Published online Oct 14, 2019. doi: 10.3748/wjg.v25.i38.5789
Peer-review started: July 29, 2019
First decision: August 17, 2019
Revised: August 30, 2019
Accepted: September 13, 2019
Article in press: September 13, 2019
Published online: October 14, 2019
Processing time: 77 Days and 1.7 Hours
Hepatocellular carcinoma (HCC) has become a great threat for people’s health. Many long noncoding RNAs are involved in the pathogenesis of HCC. SNHG15, as a tissue specific long noncoding RNAs, has been studied in many human cancers, except HCC.
To explore the regulatory mechanism of SNHG15 in HCC.
In the present research, 101 HCC patient samples, two HCC cell lines and one normal liver cell line were used. RT-qPCR and Western blot analysis were applied to detect SNHG15, miR-490-3p and histone deacetylase 2 (HDAC2) expression. The regulatory mechanism of SNHG15 was investigated using CCK-8, Transwell and luciferase reporter assays.
Our research showed that up-regulation of SNHG15 was found in HCC and was related to aggressive behaviors in HCC patients. Moreover, knockdown of SNHG15 restrained HCC cell proliferation, migration and invasion. In addition, SNHG15 served as a molecular sponge for miR-490-3p. Further, miR-490-3p directly targets HDAC2. HDAC2 was involved in HCC progression by interacting with the SNHG15/miR-490-3p axis.
In conclusion, long noncoding RNA SNHG15 promotes HCC progression by mediating the miR-490-3p/HDAC2 axis in HCC.
Core tip: Long noncoding RNA (lncRNA)-SNHG15 is up-regulated in hepatocellular carcinoma (HCC) tissue and cell lines. HCC patients with up-regulated lncRNA-SNHG15 level were more likely to have larger tumor sizes and advanced clinical stage. lncRNA-SNHG15 promotes cell proliferation, migration and invasion in HCC cell lines. The miR-490-3p/histone deacetylase 2 axis was determined to be the target regulated by lncRNA-SNHG15 in HCC, and the regulatory mechanism of lncRNA-SNHG15 has been preliminarily illuminated.
- Citation: Dai W, Dai JL, Tang MH, Ye MS, Fang S. lncRNA-SNHG15 accelerates the development of hepatocellular carcinoma by targeting miR-490-3p/ histone deacetylase 2 axis. World J Gastroenterol 2019; 25(38): 5789-5799
- URL: https://www.wjgnet.com/1007-9327/full/v25/i38/5789.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i38.5789
Hepatocellular carcinoma (HCC) is a high-risk, highly harmful malignant tumor. Recently, HCC has jumped to the top three cancers in the Asia-Pacific region including China, posing a threat to human health[1]. Moreover, the pathogenesis of HCC is not fully understood. It has been reported that environmental and dietary factors affect the occurrence of HCC[2]. At present, surgery is still the first choice for the treatment of HCC. However, the recurrence rate of HCC is still high, and the 5-year survival rate is generally below 50%[3]. Thus, exploring the pathogenesis of HCC to screen for effective diagnostic markers and therapeutic targets is very urgent for HCC patients.
Long non-coding RNAs (lncRNAs) are involved in the occurrence of human diseases and cancers[4]. Recently, the presence of increased numbers of lncRNAs have been demonstrated to be involved in HCC progression. For instance, lncRNA HOXD-AS1 was overexpressed in HCC and accelerated cell proliferation and cell cycle progression through the MEK/ERK pathway[5]. On the contrary, lncRNA TPTEP1 was down-regulated in HCC and restrained HCC progression by suppressing STAT3 phosphorylation[6].
The specific functions of lncRNA SNHG15 in human cancers have caught our attention. It had been reported that SNHG15 was down-regulated and served as a tumor suppressor in thyroid cancer[7]. However, SNHG15 was up-regulated in prostate cancer and acted as a tumor promoter by mediating miR-338-3p[8]. These results indicate that expression of SNHG15 is tissue specific. In particular, SNHG15 expression was increased in HCC and predicted poor survival in HCC patients[9]. However, the specific role of SNHG15 is still unclear and needs to be elucidated.
In addition, it is well-known that lncRNAs exert an effect in human diseases by inhibiting the expression and biological functions of miRNAs[10]. SNHG15 was found to enhance colorectal cancer cell viability through down-regulation of miR-338-3p[11]. In this study, miR-490-3p was predicted to have binding sites with SNHG15. Moreover, miR-490-3p sponging by CircSLC3A2 was reported to regulate HCC progression[12]. It had been shown that miR-490-3p expression was reduced in HCC and restrained autophagy[13]. However, whether SNHG15 regulates HCC progression via regulating miR-490-3p remains unknown.
Besides that, histone deacetylase 2 (HDAC2) was predicted as a target of miR-490-3p in this study. Moreover, HDAC2 expression had been reported to be increased in HCC tissues, which was related to adverse prognosis[14]. Additionally, miR-145 was proposed to act as a tumor inhibitor via binding to HDAC2 in liver cancer[15]. However, the interaction between miR-490-3p and HDAC2 has not been reported in previous studies. Therefore, their relationship in HCC was investigated in our study. Further, the regulatory mechanism of lncRNA SNHG15 with the miR-490-3p/HDAC2 axis was elucidated in HCC progression. Our results will contribute to better understand the role of lncRNA SNHG15 in HCC progression.
One hundred and one HCC patients in the Affiliated Hospital of Guangdong Medical University, the second Affiliated Hospital of Guangdong Medical University and the Seventh Affiliated Hospital of Sun Yat-sen University participated in this research. The clinical features of the patients were shown in Table 1. Among them, 33 randomly selected HCC tissues and paired adjacent non-neoplastic liver tissues were applied for further experiments. Before the experiment, written informed consent was collected from all HCC patients. Moreover, patients with HCC did not receive any treatment except for surgery. The permission of this study was obtained from the Institutional Ethics Committee of the Affiliated Hospital of Guangdong Medical University, the second Affiliated Hospital of Guangdong Medical University and the Seventh Affiliated Hospital of Sun Yat-sen University.
HCC cell lines HuH-1, HuH-7 and normal human liver cells L-O2 were obtained from the Japanese Collection of Research Bioresources Cell Bank (JCRB, Shanghai, China). The growth conditions of these cells are 5% CO2, 37 °C and culture solution (90% DMEM + 10% fetal bovine serum).
SNHG15 siRNA (si-SNHG15), pcDNA3.1-SNHG15 vectors, HDAC2 siRNA (si-HDAC2) and miR-490-3p mimics or inhibitor were purchased from Genepharma (Shanghai, China). Next, the siRNAs, vectors, mimics or inhibitors (20 nM) were transfected into HuH-1 or L-O2 cells using Lipofectamine 2000 (Invitrogen). Untreated cells were used as the controls.
Total RNA isolation was performed with TRIzol reagent (Sigma, United States). The cDNA was reverse transcribed using microRNA reverse transcription kit (TAKARA, Dalian, China). RT-qPCR assay was performing using SYBR Green Master Mix II (TAKARA). U6 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference for RNA or protein. Relative expression of SNHG15, miR-490-3p and HDAC2 were detected with the 2-ΔΔct method.
Protein samples were lysed using RIPA buffer (Beyotime, Shanghai, China). Next, 10% SDS-PAGE was used to separate protein. Protein samples were transferred to PVDF membranes and blocked with 5% nonfat milk. Next, protein samples were incubated with HDAC2 and GAPDH primary antibodies (Abcam, Shanghai, China) overnight at 4 °C. After washing, secondary antibodies (Abcam, United States) were incubated with the protein samples for 1 h. Finally, ECL kit (Beyotime) was used to assess protein bands.
Transfected HuH-1 cells were incubated for 24 h (at 37 °C, 5% CO2) in 96-well plates. Next, HuH-1 cells (3 × 103/well) were incubated in DMEM medium for 24, 48, 72 and 96 h. CCK-8 (Dojindo, Kumamoto, Japan) was added and incubated with the cells for 4 h. Finally, the optical density at 450 nm was observed by a microplate reader (Molecular Devices) to assess cell viability.
First, cell invasion was detected in the upper chamber with Matrigel (BD Biosciences, United States). After 30 min, Transwell upper chamber was added with HuH-1 or L-O2 cell suspension (5 × 103 cells/well). Next, lower chamber was added with DMEM medium (10% fetal bovine serum). After 24 h, 0.1% crystal violet was applied to stain the moved cells. For cell migration, 5 × 103 transfected cells were placed in the upper chambers without Matrigel. Observation and photographing were performed by a light microscope.
Reporter plasmids of SNHG15 (wt-SNHG15 and mut-SNHG15) and HDAC2 (wt-HDAC2 and mut-HDAC2) were cloned into empty pGL3 vectors (GenePharma, Shanghai, China. Then, the above reporter vectors were transfected into HuH-1 cells with miR-490-3p mimics. After 48 h, luciferase activities were examined by dual-luciferase reporter assay system (Promega, United States). HuH-1 cells with empty pGL3 vectors were used as the control.
Data were analyzed by SPSS 17.0 or Graphpad Prism 6, which are shown as mean ± standard deviation. One-way ANOVA and Student’s t-test were employed to compare differences among multiple groups. Chi-squared test was used to analyze the association between SNHG15 and clinical features in HCC patients. P < 0.05 indicated significant difference.
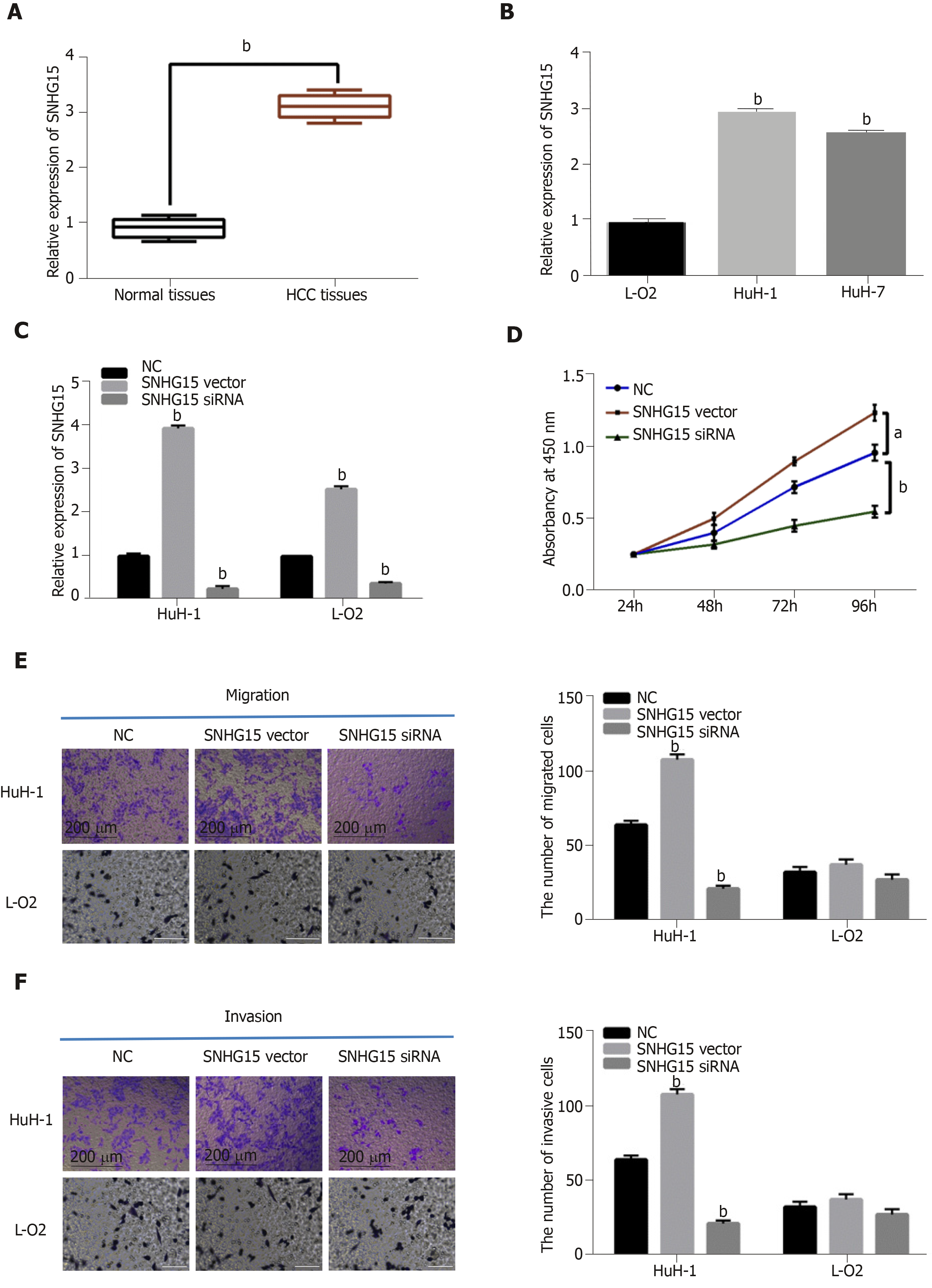
First, a difference in SNHG15 expression was detected in HCC tissues. We found higher SNHG15 expression in HCC tissues than normal tissues (Figure 1A). Similarly, up-regulation of SNHG15 was found in HuH-1 and HuH-7 cells compared to L-O2 cells (Figure 1B). The expression of SNHG15 in HuH-1 cells is higher than HuH-7 cells. Therefore, HuH-1 cells were used for further experiments. In addition, the association between HCC clinical features and abnormal expression of SNHG15 was analyzed. Tumor size, TNM stage and degrees of differentiation in HCC under Edmondson-Steiner grading system were associated with abnormal SNHG15 expression in HCC patients (Table 1). These results suggested that SNHG15 may affect tumorigenesis of HCC.
Next, SNHG15 siRNA or SNHG15 overexpression vector was transfected into HuH-1 cells to explore its role in HCC. The transfection efficiency was confirmed by RT-qPCR (Figure 1C). CCK-8 assay suggested that knockdown of SNHG15 suppressed proliferation of HuH-1 cells, while up-regulation of SNHG15 accelerated cell proliferation in HuH-1 cells (Figure 1D). In addition, the Transwell assay showed that cell migration was also promoted by up-regulation of SNHG15 and restrained by knockdown of SNHG15 (Figure 1E). We also found that SNHG15 siRNA decreased its expression in L-O2 cells, whereas SNHG15 overexpression vector enhanced its expression in L-O2 cells (Figure 1C). However, up-regulation or down-regulation of SNHG15 had little effect on cell migration and invasion in normal human liver cells L-O2 (Figure 1E and F). These findings indicated the potential carcinogenesis of SNHG15 in HCC.
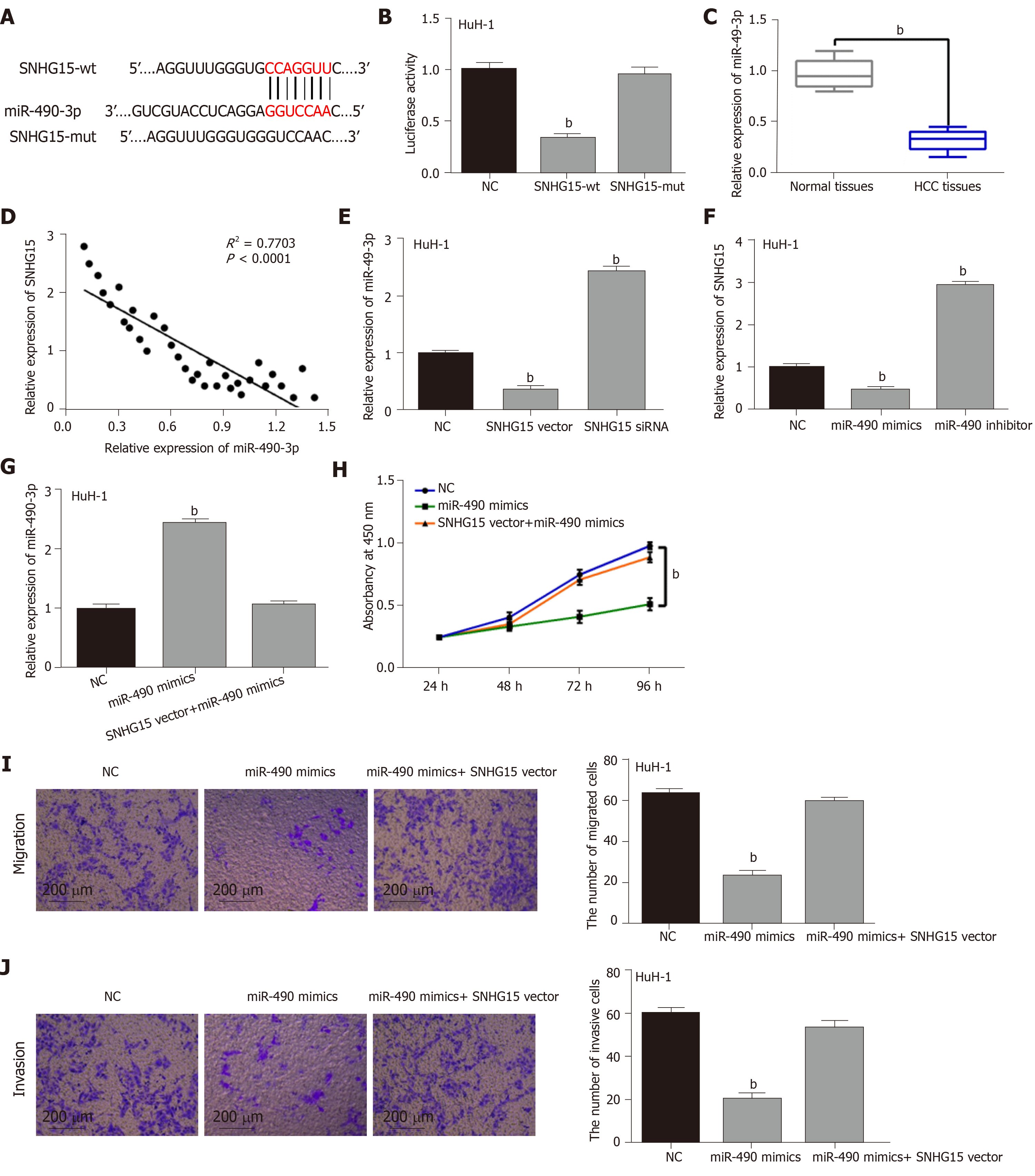
To explore the regulatory mechanism of SNHG15, its target miRNA was searched in starBASEv2.0 database (http://starbase.sysu.edu). It predicted that SNHG15 has potential binding sites with miR-490-3p (Figure 2A). Luciferase reporter assay showed that miR-490-3p mimics decreased SNHG15-wt luciferase activity, but not SNHG15-mut (Figure 2B). Next, lower miR-490-3p expression was identified in HCC tissues compared to normal tissues (Figure 2C). Furthermore, SNHG15 was negatively associated with miR-490-3p expression in HCC tissues (Figure 2D). In HuH-1 cells, knockdown of SNHG15 enhanced miR-490-3p expression, while up-regulation of SNHG15 reduced miR-490-3p expression (Figure 2E). Interestingly, down-regulation or overexpression of miR-490-3p could also inversely regulate SNHG15 expression in HuH-1 cells (Figure 2F). To further explain their interaction, the SNHG15 vector was transfected into HuH-1 cells containing miR-490-3p mimics. Moreover, the increased miR-490-3p expression mediated by its mimics was weakened by up-regulation of SNHG15 (Figure 2G). Functionally, miR-490-3p induced inhibition of cell proliferation was also restored by SNHG15 up-regulation (Figure 2H). Similarly, up-regulation of SNHG15 also weakened the suppressive effect of miR-490-3p on migration and invasion of HCC cells (Figure 2I and 2J). Based on the results, we hypothesized that SNHG15 may accelerate HCC progression via molecular sponging of miR-490-3p.
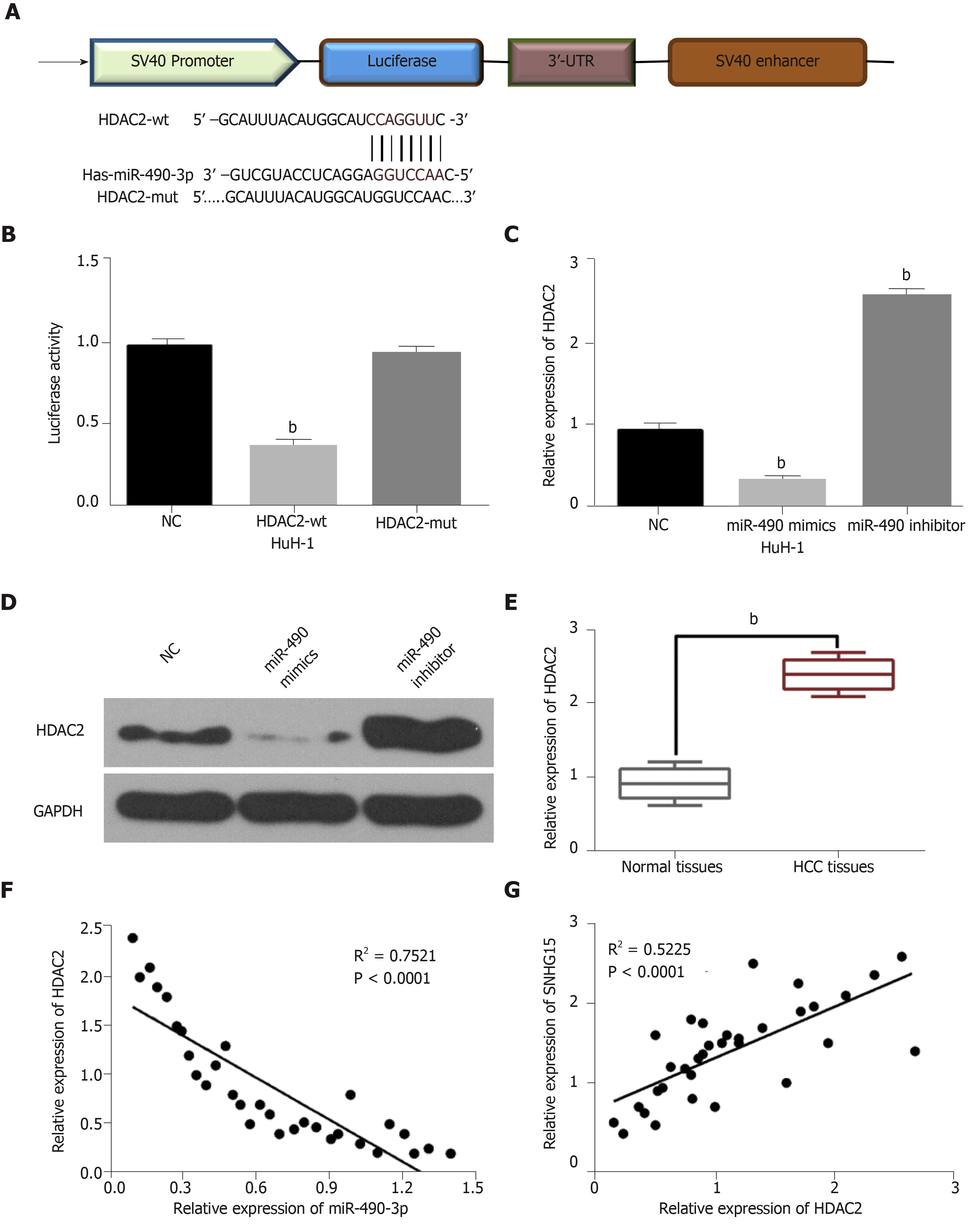
TargetScan (http://www.targetscan.org) predicted that miR-490-3p has a binding site to HDAC2 (Figure 3A). Dual-luciferase reporter assay indicated that miR-490-3p mimics reduced HDAC2-wt luciferase activity, but not HDAC2-mut (Figure 3B). Moreover, miR-490-3p mimics were found to decrease HDAC2 expression, while a miR-490-3p inhibitor up-regulated HDAC2 in HuH-1 cells (Figure 3C and D). These studies indicated that miR-490-3p directly targets HDAC2. In addition, the dysregulation of HDAC2 was identified in HCC tissues. Furthermore, HDAC2 was up-regulated in HCC tissues compared with normal tissues (Figure 3E), and miR-490-3p had an inverse correlation with HDAC2 expression in HCC tissues (Figure 3F). On the contrary, a positive correlation between SNHG15 and HDAC2 expression was identified in HCC tissues (Figure 3G). According to these results, we suspected that HDAC2 may be involved in HCC progression by affecting the SNHG15/miR-490-3p axis.
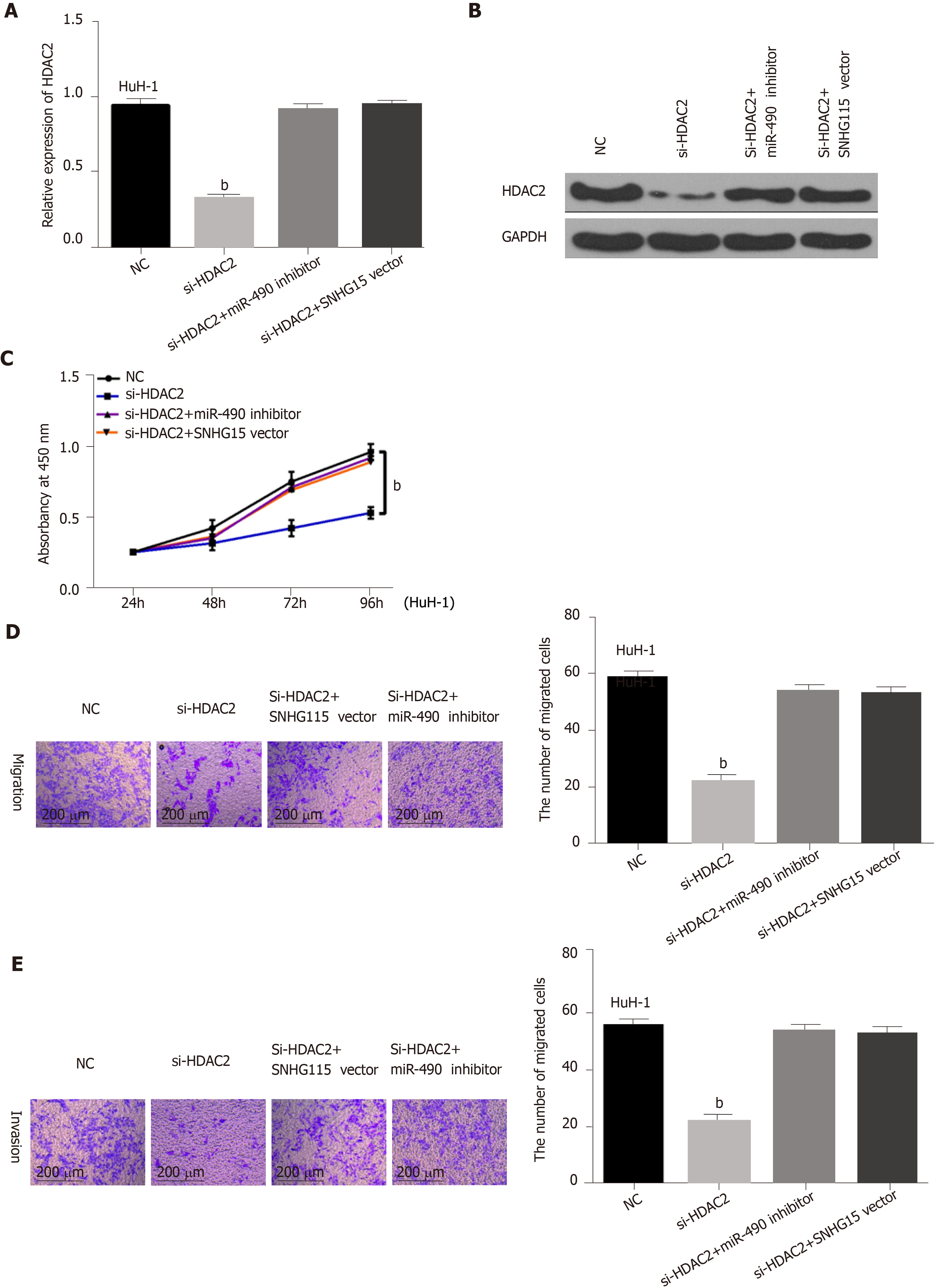
Finally, HuH-1 cells containing HDAC2 siRNA were transfected with a SNHG15 vector or a miR-490-3p inhibitor to further explain their interaction. First, we found that mRNA and protein expression of HDAC2 was down-regulated by HDAC2 siRNA. However, the decreased expression of HDAC2 was reversed by the miR-490-3p inhibitor or SNHG15 vector (Figure 4A and B). Functionally, the inhibition of cell proliferation induced by HDAC2 siRNA was restored by miR-490-3p down-regulation or SNHG15 up-regulation (Figure 4C). Similarly, the reverse effects of the miR-490-3p inhibitor or SNHG15 vector on migration and invasion were also identified in HuH-1 cells with HDAC2 siRNA (Figure 4D and 4E). These results showed that the SNHG15/miR-490-3p axis exerts an effect in the development of HCC progression by interacting with HDAC2.
As potential therapeutic targets, many lncRNAs have been found to regulate HCC progression. For example, lncRNA-MNX1-AS1 was found to accelerate HCC development via regulating the miR-218-5p/COMMD8 axis[16]. In our research, lncRNA SNHG15 also served as an oncogene in HCC. In particular, up-regulation of SNHG15 was identified in HCC, which was related to adverse clinical outcomes in HCC patients. Functionally, knockdown of SNHG15 decreased migration, invasion and proliferation of HCC cells. At the same time, SNHG15 was found to accelerate HCC progression by targeting the miR-490-3p/HDAC2 axis, indicating that SNHG15 may be a therapeutic target for HCC patients.
Consistent with our results, up-regulation of SNHG15 was also detected in colorectal carcinoma and lung cancer[17,18]. Functionally, up-regulated expression of SNHG15 accelerated proliferation and invasion of gastric cancer cells[19]. In addition, Kong et al[20] reported that SNHG15 facilitated human breast cancer cell migration by sponging miR-211-3p. The same effect of SNHG15 was also identified in HCC, which was consistent with previous studies. Besides that, Zhang et al[9] demonstrated that abnormal expression of SNHG15 was related to TNM stage and vein invasion in HCC patients. Similarly, tumor size, Edmondson-Steiner grading and TNM stage were also related to SNHG15 expression in our research. Further, SNHG15 was confirmed as a molecular sponge for miR-490-3p in this study, which has not been found in previous studies.
The dysregulation of miR-490-3p has been identified in the tumorigenesis of human cancers. Down-regulation of miR-490-3p had been found in ovarian epithelial carcinoma and colorectal cancer[21,22]. In this research, miR-490-3p was also down-regulated in HCC. Furthermore, miR-490-3p overexpression was found to decrease migration, invasion and proliferation of HCC cells. Consistently, the inhibitory role of miR-490-3p had been also identified in lung cancer and colorectal cancer[23,24]. miR-490-3p promoted viability and epithelial to mesenchymal transition of HCC cells[25], which also supports the accuracy of our results. In addition, reciprocal suppression between SNHG15 and miR-490-3p was identified in HCC. Furthermore, SNHG15 up-regulation abolished the suppressive effect of miR-490-3p in HCC progression. These findings imply that SNHG15 accelerated HCC development by sponging miR-490-3p.
We explored the downstream mechanism of miR-490-3p in HCC. We found that miR-490-3p directly targets HDAC2. Moreover, up-regulation of HDAC2 was found in HCC, and a negative association between their expressions was detected in HCC. Meanwhile, a positive correlation between the expression of SNHG15 and HDAC2 was observed in HCC. Previous studies suggested that HDAC2 was up-regulated in breast cancer and colorectal cancer, acting as an oncogene[26,27]. In our research, knockdown of HDAC2 was also found to inhibit HCC progression, serving as a tumor promoter. More importantly, down-regulation of miR-490-3p or up-regulation of SNHG15 was identified to recover the inhibitory effect of HDAC2 silencing in HCC. Taken together, we for the first time demonstrated that HDAC2 regulated by the SNHG15/miR-490-3p axis promoted the tumorigenesis of HCC.
Among all cancers, hepatocellular carcinoma (HCC) related mortality is one of the highest and has seen a dramatic increase in annual global incidence rate. Many recent studies have demonstrated how transcriptional regulation affects HCC. Long non-coding RNAs (lncRNA) play a role in the initiation and progression of HCC, such as maintenance of cell growth, evasion of apoptosis, promotion of invasion and metastasis, stemness maintenance and epithelial to mesenchymal transition.
To discover biomarkers for the diagnosis and treatment of HCC.
To investigate the underlying mechanisms of lncRNA-SNHG15 in HCC.
lncRNA-SNHG15 expression was observed by qRT-PCR assay in HCC tissue and cell lines. Clinicopathological characteristics were collected, arranged and combined with expression analysis of HCC to evaluate the functions of lncRNA-SNHG15. Moreover, cell function assays and western blot were performed to explore the functions of lncRNA-SNHG15 and targets regulated by lncRNA-SNHG15 in HCC cell lines.
We found that lncRNA-SNHG15 was increased in HCC tissues and cell lines and exhibited a significantly positive relationship with tumor sizes, TNM stage and Edmondson-Steiner grading. Cell experiments showed SNHG15 increased the proliferation and invasion capacity of HCC cell lines, and miR-490-3p/histone deacetylase 2 may be the target regulated by lncRNA-SNHG15 in HCC cells.
Our study demonstrated that lncRNA-SNHG15 can significantly promote cell growth, migration and invasion of HCC. Furthermore, it can work through miR-490-3p/histone deacetylase 2. Therefore, our study provides some molecular mechanism and three new biomarkers for HCC.
In the future, research may reveal the important role of lncRNA-SNHG15 that enhances the sensitivity of HCC detection and further develop its application in anti-cancer treatments.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Grade E (Poor): 0
P-Reviewer: El-Hawary AK, Tanabe S S-Editor: Wang J L-Editor: Filipodia E-Editor: Liu MY
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 2. | Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 363] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 3. | Liu PH, Hsu CY, Hsia CY, Lee YH, Su CW, Huang YH, Lee FY, Lin HC, Huo TI. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J Hepatol. 2016;64:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 4. | Harries LW. Long non-coding RNAs and human disease. Biochem Soc Trans. 2012;40:902-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Sun J, Guo Y, Bie B, Zhu M, Tian H, Tian J, Li J, Yang Y, Ji F, Kong G, Li Z. Silencing of long noncoding RNA HOXD-AS1 inhibits proliferation, cell cycle progression, migration and invasion of hepatocellular carcinoma cells through MEK/ERK pathway. J Cell Biochem. 2019; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Ding H, Liu J, Zou R, Cheng P, Su Y. Long non-coding RNA TPTEP1 inhibits hepatocellular carcinoma progression by suppressing STAT3 phosphorylation. J Exp Clin Cancer Res. 2019;38:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Liu Y, Li J, Li F, Li M, Shao Y, Wu L. SNHG15 functions as a tumor suppressor in thyroid cancer. J Cell Biochem. 2019;120:6120-6126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Zhang D, Lv J, Wang S, Zhang Q. LncRNA SNHG15 acts as an oncogene in prostate cancer by regulating miR-338-3p/FKBP1A axis. Gene. 2019;705:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Zhang JH, Wei HW, Yang HG. Long noncoding RNA SNHG15, a potential prognostic biomarker for hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2016;20:1720-1724. [PubMed] |
| 10. | Keller C, Kulasegaran-Shylini R, Shimada Y, Hotz HR, Bühler M. Noncoding RNAs prevent spreading of a repressive histone mark. Nat Struct Mol Biol. 2013;20:994-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Li M, Bian Z, Jin G, Zhang J, Yao S, Feng Y, Wang X, Yin Y, Fei B, You Q, Huang Z. LncRNA-SNHG15 enhances cell proliferation in colorectal cancer by inhibiting miR-338-3p. Cancer Med. 2019;8:2404-2413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Wang H, Chen W, Jin M, Hou L, Chen X, Zhang R, Zhang J, Zhu J. CircSLC3A2 functions as an oncogenic factor in hepatocellular carcinoma by sponging miR-490-3p and regulating PPM1F expression. Mol Cancer. 2018;17:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Ou Y, He J, Liu Y. MiR-490-3p inhibits autophagy via targeting ATG7 in hepatocellular carcinoma. IUBMB Life. 2018;70:468-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Noh JH, Bae HJ, Eun JW, Shen Q, Park SJ, Kim HS, Nam B, Shin WC, Lee EK, Lee K, Jang JJ, Park WS, Lee JY, Nam SW. HDAC2 provides a critical support to malignant progression of hepatocellular carcinoma through feedback control of mTORC1 and AKT. Cancer Res. 2014;74:1728-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Noh JH, Chang YG, Kim MG, Jung KH, Kim JK, Bae HJ, Eun JW, Shen Q, Kim SJ, Kwon SH, Park WS, Lee JY, Nam SW. MiR-145 functions as a tumor suppressor by directly targeting histone deacetylase 2 in liver cancer. Cancer Lett. 2013;335:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Ji D, Wang Y, Sun B, Yang J, Luo X. Long non-coding RNA MNX1-AS1 promotes hepatocellular carcinoma proliferation and invasion through targeting miR-218-5p/COMMD8 axis. Biochem Biophys Res Commun. 2019;513:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Saeinasab M, Bahrami AR, González J, Marchese FP, Martinez D, Mowla SJ, Matin MM, Huarte M. SNHG15 is a bifunctional MYC-regulated noncoding locus encoding a lncRNA that promotes cell proliferation, invasion and drug resistance in colorectal cancer by interacting with AIF. J Exp Clin Cancer Res. 2019;38:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Cui HX, Zhang MY, Liu K, Liu J, Zhang ZL, Fu L. LncRNA SNHG15 promotes proliferation and migration of lung cancer via targeting microRNA-211-3p. Eur Rev Med Pharmacol Sci. 2018;22:6838-6844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 19. | Chen SX, Yin JF, Lin BC, Su HF, Zheng Z, Xie CY, Fei ZH. Upregulated expression of long noncoding RNA SNHG15 promotes cell proliferation and invasion through regulates MMP2/MMP9 in patients with GC. Tumour Biol. 2016;37:6801-6812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Kong Q, Qiu M. Long noncoding RNA SNHG15 promotes human breast cancer proliferation, migration and invasion by sponging miR-211-3p. Biochem Biophys Res Commun. 2018;495:1594-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Xu X, Chen R, Li Z, Huang N, Wu X, Li S, Li Y, Wu S. MicroRNA-490-3p inhibits colorectal cancer metastasis by targeting TGFβR1. BMC Cancer. 2015;15:1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Chen S, Chen X, Xiu YL, Sun KX, Zhao Y. MicroRNA-490-3P targets CDK1 and inhibits ovarian epithelial carcinoma tumorigenesis and progression. Cancer Lett. 2015;362:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Gu H, Yang T, Fu S, Chen X, Guo L, Ni Y. MicroRNA-490-3p inhibits proliferation of A549 lung cancer cells by targeting CCND1. Biochem Biophys Res Commun. 2014;444:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Zheng K, Zhou X, Yu J, Li Q, Wang H, Li M, Shao Z, Zhang F, Luo Y, Shen Z, Chen F, Shi F, Cui C, Zhao D, Lin Z, Zheng W, Zou Z, Huang Z, Zhao L. Epigenetic silencing of miR-490-3p promotes development of an aggressive colorectal cancer phenotype through activation of the Wnt/β-catenin signaling pathway. Cancer Lett. 2016;376:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Zhang LY, Liu M, Li X, Tang H. miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3). J Biol Chem. 2013;288:4035-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Shan W, Jiang Y, Yu H, Huang Q, Liu L, Guo X, Li L, Mi Q, Zhang K, Yang Z. HDAC2 overexpression correlates with aggressive clinicopathological features and DNA-damage response pathway of breast cancer. Am J Cancer Res. 2017;7:1213-1226. [PubMed] |
| 27. | Tang W, Zhou W, Xiang L, Wu X, Zhang P, Wang J, Liu G, Zhang W, Peng Y, Huang X, Cai J, Bai Y, Bai L, Zhu W, Gu H, Xiong J, Ye C, Li A, Liu S, Wang J. The p300/YY1/miR-500a-5p/HDAC2 signalling axis regulates cell proliferation in human colorectal cancer. Nat Commun. 2019;10:663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |