Published online Apr 14, 2019. doi: 10.3748/wjg.v25.i14.1729
Peer-review started: February 18, 2019
First decision: February 26, 2019
Revised: March 11, 2019
Accepted: March 24, 2019
Article in press: March 25, 2019
Published online: April 14, 2019
Processing time: 55 Days and 19.7 Hours
Few studies have examined intestinal motility disorders, which are disabling conditions associated with chronic functional constipation, whose pathogenesis is actually not well-defined.
To investigate the relationship between serum 25-hydroxyvitamin D levels and functional chronic constipation associated to intestinal motility disorders.
We performed a prospective case-control study, from May-June to November 2017. Glucose/lactulose breath tests, radiopaque markers (multiple capsule techniques) and wireless motility capsule analysis were used to assess colonic and oro-cecal transit time, after excluding small-intestinal bacterial overgrowth condition. Then, we measured 25-hydroxyvitamin D levels in patients with intestinal motility disorders and we further evaluated the influence of intestinal motility disorders on psychological symptoms/quality of life using validated questionnaires, the Irritable Bowel Syndrome Quality of life (IBS-QOL), the Short Form Health Survey 12, and the Hospital Anxiety and Depression Scale 14 (HADS-14 A and HADS-14 D).
We enrolled 86 patients with chronic functional constipation associated to intestinal motility disorders and 86 matched healthy subjects. Patients with intestinal motility disorders had lower 25-hydroxyvitamin D levels (P < 0.001), and they showed a significant impairment of all health-related quality of life and psychological tests (IBS-QOL, Short Form Health Survey 12-Physical Component Summary, Short Form Health Survey 12-Mental Component Summary, HADS-14 A and HADS-14 D), as compared to the control group (P < 0.001), which significantly correlated with low vitamin D levels (r = - 0.57, P < 0.001; r = 0.21, P = 0.01; r = - 0.48, P < 0.001; r = - 0.57, P < 0.001; r = - 0.29, P < 0.001, respectively). At multivariate analysis vitamin D low levels remained a significant independent risk factor for the occurrence of intestinal motility disorder (odds ratio = 1.19; 95% confidence interval: 1.14-1.26, P < 0.001).
Vitamin D deficiency, anxiety and depression symptoms are commonly associated with chronic functional constipation induced by intestinal motility disorders. Vitamin D serum levels should be routinely measured in these patients.
Core tip: Intestinal motility disorders, which are disabling conditions associated with chronic constipation, have been examined in only A few studies. Patients with intestinal motility disorders are frequently affected by vitamin D deficiency, which is strongly associated to anxiety, depression symptoms and to severe impairment of quality of life. These data suggest that vitamin D serum levels should be routinely measured, and its supplementation should be evaluated in patients with intestinal motility disorders.
- Citation: Panarese A, Pesce F, Porcelli P, Riezzo G, Iacovazzi PA, Leone CM, De Carne M, Rinaldi CM, Shahini E. Chronic functional constipation is strongly linked to vitamin D deficiency. World J Gastroenterol 2019; 25(14): 1729-1740
- URL: https://www.wjgnet.com/1007-9327/full/v25/i14/1729.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i14.1729
The term “Constipation” has a broad definition both for physicians and patients[1]. The most common form is functional chronic constipation, which is a gastrointestinal disorder defined by symptom criteria, after the exclusion of secondary causes[2-4]. This clinical condition usually affects women, older subjects, particularly those of lower socioeconomic status[5], and has a prevalence ranging between 2% and 27% in western countries with a high burden on global health-care system[2,6,7]. The main symptoms of functional chronic constipation are difficulty with evacuation, bloating, abdominal pain, discomfort or hard stools that significantly impair health-related quality of life[2,8]. Since depression has been commonly observed in patients with the constipation variant of irritable bowel syndrome (IBS), specific questionnaires have been endorsed to evaluate some aspects of the quality of life of these patients, especially the IBS Quality of life (IBS-QOL), the Short Form Health Survey 12 (SF-12), and the Hospital Anxiety and Depression Scale 14 (HADS-14-A/D)[9-12]. Moreover, previous studies showed a possible role of vitamin D deficiency in the pathophysiology of IBS and depression, and also beneficial effects of vitamin D supplementation in alleviating depression and certain gastrointestinal symptoms in a population of study prevalently affected by diarrhea or by alternating subtype, and the minority by constipation symptoms[13-20]. Normal transit constipation is probably the most common form, and it frequently overlaps with the constipation variant of IBS[9].
Among patients with functional chronic constipation, intestinal motility disorders have been reported. Although the overall prevalence of intestinal motility disorders is not currently well-defined[2], it involves more commonly patients affected by slow transit constipation (STC)[2], and rarely subjects with delayed oro-cecal transit time[21], In addition, defecation disorders significantly overlap with normal and STC[9,22]. While STC is characterized by an altered colonic motor activity, and reduced reaction after a meal and when waking up, decreased “high amplitude propagated contractions”, which have been associated to altered regulation of enteric nervous system, reduction of neurons and axons in the myenteric plexus[2,22-24], delayed oro-cecal transit time could be partially associated with an underlying small-intestinal bacterial overgrowth (SIBO)[25].
Moreover, the most severe form of intestinal motility disorder may be considered chronic intestinal pseudo-obstruction, which is a symptomatic and disabling disease, related to a visceral myopathy or/and neuropathy[26]. Hence, colonic transit time could be objectively measured by radiopaque markers (single or multiple capsule techniques), and this diagnostic tool is also used to rule out dyssynergia defecation[2,23]. Radiopaque markers studies are useful, inexpensive and widely available[2,23]. In addition, even if not definitively recommended by guidelines, lactulose breath test (LBT) and glucose breath test (GBT), are commonly used in clinical practice to evaluate the presence of SIBO as well as small-bowel motility by estimating oral-cecal transit time[25,27]. Other expensive tests such as colonic scintigraphy and wireless ingestible motility capsule, measure more accurately overall transit time[23,27], whereas advanced physiologic tests identify anorectal dysfunctions in patients not responding to initial therapy[2,27]. In this paper we hypothesized that serum vitamin D deficiency could be associated with chronic functional constipation secondary to delayed intestinal transit time and consequently we investigated this relationship and the related psychological aspects.
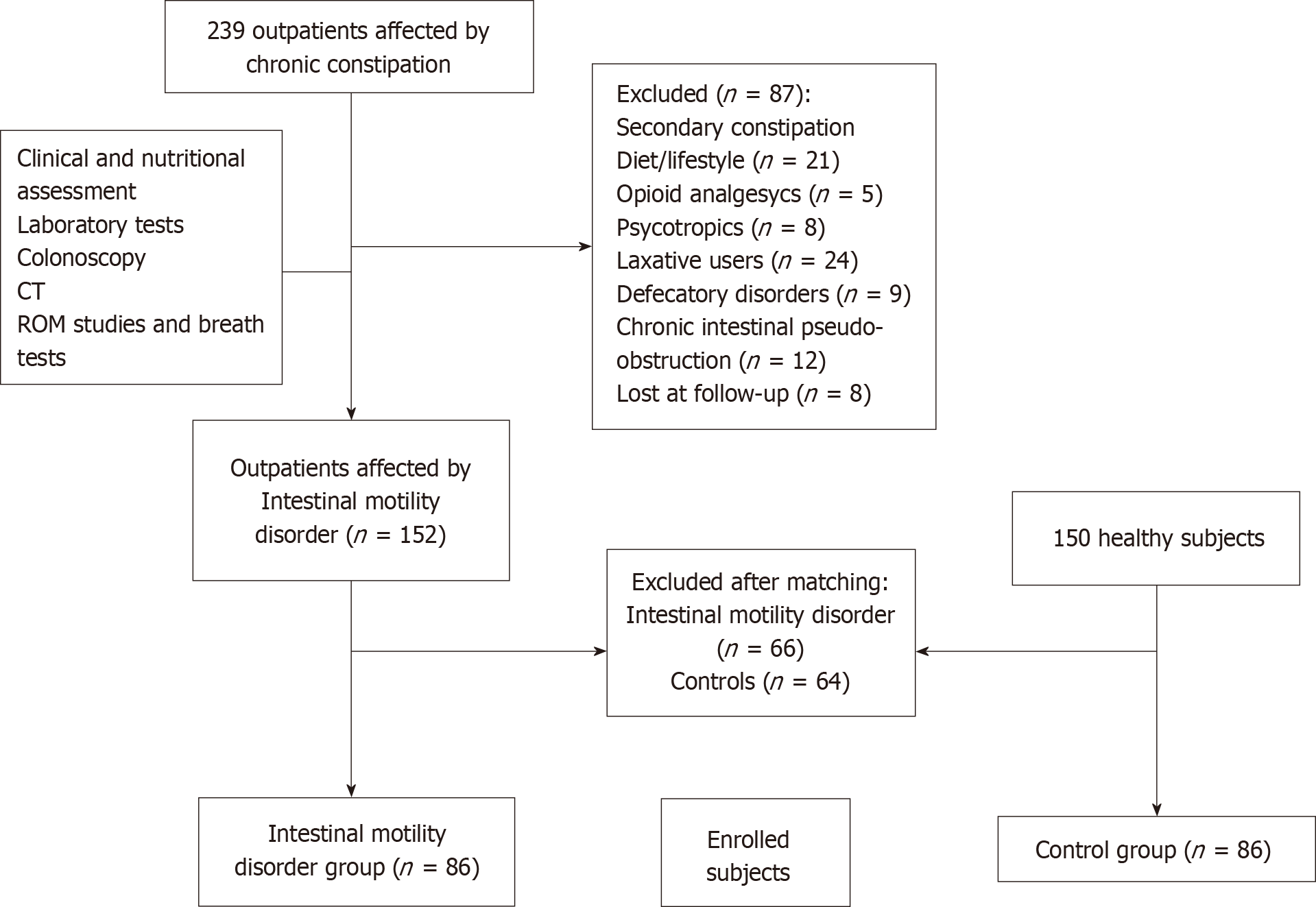
This case-control study was performed from May-June to November 2017, in the Outpatients Clinic of our Institution, in Apulia, a Mediterranean region located in southeast of Italy. As shown in Figure 1, we enrolled 86 subjects from a population of 152 consecutive constipated patients affected by intestinal motility disorders.
Inclusion criteria were the following: Caucasian subjects ≥ 18 years old, body mass index (BMI) ≥ 18.5 kg/m2, affected by functional chronic constipation associated to delayed intestinal transit time, supported by Roma IV criteria[8,26,28]. Exclusion criteria were the following: Subjects > 75 years old, IBS, pregnancy, significant comorbidities such as cardiac, respiratory, chronic renal insufficiency, anorectal and pelvic floor dysfunctions, metabolic/endocrine (diabetes mellitus, hypothyroidism, hypercalcaemia, panhypopituitarism), medications (opiates, antihypertensive agents, iron preparations, anti-epileptic drugs, tryciclic antidepressants, anticholinergics or dopaminergics), organic (extra-intestinal mass, colorectal cancer, ischaemic or surgical stenosis, anal fissure, anal strictures, inflammatory bowel disease, intestinal malabsorption and diverticular diseases), neurological (Parkinson disease, multiple sclerosis, paraplegia, autonomic neuropathy, chronic intestinal pseudo-obstruction, gastroparesis, Hirschsprung disease and stroke), past psychiatric disease, and myogenic (scleroderma, amyloidosis and myotonic dystrophy dermatomyositis)[2,21,26]. In addition, we also assessed a control group of 86 healthy subjects (sex, age and BMI matched), recruited from a population-based cohort study of 150 healthy subjects, which underwent routine clinical exams in our Institute to check their healthy status, after excluding the presence of a functional chronic constipation during the clinical interview.
Our research was carried out in compliance with the Helsinki Declaration and procedures received approval by the Institutional review board of the National Institute of Gastroenterology “S. De Bellis” Research Hospital (0807/16) and by the Research Ethics Committee of the National Oncological Institute of Bari (CAAE:147/16) (Trial registration number: NCT03096704). Informed consent was obtained from all participants of the study. The statistical review of the study was performed by a biomedical statistician.
All selected patients were inquired, before, for any possible secondary causes of functional chronic constipation, including alarm features, lifestyle factors and medical treatments and we also identified the response (or lack of) to previous treatment about constipation symptoms, by interviewing patients. Then, they were considered affected by functional chronic constipation only when they showed a frequency of evacuation less than twice a week with increased stool consistency[8,26,28]. All the selected patients underwent blood tests, colonoscopy, nutritional assessment (with daily food frequency questionnaires), stool frequency diary, LBT, GBT, wireless motility testing, radiopaque markers studies, and when required, tests for pelvic floor dysfunction, and psychological tests exploring patient’s quality of life.
Accordingly, when not eligible, patients were excluded (Figure 1): 152 consecutive subjects affected by functional chronic constipation with intestinal motility disorders met the eligibility criteria, along with 150 healthy subjects. Patients and controls in each group were first randomly sampled from the pool of available individuals (152 with intestinal motility disorders and 150 controls) and, then matched for age, sex and BMI, using MatchIt[29]. This analysis resulted in 86 patients and 86 matched controls that were enrolled for the study. Our patients did not receive any pharmacological treatment before all evaluations.
The colon transit time was measured with radiopaque markers, using multiple capsule techniques (P and A Mauch CH-4142 Munchenstein). The patient ingested 1 capsule a day (at 9.00 am, during breakfast) for 3 consecutive days and each capsule with 24 different shaped radio-opaque polyurethane markers, and then performed abdominal x-rays (100KV) on day 7, at 9.00 am, to reconstruct the colon activity during the last week[30,31]. Normal transit time was considered about 30-40 h, whereas the upper limit, above 70 h. Markers scattered about the colon was due most likely to STC, whereas markers gathered in the recto-sigmoid tract was considered a defecatory disorder[5,27] (Supplementary material).
Moreover, LBT and GBT were used for measuring oro-cecal transit time. A rise in hydrogen of ≥ 20 ppm by 90 min was considered the cut-off value used to exclude SIBO for both tests[25]. Time to the second peak and rise of 5-10 ppm of hydrogen, or a level of positivity for methane ≥ 10 ppm on a breath test, were considered useful in estimating oro-cecal transit time[25,27,32] (Supplementary material). Wireless motility analysis was also used to quantify oro-cecal transit time (Smartpill, Medtronic, Sunnyvale, California, United States), in order to define the presence of a delayed small intestinal transit time. Normal small-bowel transit should be 6 hours or less[25] (Supplementary material). Finally, STC and/or delayed oro-cecal transit time were definitively endorsed when radiopaque markers and/or breath tests with wireless motility analysis identified a specific intestinal motility disorder[27].
The IBS-QOL questionnaire is a 34-item tool validated to quantify quality of life in non-subtyped IBS patients, but also used to assess the severity of IBS-like symptoms, and psychological factors[10]. The SF-12 includes a subset of 12 items, used to assess the physical and mental health domain in many diseases. All these scores are converted into a standardized 0-100 score. Higher scores indicate a better self-reported health status[11]. Furthermore, the HADS-14 represents a global measure of psychological distress and includes 14 items, 7 of them evaluate anxiety symptoms, and 7 depressive ones. Each item is coded from 0 to 3. The total scores for anxiety and depression can range between 0-21, based on symptoms characteristics[12].
Serum 25-hydroxyvitamin D [25-(OH)-D] (Supplementary material) and parathyroid hormone (PTH) levels were measured in patients and healthy controls within 1 hour from blood draw. Overall subjects were enrolled in the interval time of the year with major sunlight exposition, when 25-(OH)-D values are usually higher[13]. The 25-(OH)-D levels status was categorized as usually proposed by experts in literature: using a standardized cut-off (deficiency less than 20 ng/mL, insufficiency from 20 to 29 ng/mL and sufficient when equal or higher than 30 ng/mL)[13].
Screened patients and controls in each group were matched for age, sex and BMI using the nearest neighbor matching algorithm implemented in MatchIt[29]. Normal distribution of continuous variables was assessed with the Shapiro-Wilk test and data were expressed as mean and standard deviation and compared using Student’s t-test. Categorical variables were reported as percentages and compared using the Chi-squared test or Fisher’s exact test, when needed.
Spearman’s test was performed to evaluate possible correlations of vitamin D values with quality of life scores and psychological functions (IBS-QOL, SF12-PCS, SF12-MCS, HADS-14 A and HADS-14 D). The impact of the vitamin D levels on patient’s risk to have intestinal motility disorders was analysed using univariate and multivariate logistic regression analyses. The association between each explanatory variable and the outcome (intestinal motility disorders occurrence) was tested using the likelihood ratio test. We included in the multivariate model all explanatory variables showing a P < 0.05 at univariate analysis. For each variable included in the multivariate model, we calculated both unadjusted and adjusted odds ratios (OR), with their 95% confidence intervals (CI), and the level of significance (using the likelihood ratio test). Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS 23.0 software (SPSS, Chicago, IL, United States) and R version 3.4.3 (http://www.R-project.org/).
As shown in Figure 1, 86 patients along with 86 healthy matched subjects, were finally enrolled. The characteristics of patients are shown in Table 1. About 85% of patients suffering from intestinal motility disorders were female, and mean age was 49.9 ± 17.4 years. Patients with intestinal motility disorders had significantly lower intermediate and bachelor schooling degree, as compared to the control group (P = 0.001 and P < 0.001, respectively).
| Variables | Intestinal motility disorders (n = 86) | Controls (n = 86) |
| Age, mean ± SD , yr | 49.9 (17.4) | 50 (17.1) |
| Sex, n (%) | ||
| Male | 13 (15.1) | 13 (15.1) |
| Female | 73 (84.9) | 73 (84.9) |
| BMI, mean ± SD, kg/m2 | 23.7 (2.8) | 24.6 (3.4) |
| Education, n (%) | ||
| Primary | 5 (5.8) | 5 (5.8) |
| Intermediate | 33 (38.4)a | 14 (16.3)a |
| High school | 38 (44.2) | 37 (43) |
| Bachelor degree | 10 (11.6)a | 28 (32.6)a |
| No data | 0 | 2 (2.3) |
| Marital status | ||
| Single | 30 (34.9) | 22 (25.6) |
| Married | 53 (61.6) | 58 (67.4) |
| Widower | 3 (3.5) | 4 (4.7) |
| Divorced | 0 | 1 (1.2) |
| No data | 0 | 1 (1.2) |
| Symptoms | ||
| Constipation | 78 (90.1)a | 3 (3.5)a |
| Abdominal pain | 73 (84.9)a | 2 (2.3)a |
| Swelling | 79 (91.9)a | 14 (16)a |
| IBS-QOL (total score) | 108.4 (23.7)a | 39.8 (7.7)a |
| Dysphoria | 24.1 (7.7)a | 8.7 (1.5)a |
| Interference | 15.9 (5.5)a | 8 (1.6)a |
| Body image | 15 (2.1)a | 4.8 (1.4)a |
| Anxiety health | 13.5 (1.8)a | 3.7 (1.4)a |
| Food avoidance | 13.6 (1.6)a | 3.7 (1.4)a |
| Social reaction | 10.8 (3.2)a | 5 (1.1)a |
| Interpersonal relationships | 6.3 (3.3)a | 3.3 (0.6)a |
| Sexuality | 4.6 (2.2)a | 2 (0.1)a |
| HADS-14 A | 14.6 (4.3)a | 2.4 (1.8)a |
| HADS-14 D | 14.6 (3.1)a | 4.5 (2.6)a |
| SF12-PCS | 37.3 (9.8)a | 47.2 (7.6)a |
| SF12-MCS | 29.1 (7.4)a | 56 (7.1)a |
| PTH, mean ± SD, 10-70 pg/mL | 51.1 (12.1) | - |
| Vitamin D, mean ± SD, IU/mL | 14.6 (7.7)a | 28.4 (8.8)a |
The proportion of patients with intestinal motility disorders was more affected by constipation, abdominal pain, swelling, anxiety and depression symptoms as well as by quality of life alterations, as compared to healthy subjects (P < 0.001). The results of the various questionnaires compiled by patients (IBS-QOL, HADS-14 A, HADS-14 D, SF12-PCS and SF12-MCS) showed a significant impairment in all health-related domains (P < 0.001) (Table 1). Patients with intestinal motility disorders had lower vitamin D serum levels, as compared to the control group (P < 0.001). Moreover, the poor results of psychological tests (IBS-QOL, SF12-PCS, SF12-MCS, HADS-14 A and HADS-14 D) significantly correlated with low vitamin D levels (r = - 0.57, P < 0.001; r = 0.21, P = 0.01; r = - 0.48, P < 0.001; r = - 0.57, P < 0.001; r = - 0.29, P < 0.001, respectively). Other parameters, such as the age, sex and marital status did not differ between the two groups (Table 1).
In the groups with intestinal motility disorders, 32, 28 and 26 patients, after completing all diagnostic tests, received a diagnosis of delayed oro-cecal transit time (37.2%), STC (32.6%) or delayed oro-cecal transit time with STC (30.2%), respectively. In the group with delayed oro-cecal transit time and with STC, the proportion of patients who suffered constipation, swelling, abdominal pain and vitamin D deficiency (< 20 ng/mL) was higher than groups with delayed oro-cecal transit time or with STC (P = 0.03, P = 0.02, P = 0.03 and P = 0.04 respectively) (Figure 2). When we considered the three groups: subjects with delayed transit time in both intestinal tracts (26), to patients with delayed transit time in only one tract (60) and to controls (86), the quality of life showed worsening functions. Moreover, vitamin D and BMI were significantly reduced with decreasing values as following for the variables: delayed oro-cecal transit time with STC group less than delayed oro-cecal transit time or STC group, less than controls (P < 0.001, respectively) (Supplementary material).
Patients with delayed transit time of both intestinal tracts, as compared to patients with delayed transit time involving only one tract, showed significantly reduced levels of vitamin D and higher PTH levels (for both P < 0.001), and showed worsening quality of life (P < 0.001) (Supplementary material). Patients with delayed transit time in both intestinal tracts showed significantly reduced serum levels of vitamin D and higher PTH serum levels (for both P < 0.001), and showed worsening quality of life (P < 0.001) than patients with delayed transit time in only one tract (Supplementary material).
Results from linear regression analysis are shown in Table 2. At univariate and multivariate analysis adjusted for BMI, vitamin D levels remained a significant independent risk factor for intestinal motility disorders occurrence (OR = 1.19; 95%CI: 1.14-1.26, P < 0.001).
| Variable | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR1(95%CI) | P value | |
| Age, yr | 1.0 (0.98-1.01) | 0.76 | - | - |
| BMI, kg/m2 | 0.91 (0.82-1.0) | 0.04 | 0.98 (0.86-1.11) | 0.72 |
| Vitamin D, IU/mL | 0.82 (0.78-0.87) | < 0.001 | 0.83 (0.78-0.87) | < 0.001 |
In this study, we demonstrated for the first time that serum vitamin D deficiency could be associated to functional chronic constipation induced by intestinal motility disorders. Furthermore, patients showing the latter clinical condition are frequently affected by anxiety and depression symptoms which severely impair their quality of life.
About 1 billion people have vitamin D insufficiency or deficiency[13]. Nowadays, this phenomenon is associated with significant disability and healthcare costs[14,15]. Several studies showed that hypovitaminosis D may be a risk factor for many chronic diseases and for mortality[16,33-36]. Notably, vitamin D deficiency has been involved in the pathophysiology of inflammatory bowel disease, IBS and also depression[13-17,19]
Some multicentric studies, reported a higher prevalence of hypovitaminosis D in the southern Europe[37,38]. Interestingly, Italian elderly subjects showed the lowest levels of vitamin D in Europe in the winter season[37,38]. In our study, we found low serum levels of vitamin D, mainly checked during the summer season, among patients affected by intestinal motility disorders during an interval time when vitamin D values are usually high, due to beta-ultraviolet rays exposition[13,36]. Moreover, our patients were prevalently female (84.9%) and relatively younger, although, vitamin D deficiency has not been limited to the elderly age[35-38]. While low levels of vitamin D have been also observed in winter in 33% of pre-menopausal women, especially if obese[39], in our study the majority of patients with intestinal motility disorders had normal BMI.
How vitamin D deficiency and intestinal motility disorders are linked remains an open question. One intriguing hypothesis may be that STC and/or delayed small intestinal transit time could negatively modify the gut microbiota[36], or conversely altered microbiome could primarily affect mucosal barrier and gut motility due to microbial-derived metabolites[40]. In addition, some studies suggested that vitamin D deficiency could predispose to gastrointestinal infections[16], which could be responsible of the “leaky gut” alteration and of loss of immune homeostasis[40,41]. However, in our patients we also performed LBT and GBT, to exclude SIBO. When the clinical suspicion of the latter condition remained, we treated them “ex juvantibus”, and we further excluded SIBO as the main cause of bowel symptoms, especially when they did not disappear after a gut-targeted antibiotic therapeutic cycle.
Anyway, the presence of vitamin D receptor on gut epithelial cells, macrophages and lymphocytes surface, has suggested a possible link between vitamin D deficiency, the dysfunction of its receptor and gut microbiota composition, leading to the onset of autoimmune diseases[40,42,43]. Finally, the influence of vitamin D deficiency on human immune system is also supported by its involvement in the development of multiple sclerosis[44]. As concerning this aspect, an interesting study supported a role of autoantibodies against enteric nervous system targets in B cell-deficient mice with experimental autoimmune encephalomyelitis model of multiple sclerosis, showing that serum immunoreactivity (idiopathically or secondary to another autoimmune disease), could be implicated in the induction of autoimmune gastrointestinal dysmotility[45]. In fact, in humans with multiple sclerosis, whose pathogenesis has been linked to vitamin D deficiency, a slow colonic motility in the proximal tract as well as autonomic rectal dysfunction has been observed[44-48].
We may suppose that the intestinal motility disorder could be the “primum movens” of an underlying autoimmune process in a specific genetic background and unmasked by chronic vitamin D deficiency, which could exert metabolic/immunologic damage on epithelial and neuromuscular structures of the gut. The latter alterations could include gut hyper-permeability and bacterial translocation, whose degree of injury and extension could be influenced by the severity of vitamin D deficiency[22,45]. This hypothesis may be supported by our results, which showed that levels of vitamin D were independently associated with intestinal motility disorders and by the fact that the prevalence of symptoms associated to functional chronic constipation grew up in concordance with the worsening of vitamin D levels, especially in patients involving more extensively the bowel tract. Furthermore, patients with intestinal motility disorders had high levels of psychological distress with impaired quality of life, and hypovitaminosis D significantly correlated with the worsening of the psychological functions. In our study, a potential bias could derive from disease misclassification, which could be a negligible factor since intestinal motility disorder was diagnosed following standardized criteria, and from the small sample size of our population. Moreover, we did not check fecal microbiota composition.
In conclusion, we demonstrated that vitamin D deficiency could be strongly related to intestinal motility disorders. Moreover, patients with intestinal motility disorders are very commonly affected by anxiety and depression symptoms which severely influence their quality of life. If the latter two psychiatric symptoms are caused by intestinal factors, it will be confirmed only through a cross-sectional study. Therefore, we suggest that vitamin D serum levels should be routinely measured and vitamin D supplementation should be considered, to better evaluate its effects on intestinal motility and quality of life of patients with intestinal motility disorders.
Functional chronic constipation is a gastrointestinal disorder that affects more commonly women and older subjects, with a deep impact on global health-care system. Nowadays, only few studies have examined intestinal motility disorders, which are severe clinical conditions associated with chronic functional constipation, whose pathogenesis and prevalence are actually partially known. In this subgroup are inclued patients with slow transit constipation, as well as with slow oro-cecal transit time, whereas their extreme clinical form, could be considered chronic intestinal pseudo-obstruction, which has been related to the structural damage of neural and smooth muscle cells of gut.
Although some studies have shown a possible link between vitamin D deficiency and irritable bowel syndrome (IBS), as well as with depression (and with several other diseases), the same link has never been detected before in patients affected by intestinal motility disorders, and the indications to look for vitamin D in these patients relied only of opinions of experts in the field. Therefore we investigated this relationship and the psychological aspects in this subgroup of patients.
To investigate the relationship between serum 25-hydroxyvitamin D levels and functional chronic constipation linked to intestinal motility disorders.
Herein, we applied rigorous statistical methods to elucidate this relationship. We performed a prospective case-control study, from May-June to November 2017. We used Glucose/lactulose breath tests, radiopaque markers (multiple capsule techniques) and wireless motility capsule analysis to estimate both oro-cecal and colonic transit time. After receiving a diagnosis of intestinal motility disorders, patients underwent to blood sampling, for checking 25-hydroxyvitamin D levels. Furthermore, we evaluated for these patients the influence on psychological features and on their quality of life, which were estimated by using validated questionnaires, the IBS Quality of life (IBS-QOL), the Short Form Health Survey 12 (SF-12), and the Hospital Anxiety and Depression Scale 14 (HADS-14 A and HADS-14 D).
Our cohort included 86 patients with chronic functional constipation associated to intestinal motility disorders and 86 age, sex, body mass index (BMI)-matched healthy subjects. Patients with intestinal motility disorders had lower 25-hydroxyvitamin D levels (P < 0.001), and they showed a significant impairment of all health-related quality of life domains and psychological tests (IBS-QOL, SF12-PCS, SF12-MCS, HADS-14 A and HADS-14 D), as compared to the control group (P < 0.001). Moreover, the latter tests significantly correlated with reduced vitamin D levels (r = - 0.57, P < 0.001; r = 0.21, P = 0.01; r = - 0.48, P < 0.001; r = - 0.57, P < 0.001; r = - 0.29, P < 0.001, respectively). In multivariate analysis, vitamin D low levels remained significantly associated with the occurrence of intestinal motility disorder, after adjusting for BMI (odds ratio = 1.19; 95% confidence interval: 1.14-1.26, P < 0.001).
We demonstrated for the first time a strong association between vitamin D deficiency and intestinal motility disorders. Moreover, these patients are very commonly affected by anxiety and depression symptoms which deeply impact on their quality of life. These findings suggest that vitamin D serum levels should be routinely measured in this category of patients and consequently vitamin D supplementation could represent a further therapeutic aid for this clinical condition.
Our findings may confirm how vitamin D deficiency could exert a wide spectrum of action in many gastrointestinal (or not) diseases, being highly associated with intestinal motility disorders and with certain neuropsychiatric symptoms, but remains unclear if it could have a causative role in this process, and for this reason, future cross-sectional studies are needed, also to investigate if anxiety and depression symptoms are caused by intestinal factors.
We thank to Vanessa Terenzio and Domenico Flavio Terenzio, native English speakers, for the language revision of this manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feretis M, Luthin DR, Negreanu L S-Editor: Yan JP L-Editor: A E-Editor: Song H
| 1. | Pare P, Ferrazzi S, Thompson WG, Irvine EJ, Rance L. An epidemiological survey of constipation in canada: Definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001;96:3130-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 305] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Tack J, Müller-Lissner S, Stanghellini V, Boeckxstaens G, Kamm MA, Simren M, Galmiche JP, Fried M. Diagnosis and treatment of chronic constipation--a European perspective. Neurogastroenterol Motil. 2011;23:697-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Palsson OS, Baggish JS, Turner MJ, Whitehead WE. IBS patients show frequent fluctuations between loose/watery and hard/lumpy stools: Implications for treatment. Am J Gastroenterol. 2012;107:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Palsson OS, Baggish J, Whitehead WE. Episodic nature of symptoms in irritable bowel syndrome. Am J Gastroenterol. 2014;109:1450-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Wald A, Scarpignato C, Kamm MA, Mueller-Lissner S, Helfrich I, Schuijt C, Bubeck J, Limoni C, Petrini O. The burden of constipation on quality of life: Results of a multinational survey. Aliment Pharmacol Ther. 2007;26:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. Am J Gastroenterol. 2011;106:1582-1591; quiz 1581, 1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 575] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 7. | Cottone C, Tosetti C, Disclafani G, Ubaldi E, Cogliandro R, Stanghellini V. Clinical features of constipation in general practice in Italy. United European Gastroenterol J. 2014;2:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Belsey J, Greenfield S, Candy D, Geraint M. Systematic review: Impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010;31:938-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 9. | Shekhar C, Monaghan PJ, Morris J, Issa B, Whorwell PJ, Keevil B, Houghton LA. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterology. 2013;145:749-757; quiz e13-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, Jia H, Bangdiwala SI. Further validation of the IBS-QOL: A disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 299] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L, Sullivan M. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Pallant JF, Tennant A. An introduction to the Rasch measurement model: an example using the Hospital Anxiety and Depression Scale (HADS). Br J Clin Psychol. 2007;46:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 647] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 13. | Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9399] [Cited by in RCA: 9410] [Article Influence: 522.8] [Reference Citation Analysis (1)] |
| 14. | Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006;367:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3532] [Cited by in RCA: 3494] [Article Influence: 183.9] [Reference Citation Analysis (0)] |
| 15. | Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9:941-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 329] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 16. | Autier P, Mullie P, Macacu A, Dragomir M, Boniol M, Coppens K, Pizot C, Boniol M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (1)] |
| 17. | Tazzyman S, Richards N, Trueman AR, Evans AL, Grant VA, Garaiova I, Plummer SF, Williams EA, Corfe BM. Vitamin D associates with improved quality of life in participants with irritable bowel syndrome: Outcomes from a pilot trial. BMJ Open Gastroenterol. 2015;2:e000052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Abbasnezhad A, Amani R, Hajiani E, Alavinejad P, Cheraghian B, Ghadiri A. Effect of vitamin D on gastrointestinal symptoms and health-related quality of life in irritable bowel syndrome patients: A randomized double-blind clinical trial. Neurogastroenterol Motil. 2016;28:1533-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 535] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 20. | Li G, Mbuagbaw L, Samaan Z, Falavigna M, Zhang S, Adachi JD, Cheng J, Papaioannou A, Thabane L. Efficacy of vitamin D supplementation in depression in adults: A systematic review. J Clin Endocrinol Metab. 2014;99:757-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 21. | Rao SS, Rattanakovit K, Patcharatrakul T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol. 2016;13:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 22. | De Giorgio R, Camilleri M. Human enteric neuropathies: Morphology and molecular pathology. Neurogastroenterol Motil. 2004;16:515-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Rao SS, Kuo B, McCallum RW, Chey WD, DiBaise JK, Hasler WL, Koch KL, Lackner JM, Miller C, Saad R, Semler JR, Sitrin MD, Wilding GE, Parkman HP. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Parthasarathy G, Chen J, Chen X, Chia N, O'Connor HM, Wolf PG, Gaskins HR, Bharucha AE. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology. 2016;150:367-379.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 282] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 25. | Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, Schmulson M, Valdovinos M, Zakko S, Pimentel M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol. 2017;112:775-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 515] [Article Influence: 64.4] [Reference Citation Analysis (1)] |
| 26. | Di Nardo G, Karunaratne TB, Frediani S, De Giorgio R. Chronic intestinal pseudo-obstruction: Progress in management? Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Rao SS, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, Scott MS, Simren M, Soffer E, Szarka L. Evaluation of gastrointestinal transit in clinical practice: Position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23:8-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 28. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;pii:S0016-5085(16)00222-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1886] [Article Influence: 209.6] [Reference Citation Analysis (3)] |
| 29. | Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric reprocessing for parametric causal inference. J Stat Softw. 2011;42:1-28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1462] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 30. | Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 640] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 31. | Chaussade S, Roche H, Khyari A, Couturier D, Guerre J. Measurement of colonic transit time: Description and validation of a new method. Gastroenterol Clin Biol. 1986;10:385-389. [PubMed] |
| 32. | Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Hess AF, Unger LJ. The cure of infantile rickets by sunlight. JAMA. 1921;77:39-41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Stamp TC. Sources of vitamin D nutrition. Lancet. 1980;1:316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | van der Wielen RP, Löwik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 485] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 36. | Vaes AMM, Brouwer-Brolsma EM, van der Zwaluw NL, van Wijngaarden JP, Berendsen AAM, van Schoor N, van der Velde N, Uitterlinden A, Lips P, Dhonukshe-Rutten RAM, de Groot LCPGM. Food sources of vitamin D and their association with 25-hydroxyvitamin D status in Dutch older adults. J Steroid Biochem Mol Biol. 2017;173:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Mølgaard C, Jorde R, Grimnes G, Moschonis G, Mavrogianni C, Manios Y, Thamm M, Mensink GB, Rabenberg M, Busch MA, Cox L, Meadows S, Goldberg G, Prentice A, Dekker JM, Nijpels G, Pilz S, Swart KM, van Schoor NM, Lips P, Eiriksdottir G, Gudnason V, Cotch MF, Koskinen S, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Kiely M. Vitamin D deficiency in Europe: Pandemic? Am J Clin Nutr. 2016;103:1033-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 874] [Cited by in RCA: 902] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 38. | Carnevale V, Modoni S, Pileri M, Di Giorgio A, Chiodini I, Minisola S, Vieth R, Scillitani A. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: Seasonal and gender differences. Osteoporos Int. 2001;12:1026-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Adami S, Bertoldo F, Braga V, Fracassi E, Gatti D, Gandolini G, Minisola S, Battista Rini G. 25-hydroxy vitamin D levels in healthy premenopausal women: Association with bone turnover markers and bone mineral density. Bone. 2009;45:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Clark A, Mach N. Role of Vitamin D in the Hygiene Hypothesis: The Interplay between Vitamin D, Vitamin D Receptors, Gut Microbiota, and Immune Response. Front Immunol. 2016;7:627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 41. | Ge X, Zhao W, Ding C, Tian H, Xu L, Wang H, Ni L, Jiang J, Gong J, Zhu W, Zhu M, Li N. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci Rep. 2017;7:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 42. | He L, Liu T, Shi Y, Tian F, Hu H, Deb DK, Chen Y, Bissonnette M, Li YC. Gut Epithelial Vitamin D Receptor Regulates Microbiota-Dependent Mucosal Inflammation by Suppressing Intestinal Epithelial Cell Apoptosis. Endocrinology. 2018;159:967-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 43. | Shahini E, Iannone A, Romagno D, Armandi A, Carparelli S, Principi M, Viggiani MT, Ierardi E, Di Leo A, Barone M. Clinical relevance of serum non-organ-specific antibodies in patients with HCV infection receiving direct-acting antiviral therapy. Aliment Pharmacol Ther. 2018;48:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 1267] [Article Influence: 181.0] [Reference Citation Analysis (2)] |
| 45. | Spear ET, Holt EA, Joyce EJ, Haag MM, Mawe SM, Hennig GW, Lavoie B, Applebee AM, Teuscher C, Mawe GM. Altered gastrointestinal motility involving autoantibodies in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neurogastroenterol Motil. 2018;30:e13349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Chia YW, Gill KP, Jameson JS, Forti AD, Henry MM, Swash M, Shorvon PJ. Paradoxical puborectalis contraction is a feature of constipation in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996;60:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Li Q, Michel K, Annahazi A, Demir IE, Ceyhan GO, Zeller F, Komorowski L, Stöcker W, Beyak MJ, Grundy D, Farrugia G, De Giorgio R, Schemann M. Anti-Hu antibodies activate enteric and sensory neurons. Sci Rep. 2016;6:38216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | De Giorgio R, Bovara M, Barbara G, Canossa M, Sarnelli G, De Ponti F, Stanghellini V, Tonini M, Cappello S, Pagnotta E, Nobile-Orazio E, Corinaldesi R. Anti-HuD-induced neuronal apoptosis underlying paraneoplastic gut dysmotility. Gastroenterology. 2003;125:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |