Published online Jul 14, 2018. doi: 10.3748/wjg.v24.i26.2806
Peer-review started: March 28, 2018
First decision: May 9, 2018
Revised: June 3, 2018
Accepted: June 22, 2018
Article in press: June 22, 2018
Published online: July 14, 2018
Processing time: 107 Days and 9.7 Hours
Gastrointestinal stromal tumors (GISTs) are the most common malignant subepithelial lesions (SELs) of the gastrointestinal tract. They originate from the interstitial cells of Cajal located within the muscle layer and are characterized by over-expression of the tyrosine kinase receptor KIT. Pathologically, diagnosis of a GIST relies on morphology and immunohistochemistry [KIT and/or discovered on gastrointestinal stromal tumor 1 (DOG1) is generally positive]. The prognosis of this disease is associated with the tumor size and mitotic index. The standard treatment of a GIST without metastasis is surgical resection. A GIST with metastasis is usually only treated by tyrosine kinase inhibitors without radical cure; thus, early diagnosis is the only way to improve its prognosis. However, a GIST is usually detected as a SEL during endoscopy, and many benign and malignant conditions may manifest as SELs. Conventional endoscopic biopsy is difficult for tumors without ulceration. Most SELs have therefore been managed without a histological diagnosis. However, a favorable prognosis of a GIST is associated with early histological diagnosis and R0 resection. Endoscopic ultrasonography (EUS) and EUS-guided fine needle aspiration (EUS-FNA) are critical for an accurate diagnosis of SELs. EUS-FNA is safe and effective in enabling an early histological diagnosis and adequate treatment. This review outlines the current evidence for the diagnosis and management of GISTs, with an emphasis on early management of small SELs.
Core tip: Potentially malignant gastrointestinal stromal tumors are the most common subepithelial lesions (SELs) of the gastrointestinal tract. SELs include a broader range of differential diagnoses from benign to malignant lesions. The possibility of having a malignant lesion may cause anxiety and discomfort in patients and gastroenterologists. Early and accurate diagnosis of SELs using endoscopic ultrasonography (EUS) and/or EUS-guided fine needle aspiration is vital to guide selection of early appropriate management.
- Citation: Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 2018; 24(26): 2806-2817
- URL: https://www.wjgnet.com/1007-9327/full/v24/i26/2806.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i26.2806
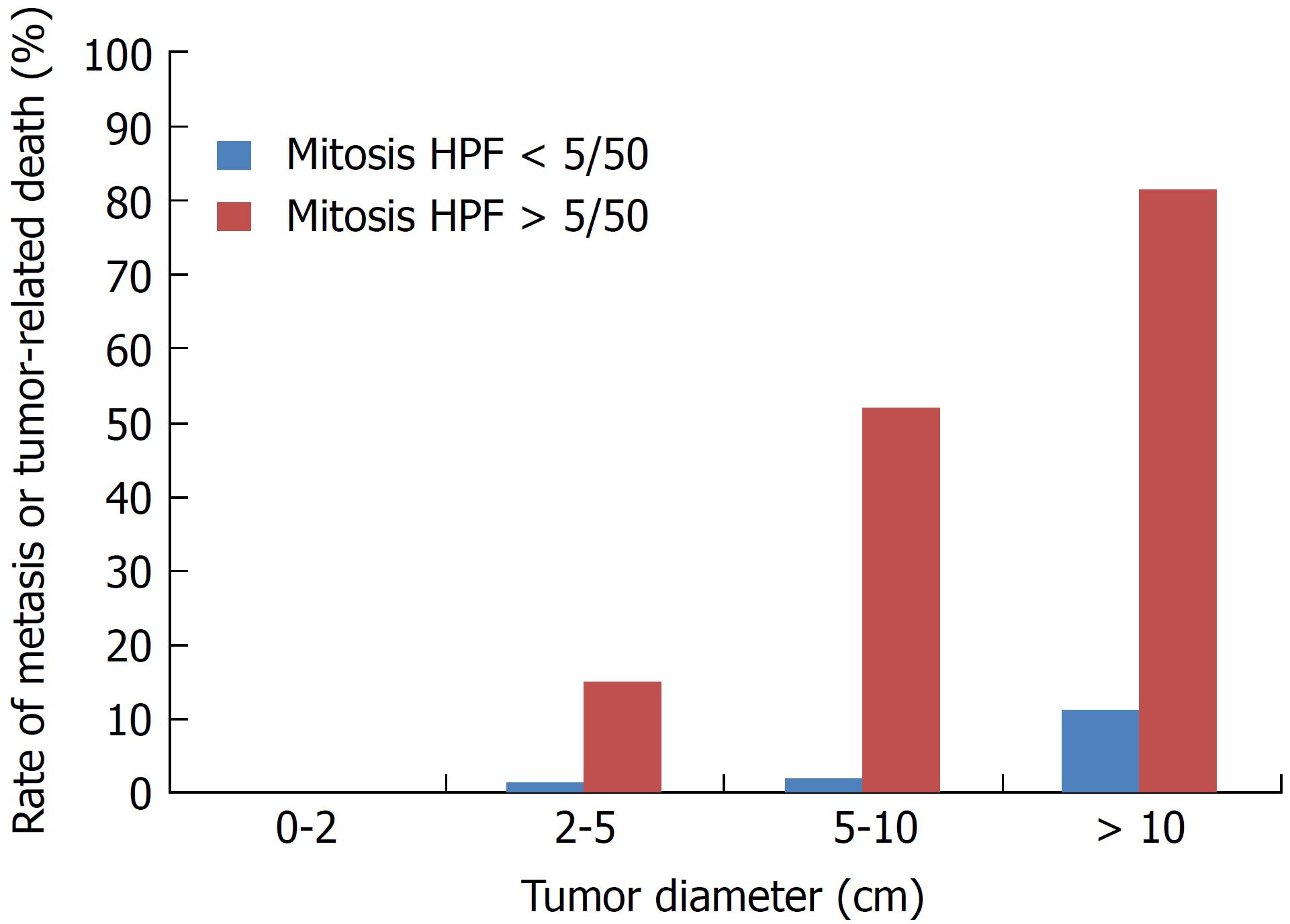
Gastrointestinal stromal tumors (GISTs) are the most common malignant subepithelial lesions (SELs) of the gastrointestinal tract in the daily clinical setting[1,2]. GISTs are thought to originate from the interstitial cells of Cajal, which are the pacemaker cells of gastrointestinal movement[3]. GISTs are largely caused by oncogenic mutations in the tyrosine kinase receptor KIT[4] and/or platelet-derived growth factor receptor-α (PDGFR-α)[5]. Approximately 10% to 30% of GISTs have a malignant clinical course[1,6,7]. Additionally, it has been reported that not only large GISTs with a high mitotic index frequently exhibit a malignant clinical course, but also small GISTs with a low mitotic index rarely show a malignant course with metastasis. Thus, a GIST is considered to be a potentially malignant tumor. GISTs are not classified as either benign or malignant but are rather stratified by their clinical risk of malignancy: Very low, low, intermediate, or high[7]. Mietinenn reported that the metastatic risk of GISTs increases according to the tumor size irrespective of the mitotic count[6] (Figure 1). Surgical resection is the primary approach to management of localized GISTs[8]. Despite complete resection, postoperative recurrence occurs in at least half of all patients with GISTs[2,9]. Although tyrosine kinase inhibitors have been shown to provide sustained disease management in patients with metastasis[10-16], surgical R0 resection of small GISTs without metastasis is the only promising treatment for a permanent cure[8,17]. The best treatment strategy for GISTs is early diagnosis and early resection. However, GISTs are frequently detected as SELs during endoscopy[8,18-20]. The differential diagnoses of SELs are quite broad and can include extra-gastrointestinal tract compression, varices, an ectopic pancreas, and various tumors including GIST, SEL-like cancer, leiomyoma, schwannoma, and lipoma[8,20,21]. GISTs should be diagnosed by immunohistochemical analysis including assessment of KIT, CD34, and/or discovered on gastrointestinal stromal tumor 1 (DOG1)[8,22,23]. However, it is more difficult to obtain a conclusive histologic diagnosis of a GIST than gastrointestinal cancer by standard endoscopic forceps biopsy because a GIST is covered by normal mucosa. Although imaging tests including endoscopic ultrasonography (EUS) and computed tomography (CT) are useful for narrowing down the differential diagnoses of SELs, these techniques are unable to provide a conclusive diagnosis. At present, EUS-guided fine needle aspiration (EUS-FNA) is the most accurate, safe, and reliable preoperative immunohistological test to secure a definitive diagnosis of SELs[8,18,19,23]. Aggressive use of EUS and EUS-FNA for SELs is the key to facilitating early intervention of GISTs[21,23].
This paper provides an overview of the diagnosis and treatment of GISTs, with an emphasis on early diagnosis and management of GISTs using EUS-FNA.
In epidemiological surveys of GISTs, the estimated mean age at diagnosis is in the sixth decade of life, and the frequency of occurrence is 6.8 to 14.5 cases per million individuals per year[24-26]. GISTs most commonly occur in the stomach (51%), followed by the small intestine (36%), colon (7%), rectum (5%), and esophagus (1%)[24].
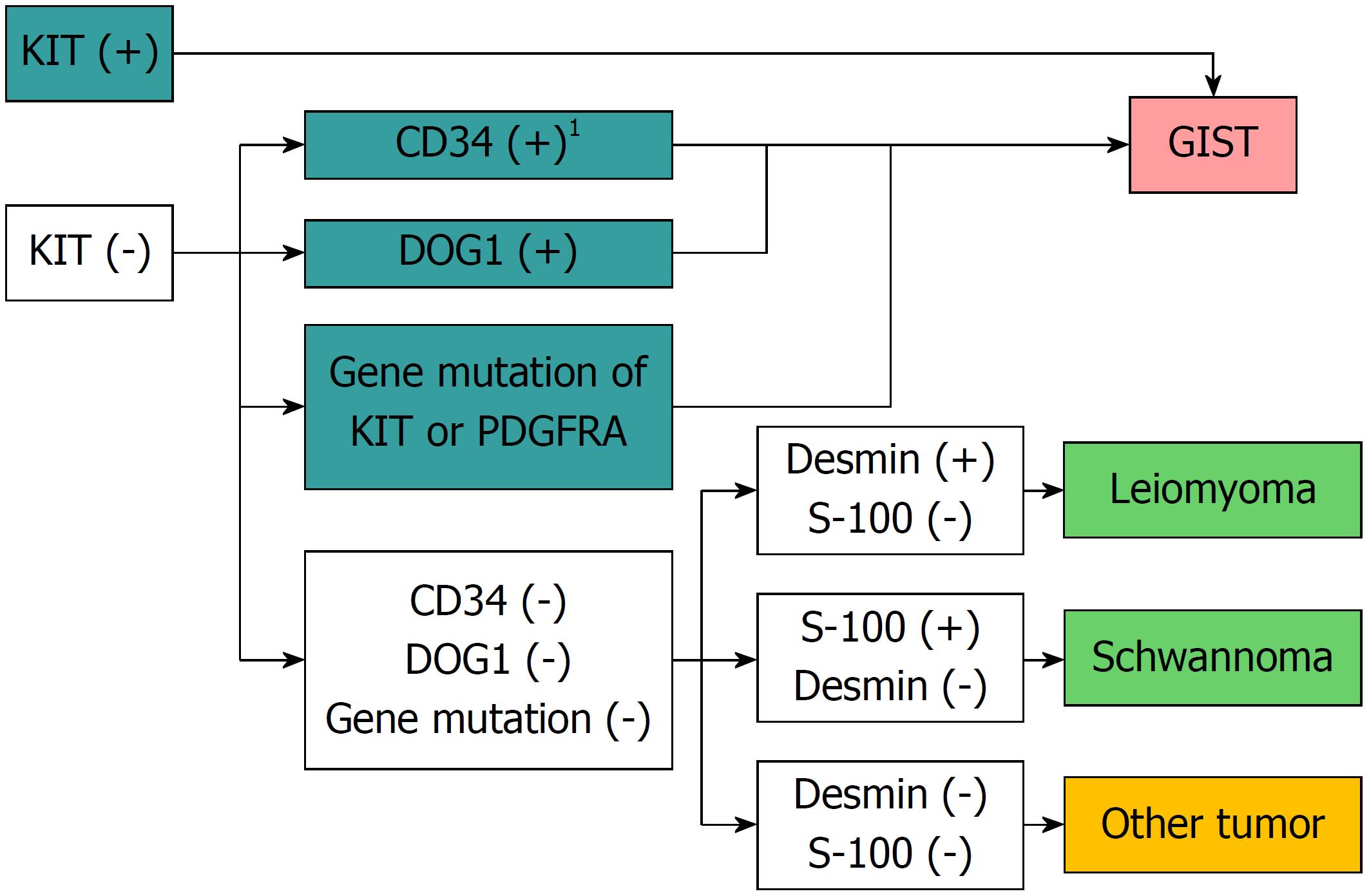
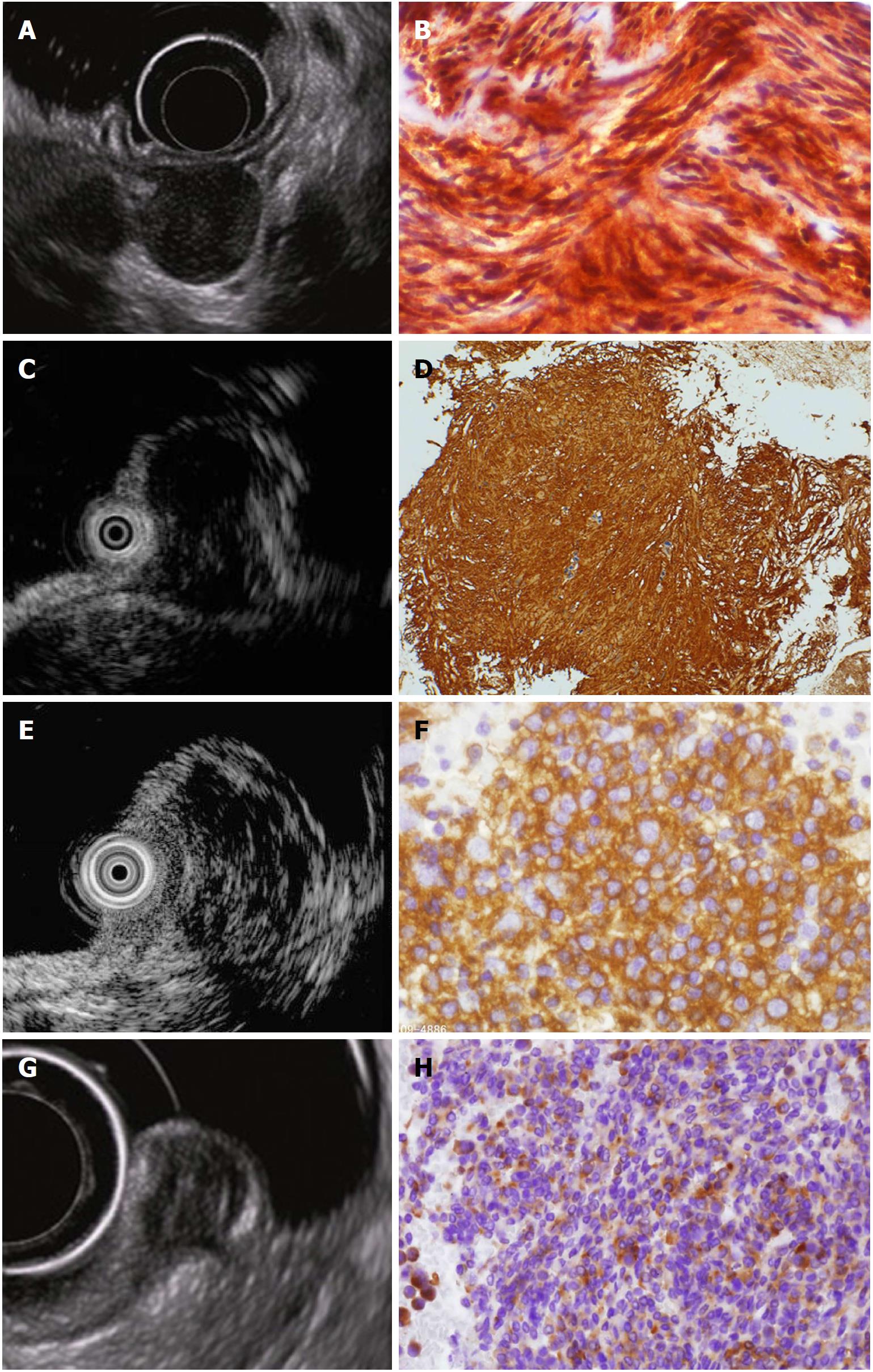
The main morphologic types of GISTs are the spindle-shaped cell type (70%), epithelial cell type (20%), and mixed type (10%)[27]. It is difficult to differentiate between leiomyomas and neurinomas, two other mesenchymal tumors, using only hematoxylin and eosin staining; differentiation using immunostaining is indispensable[8,22,23]. A GIST is diagnosed in the presence of KIT or CD34 positivity. If the tumor is negative for KIT, CD34, desmin, and S-100, additional tests including DOG1 staining or a mutation search of the KIT or PDGFRA gene are useful for diagnosis of GISTs[28] (Figure 2).
The most common symptoms of GISTs are gastrointestinal bleeding, including acute melena and hematemesis with subsequent anemia; weakness; and abdominal pain, distension, and discomfort due to a tumor-induced mass effect[29]. Previous studies have shown that 15% to 30% of patients with GISTs are asymptomatic, and their GISTs are found incidentally during postmortem autopsy or surgery for treatment of other diseases[6,25,30]. Many pathological studies have highlighted the existence of subclinical microscopic or so-called mini (< 1 cm) GISTs[31-36]. Kawanowa et al[31] reported that microscopic GISTs were present in 35% of patients who underwent gastrectomy for treatment of gastric adenocarcinoma. Agaimy et al[32] reported that microscopic gastric GISTs were found in 22.5% of consecutive autopsies of patients aged ≥ 50 years. The reported incidence of mini-GISTs according to the affected organ is 3% to 10% in the stomach, 0.2% in the colon, and 0.01% in the rectum[31-36]. The detection of incidental SELs during gastrointestinal endoscopy has recently increased with the more widespread performance of endoscopic examinations. Gastric SELs are found in 0.36% of middle-aged adults during health examinations, and half of these tumors are considered to be neoplastic[37]. Most gastric SELs found during physical check-ups are small and asymptomatic. GISTs are considered to account for half of these incidentally found SELs in the stomach[31,34]. Based on these studies, GISTs are presumed to be much more common than previously recognized[18].
SELs are frequently found during ordinary optical endoscopy. The main endoscopic finding of GISTs is common to all SELs: A nonspecific smooth bulge covered with normal mucosa[11,21,38] (Figure 3). Therefore, endoscopic examination provides insufficient information for differential diagnosis of SELs. Irregular borders, ulceration, and/or growth during endoscopic follow-up are considered clinically malignant features on endoscopy[36]. GISTs are usually hard and the cushion sign is negative. When GISTs increase in size, ulceration may be seen on the top of the tumor[19].
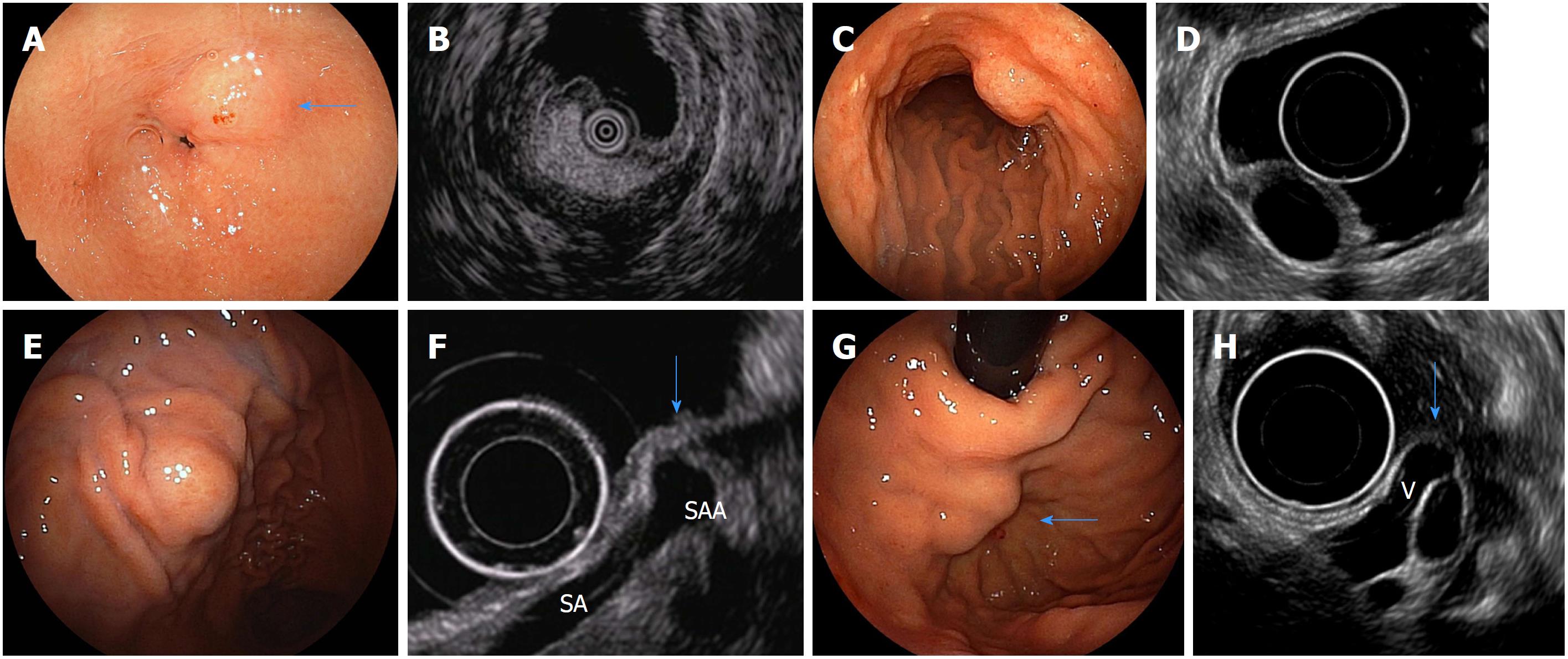
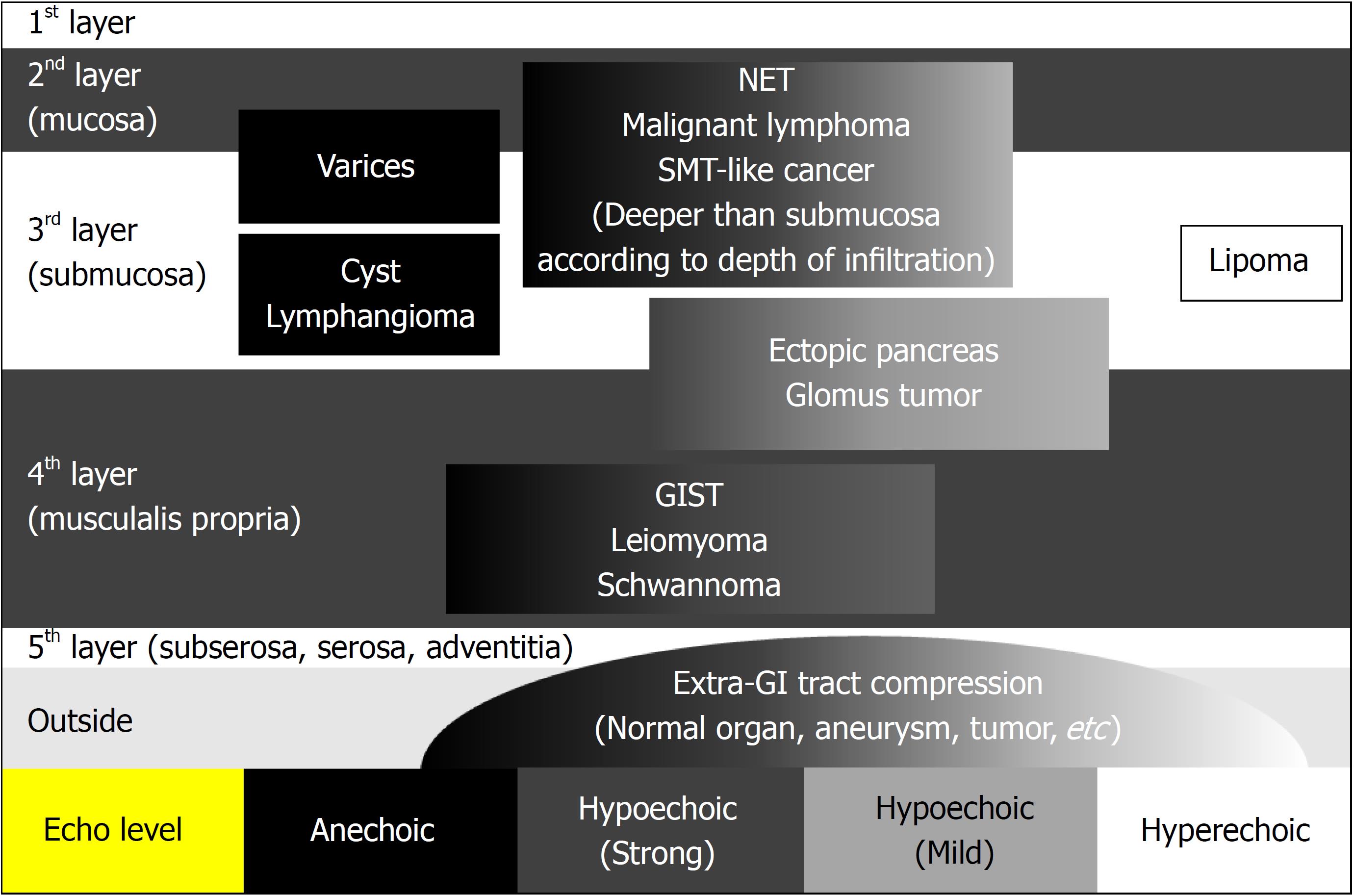
EUS is a key test for differential diagnosis of SELs because it provides high-resolution tomographic imaging using high-frequency ultrasound. EUS provides the following information regarding SELs[39] (Figure 4): The gastrointestinal wall layer from which it originates (within the submucosal layer, in continuity with the muscularis propria, or outside the wall), the nature of the lesion (liquid, fat, solid tumor, or blood vessel), and the true size of the SEL from a cross-sectional image[39]. Thus, EUS is the safest and most useful modality for differential diagnosis and follow-up of SELs[21,40,41]. EUS allows for the conclusive diagnosis of many lesions using echo findings only, such as lipomas (highly echoic masses) (Figure 3A and B), cysts (anechoic masses) (Figure 3C and D), extraluminal compression by surrounding normal organs or lesions[42] (Figure 3E and F), and varices (Figure 3G and H)[21,38,43]. The typical EUS imaging feature of a GIST is a hypoechoic solid mass. EUS can accurately discriminate a SEL suspected to be a GIST (hypoechoic solid mass) from other SELs, including lipomas, cysts, varices, and extra-gastrointestinal compression. According to previous reports, possible high-risk EUS features for GISTs are a size of > 2 cm, irregular borders, heterogeneous echo patterns, anechoic spaces, echogenic foci, and growth during follow-up[44,45]. However, Kim et al[46] reported that tumor size and EUS features cannot be used to preoperatively predict the risk of malignancy of medium-sized (2-5 cm) gastric GISTs. At present, estimation of the risk of malignancy of GISTs of < 5 cm by EUS imaging alone seems to be difficult. The finding of a hypoechoic solid mass by EUS is also seen in malignant tumors such as malignant lymphoma, metastatic cancer, neuroendocrine tumor, and SEL-like cancer and in benign conditions such as leiomyoma, neurinoma, and an aberrant pancreas[21]. It is difficult to distinguish among these lesions using EUS findings only. The accuracy of differential diagnosis of SELs by EUS is extremely poor and ranges from 45.5% to 48.0%[47,48]. Because current EUS imaging characteristics alone provide insufficient accuracy in the diagnosis of GISTs, tissue sampling for immunohistochemical analysis using EUS-FNA or biopsy is required for a definite diagnosis before surgery or chemotherapy[18-21].
Conventional endoscopic forceps biopsy is limited because these forceps usually cannot reach the tumor beyond the overlying normal mucosa and submucosa[49]. When ulceration is present, a biopsy within the ulcer is effective for a conclusive diagnosis[18,49,50]. Although special methods such as “jumbo” or “bite-on-bite” biopsy are available, the diagnostic yield of these approaches is poor, ranging from 17% to 59%[51-53]. Additionally, one study showed that significant bleeding occurred in 35.7% of patients after jumbo biopsy, and 34.9% of patients needed subsequent endoscopic hemostasis[53].
EUS-FNA is the most established tissue sampling method for SELs and can provide a conclusive immunohistochemical diagnosis safely and accurately (Figure 5) (Video 1). Typical EUS-FNA findings of GISTs are KIT- or CD34-positive spindle-shaped cells or epithelial cells. The diagnostic rate of SELs using EUS-FNA ranges from 62.0% to 93.4%[23,54-56]. The diagnostic rate according to tumor diameter is 71% for 1-cm to 2-cm tumors, 86% for 2-cm to 4-cm tumors, and 100% for > 4-cm tumors[23]. The diagnostic rate tends to be higher as the tumor diameter increases. Unfortunately, EUS-FNA for a subepithelial hypoechoic solid mass of < 1 cm is technically difficult using a standard EUS-FNA scope; thus, EUS-FNA is recommended for masses of > 1 cm[56,57]. However, forward-viewing and curved linear-array echoendoscopes [58] and drill needles[59] have recently been developed and are expected to improve the diagnostic rate of small SELs. The rate of adverse events associated with EUS-FNA using a 22-gauge needle is reportedly close to 0[54-56].
Evaluation of mitosis is important to determine the metastatic risk of GISTs. Unfortunately, the tissue sample volume obtained by EUS-FNA is usually small. Therefore, assessment of mitosis by EUS-FNA is difficult. Ando et al[60] reported that the MIB-1 labeling index is accurate (100%) for diagnosis of malignant GISTs because Ki-67-positive cells can be easily recognized in the small specimens obtained by EUS-FNA.
Invasive endoscopic tissue acquisition to obtain a higher tissue volume was recently developed and clinically applied[56-59]. Various endoscopic tissue-obtaining methods using endoscopic submucosal dissection (ESD) techniques or endoscopic snare resection techniques significantly increase the diagnostic yield when compared with standard forceps biopsy; the reported diagnostic rates range from 85% to 94%[61-64]. An additional advantage of these methods is the ability to evaluate the risk classification of GISTs using the mitotic count per 50 high-power fields[65,66]. However, ESD and endoscopic snare resection are invasive procedures; therefore, endoscopists should pay special attention to intraoperative bleeding and perforation while performing these techniques because such complications may cause severe hypotension or tumor cell seeding. Lee et al[63] reported that minor hemorrhage occurred in 56% of patients who underwent endoscopic partial removal with the unroofing technique, but hemostasis was successfully achieved in all patients with argon plasma coagulation, and no perforations occurred. Furthermore, tissue sampling of SELs with an extraluminal growth pattern is difficult[64]. A potential disadvantage of these aggressive endoscopic tissue acquisition techniques using ESD or endoscopic snare resection is the development of perilesional fibrosis, which may render subsequent attempts at submucosal tunneling endoscopic resection[67,68] difficult or even impossible[69].
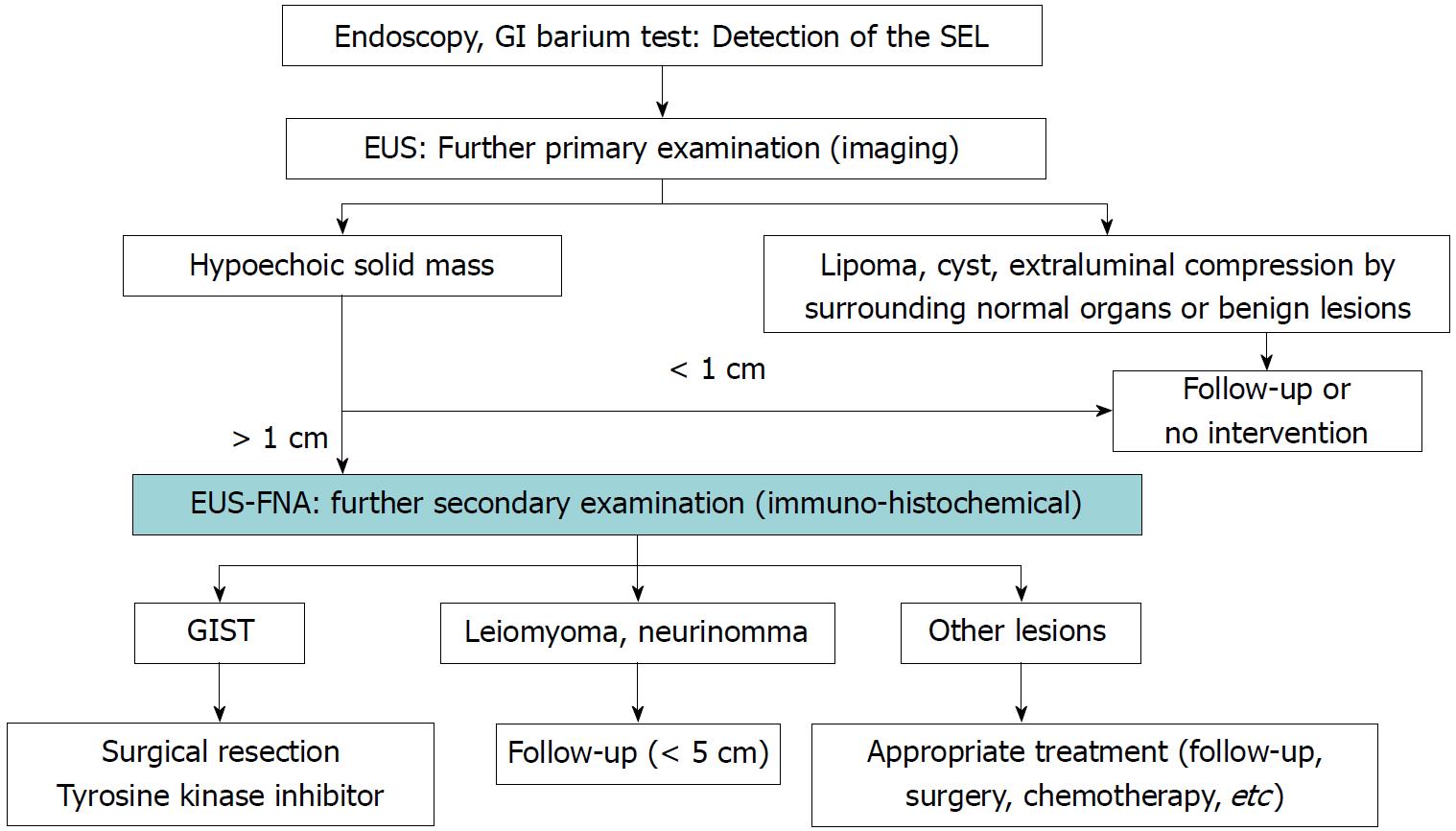
GISTs have no specific endoscopic or EUS findings, and diagnosis is difficult to achieve by histopathological examination using hematoxylin and eosin staining alone. Immunohistochemical analysis such as that involving KIT, CD34, or DOG1 measurement is essential for a definitive diagnosis[8,21]. However, because not all SELs are GISTs, it is necessary to identify those SELs that are suspicious for GISTs and perform immunohistochemical analysis of these SELs in clinical practice. Figure 6 shows our institutional algorithm for the detection and management of SELs as discussed herein[21]. First, all SELs are examined by EUS, and the SELs mentioned in the EUS section (Figure 3) that are conclusively diagnosed by EUS findings only are excluded. Second, EUS-FNA using immunohistochemical analysis is performed for the remaining hypoechoic solid masses to differentiate GISTs from other tumors. Narrowing down of SELs by EUS is important for efficient performance of EUS-FNA in the diagnosis of GISTs. In the Japanese clinical practice guidelines for GISTs, biopsy is recommended for exclusion of SEL-like cancer when an SEL is endoscopically diagnosed[70]. However, because SELs also include vascular diseases for which biopsy is contraindicated, such as varices (Figure 3G and H), it is desirable to perform EUS before biopsy.
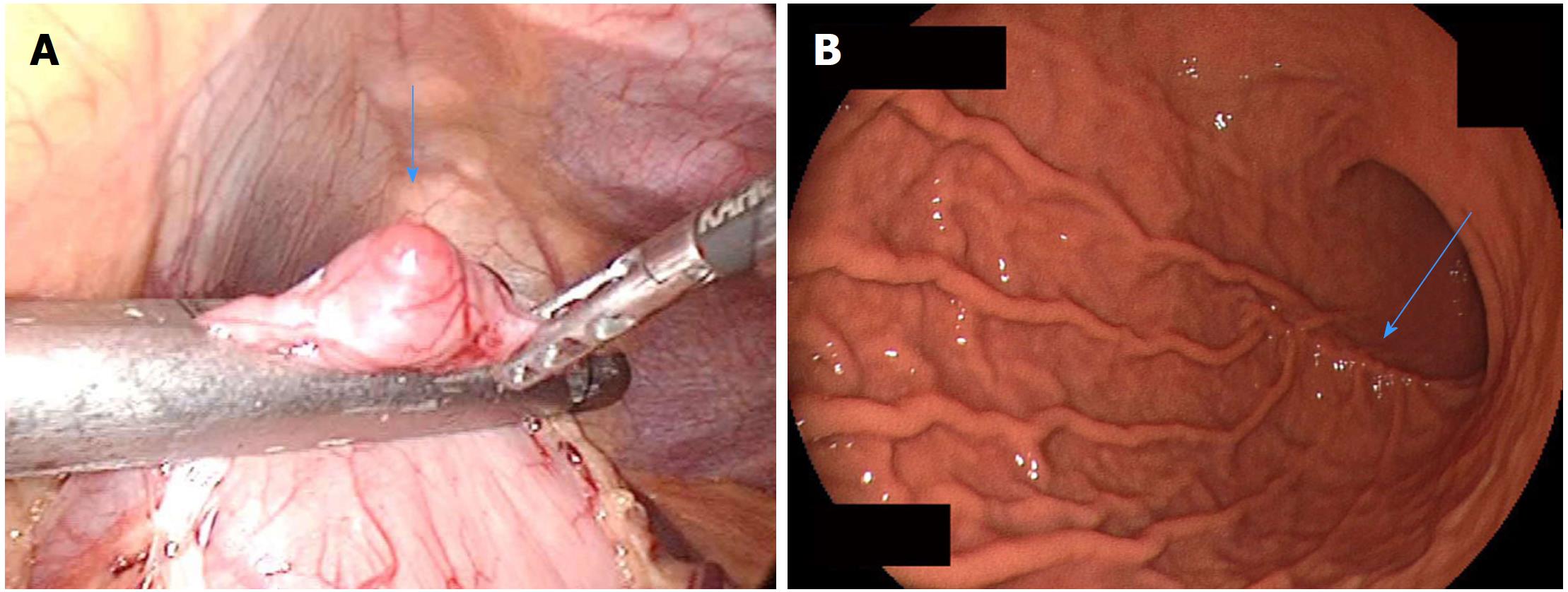
The principle treatment strategy for immunohistologically confirmed GISTs is as follows: (1) Surgical resection is the first choice for resectable GISTs without metastasis; and (2) Administration of tyrosine kinase inhibitors such as imatinib is the primary approach for unresectable, metastatic, or recurrent GISTs[70-73]. The objective of surgery is to achieve R0 resection to the greatest extent possible. Lymph node dissection is not recommended except when lymph node metastasis is clinically suspected; most metastasis of GIST is liver metastasis or peritoneal seeding, and lymph node metastasis is extremely rare[74,75]. Therefore, wedge or segmental resection with preservation of organs and organ functions and maintenance of a good quality of life after surgery is recommended[76]. Previous studies have shown that laparoscopic resection is feasible and safe for gastric GISTs and is less invasive than traditional open surgery, with similar oncological outcomes (Figure 7)[77-79]. Other minimally invasive techniques such as submucosal tunneling endoscopic resection[67,68], endoscopic fullthickness resection[80], and laparoscopic endoscopic cooperative surgery[81] have recently shown good clinical outcomes; however, there are still insufficient studies concerning their long-term safety, and they are still at clinical research levels.
In contrast, the introduction of imatinib (first-line tyrosine kinase inhibitor) has dramatically improved the management of GISTs, prolonging recurrence-free survival after surgery[82] and extending overall survival in metastatic or unresectable cases[14]. Three years of adjuvant therapy with imatinib for patients with high-risk GISTs who have undergone macroscopic complete tumor resection (R0 and R1) is recommended because it improves overall survival and recurrence-free survival[82]. Sunitinib (second-line tyrosine kinase inhibitor)[83] and regorafenib (third-line multikinase inhibitor)[84] can be used in advanced GISTs after treatment failure with imatinib. However, it is difficult to obtain a permanent cure by tyrosine kinase inhibitors. Therefore, early diagnosis (early GISTs without metastasis) with early surgical resection is the only promising way to obtain complete cure of this disease[20,21,56].
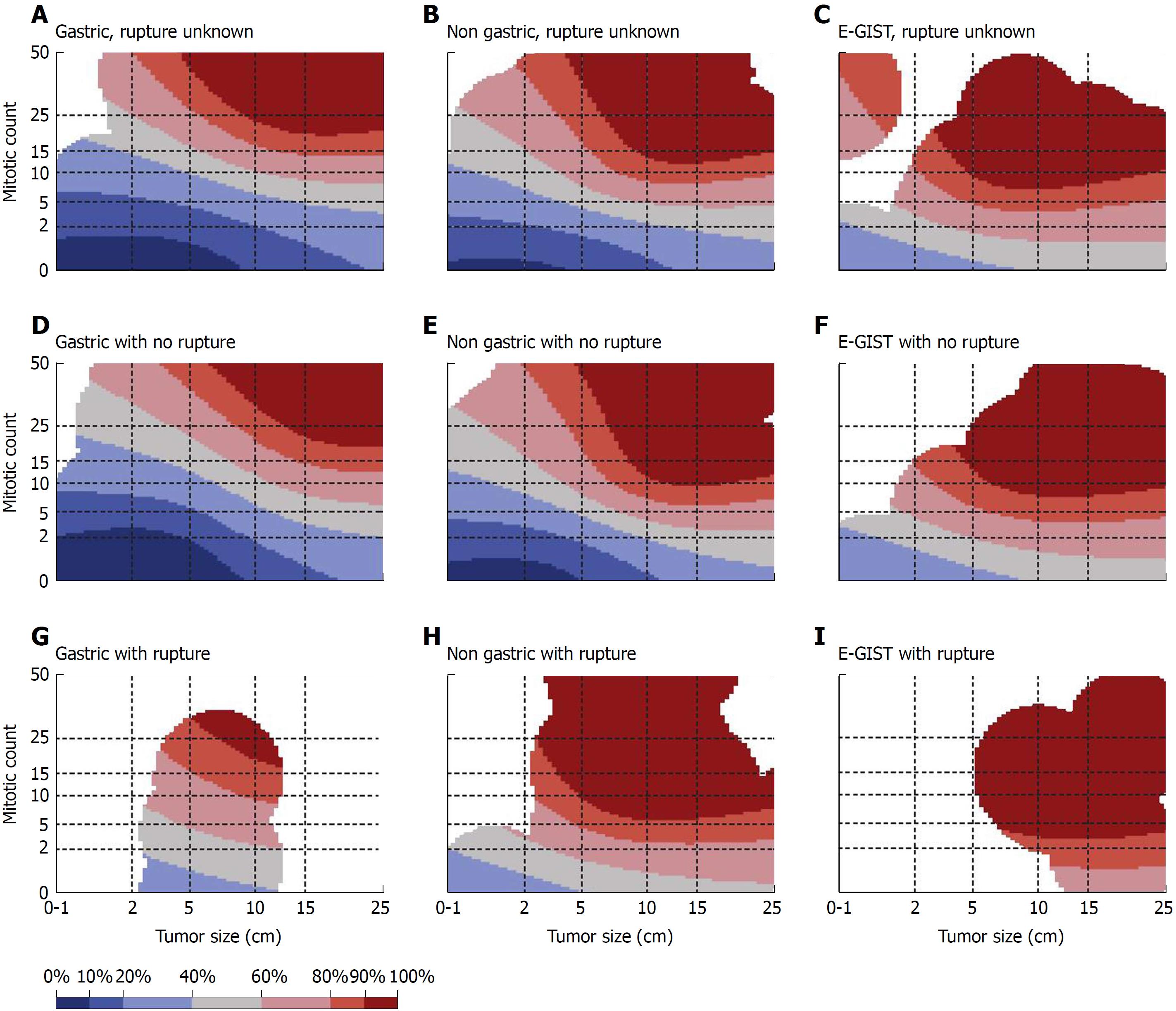
Differentiation between a benign and malignant GIST is difficult even using postoperative histopathological findings. Thus, even if the tumor diameter is small and/or the mitotic rate is low, postoperative metastasis is possible. GISTs are currently regarded as potentially malignant tumors. Discrimination of a benign GIST from a malignant GIST by postoperative histological analysis (tumor diameter, mitotic index, and Ki67 expression level) is difficult; therefore, risk classifications to predict postoperative metastasis have been introduced[7,85,86]. Currently, the modified Fletcher classification (Joensuu classification) is widely used (Table 1)[7]. In addition, contour maps (Figure 8) can be created based on investigation of the prognosis of many cases worldwide. In these maps, the risk of recurrence at the 10th year after surgical treatment of a GIST is calculated using the maximum diameter of the tumor, the number of mitoses, the tumor site, and the presence or absence of tumor capsule rupture; continuous risk assessment is also possible[87]. Using such maps, physicians and patients can predict the probability of recurrence in the 10th postoperative year. This is useful for individual decision-making with respect to adjuvant therapy.
| Risk category | Tumor size (cm) | Mitotic index (per 50 HPFs) | Primary tumor site |
| Very low risk | < 2.0 | ≤ 5 | Any |
| Low risk | 2.1-5.0 | ≤ 5 | Any |
| Intermediate risk | 2.1-5.0 | > 5 | Gastric |
| < 5 | 6-10 | Any | |
| 5.1-10.0 | ≤ 5 | Gastric | |
| High risk | Any | Any | Tumor rupture |
| > 10 cm | Any | Any | |
| Any | > 10 | Any | |
| > 5.0 | > 5 | Any | |
| 2.1-5.0 | > 5 | Non-gastric | |
| 5.1-10 | ≤ 5 | Non-gastric |
The goal of postoperative follow-up is early detection and management of recurrence. Because the targets of postoperative follow-up observation are local recurrence, liver metastasis, and peritoneal dissemination, abdominal contrast CT, which can be sufficiently evaluated from the diaphragm to the inguinal region, is recommended as a follow-up examination method according to the Japanese clinical practice guideline for GISTs[70]. Based on the above-mentioned risk classification, the following observation intervals are recommended[70]: GISTs with very low, low, and moderate risks are followed up by CT every 6 mo to 1 year, and high-risk and clinically malignant GISTs (those with metastasis, injury to the pseudocapsule, peritoneal dissemination, or infiltration of other organs) are followed up by CT every 4 to 6 mo. The natural history of GISTs is unknown. Previous studies have shown that the estimated 5-year recurrence-free survival rate after surgery is 59.9%; few recurrences occurred after the first 10 years of follow-up[7,88]. Follow-up observation after surgery is considered necessary for more than 10 years.
The detection rate of small GISTs has continuously increased with advancements in endoscopy[89,90]. However, the surveillance and management of GISTs smaller than 2 cm is controversial or lacks evidence-based approaches[41,56,89-92]. Most small GISTs are discovered incidentally and usually show a benign or indolent clinical course. Conversely, strict discrimination between benign and malignant GISTs is considered to be very difficult using both clinical and pathological examinations. Thus, the European Society for Medical Oncology[72], Japanese[70], and Chinese Society of Clinical Oncology[73] GIST guidelines recommend surgical resection when an SEL is immunohistologically diagnosed as a GIST, even when smaller than 2 cm. In contrast, the National Comprehensive Cancer Network[71] guidelines recommend that small GISTs of < 2 cm may be periodically followed up by EUS when they lack high-risk features including an irregular border, cystic spaces, ulceration, echogenic foci, and heterogeneity. However, in the examination of 378 histologically diagnosed GISTs of < 2 cm registered in the National Cancer Institute’s Surveillance Epidemiology and End Results database, which is a cancer database in the United States, 11.4% of the patients had regional/distant metastatic disease and the 5-year GIST-specific mortality rate was 12.9%[93]. In a study of 43 surgically resected small GISTs of < 2 cm with immunohistochemical analysis, 23% of lesions were classified as having intermediate risk according to the Joensuu risk stratification[56]. Although GISTs of ≤ 2 cm are reportedly metastatic at a low frequency (but not 0%)[89,90,93], early tissue diagnosis and early resection with postoperative follow-up are desired. Importantly, therefore, gastroenterologists should consider early interventions such as EUS for incidentally detected small SELs. Active performance of EUS is effective even for small SELs of ≤ 2 cm to ensure early detection of hypoechoic solid masses suspected to be GISTs[56]. If EUS imaging of a SEL with an endoscopically negative biopsy shows a hypoechoic solid mass of > 1 cm, subsequent EUS-FNA is needed to obtain a conclusive tissue diagnosis of a GIST[21,56]. Small SELs of < 1 cm are currently recommended to undergo periodic EUS follow-up (every 6 mo or 1 year)[56,91] because EUS-FNA for small SELs of < 1 cm is technically difficult. These aggressive approaches for early diagnosis and early treatment of small SELs, similar to the approaches for gastrointestinal tract cancer, seem to be the only promising way to improve patients’ quality of life and prognosis.
GISTs are the most common malignant SELs of the digestive tract. According to previous studies, early histologic diagnosis and early surgical resection of small localized disease is currently the most reliable and curative treatment technique for GISTs. However, sufficient prospective studies including small GISTs have not been performed to improve the current clinical GIST management. Further studies are needed to focus on early tissue diagnosis and therapeutic approaches, especially for small SELs.
Manuscript Source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Adachi Y, Akbulut S, Contini S, Caboclo JF, Rabago LR, Yan SL S- Editor: Wang JL L- Editor: A E- Editor: Yin SY
| 1. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [PubMed] |
| 2. | Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 3. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 4. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3115] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 5. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1723] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 6. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 867] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 7. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 865] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 8. | Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 9. | Plaat BE, Hollema H, Molenaar WM, Torn Broers GH, Pijpe J, Mastik MF, Hoekstra HJ, van den Berg E, Scheper RJ, van der Graaf WT. Soft tissue leiomyosarcomas and malignant gastrointestinal stromal tumors: differences in clinical outcome and expression of multidrug resistance proteins. J Clin Oncol. 2000;18:3211-3220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3203] [Cited by in RCA: 3111] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 11. | Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD, Roberts PJ, Heinz D. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 751] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 12. | Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki RG. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 770] [Article Influence: 45.3] [Reference Citation Analysis (1)] |
| 13. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1326] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 14. | Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1207] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 15. | Verweij J, van Oosterom A, Blay JY, Judson I, Rodenhuis S, van der Graaf W, Radford J, Le Cesne A, Hogendoorn PC, di Paola ED. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 274] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, Martens M, Webb A, Sciot R, Van Glabbeke M. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358:1421-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 885] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 17. | Choi SM, Kim MC, Jung GJ, Kim HH, Kwon HC, Choi SR, Jang JS, Jeong JS. Laparoscopic wedge resection for gastric GIST: long-term follow-up results. Eur J Surg Oncol. 2007;33:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 18. | Sepe PS, Brugge WR. A guide for the diagnosis and management of gastrointestinal stromal cell tumors. Nat Rev Gastroenterol Hepatol. 2009;6:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 19. | Faigel DO, Abulhawa S. Gastrointestinal stromal tumors: the role of the gastroenterologist in diagnosis and risk stratification. J Clin Gastroenterol. 2012;46:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Mullady DK, Tan BR. A multidisciplinary approach to the diagnosis and treatment of gastrointestinal stromal tumor. J Clin Gastroenterol. 2013;47:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Akahoshi K, Oya M. Gastrointestinal stromal tumor of the stomach: How to manage? World J Gastrointest Endosc. 2010;2:271-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008;3:557-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 23. | Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077-2082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 159] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 24. | Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 437] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 25. | Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 875] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 26. | Tzen CY, Wang JH, Huang YJ, Wang MN, Lin PC, Lai GL, Wu CY, Tzen CY. Incidence of gastrointestinal stromal tumor: a retrospective study based on immunohistochemical and mutational analyses. Dig Dis Sci. 2007;52:792-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Graadt van Roggen JF, van Velthuysen ML, Hogendoorn PC. The histopathological differential diagnosis of gastrointestinal stromal tumours. J Clin Pathol. 2001;54:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 457] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 29. | Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 30. | Tryggvason G, Kristmundsson T, Orvar K, Jónasson JG, Magnússon MK, Gíslason HG. Clinical study on gastrointestinal stromal tumors (GIST) in Iceland, 1990-2003. Dig Dis Sci. 2007;52:2249-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Kawanowa K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, Hosoya Y, Nakajima T, Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | Agaimy A, Wünsch PH, Hofstaedter F, Blaszyk H, Rümmele P, Gaumann A, Dietmaier W, Hartmann A. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 33. | Agaimy A, Wünsch PH, Dirnhofer S, Bihl MP, Terracciano LM, Tornillo L. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: a clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am J Surg Pathol. 2008;32:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Abraham SC, Krasinskas AM, Hofstetter WL, Swisher SG, Wu TT. “Seedling” mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol. 2007;31:1629-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Muenst S, Thies S, Went P, Tornillo L, Bihl MP, Dirnhofer S. Frequency, phenotype, and genotype of minute gastrointestinal stromal tumors in the stomach: an autopsy study. Hum Pathol. 2011;42:1849-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 37. | Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 212] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 38. | Akahoshi K, Inoue K, Oya M, Tamura S, Takaki M, Tatsushima S, Shiratsuchi Y, Kubokawa M, Gibo J, Yodoe K. Endoscopic ultrasonography-guided fine needle aspiration for gastrointestinal lesion. Endoscopia Digestiva. 2016;28:1581-1590. |
| 39. | Akahoshi K, Oya M, Motomura Y, Kubokawa M, Itaba S, Osoegawa T, Nakama N, Komori K, Gibo J, Yamada M. Diagnosis of gastric submucosal tumor and submucosal tumor like lesion by endoscopic ultrasonography-guided fine needle aspiration. Endoscopia Digestiva. 2011;23:1530-1536. |
| 40. | Hwang JH, Rulyak SD, Kimmey MB; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2217-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 41. | Sekine M, Imaoka H, Mizuno N, Hara K, Hijioka S, Niwa Y, Tajika M, Tanaka T, Ishihara M, Ito S. Clinical course of gastrointestinal stromal tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Dig Endosc. 2015;27:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Higuchi N, Akahoshi K, Honda K, Matsui N, Kubokawa M, Motomura Y, Nakamura K, Takayanagi R. Diagnosis of a small splenic artery aneurysm mimicking a gastric submucosal tumor on endoscopic ultrasound. Endoscopy. 2010;42 Suppl 2:E107-E108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (2)] |
| 43. | Boyce GA, Sivak MV Jr, Rösch T, Classen M, Fleischer DE, Boyce HW Jr, Lightdale CJ, Botet JF, Hawes RH, Lehman GA. Evaluation of submucosal upper gastrointestinal tract lesions by endoscopic ultrasound. Gastrointest Endosc. 1991;37:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 45. | Chak A, Canto MI, Rösch T, Dittler HJ, Hawes RH, Tio TL, Lightdale CJ, Boyce HW, Scheiman J, Carpenter SL. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997;45:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 206] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Kim MN, Kang SJ, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Prediction of risk of malignancy of gastrointestinal stromal tumors by endoscopic ultrasonography. Gut Liver. 2013;7:642-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 49. | Kaneko E, Kumagai J, Honda N, Nakamura S, Kino I. Evaluation of the new giant-biopsy forceps in the diagnosis of mucosal and submucosal gastric lesions. Endoscopy. 1983;15:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Scarpa M, Bertin M, Ruffolo C, Polese L, D’Amico DF, Angriman I. A systematic review on the clinical diagnosis of gastrointestinal stromal tumors. J Surg Oncol. 2008;98:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Cantor MJ, Davila RE, Faigel DO. Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc. 2006;64:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc. 2003;57:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Buscaglia JM, Nagula S, Jayaraman V, Robbins DH, Vadada D, Gross SA, DiMaio CJ, Pais S, Patel K, Sejpal DV. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012;75:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Mekky MA, Yamao K, Sawaki A, Mizuno N, Hara K, Nafeh MA, Osman AM, Koshikawa T, Yatabe Y, Bhatia V. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 55. | Larghi A, Fuccio L, Chiarello G, Attili F, Vanella G, Paliani GB, Napoleone M, Rindi G, Larocca LM, Costamagna G. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy. 2014;46:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Akahoshi K, Oya M, Koga T, Koga H, Motomura Y, Kubokawa M, Gibo J, Nakamura K. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J Gastrointestin Liver Dis. 2014;23:405-412. [PubMed] |
| 57. | Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 58. | Yamabe A, Irisawa A, Bhutani MS, Shibukawa G, Abe Y, Saito A, Imbe K, Hoshi K, Igarashi R. Usefulness of endoscopic ultrasound-guided fine-needle aspiration with a forward-viewing and curved linear-array echoendoscope for small gastrointestinal subepithelial lesions. Endosc Int Open. 2015;3:E161-E164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Uesato M, Tamachi T, Hanari N, Muto Y, Kagaya A, Urahama R, Ogura Y, Suito H, Nakano A, Aikawa M. Drill needle aspiration biopsy for submucosal tumors in an experimental study. Gastric Cancer. 2017;20:475-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 61. | de la Serna-Higuera C, Pérez-Miranda M, Díez-Redondo P, Gil-Simón P, Herranz T, Pérez-Martín E, Ochoa C, Caro-Patón A. EUS-guided single-incision needle-knife biopsy: description and results of a new method for tissue sampling of subepithelial GI tumors (with video). Gastrointest Endosc. 2011;74:672-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Mimura T, Kuramoto S, Hashimoto M, Yamasaki K, Kobayashi K, Kobayashi M, Oohara T. Unroofing for lymphangioma of the large intestine: a new approach to endoscopic treatment. Gastrointest Endosc. 1997;46:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Lee CK, Chung IK, Lee SH, Lee SH, Lee TH, Park SH, Kim HS, Kim SJ, Cho HD. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010;71:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 64. | Ihara E, Matsuzaka H, Honda K, Hata Y, Sumida Y, Akiho H, Misawa T, Toyoshima S, Chijiiwa Y, Nakamura K. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J Gastrointest Endosc. 2013;5:191-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Dolak W, Beer A, Kristo I, Tribl B, Asari R, Schöniger-Hekele M, Wrba F, Schoppmann SF, Trauner M, Püspök A. A retrospective study on the safety, diagnostic yield, and therapeutic effects of endoscopic unroofing for small gastric subepithelial tumors. Gastrointest Endosc. 2016;84:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Kobara H, Mori H, Nishimoto N, Fujihara S, Nishiyama N, Ayaki M, Yachida T, Matsunaga T, Chiyo T, Kobayashi N. Comparison of submucosal tunneling biopsy versus EUS-guided FNA for gastric subepithelial lesions: a prospective study with crossover design. Endosc Int Open. 2017;5:E695-E705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 68. | Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 69. | Standards of Practice Committee, Faulx AL, Kothari S, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Gurudu SR, Khashab MA, Lightdale JR, Muthusamy VR, Shaukat A, Qumseya BJ, Wang A, Wani SB, Yang J, DeWitt JM. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017;85:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 70. | Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F, Kubota T; GIST Guideline Subcommittee. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 71. | National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: soft tissue sarcoma, version 1. Available from: http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf. |
| 72. | ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii21-iii26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 73. | Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y, He Y, Liang X, Liu X, Zhou Y. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. 2017;29:281-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 74. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-41; quiz S42-4. [PubMed] |
| 75. | Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 358] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 76. | Koga T, Hirayama Y, Yoshiya S, Taketani K, Nakanoko T, Yoshida R, Minagawa R, Kai M, Kajiyama K, Akahoshi K. Necessity for resection of gastric gastrointestinal stromal tumors ≤ 20 mm. Anticancer Res. 2015;35:2341-2344. [PubMed] |
| 77. | Akahoshi K, Oya M, Koga T. Diagnosis and treatment of gastrointestinal stromal tumor (GIST). Endoscopia Digestiva. 2012;24:626-632. |
| 78. | Chen K, Zhou YC, Mou YP, Xu XW, Jin WW, Ajoodhea H. Systematic review and meta-analysis of safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors of the stomach. Surg Endosc. 2015;29:355-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Koh YX, Chok AY, Zheng HL, Tan CS, Chow PK, Wong WK, Goh BK. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol. 2013;20:3549-3560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 80. | Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 81. | Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. 2015;27:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 82. | Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 682] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 83. | Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 1919] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 84. | Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M, Joensuu H. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 1033] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 85. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 86. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1305] [Article Influence: 72.5] [Reference Citation Analysis (33)] |
| 87. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 672] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 88. | Joensuu H, Martin-Broto J, Nishida T, Reichardt P, Schöffski P, Maki RG. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer. 2015;51:1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Tanaka J, Oshima T, Hori K, Tomita T, Kim Y, Watari J, Oh K, Hirota S, Matsumoto T, Miwa H. Small gastrointestinal stromal tumor of the stomach showing rapid growth and early metastasis to the liver. Dig Endosc. 2010;22:354-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 90. | Aso A, Ihara E, Kubo H, Osoegawa T, Oono T, Nakamura K, Ito T, Kakeji Y, Mikako O, Yamamoto H. Gastric gastrointestinal stromal tumor smaller than 20 mm with liver metastasis. Clin J Gastroenterol. 2013;6:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Gao Z, Wang C, Xue Q, Wang J, Shen Z, Jiang K, Shen K, Liang B, Yang X, Xie Q. The cut-off value of tumor size and appropriate timing of follow-up for management of minimal EUS-suspected gastric gastrointestinal stromal tumors. BMC Gastroenterol. 2017;17:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 92. | Kim MC, Yook JH, Yang HK, Lee HJ, Sohn TS, Hyung WJ, Ryu SW, Kurokawa Y, Kim YW, Han SU. Long-Term Surgical Outcome of 1057 Gastric GISTs According to 7th UICC/AJCC TNM System: Multicenter Observational Study From Korea and Japan. Medicine (Baltimore). 2015;94:e1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 93. | Coe TM, Fero KE, Fanta PT, Mallory RJ, Tang CM, Murphy JD, Sicklick JK. Population-Based Epidemiology and Mortality of Small Malignant Gastrointestinal Stromal Tumors in the USA. J Gastrointest Surg. 2016;20:1132-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |