Published online Jul 14, 2019. doi: 10.3748/wjg.v25.i26.3370
Peer-review started: April 25, 2019
First decision: May 16, 2019
Revised: May 26, 2019
Accepted: May 31, 2019
Article in press: June 1, 2019
Published online: July 14, 2019
Processing time: 80 Days and 20.2 Hours
The treatment of difficult common bile duct stones (CBDS) remains a big challenge around the world. Biliary stenting is a widely accepted rescue method in patients with failed stone extraction under endoscopic retrograde cholangiopancreatography. Fully covered self-expanding metal stent (FCSEMS) has gained increasing attention in the management of difficult CBDS.
To manufacture a drug-eluting FCSEMS, which can achieve controlled release of stone-dissolving agents and speed up the dissolution of CBDS.
Customized covered nitinol stents were adopted. Sodium cholate (SC) and disodium ethylene diamine tetraacetic acid (EDTA disodium, EDTA for short) were used as stone-dissolving agents. Three different types of drug-eluting stents were manufactured by dip coating (Stent I), coaxial electrospinning (Stent II), and dip coating combined with electrospinning (Stent III), respectively. The drug-release behavior and stone-dissolving efficacy of these stents were evaluated in vitro to sort out the best manufacturing method. And the selected stone-dissolving stents were further put into porcine CBD to evaluate their biosecurity.
Stent I and Stent II had obvious burst release of drugs in the first 5 d while Stent III presented controlled and sustainable drug release for 30 d. In still buffer, the final stone mass-loss rate of each group was 5.19% ± 0.69% for naked FCSEMS, 20.37% ± 2.13% for Stent I, 24.57% ± 1.45% for Stent II, and 33.72% ± 0.67% for Stent III. In flowing bile, the final stone mass-loss rate of each group was 5.87% ± 0.25% for naked FCSEMS, 6.36% ± 0.48% for Stent I, 6.38% ± 0.37% for Stent II, and 8.15% ± 0.27% for Stent III. Stent III caused the most stone mass-loss no matter in still buffer or in flowing bile, which was significantly higher than those of other groups (P < 0.05). In vivo, Stent III made no difference from naked FCSEMS in serological analysis (P > 0.05) and histopathological examination (P > 0.05).
The novel SC and EDTA-eluting FCSEMS is efficient in diminishing CBDS in vitro. When conventional endoscopic techniques fail to remove difficult CBDS, SC and EDTA-eluting FCSEMS implantation may be considered a promising alternative.
Core tip: The idea of delivering stone-dissolving agents to the location of common bile duct stones (CBDS) via biliary stent is first introduced by our research group. Based on our previous work and updated progress in the endoscopic field, we have further modified our previous version and present a brand-new stone-dissolving fully covered self-expanding metal stent, which is expected to serve as an alternative in the management of difficult CBDS.
- Citation: Huang C, Cai XB, Guo LL, Qi XS, Gao Q, Wan XJ. Drug-eluting fully covered self-expanding metal stent for dissolution of bile duct stones in vitro. World J Gastroenterol 2019; 25(26): 3370-3379
- URL: https://www.wjgnet.com/1007-9327/full/v25/i26/3370.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i26.3370
Biliary stenting is a widely accepted rescue method in patients with failed stone extraction under endoscopic retrograde cholangiopancreatography (ERCP) and plastic biliary stents are commonly used around the world[1,2]. It is worth noting that fully covered self-expanding metal stents (FCSEMS) have been used in the management of difficult common bile duct stones (CBDS) and have gained satisfactory clinical outcome[3-7]. In the research of Garcia-Cano’s group[3], FCSEMS implantation was adopted after an ERCP session without complete CBDS extraction. Successful biliary drainage was obtained in all 29 cases. After a median of 199.5 d of follow-up, FCSEMS was removed in 16 patients with a complete CBDS extraction in 15 (93.7%) cases. Another research reported that after 6.4-wk FCSEMS insertion, stent patency rate was 100% (36/36) and success rate of first attempt to clear CBDS was 80.6% (29/36)[4].
Both sodium cholate (SC) and disodium ethylene diamine tetraacetic acid (EDTA) have been found to be able to dissolve biliary stones[8-11]. In our previous studies, we have developed a drug-eluting plastic stent (PS) by the method of dip coating, which is able to release EDTA and SC, and the novel stent has been proved to effectively dissolve CBDS ex vivo and in a live porcine CBDS model[12,13]. But we also observed that the drug-loaded coating outside the PS might fall off during application, which could weaken the stone-dissolving effect of the stent. We have tried coaxial electrospinning, one method to produce drug-loaded nanofiber film, instead of dip coating to manufacture drug-loaded stent; however, burst release of drug became another issue and the stone-dissolving effect was barely satisfactory[14].
Sustainable drug release and unobstructed bile drainage are desired in stone-dissolving stents. In this study, we aimed to modify our stone-dissolving stents. PS was replaced by FCSEMS since FCSEMS can provide more effective drainage and is becoming a promising alternative in the bridge therapy for difficult CBDS. And a new method of manufacturing drug-loaded stent was tested to overcome the problem of coating falling off and burst release of drug.
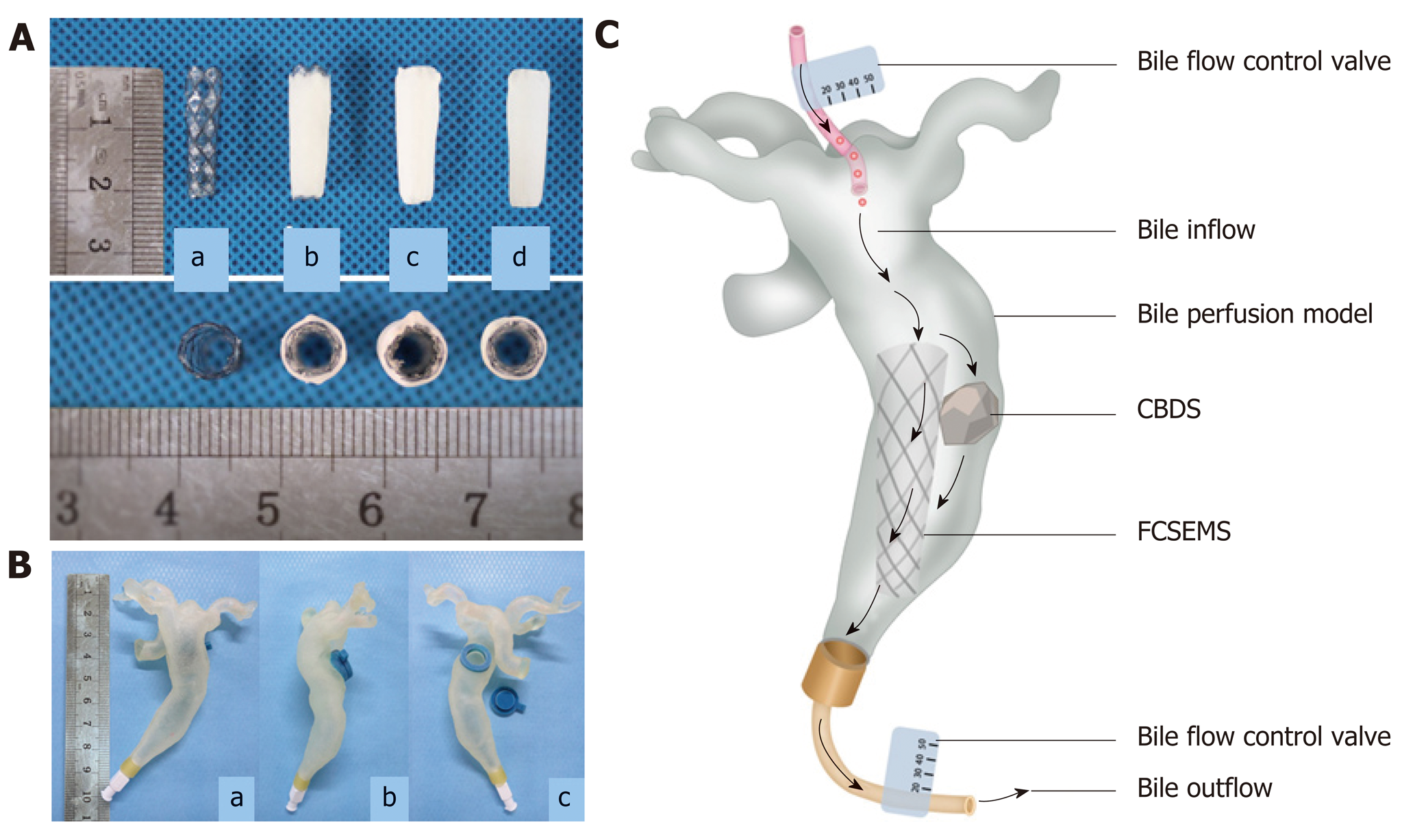
Customized nitinol stents (Micro-Tech Co., Ltd., Nanjing, China), which are 2 cm long, 6 mm wide at the proximal end, 5 mm wide at the waist, and 4 mm wide at the distal end and covered with silicone membrane, were used in this study (Figure 1A). Polycaprolactone (PCL) was adopted as drug carrier and 100 mg SC and EDTA at a molar ratio of 1:1 were loaded on stents. Three different types of SC and EDTA-eluting stents were manufactured by dip coating, coaxial electrospinning, or dip coating combined with electrospinning, which were assigned as Stent I, Stent II, and Stent III, respectively (Figure 1A). Naked nitinol stent served as a control. By dip coating, naked nitinol stents were immersed in mixed solution of PCL and SC and EDTA, and air dried in fuming cupboard. Then, the above-mentioned steps were repeated for several times until the stents reached the expected drug-loading. The steps of coaxial electrospinning was the same as we described in our previous study[14]. For dip coating combined with electrospinning, after dip coating, another super-thin layer of PCL was coated outside the drug-loaded stents by electrospinning. Every drug-eluting stent was weighed before and after they were coated. The mass of coating (mg) was calculated as mass of coated FCSEMS (mg) – mass of naked FCSEMS (mg), and the drug-loading efficiency (%) was calculated as drug loading/coating mass × 100%.
Three kinds of drug-eluting stents loaded with 100 mg SC and EDTA and naked nitinol stents (stents without drugs) were immersed in 10 mL phosphate-buffered saline (PBS, 0.01 M, pH 7.4) in 15 mL tubes (n = 6 in each group). All the tubes were placed in a shaker incubator at an oscillator frequency of 60 rpm at 37 °C. After predetermined incubation time of 1, 2, 3, 4, 5, 10, 15, 20, 25, or 30 d, 1 mL of the buffer in each tube was collected and replaced with 1 mL of fresh PBS. The collected buffer was measured by high-performance liquid chromatography (HPLC) to figure out how much SC and EDTA were released into PBS. Cumulative drug release percentage (%) was calculated as actual drug release/drug loading capacity × 100%.
CBDS were obtained from patients with choledocholithiasis who underwent choledocholithotomy at Shanghai General Hospital, and informed consent was obtained from all patients. The collected stones were rinsed with saline and then air-dried for one month. CBDS (mass in 100 mg) together with one of four FCSEMS were placed into 10 mL PBS in 15 mL tubes (n = 6 in each group) and the PBS in each tube was changed daily. All the tubes were also placed in a shaker incubator at an oscillator frequency of 60 rpm at 37 °C. Stone-dissolving efficacy of drug-eluting stents was assessed by calculating mass loss ratio of stones at predetermined incubation time of 1, 2, 3, 4, 5, 10, 15, 20, 25, or 30 d. The stones were dried in a homeothermal dryer at 60 °C for 24 h before they were weighed. Weight loss ratio (%) was calculated as [(original weight – residual weight)/original weight] × 100%.
In order to mimic the biological environment in the CBD with bile flow, we collected some computed tomography and MRCP images of patients with CBDS and made an in vitro human-scale simulated CBD as bile perfusion model with the help of 3D-printing (Figure 1B). Human bile was obtained from patients who underwent endoscopic nasobiliary drainage. One stent and one CBDS (mass in 100 mg) were put into the CBD model together and a total of 1000 mL of human bile was perfused into each CBD model every day (Figure 1C, n = 6 in each group). After 5, 10, 15, 20, 25, or 30 d of bile perfusion, mass loss ratio of stones in each group was calculated as described above.
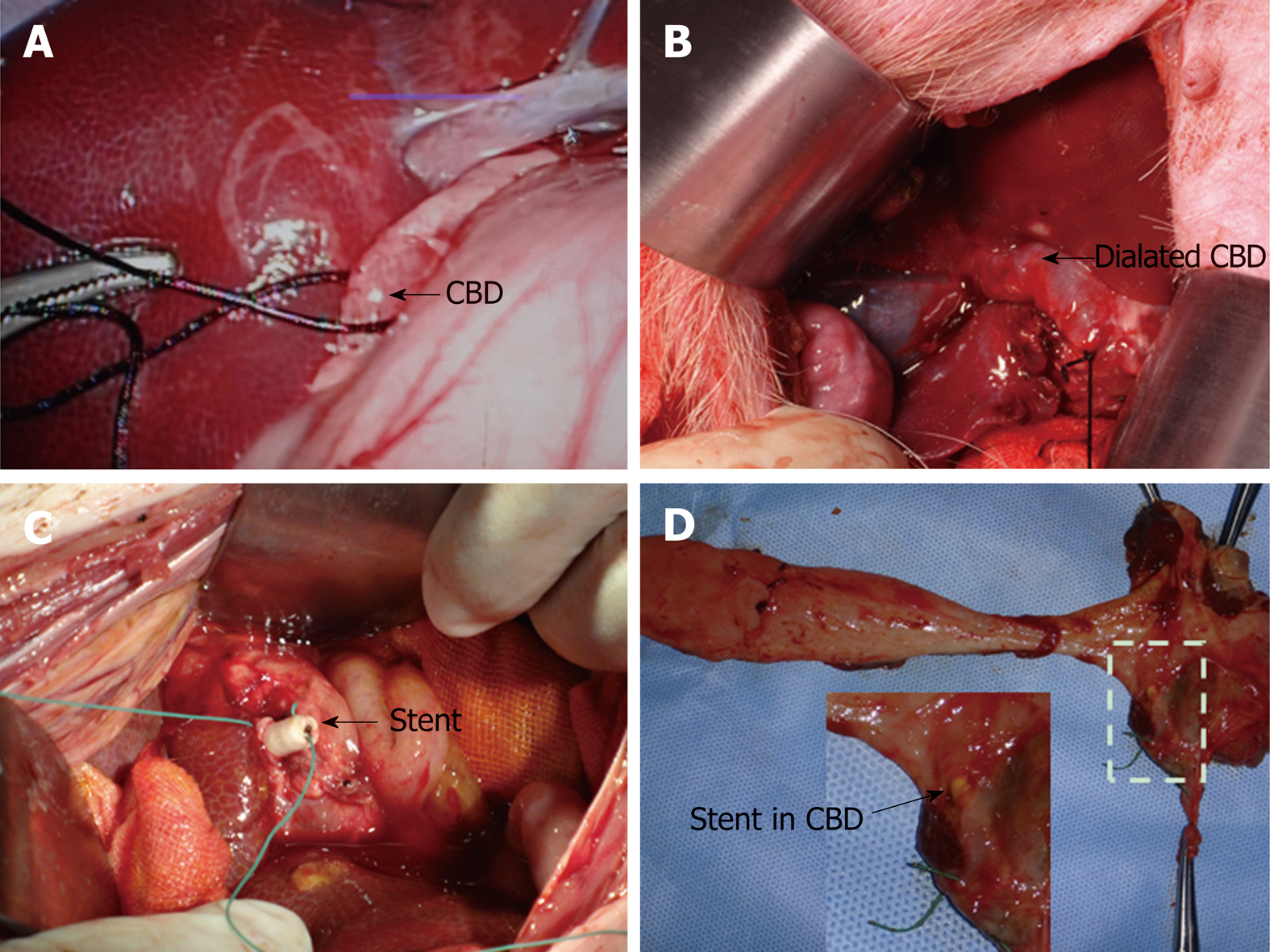
All experimental procedures were approved by the Animal Care and Use Committee of Shanghai Jiaotong University School of Medicine. A total of 10 male miniature pigs aged 1-2 years and weighing 15-20 kg were included in the study and randomly assigned to two groups (n = 5 each). The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk prior to experimentation. Naked FCSEMS and Stent III were involved in this part. The porcine CBD was partially ligated for one week via laparoscopy and one stent was put into the dilated porcine CBD above the papilla through open surgery (Figure 2). All the procedures were conducted under general anesthesia. Serum samples were collected before stent insertion, 15 and 30 d after stent insertion and tested. After 1-mo follow-up, all the animals were sacrificed. Specimens, including the gallbladder, CBD, duodenal wall, liver, and kidney were collected. Hematoxylin and eosin (HE) staining was conducted on all the specimens for histopathological examination. And two senior pathologists helped read the HE slides and grade the severity of inflammation on a scale from mild (+), moderate (++) to severe (+++), according to the numbers and range of infiltrated inflammatory cells.
Statistical analyses were performed using SAS 8e (SAS Institute, Cary, NC, United States). Data are presented as the mean ± standard deviation (SD). Student’s t-test was used to compare continuous quantitative data between two groups and difference among multiple groups was detected by analysis of variance (ANOVA). For ranked data, rank sum test was applied. P < 0.05 was considered statistically significant.
All the drug-eluting FCSEMS possessed a smooth surface and good patency (Figure 1A). In terms of coating mass and thickness, there was a significant difference among the three groups (P < 0.05). As shown in Table 1, with the same drug loading and drug-loaded material, the average coating mass and thickness of Stent II were both greater than those of Stent I and Stent III (P < 0.05), while there was no significant difference between Stent I and Stent III (P > 0.05). The drug-loading efficiency was much lower in Stent II than in another two groups. In other words, given the same coating mass, Stent II had the least drug loading capacity.
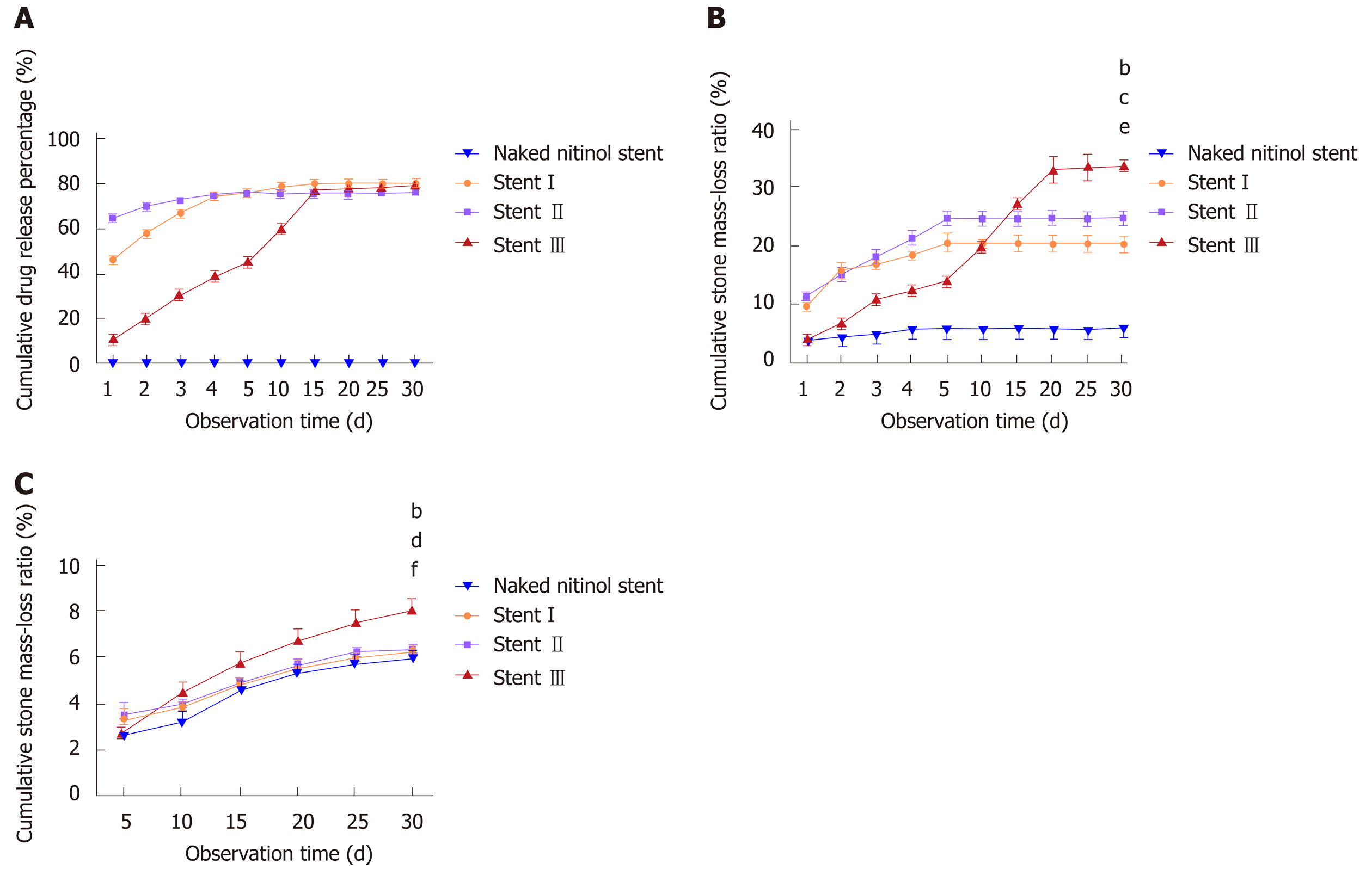
Release behaviors of SC and EDTA-eluting FCSEMS and naked FCSEMS are presented in Figure 3A. Naked FCSEMS were not loaded with drugs, so neither SC nor EDTA was detected. In terms of Stent I, the cumulative release of SC and EDTA reached 46.50% ± 2.50% of the total loaded drug on day 1, 76.72% ± 1.30% on day 5, and 80.12% ± 1.80% on day 15; however, no more drugs were released thereafter. In terms of Stent II, the cumulative release of SC and EDTA reached 65.20% ± 2.38% of the total loaded drug on day 1 and 76.51% ± 1.97% on day 5, then no more drugs were released. By contrast, Stent III only released 11.29% ± 1.88% of the total loaded drug on day 1, 45.21% ± 2.77% on day 5, and 80.14% ± 1.86% on day 30. In sum, Stent I and Stent II had obvious burst release of drugs while Stent III presented controlled drug release as we expected.
The dissolution curve is showed in Figure 3B. Naked FCSEMS only caused a little loss of stone mass (5.19% ± 0.69%) at the end of observation, which might be due to the dissolution of bile pigments of stones into the buffer. Stone mass-loss rate of Stent I reached 9.74% ± 1.14% on day 1, which gradually increased to 20.37% ± 2.13% on day 5; however, no more mass loss was seen thereafter. The situation was similar in Stent II. Stone mass loss was detected only in the first 5 d and the maximum stone mass-loss rate of Stent II was 24.57% ± 1.45%. As to Stent III, stone mass-loss rate on day 1 only reached 4.11% ± 1.67%, and stone mass loss in the first five days was lower than those of Stent I and Stent II. However, stone mass loss of Stent III gradually increased in the next 25 d and come to a final stone mass-loss rate of 33.72% ± 0.67%. In terms of final stone mass-loss rate, there were differences among the three groups (P < 0.05). The final stone mass-loss rate of Stent III was significantly higher than those of Stent I and Stent II (P < 0.05), and there existed no difference between Stent I and Stent II. In other words, with the same drug loading, Stent III possessed the best stone-dissolving effect in still buffer.
Stone mass loss could be observed throughout the whole experiment in all the groups (Figure 3C). In the first 5 d, naked FCSEMS gained 2.61% ± 0.58% of stone mass loss, while Stent I, Stent II, and Stent III gained 3.32% ± 0.62%, 3.47% ± 0.83%, and 2.82% ± 0.37%, respectively. The stone mass loss ratio of naked FCSEMS was statistically lower than those of Stent I and Stent II (P < 0.05), but similar to that of Stent III (P > 0.05). However, there existed no difference in stone mass loss ratio between any two of Stent I, Stent II, and Stent III. At the end of observation, the stone mass loss ratio of naked FCSEMS, Stent I, Stent II, and Stent III was 5.87% ± 0.25%, 6.36% ± 0.48%, 6.38% ± 0.37%, and 8.15% ± 0.27%, respectively. The final stone mass loss ratio of naked FCSEMS was lower than those of the other three groups (naked FCSEMS vs Stent I, P < 0.05; naked FCSEMS vs Stent II, P < 0.01; naked FCSEMS vs Stent III, P < 0.01). Stent I and Stent II had similar stone mass loss ratio (P > 0.05), both of which were remarkably lower than that of Stent III (P < 0.01).
In view of better stone-dissolving effect in flowing bile, Stent III was included in the animal experiment and naked FCSEMS acted as a control. Since we failed to place one stent together with one stone into porcine CBD through choledochotomy due to high mortality of miniature pigs (3/3) in our preliminary experiment, only the stents were placed into CBD in this study. All animals survived well after the stent placement. Comparing all the observed serological indicators (listed in Table 2) between the two groups, there were no difference before nor after the stent placement (P > 0.05 for all); comparing all the indicators before and after the stent placement in the same group, no difference was observed in both groups (P > 0.05 for all).
| Naked FCSEMS (n = 6) | Stent III (n = 6) | |||||
| Day 0 | Day 15 | Day 30 | Day 0 | Day 15 | Day 30 | |
| ALT (U/L) | 33.06 ± 7.31 | 29.93 ± 8.22 | 32.10 ± 5.97 | 33.16 ± 6.14 | 30.50 ± 3.41 | 35.66 ± 5.51 |
| AST (U/L) | 29.60 ± 3.82 | 34.86 ± 7.28 | 34.66 ± 8.73 | 48.64 ± 18.81 | 28.06 ± 4.00 | 29.37 ± 3.33 |
| Alb (g/L) | 30.80 ± 5.33 | 31.50 ± 6.07 | 32.56 ± 5.43 | 32.44 ± 5.37 | 34.6 ± 0.81 | 34.54 ± 6.26 |
| TBIL (umol/L) | 1.32 ± 0.38 | 1.95 ± 0.54 | 1.80 ± 0.37 | 1.68 ± 0.26 | 2.14 ± 0.65 | 1.74 ± 0.55 |
| TBA (umol/L) | 16.43 ± 7.24 | 16.83 ± 9.50 | 13.30 ± 3.55 | 16.56 ± 8.58 | 9.21 ± 1.90 | 16.90 ± 9.35 |
| γ-GT (U/L) | 44.04 ± 9.29 | 41.36 ± 3.59 | 42.62 ± 7.18 | 47.40 ± 7.60 | 42.38 ± 3.74 | 41.16 ± 9.57 |
| ALP (U/L) | 167.02 ± 42.32 | 138.33 ± 47.60 | 144.12 ± 40.23 | 158.4 ± 60.56 | 124.20 ± 31.39 | 163.00 ± 28.91 |
| Cre (umol/L) | 47.90 ± 12.16 | 46.06 ± 9.27 | 48.00 ± 13.94 | 39.12 ± 12.96 | 49.72 ± 10.50 | 60.02 ± 19.71 |
| Ca (mmol/L) | 2.89 ± 0.28 | 2.57 ± 0.01 | 2.86 ± 0.33 | 2.60 ± 0.52 | 2.75 ± 0.53 | 2.81 ± 0.29 |
| Mg (mmol/L) | 1.01 ± 0.08 | 0.93 ± 0.06 | 1.14 ± 0.27 | 1.28 ± 0.21 | 1.00 ± 0.22 | 1.04 ± 0.25 |
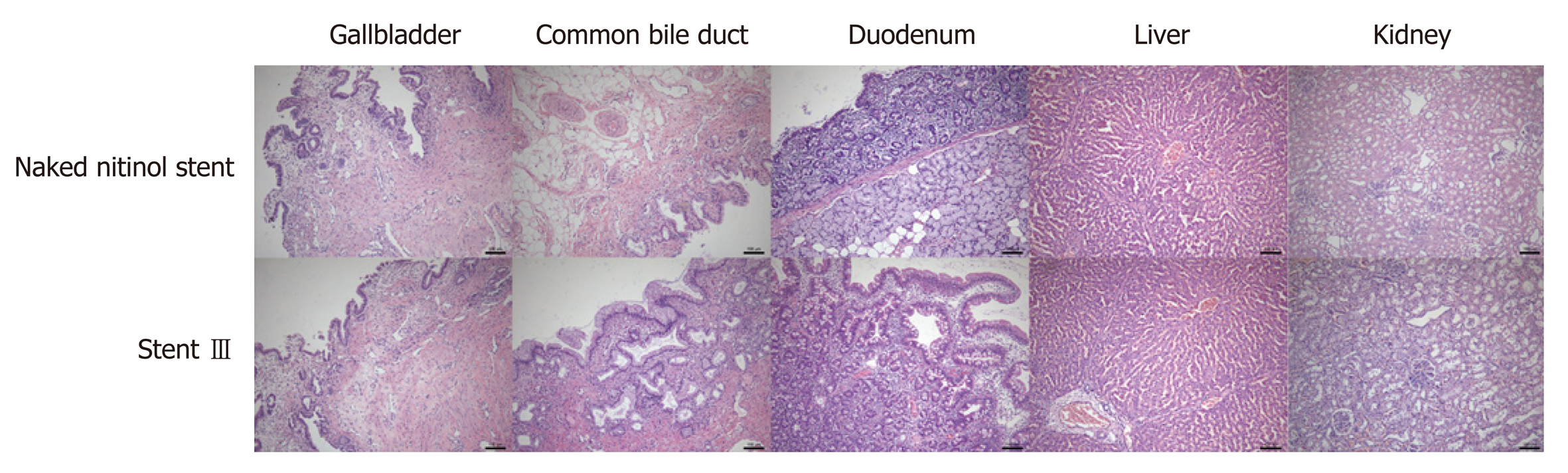
HE staining demonstrated that the gallbladder in both groups presented mild to moderate inflammatory cell infiltration and the CBD in both groups had mild to severe inflammatory cell infiltration. No necrosis or fibrosis was seen in the gallbladder and CBD. The inflammatory response rankings of the gallbladder and CBD in both groups are listed in Table 3, and there existed no statistical difference between the two groups (P > 0.05). Besides, the duodenal wall, liver, and kidney were normal in both groups (Figure 4).
| Gallbladder | CBD | |||
| Naked FCSEMS | Stent III | Naked FCSEMS | Stent III | |
| (n = 5) | (n = 5) | (n = 5) | (n = 5) | |
| - | 0 | 0 | 0 | 0 |
| + | 2 | 3 | 1 | 1 |
| ++ | 3 | 2 | 3 | 2 |
| +++ | 0 | 0 | 1 | 2 |
| Z-value | 0.48 | -0.34 | ||
| P-value | 0.36 | 0.65 | ||
The treatment of difficult CBDS remains a big challenge around the world. The idea of delivering stone-dissolving agents to the location of CBDS via biliary stent is first introduced by our research group. Based on our previous work and updated progress in the endoscopic field, we presented a brand-new stone-dissolving FCSEMS.
The primary goal of stent implantation in patients with CBDS is to release biliary obstruction. In this study, stone mass loss in flowing bile was observed throughout the whole experiment in all four groups, which indicated that unobstructed bile duct with flowing bile might contribute to the dissolution of CBDS. PS are usually deployed across the duodenal papilla and the incidence of duodenobiliary reflux is not rare, leading to recurrent choledocholithiasis and bile infection. Limited drainage lumen and biliary infection make PS clogged easily[15,16] so that PS needs to be replaced periodically. Compared with PS, FCSEMS have several advantages as follows. First, FCSEMS have a bigger inner diameter, providing larger drainage volume and decreasing the possibility of stent occlusion. Second, FCSEMS with a retrieval lasso can be put above the duodenal papilla without disturbing the physiological function of the Oddi’s sphincter, thus reducing the chance of biliary infection caused by duodenal-biliary reflux[6,17]. What is more, the self-expanding performance and flexibility of nitinol stents allow larger contact area between stent and stone(s), which might increase the friction between them and speed up the disintegration of CBDS, thus facilitating later bile duct clearance. The research of Kwon et al[18] also suggested that stents with a wide contact surface with the stones were clinically more effective in reducing the stone size. In short, we presumed that FCSEMS exceed PS in maintaining fluent bile flow and disintegrating CBDS. Unfortunately, this idea cannot be verified in this study and no one has made a comparison between PS and FCSEMS in the management of CBDS yet. It is also worth noting that when the FCSEMS is put entirely in the CBD, there exists the problem of stent migration[4]. Risks related to FCSEMS insertion above the papilla need to be further evaluated.
Stent I and Stent II had most of their drugs released in the first 5 d, and stone mass loss in still buffer was only detected in the first 5 d. Stent III performed continuous and steady release of drugs for 30 d, and stone mass loss in still buffer gradually increased with time. What is more, Stent III gained the highest stone mass-loss rate among all the groups in flowing bile. It is not difficult to draw the conclusion that better stone-dissolving outcome is related to the sustainable drug release.
Another super-thin layer of PCL was the only difference between Stent I and Stent III, therefore, we deemed that this layer of PCL contributed to the controlled drug release. Another compact layer of PCL means that drug molecules have a longer way to go to get into the water surrounding the stent, in other words, the release of drugs slows down to some extent. Comparing with burst drug release, gradual release of drugs in each day let the CBDS have more contact time with the drugs, and the litholytic effect accumulates day by day.
With the same drug loading, the drug-loaded coating of Stent I and Stent III was thinner, which made Stent I and Stent III lighter and more practical. As set forth, drug-loaded stent manufactured by dip coating had the problem of coating falling off, however, this phenomenon was not observed on Stent I. And we believe that one well-knit layer of PCL by electrospinning could prevent this potential trouble. And the further in vivo experiment demonstrated that Stent III as well as naked FCSEMS was characterized with good biosecurity in mini-pigs.
The present study was subject to several limitations. First, the stones in this study were obtained from human CBD, however, we did not tell cholesterol stones, pigmented stones, or mixed stones apart. If we want to figure out the composition of one specific stone, this stone needs to be grinded down into fine power and be tested, which made the stone classification impossible before all the experiments. Second, we failed to place one stent together with one stone into porcine CBD through choledochotomy due to high mortality of miniature pigs (3/3) in our preliminary experiment and only the stents were placed into CBD in this study. The disintegration of stone caused by mechanical friction between FCSEMS and stone could not be fully evaluated.
In conclusion, EDTA and SC-eluting stent can speed up the disintegration of CBDS in vitro and it works better when the drugs are controlled released. Given the successful application of FCSEMS in the management of difficult CBDS, the combination of controlled EDTA and SC release and FCSEMS might be a promising alternative.
The treatment of difficult common bile duct stones (CBDS) remains a big challenge around the world and there is no consensus on the management of difficult CBDS.
In our previous studies, we have developed a drug-eluting plastic stent (PS) which is able to release disodium ethylene diamine tetraacetic acid (EDTA) and sodium cholate (SC), and the stent has been proved to effectively dissolve CBDS ex vivo and in a live porcine CBDS model. However, there are several shortcomings in our previous version, thus we aimed to modify our stone-dissolving stents in this study.
This study aimed to manufacture a drug-eluting metal stent, which can achieve controlled release of stone-dissolving agents and speed up the dissolution of CBDS, thus providing a promising alternative for the management of difficult CBDS.
In this study, three different methods were used to manufacture the drug-eluting stents. The drug-release behavior and stone-dissolving efficacy of these stents was evaluated in vitro to sort out the best manufacturing method. And the selected stone-dissolving stents were further put into porcine CBD to evaluate their biosecurity.
We found that the stent manufactured by dip coating combined with electrospinning was characterized by sustainable drug release, better stone-dissolving efficacy, and good biosecurity. However, we failed to establish the CBDS model in miniature pigs and the disintegration of stone caused by mechanical friction between fully covered self-expanding metal stent (FCSEMS) and stone could not be fully evaluated.
The novel SC and EDTA-eluting FCSEMS is efficient in diminishing CBDS in vitro and is characterized by good biosecurity. The idea of delivering stone-dissolving agents to the location of CBDS via biliary stent is feasible. When conventional endoscopic techniques fail to remove difficult CBDS, SC and EDTA-eluting FCSEMS implantation may be considered a promising alternative.
In the future research, a live porcine CBDS model needs to be established so that the disintegration of stone caused by mechanical friction between FCSEMS and stone could be fully evaluated. And we can compare which stent works better in disintegrating CBDS, PS, or FCSEMS.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali FEM, Sultan AM S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Yasuda I, Itoi T. Recent advances in endoscopic management of difficult bile duct stones. Dig Endosc. 2013;25:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Ogura T, Higuchi K. A review of treatment options for bile duct stones. Expert Rev Gastroenterol Hepatol. 2016;10:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | García-Cano J; Reyes-Guevara AK, Martínez-Pérez T, Valiente-González L, Martínez-Fernández R, Viñuelas-Chicano M, Gómez-Ruiz CJ, Morillas-Ariño J, Pérez-Vigara G, Pérez-García JI, Pérez-Sola Á. Fully covered self-expanding metal stents in the management of difficult common bile duct stones. Rev Esp Enferm Dig. 2013;105:7-12. [PubMed] |
| 4. | Hartery K, Lee CS, Doherty GA, Murray FE, Cullen G, Patchett SE, Mulcahy HE. Covered self-expanding metal stents for the management of common bile duct stones. Gastrointest Endosc. 2017;85:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Cho E, Park CH, Jun CH, Kim HS, Choi SK, Rew JS. Self-expandable metal stents for the extraction of common bile duct stones in patients receiving dual antiplatelet agents: A pilot study. Surg Endosc. 2018;32:1077-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Ueda T, Kikuyama M, Kodama Y, Kurokami T. Short-Term Biliary Stent Placement Contributing Common Bile Duct Stone Disappearance with Preservation of Duodenal Papilla Function. Gastroenterol Res Pract. 2016;2016:6153893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Jun CH, Park CH, Jeon J, Park IH, Lee HJ, Park SY, Kim HS, Choi SK, Rew JS. Feasibility of self-expandable metal stents for preservation of sphincter of Oddi function in patients with common bile duct stones: A pilot study. Gastrointest Endosc. 2015;82:719-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Sohrabi A, Max MH, Hershey CD. Cholate sodium infusion for retained common bile duct stones. Arch Surg. 1979;114:1169-1172. [PubMed] |
| 9. | Dai KY, Montet JC, Zhao XM, Amic J, Montet AM. Dissolution of human brown pigment biliary stones. J Hepatol. 1989;9:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Klueppelberg U, Baumgaertel H, Schusdziarra V, Swobodnik W. Dissolution of bile duct stones by a hydrophilized glyceromonooctanoin-bile-acid-EDTA emulsion. Klin Wochenschr. 1991;69:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Wosiewitz U, Sabinski F, Haus C, Güldütuna S, Leuschner U. Experimental dissolution of pigment gallstone material using alkaline EDTA and adjuvant bile salts/non-bile salt detergents, thiols and urea, with respect to local chemolitholysis. J Hepatol. 1992;14:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Cai XB, Zhang WX, Wan XJ, Yang Q, Qi XS, Wang XP, Lu LG. The effect of a novel drug-eluting plastic stent on biliary stone dissolution in an ex vivo bile perfusion model. Gastrointest Endosc. 2014;79:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Cai XB, Zhang WX, Zhang RL, Dong Yuan X, Yang Q, Qi XS, Li BW, Qin Qian Y, Wang XP, Lu LG, Xu ZJ, Wan XJ. Safety and efficacy of a novel plastic stent coated with stone-dissolving agents for the treatment of biliary stones in a porcine model. Endoscopy. 2015;47:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Gao Q, Huang C, Sun B, Aqeel BM, Wang J, Chen W, Mo X, Wan X. Fabrication and characterization of metal stent coating with drug-loaded nanofiber film for gallstone dissolution. J Biomater Appl. 2016;31:784-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Donelli G, Guaglianone E, Di Rosa R, Fiocca F, Basoli A. Plastic biliary stent occlusion: Factors involved and possible preventive approaches. Clin Med Res. 2007;5:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Sung JY, Leung JW, Shaffer EA, Lam K, Olson ME, Costerton JW. Ascending infection of the biliary tract after surgical sphincterotomy and biliary stenting. J Gastroenterol Hepatol. 1992;7:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Hu B, Leung JW, Gao DJ, Wang TT, Wu J. Management of benign biliary strictures with a novel retrievable self-expandable metal stent. J Dig Dis. 2014;15:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Kwon CI, Kim G, Jeong S, Choi SH, Ko KH, Lee DH, Cho JY, Hong SP. Experimental study on the friction effect of plastic stents for biliary stone fragmentation (with video). Dig Endosc. 2018;30:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |