negative | ESMO 2012 | JCO 2015[7] | Zhu AX | ||
| 3 Sorafenib vs Brivanib | BRISK-FL | Negative | AASLD 2012 | JCO 2013[8] | Johnson PJ | ||
| 4 Sorafenib vs Linifanib | LiGHT | Negative | ASCO-GI 2013 | JCO 2015[9] | Cainap C | ||
| 5 Sorafenib +/- Doxorubicin | CALGB 80802 | Negative | ASCO-GI 2016 | ||||
| 6 Sorafenib +/- HAIC | SILIUS | Negative | EASL 2016 | Lancet GH 2018[10] | Kudo M | ||
| 7 Sorafenib +/- Y90 | SARAH | Negative | EASL 2017 | Lancet-O 2017[11] | Vilgrain V | ||
| 8 Sorafenib +/- Y90 | SIRveNIB | Negative | ASCO 2017 | JCO 2018[12] | Chow P | ||
| 9 Sorafenib vs Lenvatinib | REFLECT | Positive | ASCO 2017 | Lancet 2018[34] | Kudo M | ||
| 10 Sorafenib vs Nivolumab | CheckMate-459 | Ongoing | |||||
| 11 Sorafenib vs Durvalumab + Tremelimumab vs Durva | HIMALAYA | Ongoing | |||||
| 12 Sorafenib vs Atezolizumab + Bevacizumab | Imbrave 150 | Ongoing | |||||
| 13 Sorafenib vs Tislelizumab | Ongoing | ||||||
| Second line | 1 Brivanib vs Placebo | BRISK-PS | Negative | EASL 2012 | JCO 2013[13] | Llovet JM | |
| 2 Everolimus vs Placebo | EVOLVE-1 | Negative | ASCO-GI 2014 | JAMA 2014[14] | Zhu AX | ||
| 3 Ramucirumab vs Placebo | REACH | Negative | ESMO 2014 | Lancet-O 2015[15] | Zhu AX | ||
| 4 S-1 vs Placebo | S-CUBE | Negative | ASCO 2015 | Lancet GH 2017[16] | Kudo M | ||
| 5 ADI-PEG 20 vs Placebo | NA | Negative | ASCO 2016 | Ann Oncol 2018[17] | Abou-Alfa G | ||
| 6 Regorafenib vs Placebo | RESORCE | Positive | WCGC 2016 | Lancet 2017[41] | Bruix J | ||
| 7 Tivantinib vs Placebo | METIV-HCC | Negative | ASCO 2017 | Lancet-O 2018[18] | Rimassa L | ||
| 8 Tivantinib vs Placebo | JET-HCC | Negative | ESMO 2017 | ||||
| 9 DT vs Placebo | ReLive | Negative | ILCA 2017 | ||||
| 10 Cabozantinib vs Placebo | CELESTIAL | Positive | ASCO-GI 2018 | NEJM 2018[45] | Abou-Alfe G | ||
| 11 Ramucirumab vs Placebo | REACH-2 | Positive | ASCO 2018 | Lancet-O 2019[30] | Zhu AX | ||
| 12 Pembrolizumab vs Placebo | KEYNOTE-240 | Negative | |||||
| Target population | Design | Trial name | Result | Presentation | Publication | 1st author | |
| Early | Adjuvant (prevention of recurrence) | 1 Vitamin K2 vs Placebo | Negative | Hepatology 2011[21] | Yoshida H | ||
| 2 Peretinoin vs Placebo | NIK-333 | Negative | ASCO 2010 | JG 2014[22] | Okita K | ||
| 3 Sorafenib vs Placebo | STORM | Negative | ASCO 2014 | Lancet-O 2015[23] | Bruix J | ||
| 4 Peretinoin vs Placebo | NIK-333/K-333 | Ongoing | |||||
| Improvement of RFA | 1 RFA +/- LTLD | HEAT | Negative | ILCA 2013 | CCR 2017[24] | Tak WY | |
| 2 RFA +/- LTLD | OPTIMA | ||||||
| Intermediate | Improvement of TACE | 1 TACE +/- Sorafenib | Post-TACE | Negative | ASCO-GI 2010 | EJC 2011[25] | Kudo M |
| 2 TACE +/- Sorafenib | SPACE (Ph II) | Negative | ASCO-GI 2012 | J Hepatol 2016[26] | Lencioni R | ||
| 3 TACE +/- Brivanib | BRISK-TA | Negative | ILCA 2013 | Hepatol 2014[27] | Kudo M | ||
| 4 TACE +/- Orantinib | ORIENTAL | Negative | EASL 2015 | Lancet GH 2017[28] | Kudo M | ||
| 5 TACE +/- Sorafenib | TACE-2 | Negative | ASCO 2016 | Lancet GH 2017[29] | Meyer T | ||
| 6 TACE +/- Sorafenib | TACTICS (Ph II) | Positive | ASCO-GI 2018[30] | Kudo M | |||
Sorafenib is an oral drug that suppresses tumor growth by inhibiting the serine-threonine kinases C-Raf, wild-type B-Raf, and mutant (V600E) B-Raf, all of which are components of the Raf/MEK/ERK pathway (mitogen-activated proteins kinase pathway). This pathway acts downstream of the vascular endothelial growth factor receptor (VEGFR), the platelet-derived growth factor receptor (PDGFR), and the epidermal growth factor receptor. It also exerts anti-tumor effects by suppressing neovascularization. It achieves tumor neovascularization by inhibiting the tyrosine kinases VEGFR1, VEGFR2, VEGFR3, PDGFRβ, RET, and fms-related tyrosine kinase 3 (FLT-3). Two large-scale pivotal trials (the SHARP and Asia-Pacific trials) of sorafenib reported significant prolongation of overall survival (OS) compared with placebo[31,32]; indeed, sorafenib is now the standard therapeutic agent for advanced HCC. However, its ability to shrink tumors is weak and its systemic toxicity is relatively high. Therefore, novel molecular targeted agents with more potency or similar effects, but less toxicity, have been unmet need.
Although eight clinical trials with various agents/modalities comparing with sorafenib conducted in the last decade has shown negative outcomes, the results of the REFLECT trial with use of lenvatinib met its primary endpoint of non-inferiority of prolonging OS compared with sorafenib. Lenvatinib is an oral kinase inhibitor that selectively inhibits receptor tyrosine kinases involved in neovascularization and progression to high malignancy grade tumors and a poor prognosis; targeted kinases include VEGFR1, VEGFR2, VEGFR3, fibroblast growth factor receptor (FGFR) 1, FGFR2, FGFR3, FGFR4, PDGFRα, KIT, and RET. In particular, strong inhibition of FGFR4 is considered important for preventing aggressive growth or progression to a higher malignancy grade of HCC. The drug also suppresses invasion and metastasis. A single-arm phase II study of lenvatinib as a treatment for advanced HCC reported a time to progression (TTP) of 7.4 mo and an OS of 18.7 mo, which are very favorable[33]. Subsequently, a phase III study comparing sorafenib with lenvatinib, the REFLECT trial, was conducted[34].
The REFLECT trial was a global phase III study to show the non-inferiority of lenvatinib to sorafenib, in which patients with unresectable HCC, not previously treated with systemic chemotherapy, were allocated randomly to the lenvatinib or sorafenib arms at a 1:1 ratio. Stratification factors were Asian/non-Asian, vascular invasion and/or extrahepatic spread (presence or absence), Eastern Cooperative Oncology Group performance status 0 or 1, and body weight < 60 kg or ≥ 60 kg. Administration was continued until disease progression or development of a non-tolerable adverse event. The primary endpoint was verification of non-inferiority in terms of OS; the non-inferiority margin of OS was set at 1.08, which is very strict. PFS, TTP, objective response rate (ORR), and safety were evaluated as secondary endpoints.
Of the 954 patients registered, 478 and 476 were allocated to the lenvatinib and sorafenib groups, respectively. Overall, 67% of patients in the lenvatinib group were from the Asia-Pacific region and 33% were from western countries. Of these, 32% weighed less than 60 kg and 68% weighed 60 kg or more in lenvatinib arm. Vascular invasion and/or extrahepatic spread were noted in 69%, and BCLC stage C HCCs accounted for 78% in the lenvatinib arm. Regarding the cause of disease, HCC due to hepatitis C accounted for 19% in the lenvatinib arm and 27% in the sorafenib arm, suggesting an advantageous imbalance toward sorafenib[34]. In contrast, HCC due to hepatitis B accounted for 53% of cases in the lenvatinib arm and for 48% of cases in the sorafenib arm. In the lenvatinib arm, 46% of patients had an alpha fetoprotein (AFP) level exceeding 200 ng/mL vs 39% in the sorafenib group, also indicating an advantageous imbalance toward sorafenib.
The primary endpoint OS in the lenvatinib and sorafenib arms was 13.6 and 12.3 mo, respectively, and the hazard ratio (HR) was 0.92 (0.79-1.06); this was lower than the non-inferiority margin with a specified upper limit of a 95% confidence interval (CI) of 1.08. These data suggest that lenvatinib was not inferior to sorafenib in terms of OS[34]. In addition, the PFS reported by institutional investigators according to the modified Response Evaluation Criteria in Solid Tumors (modified RECIST) criteria was 7.3 and 3.6 mo for the lenvatinib and sorafenib arms, respectively. TTP was 7.4 and 3.7 mo, respectively, and the ORR was 40.6% and 12.6% according to the results of Masked Independent Review Committee, respectively. Thus, lenvatinib had significantly more favorable anti-tumor effects than sorafenib (Table 3)[34]. Evaluation by Masked Independent Review Committee based on RECIST1.1 also confirmed that lenvatinib had favorable anti-tumor effects with regard to PFS, TTP, and ORR as well. Furthermore, when analysis was confined to intermediate stage HCC, the PFS in the lenvatinib arm was 9.1 mo, which is fairly long, and the ORR of BCLC B-stage HCC in the lenvatinib arm for the Japanese population was 61.3%, which is extremely high[35].
| Lenvatinib (n = 478) | Sorafenib (n = 476) | HR, P-value | |
| OS (M, 95% CI) | 13.6 (12.1-14.9) | 12.3 (10.4-13.9) | HR 0.92 (0.79-1.06) |
| PFS (M, 95% CI) | 7.3 (5.6-7.5) | 3.6 (3.6-3.7) | HR 0.64 (0.55-0.75) P < 0.0001 |
| TTP (M, 95% CI) | 7.4 (7.2-9.1) | 3.7 (3.6-3.9) | HR 0.60 (0.51-0.71) P < 0.0001 |
| Objective response (independent review, mRECIST) | |||
| CR | 10 (2%) | 4 (1%) | |
| PR | 184 (38%) | 55 (12%) | |
| SD | 159 (33%) | 219 (46%) | |
| PD | 79 (17%) | 152 (32%) | |
| ORR | 194 (40.6%) | 59 (12.4%) | P < 0.0001 |
| DCR | 353 (73.8%) | 278 (58.4%) | P < 0.0001 |
Because AFP was not included as a stratification factor, more patients with an AFP level exceeding 200 ng/mL were enrolled in the lenvatinib group. When this was corrected by analysis of covariance, the lenvatinib arm showed a superior OS (HR = 0.856; 95%CI = 0.736-0.995; nominal P-value = 0.0342)[34]. Based on these findings, if AFP was included as a stratified factor, the study would likely have confirmed the superiority of lenvatinib over sorafenib[36,37]. The probability that lenvatinib was superior to sorafenib after adjustment for AFP is as high as 0.743[38].
Sub-analysis of OS revealed that the OS-prolonging effects of lenvatinib were superior to those of sorafenib in almost all subsets. It should be noted that specifically that in the group with body weight < 60 kg, the OS benefits of lenvatinib were superior to those of sorafenib at a dose of 8 mg, and the HR was more favorable than that in the group weighing ≥ 60 kg or higher at a dose of 12 mg (HR, 0.85 vs 0.95, respectively). This suggests that weight-based dosing is successful[37,39]. In addition, the HR in the group with a baseline AFP of ≥ 200 ng/mL was 0.78 (95%CI, 0.63-0.98), confirming that lenvatinib has favorable OS benefits even in a group with a high AFP level and a poor prognosis. The treatment durations for the lenvatinib and sorafenib arms were 5.7 and 3.7 mo, respectively; therefore, lenvatinib can be given orally for a longer duration and the frequency of subjective AEs such as hand foot skin reaction and diarrhea is lower, indicating superior tolerability.
Based on the above findings, lenvatinib is not inferior to sorafenib in terms of OS; indeed, lenvatinib showed statistically significant and clinically meaningful improvement in all secondary endpoints (PFS, TTP, and ORR), confirming its efficacy as a first-line agent for patients with unresectable HCC. Regarding the AEs, hypertension, proteinuria and hypothyroidism were more frequently observed in lenvatinib arm although hand-foot skin reaction, diarrhea and alopecia were less frequently observed in lenvatinib arm as compared with sorafenib arm[34]. This AE profile suggests that AEs in lenvatinib arm are more favorable and tolerable than sorafenib since most of AEs observed in lenvatinib arm are asymptomatic and can be manageable by medication (hypertension, hypothyroidism) or by dose reduction (proteinuria). Based on these results, lenvatinib has been approved to treat HCC on March 23, 2018 in Japan. It was also approved in HCC by the Food and Drug Administration on August 16, 2018, in Europe on August 23, 2018, in South Korea on September 4, 2018, in China on August 29, 2018 and in Taiwan on November 28, 2018.
Regorafenib is an oral kinase inhibitor. It targets protein kinases such as VEGFR1, VEGFR2, VEGFR3, TIE2, PDGFRβ, FGFR, KIT, RET, RAF-1, and BRAF[40]. Because it is synthesized simply by binding fluorine to sorafenib, it has almost the same molecular structure as sorafenib and a similar, but stronger toxicity profile. Therefore, unlike other drugs, a placebo-controlled phase III study (RESORCE trial) was performed that included only patients who progressed under sorafenib treatment and those intolerant to sorafenib patients were excluded. The primary endpoint, OS, in the regorafenib arm was 10.6 mo and that in the placebo arm was 7.8 mo, which is a significant improvement[41]. PFS and TTP were also significantly more favorable (Table 4). This is the first drug for which efficacy was proved as a second-line systemic agent who progressed on sorafenib.
| Regorafenib (n = 379) | Placebo (n = 194) | HR, P-value | |
| OS (M, 95%CI) | 10.6 (9.1-12.1) | 7.8 (6.3-8.8) | HR 0.63 (95%CI 0.50-0.79) P < 0.0001 |
| PFS (M, 95%CI) | 3.1 (2.8-4.1) | 10.6 (1.4-1.6) | HR 0.46 (95%CI 0.37-0.56) P < 0.0001 |
| TTP (M, 95%CI) | 3.2 (2.9-4.2) | 10.6 (1.4-1.6) | HR 0.44 (95%CI 0.36-0.55) P < 0.0001 |
| Objective response(investigator assessed, mRECIST) | |||
| CR | 2 (1%) | 0 | |
| PR | 38 (10%) | 8 (4%) | |
| SD | 206 (54%) | 62 (32%) | |
| PD | 86 (23%) | 108 (56%) | |
| ORR | 40 (11%) | 8 (4%) | P = 0.0047 |
| DCR | 247 (65%) | 70 (36%) | P < 0.0001 |
Regarding the AE profiles of regorafenib is as follows; grade 3/4 drug-related treatment-emergent AEs include hand-foot skin reaction in 13%, fatigue in 6%, hypertension in 13% and diarrhea in 2%[41]. However, most these AEs were manageable by medication or dose reduction/interruption. Based on these results, its use as a second-line agent for HCC in Japan was approved in May 2017. However, the drug is unsuitable for patients with sorafenib intolerance; this means that there is an unmet need for a second-line treatment in this group. However, this was soon solved by successful trials of cabozantinib and ramucirumab in 2018 as mentioned later.
The RESORCE trial was successful for the following reasons: (1) For second-line treatment with regorafenib, patients that discontinued sorafenib due to AEs were excluded, in other words, only patients with progressive disease (PD) under sorafenib treatment were included; (2) vascular invasion and extrahepatic spread were set as independent stratification factors to prevent imbalanced distribution between the two arms (active drug and placebo arms); (3) AFP, a strong poor prognostic factor, was also included as a stratification factor; and (4) only patients with sufficient sorafenib tolerance were selected (limited to patients who were able to tolerate sorafenib doses of 400 mg or higher for 20 d or longer during the 28-d period before PD). This study design prevented dropout due to AEs caused by regorafenib since molecular structure of regorafenib is very similar to sorafenib, and minimized the influence of post-treatment effects following PD after regorafenib treatment[41]. According to the RESORCE trial, the median survival time for patients treated with regorafenib was 10.6 mo (placebo: 7.8 mo; HR = 0.63; P < 0.0001). Sub-analysis of OS revealed that it was significantly more favorable for patients when sorafenib was first used at a Child-Pugh score of 5 than for patients introduced to sorafenib at a score of 6; this suggests that early switching from TACE to sorafenib for patients who become refractory to TACE at a Child-Pugh score of 5, and early switching to regorafenib for patients who become refractory to sorafenib, are important for extending survival. Furthermore, the median duration of pre-treatment with sorafenib before the RESORCE trial was 7.8 mo, which is relatively long. Many patients with long SD that responded to sorafenib well were enrolled in this trial. Thus, the effects on patients with rapid PD have been unclear. However, a recent report of sub-analysis of the RESORCE trial showed that the HR for OS in patients with rapid PD on sorafenib (TTP: 2.3 mo) was 0.66, thereby clarifying that regorafenib has OS benefit, even in patients showing rapid PD on sorafenib[42].
Based on the results of the RESORCE trial, sorafenib-regorafenib sequential therapy extended OS from the time of sorafenib treatment initiation to 26 mo (placebo group: 19.2 mo), thereby providing a favorable outcome[42,43]. This is a very important message to clinical practice. This outcome (OS = 26 mo) is almost comparable with that for intermediate stage HCC patients treated with conventional TACE[27]. At present, the BRISK-TA trial, a prospective phase III study involving the largest number of patients in the world, is the world largest TACE combination trial. Therefore, the outcome of the placebo arm of this trial is considered to be the world standard outcome of TACE treatment with no selection bias. In addition, the target groups of this trial comprised patients with BCLC B, BCLC A, and BCLC C (59%, 23%, and 17% of patients, respectively). Thus, 82% of all patients had early/intermediate stage disease and only 17% had advanced stage disease. In contrast, patients with advanced stage BCLC C accounted for 86% of patients enrolled in the RESORCE trial. When these two cohorts were compared, the OS of TACE-treated patients were 26.1 mo and that of patients who received sorafenib-regorafenib sequential therapy was 26 mo. Although it may be inappropriate to compare the results of the different randomized controlled trials (RCTs), it should be justified because there was no selection bias in the placebo group from the well-designed RCT. Considering that sorafenib-regorafenib sequential therapy targets far more advanced cases of HCC, the finding of a comparable OS result for TACE- and sorafenib-regorafenib-treated patients implicates very important issue. Although it was a highly selected patient population, the survival benefit of sorafenib-regorafenib sequential therapy for advanced stage HCC were comparable with those of TACE for intermediate stage HCC[27,42-44]. Therefore, sorafenib-regorafenib sequential therapy is expected to achieve a favorable outcome in the real-world clinical practice as well, suggesting that the timing of sorafenib introduction should be re-considered. Previously, a patient was switched from TACE to systemic therapy when they became refractory to TACE. However, it may be more important to identify a subgroup likely to become refractory to TACE and then introduce systemic therapy at an earlier time when the liver function reserve is maintained at a Child-Pugh score of 5 before becoming refractory to TACE[43,44].
Cabozantinib is an oral multi-kinase inhibitor that inhibits the activity of VEGF, c-MET, RET, AXL, TIE2, and FLT3. The survival-prolonging effects of cabozantinib as a second-line agent for patients with HCC refractory/intolerant to sorafenib treatment compared with placebo control were presented at the American Society of Clinical Oncology Gastrointestinal Cancer Symposium (ASCO-GI), held in January 2018. In total, 707 patients with unresectable HCC were allocated to the cabozantinib and placebo groups (2:1 ratio). The median OS for the cabozantinib group (n = 470) was 10.2 mo (95%CI, 9.1-12.0), demonstrating significant survival benefit when compared with the placebo group [8.0 mo (95%CI, 6.8-9.4)] (Table 5)[45,46]. Systemic chemotherapy with a drug other than sorafenib was allowed during this trial, with the drug positioned as the second- or third-line therapy.
| Cabozantinib (n = 470) | Placebo (n = 237) | HR, P-value | |
| OS (M, 95%CI) | 10.2 (9.1-12.0) | 8.0 (6.8-9.4) | HR 0.76 (95%CI 0.63-0.92) P = 0.0049 |
| PFS (M, 95%CI) | 5.2 (4.0-5.5) | 1.9 (1.9-1.9) | HR 0.44 (95%CI 0.36-0.52) P < 0.0001 |
| Objective response (investigator assessed, RECIST 1.1) | |||
| CR (%) | 0 | 0 | |
| PR (%) | 4 | 0.4 | |
| SD (%) | 60 | 33 | |
| PD (%) | 21 | 55 | |
| NE (%) | 15 | 11 | |
| ORR (%, 95CI) | 4 (2.3-6.0) | 0.4 (0.0-2.3) | P = 0.0086 |
| DCR (%) | 64 | 33.4 | |
Regarding the AE profiles, grade 3/4 AEs include diarrhea (10%), decreased appetite (6%), hand-foot skin reaction (17%), fatigue (10%) and hypertension (16%)[45]. However, most of AEs were manageable by medication or dose reduction/interruption. As this trial was not performed in Japan, a bridging phase 2 study is now underway; therefore, its approval as a second-line agent is expected in the near future in Japan as well.
Ramucirumab is a recombinant monoclonal human immunoglobulin IgG1 antibody specific for VEGFR-2. It is injected intravenously and inhibits VEGFR-2 activity by blocking its binding to VEGF-A, VEGF-C, and VEGF-D. Thus, it exerts anti-tumor effects by inhibiting endothelial cell proliferation, migration, and survival, thereby preventing tumor neovascularization.
The previous phase of the REACH trial examined the survival benefit of ramucirumab compared with placebo in patients with unresectable advanced HCC that was refractory/intolerant to sorafenib treatment. No survival benefit was proven in this trial[15]. However, sub-analysis limited to patients with an AFP level ≥ 400 ng/mL revealed improved survival; this result was reproducible regardless of the geographic region[47,48]. Thus, the REACH-2 trial was planned and conducted. The study design was not markedly different from that of the original REACH trial except that the subjects were limited to patients with an AFP level of ≥ 400 ng/mL, and macroscopic vascular invasion was included as a stratification factor. The results were presented at ASCO in June 2018. The study results were positive in terms of the primary endpoint: improvement of OS[49,50]. The median OS in the ramucirumab group was 8.5 mo and that in the placebo group was 7.3 mo, confirming a significant survival benefit over placebo (HR = 0.710; P = 0.0199) (Table 6).
| Ramucirumab (n = 197) | Placebo (n = 95) | HR, P-value | |
| OS (M, 95%CI) | 8.5 | 7.3 | HR 0.710 (95%CI 0.531-0.949) P = 0.0199 |
| PFS (M, 95%CI) | 2.8 | 1.6 | HR 0.452 (95%CI 0.339-0.603) P < 0.0001 |
| Objective response (RECIST 1.1) | |||
| CR (n, %) | 0 (0.0) | 0 (0.0) | |
| PR (n, %) | 9 (4.6) | 1 (1.1) | |
| SD (n, %) | 109 (55.3) | 36 (37.9) | |
| PD (n,%) | 66 (33.5) | 48 (50.5) | |
| NE (n, %) | 13 (6.6) | 10 (10.5) | |
| ORR (%, 95CI) | 9 (4.6) | 1 (1.1) | P = 0.1697 |
| DCR (%) | 118 (59.9) | 37 (38.9) | P = 0.0006 |
Regarding the AE profiles, grade 3/4 AEs include hypertension (12%), thrombocytopenia (5%), hepatic encephalopathy (3%) and neutropenia (3%). These AEs were manageable by medication and/or dose reduction/interruption. Most importantly, relative dose intensity was as high as 98.5%, which suggests tolerability of this drug is fairly high[49]. Based on these results, ramucirumab is expected to be approved for patients refractory or intolerant to sorafenib treatment and with an AFP level ≥ 400 ng/mL.
The TACTICS trial was a multicenter prospective RCT comparing TACE plus sorafenib with TACE alone that was conducted at 33 sites in Japan[30,51]. A total of 156 patients with unresectable HCC were assigned to receive sorafenib plus TACE (n = 80) or TACE alone (n = 76) at a 1: 1 ratio. The inclusion criteria were Child-Pugh score ≤ 7, a maximum of two previous TACE sessions, and ≤ 10 HCCs with none exceeding 10 cm in size. The exclusion criteria were extrahepatic spread and vascular invasion. Patients in the TACE plus sorafenib arm started sorafenib 2-3 wk before TACE at a dose of 400 mg once daily. The purpose of this sequential pretreatment with sorafenib was to assess tolerability to sorafenib, normalize the tumor vasculature to improve TACE effectiveness, and attenuate VEGF upregulation after the TACE procedure. Sorafenib was temporarily suspended 2 d before and after TACE. In patients showing sorafenib tolerance, the dose was increased to 800 mg daily when possible. TACE was performed on demand, and repeated TACE was generally performed in cases with viable lesions that grew by ≥ 50% over baseline. Response was assessed using computed tomography, magnetic resonance imaging, or other related modalities every 8 wk. The study had two co-primary endpoints, namely, PFS and OS, and adopted a gatekeeping strategy. The secondary endpoints were the time until TACE was no longer feasible or no longer showed any benefit (time to un-TACEable progression: TTUP), TTP, response rate, and safety. As further explained below, the development of new intrahepatic lesions was not defined as tumor progression. This criterion was introduced to maximize the duration of sorafenib administration and to keep the progression criteria for TACE as consistent as possible with those currently used in clinical practice. Use of the RECIST criteria as response evaluation criteria/a stopping rule is inappropriate because repeated TACE is generally performed after detecting a new intrahepatic lesion, which does not qualify as treatment failure requiring a switch to a next line of treatment. Therefore, the TACE progression criteria were created specifically for the TACTICS trial and were consistent with those used in clinical practice.
The criteria for progression with TACE (unTACEable progression) were: (1) ≥ 25% increase in intrahepatic viable lesions; (2) decline in hepatic functional reserve to Child-Pugh class C; (3) appearance of extrahepatic lesions; (4) appearance of vascular invasion; or (5) meeting the Japan Society of Hepatology criteria for TACE-refractory disease[52]. Therefore, PFS was defined as the time to either unTACEable progression or death. The most important feature of the TACTICS trial design is that the RECIST criteria were not used, and consequently the development of new intrahepatic lesions was not considered progression. This enabled long-term administration of sorafenib.
The results for the primary endpoint of PFS were very favorable, with a median of 25.2 mo in the TACE plus sorafenib arm and 13.5 mo in the TACE alone arm (HR, 0.59; P = 0.006; Table 7)[30,51]. TTUP results were also favorable, with a median of 26.7 mo in the TACE plus sorafenib arm and 20.6 mo in the TACE alone arm (HR, 0.57; P = 0.02; Table 7). Similarly, TTP results were favorable, with a median of 26.7 mo in the TACE plus sorafenib arm and 16.4 mo in the TACE alone arm (HR, 0.54; P = 0.005). PFS results were also better for the TACE plus sorafenib arm in all subgroup analyses[30]. The response rates after the first TACE session did not differ significantly between the arms. There were no unexpected AEs. The median duration of sorafenib administration was long at 38.7 mo, and the median daily dose was somewhat low at 355.2 mg. The interval between TACE sessions was 21.1 wk in the TACE plus sorafenib arm, which was significantly longer than the interval of 16.9 wk in the TACE alone arm (P = 0.018). Other parameters that were significantly longer in the TACE plus sorafenib arm than in the TACE alone arm were time to detection of vascular invasion (31.3 mo vs 4.0 mo), time to detection of extrahepatic spread (15.7 mo vs 6.9 mo), and time to stage progression (22.5 mo vs 6.3 mo) (Table 7)[30,51].
| TACE with sorafenib median (M) | TACE alone median (M) | HR (95% CI) | P value | |
| PFS | 25.2 | 13.5 | 0.59 (0.41-0.87) | 0.006 |
| TTUP | 26.7 | 20.6 | 0.57 (0.36-0.92) | 0.02 |
| TTP | 26.7 | 16.4 | 0.54 (0.35-0.83) | 0.005 |
| TTVI | 31.3 | 4.0 | 0.26 (0.09-0.75) | 0.005 |
| TTEHS | 15.7 | 6.9 | 0.21 (0.06-0.70) | 0.006 |
| TTSP | 22.5 | 6.3 | 0.31 (0.15-0.63) | 0.001 |
In 2018, sorafenib and lenvatinib became first-line molecular targeted agents for HCC available worldwide. Regorafenib is also available as a second-line agent worldwide, but the following strict conditions are applicable: (1) Disease progression under sorafenib treatment; (2) exclusion of patients intolerant to sorafenib; (3) confirmation of sufficient tolerance to sorafenib (patients must tolerate ≥ 400 mg of sorafenib for 20 d or longer during the 28-d period before PD); and (4) Child-Pugh A liver function.
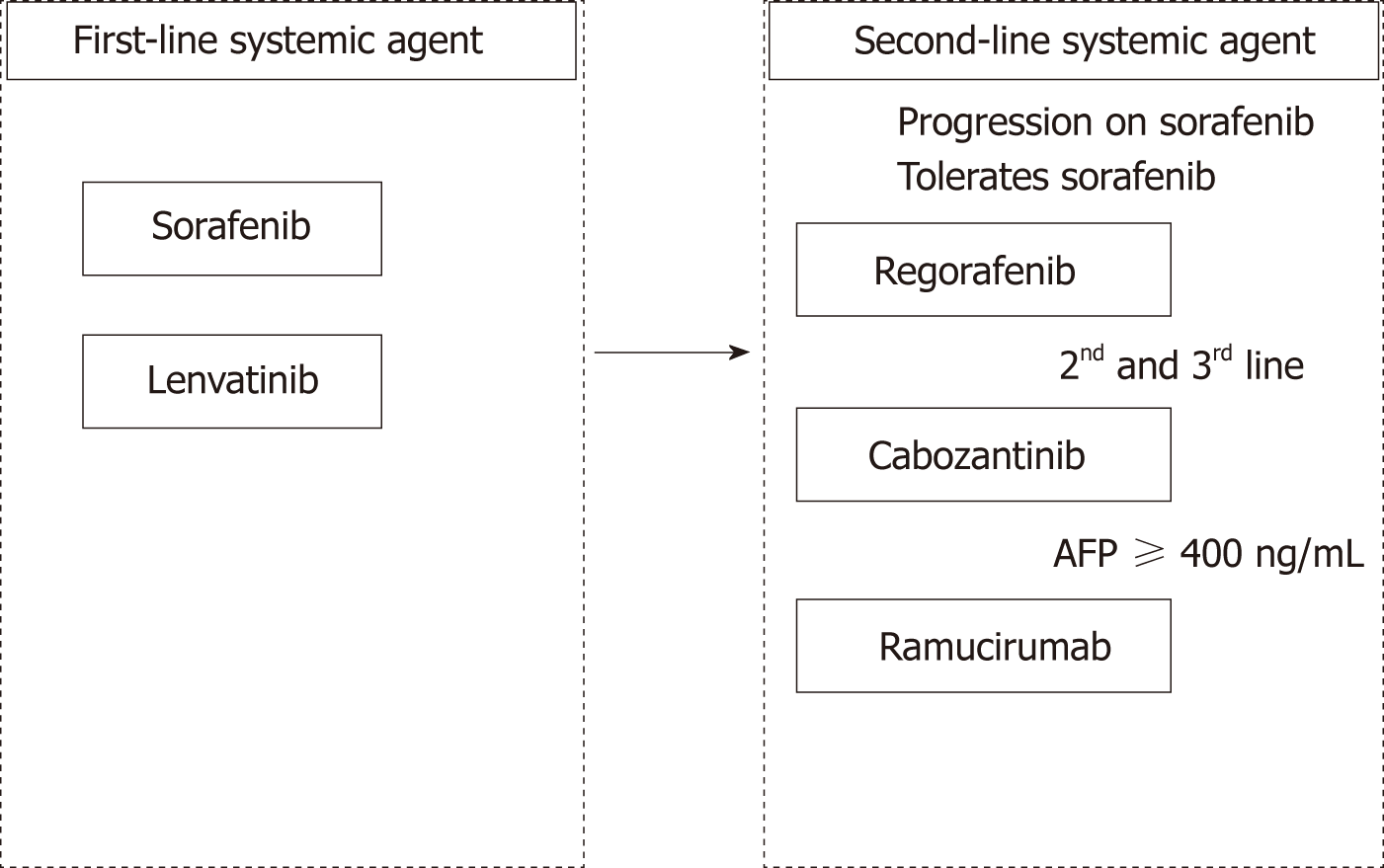
A successful outcome for the cabozantinib bridging study in Japan will result in an application for approval. Also, ramucirumab for use in patients with an AFP ≥ 400 ng/mL will be expected to be approved. This will open up an era in which we have access to two first-line drugs and three second-line drugs(Figure 1)[53]. The HCC practice guideline published by the European Association of Study of the Liver revised in 2018[3] described regorafenib, cabozantinib, and ramucirumab as second-line agents to be used when a patient becomes refractory to sorafenib; however, no data are available regarding their use as second-line agents after lenvatinib treatment, which is an unmet need. In contrast, the guidelines published by the American Association for the Study of Liver Diseases revised in August 2018 state that although there is no clear evidence for their use as second-line agents after patients become refractory to lenvatinib, use of multi-kinase inhibitors, such as sorafenib-regorafenib, cabozantinib or ramucirmab may be considered[4].
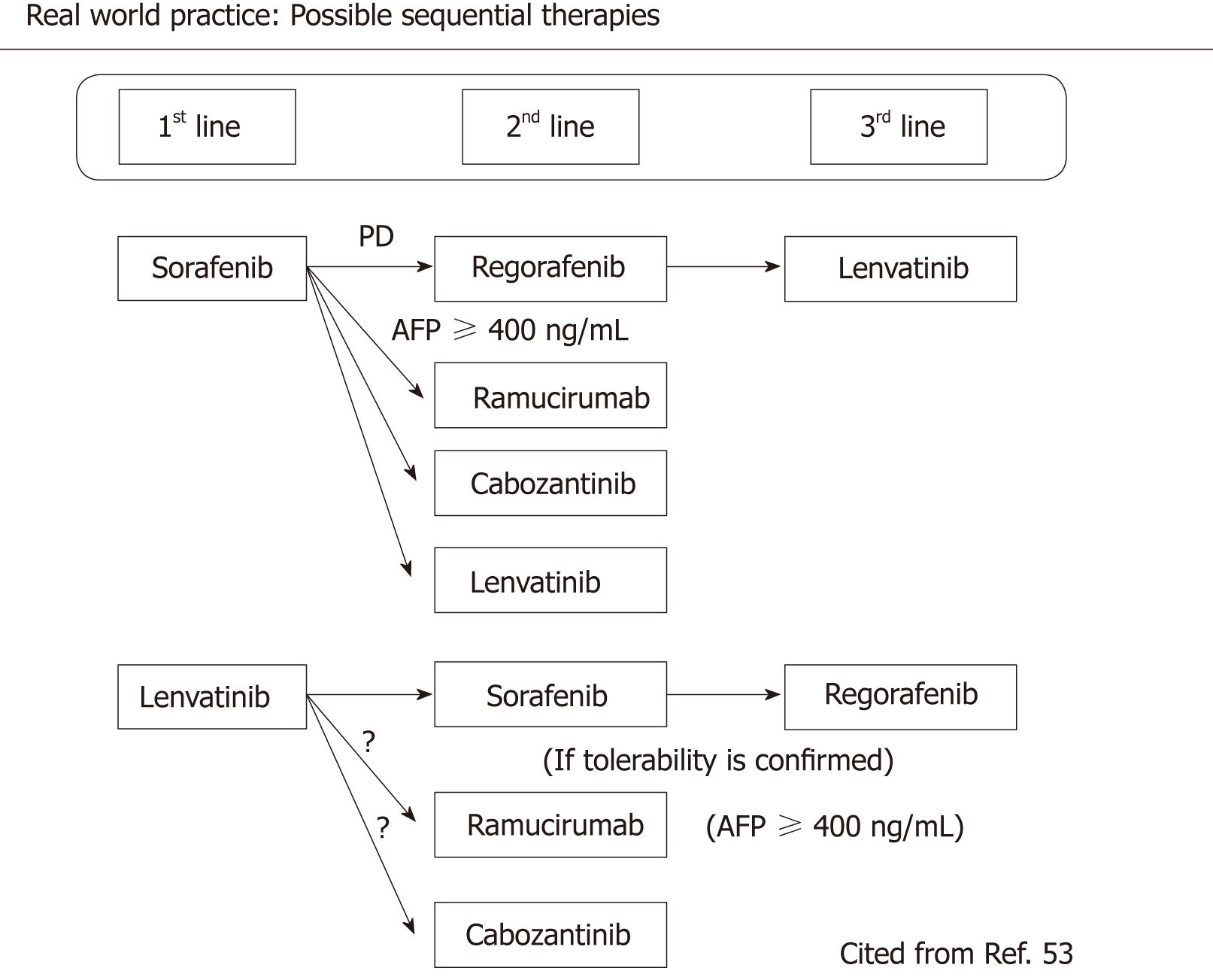
Indeed, after its approval for use in HCC patients in Japan, lenvatinib was administered to more than 8000 patients over 11 mo. Based on recent reports of early experiences, lenvatinib was administered patients not only as a first-line agent, but also as a second-line agent after sorafenib, and as a third-line agent after sorafenib-regorafenib sequential therapy in approximately half of patients (Figure 2). In addition, when the drug is used in this manner the response rate is as high as 40%-50% when assessed by mRECIST, a percentage similar to that reported in the REFLECT trial, in a real world clinical practice. Thus, lenvatinib is expected to have positive effects in the real world clinical practice setting even after treatment with sorafenib and even regorafenib. In addition, it is possible that sorafenib, regorafenib, ramucirumab, and cabozantinib are effective even after PD on lenvatinib. However, regorafenib should be administered only when tolerance to sorafenib is confirmed (Figure 2).
One important issue is whether sorafenib or lenvatinib should be used as the first option among 2 first-line agents. From an oncological and scientific viewpoint, the drug with the higher response rate should be selected for patients with advanced cancer who do not have much time left. In the oncology field, it is common sense that a survival benefit is obtained when the patient responds to the agent. However, there used to be some confusion between survival benefit in “responders” in individual patients, and a correlation between “response rate” and the result in the clinical trial. Even if the response rate of a testing drug is high, this does not always mean that the trial meets the primary endpoint of OS benefit. However, OS in responders were always better than non-responders even primary endpoint was negative as shown in previous trials[10,26,29]. The response rate for sorafenib is not high, but the drug reliably provides a survival benefit by stabilizing disease progression, the SD effect. However, even sorafenib shows a clear survival benefit in responders compared with non-responders according to mRECIST used to evaluate a database from prospective clinical trials with no selection bias[10,26,29], indicating that lenvatinib with its higher response rate than sorafenib increases the percentage of patients who benefit from survival-prolonging effects. Thus, oncologically speaking, agent with higher response rate may be used as the first choice of treatment among the first-line agents.
High response rates on imaging lead to both physicians and patients to be motivated to continue treatment. This high tumor response obtained in many patients will increase patient compliance and possibly increase the conversion rate to more curative modalities such as resection, ablation, and curative TACE[35]. Furthermore, the recently published response rate for Japanese BCLC B patients was 61.3%, which is fairly high and is superior to the world standard response rate of TACE (42%)[27,54,55] and initial TACE (52%) in the Japanese subgroup[56] as reported in the OPTIMIS trial. The response rate was particularly high when compared with that for the 2nd and later TACE session in the OPTIMIS trial, and for Japanese patients whose disease became refractory to TACE, in which ORR was just 13.9%[57].
Therefore, drugs with a high response rate may be used as the first choice for BCLC B patients unsuitable for TACE, i.e., patients likely to become refractory. This statement does not entirely rule out the usefulness of TACE; indeed, as reported by the TACTICS trial[30], when a tumor enlarges during targeted therapy then treatment of the enlarged tumor with super-selective TACE to control growth while preserving liver function may be a good strategy in a real world clinical practice.
Nivolumab is the first recombinant monoclonal human immunoglobulin IgG4 antibody specific for human PD-1. A phase I/II study of advanced HCC, the Checkmate-040 trial, reported a response rate of 20%, which included three complete responders. The disease control rate was 64%, which is very promising. The drug also showed a durable and long-lasting response effect in responders[58]. AE profiles are fairly mild; grade 3 AE was only observed in 1 % (fatigue). Grade 1/2 AEs include rash (19%), pruritus (10%), diarrhea (10%), decreased appetite (10%) and fatigue (8%)[58]. Subsequently, a trial involving an increased number of patients was performed and the updated results were reported at ASCO in 2017. The median survival of patients treated with nivolumab as a first-line therapy was 28.6 mo whereas that of patients treated with nivolumab as a second-line was 15 mo; again, very promising. Based on the results of the phase I/II study described above, the United States marked nivolumab for priority review as a second-line agent after sorafenib; it was approved by the Food and Drug Administration in September 2017. The head-to-head phase III study (CheckMate-459) of nivolumab vs sorafenib as a first-line agent is now ongoing and the results are eagerly awaited. If this study is positive, 1st option among 1st line agents will undoubtedly become nivolumab because of its durable long-lasting response in responders.
Similar to nivolumab pembrolizumab is a recombinant monoclonal human immunoglobulin IgG4 antibody specific for human PD-1. A phase II study of HCC patients reported a response rate of 17%, indicating that the effects are comparable with those of nivolumab[59]. AE profiles are also mild and comparable with those of nivolumab: grade 3 AEs were observed only in 4% (fatigue) and 1% (decreased appetite). Grade 1/2 AEs include fatigue (17%), pruritus (12%), diarrhea (11%) and rash (10%)[59]. A placebo-controlled phase III study of pembrolizumab as a second-line treatment for patients refractory/intolerant to sorafenib was conducted[60], however, the information was press released on Feb 19, 2019 that this trial did not meet its primary endpoints of prolonging PFS nor OS.
The results of a phase 1b study of combination therapy with an anti-PD-L1 antibody, atezolizumab, and another antibody to VEGF, bevacizumab, were reported at ASCO in 2018. Although there were only 23 patients, the response rate according to a RECIST 1.1-based blind review reached 65%, with combination therapy showing a synergistic effect[61]. Based on this result, FDA designated this combination trial as a breakthrough therapy in July 2018. However, updated results presented at ESMO 2018 on October 21, 2018 revealed ORR according to modified RECIST- based independent review assessment dropped to 34% as a result of increased numbers of patients to 73 (Table 8)[62]. Therefore, we have to be cautious on the results derived from small numbers of patients in phase 1/2 trial. A randomized controlled phase III study of combination therapy versus sorafenib is underway (the IMbrave150 trial) (Table 1)[60,63]. Other combination cancer immunotherapies in HCC are also ongoing (Table 8)[64,65].
| Nivolumab[58] | Pembrolizumab[59] | Pembrolizumab plus Lenvatinib[67] | Atezolizumab plus Bevacizumab1[62] | SHR-1210 plus Apatinib[64] | Durvalumab plus Tremelimumab[65] | |
| (n = 214) | (n = 104) | (n = 26) | (n = 73) | (n = 18) | (n = 40) | |
| ORR (%, 95%CI) | 20 (15-26)2 | 17 (11-26)2 | 42.3 (23.4-63.1)3 | 343 | 38.9 3 | 252 |
| DCR (%, 95%CI) | 64 (58-71) | 62 (52-71) | 100 | 75 | 83.3 | 57.5 (> 16 wk) |
| PFS (M, 95%CI) | 4.0 (2.9-5.4) | 4.9 (3.4-7.2) | 9.7 (5.6-NE) | 7.5 (0.4-23.9) | 7.2 (2.6-NE) | NA |
| OS (M, 95%CI) | NR (9M, 74%) | 12.9 (9.7-15.5) | NR | NR | NR | NA |
| DOR (M) | 9.9 (8.3-NE) | ≤ 9 (77%) | NE | NR | NE | NA |
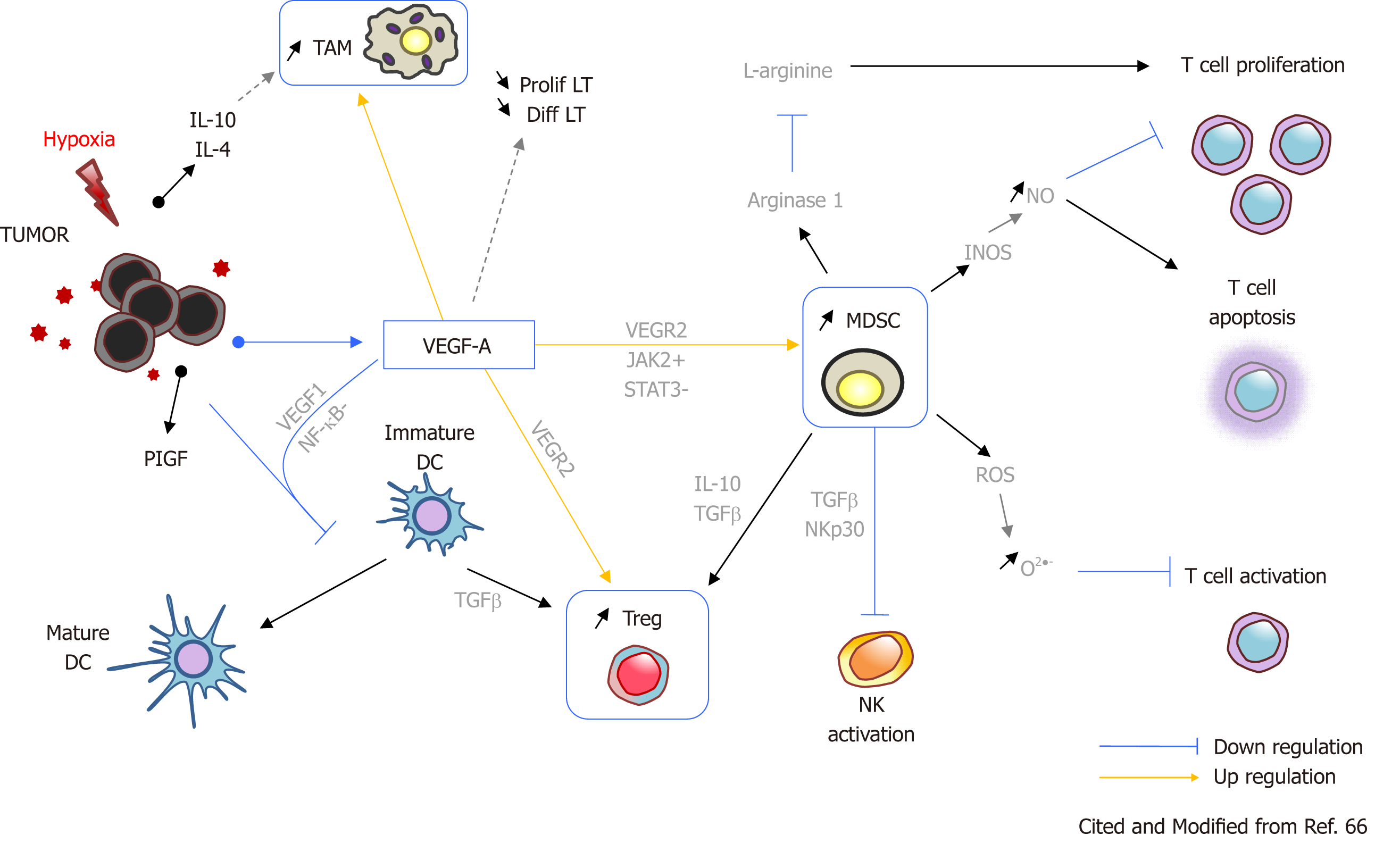
VEGF released by cancer cells suppresses anti-tumor immune responses (Figure 3). VEGF activates the main players in an immunosuppressive tumor microenvironment; these include regulatory T cells (Tregs), tumor-associated macrophages, and myeloid-derived suppressor cells. Cytokines released by these cells inhibit dendritic cell maturation and activation and proliferation of NK cells and CD8-positive cells, thereby driving an immunosuppressive microenvironment (Figure 3)[66]. Accordingly, combination treatment with a molecular targeted drug plus an anti-VEGF agent, bevacizumab, is appropriate to induce synergistic effects.
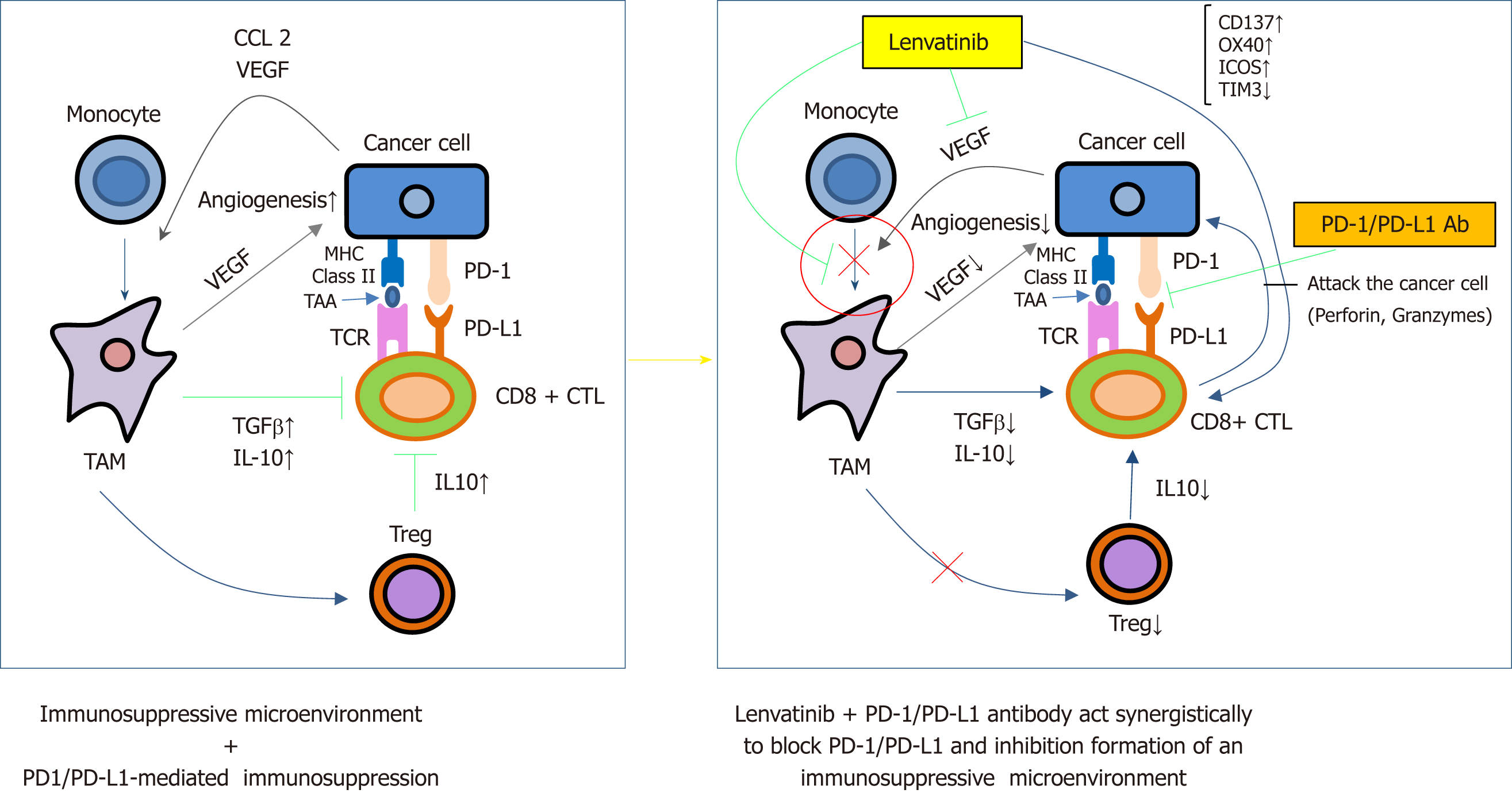
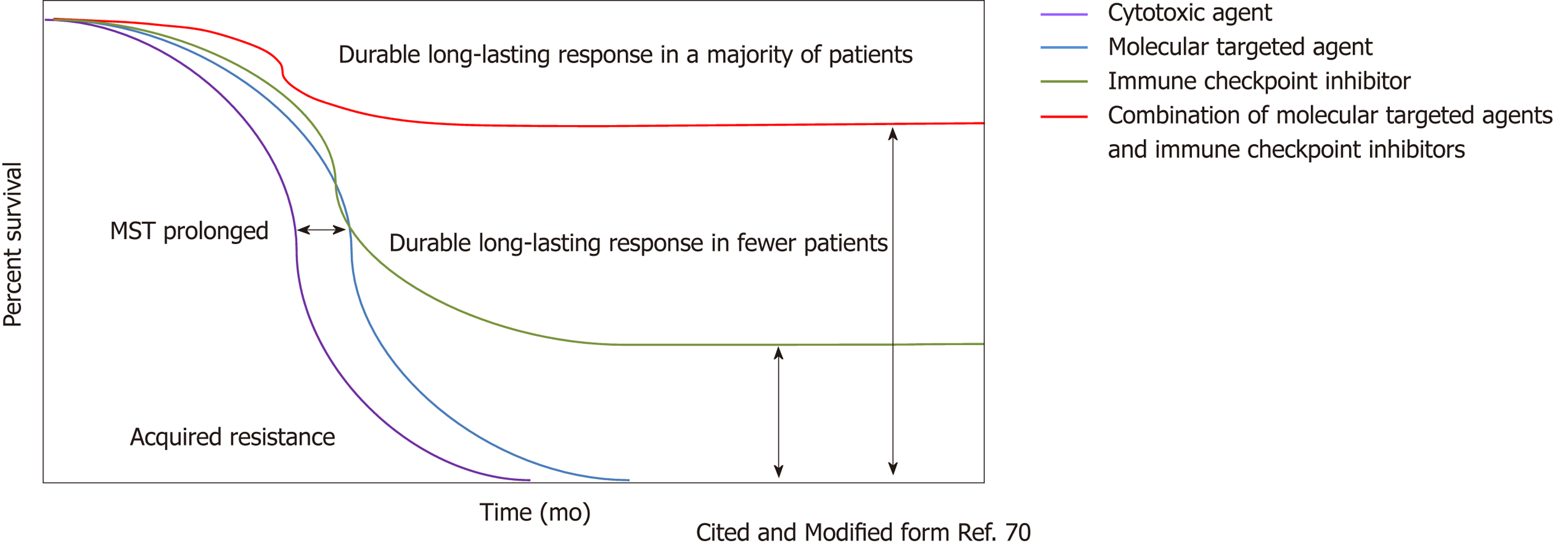
The results of a phase 1b study of combination treatment with lenvatinib plus pembrolizumab were reported at the same ASCO meeting in 2018. The results of this study were also promising. The effects were evaluated in 26 patients; although the mean duration of follow-up was less than 3 mo, the response rate was 42.3%, with no PD patients (Table 8)[67]. However, again we have to be cautious on the results derived from small numbers of patients in phase 1/2 trial similar to atezolizumab/bevacizumab combination trial. The synergistic effects of lenvatinib plus an anti-PD-1 antibody were also suggested in a mouse model (Figure 4)[68,69]. Thus, combination therapy with immune checkpoint inhibitor/molecular targeted drugs may play an important role in the HCC treatment paradigm in the very near future (Figure 5)[70,71]. Biomarker that predicts response to immunotherapy or combination immunotherapy is still an unmet need in immunotherapy of HCC and extensive effort to identify such biomarkers is warranted.
Here, the latest results of trials of systemic therapy for HCC are reviewed. With respect to molecular targeted agents, lenvatinib and regorafenib have been approved for treatment of HCC in addition to sorafenib. Cabozantinib and ramucirumab may also be approved in 2019. Many HCC patients will benefit from increased treatment options and their sequential use for HCC; however, selection of therapeutic drugs may become more complex. When the immune checkpoint inhibitors nivolumab and pembrolizumab become available, the benefits of combination therapy with a molecular targeted agent can also be expected. Indeed, studies of combination therapy with several molecular targeted agents and immune checkpoint inhibitors are ongoing (Table 1, 2, 8) and the results are eagerly awaited. These novel treatment strategies will benefit patients with HCC at all stages from early, intermediate and advanced stage HCCs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Du Z, Gorrell MD, Grassi G, Ji G S- Editor: Yan JP L- Editor: A E- Editor: Huang Y
| 1. | Japan Soceity of Hepatology. Clinical Practice Guidelines for Hepatocellular Carcinoma. Tokyo: Kanehara Co 2017; (in Japanese). |
| 2. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1646] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6064] [Article Influence: 866.3] [Reference Citation Analysis (3)] |
| 4. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3243] [Article Influence: 463.3] [Reference Citation Analysis (1)] |
| 5. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 6. | Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G, Omata M, Pitman Lowenthal S, Lanzalone S, Yang L, Lechuga MJ, Raymond E. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 594] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 7. | Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, Bruix J, Qin S, Thuluvath PJ, Llovet JM, Leberre MA, Jensen M, Meinhardt G, Kang YK. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 430] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 8. | Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J, Lu L, Chao Y, Boucher E, Han KH, Paik SW, Robles-Aviña J, Kudo M, Yan L, Sobhonslidsuk A, Komov D, Decaens T, Tak WY, Jeng LB, Liu D, Ezzeddine R, Walters I, Cheng AL. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517-3524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 9. | Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, Kudo M, Kang YK, Chen PJ, Toh HC, Gorbunova V, Eskens FA, Qian J, McKee MD, Ricker JL, Carlson DM, El-Nowiem S. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2015;33:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 483] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 10. | Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, Aikata H, Nagano H, Hatano E, Sasaki Y, Hino K, Kumada T, Yamamoto K, Imai Y, Iwadou S, Ogawa C, Okusaka T, Kanai F, Akazawa K, Yoshimura KI, Johnson P, Arai Y; SILIUS study group. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 11. | Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, Sibert A, Bouattour M, Lebtahi R, Allaham W, Barraud H, Laurent V, Mathias E, Bronowicki JP, Tasu JP, Perdrisot R, Silvain C, Gerolami R, Mundler O, Seitz JF, Vidal V, Aubé C, Oberti F, Couturier O, Brenot-Rossi I, Raoul JL, Sarran A, Costentin C, Itti E, Luciani A, Adam R, Lewin M, Samuel D, Ronot M, Dinut A, Castera L, Chatellier G; SARAH Trial Group. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 594] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 12. | Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, Choo SP, Cheow PC, Chotipanich C, Lim K, Lesmana LA, Manuaba TW, Yoong BK, Raj A, Law CS, Cua IHY, Lobo RR, Teh CSC, Kim YH, Jong YW, Han HS, Bae SH, Yoon HK, Lee RC, Hung CF, Peng CY, Liang PC, Bartlett A, Kok KYY, Thng CH, Low AS, Goh ASW, Tay KH, Lo RHG, Goh BKP, Ng DCE, Lekurwale G, Liew WM, Gebski V, Mak KSW, Soo KC; Asia-Pacific Hepatocellular Carcinoma Trials Group. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol. 2018;36:1913-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 473] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 13. | Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V, Mathurin P, Fartoux L, Lin DY, Bruix J, Poon RT, Sherman M, Blanc JF, Finn RS, Tak WY, Chao Y, Ezzeddine R, Liu D, Walters I, Park JW. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: Results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 486] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 14. | Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RT, Blanc JF, Vogel A, Chen CL, Dorval E, Peck-Radosavljevic M, Santoro A, Daniele B, Furuse J, Jappe A, Perraud K, Anak O, Sellami DB, Chen LT. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: The EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 15. | Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, Okusaka T, Kubackova K, Trojan J, Sastre J, Chau I, Chang SC, Abada PB, Yang L, Schwartz JD, Kudo M; REACH Trial Investigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 655] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 16. | Kudo M, Moriguchi M, Numata K, Hidaka H, Tanaka H, Ikeda M, Kawazoe S, Ohkawa S, Sato Y, Kaneko S, Furuse J, Takeuchi M, Fang X, Date Y, Takeuchi M, Okusaka T. S-1 versus placebo in patients with sorafenib-refractory advanced hepatocellular carcinoma (S-CUBE): A randomised, double-blind, multicentre, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Abou-Alfa GK, Qin S, Ryoo BY, Lu SN, Yen CJ, Feng YH, Lim HY, Izzo F, Colombo M, Sarker D, Bolondi L, Vaccaro G, Harris WP, Chen Z, Hubner RA, Meyer T, Sun W, Harding JJ, Hollywood EM, Ma J, Wan PJ, Ly M, Bomalaski J, Johnston A, Lin CC, Chao Y, Chen LT. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol. 2018;29:1402-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 18. | Rimassa L, Assenat E, Peck-Radosavljevic M, Pracht M, Zagonel V, Mathurin P, Rota Caremoli E, Porta C, Daniele B, Bolondi L, Mazzaferro V, Harris W, Damjanov N, Pastorelli D, Reig M, Knox J, Negri F, Trojan J, López López C, Personeni N, Decaens T, Dupuy M, Sieghart W, Abbadessa G, Schwartz B, Lamar M, Goldberg T, Shuster D, Santoro A, Bruix J. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): A final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19:682-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 306] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 19. | Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Cucchetti A, Piscaglia F, Pinna AD, Djulbegovic B, Mazzotti F, Bolondi L. Efficacy and Safety of Systemic Therapies for Advanced Hepatocellular Carcinoma: A Network Meta-Analysis of Phase III Trials. Liver Cancer. 2017;6:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Yoshida H, Shiratori Y, Kudo M, Shiina S, Mizuta T, Kojiro M, Yamamoto K, Koike Y, Saito K, Koyanagi N, Kawabe T, Kawazoe S, Kobashi H, Kasugai H, Osaki Y, Araki Y, Izumi N, Oka H, Tsuji K, Toyota J, Seki T, Osawa T, Masaki N, Ichinose M, Seike M, Ishikawa A, Ueno Y, Tagawa K, Kuromatsu R, Sakisaka S, Ikeda H, Kuroda H, Kokuryu H, Yamashita T, Sakaida I, Katamoto T, Kikuchi K, Nomoto M, Omata M. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology. 2011;54:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Okita K, Izumi N, Matsui O, Tanaka K, Kaneko S, Moriwaki H, Ikeda K, Osaki Y, Numata K, Nakachi K, Kokudo N, Imanaka K, Nishiguchi S, Okusaka T, Nishigaki Y, Shiomi S, Kudo M, Ido K, Karino Y, Hayashi N, Ohashi Y, Makuuchi M, Kumada H; Peretinoin Study Group. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: A randomized double-blind placebo-controlled study. J Gastroenterol. 2015;50:191-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 788] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 24. | Tak WY, Lin SM, Wang Y, Zheng J, Vecchione A, Park SY, Chen MH, Wong S, Xu R, Peng CY, Chiou YY, Huang GT, Cai J, Abdullah BJJ, Lee JS, Lee JY, Choi JY, Gopez-Cervantes J, Sherman M, Finn RS, Omata M, O'Neal M, Makris L, Borys N, Poon R, Lencioni R. Phase III HEAT Study Adding Lyso-Thermosensitive Liposomal Doxorubicin to Radiofrequency Ablation in Patients with Unresectable Hepatocellular Carcinoma Lesions. Clin Cancer Res. 2018;24:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 25. | Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, Kaneko S, Tsubouchi H, Suh DJ, Furuse J, Okusaka T, Tanaka K, Matsui O, Wada M, Yamaguchi I, Ohya T, Meinhardt G, Okita K. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 459] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 26. | Lencioni R, Montal R, Torres F, Park JW, Decaens T, Raoul JL, Kudo M, Chang C, Ríos J, Boige V, Assenat E, Kang YK, Lim HY, Walters I, Llovet JM. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol. 2017;66:1166-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 27. | Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, Yang J, Lu L, Tak WY, Yu X, Lee JH, Lin SM, Wu C, Tanwandee T, Shao G, Walters IB, Dela Cruz C, Poulart V, Wang JH. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology. 2014;60:1697-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 28. | Kudo M, Cheng AL, Park JW, Park JH, Liang PC, Hidaka H, Izumi N, Heo J, Lee YJ, Sheen IS, Chiu CF, Arioka H, Morita S, Arai Y. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): A randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol. 2018;3:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 29. | Meyer T, Palmer DH, Cheng AL, Hocke J, Loembé AB, Yen CJ. mRECIST to predict survival in advanced hepatocellular carcinoma: Analysis of two randomised phase II trials comparing nintedanib vs sorafenib. Liver Int. 2017;37:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Kudo M, Ueshima K, Torimura T, Tanabe N, Ikeda M, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Isoda N, Yasui K, Kuzuya T, Okusaka T, Furuse J, Kokudo N, Okita K, Yoshimura K, Arai Y; TACTICS Study Group. Randomized, open label, multicenter, phase II trial of transcatheter arterial chemoembolization (TACE) therapy In combination with sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. J Clin Oncol. 2018;36 suppl:Abstract 4017. |
| 31. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10271] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 32. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4653] [Article Influence: 273.7] [Reference Citation Analysis (0)] |
| 33. | Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, Tamai T, Suzuki T, Hisai T, Hayato S, Okita K, Kumada H. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 34. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans J, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3831] [Article Influence: 547.3] [Reference Citation Analysis (1)] |
| 35. | Kudo M. Extremely High Objective Response Rate of Lenvatinib: Its Clinical Relevance and Changing the Treatment Paradigm in Hepatocellular Carcinoma. Liver Cancer. 2018;7:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Kudo M. Lenvatinib in Advanced Hepatocellular Carcinoma. Liver Cancer. 2017;6:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver cancer. 2018;7:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 38. | Ohashi Y. Non-inferiority and change of endpoints in cancer clinical trials. Kan-Tan-Sui. 2018;77:271-277 (in Japanese). |
| 39. | Tamai T, Hayato S, Hojo S, Suzuki T, Okusaka T, Ikeda K, Kumada H. Dose Finding of Lenvatinib in Subjects With Advanced Hepatocellular Carcinoma Based on Population Pharmacokinetic and Exposure-Response Analyses. J Clin Pharmacol. 2017;57:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 40. | Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1022] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 41. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2717] [Article Influence: 339.6] [Reference Citation Analysis (0)] |
| 42. | Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Gerolami R, Caparello C, Cabrera R, Chang C, Sun W, LeBerre MA, Baumhauer A, Meinhardt G, Bruix J. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 275] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 43. | Kudo M. Regorafenib as Second-Line Systemic Therapy May Change the Treatment Strategy and Management Paradigm for Hepatocellular Carcinoma. Liver Cancer. 2016;5:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Kudo M. A New Era of Systemic Therapy for Hepatocellular Carcinoma with Regorafenib and Lenvatinib. Liver Cancer. 2017;6:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1771] [Article Influence: 253.0] [Reference Citation Analysis (0)] |
| 46. | Kudo M. Cabozantinib as a Second-Line Agent in Advanced Hepatocellular Carcinoma. Liver Cancer. 2018;7:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Kudo M, Hatano E, Ohkawa S, Fujii H, Masumoto A, Furuse J, Wada Y, Ishii H, Obi S, Kaneko S, Kawazoe S, Yokosuka O, Ikeda M, Ukai K, Morita S, Tsuji A, Kudo T, Shimada M, Osaki Y, Tateishi R, Sugiyama G, Abada PB, Yang L, Okusaka T, Zhu AX. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: Japanese subgroup analysis of the REACH trial. J Gastroenterol. 2017;52:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Park JO, Ryoo BY, Yen CJ, Kudo M, Yang L, Abada PB, Cheng R, Orlando M, Zhu AX, Okusaka T. Second-line ramucirumab therapy for advanced hepatocellular carcinoma (REACH): An East Asian and non-East Asian subgroup analysis. Oncotarget. 2016;7:75482-75491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M. Ramucirumab in advanced hepatocellular carcinoma and elevated alpha-fetoprotein following sorafenib (REACH-2): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2019;In press. |
| 50. | Kudo M. Ramucirumab as Second-Line Systemic Therapy in Hepatocellular Carcinoma. Liver Cancer. 2018;7:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Kudo M. Proposal of Primary Endpoints for TACE Combination Trials with Systemic Therapy: Lessons Learned from 5 Negative Trials and the Positive TACTICS Trial. Liver Cancer. 2018;7:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T; Liver Cancer Study Group of Japan. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87 Suppl 1:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 53. | Kudo M. Systemic therapy for hepatocellular carcinoma: Recent advances. Kanzo. 2018;59:587-603. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 54. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del Arbol LR, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 544] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 55. | Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, Stubbs C, Stocken DD, Wall L, Watkinson A, Hacking N, Evans TRJ, Collins P, Hubner RA, Cunningham D, Primrose JN, Johnson PJ, Palmer DH. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 378] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 56. | Kudo M, Raoul JL, Peck-Radosavljevic M, Lee HC, Nakajima K, Cheng AL; on behalf of the OPTIMIS Investigators. An international observational study to assess the real-world use of transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma (HCC): The final analysis and Japanese subpopulation analysis of OPTIMIS. The 54th Annual Meeting of Liver Cancer Study Group of Japan; 2018, June 28-29; Kurume. |
| 57. | Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, Tawada A, Kanai F, Yoshikawa M, Yokosuka O. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 58. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3314] [Article Influence: 414.3] [Reference Citation Analysis (1)] |
| 59. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1900] [Article Influence: 271.4] [Reference Citation Analysis (0)] |
| 60. | Kudo M. Immune checkpoint blockade in hepatocellular carcinoma: 2017 update. Liver cancer. 2016;6:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Stein S, Pishvaian MJ, Lee MS, Lee KH, Hernandez S, Kwan A. Safety and clinical activity of 1L atezolizumab + bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC). J Clin Oncol. 2018;36:4074. [RCA] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 62. | Pishvaian MJ, Lee MS, Ryoo B, Stein S, Lee K, Verret W, Spahn J, Shao H, Liu B, Iizuka K, Hsu C. Updated safety and clinical activity results from a Phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). Ann Oncol. 2018;29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Finn RS, Ducreux M, Qin S, Galle PR, Zhu A, Ikeda M, Kim TY, Xu DZ, Verret W, Liu J, Grossman W, Cheng AL. IMbrave150: A randomized phase III study of 1L atezolizumab plus bevacizumab vs sorafenib in locally advanced or metastatic hepatocellular carcinoma. J Clin Oncol. 2018;36:TPS4141. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 64. | Xu JM, Zhang Y, Jia R, Wang Y, Liu R, Zhang G, Zhao C, Zhang Y, Zhou J, Wang Q. Anti-programmed death-1 antibody SHR-1210 (S) combined with apatinib (A) for advanced hepatocellular carcinoma (HCC), gastric cancer (GC) or esophagogastric junction (EGJ) cancer refractory to standard therapy: A phase 1 trial. J Clin Oncol. 2018;36:4075. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Kelley RK, Abou-Alfa GK, Bendell JC, Kim TY, Borad MJ, Yong WP, Morse M, Kang YK, Rebelatto M, Makowsky M, Xiao F, Morris SR, Sangro B. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma(HCC): Phase I safety and efficacy analyses. J Clin Oncolol. 2017;35:4073 [10.1200/JCO.2017.35.15_suppl.4073]. |
| 66. | Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, Terme M. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 67. | Ikeda M, Sung MW, Kudo M, Kobayashi M, Baron AD, Finn RS, Kaneko S, Zhu A, Kubota T, Kraljevic S, Ishikawa K, Siegel AB, Kumada H, Okusaka T. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. 2018;36:4076. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 68. | Kato Y, Bao X, MacGrath S, Tabata K, Hori Y, Tachino S, Matijevici M, Funahashi Y, Funahashi J. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Kudo M. Immune Checkpoint Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology. 2017;92 Suppl 1:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 70. | Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell. 2015;161:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1791] [Article Influence: 179.1] [Reference Citation Analysis (0)] |
| 71. | Kudo M. Combination Cancer Immunotherapy in Hepatocellular Carcinoma. Liver Cancer. 2018;7:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |