Copyright
©The Author(s) 2019.
World J Gastroenterol. Jul 14, 2019; 25(26): 3370-3379
Published online Jul 14, 2019. doi: 10.3748/wjg.v25.i26.3370
Published online Jul 14, 2019. doi: 10.3748/wjg.v25.i26.3370
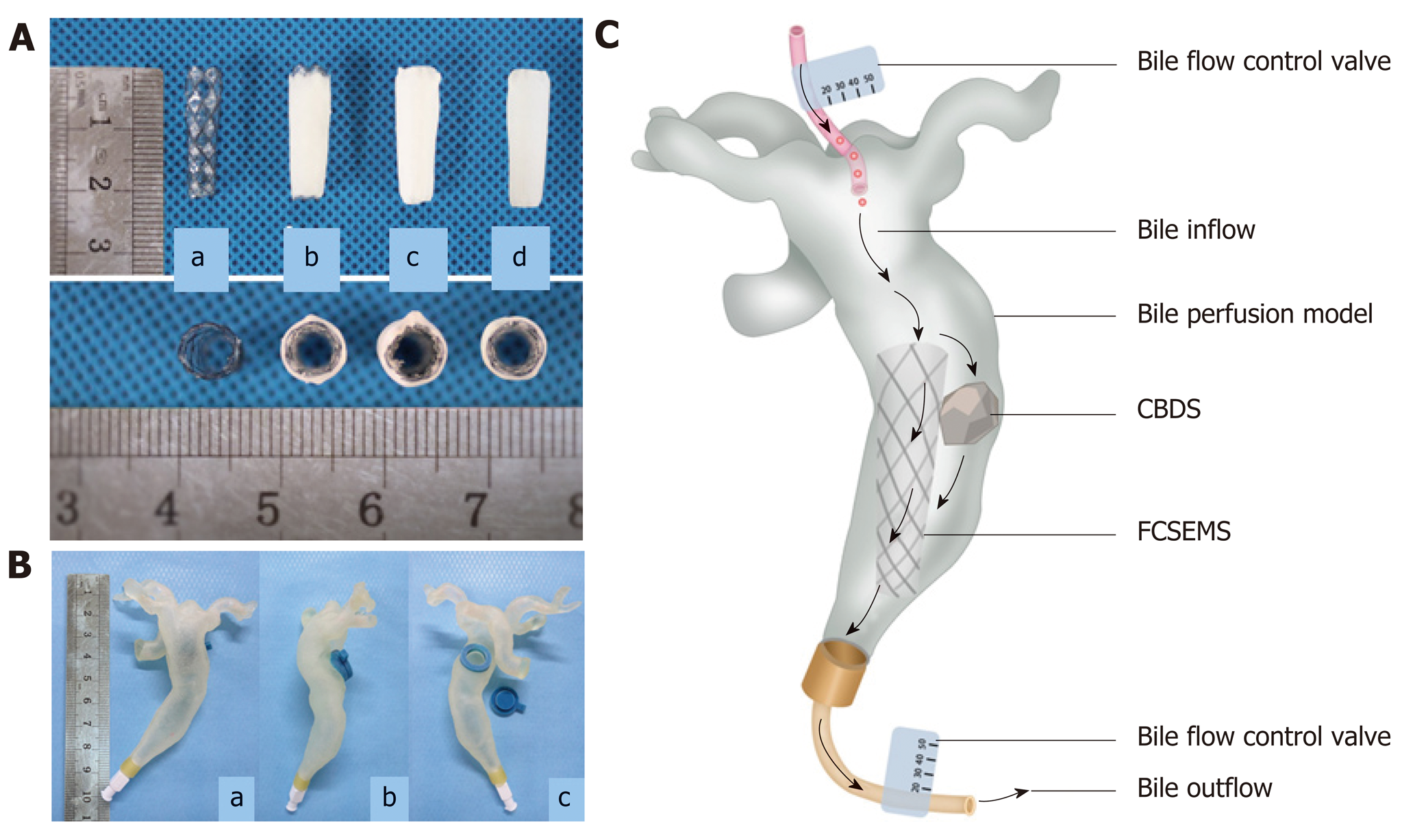
Figure 1 Pictures of stents and common bile duct model.
A: Picture of covered nitinol stent and drug-eluting stents manufactured by dip coating, coaxial electrospinning, and dip coating combined with electrospinning (a: Covered nitinol stent without drug load; b: Drug-eluting stent manufactured by dip coating; c: Drug-eluting stent manufactured by coaxial electrospinning; d: Drug-eluting stent manufactured by dip coating combined with electrospinning); B: In vitro common bile duct (CBD) model manufactured by 3D printing technology (a: Front view; b: Lateral view; c: Back view, one stent and one stone can be put inside the model through the hole on the back); C: Picture illustrating dissolution of biliary stone in flowing bile in bile perfusion model. One stent and one CBD stone (mass in 100 mg) were put into the CBD model together and a total of 1000 mL human bile was perfused into each CBD model every day, that is, 1000 mL human bile will flow through the stent and stone every day. In this way, we get to know whether our stent can exert the stone-dissolving effect in flowing bile. CBD: Common bile duct; CBDS: Common bile duct stone.
- Citation: Huang C, Cai XB, Guo LL, Qi XS, Gao Q, Wan XJ. Drug-eluting fully covered self-expanding metal stent for dissolution of bile duct stones in vitro. World J Gastroenterol 2019; 25(26): 3370-3379
- URL: https://www.wjgnet.com/1007-9327/full/v25/i26/3370.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i26.3370