Published online Sep 14, 2019. doi: 10.3748/wjg.v25.i34.5049
Peer-review started: May 15, 2019
First decision: July 21, 2019
Revised: July 28, 2019
Accepted: August 19, 2019
Article in press: August 19, 2019
Published online: September 14, 2019
Processing time: 121 Days and 0.6 Hours
Esophageal squamous cell carcinoma (ESCC) and esophagogastric junction adenocarcinoma (EGJA) are the two main types of gastrointestinal cancers that pose a huge threat to human health. ESCC remains one of the most common malignant diseases around the world. In contrast to the decreasing prevalence of ESCC, the incidence of EGJA is rising rapidly. Early detection represents one of the most promising ways to improve the prognosis and reduce the mortality of these cancers. Current approaches for early diagnosis mainly depend on invasive and costly endoscopy. Non-invasive biomarkers are in great need to facilitate earlier detection for better clinical management of patients. Tumor-associated autoantibodies can be detected at an early stage before manifestations of clinical signs of tumorigenesis, making them promising biomarkers for early detection and monitoring of ESCC and EGJA. In this review, we summarize recent insights into the iden-tification and validation of tumor-associated autoantibodies for the early detection of ESCC and EGJA and discuss the challenges remaining for clinical validation.
Core tip: The current protocol for early diagnosis of esophageal squamous cell carcinoma and esophagogastric junction adenocarcinoma is endoscopic imaging followed by biopsy confirmation. However, the invasive nature of the procedure and high cost of endoscopy limit it as a tool for screening the general population. This review highlights autoantibodies as non-invasive biomarkers in the early detection of esophageal squamous cell carcinoma and esophagogastric junction adenocarcinoma.
- Citation: Xu YW, Peng YH, Xu LY, Xie JJ, Li EM. Autoantibodies: Potential clinical applications in early detection of esophageal squamous cell carcinoma and esophagogastric junction adenocarcinoma. World J Gastroenterol 2019; 25(34): 5049-5068
- URL: https://www.wjgnet.com/1007-9327/full/v25/i34/5049.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i34.5049
Esophageal cancer is the eighth leading malignant disease and the sixth most common cause of cancer-related death worldwide. It represents a serious health problem globally[1]. Esophageal cancer is mainly composed of two epidemiologically and histopathologically distinct sub-types designated as esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma. In China, esophageal cancer is the third leading cause of cancer death with an estimated 246000 new cases and 188000 deaths in 2015[2]. Although ESCC, which accounts for 70% of cases, remains the most prevalent form of esophageal cancer, the prevalence of ESCC has declined substantially in recent years. In contrast to the decreasing prevalence of ESCC, an alarming rise of the incidence in esophagogastric junction adenocarcinoma (EGJA) has been observed in both developed and developing countries with 260000 new cases diagnosed in 2012[2-4]. Interestingly, in China the incidence of EGJA appears to be high in areas where the prevalence of ESCC is also high[5]. This similar geographic distribution suggests similar environmental factors, similar dietary habits and even similar molecular alterations are involved in both ESCC and EGJA[6].
The prognosis of ESCC is poor with an overall 5-year incidence of survival ranging from 15% to 25%[7,8]. The high mortality in ESCC and EGJA mostly results from diagnosis at late stages due to the lack of specific symptoms of patients in early stage disease, but the prognosis is substantially better for patients diagnosed in the early stage (e.g., 5-year survival of more than 85% for ESCC patients diagnosed in early stage and more than 90% for EGJA patients with node-negative T1 tumors)[9,10]. However, effective strategies are lacking for screening or detection of pre-cancerous lesions in early-stage ESCC and EGJA. Although endoscopy is used as a primary screening technique and can identify ESCC and EGJA at an early stage, its extensive utilization is limited by the invasive nature, serious side effects and dependence on the skill of the endoscopist. Moreover, some individuals are unwilling to undergo endoscopy, whereas a simple blood test might be more acceptable. Thus, identification and validation of novel non-invasive, blood-based biomarkers can fulfill a great need for early detection of ESCC and EGJA.
Tumor-associated (TA) autoantibodies are emerging as strong candidates for clinically useful cancer biomarkers because they are produced early in tumorigenesis and can be detectable up to five years before the clinical manifestations of cancer[11-13]. Moreover, autoantibodies are also reported as biomarkers used in cancer prognosis and therapeutic monitoring (Table 1)[11,14-21]. A large number of articles have evaluated the potential use of TA autoantibodies for early ESCC detection. Therefore, a systematic review is warranted to assess the current potential of TA autoantibodies for the early diagnosis of patients with ESCC. Considering the similarity of the etiology and epidemiology in EGJA and ESCC, we believe that it would be much desirable to provide together a review of TA autoantibodies in EGJA and ESCC at this current time. We focus on the key aspects of the study designs and participant characteristics, the sensitivity, specificity and area under the receiver operating characteristic curve of the TA autoantibody biomarkers to help identify the most promising candidates for future clinical screening tests.
| Representative tumor-associated autoantigens | Authors, year | Tumor type | Biological significance |
| p53 | Chapman et al[11], 2012 | Lung | Early detection |
| Takeda et al[14], 2001 | Colorectal | Increased recurrence | |
| Anderson et al[15], 2010 | Ovarian | Increased survival | |
| NY-ESO-1 | Shan et al[16], 2013 | Lung | Early detection |
| Fosså et al[17], 2004 | Prostate | Decreased survival | |
| Elke et al[18], 1999 | Melanoma | Therapeutic monitoring | |
| MUC1 | Pedersen et al[19], 2014 | Ovarian | Early detection |
| Kurtenkov et al[20], 2007 | Gastric | Increased survival | |
| Hu | Chapman et al[11], 2011 | Lung | Early detection |
| Graus et al[21], 1997 | Lung | Therapeutic monitoring and increased survival |
As early as the 1960s, Robert W. Baldwin showed that the immune system could react to a developing tumor[22-24]. Most studies have mainly focused on evaluating TA autoantibodies as early cancer biomarkers since their discovery. On the other hand, investigation of the underlying causes of TA autoantibody production may contribute to a clearer understanding of mechanisms concerning the rendering of autologous proteins immunogenic and also reveal novel therapeutic targets for potential clinical use. It is commonly accepted that autologous cellular antigens expressed in tumors, also referred to as TA antigens (TAA), can be recognized early by the immune system and thus trigger a reaction known as cancer immunoediting, which consists of three phases: Elimination, equilibration and escape[25,26]. Immunosurveillance occurs during the elimination phase when the first few transformed cells are recognized by the immune system and targeted by natural killer cells that secrete certain cytokines to let other immune cells convene to the tumor[27]. The ensuing disruption of certain transformed cells and the uptake and disposal of the corresponding fragments by the recruited immune cells activate the appropriate immune response. A cascade of dynamic events further boosts the activation of innate immunity and facilitates the expansion and generation of T and B cells (the latter produces antibodies)[28]. Tumor cells that escape elimination and are permitted to grow will enter into the equilibrium phase during which tumor cell variants emerge with increasing ability to survive an immune attack. The equilibrium phase is the longest among these three phases and may persist for many years. Escape eventually occurs if the host immune defenses are breached and tumor cell variants grow and proliferate in an uncontrolled manner[29].
It is clear that the generation of many abnormal antigens during tumorigenesis can induce the host immune response to produce autoantibodies. However, how factors exactly facilitate an enhancement or disorder of immune surveillance in cancer resulting in the TA autoantibody production response is still unclear. The generation of TA autoantibodies is thought to occur in response to mutations[30,31], over-expression[32,33] or abnormal processing[34,35], which lead to the formation of altered or novel epitopes, aberrantly high expression levels resulting in loss of tolerance and abnormal post-translational modifications, such as acetylation, glycosylation and phosphorylation, all of which could create a neoepitope, enhance self-epitope presentation or expose antigens normally located in immune-privileged sites (e.g., cancer-testis antigens). With these mechanisms, extracellular and intracellular host proteins could be recognized by B cells to produce TA autoantibodies. Recent research has estimated that most TA autoantigens are mutated or overexpressed proteins among which 42% are cytoplasmatic, 26.1% are expressed predominantly in the nucleus, 21.4% are membrane-bound and 10.3% are extracellular[36]. It is surprising that TA autoantibodies seem to be more specific to intracellular molecules rather than their more common cell surface targets. This may be explained by greater vascular permeability for cytoplasmic proteins and enhancement of autoantibody generation by the proinflammatory environment[37,38]. Although the exact role of TA auto-antibodies in cancer is largely undefined, that secreted TA autoantibodies reflect tumor burden makes them attractive and promising biomarkers.
Although TA autoantibodies have been described in a wide variety of human malignancies in the past several decades and have shown early diagnostic relevance, most studies evaluating autoantibodies in patients with ESCC and normal controls have emerged within the last 20 years. At least 49 individual TA autoantibodies have been assessed with diagnostic parameters in ESCC. Table 2 represents a list of single TA autoantibodies reported in the literature that could serve as potential serum/ plasma biomarkers for ESCC. The diagnostic value of the TA autoantibodies, whether for the same autoantibody or for different types of autoantibodies, shows large variation for ESCC in terms of sensitivity and specificity, which might be due to sample size, the ethnic group studied or the test method of evaluation.
| Target antigen of auto-anti-bodies | Authors, year | ESCC cases, n | Stage, n | Con-trols, n | P value | Sensiti-vity, all stages/ early stage | Specifi-city, all stages/ early stage | AUC, all stages/ early stage | Method | ||||
| 0/I | II | III | IV | Tx | |||||||||
| Survivin | Xiu et al[57], 2018 | 159 | - | - | - | - | 159 | 362 | 0.524 | 14.5%/- | 90.0%/- | 0.327/- | ELISA |
| Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 12.1%/- | 99.6%/- | 99.6/- | ELISA | |
| Zhou et al[44], 2014 | 88 | - | - | - | - | 88 | 200 | 0.06 | 9.0%/- | 96.0%/- | - | ELISA | |
| Meglior-ino et al[59], 2005 | 77 | - | - | - | - | 77 | 82 | < 0.05 | 10.4%/- | 97.6%/- | - | ELISA | |
| TOPO48 | Zhang et al[60], 2018 | 112 | 29 | 28 | 28 | 27 | - | 112 healthy volunte-ers and 75 esopha-geal benign tumor patients | 0.001 | 49.1%/ 61.4% | 100%/ 100% | -/0.860 | ELISA |
| L1CAM | Xu et al[61], 2017 | 191 (Cohort 1) | 10 | 104 | 77 | - | - | 94 (Cohort 1) | 0.005 | 26.2%/ 25.2% | 90.4%/ 90.4% | 0.603/ 0.611 | ELISA |
| 47 (Cohort 2) | 5 | 18 | 24 | - | - | 47 (Cohort 2) | 0.032 | 27.7%/ 33.3% | 91.5%/ 91.5% | 0.628/ 0.636 | ELISA | ||
| Ezrin | Li et al[62], 2017 | 149 | 4/14 | 57 | 71 | 3 | 149 | 98 | < 0.0001 | 27.5%/ 27.8% | 95.9%/ 95.9% | - | ELISA |
| STIP1 | Xu et al[63], 2017 | 148 (Train-ing) | 3/17 | 48 | 76 | 4 | 148 | 111 (Train-ing) | < 0.001 | 41.9%/ 35.7% | 90.1%/ 90.1% | 0.682/ 0.684 | ELISA |
| 60 (Valida-tion) | 1/8 | 20 | 30 | 1 | 60 | 40 (Valida-tion) | < 0.001 | 40.0%/ 38.5% | 92.5%/ 92.5% | 0.710/ 0.756 | ELISA | ||
| Fascin | Chen et al[64], 2017 | 149 | 4/14 | 57 | 71 | 3 | 149 | 98 | < 0.001 | 24.8%/ 20.6% | 99.0%/ 99.0% | 0.636/ 0.632 | ELISA |
| DKK-1 | Peng et al[65], 2016 | 185 (Train-ing) | 4/23 | 69 | 85 | 4 | 185 | 97 (Train-ing) | < 0.0001 | 33.5%/ 34.6% | 91.8%/ 91.8% | 0.643/ 0.640 | ELISA |
| 104 (Valida-tion) | 1/12 | 35 | 53 | 3 | 104 | 53 (Valida-tion) | < 0.0001 | 33.7%/ 26.9% | 92.5%/ 92.5% | 0.629/ 0.603 | ELISA | ||
| P16 | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | < 0.001 | 29.3%/- | 81.8%/- | 0.60/- | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | < 0.01 | - | - | - | ELISA | ||
| Jin et al[66], 2015 | 88 | 24 | 42 | 15 | 2 | 5 | 208 | 0.0052 | 5.7%/- | 99.1%/- | - | ELISA | |
| Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 18.4%/- | 98.8%/- | 0.6/- | ELISA | |
| Zhou et al[44], 2014 | 88 | - | - | - | - | 88 | 200 | 0.004 | 11.0%/- | 97.0%/- | - | ELISA | |
| Looi et al[67], 2006 | 71 | - | - | - | - | - | 82 | < 0.05 | 14.1%/- | 98.8%/- | - | ELISA | |
| p53 | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | < 0.001 | 55.9%/- | 89.5%/- | 0.784/- | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | < 0.001 | -/- | -/- | -/- | ELISA | ||
| Xu et al[43], 2014 | 388 (Test) | 2/29 | 96 | 229 | 27 | 5 | 125 (Test) | < 0.0001 | 30.0%/- | 98.0%/- | - | ELISA | |
| 237 (Valida-tion) | 2/31 | 114 | 90 | - | - | 134 (Valida-tion) | < 0.0001 | 29.0%/- | 97.0%/- | - | ELISA | ||
| Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 21.8%/- | 96.3%/- | 0.6/- | ELISA | |
| Chai et al[68], 2014 | 157 | - | - | - | - | 157 | 85 | < 0.01 | 22.9%/- | 100%/- | - | ELISA | |
| Zhou et al[44], 2014 | 88 | - | - | - | - | 88 | 200 | < 0.001 | 22.0%/- | 98.0%/- | - | ELISA | |
| Cai et al[69], 2008 | 46 | 10 | 17 | 14 | 5 | - | 30 | < 0.0001 | 39.1%/ 22.2% | 100%/ 100% | - | ELISA | |
| Looi et al[67], 2006 | 71 | - | - | - | - | 71 | 82 | < 0.05 | 7%/- | 98.8%/- | - | ELISA | |
| Müller et al[70], 2006 | 50 | - | - | - | - | 50 | 436 | < 0.05 | 20.0%/- | 100%/- | - | Western blot | |
| Meglior-ino et al[59], 2005 | 77 | - | - | - | - | 77 | 82 | < 0.01 | 14.3%/- | 97.6%/- | - | ELISA | |
| Shimada et al[71], 2003 | 301 | - | - | - | - | 301 | 205 | < 0.05 | 30.0%/- | 95.5%/- | - | ELISA | |
| Shimada et al[72], 2002 | 105 | 50 | 24 | 21 | 10 | - | 153 | < 0.001 | 26.7%/ 20.0% | 95.5%/ 95.5% | - | ELISA | |
| Ralhan et al[73], 2000 | 60 | - | - | - | - | 60 | 50 | < 0.05 | 60.0%/- | 92.0%/- | - | ELISA | |
| Shimada et al[74], 2000 | 35 | - | - | - | - | 35 | 69 | < 0.0001 | 40.0%/- | 100.0%/- | - | ELISA | |
| Hagi-wara et al[75], 2000 | 46 | 6 | 15 | 24 | 2 | - | 13 | < 0.05 | 28.0%/ 28.6% | 100%/ 100% | - | ELISA | |
| Shimada et al[76], 1998 | 57 | 6/9 | 9 | 11 | 11 | 1 | 208 | < 0.05 | 58.0%/- | 99.0%/- | - | ELISA | |
| Sobti et al[77], 1998 | 20 | - | - | - | - | 20 | 20 | 0.0202 | 30.0%/- | 100%/- | - | ELISA | |
| Cawley et al[78], 1988 | 23 | - | - | - | - | 23 | 19 | 0.0372 | 34.8%/- | 94.7%/- | - | ELISA | |
| NY-ESO-1 | Xu et al[43], 2014 | 388 (Test) | 2/29 | 96 | 229 | 27 | 5 | 125 (Test) | < 0.0001 | 26.0%/- | 100%/- | - | ELISA |
| 237 (Valida-tion) | 2/31 | 114 | 90 | - | - | 134 (Valida-tion) | < 0.0001 | 24.0%/- | 99.0%/- | - | ELISA | ||
| Oshima et al[79], 2016 | 172 | - | - | - | - | 172 | 74 | < 0.001 | 32.0%/ 16.0% | 100%/ 100% | - | ELISA | |
| Fujita et al[80], 2004 | 51 | - | - | - | - | 51 | 29 | 0.532 | 3.9%/- | 100%/- | - | ELISA | |
| P90 | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | < 0.001 | 31.5%/- | 84.9%/- | 0.617/- | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | < 0.001 | - | - | - | ELISA | ||
| Mmp-7 | Xu et al[43], 2014 | 388 (Test) | 2/29 | 96 | 229 | 27 | 5 | 125 (Test) | < 0.001 | 9.0%/- | 100%/- | - | ELISA |
| 237 (Valida-tion) | 2/31 | 114 | 90 | - | - | 134 (Valida-tion) | < 0.001 | 10.0%/- | 100%/- | - | ELISA | ||
| Zhou et al[81], 2011 | 50 | - | - | - | - | 50 | 58 | < 0.001 | 78.0% | 81.0% | 0.87/- | ELISA | |
| Hsp70 | Xu et al[43], 2014 | 388 (Test) | 2/29 | 96 | 229 | 27 | 5 | 125 (Test) | < 0.001 | 11.0%/- | 99.0%/- | - | ELISA |
| 237 (Valida-tion) | 2/31 | 114 | 90 | - | - | 134 (Valida-tion) | < 0.01 | 8.0%/- | 99.0%/- | - | ELISA | ||
| Zhang et al[82], 2011 | 69 | - | - | - | - | 69 | 76 | > 0.01 | 39.1%/- | 92.3%/- | - | ELISA | |
| Fujita et al[40], 2008 | 16 | 2 | 7 | 4 | 3 | - | 13 | < 0.001 | 93.7%/- | 100%/- | - | ELISA | |
| PRDX 6 | Xu et al[43], 2014 | 388 (Test) | 2/29 | 96 | 229 | 27 | 5 | 125 (Test) | < 0.001 | 11.0%/- | 100%/- | - | ELISA |
| 237 (Valida-tion) | 2/31 | 114 | 90 | - | - | 134 (Valida-tion) | < 0.001 | 10.0%/- | 100%/- | - | ELISA | ||
| Fujita et al[83], 2006 | 30 | 7 | 8 | 11 | 4 | - | 30 | < 0.05 | 50.0%/ 53.5% | 93.4%/ 93.4% | - | Western blot | |
| Bmi-1 | Xu et al[43], 2014 | 388 (Test) | 2/29 | 96 | 229 | 27 | 5 | 125 (Test) | < 0.01 | 11.0%/- | 98.0%/- | - | ELISA |
| 237 (Valida-tion) | 2/31 | 114 | 90 | - | - | 134 (Valida-tion) | < 0.01 | 8.0%/- | 100%/- | - | ELISA | ||
| Liu et al[84], 2010 | 159 | 6 | 72 | 69 | 12 | - | 102 | < 0.001 | 39.0%/- | 100%/- | - | ELISA | |
| Imp1 | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | < 0.001 | 26.9%/- | 81.2%/- | 0.576/- | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | < 0.01 | - | - | - | ELISA | ||
| Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 16.1%/- | 98.3%/- | 0.6/- | ELISA | |
| Zhou et al[44], 2014 | 88 | - | - | - | - | 88 | 200 | < 0.001 | 14.0%/- | 99.0%/- | - | ELISA | |
| Cyclin B1 | Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 16.1%/- | 97.9%/- | 0.6/- | ELISA |
| Zhou et al[44], 2014 | 88 | - | - | - | - | 88 | 200 | 0.02 | 10.0%/- | 97.0%/- | - | ELISA | |
| C-Myc | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | < 0.001 | 49.1%/- | 81.5%/- | 0.699/- | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | < 0.001 | - | - | - | ELISA | ||
| Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 15.5%/- | 98.8%/- | 0.6/- | ELISA | |
| Zhou et al[44], 2014 | 88 | - | - | - | - | 88 | 200 | < 0.001 | 18.0%/- | 96.0%/- | - | ELISA | |
| Looi et al[67], 2006 | 71 | - | - | - | - | - | 82 | < 0.05 | 7%/- | 100%/- | - | ELISA | |
| Meglio-rino et al[59], 2005 | 77 | - | - | - | - | 77 | 82 | < 0.01 | 11.7%/- | 100%/- | - | ELISA | |
| RalA | Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 15.5%/- | 96.7%/- | 0.6/- | ELISA |
| P62 | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | < 0.001 | 29.3%/- | 81.8%/- | 0.60/- | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | < 0.001 | - | - | - | ELISA | ||
| Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 12.1%/- | 95.9%/- | 0.5/- | ELISA | |
| Zhou et al[44], 2014 | 88 | - | - | - | - | 88 | 200 | 0.001 | 13.0%/- | 98.0%/- | - | ELISA | |
| Koc | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | < 0.001 | 35.8%/- | 82.1%/- | 0.63/- | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | < 0.05 | - | - | - | ELISA | ||
| Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 11.5%/- | 97.9%/- | 0.5/- | ELISA | |
| Zhou et al[44], 2014 | 88 | - | - | - | - | 88 | 200 | 0.05 | 10.0%/- | 96.0%/- | - | ELISA | |
| Cyclin D1 | Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 10.3%/- | 96.3%/- | 0.5/- | ELISA |
| Cyclin E | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | < 0.001 | 26.5%/- | 83.0%/- | 0.581/- | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | < 0.05 | - | - | - | ELISA | ||
| Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | < 0.05 | 10.3%/- | 99.2%/- | 0.5/- | ELISA | |
| HCCR | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | < 0.001 | 34.6%/- | 80.0%/- | 0.596/- | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | < 0.001 | - | - | - | ELISA | ||
| GSTO1 | Li et al[85], 2014 | 67 | - | - | - | - | 67 | 90 | < 0.01 | 44.8%/- | 93.3%/- | - | ELISA |
| MDM2 | Chai et al[68], 2014 | 157 | - | - | - | - | 157 | 85 | < 0.01 | 14.0%/- | 98.8%/- | - | ELISA |
| HSP105 | Gao et al[51], 2014 | 46 | 7 | - | - | - | 39 | 40 | < 0.01 | 39.1%/ 42.9% | 95%/95% | 0.794/- | Western blot |
| TIM | Gao et al[51], 2014 | 46 | 7 | - | - | - | 39 | 40 | < 0.01 | 34.8%/ 28.6% | 95%/95% | 0.786/- | Western blot |
| Prdx1 | Ren et al[86], 2013 | 68 | - | - | - | - | 68 | 89 | < 0.01 | 13.2%/- | 100%/- | - | ELISA, Western blot |
| FOXP3 | Ye et al[87], 2013 | 97 | 26 | 45 | 19 | 2 | 5 | 227 | < 0.0001 | 22.7%/- | 95.2%/- | 0.70/- | ELISA, |
| CD25 | Guan et al[88], 2013 | 97 | 26 | 45 | 17 | 3 | 6 | 226 | < 0.001 | 37.2%/- | 90.0%/- | 0.69/- | ELISA |
| ABCC3 (IgA) | Cheng et al[89], 2013 | 114 | - | - | - | - | - | 226 | < 0.001 | 13.2%/- | >95%/- | 0.65/- | ELISA |
| LY6K | Zhang et al[90], 2012 | 62 | 13 | 27 | 22 | - | 58 | < 0.001 | 80.6%/ 73.2% | 78.7%/ 78.7% | 0.85/- | ELISA | |
| HMGB1 | Zhang et al[82], 2011 | 69 | - | - | - | - | 69 | 76 | > 0.05 | 7.2%/- | 98.7%/- | - | ELISA |
| ESCA-1 | Kagaya et al[91], 2011 | 146 | 32 | 29 | 42 | 43 | - | 118 | 0.0001 | 21.2%/- | 98.3%/- | - | ELISA |
| ESCA-2 | Kagaya et al[91], 2011 | 72 | 37 | 35 | 72 | 98 | 0.0026 | 15.3%/ 8.1% | 99.0%/ 99.0% | - | ELISA | ||
| ESCA-3 | Kagaya et al[91], 2011 | 68 | - | - | - | - | 68 | 74 | 0.0079 | 16.2%/- | 98.6%/- | - | ELISA |
| CDC25B | Dong et al[92], 2010 | 134 | 80 | 54 | - | 134 | < 0.001 | 56.7%/- | 91.0% | 0.87/- | ELISA | ||
| Liu et al[93], 2008 | 124 | - | - | - | - | 123 | 102 | < 0.05 | 36.3%/- | 100%/- | - | ELISA | |
| GRP78 | Tsunemi et al[94], 2010 | 15 | - | - | - | - | 15 | 20 | < 0.05 | 26.7%/- | 100%/- | - | Western blot |
| Makorin 1 | Shimada et al[95], 2009 | 73 | 1/13 | 21 | 27 | 11 | - | 43 | < 0.05 | 25.0%/ 22.9% | 100%/ 100% | - | Western blot |
| CUEC-23 | Shimada et al[96], 2009 | 54 | 7 | 13 | 18 | 16 | - | 46 | < 0.05 | 26.0%/ 33.3% | 96.0%/- | - | Western blot |
| Shimada et al[96], 2009 | 29 | 1 | 11 | 8 | 9 | - | 46 | 0.036 | 17.0%/- | 100%/- | - | ELISA | |
| MMGL | Shimada et al[97], 2007 | 91 | 21 | 22 | 35 | 13 | - | 45 | < 0.05 | 47.0%/ 38.0% | 97.8%/ 97.8% | - | Western blot |
| TRIM21 | Kubo-shima et al[98], 2006 | 91 | 39 | 52 | - | 42 | < 0.05 | 20.0%/ 13.0% | 100%/ 100% | - | Western blot | ||
| Kubo-shima et al[98], 2006 | 54 | - | - | - | - | 54 | 42 | 0.013 | 15.0%/- | 98.0%/- | - | ELISA | |
| SLC2A1 | Kubo-shima et al[99], 2006 | 57 | 19 | 6 | 13 | 19 | - | 31 | < 0.001 | 21.0%/ 22.0% | 100%/ 100% | - | ELISA |
| SURF1 | Shimada et al[50], 2005 | 21 | - | 3 | 13 | 5 | - | 37 | 0.0003 | 48%/- | 95%/- | - | ELISA |
| LOC 146223 | Shimada et al[50], 2005 | 21 | - | 3 | 13 | 5 | - | 37 | 0.0028 | 38%/- | 95%/- | - | ELISA |
| HOOK2 | Shimada et al[50], 2005 | 21 | - | 3 | 13 | 5 | - | 37 | 0.0431 | 14%/- | 100%/- | - | ELISA |
| AGENCOURT_7565913 | Shimada et al[50], 2005 | 21 | - | 3 | 13 | 5 | - | 37 | 0.0431 | 14%/- | 100%/- | - | ELISA |
| TROP2 | Naka-shima et al[100], 2004 | 75 | 14 | 14 | 24 | 23 | - | 43 | < 0.05 | 31.0%/ 21.0% | 97.7%/ 97.7% | - | Western blot |
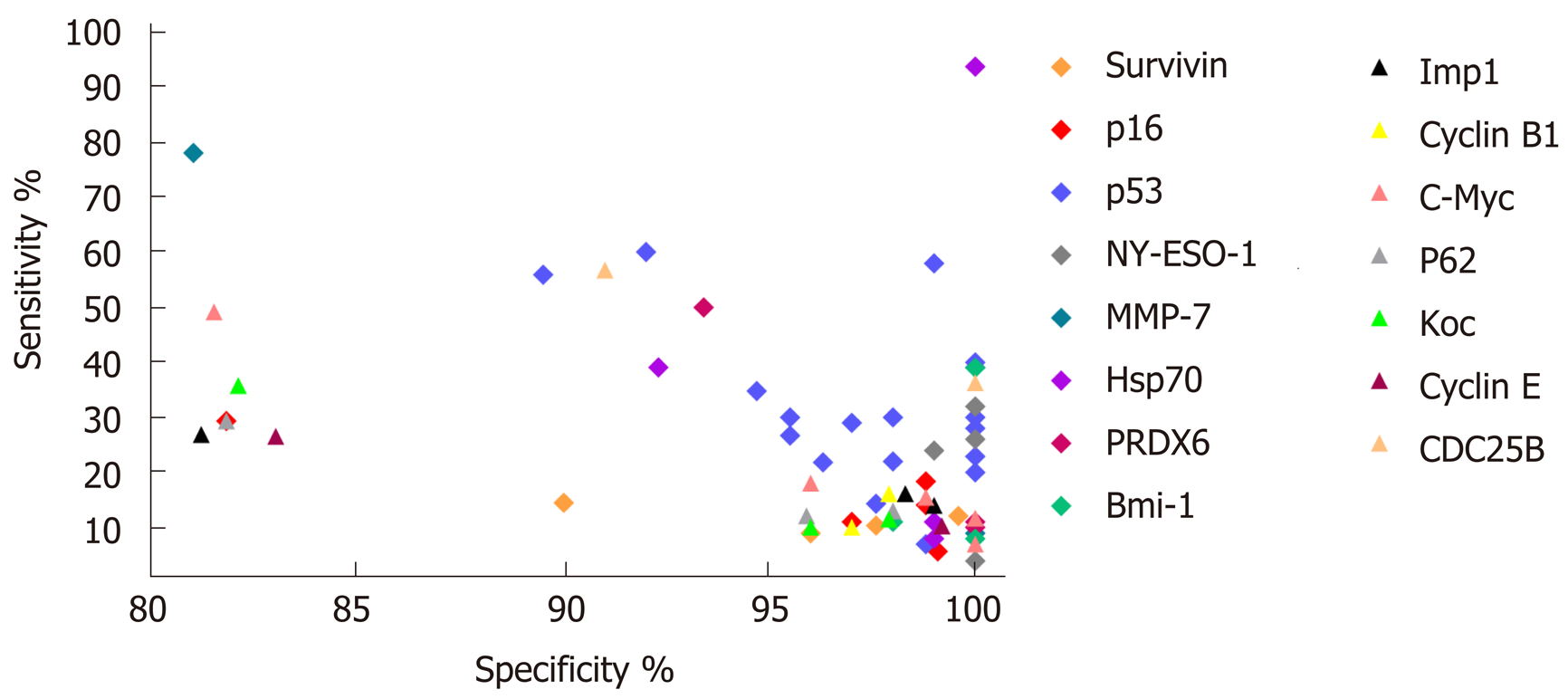
In general, the majority of TA autoantibody biomarkers show relatively low sensitivity but high specificity. The sensitivities and specificities for ESCC range from 3.9% to 93.7% and from 78.7% to 100%, respectively (Table 2). Receiver operating characteristic curves as a summary measure will not set cutoff values artificially but rather considers sensitivity and specificity simultaneously. Nevertheless, only a few studies used receiver operating characteristic curve analysis and area under the curve values to evaluate the diagnostic performance of TA autoantibodies (Table 2). The graphical representation of the sensitivities and specificities for autoantibodies in ESCC evaluated in more than one study is shown in Figure 1. Of note, the diagnostic ability of the great majority of TA autoantibodies lack independent validation, and the numbers of cases in some of the studies are very small. It is also noteworthy that most of the single TA autoantibodies lack diagnostic assessment in patients with early stage ESCC, which is one of the most important elements for biomarker development and application in early cancer diagnosis.
The most comprehensively investigated TA autoantibodies in ESCC have been p53 autoantibodies followed by autoantibodies against P16 and c-Myc. Given the prominent feature of p53 in cancers it is not unexpected that this is the most widely studied autoantibody in ESCC. Autoantibodies against p53 in the diagnosis of ESCC have been evaluated in 17 studies (Table 2), and the sensitivities vary largely between reports (7%-60%) while less variance is observed in the specificity (range 89.5%-100%, Table 2). A meta-analysis by Zhang et al[39] showed the overall sensitivity and specificity of p53 autoantibody for esophageal cancer are 29.6% and 97.9%, respectively. Autoantibodies against P16 and c-Myc were each analyzed in five studies, and both exhibited high specificity but poor sensitivity (Table 2). Therefore, despite the high specificity, all studies show that use of a single autoantibody provides low sensitivity indicating limited clinical application. The sensitivity and specificity for Hsp70 autoantibodies reported by Fujita et al[40] can be up to 93.7% and 100%, respectively. However, the very small sample size of this study reduces the stability and power of the results. Overall, quite apparent is the fact that the diagnostic value of individual TA autoantibody biomarkers in ESCC is quite limited.
Very few clinical or translational studies have treated EGJA as a separate entity, which have been generally divided between those targeting esophageal cancer and those targeting gastric cancer. Likewise, a similar phenomenon has been observed in the studies on autoantibodies for the diagnosis of EGJA. As can be seen from Table 3, a total of 13 autoantibodies were investigated in two studies[41,42], all of which were initially assessed in ESCC by Xu et al[43] and Zhou et al[44]. As anticipated, the presence of TA autoantibodies indicates early diagnostic potential for EGJA. The sensitivity of single TA autoantibody biomarkers for EGJA ranged from 11.0% to 54.3% with generally high specificity ranging from 86.3% to 97% (Table 3). From the list of autoantibodies shown in Table 3, there is no good way of forecasting which TA autoantibodies may work. Like ESCC, the most commonly tested TA autoantibody in EGJA is the p53 autoantibody, which has the highest area under the curve value (0.799) with moderate sensitivity and specificity in the diagnosis of early stage EGJA (Table 3). However, it remains fact that the capability of a single TA autoantibody biomarker to identify EGJA patients is limited. It also should be pointed out that research on autoantibodies is still in its infancy. Thus, more autoantibody biomarkers need to be identified and evaluated to enlarge the autoantibody pool for EGJA.
| Target antigen of auto-anti-bodies | Authors, year | EGJA cases, n | Stage, n | Con-trols, n | P value | Sensiti-vity, all stages/ early stage | Specifi-city, all stages/ early stage | AUC, all stages/ early stage | Method | ||||
| I | II | III | IV | Tx | |||||||||
| p53 | Xu et al[42], 2019 | 122 (Train-ing) | 2 | 16 | 87 | 17 | 122 | 169 (Valida-tion) | < 0.0001 | 35.2%/ 33.3% | 90.5%/ 90.5% | 0.718/ 0.648 | ELISA |
| 70 (Train-ing) | 11 | 14 | 30 | 15 | 80 | 80 (Valida-tion) | < 0.0001 | 35.7%/ 40.0% | 96.3%/ 96.3% | 0.766/ 0.799 | ELISA | ||
| Zhou et al[41], 2015 | 75 | - | - | - | - | 75 | 140 | < 0.001 | 24.0%/- | 92%/- | 0.67/- | ELISA | |
| NY-ESO-1 | Xu et al[42], 2019 | 122 (Train-ing) | 2 | 16 | 87 | 17 | 122 | 169 (Valida-tion) | < 0.0001 | 37.7%/ 27.8% | 90.5%/ 90.5% | 0.718/ 0.654 | ELISA |
| 70 (Train-ing) | 11 | 14 | 30 | 15 | 80 | 80 (Valida-tion) | < 0.0001 | 34.3%/ 28.0% | 95.0%/ 95.0% | 0.747/ 0.714 | ELISA | ||
| PRDX6 | Xu et al[42], 2019 | 122 (Train-ing) | 2 | 16 | 87 | 17 | 122 | 169 (Valida-tion) | 0.033 | 34.4%/ 38.9% | 90.5%/ 90.5% | 0.573/ 0.602 | ELISA |
| 70 (Train-ing) | 11 | 14 | 30 | 15 | 80 | 80 (Valida-tion) | 0.002 | 30.0%/ 28.0% | 90.0%/ 90.0% | 0.647/ 0.629 | ELISA | ||
| MMP-7 | Xu et al[42], 2019 | 122 (Train-ing) | 2 | 16 | 87 | 17 | 122 | 169 (Valida-tion) | 0.005 | 30.3%/ 33.3% | 90.5%/ 90.5% | 0.597/ 0.575 | ELISA |
| 70 (Train-ing) | 11 | 14 | 30 | 15 | 80 | 80 (Valida-tion) | 0.036 | 24.3%/ 28.0% | 95.0%/ 95.0% | 0.599/ 0.609 | ELISA | ||
| Hsp70 | Xu et al[42], 2019 | 122 (Train-ing) | 2 | 16 | 87 | 17 | 122 | 169 (Valida-tion) | < 0.0001 | 18.0%/ 16.7% | 90.5%/ 90.5% | 0.652/ 0.697 | ELISA |
| 70 (Train-ing) | 11 | 14 | 30 | 15 | 80 | 80 (Valida-tion) | < 0.0001 | 28.6%/ 32.0% | 86.3%/ 86.3% | 0.686/ 0.702 | ELISA | ||
| Bmi-1 | Xu et al[42], 2019 | 122 (Train-ing) | 2 | 16 | 87 | 17 | 122 | 169 (Valida-tion) | < 0.0001 | 22.1%/ 27.8% | 90.5%/ 90.5% | 0.686/ 0.685 | ELISA |
| 70 (Train-ing) | 11 | 14 | 30 | 15 | 80 | 80 (Valida-tion) | < 0.0001 | 54.3%/ 40.0% | 90.0%/ 90.0% | 0.711/ 0.682 | ELISA | ||
| Koc | Zhou et al[41], 2015 | 75 | - | - | - | - | 75 | 140 | 0.05 | 19.0%/- | 91%/- | - | ELISA |
| P62 | Zhou et al[41], 2015 | 75 | - | - | - | - | 75 | 140 | 0.02 | 16.0%/- | 94%/- | - | ELISA |
| C-Myc | Zhou et al[41], 2015 | 75 | - | - | - | - | 75 | 140 | 0.18 | 11.0%/- | 94%/- | - | ELISA |
| IMP1 | Zhou et al[41], 2015 | 75 | - | - | - | - | 75 | 140 | 0.04 | 13.0%/- | 95%/- | - | ELISA |
| Survivin | Zhou et al[41], 2015 | 75 | - | - | - | - | 75 | 140 | 0.002 | 17.0%/- | 96%/- | - | ELISA |
| P16 | Zhou et al[41], 2015 | 75 | - | - | - | - | 75 | 140 | 0.01 | 15.0%/- | 96%/- | - | ELISA |
| Cyclin B1 | Zhou et al[41], 2015 | 75 | - | - | - | - | 75 | 140 | 0.01 | 12.0%/- | 97%/- | - | ELISA |
Over the past few years, as single TA autoantibodies do not appear to demonstrate enough diagnostic sensitivity to set up a reliable test for early detection, studies have aimed to identify a suitable panel of TA autoantibodies. These predicaments are presumably due to cancer heterogeneity. In fact, it is unlikely that most patients will respond to the same immunodominant antigens. Even tumors of the same kind are comprised of a diverse mix of biological subtypes; accordingly, cancer patients are more likely to induce an immunoreaction to different sets of TAA, and not all cancers are likely to be detected by autoantibodies against a single antigen. Tables 4 and 5 give an overview of different combinations of multiple autoantibodies as potential blood-based biomarkers for ESCC and EGJA that have been described in the literature by various research groups.
| Target antigen of auto-anti-bodies | Authors, year | ESCC cases, n | Stage, n | Con-trols, n | Sensiti-vity, all stages/ early stage | Specifi-city, all stages/ early stage | AUC, all stages/ early stage | Method | ||||
| 0/I | II | III | IV | Unknown | ||||||||
| c-Myc, HCCR, IMP1, Koc, p53 and p62 | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | 67.9%/ 66.9% | 86.7%/ 86.7% | 0.838/ 0.829 | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | 67.7%/ 56.7% | 85.5%/ 85.5% | 0.859/ 0.818 | ELISA | ||
| c-Myc, HCCR, p53 and p62 | Zhang et al[55], 2016 | 324 (Train-ing) | 5/13 | 130 | 50 | 39 | 87 | 324 (Train-ing) | 67.6%/ 67.6% | 86.4%/ 86.4% | 0.838/ 0.831 | ELISA |
| 186 (Valida-tion) | 1 | 29 | 14 | 46 | 96 | 186 (Valida-tion) | 72.0%/ 63.3% | 85.0%/ 85.0% | 0.872/ 0.837 | ELISA | ||
| MAGEA4, CTAG1, TP53, SDCCAG8 and ERBB2_C | Werner et al[101], 2016 | 31 | - | - | - | - | 31 | 321 | 26.0%/- | 88.5%/- | - | Bead-based multiplex serology |
| p53 and MDM2 | Chai et al[68], 2014 | 157 | - | - | - | - | 157 | 85 | 35.0%/- | 98.8%/- | - | ELISA |
| p53, pl6, Imp-l, CyclinB1, c-Myc, RalA, p62, Survivin, Koc, Cyclin D1 and Cyclin E | Qin et al[58], 2014 | 174 | 3/8 | 79 | 52 | 18 | - | 242 | 75.3%/- | 81.0%/- | 0.78/- | ELISA |
| p53, NY-ESO-1, MMP-7, Hsp70, PRDX 6 and Bmi-1 | Xu et al[43], 2014 | 388 (Test) | 2/29 | 96 | 229 | 27 | 5 | 125 (Test) | 57.0%/ 45.0% | 95.0%/ 95.0% | - | ELISA |
| 237 (Valida-tion) | 2/31 | 114 | 90 | - | - | 134 (Valida-tion) | 51.0%/ 46.0% | 96.0%/ 96.0% | - | ELISA | ||
| p53, NY-ESO-1, Hsp70 and PRDX 6 | Xu et al[43], 2014 | 388 (Test) | 2/29 | 96 | 229 | 27 | 5 | 125 (Test) | 55.0%/ 45.0% | 98.0%/ 98.0% | - | ELISA |
| 237 (Valida-tion) | 2/31 | 114 | 90 | - | - | 134 (Valida-tion) | 48.0%/ 45.0% | 96.0%/ 96.0% | - | ELISA | ||
| p53, IMP1, P16, Cyclin B1, P62, and C-myc | Zhou et al[44], 2014 | 88 | - | - | - | - | 88 | 200 | 64.0%/- | 94.0%/- | 0.78/- | ELISA |
| HSP105 and TIM | Gao et al[51], 2014 | 46 | 7 | - | - | - | 39 | 40 | 54.3%/- | 95.0%/- | 0.823/- | Western blot |
| p16, c-Myc and p53 | Looi et al[67], 2006 | 71 | - | - | - | - | 71 | 82 | 7%/- | 100%/- | - | ELISA |
| SURF1, LOC146223, HOOK2 and AGENCOURT_7565913 | Shimada et al[50], 2005 | 21 | - | 3 | 13 | 5 | - | 37 | 86%/- | 100%/- | - | ELISA |
| Survivin, p53 and C-myc | Meglio-rino et al[59], 2005 | 77 | - | - | - | - | 77 | 82 | 29.9%/- | 95.1%/- | - | ELISA |
| Target antigen of auto-anti-bodies | Authors, year | EGJA cases, n | Stage, n | Con-trols, n | Sensiti-vity, all stages/ early stage | Specifi-city, all stages/ early stage | AUC, all stages/ early stage | Method | ||||
| 0/I | II | III | IV | Unknown | ||||||||
| p53, NY-ESO-1, MMP-7, Hsp70, PRDX6 and Bmi-1 | Xu et al[42], 2019 | 122 (Train-ing) | 2 | 16 | 87 | 17 | 122 | 169 (Valida-tion) | 59.0%/ 50.0% | 90.5%/ 90.5% | 0.818/ 0.786 | ELISA |
| 70 (Valida-tion) | 11 | 14 | 30 | 15 | 80 | 80 (Valida-tion) | 61.4%/ 56.0% | 90.0%/ 90.0% | 0.815/ 0.786 | ELISA | ||
| p53, NY-ESO-1 and Bmi-1 | Xu et al[42], 2019 | 122 (Train-ing) | 2 | 16 | 87 | 17 | 122 | 169 (Valida-tion) | 53.5%/ 55.6% | 90.5%/ 90.5% | 0.814/ 0.744 | ELISA |
| 70 (Valida-tion) | 11 | 14 | 30 | 15 | 80 | 80 (Valida-tion) | 60.0%/ 52.0% | 93.7%/ 93.7% | 0.823/ 0.773 | ELISA | ||
| p53, Koc, P62, c-Myc, IMP1, Survivin and P16 | Zhou et al[41], 2015 | 75 | - | - | - | - | 75 | 140 | 64.0%/- | 87.0%/- | 0.73/- | ELISA |
With improvements in technology, several high-throughput methods, such as proteomics platforms, have enabled the uncovering of autoantibodies and the generation of a panel of TAA. These discovery techniques encompass serological analysis of tumor antigens by recombinant cDNA expression cloning[45], serological proteome analysis[46], phage display[47], protein microarrays[48] and multiple affinity protein profiling[49]. Shimada et al[50] were the first to use the high-throughput method of serological analysis of tumor antigens by recombinant cDNA expression cloning in ESCC. They showed that several TAA that could elicit a humoral immune response that could be detected simultaneously, and the technique enabled the generation of an autoantibody panel that exhibited better diagnostic value (86% sensitivity and 100% specificity) than a single TA autoantibody. Subsequently, a study using serological proteome analysis identified some novel TAA associated with ESCC, and the combination of two TAA (HSP105 and TIM) can give 54.3% sensitivity and 95% specificity in distinguishing ESCC from controls[51]. These studies all show that the combined detection of autoantibodies against several antigens in the panel can greatly increase sensitivity in the diagnosis of ESCC. However, except for the two above-mentioned studies, no other relevant literature applying proteomic technology to identify a TAA panel has appeared. This indicates, to some extent, that the identification and development of novel autoantibodies by proteomics platforms for ESCC is limited and behindhand especially compared with other tumor types, such as lung, breast and liver tumors[52-54].
On the other hand, researchers have been more inclined to evaluate the diagnostic performance of combinations of several known TAA. In accord with such thinking, eight studies reported the diagnostic value of different combinations of autoantibodies for ESCC (Table 4). From the list of autoantibodies examined in the panel (Table 4), p53 autoantibodies were the most common choice for inclusion in the biomarker combinations. As is known, p53 as a tumor suppressor gene has been linked to many cancers, including ESCC, and thus would be a rational biomarker to be investigated. Zhang et al[55] assessed a combination of six immunoreactive TAA in ESCC samples and normal controls with independent validation. Then, they sought to identify which biomarkers used in combination were more informative and allowed a similar discrimination between groups. They finally found a restricted panel of four TAA that gave similar sensitivity and specificity in early stage ESCC. Indeed, a similar research strategy had been previously performed by Xu et al[43] who used two independent cohorts to investigate the combination of autoantibodies against p53, NY-ESO-1, MMP-7, Hsp70, Prx VI and Bmi-1. This panel distinguished early stage ESCC from normal controls with a sensitivity/specificity of 45%/95% and 46%/96%, respectively in the test and validation cohorts. Interestingly, the authors also determined a simplified autoantibody panel retaining four out of six biomarkers that exhibited almost the same diagnostic efficacy (Table 4). Although it is reported that a majority of biomarkers with desirable outcomes in a first data set often result in less promising results in additional independent data sets[56], the two above studies with the combinations of known TAA all showed satisfactory diagnostic value in independent validation cohorts. This suggests potential clinical applications for autoantibody combinations to diagnose ESCC. However, we can see from Table 4 that most of the studies reviewed lack validation in an independent population. In practice, the results of biomarkers need to be validated in larger multicenter cohorts and evaluated as a screening test in high-risk populations. However, no study on the evaluation of autoantibodies in ESCC diagnosis has been able to do so. All previously identified autoantibody panels for ESCC should be validated by these procedures to evaluate their true clinical relevance and diagnostic power.
As the combined detection of selected autoantibodies as a panel could generally increase diagnostic sensitivity while keeping relatively high specificity in ESCC, two studies have attempted to evaluate the same panels of autoantibodies identified in ESCC for early detection of EGJA and have shown promising results. They demonstrated sensitivities above 50% and specificities above 86% (Table 5). Zhou et al[41] detected autoantibodies to eight TAA, comprised of p53, IMP1, P16, cyclin B1, P62, c-Myc, survivin and Koc and suggested that successive addition of seven TAA (p53, Koc, P62, c-Myc, IMP1, survivin and P16) led to stepwise increases in sensitivity and specificity, ultimately achieving a sensitivity of 64.0% with a specificity of 87.0%. This optimized combination is somewhat different from an optimized panel identified for ESCC (p53, IMP1, P16, cyclin B1, P62 and c-Myc) studied by the same research team. Subsequently, Xu et al[42] showed that autoantibodies against a combination of p53, NY-ESO-1, MMP-7, Hsp70, PRDX6 and Bmi-1, which is the same as the panel used for evaluation of ESCC, could be potentially used for early diagnosis of EGJA. When comparing stage I and II patients to normal controls, the authors showed sensitivities and specificities of 50.0% and 90.5% and 56.0% and 90.0%, respectively, in the training and validation cohorts. It should be noted that a strict panel of p53, NY-ESO-1 and Bmi-1 to comprise informative biomarkers for EGJA gives similar diagnostic performance. Interestingly, as discussed above, a different restricted combination (p53, NY-ESO-1, PRDX6 and Hsp70) from the same autoantibody panel in early stage ESCC retains high sensitivity and specificity.
These studies suggest that the importance of individual autoantibodies in the panel assay varies in different types of cancers. However, we still need to determine which TA autoantibodies applied in combination are more informative and allow a better diagnostic value. In future work, more TA autoantibodies need to be discovered and characterized to identify the best combination for EGJA. Meanwhile, the identified signatures for EGJA should be verified in larger multicenter-appropriated cohorts of early stage patients and controls to test the diagnostic power.
Endoscopic examination is a current but invasive diagnostic and screening procedure for early detection of ESCC and EGJA. The development and validation of non-invasive biomarkers is of great need for ESCC and EGJA screening. In recent decades, a large number of blood-based cancer biomarkers, such as cell-free circulating tumor DNAs, various non-coding RNAs, proteins and TA autoantibodies, have been identified and indicate the potential for early detection of esophageal cancer. Among these biomarkers, TA autoantibodies are a promising biomarker entity in early cancer detection as they are capable of identifying cancer in high-risk individuals. Moreover, they are highly stable and can be easily detected by routine methods (e.g., ELISA). Recently, a TA autoantibody assay named EarlyCDT-Lung (against p53, NY-ESO-1, CAGE, GBU4-5, MAGE A4, SOX2 and Hu-D) approved by the FDA has been clinically and analytically validated. An ongoing prospective randomized trial is evaluating the clinical utility of this TA autoantibody panel and its use in a clinical setting of which the results are expected to be announced in the near future. Once this assay is successful for lung cancer, we would predict that tests for all solid tumors, including ESCC and EGJA, will follow.
Biomarker development needs several gradual steps covering preclinical studies, retrospective studies of stored specimens, multicenter validation studies and prospective screening studies. However, in ESCC and EGJA, autoantibody studies on early detection are hampered by several issues. First, the availability of sera from early stage patients seems limited. Only few studies have investigated the diagnostic value of TA autoantibody panels in patients with early stage tumors. Access to large early stage sample cohorts is an essential and necessary issue to examine a test’s value for early stage disease. Moreover, few patients with pre-diagnostic serum samples or high-risk ESCC or EGJA cohorts are available, and up to now no study has reported on the immune response in the form of autoantibodies in these populations. Thus, an investigation of TA autoantibodies for the early detection of ESCC and EGJA will be limited mainly by the availability of human samples. On the other hand, current studies (Tables 4 and 5) investigating autoantibodies show promise but still lack the necessary validation stages. These studies need clinical multicenter validation through use of a broader population to further determine diagnostic value.
It seems that there are different patterns of TA autoantibody frequencies in different types of cancers. Thus, one encountered difficulty is the definition of the panel. This leads to the question of how to choose the optimized combination that works best in terms of sensitivity, specificity and predictive value. At this moment, these is no good guiding principle, but more advanced high-throughput proteome technology might be helpful. On the other hand, it should also be pointed out that TA autoantibodies may not be unique for specific types of cancers. Therefore, TA autoantibody panels identified for ESCC or EGJA are likely to be used as a screening test to discover the existence of cancer, and in general more specific diagnostic tools, such as endoscopy, should be carried out in the event of a positive result.
In conclusion, this review suggests that TA autoantibodies have the potential to serve as diagnostic biomarkers for ESCC and EGJA possibly as part of a general cancer screen. However, present studies in ESCC and EGJA remain at an early stage. It is clear that extensive efforts are needed to uncover promising autoantibody signatures to detect these cancers especially at the early stage. Moreover, it is too early to evaluate the diagnostic value of the autoantibodies reviewed here for clinical use. Standardized assay protocols facilitating the establishment of autoantibodies as highly accurate biomarkers is of great need in ESCC and EGJA. Finally, future studies performed with precise design and collaborative efforts among groups to build standardized guidelines to report results will contribute greatly in this research area.
We thank Professor Stanley Li Lin who re-read this manuscript carefully.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carbone J, Gheita TA, Matsui K, Mavridis K S-Editor: Yan JP L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 2. | Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 3. | Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 4. | Zhou Y, Zhang Z, Zhang Z, Wu J, Ren D, Yan X, Wang Q, Wang Y, Wang H, Zhang J, Zhu X, Yang Y, Luo C, Guo X, Tang C, Qiao L. A rising trend of gastric cardia cancer in Gansu Province of China. Cancer Lett. 2008;269:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 5. | Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, Dong ZW, Mark SD, Qiao YL, Taylor PR. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 526] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 6. | Chen H, Wang LD, Guo M, Gao SG, Guo HQ, Fan ZM, Li JL. Alterations of p53 and PCNA in cancer and adjacent tissues from concurrent carcinomas of the esophagus and gastric cardia in the same patient in Linzhou, a high incidence area for esophageal cancer in northern China. World J Gastroenterol. 2003;9:16-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2219] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 8. | Kim T, Grobmyer SR, Smith R, Ben-David K, Ang D, Vogel SB, Hochwald SN. Esophageal cancer--the five year survivors. J Surg Oncol. 2011;103:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Wang GQ, Jiao GG, Chang FB, Fang WH, Song JX, Lu N, Lin DM, Xie YQ, Yang L. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann Thorac Surg. 2004;77:1740-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 10. | Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M, Stolte M, Ell C. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut. 2008;57:1200-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 11. | Chapman CJ, Thorpe AJ, Murray A, Parsy-Kowalska CB, Allen J, Stafford KM, Chauhan AS, Kite TA, Maddison P, Robertson JF. Immunobiomarkers in small cell lung cancer: Potential early cancer signals. Clin Cancer Res. 2011;17:1474-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Zhong L, Coe SP, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol. 2006;1:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Trivers GE, De Benedetti VM, Cawley HL, Caron G, Harrington AM, Bennett WP, Jett JR, Colby TV, Tazelaar H, Pairolero P, Miller RD, Harris CC. Anti-p53 antibodies in sera from patients with chronic obstructive pulmonary disease can predate a diagnosis of cancer. Clin Cancer Res. 1996;2:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Takeda A, Shimada H, Nakajima K, Imaseki H, Suzuki T, Asano T, Ochiai T, Isono K. Monitoring of p53 autoantibodies after resection of colorectal cancer: Relationship to operative curability. Eur J Surg. 2001;167:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Anderson KS, Wong J, Vitonis A, Crum CP, Sluss PM, Labaer J, Cramer D. p53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:859-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Shan Q, Lou X, Xiao T, Zhang J, Sun H, Gao Y, Cheng S, Wu L, Xu N, Liu S. A cancer/testis antigen microarray to screen autoantibody biomarkers of non-small cell lung cancer. Cancer Lett. 2013;328:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Fosså A, Berner A, Fosså SD, Hernes E, Gaudernack G, Smeland EB. NY-ESO-1 protein expression and humoral immune responses in prostate cancer. Prostate. 2004;59:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Jäger E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jäger D, Arand M, Ritter G, Old LJ, Knuth A. Humoral immune responses of cancer patients against "Cancer-Testis" antigen NY-ESO-1: Correlation with clinical events. Int J Cancer. 1999;84:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Pedersen JW, Gentry-Maharaj A, Nøstdal A, Fourkala EO, Dawnay A, Burnell M, Zaikin A, Burchell J, Papadimitriou JT, Clausen H, Jacobs I, Menon U, Wandall HH. Cancer-associated autoantibodies to MUC1 and MUC4--a blinded case–control study of colorectal cancer in UK collaborative trial of ovarian cancer screening. Int J Cancer. 2014;134:2180-2188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Kurtenkov O, Klaamas K, Mensdorff-Pouilly S, Miljukhina L, Shljapnikova L, Chuzmarov V. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: Relation to survival. Acta Oncol. 2007;46:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Graus F, Dalmou J, Reñé R, Tora M, Malats N, Verschuuren JJ, Cardenal F, Viñolas N, Garcia del Muro J, Vadell C, Mason WP, Rosell R, Posner JB, Real FX. Anti-Hu antibodies in patients with small-cell lung cancer: Association with complete response to therapy and improved survival. J Clin Oncol. 1997;15:2866-2872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 253] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Baldwin RW. Tumour-specific immunity against spontaneous rat tumours. Int J Cancer. 1966;1:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Baldwin RW. An immunological approach to cancer. Lav Ist Anat Istol Patol Univ Studi Perugia. 1968;28:65-85. [PubMed] |
| 24. | Baldwin RW. Tumour-associated antigens and tumour-host interactions. Proc R Soc Med. 1971;64:1039-1042. [PubMed] |
| 25. | Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3425] [Cited by in RCA: 3522] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 26. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4542] [Article Influence: 324.4] [Reference Citation Analysis (0)] |
| 27. | Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 639] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 28. | Finn OJ. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23 Suppl 8:viii6-viii9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 413] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 29. | Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527-5536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 332] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 30. | Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdar AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52:4168-4174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 511] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 32. | Chen YT, Scanlan MJ, Sahin U, Türeci O, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 932] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 33. | Goodell V, Waisman J, Salazar LG, de la Rosa C, Link J, Coveler AL, Childs JS, Fintak PA, Higgins DM, Disis ML. Level of HER-2/neu protein expression in breast cancer may affect the development of endogenous HER-2/neu-specific immunity. Mol Cancer Ther. 2008;7:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Plotz PH. The autoantibody repertoire: Searching for order. Nat Rev Immunol. 2003;3:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Burford B, Gentry-Maharaj A, Graham R, Allen D, Pedersen JW, Nudelman AS, Blixt O, Fourkala EO, Bueti D, Dawnay A, Ford J, Desai R, David L, Trinder P, Acres B, Schwientek T, Gammerman A, Reis CA, Silva L, Osório H, Hallett R, Wandall HH, Mandel U, Hollingsworth MA, Jacobs I, Fentiman I, Clausen H, Taylor-Papadimitriou J, Menon U, Burchell JM. Autoantibodies to MUC1 glycopeptides cannot be used as a screening assay for early detection of breast, ovarian, lung or pancreatic cancer. Br J Cancer. 2013;108:2045-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 36. | Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 37. | Matsumoto I, Maccioni M, Lee DM, Maurice M, Simmons B, Brenner M, Mathis D, Benoist C. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol. 2002;3:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, Weissleder R, Mathis D, Benoist C. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Zhang H, Xia J, Wang K, Zhang J. Serum autoantibodies in the early detection of esophageal cancer: A systematic review. Tumour Biol. 2015;36:95-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 40. | Fujita Y, Nakanishi T, Miyamoto Y, Hiramatsu M, Mabuchi H, Miyamoto A, Shimizu A, Takubo T, Tanigawa N. Proteomics-based identification of autoantibody against heat shock protein 70 as a diagnostic marker in esophageal squamous cell carcinoma. Cancer Lett. 2008;263:280-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Zhou SL, Ku JW, Fan ZM, Yue WB, Du F, Zhou YF, Liu YL, Li Y, Tang S, Hu YL, Hu XP, Hou ZC, Liu J, Liu Y, Feng XS, Wang LD. Detection of autoantibodies to a panel of tumor-associated antigens for the diagnosis values of gastric cardia adenocarcinoma. Dis Esophagus. 2015;28:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Xu YW, Chen H, Guo HP, Yang SH, Luo YH, Liu CT, Huang XY, Tang XM, Hong CQ, Li EM, Xu LY, Peng YH. Combined detection of serum autoantibodies as diagnostic biomarkers in esophagogastric junction adenocarcinoma. Gastric Cancer. 2019;22:546-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Xu YW, Peng YH, Chen B, Wu ZY, Wu JY, Shen JH, Zheng CP, Wang SH, Guo HP, Li EM, Xu LY. Autoantibodies as potential biomarkers for the early detection of esophageal squamous cell carcinoma. Am J Gastroenterol. 2014;109:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 44. | Zhou SL, Yue WB, Fan ZM, Du F, Liu BC, Li B, Han XN, Ku JW, Zhao XK, Zhang P, Cui J, Zhou FY, Zhang LQ, Fan XP, Zhou YF, Zhu LL, Liu HY, Wang LD. Autoantibody detection to tumor-associated antigens of P53, IMP1, P16, cyclin B1, P62, C-myc, Survivn, and Koc for the screening of high-risk subjects and early detection of esophageal squamous cell carcinoma. Dis Esophagus. 2014;27:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Sahin U, Türeci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A. 1995;92:11810-11813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 785] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 46. | Klade CS, Voss T, Krystek E, Ahorn H, Zatloukal K, Pummer K, Adolf GR. Identification of tumor antigens in renal cell carcinoma by serological proteome analysis. Proteomics. 2001;1:890-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | Mintz PJ, Kim J, Do KA, Wang X, Zinner RG, Cristofanilli M, Arap MA, Hong WK, Troncoso P, Logothetis CJ, Pasqualini R, Arap W. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat Biotechnol. 2003;21:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 267] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 48. | Kijanka G, Murphy D. Protein arrays as tools for serum autoantibody marker discovery in cancer. J Proteomics. 2009;72:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Hardouin J, Lasserre JP, Sylvius L, Joubert-Caron R, Caron M. Cancer immunomics: From serological proteome analysis to multiple affinity protein profiling. Ann N Y Acad Sci. 2007;1107:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Shimada H, Nakashima K, Ochiai T, Nabeya Y, Takiguchi M, Nomura F, Hiwasa T. Serological identification of tumor antigens of esophageal squamous cell carcinoma. Int J Oncol. 2005;26:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Gao H, Zheng Z, Mao Y, Wang W, Qiao Y, Zhou L, Liu F, He H, Zhao X. Identification of tumor antigens that elicit a humoral immune response in the sera of Chinese esophageal squamous cell carcinoma patients by modified serological proteome analysis. Cancer Lett. 2014;344:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Macdonald IK, Parsy-Kowalska CB, Chapman CJ. Autoantibodies: Opportunities for Early Cancer Detection. Trends Cancer. 2017;3:198-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 53. | Qiu J, Keyser B, Lin ZT, Wu T. Autoantibodies as Potential Biomarkers in Breast Cancer. Biosensors (Basel). 2018;8:pii: E67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Hong Y, Huang J. Autoantibodies against tumor-associated antigens for detection of hepatocellular carcinoma. World J Hepatol. 2015;7:1581-1585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Zhang HF, Qin JJ, Ren PF, Shi JX, Xia JF, Ye H, Wang P, Song CH, Wang KJ, Zhang JY. A panel of autoantibodies against multiple tumor-associated antigens in the immunodiagnosis of esophageal squamous cell cancer. Cancer Immunol Immunother. 2016;65:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Mischak H, Allmaier G, Apweiler R, Attwood T, Baumann M, Benigni A, Bennett SE, Bischoff R, Bongcam-Rudloff E, Capasso G, Coon JJ, D'Haese P, Dominiczak AF, Dakna M, Dihazi H, Ehrich JH, Fernandez-Llama P, Fliser D, Frokiaer J, Garin J, Girolami M, Hancock WS, Haubitz M, Hochstrasser D, Holman RR, Ioannidis JP, Jankowski J, Julian BA, Klein JB, Kolch W, Luider T, Massy Z, Mattes WB, Molina F, Monsarrat B, Novak J, Peter K, Rossing P, Sánchez-Carbayo M, Schanstra JP, Semmes OJ, Spasovski G, Theodorescu D, Thongboonkerd V, Vanholder R, Veenstra TD, Weissinger E, Yamamoto T, Vlahou A. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med. 2010;2:46ps42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 57. | Xiu Y, Sun B, Jiang Y, Wang A, Liu L, Liu Y, Sun S, Huangfu M. Diagnostic Value of the Survivin Autoantibody in Four Types of Malignancies. Genet Test Mol Biomarkers. 2018;22:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Qin JJ, Wang XR, Wang P, Ren PF, Shi JX, Zhang HF, Xia JF, Wang KJ, Song CH, Dai LP, Zhang JY. Mini-array of multiple tumor-associated antigens (TAAs) in the immunodiagnosis of esophageal cancer. Asian Pac J Cancer Prev. 2014;15:2635-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Megliorino R, Shi FD, Peng XX, Wang X, Chan EK, Tan EM, Zhang JY. Autoimmune response to anti-apoptotic protein survivin and its association with antibodies to p53 and c-myc in cancer detection. Cancer Detect Prev. 2005;29:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Zhang JB, Cao M, Chen J, Ye SR, Xie K, He X, Ma XL, Zhang J, Yie SM. Serum anti-TOPO48 autoantibody as a biomarker for early diagnosis and prognosis in patients with esophageal squamous cell carcinoma. Clin Res Hepatol Gastroenterol. 2018;42:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Xu YW, Peng YH, Ran LQ, Zhai TT, Guo HP, Qiu SQ, Chen HL, Wu ZY, Li EM, Xie JJ. Circulating levels of autoantibodies against L1-cell adhesion molecule as a potential diagnostic biomarker in esophageal squamous cell carcinoma. Clin Transl Oncol. 2017;19:898-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Li L, Liu M, Lin JB, Hong XB, Chen WX, Guo H, Xu LY, Xu YW, Li EM, Peng YH. Diagnostic Value of Autoantibodies against Ezrin in Esophageal Squamous Cell Carcinoma. Dis Markers. 2017;2017:2534648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Xu YW, Liu CT, Huang XY, Huang LS, Luo YH, Hong CQ, Guo HP, Xu LY, Peng YH, Li EM. Serum Autoantibodies against STIP1 as a Potential Biomarker in the Diagnosis of Esophageal Squamous Cell Carcinoma. Dis Markers. 2017;2017:5384091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Chen WX, Hong XB, Hong CQ, Liu M, Li L, Huang LS, Xu LY, Xu YW, Peng YH, Li EM. Tumor-associated autoantibodies against Fascin as a novel diagnostic biomarker for esophageal squamous cell carcinoma. Clin Res Hepatol Gastroenterol. 2017;41:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Peng YH, Xu YW, Guo H, Huang LS, Tan HZ, Hong CQ, Li SS, Xu LY, Li EM. Combined detection of serum Dickkopf-1 and its autoantibodies to diagnose esophageal squamous cell carcinoma. Cancer Med. 2016;5:1388-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Jin Y, Guan S, Liu L, Sun S, Lee KH, Wei J. Anti-p16 autoantibodies may be a useful biomarker for early diagnosis of esophageal cancer. Asia Pac J Clin Oncol. 2015;11:e37-e41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Looi K, Megliorino R, Shi FD, Peng XX, Chen Y, Zhang JY. Humoral immune response to p16, a cyclin-dependent kinase inhibitor in human malignancies. Oncol Rep. 2006;16:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 68. | Chai Y, Peng B, Dai L, Qian W, Zhang Y, Zhang JY. Autoantibodies response to MDM2 and p53 in the immunodiagnosis of esophageal squamous cell carcinoma. Scand J Immunol. 2014;80:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Cai HY, Wang XH, Tian Y, Gao LY, Zhang LJ, Zhang ZY. Changes of serum p53 antibodies and clinical significance of radiotherapy for esophageal squamous cell carcinoma. World J Gastroenterol. 2008;14:4082-4086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Müller M, Meyer M, Schilling T, Ulsperger E, Lehnert T, Zentgraf H, Stremmel W, Volkmann M, Galle PR. Testing for anti-p53 antibodies increases the diagnostic sensitivity of conventional tumor markers. Int J Oncol. 2006;29:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 71. | Shimada H, Ochiai T, Nomura F; Japan p53 Antibody Research Group. Titration of serum p53 antibodies in 1,085 patients with various types of malignant tumors: A multiinstitutional analysis by the Japan p53 Antibody Research Group. Cancer. 2003;97:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Shimada H, Nabeya Y, Okazumi S, Matsubara H, Funami Y, Shiratori T, Hayashi H, Takeda A, Ochiai T. Prognostic significance of serum p53 antibody in patients with esophageal squamous cell carcinoma. Surgery. 2002;132:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Ralhan R, Arora S, Chattopadhyay TK, Shukla NK, Mathur M. Circulating p53 antibodies, p53 gene mutational profile and product accumulation in esophageal squamous-cell carcinoma in India. Int J Cancer. 2000;85:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 74. | Shimada H, Takeda A, Arima M, Okazumi S, Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T, Kobayashi S, Ochiai T. Serum p53 antibody is a useful tumor marker in superficial esophageal squamous cell carcinoma. Cancer. 2000;89:1677-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 75. | Hagiwara N, Onda M, Miyashita M, Sasajima K. Detection of circulating anti-p53 antibodies in esophageal cancer patients. J Nippon Med Sch. 2000;67:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Shimada H, Nakajima K, Ochiai T, Koide Y, Okazumi SI, Matsubara H, Takeda A, Miyazawa Y, Arima M, Isono K. Detection of serum p53 antibodies in patients with esophageal squamous cell carcinoma: Correlation with clinicopathologic features and tumor markers. Oncol Rep. 1998;5:871-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Sobti RC, Parashar K. A study on p53 protein and anti-p53 antibodies in the sera of patients with oesophageal cancer. Mutat Res. 1998;422:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Cawley HM, Meltzer SJ, De Benedetti VM, Hollstein MC, Muehlbauer KR, Liang L, Bennett WP, Souza RF, Greenwald BD, Cottrell J, Salabes A, Bartsch H, Trivers GE. Anti-p53 antibodies in patients with Barrett's esophagus or esophageal carcinoma can predate cancer diagnosis. Gastroenterology. 1998;115:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 79. | Oshima Y, Shimada H, Yajima S, Nanami T, Matsushita K, Nomura F, Kainuma O, Takiguchi N, Soda H, Ueda T, Iizasa T, Yamamoto N, Yamamoto H, Nagata M, Yokoi S, Tagawa M, Ohtsuka S, Kuwajima A, Murakami A, Kaneko H. NY-ESO-1 autoantibody as a tumor-specific biomarker for esophageal cancer: Screening in 1969 patients with various cancers. J Gastroenterol. 2016;51:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 80. | Fujita S, Wada H, Jungbluth AA, Sato S, Nakata T, Noguchi Y, Doki Y, Yasui M, Sugita Y, Yasuda T, Yano M, Ono T, Chen YT, Higashiyama M, Gnjatic S, Old LJ, Nakayama E, Monden M. NY-ESO-1 expression and immunogenicity in esophageal cancer. Clin Cancer Res. 2004;10:6551-6558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Zhou JH, Zhang B, Kernstine KH, Zhong L. Autoantibodies against MMP-7 as a novel diagnostic biomarker in esophageal squamous cell carcinoma. World J Gastroenterol. 2011;17:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Zhang J, Wang K, Zhang J, Liu SS, Dai L, Zhang JY. Using proteomic approach to identify tumor-associated proteins as biomarkers in human esophageal squamous cell carcinoma. J Proteome Res. 2011;10:2863-2872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 83. | Fujita Y, Nakanishi T, Hiramatsu M, Mabuchi H, Miyamoto Y, Miyamoto A, Shimizu A, Tanigawa N. Proteomics-based approach identifying autoantibody against peroxiredoxin VI as a novel serum marker in esophageal squamous cell carcinoma. Clin Cancer Res. 2006;12:6415-6420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 84. | Liu WL, Guo XZ, Zhang LJ, Wang JY, Zhang G, Guan S, Chen YM, Kong QL, Xu LH, Li MZ, Song LB, Zeng MS. Prognostic relevance of Bmi-1 expression and autoantibodies in esophageal squamous cell carcinoma. BMC Cancer. 2010;10:467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Li Y, Zhang Q, Peng B, Shao Q, Qian W, Zhang JY. Identification of glutathione S-transferase omega 1 (GSTO1) protein as a novel tumor-associated antigen and its autoantibody in human esophageal squamous cell carcinoma. Tumour Biol. 2014;35:10871-10877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Ren P, Ye H, Dai L, Liu M, Liu X, Chai Y, Shao Q, Li Y, Lei N, Peng B, Yao W, Zhang J. Peroxiredoxin 1 is a tumor-associated antigen in esophageal squamous cell carcinoma. Oncol Rep. 2013;30:2297-2303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Ye L, Guan S, Zhang C, Lee KH, Sun S, Wei J, Liu B. Circulating autoantibody to FOXP3 may be a potential biomarker for esophageal squamous cell carcinoma. Tumour Biol. 2013;34:1873-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Guan S, Liu B, Zhang C, Lee KH, Sun S, Wei J. Circulating autoantibody to CD25 may be a potential biomarker for early diagnosis of esophageal squamous cell carcinoma. Clin Transl Oncol. 2013;15:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Cheng Y, Xu J, Guo J, Jin Y, Wang X, Zhang Q, Liu L. Circulating autoantibody to ABCC3 may be a potential biomarker for esophageal squamous cell carcinoma. Clin Transl Oncol. 2013;15:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Zhang B, Zhang Z, Zhang X, Gao X, Kernstine KH, Zhong L. Serological antibodies against LY6K as a diagnostic biomarker in esophageal squamous cell carcinoma. Biomarkers. 2012;17:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Kagaya A, Shimada H, Shiratori T, Kuboshima M, Nakashima-Fujita K, Yasuraoka M, Nishimori T, Kurei S, Hachiya T, Murakami A, Tamura Y, Nomura F, Ochiai T, Matsubara H, Takiguchi M, Hiwasa T. Identification of a novel SEREX antigen family, ECSA, in esophageal squamous cell carcinoma. Proteome Sci. 2011;9:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Dong J, Zeng BH, Xu LH, Wang JY, Li MZ, Zeng MS, Liu WL. Anti-CDC25B autoantibody predicts poor prognosis in patients with advanced esophageal squamous cell carcinoma. J Transl Med. 2010;8:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Liu WL, Zhang G, Wang JY, Cao JY, Guo XZ, Xu LH, Li MZ, Song LB, Huang WL, Zeng MS. Proteomics-based identification of autoantibody against CDC25B as a novel serum marker in esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 2008;375:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Tsunemi S, Nakanishi T, Fujita Y, Bouras G, Miyamoto Y, Miyamoto A, Nomura E, Takubo T, Tanigawa N. Proteomics-based identification of a tumor-associated antigen and its corresponding autoantibody in gastric cancer. Oncol Rep. 2010;23:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Shimada H, Shiratori T, Yasuraoka M, Kagaya A, Kuboshima M, Nomura F, Takiguchi M, Ochiai T, Matsubara H, Hiwasa T. Identification of Makorin 1 as a novel SEREX antigen of esophageal squamous cell carcinoma. BMC Cancer. 2009;9:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Shimada H, Kagaya A, Shiratori T, Nomura F, Takiguchi M, Matsubara H, Hiwasa T. Detection of anti-CUEC-23 antibodies in serum of patients with esophageal squamous cell carcinoma: A possible new serum marker for esophageal cancer. J Gastroenterol. 2009;44:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | Shimada H, Kuboshima M, Shiratori T, Nabeya Y, Takeuchi A, Takagi H, Nomura F, Takiguchi M, Ochiai T, Hiwasa T. Serum anti-myomegalin antibodies in patients with esophageal squamous cell carcinoma. Int J Oncol. 2007;30:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Kuboshima M, Shimada H, Liu TL, Nomura F, Takiguchi M, Hiwasa T, Ochiai T. Presence of serum tripartite motif-containing 21 antibodies in patients with esophageal squamous cell carcinoma. Cancer Sci. 2006;97:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Kuboshima M, Shimada H, Liu TL, Nakashima K, Nomura F, Takiguchi M, Hiwasa T, Ochiai T. Identification of a novel SEREX antigen, SLC2A1/GLUT1, in esophageal squamous cell carcinoma. Int J Oncol. 2006;28:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 100. | Nakashima K, Shimada H, Ochiai T, Kuboshima M, Kuroiwa N, Okazumi S, Matsubara H, Nomura F, Takiguchi M, Hiwasa T. Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int J Cancer. 2004;112:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 101. | Werner S, Chen H, Butt J, Michel A, Knebel P, Holleczek B, Zörnig I, Eichmüller SB, Jäger D, Pawlita M, Waterboer T, Brenner H. Evaluation of the diagnostic value of 64 simultaneously measured autoantibodies for early detection of gastric cancer. Sci Rep. 2016;6:25467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |