Published online Nov 28, 2018. doi: 10.3748/wjg.v24.i44.4962
Peer-review started: September 10, 2018
First decision: October 24, 2018
Revised: October 30, 2018
Accepted: November 7, 2018
Article in press: November 8, 2018
Published online: November 28, 2018
Processing time: 80 Days and 14 Hours
The mononuclear phagocyte system (MPS), which consists of monocytes, dendritic cells (DCs), and macrophages, plays a vital role in the innate immune defense against pathogens. Hepatitis C virus (HCV) is efficient in evading the host immunity, thereby facilitating its development into chronic infection. Chronic HCV infection is the leading cause of end-stage liver diseases, liver cirrhosis, and hepatocellular carcinoma. Acquired immune response was regarded as the key factor to eradicate HCV. However, innate immunity can regulate the acquired immune response. Innate immunity-derived cytokines shape the adaptive immunity by regulating T-cell differentiation, which determines the outcome of acute HCV infection. Inhibition of HCV-specific T-cell responses is one of the most important strategies for immune system evasion. It is meaningful to illustrate the role of innate immune response in HCV infection. With the MPS being the important factor in innate immunity, therefore, understanding the role of the MPS in HCV infection will shed light on the pathophysiology of chronic HCV infection. In this review, we outline the impact of HCV infection on the MPS and cytokine production. We discuss how HCV is detected by the MPS and describe the function and impairment of MPS components in HCV infection.
Core tip: Hepatitis C virus (HCV) infection is efficient to develop into chronic infection. Innate immune system can shape the acquired immune response, which can eradicate HCV directly. As the main component of innate immunity, the mononuclear phagocyte system (MPS) plays a vital role in HCV infection. In this review, we discuss the interaction between the HCV and MPS. MPS can detect HCV to promote virus eradication, and HCV can shape the MPS to facilitate HCV persistence. We hope that this review will enable us to better understand HCV infection.
- Citation: Yang Y, Tu ZK, Liu XK, Zhang P. Mononuclear phagocyte system in hepatitis C virus infection. World J Gastroenterol 2018; 24(44): 4962-4973
- URL: https://www.wjgnet.com/1007-9327/full/v24/i44/4962.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i44.4962
Hepatitis C virus (HCV) is a positive sense single-stranded RNA virus that belongs to the family Flaviviridae[1]. HCV infection affects more than 170 million people worldwide and is regarded as a leading cause of chronic liver disease[2]. The viral genome is approximately 9.6 kb, encoding a single 3011-amino acid-long polyprotein. The polyprotein is cleaved into three structural proteins (core, E1, and E2) and seven non-structural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B)[3]. HCV is classified into seven genotypes as well as 67 subtypes, and it shows significant genetic diversity among different nations[4]. Even within the same patient, HCV usually exists in blood as a group of related quasispecies[5]. Acute HCV infections are anicteric and asymptomatic[6]. Nevertheless, 15%-20% of HCV-infected patients can recover from an acute infection, whereas the remaining 80%-85% of patients will progress to chronic infection[6-8]. Chronic HCV infection is a leading cause of end-stage liver diseases, liver failure, and hepatocellular carcinoma, resulting in approximately 350000 deaths per year[9,10]. HCV infection is usually diagnosed via the detection of both HCV antibody and HCV RNA. In the absence of viral RNA, the detection of HCV antibody indicates a spontaneously resolved or cured infection[10]. The combination of subcutaneous pegylated interferon (peginterferon) alpha and oral ribavirin was once the standard treatment for chronic HCV infection. However, this combination results in a sustained virological response (SVR) in only approximately 50% of patients[11]. In 2011, the United States Food and Drug Administration approved a novel HCV therapy including direct-acting antiviral drugs and protease inhibitor drugs. These drugs significantly increased the response rate, thereby revealing a new era of HCV treatment[12,13].
The term mononuclear phagocyte system (MPS) was developed in the late 1960s and early 1970s by van Furth[14]. The MPS encompasses monocytes, dendritic cells (DCs), and macrophages, and altogether they play vital roles in tissue development, maintenance of homeostasis, inflammation, and the innate immune defense against pathogens.
Monocytes constitute 5%-10% of the peripheral blood leukocytes in humans and are generated in the bone marrow and spleen[14]. During inflammation, monocytes can differentiate into macrophages and DCs[15-19], and they play important roles in both innate and adaptive immunity[20-24]. Circulating monocytes can traffic through the sinusoids, and thus, it has been proposed that liver-resident monocytes and circulating monocytes should be distinguished[25]. However, blood monocytes pass through the liver numerous times, and therefore, we will consider circulating monocytes with liver-resident monocytes as one entity in this review.
Human blood DCs are major histocompatibility complex (MHC) class II [human leukocyte antigen D-related (HLA-DR)] positive and can be divided into myeloid DCs (mDCs) and plasmacytoid DCs (pDCs)[26]. pDCs are CD11c negative and are distinguished from mDCs using positive markers such as CD123, CD303, and CD304[26]. Alternatively, mDCs can be subdivided according to CD1c and CD141 expression[26]. Accordingly, DCs exist in CD303+ pDCs, CD11c+ CD1c+ mDCs, and CD11c+ CD141+ mDCs populations. It is worth mentioning that all these subsets are present in the liver[25], and the CD1c+ mDC population is the most prevalent liver DC subset[27]. Compared to blood DCs, hepatic DCs present an immature phenotype and have a lower capacity to stimulate T cells[27-29]. Furthermore, hepatic DCs produce more interleukin (IL)-10 and less IL-12p70[30,31], highlighting the tolerogenic peculiarity of hepatic DCs.
Macrophages are large phagocytic cells with multifunctional roles in development, homeostasis, and diseases[32]. Kupffer cells (KCs) are tissue-resident macrophages of the liver that have important functions in both the innate and acquired immune responses[32-34]. However, owing to their stationary state, they are not as potent as DCs in stimulating T cells[35]. Additionally, KCs can also regulate the functions of other hepatic cells[36,37]. As early as the 1990s, the interaction between KCs with natural killer (NK) cells and liver stellate cells was identified by electron microscopy, implying that the functions of NK cells and stellate cells may be shaped by KCs[38]. In our lab, we previously identified Toll-like receptor (TLR)-dependent crosstalk between human KCs and NK cells[39].
HCV infection is notorious for its propensity to become chronic due to the lack of robust acquired immune responses. The immune response against HCV infection is primarily controlled by the adaptive immune system; however, a robust acquired immune response is determined by the innate immune response[40]. In other words, proper innate immunity is essential for the initiation of the acquired immune response. Mounting evidence confirms that the MPS is crucial for innate immunity and plays an important role in multiple infections, including parasitic infections[41], tuberculosis[42], human immunodeficiency virus (HIV) infection[43,44], and respiratory syncytial virus infection[45]. Therefore, it is necessary to clarify the interaction between HCV and the MPS. The immunophenotype of the MPS in normal liver has been previously reviewed[25]. However, the impact of HCV infection on the MPS has not been reviewed yet. Therefore, in this review, we summarize recent findings regarding the role of the MPS in HCV infection, and we focus on the function and impairment of MPS components following HCV infection.
Pathogen-associated molecular patterns (PAMPs) on HCV can be detected by three classes of pattern recognition receptors (PRRs): RIG I-like receptors (RLRs), TLRs, and NOD-like receptors (NLRs)[46]. These PRRs function early after infection, thereby restricting HCV replication[46].
RIG-I, representative of RLRs, can sense HCV RNA as non-self through the 5′-triphosphate (5′-ppp) found on the viral RNA in addition to the 3′ poly-U/UC tract[47,48]. Blocking of the signaling pathway of melanoma differentiation-associated gene 5 (MDA5), another member of the RLRs, led to enhanced HCV replication[49]. Both RIG-I and MDA5 utilize the adaptor protein mitochondrial antiviral signaling (MAVS) to initiate immune signaling, and they recognize different PAMPs, indicating that they may function complementarily[50-52]. In West Nile virus infection, RIG-I was found to play an important role in the early immune response after infection, whereas MDA5 was more important in the later period of infection[53].
Endosomal TLRs are the main sensors that detect HCV. Among them, TLR3 can sense double-stranded (ds)RNA[54,55], whereas the GU-rich sequences in HCV RNA can be recognized by TLR7 and TLR8[56,57]. Additionally, TLR2 is specialized in HCV protein detection[58]. Wang et al[54] previously demonstrated that interferon (IFN)-stimulated genes (ISGs) are upregulated in primary human hepatocytes after polyinosinic: polycytidylic acid (polyI:C) stimulation, owing to the expression of TLR3. However, the authors observed that HCV infection weakened the ability of hepatocytes to induce ISG expression compared to the polyI:C stimulation[54], indicating that TLR3 signaling may be impaired by HCV. Consistently, it was previously established that TIR-domain-containing adapter-inducing interferon-β (TRIF), an adaptor protein of TLR3 signaling, can also be cleaved by the NS3/4A protease[59,60].
It is worth mentioning that the results described above were derived from primary human hepatocytes or hepatocyte cell lines infected by HCV. In vivo, uninfected hepatocytes were able to sense the adjacent infected cells by TLR3[55]. Extracellular dsRNA was detected by the uninfected hepatocytes in a macrophage scavenger receptor 1 (MSR1)-dependent manner[55]. MSR1 can bind to the viral dsRNA and transport it to the endosome, within which TLR3 is engaged[55]. This mechanism may be employed by the MPS to trigger an antiviral state in a TLR3-dependent manner. Furthermore, HCV-infected cells can induce the production of type I IFN from pDCs[61]. Additionally, HCV RNA activates the MPS populations like mDCs and pDCs to produce proinflammatory cytokines and chemokines, including IL-1β, tumor necrosis factor (TNF)-α, IL-6, IL-12, IL-10, CXCL9, and CXCL10[57]. Particularly, the GU-rich sequences induce type I IFN from monocytes and pDCs[57]. In contrast, the polyU/UC sequences of HCV RNA activate IL-1β production from the nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome of macrophages, resulting in persistent liver inflammation[62,63].
In addition, HCV proteins can also activate the MPS. It was identified that HCV core protein (HCVc) and NS3 activate monocytes[64] and macrophages[58], thereby triggering inflammatory pathways in a TLR2-dependent manner[58]. Additionally, HCVc and NS3 inhibit DC differentiation[64]. Furthermore, TLR1 and TLR6, co-receptors of TLR2, are also involved in HCVc and NS3-induced macrophage activation[65].
Compared to the HCV RNA, HCV viral particles are less efficient in stimulating the MPS[57]. Nevertheless, they can activate macrophages, leading to production of proinflammatory cytokines like IL-6, IL-1β, and TNF-α rather than the antiviral cytokines including IL-12 and type I IFN[57].
Effect of HCV on TLR signaling: TLR signaling is associated with the outcome of acute HCV infection as well as the therapeutic outcome[66]. Accumulating evidence suggests that HCV infection can influence the expression of TLRs[67-69]. Particularly, the expression levels of TLR2 and TLR4 are elevated after HCV infection in monocytes[67-69]. The expression of TLR2 is significantly correlated with serum TNF-α and alanine transaminase (ALT) levels[67], indicating that the inflammation associated with HCV infection is partially attributed to production of proinflammatory cytokines in a TLR2-dependent manner. Similarly, HCVc can activate the MPS in a TLR2-dependent manner[58]. In contrast, TLR3 and TLR4 in monocytes are compromised after HCV infection[70]. In healthy individuals, the repeated stimulation of monocytes via the TLR ligands leads to tolerance, thereby providing a protective mechanism to limit inflammation. However, this tolerance is disrupted in HCV-infected patients[71]. Therefore, monocytes from HCV-infected patients are hyper-responsive, and their expression of TNF-α is upregulated. The loss of TLR tolerance can be attributed to IFN-γ[71]. Alternatively, other reports demonstrated that HCVc can induce downregulation of IL-6 production after stimulation with TLR2 and TLR4 ligands[72,73]. We hypothesize that HCVc induces hyporesponsiveness, leading to the evasion of immunity in the early period of infection, whereas IFN-γ-induced loss of tolerance may contribute to inflammation and subsequent liver damage in chronic infection.
Impact of HCV on cytokine production from monocytes: IL-10, an anti-inflammatory cytokine, can be produced by monocytes[74]. IL-10 has several immunoregulatory functions after HCV infection. It is involved in HCV-specific CD8+ T cell regulation; specifically, IL-10 can reduce the frequency of CD8+ T cells and impair their differentiation[75]. Furthermore, IL-10 preferentially targets TLR4 signaling[76]. The inhibitory role of IL-10 against the production of proinflammatory cytokines was preferentially mediated by TLR4 signaling, i.e., the stimulation of chronic hepatitis C (CHC) patient-derived monocytes by lipopolysaccharide (LPS) (a TLR4 ligand) rather than R848 (a TLR8 agonist) led to lower TNF-α and IL-12 production[76].
Analysis of serum samples collected from CHC patients often shows higher IL-10 levels either produced spontaneously or after stimulation with HCV antigens[77,78]. Particularly, CHC patients have high IL-10 levels and relatively low levels of IFN-γ and IL-2[79], whereas patients with the self-limiting HCV produce lower IL-10 levels in response to both viral antigens and unspecific stimulation[80].
HCV NS4 can stimulate peripheral blood mononuclear cells (PBMCs) to produce IL-10 and transforming growth factor (TGF)-β[81]. TGF-β cooperates with IL-10 to inhibit the host-protective immune responses[82]. Additionally, supernatants of NS4-stimulated monocytes can inhibit DC maturation and DC stimulatory function[81].
In our lab, we studied the network of cytokines that regulate IL-10 production and the cytokines regulated by IL-10 upon HCV infection[74]. The stimulation of monocytes with HCVc and polyI:C induces the secretion of TNF-α, IL-1β, IL-10, and type I IFN. Interestingly, TNF-α, IL-1β, and IFN promote the IL-10 production, whereas high IL-10 levels inhibit TNF-α, IL-1β, and IFN production[74]. Furthermore, receptors for IL-10 on monocytes are also elevated during HCV infection and the type I as well as type III IFNs upregulate the IL-10 monocyte receptors, leading to higher sensitivity of monocytes to IL-10[83].
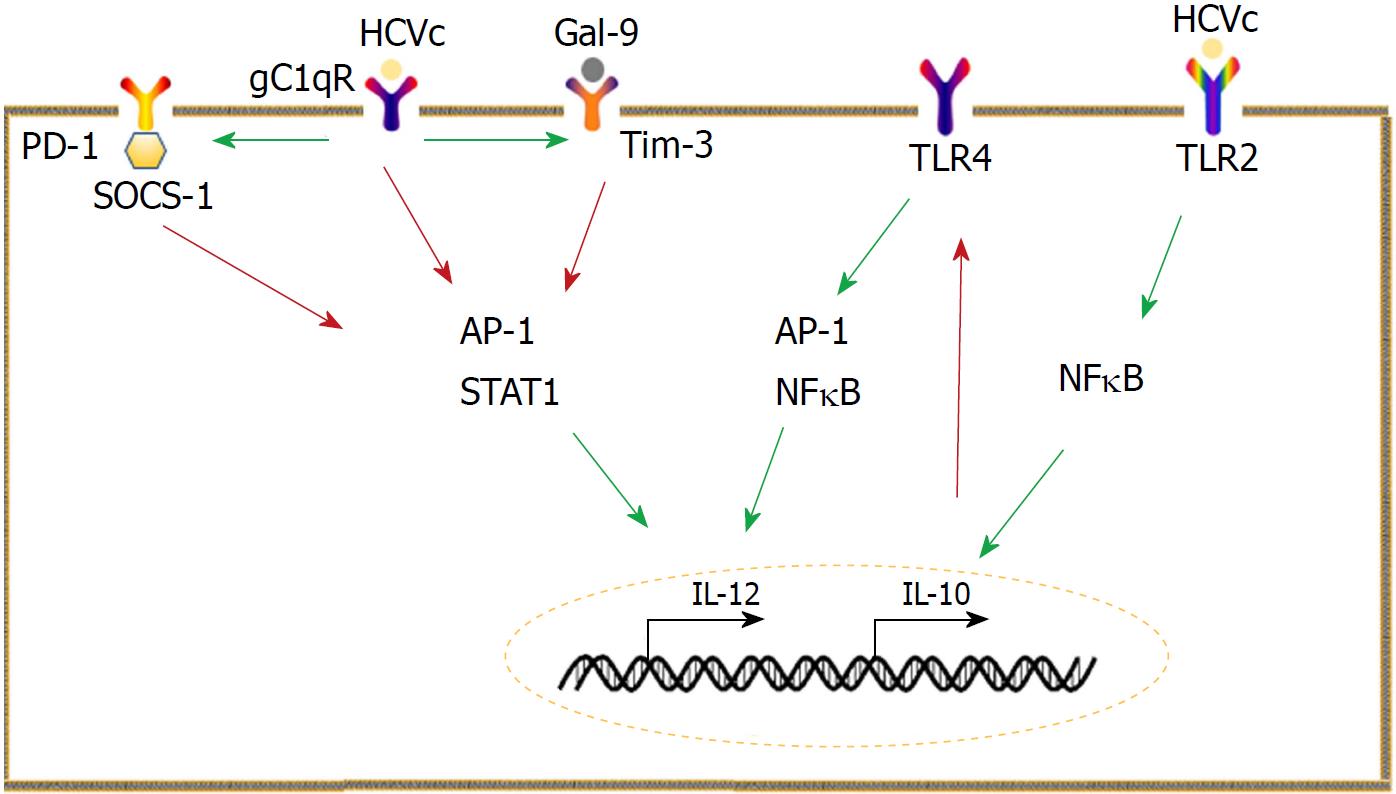
Programmed cell death-1 (PD-1) is primarily expressed on activated lymphocytes, whereas its ligand (PD-L) is widely expressed by many cells[84]. PD-1/PD-L interactions can affect responses against self and foreign antigens[84]. Consistently, PD-1/PD-L1 signaling in monocytes has critical roles in HCV infection. Monocytes from CHC patients are endowed with high levels of PD-L1, which enables the suppression of T cell proliferation, reduces the frequency of HCV-specific effector T cells, and downregulates the production of type 1 help T cell (Th1) cytokines as well[85]. PD-L1 signaling downregulates IL-12 expression, leading to low Th1 cytokine production[86]. HCVc interacts with the receptor for the globular heads of C1q (gC1qR) to increase PD-1 expression by monocytes[87]. PD-1 is associated with suppressor of cytokine signaling 1 (SOCS-1), and they work together to inhibit the activation of signal transducer and activator of transcription (STAT)-1 and the subsequent IL-12 production[87].
The galectin-9 (Gal-9) and T cell immunoglobulin and mucin domain 3 (Tim-3) pathway in monocytes is also vital for HCV infection. Monocytes express Gal-9 upon exposure to HCV-infected cells or the subgenomic replicon cells and exosomes from infected cells[88]. Consistently, Tim-3, receptor of Gal-9, is constitutively expressed on resting monocytes and can be up-regulated in CHC patients[89]. HCVc upregulates Tim-3 in a c-Jun N-terminal kinase (JNK) and T-bet-dependent manner[90]. The Gal-9/Tim-3 pathway is involved in the dysfunction of IL-12, IL-23, and IL-17[89,91]. Crosstalk between PD-1 and SOCS-1, Gal-9, and Tim-3 inhibits IL-12 production by limiting STAT-1 phosphorylation[89].
In conclusion, imbalance between IL-10 and IL-12 is a key feature of HCV infection. High levels of IL-10 combined with low IL-12 levels lead to a poor antiviral microenvironment. To make matters worse, HCV-infected patients and healthy controls show different responses to IL-10 and IL-12, i.e., IL-10 can suppress IFN-γ production in both HCV-infected patients and healthy controls, whereas the stimulatory effect of IL-12 on IFN-γ is compromised in HCV-infected patients[92] (Figure 1).
Regulatory function of monocytes following HCV infection: Following HCV infection, monocytes modulate the functions of other immune cells, such as NK cells and T cells. Additionally, NS5A can upregulate IL-10 and TGF-β expression in monocytes, and in turn, these cytokines suppress NK cell function by downregulating the expression of NKG2D, an activating receptor expressed on the surface of NK cells[93]. Furthermore, monocytes secrete the IL-18 and IL-36 inhibitory proteins, which can reduce NK cell activation, TNF-related apoptosis-inducing ligand (TRAIL) expression, and the ability to kill target cells[94]. Monocyte-derived Gal-9 upregulates the cytotoxicity of NK cells, leading to HCV-specific T cell apoptosis and liver injury[95]. Co-culture of monocytes with T cells leads to elevated mortality rate of T cells[96]. In addition to these detrimental functions, monocytes were found to be beneficial in the following situation: elevated OX40L expression, which is involved in the CD4+ T cell response. Blocking OX40L expression from monocytes leads to HCV-specific CD4+ T cell impairment[97]. Upon co-culture with JFH-1/HuH7.5 cells, NK cells from PBMCs produce high levels of IFN-γ. pDC-derived IFN-α is indispensable for IFN-γ production, whereas the monocyte-derived IL-15 can augment IFN-γ production to the maximum[98].
Impaired functions of DCs following HCV infection: In vivo study showed that gene expression in DCs from acute HCV resolving patients and from patients who become chronically infected is different[99]. The same result is also confirmed in healthy controls and CHC patients[99]. All these indicate that DCs play an important role in HCV infection.
DCs derived from peripheral blood progenitors in vitro enabled the extensive study of DC populations. Compared to healthy control DCs, HCV-DCs (derived from CHC patients) exhibit a normal phenotype and morphology but stimulate allogeneic T cells poorly[100,101]. Owing to the low expression of IL-12 in HCV-DCs, they induce lower amounts of IFN-γ from T cells compared with control DCs in co-cultures of allogeneic DCs and T cells[102]. Additionally, HCV-DCs are refractory to maturation stimuli and maintain an immature phenotype[103]. Interestingly, the observed defects in HCV-DCs are improved after viral clearance[100,103]. In agreement, transfection of DCs from a healthy donor with adenovirus encoding HCV E1 and HCVc resulted in poor ability to stimulate the allogeneic and autologous T cells[104].
To confirm the results obtained from in vitro generated DCs, researchers evaluated the functions and phenotypes of blood DCs ex vivo directly during chronic HCV infection[105-109]. Compared to those among healthy controls, the frequencies of mDCs, pDCs, and DC progenitors are significantly lower in HCV-infected patients[106,108-110]. DCs from HCV-infected patients have a reduced ability to stimulate allogeneic CD4+ T cells[105,107,110]. Additionally, they show abnormalities in the production of cytokines, such as reduced IFN-α and IL-12 levels[107,110] and increased IL-10 production[107,108]. Interestingly, these defects are resolved after viral elimination, indicating that HCV can indeed infect DCs and alter their function[106,108,109]. Additionally, the tryptophan-catabolizing enzyme indolamine 2,3-dioxygenase (IDO), an inducer of immune tolerance, was found to be significantly increased in mDCs of CHC patients[111]. Moreover, HCV-infected patient monocyte-derived DCs and infected control monocyte-derived DCs (infected ex vivo with HCV) show an inability to mature, and this impairment can be reversed by IDO inhibitors[111].
The anti-HCV immune response mainly occurs in the liver; therefore, it is reasonable to speculate that the behavior of circulating DCs can be different from that of liver-resident DCs. Therefore, studies were designed to isolate and characterize human liver DCs[112]. In contrast to the circulating DCs, mDCs from livers of HCV-infected patients did not show noticeable defects in stimulating T cells and produced lower levels of IL-10 than mDCs from healthy individuals[112]. However, the livers of HCV-infected patients harbored decreased numbers of pDCs compared to the livers of healthy individuals[112], and thus, the amount of IFN-α was lower in the HCV-infected patients[112]. In summary, lower amount of IFN-α and lower levels of IL-10 can contribute to persistent viral infection and inflammation in HCV infection, respectively[112].
Additionally, DCs from HCV-infected patients showed lower production of IFN-λ[113], abolished cytotoxic activity[114], upregulated levels of Fas ligand as well as PD-L2[115], and imbalanced expression between the co-stimulatory and co-inhibitory markers[116,117].
HCV-derived mechanisms underlying DC impairment: The mechanisms underlying DC impairment as well as the HCV proteins modulating DC functions have been previously investigated[118]. HCVc and NS3 proteins are involved in the impairment of DC maturation, lower levels of T cell stimulation as well as higher levels of IL-10 production from DCs in HCV-infected patients[64] (Table 1). Additionally, HCVc protein can engage gC1qR to inhibit IL-12 production and further restrain Th1 responses[119]. HCV E2 protein interacts with CD81 of DCs to alter DC migratory behavior, thereby incapacitating the recirculation of DCs to the lymphoid tissue, which can cause impairment of T cell priming[120] (Table 1). In our lab, we isolated liver-derived pDCs from normal liver tissues collected from benign tumor dissections and liver transplant donors. We observed that the interaction of E2 with CD81 inhibits pDC maturation, activation, and IFN-α production[121]. HCV NS4 protein can change the DC phenotype and is involved in the reduction of Th1 cytokine production and impairment of T cell stimulation[122]. NS3 and E2 proteins can hinder IFN-λ production from DCs[113]. NS5A increases IL-8 production from DCs and influences the phosphorylation of STAT1 and STAT2[123] (Table 1).
| HCV protein | Target cells | Functional change | Mechanism | Ref. |
| HCV core and NS3 | mDCs | Impaired maturation | Increased IL-10 and decreased IL-12 production | [64] |
| Impaired T-cell stimulation | ||||
| E2 | mDCs | Alter DC migratory behavior | Interacts with CD81 | [120] |
| pDCs | Inhibited maturation | [121] | ||
| Impaired activation | ||||
| Decreased IFN-α production | ||||
| E2 andNS3 | mDCs | Impaired IFN-λ production | Not shown | [113] |
| NS4 | mDCs | Th1 cytokine reduction | Not shown | [122] |
| T-cell stimulatory impairment | ||||
| NS5A | mDCs | Increased IL-8 production | Not shown | [123] |
| Impaired interferon signaling | Influence the phosphorylation of STAT1 and STAT2 |
On the other hand, a number of studies failed to find defects in DCs during HCV infection[124-128]. It was reported that both HCV patients and chimpanzees infected with HCV harbor phenotypic and functional intact mDCs and pDCs[124,125]. DCs (both pDCs and monocyte-derived DCs) from healthy donors and HCV patients show comparable functions[127]. These discrepancies can be attributed to the inhomogeneous disease state of the patient cohorts, technicalities in methods used for DC purification, stimuli used to induce maturation, and the evaluation of discrepant effector functions.
Fundamental functions of macrophages after HCV infection: The number of proinflammatory macrophages is increased significantly in HCV-infected livers, highlighting the importance of macrophages in HCV infection[129-131]. This increase is dependent on the proliferation of resident KCs and recruitment of monocytes[129]. Macrophages express TRAIL, Fas-ligand, granzyme B, perforin, and reactive oxygen species, which cause direct cytotoxicity to the infected hepatocytes[132,133]. Furthermore, macrophage-derived IL-6 and IL-1β can inhibit HCV replication[134,135]. Moreover, TLR3 and TLR4 ligands can activate KCs to secrete IFN-β, therefore restricting HCV replication[136]. This observation is in agreement with the results obtained by our group. We isolated KCs from living donor allografts and stimulated them with TLR ligands and/or HCVc. Indeed, we observed that TLR3 induced KCs to secrete type I IFNs, and this effect was blocked by HCVc[133]. Additionally, KCs were reported to produce TGF-β, IL-10, Gal-9, PD-L1, and PD-L2 during CHC, which suppresses the antiviral functions of T cells[133,137-139].
HCV infection can influence the macrophage phenotype: Burgio et al[140] observed that the immunophenotypes of KCs can change during HCV infection. The expression of CD80, CD40, and MHC-II was aberrantly regulated during HCV infection. Those KCs form clusters with T cells (mostly CD4+) in the livers from HCV-infected patients. In contrast, in healthy livers, the KC-T cell clusters are scarce and the T cells are mostly CD8+. Taken together, these results indicate that HCV infection can change the phenotype of KCs from efficient antigen endocytic cells to professional antigen-presenting cells[140]. Additionally, the HCV E2 protein can polarize monocyte-derived macrophages to the M2 phenotype by enhancing STAT3 and inhibiting STAT1 activation[141]. In our group, we observed that HCVc can also affect the differentiation states of cells from monocytes to macrophages. Both M1 and M2 polarization are inhibited in a TLR2-dependent manner[142].
Role of macrophages in mediating HCV-associated inflammation: HCV proteins and RNA can activate macrophages, leading to the production of proinflammatory cytokines such as IL-1β, IL-6, IL-18, and TNF-α[62,63,133,143]. It is noteworthy that upon macrophage activation with HCV viral particles, the response is proinflammatory rather than antiviral[57]. This could be attributed to the polyU/UC sequences of HCV RNA, which activate the NLRP3 inflammasome of macrophages. Additionally, macrophage-derived TNF-α was reported to promote HCV entry into polarized hepatoma cells[144]. In HCV-infected patients, LPS can induce significantly high levels of TNF-α, because macrophages of HCV-infected patients are deprived of TLR-tolerance[71,130]. The combination of increased TNF-α production along with the enhanced HCV entry may represent an important mechanism by which macrophages enhance HCV infection and infection-associated inflammation (Figure 2).
Macrophages play an important role in HCV-associated liver fibrosis and/or cirrhosis: Progressive fibrosis and/or cirrhosis is a characteristic of CHC, and macrophages play an important role in this process[145]. In CHC, the role of macrophages in fibrosis is mediated by the pro-inflammatory cytokines IL-1β and TNF-α, which have a well-established pro-fibrotic function[146-149]. Additionally, conditioned medium from HCV-exposed macrophages can modulate the primary human hepatic stellate cells (HSC) and LX2 cell line. CCL5 derived from macrophages activates HSCs, leading to the increased expression of inflammatory and profibrogenic markers such as NLRP3, IL-1β, IL-6, CCL5, TGFβ1, COL4A1, matrix metalloproteinase 2 (MMP2), and α-smooth muscle actin (SMA)[150].
HCV-infected patients have elevated serum levels of macrophage colony-stimulating factor (M-CSF) and IL-34[151], and these proteins are intensely expressed around the liver lesions. In vitro, hepatocytes produce IL-34, M-CSF, and inflammatory cytokines in response to HCV infection[151]. IL-34 and M-CSF promote the differentiation of monocytes into macrophages and endow the macrophages with profibrotic properties[151]. These profibrotic macrophages recruit monocytes to the liver and activate HSCs via platelet-derived growth factor, TGF-β, and galectin-3[151].
Components of the MPS have redundant but non-identical roles in HCV infection. Monocytes act as progenitors for DCs as well as macrophages, and they play an important role in blunting the immune system by secreting large amounts of IL-10 and decreasing IL-12 production. Altered TLR signaling is the most probable cause for abnormal cytokine production in HCV infection. Results from studies examining the impairment of DCs during HCV infection are still controversial. In this review, we adopt the argument that mDCs show a reduced ability to stimulate T cells, whereas pDCs produce decreased amounts of IFN-α in HCV infection. However, a definitive conclusion requires further investigation. Macrophages are a double-edged sword in HCV infection, with both beneficial and detrimental effects. Macrophage-derived proinflammatory cytokines can control the viral spread in acute infection. However, if HCV infection is not controlled, these proinflammatory cytokines contribute to persistent inflammation and complications, including fibrosis and cirrhosis. Persistent inflammation is a characteristic of HCV infection, and thus, the differentiation of monocytes into DCs and macrophages should happen frequently. Will the impairments of the precursor monocytes be inherited by DCs and macrophages? Or will those impairments be reversed during differentiation? These questions remain to be investigated.
The majority of previous studies focused on only one component of the MPS, and thus, data on the interplay and cooperation between MPS components are scarce[98,152,153]. For instance, the recruitment of DCs to the liver requires KCs and the majority of the recruited DCs bind to KCs. This DC-KC binding is indispensable, because KC depletion leads to the inhibition of DC migration to the liver[152]. Furthermore, monocytes produce IL-10 and TNF-α, leading to the apoptosis of pDCs and consequently inhibiting the production of IFN-α by pDCs[153]. Additionally, pDC-derived IFN-α and monocyte-derived IL-15 work together to maximize the IFN-γ induction by NK cells and NKT cells during HCV infection[98]. Other forms of interplay and cooperation involving the MPS remain to be analyzed.
In this review, we describe the impact of HCV infection on each population of the MPS. As a precursor of DCs and macrophages, monocytes are the major contributors to the regulation of the immune system following HCV infection. Monocytes produce high levels of IL-10 and low levels of IL-12, which leads to a blunted microenvironment. On the other hand, DCs demonstrate an impaired ability to stimulate T cells that inhibit efficient anti-HCV T-cell function. As tissue-resident cells, macrophages are tightly associated with HCV-induced inflammation and cirrhosis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boonstra A, Larrea S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Yin SY
| 1. | Douam F, Lavillette D, Cosset FL. The mechanism of HCV entry into host cells. Prog Mol Biol Transl Sci. 2015;129:63-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Szabó E, Lotz G, Páska C, Kiss A, Schaff Z. Viral hepatitis: new data on hepatitis C infection. Pathol Oncol Res. 2003;9:215-221. [PubMed] |
| 3. | Halliday J, Klenerman P, Barnes E. Vaccination for hepatitis C virus: closing in on an evasive target. Expert Rev Vaccines. 2011;10:659-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Pybus OG, Barnes E, Taggart R, Lemey P, Markov PV, Rasachak B, Syhavong B, Phetsouvanah R, Sheridan I, Humphreys IS. Genetic history of hepatitis C virus in East Asia. J Virol. 2009;83:1071-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Gray RR, Salemi M, Klenerman P, Pybus OG. A new evolutionary model for hepatitis C virus chronic infection. PLoS Pathog. 2012;8:e1002656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Thomson EC, Smith JA, Klenerman P. The natural history of early hepatitis C virus evolution; lessons from a global outbreak in human immunodeficiency virus-1-infected individuals. J Gen Virol. 2011;92:2227-2236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Loomba R, Rivera MM, McBurney R, Park Y, Haynes-Williams V, Rehermann B, Alter HJ, Herrine SK, Liang TJ, Hoofnagle JH. The natural history of acute hepatitis C: clinical presentation, laboratory findings and treatment outcomes. Aliment Pharmacol Ther. 2011;33:559-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Thomson EC, Fleming VM, Main J, Klenerman P, Weber J, Eliahoo J, Smith J, McClure MO, Karayiannis P. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut. 2011;60:837-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Zaltron S, Spinetti A, Biasi L, Baiguera C, Castelli F. Chronic HCV infection: epidemiological and clinical relevance. BMC Infect Dis. 2012;12 Suppl 2:S2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385:1124-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 361] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 11. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 12. | Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 13. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1862] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 14. | Jenkins SJ, Hume DA. Homeostasis in the mononuclear phagocyte system. Trends Immunol. 2014;35:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2014] [Cited by in RCA: 2427] [Article Influence: 202.3] [Reference Citation Analysis (0)] |
| 16. | Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 491] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 17. | Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1145] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 18. | Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 581] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 19. | Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 694] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 20. | Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1305] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 21. | Geissmann F. The origin of dendritic cells. Nat Immunol. 2007;8:558-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 496] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 23. | Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 887] [Cited by in RCA: 849] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 24. | Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1265] [Cited by in RCA: 1188] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 25. | Strauss O, Dunbar PR, Bartlett A, Phillips A. The immunophenotype of antigen presenting cells of the mononuclear phagocyte system in normal human liver--a systematic review. J Hepatol. 2015;62:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140:22-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 355] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 27. | Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, Gonen M, Young JW, DeMatteo RP. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol. 2009;182:1901-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Goddard S, Youster J, Morgan E, Adams DH. Interleukin-10 secretion differentiates dendritic cells from human liver and skin. Am J Pathol. 2004;164:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2721] [Cited by in RCA: 2548] [Article Influence: 169.9] [Reference Citation Analysis (0)] |
| 30. | Cabillic F, Rougier N, Basset C, Lecouillard I, Quelvennec E, Toujas L, Guguen-Guillouzo C, Corlu A. Hepatic environment elicits monocyte differentiation into a dendritic cell subset directing Th2 response. J Hepatol. 2006;44:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Tomiyama C, Watanabe H, Izutsu Y, Watanabe M, Abo T. Suppressive role of hepatic dendritic cells in concanavalin A-induced hepatitis. Clin Exp Immunol. 2011;166:258-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3468] [Article Influence: 289.0] [Reference Citation Analysis (0)] |
| 33. | Kwekkeboom J, Kuijpers MA, Bruyneel B, Mancham S, De Baar-Heesakkers E, Ijzermans JN, Bouma GJ, Zondervan PE, Tilanus HW, Metselaar HJ. Expression of CD80 on Kupffer cells is enhanced in cadaveric liver transplants. Clin Exp Immunol. 2003;132:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Guo S, Yang C, Mei F, Wu S, Luo N, Fei L, Chen Y, Wu Y. Down-regulation of Z39Ig on macrophages by IFN-gamma in patients with chronic HBV infection. Clin Immunol. 2010;136:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1215] [Cited by in RCA: 1563] [Article Influence: 130.3] [Reference Citation Analysis (0)] |
| 36. | Wahid B, Ali A, Rafique S, Saleem K, Waqar M, Wasim M, Idrees M. Role of altered immune cells in liver diseases: a review. Gastroenterol Hepatol. 2018;41:377-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 1004] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 38. | Le Bail B, Bioulac-Sage P, Senuita R, Quinton A, Saric J, Balabaud C. Fine structure of hepatic sinusoids and sinusoidal cells in disease. J Electron Microsc Tech. 1990;14:257-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 40. | Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1092] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 41. | Stijlemans B, De Baetselier P, Magez S, Van Ginderachter JA, De Trez C. African Trypanosomiasis-Associated Anemia: The Contribution of the Interplay between Parasites and the Mononuclear Phagocyte System. Front Immunol. 2018;9:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Pahari S, Kaur G, Negi S, Aqdas M, Das DK, Bashir H, Singh S, Nagare M, Khan J, Agrewala JN. Reinforcing the Functionality of Mononuclear Phagocyte System to Control Tuberculosis. Front Immunol. 2018;9:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Perry VH, Lawson LJ, Reid DM. Biology of the mononuclear phagocyte system of the central nervous system and HIV infection. J Leukoc Biol. 1994;56:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Roy S, Wainberg MA. Role of the mononuclear phagocyte system in the development of acquired immunodeficiency syndrome (AIDS). J Leukoc Biol. 1988;43:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Bohmwald K, Espinoza JA, Pulgar RA, Jara EL, Kalergis AM. Functional Impairment of Mononuclear Phagocyte System by the Human Respiratory Syncytial Virus. Front Immunol. 2017;8:1643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Sumpter R Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M Jr. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 724] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 47. | Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 588] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 48. | Uzri D, Gehrke L. Nucleotide sequences and modifications that determine RIG-I/RNA binding and signaling activities. J Virol. 2009;83:4174-4184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 49. | Andrus L, Marukian S, Jones CT, Catanese MT, Sheahan TP, Schoggins JW, Barry WT, Dustin LB, Trehan K, Ploss A. Expression of paramyxovirus V proteins promotes replication and spread of hepatitis C virus in cultures of primary human fetal liver cells. Hepatology. 2011;54:1901-1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, García-Sastre A, Katze MG. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 830] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 51. | Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1112] [Cited by in RCA: 1245] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 52. | Pichlmair A, Schulz O, Tan CP, Rehwinkel J, Kato H, Takeuchi O, Akira S, Way M, Schiavo G, Reis e Sousa C. Activation of MDA5 requires higher-order RNA structures generated during virus infection. J Virol. 2009;83:10761-10769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 366] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 53. | Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M Jr. Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol. 2008;82:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 54. | Wang N, Liang Y, Devaraj S, Wang J, Lemon SM, Li K. Toll-like receptor 3 mediates establishment of an antiviral state against hepatitis C virus in hepatoma cells. J Virol. 2009;83:9824-9834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 55. | Dansako H, Yamane D, Welsch C, McGivern DR, Hu F, Kato N, Lemon SM. Class A scavenger receptor 1 (MSR1) restricts hepatitis C virus replication by mediating toll-like receptor 3 recognition of viral RNAs produced in neighboring cells. PLoS Pathog. 2013;9:e1003345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Zhang YL, Guo YJ, Bin Li, Sun SH. Hepatitis C virus single-stranded RNA induces innate immunity via Toll-like receptor 7. J Hepatol. 2009;51:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Zhang Y, El-Far M, Dupuy FP, Abdel-Hakeem MS, He Z, Procopio FA, Shi Y, Haddad EK, Ancuta P, Sekaly RP. HCV RNA Activates APCs via TLR7/TLR8 While Virus Selectively Stimulates Macrophages Without Inducing Antiviral Responses. Sci Rep. 2016;6:29447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, Szabo G. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 59. | Ferreon JC, Ferreon AC, Li K, Lemon SM. Molecular determinants of TRIF proteolysis mediated by the hepatitis C virus NS3/4A protease. J Biol Chem. 2005;280:20483-20492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 362] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 61. | Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, Chisari FV. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA. 2010;107:7431-7436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 62. | Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 63. | Shrivastava S, Mukherjee A, Ray R, Ray RB. Hepatitis C virus induces interleukin-1β (IL-1β)/IL-18 in circulatory and resident liver macrophages. J Virol. 2013;87:12284-12290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 64. | Dolganiuc A, Kodys K, Kopasz A, Marshall C, Do T, Romics L Jr, Mandrekar P, Zapp M, Szabo G. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170:5615-5624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 66. | Lee CM, Hu TH, Lu SN, Wang JH, Hung CH, Chen CH, Yen YH. Peripheral blood toll-like receptor 4 correlates with rapid virological response to pegylated-interferon and ribavirin therapy in hepatitis C genotype 1 patients. BMC Gastroenterol. 2016;16:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Riordan SM, Skinner NA, Kurtovic J, Locarnini S, McIver CJ, Williams R, Visvanathan K. Toll-like receptor expression in chronic hepatitis C: correlation with pro-inflammatory cytokine levels and liver injury. Inflamm Res. 2006;55:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Wang JP, Zhang Y, Wei X, Li J, Nan XP, Yu HT, Li Y, Wang PZ, Bai XF. Circulating Toll-like receptor (TLR) 2, TLR4, and regulatory T cells in patients with chronic hepatitis C. APMIS. 2010;118:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Sato K, Ishikawa T, Okumura A, Yamauchi T, Sato S, Ayada M, Matsumoto E, Hotta N, Oohashi T, Fukuzawa Y. Expression of Toll-like receptors in chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:1627-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 70. | Villacres MC, Literat O, DeGiacomo M, Du W, Frederick T, Kovacs A. Defective response to Toll-like receptor 3 and 4 ligands by activated monocytes in chronic hepatitis C virus infection. J Viral Hepat. 2008;15:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627-1636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 72. | Chung H, Watanabe T, Kudo M, Chiba T. Hepatitis C virus core protein induces homotolerance and cross-tolerance to Toll-like receptor ligands by activation of Toll-like receptor 2. J Infect Dis. 2010;202:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Chung H, Watanabe T, Kudo M, Chiba T. Correlation between hyporesponsiveness to Toll-like receptor ligands and liver dysfunction in patients with chronic hepatitis C virus infection. J Viral Hepat. 2011;18:e561-e567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Pang X, Wang Z, Zhai N, Zhang Q, Song H, Zhang Y, Li T, Li H, Su L, Niu J. IL-10 plays a central regulatory role in the cytokines induced by hepatitis C virus core protein and polyinosinic acid:polycytodylic acid. Int Immunopharmacol. 2016;38:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Niesen E, Schmidt J, Flecken T, Thimme R. Suppressive effect of interleukin 10 on priming of naive hepatitis C virus-specific CD8+ T cells. J Infect Dis. 2015;211:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Liu BS, Groothuismink ZM, Janssen HL, Boonstra A. Role for IL-10 in inducing functional impairment of monocytes upon TLR4 ligation in patients with chronic HCV infections. J Leukoc Biol. 2011;89:981-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Amaraa R, Mareckova H, Urbanek P, Fucikova T. Production of interleukins 10 and 12 by activated peripheral blood monocytes/macrophages in patients suffering from chronic hepatitis C virus infection with respect to the response to interferon and ribavirin treatment. Immunol Lett. 2002;83:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Woitas RP, Petersen U, Moshage D, Brackmann HH, Matz B, Sauerbruch T, Spengler U. HCV-specific cytokine induction in monocytes of patients with different outcomes of hepatitis C. World J Gastroenterol. 2002;8:562-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Flynn JK, Dore GJ, Hellard M, Yeung B, Rawlinson WD, White PA, Kaldor JM, Lloyd AR, Ffrench RA; ATAHC Study Group. Early IL-10 predominant responses are associated with progression to chronic hepatitis C virus infection in injecting drug users. J Viral Hepat. 2011;18:549-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Martin-Blondel G, Gales A, Bernad J, Cuzin L, Delobel P, Barange K, Izopet J, Pipy B, Alric L. Low interleukin-10 production by monocytes of patients with a self-limiting hepatitis C virus infection. J Viral Hepat. 2009;16:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Brady MT, MacDonald AJ, Rowan AG, Mills KH. Hepatitis C virus non-structural protein 4 suppresses Th1 responses by stimulating IL-10 production from monocytes. Eur J Immunol. 2003;33:3448-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Rowan AG, Fletcher JM, Ryan EJ, Moran B, Hegarty JE, O'Farrelly C, Mills KH. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-beta. J Immunol. 2008;181:4485-4494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 83. | Liu BS, Janssen HL, Boonstra A. Type I and III interferons enhance IL-10R expression on human monocytes and macrophages, resulting in IL-10-mediated suppression of TLR-induced IL-12. Eur J Immunol. 2012;42:2431-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 84. | Chamoto K, Al-Habsi M, Honjo T. Role of PD-1 in Immunity and Diseases. Curr Top Microbiol Immunol. 2017;410:75-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 85. | Jeong HY, Lee YJ, Seo SK, Lee SW, Park SJ, Lee JN, Sohn HS, Yao S, Chen L, Choi I. Blocking of monocyte-associated B7-H1 (CD274) enhances HCV-specific T cell immunity in chronic hepatitis C infection. J Leukoc Biol. 2008;83:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Ma CJ, Ni L, Zhang Y, Zhang CL, Wu XY, Atia AN, Thayer P, Moorman JP, Yao ZQ. PD-1 negatively regulates interleukin-12 expression by limiting STAT-1 phosphorylation in monocytes/macrophages during chronic hepatitis C virus infection. Immunology. 2011;132:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 87. | Zhang Y, Ma CJ, Ni L, Zhang CL, Wu XY, Kumaraguru U, Li CF, Moorman JP, Yao ZQ. Cross-talk between programmed death-1 and suppressor of cytokine signaling-1 in inhibition of IL-12 production by monocytes/macrophages in hepatitis C virus infection. J Immunol. 2011;186:3093-3103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 88. | Harwood NM, Golden-Mason L, Cheng L, Rosen HR, Mengshol JA. HCV-infected cells and differentiation increase monocyte immunoregulatory galectin-9 production. J Leukoc Biol. 2016;99:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 89. | Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Jia ZS, Moorman JP, Yao ZQ. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PLoS One. 2011;6:e19664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 90. | Yi W, Zhang P, Liang Y, Zhou Y, Shen H, Fan C, Moorman JP, Yao ZQ, Jia Z, Zhang Y. T-bet-mediated Tim-3 expression dampens monocyte function during chronic hepatitis C virus infection. Immunology. 2017;150:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Wang JM, Shi L, Ma CJ, Ji XJ, Ying RS, Wu XY, Wang KS, Li G, Moorman JP, Yao ZQ. Differential regulation of interleukin-12 (IL-12)/IL-23 by Tim-3 drives T(H)17 cell development during hepatitis C virus infection. J Virol. 2013;87:4372-4383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Kakumu S, Okumura A, Ishikawa T, Iwata K, Yano M, Yoshioka K. Production of interleukins 10 and 12 by peripheral blood mononuclear cells (PBMC) in chronic hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;108:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Sène D, Levasseur F, Abel M, Lambert M, Camous X, Hernandez C, Pène V, Rosenberg AR, Jouvin-Marche E, Marche PN. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog. 2010;6:e1001184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 94. | Mele D, Mantovani S, Oliviero B, Grossi G, Lombardi A, Mondelli MU, Varchetta S. Monocytes inhibit hepatitis C virus-induced TRAIL expression on CD56bright NK cells. J Hepatol. 2017;67:1148-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Nishio A, Tatsumi T, Nawa T, Suda T, Yoshioka T, Onishi Y, Aono S, Shigekawa M, Hikita H, Sakamori R. CD14+ monocyte-derived galectin-9 induces natural killer cell cytotoxicity in chronic hepatitis C. Hepatology. 2017;65:18-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Nakamoto Y, Kaneko S, Kobayashi K. Monocyte-dependent cell death of T lymphocyte subsets in chronic hepatitis C. Immunol Lett. 2001;78:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Zhang JY, Wu XL, Yang B, Wang Y, Feng GH, Jiang TJ, Zeng QL, Xu XS, Li YY, Jin L. Upregulation of OX40 ligand on monocytes contributes to early virological control in patients with chronic hepatitis C. Eur J Immunol. 2013;43:1953-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Zhang S, Saha B, Kodys K, Szabo G. IFN-γ production by human natural killer cells in response to HCV-infected hepatoma cells is dependent on accessory cells. J Hepatol. 2013;59:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 99. | Zabaleta A, Riezu-Boj JI, Larrea E, Villanueva L, Lasarte JJ, Guruceaga E, Fisicaro P, Ezzikouri S, Missale G, Ferrari C. Gene expression analysis during acute hepatitis C virus infection associates dendritic cell activation with viral clearance. J Med Virol. 2016;88:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 100. | Bain C, Fatmi A, Zoulim F, Zarski JP, Trépo C, Inchauspé G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 101. | Ryan EJ, Stevenson NJ, Hegarty JE, O'Farrelly C. Chronic hepatitis C infection blocks the ability of dendritic cells to secrete IFN-α and stimulate T-cell proliferation. J Viral Hepat. 2011;18:840-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584-5591. [PubMed] |
| 103. | Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171-3176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 104. | Sarobe P, Lasarte JJ, Casares N, López-Díaz de Cerio A, Baixeras E, Labarga P, García N, Borrás-Cuesta F, Prieto J. Abnormal priming of CD4(+) T cells by dendritic cells expressing hepatitis C virus core and E1 proteins. J Virol. 2002;76:5062-5070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 105. | Tsubouchi E, Akbar SM, Horiike N, Onji M. Infection and dysfunction of circulating blood dendritic cells and their subsets in chronic hepatitis C virus infection. J Gastroenterol. 2004;39:754-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Wertheimer AM, Bakke A, Rosen HR. Direct enumeration and functional assessment of circulating dendritic cells in patients with liver disease. Hepatology. 2004;40:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007;123:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 108. | Della Bella S, Crosignani A, Riva A, Presicce P, Benetti A, Longhi R, Podda M, Villa ML. Decrease and dysfunction of dendritic cells correlate with impaired hepatitis C virus-specific CD4+ T-cell proliferation in patients with hepatitis C virus infection. Immunology. 2007;121:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 109. | Mengshol JA, Golden-Mason L, Castelblanco N, Im KA, Dillon SM, Wilson CC, Rosen HR; Virahep-C Study Group. Impaired plasmacytoid dendritic cell maturation and differential chemotaxis in chronic hepatitis C virus: associations with antiviral treatment outcomes. Gut. 2009;58:964-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 110. | Kanto T, Inoue M, Miyatake H, Sato A, Sakakibara M, Yakushijin T, Oki C, Itose I, Hiramatsu N, Takehara T. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 111. | Schulz S, Landi A, Garg R, Wilson JA, van Drunen Littel-van den Hurk S. Indolamine 2,3-dioxygenase expression by monocytes and dendritic cell populations in hepatitis C patients. Clin Exp Immunol. 2015;180:484-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 112. | Lai WK, Curbishley SM, Goddard S, Alabraba E, Shaw J, Youster J, McKeating J, Adams DH. Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J Hepatol. 2007;47:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 113. | Langhans B, Kupfer B, Braunschweiger I, Arndt S, Schulte W, Nischalke HD, Nattermann J, Oldenburg J, Sauerbruch T, Spengler U. Interferon-lambda serum levels in hepatitis C. J Hepatol. 2011;54:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 114. | Ciesek S, Liermann H, Hadem J, Greten T, Tillmann HL, Cornberg M, Aslan N, Manns MP, Wedemeyer H. Impaired TRAIL-dependent cytotoxicity of CD1c-positive dendritic cells in chronic hepatitis C virus infection. J Viral Hepat. 2008;15:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Zhao L, Tyrrell DL. Myeloid dendritic cells can kill T cells during chronic hepatitis C virus infection. Viral Immunol. 2013;26:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 116. | Fouad H, Raziky MS, Aziz RA, Sabry D, Aziz GM, Ewais M, Sayed AR. Dendritic cell co-stimulatory and co-inhibitory markers in chronic HCV: an Egyptian study. World J Gastroenterol. 2013;19:7711-7718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 117. | Shen T, Chen X, Chen Y, Xu Q, Lu F, Liu S. Increased PD-L1 expression and PD-L1/CD86 ratio on dendritic cells were associated with impaired dendritic cells function in HCV infection. J Med Virol. 2010;82:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 118. | Krishnadas DK, Ahn JS, Han J, Kumar R, Agrawal B. Immunomodulation by hepatitis C virus-derived proteins: targeting human dendritic cells by multiple mechanisms. Int Immunol. 2010;22:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 119. | Waggoner SN, Hall CH, Hahn YS. HCV core protein interaction with gC1q receptor inhibits Th1 differentiation of CD4+ T cells via suppression of dendritic cell IL-12 production. J Leukoc Biol. 2007;82:1407-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 120. | Nattermann J, Zimmermann H, Iwan A, von Lilienfeld-Toal M, Leifeld L, Nischalke HD, Langhans B, Sauerbruch T, Spengler U. Hepatitis C virus E2 and CD81 interaction may be associated with altered trafficking of dendritic cells in chronic hepatitis C. Hepatology. 2006;44:945-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 121. | Tu Z, Zhang P, Li H, Niu J, Jin X, Su L. Cross-linking of CD81 by HCV-E2 protein inhibits human intrahepatic plasmacytoid dendritic cells response to CpG-ODN. Cell Immunol. 2013;284:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 122. | Takaki A, Tatsukawa M, Iwasaki Y, Koike K, Noguchi Y, Shiraha H, Sakaguchi K, Nakayama E, Yamamoto K. Hepatitis C virus NS4 protein impairs the Th1 polarization of immature dendritic cells. J Viral Hepat. 2010;17:555-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 123. | Wertheimer AM, Polyak SJ, Leistikow R, Rosen HR. Engulfment of apoptotic cells expressing HCV proteins leads to differential chemokine expression and STAT signaling in human dendritic cells. Hepatology. 2007;45:1422-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 124. | Larsson M, Babcock E, Grakoui A, Shoukry N, Lauer G, Rice C, Walker C, Bhardwaj N. Lack of phenotypic and functional impairment in dendritic cells from chimpanzees chronically infected with hepatitis C virus. J Virol. 2004;78:6151-6161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 125. | Longman RS, Talal AH, Jacobson IM, Albert ML, Rice CM. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 126. | Longman RS, Talal AH, Jacobson IM, Rice CM, Albert ML. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 127. | Piccioli D, Tavarini S, Nuti S, Colombatto P, Brunetto M, Bonino F, Ciccorossi P, Zorat F, Pozzato G, Comar C. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 128. | Barnes E, Salio M, Cerundolo V, Francesco L, Pardoll D, Klenerman P, Cox A. Monocyte derived dendritic cells retain their functional capacity in patients following infection with hepatitis C virus. J Viral Hepat. 2008;15:219-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 129. | Khakoo SI, Soni PN, Savage K, Brown D, Dhillon AP, Poulter LW, Dusheiko GM. Lymphocyte and macrophage phenotypes in chronic hepatitis C infection. Correlation with disease activity. Am J Pathol. 1997;150:963-970. [PubMed] |
| 130. | Tan-Garcia A, Wai LE, Zheng D, Ceccarello E, Jo J, Banu N, Khakpoor A, Chia A, Tham CYL, Tan AT. Intrahepatic CD206+ macrophages contribute to inflammation in advanced viral-related liver disease. J Hepatol. 2017;67:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 131. | McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut. 2000;46:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 132. | Tordjmann T, Soulie A, Guettier C, Schmidt M, Berthou C, Beaugrand M, Sasportes M. Perforin and granzyme B lytic protein expression during chronic viral and autoimmune hepatitis. Liver. 1998;18:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 133. | Tu Z, Pierce RH, Kurtis J, Kuroki Y, Crispe IN, Orloff MS. Hepatitis C virus core protein subverts the antiviral activities of human Kupffer cells. Gastroenterology. 2010;138:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 134. | Zhu H, Liu C. Interleukin-1 inhibits hepatitis C virus subgenomic RNA replication by activation of extracellular regulated kinase pathway. J Virol. 2003;77:5493-5498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 135. | Zhu H, Shang X, Terada N, Liu C. STAT3 induces anti-hepatitis C viral activity in liver cells. Biochem Biophys Res Commun. 2004;324:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 136. | Broering R, Wu J, Meng Z, Hilgard P, Lu M, Trippler M, Szczeponek A, Gerken G, Schlaak JF. Toll-like receptor-stimulated non-parenchymal liver cells can regulate hepatitis C virus replication. J Hepatol. 2008;48:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 137. | Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13:316-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 410] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 138. | Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5:e9504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 139. | Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, Deeks SG, Liang TJ, Heller T. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220-1230, 1230.e1-1230.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 140. | Burgio VL, Ballardini G, Artini M, Caratozzolo M, Bianchi FB, Levrero M. Expression of co-stimulatory molecules by Kupffer cells in chronic hepatitis of hepatitis C virus etiology. Hepatology. 1998;27:1600-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 141. | Kwon YC, Meyer K, Peng G, Chatterjee S, Hoft DF, Ray R. Hepatitis C virus E2 envelope glycoprotein induces an immunoregulatory phenotype in macrophages. Hepatology. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 142. | Zhang Q, Wang Y, Zhai N, Song H, Li H, Yang Y, Li T, Guo X, Chi B, Niu J. HCV core protein inhibits polarization and activity of both M1 and M2 macrophages through the TLR2 signaling pathway. Sci Rep. 2016;6:36160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 143. | Hosomura N, Kono H, Tsuchiya M, Ishii K, Ogiku M, Matsuda M, Fujii H. HCV-related proteins activate Kupffer cells isolated from human liver tissues. Dig Dis Sci. 2011;56:1057-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 144. | Fletcher NF, Sutaria R, Jo J, Barnes A, Blahova M, Meredith LW, Cosset FL, Curbishley SM, Adams DH, Bertoletti A. Activated macrophages promote hepatitis C virus entry in a tumor necrosis factor-dependent manner. Hepatology. 2014;59:1320-1330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 145. | Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J. 2008;411:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 146. | Mancini R, Benedetti A, Jezequel AM. An interleukin-1 receptor antagonist decreases fibrosis induced by dimethylnitrosamine in rat liver. Virchows Arch. 1994;424:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 147. | Tiggelman AM, Boers W, Linthorst C, Sala M, Chamuleau RA. Collagen synthesis by human liver (myo)fibroblasts in culture: evidence for a regulatory role of IL-1 beta, IL-4, TGF beta and IFN gamma. J Hepatol. 1995;23:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 148. | Han YP, Zhou L, Wang J, Xiong S, Garner WL, French SW, Tsukamoto H. Essential role of matrix metalloproteinases in interleukin-1-induced myofibroblastic activation of hepatic stellate cell in collagen. J Biol Chem. 2004;279:4820-4828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 149. | Simeonova PP, Gallucci RM, Hulderman T, Wilson R, Kommineni C, Rao M, Luster MI. The role of tumor necrosis factor-alpha in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol Appl Pharmacol. 2001;177:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 150. | Sasaki R, Devhare PB, Steele R, Ray R, Ray RB. Hepatitis C virus-induced CCL5 secretion from macrophages activates hepatic stellate cells. Hepatology. 2017;66:746-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 151. | Preisser L, Miot C, Le Guillou-Guillemette H, Beaumont E, Foucher ED, Garo E, Blanchard S, Frémaux I, Croué A, Fouchard I. IL-34 and macrophage colony-stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology. 2014;60:1879-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 152. | Uwatoku R, Suematsu M, Ezaki T, Saiki T, Tsuiji M, Irimura T, Kawada N, Suganuma T, Naito M, Ando M. Kupffer cell-mediated recruitment of rat dendritic cells to the liver: roles of N-acetylgalactosamine-specific sugar receptors. Gastroenterology. 2001;121:1460-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 153. | Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758-6768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |