Published online Apr 21, 2018. doi: 10.3748/wjg.v24.i15.1622
Peer-review started: February 14, 2018
First decision: March 9, 2018
Revised: March 16, 2018
Accepted: March 25, 2018
Article in press: March 25,2018
Published online: April 21, 2018
To investigate whether the liver resection volume in a newly developed nonalcoholic steatohepatitis (NASH) model influences surgical outcome.
For establishment of a NASH model, mice were fed a high-fat diet for 4 wk, administered CCl4 for the last 2 wk, and administered T0901317 for the last 5 d. We divided these mice into two groups: A 30% partial hepatectomy (PH) of NASH liver group and a 70% PH of NASH liver group. In addition, a 70% PH of normal liver group served as the control. Each group was evaluated for survival rate, regeneration, apoptosis, necrosis and DNA expression after PH.
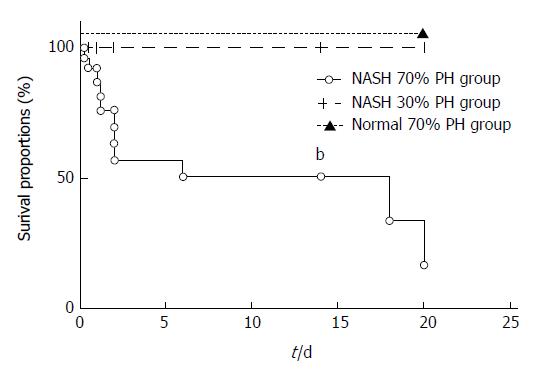
In the 70% PH of NASH group, the survival rate was significantly decreased compared with that in the control and 30% PH of NASH groups (P < 0.01). 10 of 32 mice in the NASH 70% PH group died within 48 h after PH. Serum aspartate aminotransferase (AST) levels and total bilirubin (T-Bil) in the NASH 70% PH group were significantly higher than the levels in the other two groups (AST: P < 0.05, T-Bil: P < 0.01). In both PH of NASH groups, signaling proteins involved in regeneration were expressed at lower levels than those in the control group (P < 0.01). The 70% PH of NASH group also exhibited a lower number of Ki-67-positive cells and higher rates of apoptosis and necrosis than the NASH 30% PH group (P < 0.01). In addition, DNA microarray assays showed differences in gene expression associated with cell cycle arrest and apoptosis.
The function of the residual liver is impaired in fatty liver compared to normal liver. A larger residual volume is required to maintain liver functions in mice with NASH.
Core tip: We report whether the liver resection volume in the nonalcoholic steatohepatitis (NASH) model influences surgical outcome. The population of patients with NASH has been increasing. However, few animal models fully reflect both the histopathology and pathophysiology of NASH in humans. We established a novel experimental NASH model that exhibited the same characteristics as NASH in humans. This study elucidates the metabolism of the residual liver after a hepatectomy with NASH. Compared with normal liver, the residual NASH liver function is impaired, especially its regenerative ability. Therefore, a larger residual volume is required to maintain liver function in NASH liver after partial hepatectomy.
- Citation: Ozawa Y, Tamura T, Owada Y, Shimizu Y, Kemmochi A, Hisakura K, Matsuzaka T, Shimano H, Isoda H, Ohkohchi N. Evaluation of safety for hepatectomy in a novel mouse model with nonalcoholic-steatohepatitis. World J Gastroenterol 2018; 24(15): 1622-1631
- URL: https://www.wjgnet.com/1007-9327/full/v24/i15/1622.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i15.1622
Nonalcoholic fatty liver disease (NAFLD) is observed in 20%-40% of the general population, and its incidence continues to increase in industrialized countries[1,2]. NAFLD includes several diseases, such as simple liver steatosis, nonalcoholic steatohepatitis (NASH), and cirrhosis. NASH is characterized by hepatic steatosis, lobular inflammation, and abnormal glucose tolerance. In NASH, continuous inflammation contributes to hepatocellular carcinoma (HCC)[3,4]. The cause of HCC is frequently infection with hepatitis B virus and hepatitis C virus (HCV). New antiviral medications for hepatitis are currently being used in clinics; therefore, the number of patients with virus-related HCC is expected to decrease in the future[5-9]. By contrast, the number of patients with NASH-related HCC has been increasing recently, and this trend is expected to continue because no effective treatments are available[10].
Steatosis is a risk factor for postoperative liver failure[11,12]. A number of clinical studies revealed that steatosis caused severe mortality and morbidity after liver resection compared with normal liver following liver resection[11,12]. In experimental models, hepatectomy of fatty livers resulted in suppressed liver regeneration and survival rates[13-16]. However, the influence of hepatectomy on NASH livers has not been extensively evaluated.
Hepatectomy is a standard and most effective therapy for HCC patients. Postoperative liver failure is a serious complication after hepatectomy, and its occurrence correlates with the volume and function of the residual liver[17-20]. To prevent liver failure after hepatectomy, the liver resection volume is limited according to preoperative liver function[21-23]. For promotion of regeneration and maintaining liver function preserving sufficient residual liver volume enables the prevention of liver failure[24,25]. Thus, the degree of liver regeneration is dependent on the volume of the residual liver. Although several NASH models, such as the methionine- and choline-deficient (MCD) model and high-fat (HF) diet model, have been reported, few models completely reflect the histopathology and pathophysiology of NASH in humans[26,27]. The disadvantages of the MCD model are that MCD mice exhibit severe body weight loss with the absence of insulin resistance. The HF diet model is not suitable for researching the pathogenesis of NASH because a longer period of time is required for presentation of NASH characteristics, and hepatic fibrosis is weaker than that observed in human NASH. Thus, the ability of regeneration in the NASH liver has not yet been assessed in an experimental model. For the same reasons, the effect of hepatectomy on NASH liver has not been clearly elucidated in previous reports. We established a novel experimental NASH model that indicates similar histopathological and pathophysiological characteristics as those of NASH in humans[28]. The aim of this study was to investigate whether a difference in liver resection volume in a novel NASH model influences surgical outcomes.
Six-week-old male C57BL/6J mice were obtained from Charles River Laboratories Japan, Inc. (Kanagawa, Japan) and were acclimated for one week before the start of the experiment. Mice were maintained under a 12-h light-dark cycle and had free access to standard chow and tap water. The animal experiments were performed in a humane manner after receiving approval from the Institutional University Experiment Committee of the University of Tsukuba and in accordance with the Regulations for Animal Experiments at the university and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science, and Technology.
NASH mice were fed an HF diet (60 kcal% fat; D12492, Research Diets, Inc., New Brunswick, NJ, United States) for 4 wk, intraperitoneally injected with CCl4 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) twice a week for the final 2 wk, and intraperitoneally injected with T0901317 (Cayman Chemical Co., Ann Arbor, MI, United States) solubilized in DMSO for the final 5 d. The CCl4 dose was 0.1 mL/kg, and the T0901317 dose was 2.5 mg/kg[28].
We categorized the mice into three groups: (1) 70% partial hepatectomy (PH) of normal liver mice as the control; (2) 30% PH of NASH liver group; and (3) 70% PH of NASH liver group. The normal liver mice have been not added any reagent and the histology and pathology have been not change. In 30% PH and 70% PH of the NASH liver group, liver specimens were evaluated by an experienced pathologist in a blinded fashion, the histology and pathology finding in the NASH severity of each groups have resulted in no difference in the NAFLD activity scores[28]. All mice received the hepatectomy 48 h after the final administration of CCl4 and T0901317. In the 70% PH groups, the left and middle lobes of the liver were removed by using a single ligature, whereas only the left lobe was removed in the 30% PH group[29]. Hepatectomy was performed under ether anesthesia.
Blood samples were collected from the orbital capillary and centrifuged at 3000 rpm for 10 min to isolate the serum. Each sample was stored at -80 °C until analysis. Mice of each group were sacrificed at 6 h and 12 h after PH. Then, the liver was quickly removed and weighed. The liver specimen was immediately fixed in 10% neutral-buffered formalin for further histological examination. Survival rates were evaluated in the NASH 70% PH group (n = 32) and NASH 30% PH group (n = 27).
Fixed liver tissues were processed and embedded in paraffin using standard methods. Then, liver tissues were sliced into 2-μm thick paraffin sections and stained with hematoxylin and eosin (HE) to evaluate necrosis. Necrotic areas were detected by morphological features, and the ratio of necrosis/total area was calculated in 20 random intralobular fields. Liver proliferation was assessed by Ki-67 staining. Apoptosis was detected by TUNEL staining. TUNEL staining and Ki-67 staining were performed using an antibody kit (New History Science Laboratory Co., Ltd., Tokyo, Japan). The ratio of positive/total hepatocytes was calculated in 20 random intralobular fields.
Liver tissue extracts were prepared from specimens that were frozen in liquid nitrogen. We evaluated the expression of signaling proteins involved in liver regeneration, including AKT, STAT3, and ERK1/2, by western blotting. We compared the expression levels of these proteins in each group 6 h after PH. Immunoblots were developed using polyclonal antibodies against phospho-AKT (9271), total AKT (9272), phospho-STAT3 (9131), total STAT3 (9132), phospho-ERK1/2 (9101), and total ERK1/2 (9102) (Cell Signaling Technology, Beverly, MA, United States).
Liver tissue samples were freshly collected and immediately frozen at -30 °C until investigation. Frozen liver samples were homogenized, and total RNA was isolated from whole cells using a NucleoSpin® RNA kit (Takara Bio, Inc., Otsu, Japan). RNA concentrations were determined by measuring the absorbance at 260/280 nm with a NanoDrop Spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE, United States). Synthesis of complementary DNA was performed using AMV Reverse Transcriptase (Promega, Corp., Madison, WI, United States) and random primers (Takara Bio, Inc., Otsu, Japan). Briefly, a mixture of 1 mmol/L dNTPs (Fermentas Life Sciences, Inc., Burlington, ON, Canada), 0.025 μg/mL random primers, 0.25 U/mL reverse transcriptase, and 500 ng of total RNA was incubated at 30 °C for 10 min, 37 °C for 60 min, 95 °C for 5 min and 4 °C before storage at -80 °C.
RT-PCR primers were designed using Primer Express Software for Real-time PCR ver. 3.0 (Applied Biosystems, Inc., Foster City, CA, United States) based on the sequences available in GenBank. Primers were purchased from Takara Bio, Inc. (Otsu, Japan). GADD45A primer sequences were 5’-CCTGCACTGTGTGCTGGTGA-3’ and 5’-CCACTGATCCATGTAGCGACTTTC-3’. PDE4B primer sequences were 5’-CCCATCAGCAGTTAAGGACAGGA-3’ and 5’-TGGGCAGAACTAGGGACTCAAGA-3’.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control. RT-PCR was performed using SYBR-Green Real-Time PCR Master Mix-Plus (Toyobo Co., Ltd., Osaka, Japan) and an Applied Biosystems 7300 real-time PCR system (Applied Biosystems, Inc., Foster City, CA, United States) as recommended by the manufacturer’s instructions[28].
DNA microarray analysis was conducted on RNA samples isolated from liver tissue in the control group and novel NASH model group. Labeled cRNA was synthesized from 100 ng of total RNA using a GeneChip® 3’ IVT Plus Reagent Kit (Affymetrix, Inc., Santa Clara, CA, United States) according to the manufacturer’s protocol. Fragmented and labeled cRNA (7.5 μg) was hybridized to an Affymetrix Mouse MG-430 PM Array Strip (Affymetrix) for 16 h at 45 °C. The strips were washed and stained using a GeneAtlas Fluidics Station 400 (Affymetrix), and the resulting images were scanned using a GeneAtlas Imaging Station (Affymetrix). Probe-level analysis, including background subtraction and quantile normalization, was conducted using a robust multiarray average algorithm (RMA) using Affymetrix Expression Console Software 1.4 (Affymetrix). The gene expression profile of the novel NASH model was compared with the HF group. Genes exhibiting differences in expression with an increase of greater than 1.4-fold and a decrease of less than 0.65-fold were classified as differentially expressed genes[28].
All data are expressed as the mean ± SD. Statistical analyses were conducted using PRISM. Mann-Whitney U test was used for comparing between two groups. P-values less than 0.05 were considered significant. The Kaplan-Meier estimator was used for survival rate evaluation.
The survival rate of the NASH 70% PH group was significantly lower than that of the NASH 30% PH group (P < 0.01) (Figure 1), and 10 of 32 mice in the NASH 70% PH group died within 48 h after PH. On the other hand, all mice in the NASH 30% PH group survived.
At 6 and 12 h after PH, serum aspartate aminotrans ferase (AST) and alanine aminotransferase (ALT) levels were high in all three groups. AST levels in the NASH 70% PH group were significantly higher than the levels in the other two groups (AST: P < 0.05). Total bilirubin (T-Bil) in the normal liver and NASH 30% PH groups did not change, but the values only significantly increased in the NASH 70% PH group (P < 0.01) (Table 1).
| 6 h after PH | 12 h after PH | |||||||
| AST | ALT | T-Bil | IL-6 | AST | ALT | T-Bil | IL-6 | |
| Normal 70%PH | 2343.3 ± 6160.4 | 1828.3 ± 990.4 | 1.43 ± 0.5 | 2036.0 ± 1470.9 | 2976.7 ± 1395.7 | 2053.3 ± 886.2 | 2.0 ± 0.9 | 731.5 ± 483.7 |
| NASH 30%PH | 1610.0 ± 3700.6 | 1507.1 ± 563.5 | 0.76 ± 0.2 | 1511.5 ± 284.8 | 1841.3 ± 619.1 | 1522.9 ± 537.9 | 0.9 ± 0.7 | 692.8 ± 211.1 |
| NASH 70%PH | 3064.3 ± 1289.8a | 2422.9 ± 1194.8 | 2.57 ± 1.36b | 3026.2 ± 2127.5 | 4067.5 ± 2059.2a | 2403.8 ± 1111.8 | 3.85 ± 0.96b | 987.9 ± 550.7 |
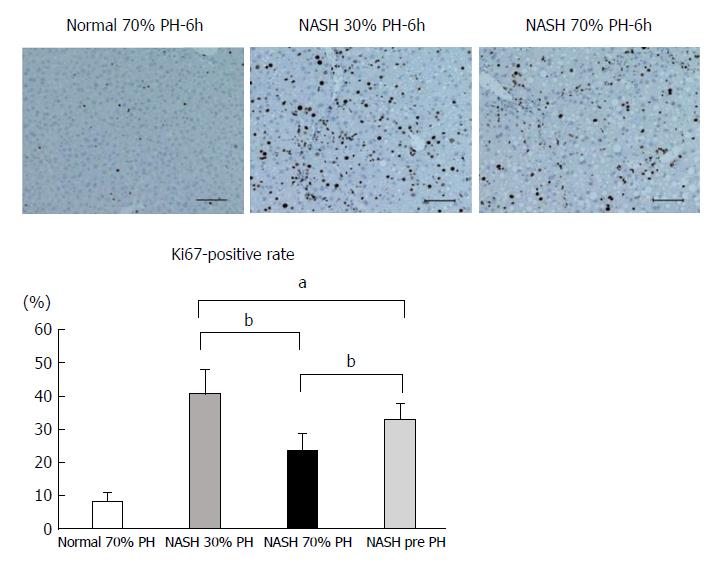
Many more Ki-67-positive hepatocytes were observed in the NASH 30% PH group than in the preoperative NASH liver (P < 0.05). On the other hand, fewer Ki-67-positive cells were noted in the NASH 70% PH group than in the preoperative NASH liver (P < 0.01). Additionally, significantly fewer Ki-67-positive cells were noted in the 70% PH group than in the NASH 30% PH group (P < 0.01) (Figure 2).
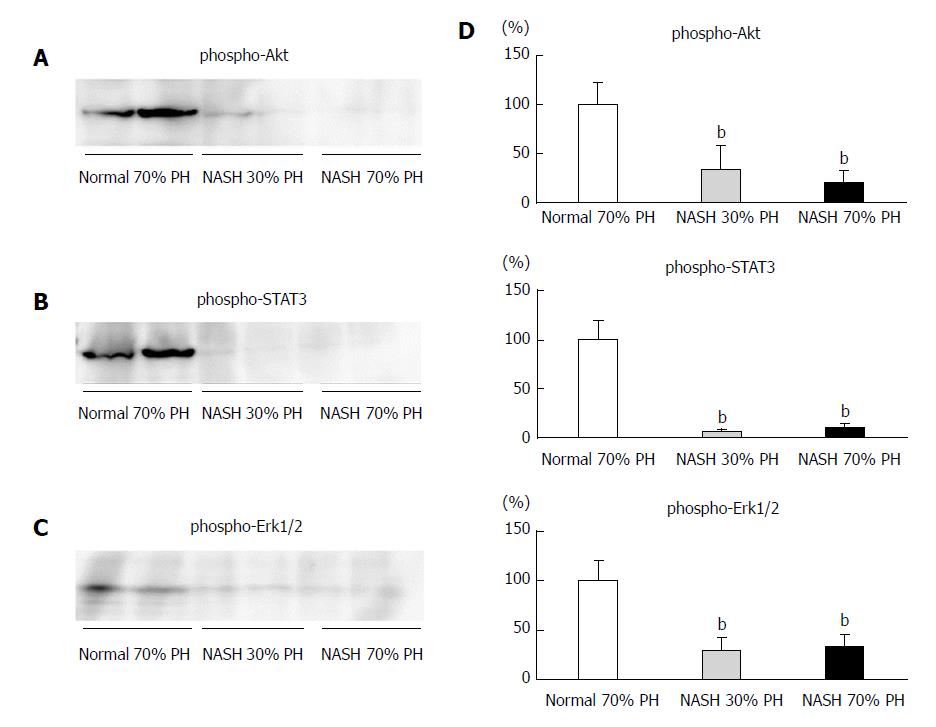
In the normal 70% PH liver group, i.e., the control group, expression of AKT, STAT3, and ERK1/2 phosphorylation was observed. In the both NASH 30% PH and 70% groups, phosphorylation of AKT, STAT3, and ERK1/2 was significantly lower than in the control group (P < 0.01) (Figure 3).
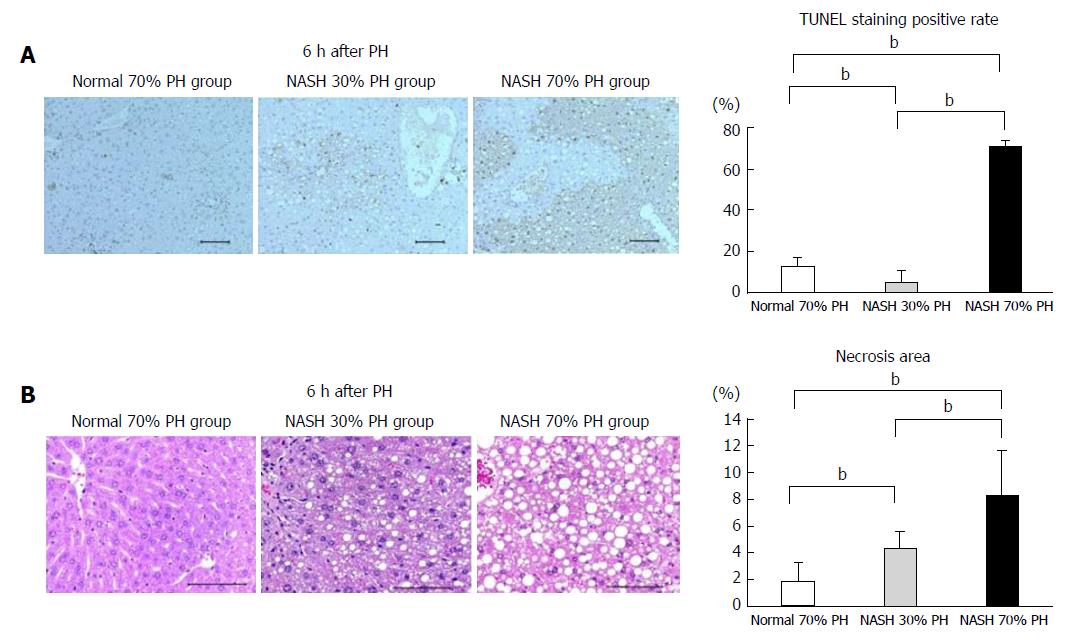
The number of TUNEL-positive cells in the NASH 70% PH group was significantly higher than in the other groups (P < 0.01). The TUNEL-positive rate of normal liver was significantly higher than that in the NASH 30% PH group (P < 0.01) (Figure 4A). The area of necrosis in the NASH 70% PH group was significantly larger than that in the NASH 30% PH group (P < 0.01). In both NASH groups, the necrotic area was significantly larger than that in the normal liver group (P < 0.01) (Figure 4B).
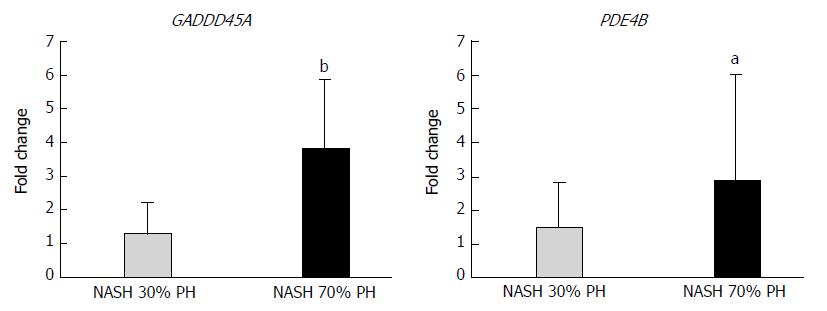
mRNAs in the NASH 70% PH group with the highest fold-change (> 1.4 or < 0.70) in expression and with P-values <0.05 were selected and compared with those in the NASH 30% PH group (Table 2). PDE4B, SLC20A1, CXADR, GADD45A, ZSWIM6, and C15orf39 were expressed at higher levels in the NASH 70% PH group. PDE4B and GADD45A are associated with cell cycle arrest and apoptosis. Using qPCR, GADD45A and PDE4B mRNA expression was significantly different between the two groups (GADD45A: P < 0.01, PDE4B: P < 0.05) (Figure 5).
| Function | Gene name | Gene abbreviation | Fold-change (> 1.4) | P value (< 0.05) |
| Regulate the cellular concentrations of cyclic nucleotides | Phosphodiesterase 4B, cAMP-specific | PDE4B | 2.102691211 | 0.0063 |
| Growth arrest | Growth arrest and DNA-damage-inducible, alpha | GADD45A | 1.672778704 | 0.013 |
| Signal transduction | Coronin 1C | CORO1C | 1.489851976 | 0.031 |
| Stimulates expression of cytokines, including IL6, MIF and VEGFA | Hypoxia inducible lipid droplet associated | HILPDA | 1.481465541 | 0.011 |
| EGF-like growth factor | Heparin binding EGF-like growth factor | HBEGF | 1.432049736 | 0.043 |
| Cell-cell junctions | Membrane associated guanylate kinase, WW And PDZ domain containing 1 | MAGI1 | 1.430522907 | 0.0222 |
| Innate immune system | Ankyrin repeat and SOCS box containing 13 | ASB13 | 0.515175325 | 0.0042 |
| Apoptosis and autophagy | TIA1 cytotoxic granule-associated RNA binding protein-like 1 | TIAL1 | 0.609948905 | 0.027 |
| Gene expression | Nucleic acid binding protein 1 | NABP1 | 0.6558797325 | 0.042 |
| Cell cycle | S-phase kinase-associated protein 2, E3 ubiquitin protein ligase | SKP2 | 0.6588204077 | 0.044 |
| Mitochondrial metabolism | Translocase of inner mitochondrial membrane 9 homolog (yeast) | TIMM9 | 0.6598542503 | 0.042 |
| Cytokine signaling in immune system | B-cell CLL/lymphoma 6 | BCL6 | 0.6760241074 | 0.0097 |
| Cell cycle | Mutated in colorectal cancers | MCC | 0.6761191711 | 0.0076 |
| Gene expression | Zinc finger protein 519 | ZNF519 | 0.6864230003 | 0.028 |
| Gene expression | RNA binding motif protein, X-linked | RBMX | 0.6983542235 | 0.012 |
NAFLD/NASH is a common hepatic disorder that causes HCC[1-4]. Recently, the population of patients with NASH and NASH-related HCC has been increasing[1,2,10]. Hepatectomy is the first-line treatment for patients with HCC[21]. After hepatectomy, the incidences of mortality and morbidity are dependent on the volume and function of the residual liver[17-20]. Previous reports have demonstrated that steatosis impaired liver regeneration and caused liver dysfunction after hepatectomy[11,12]. NASH has been proposed to cause liver failure rather than steatosis because NASH presents with not only steatosis but also fibrosis, inflammation, and insulin resistance. However, regarding NASH animal models, few models completely reflect the histopathology and pathophysiology of NASH in humans. Therefore, the effect on the residual liver under NASH conditions has not been appropriately evaluated[26,27]. In our previous study, we established a novel experimental NASH model that exhibited histopathological and pathophysiological findings similar to that of NASH in humans[28]. In this study, new NASH mice were received 30% PH or 70% PH, and the influence of liver resection volume on the residual liver function in NASH liver was investigated. Our results indicated that the survival rate after PH in NASH liver strongly correlated with resected liver volume and was attributed to the proliferative ability and the rates of apoptosis and necrosis compared with those in the normal liver. Even 30% of PH NASH residual liver could not offer sufficient liver function, and the volume of the functional residual liver significantly decreased due to less cell proliferation, apoptosis and necrosis after hepatectomy. Based on these results, we hypothesized that the residual liver volume that can support sufficient function in a normal liver could not maintain liver function in NASH. Our results suggested that to avoid liver dysfunction after hepatectomy in NASH, resection volume should be carefully determined and not the same as that in patients with normal liver.
In patients with PH, fatty liver causes a high rate of mortality and morbidity compared with normal liver[11]. In NAFLD patients, postoperative complications also increase in a manner that is similar to patients with fatty liver[12,30]. In animal models with PH, the survival rate of a fatty liver model decreased compared with a normal liver even with the same residual volume[13,15,16,31]. In this study, the survival rate after PH remarkably decreased in the NASH 70% PH group, and 30% of the deaths occurred within the first 48 h after PH. This result supported the previous reports, i.e., outcome of PH significantly influences the survival rate of fatty mice[13,15,16,31]. In NASH liver, it was assumed that other characteristics, i.e., fibrosis, inflammation, and insulin resistance, caused increased liver function deterioration. It was hypothesized that the residual liver volume of the small group, i.e., 30% of residual volume of the NASH liver, could not maintain sufficient liver function for survival after PH.
Liver regeneration occurred in cases with acute injury and/or liver resection[19]. In normal liver after PH, cell proliferation was observed in a small residual liver but not in a large residual liver[24]. Large residual livers have sufficient volume to maintain liver function, whereas small residual livers are unable to maintain liver function. Therefore, promotion of cell proliferation occurs in the small residual liver[24]. Ki-67 protein is expressed during the G1, G2, and S phases of cell division[32,33]. In this study, the number of Ki-67-positive cells in the residual liver in the 30% PH of NASH liver group was higher than in the preoperative NASH liver. On the other hand, the number of Ki-67-positive cells in the residual liver of the 70% PH of NASH liver group was significantly decreased. These results suggested that NASH hepatocytes would not have insufficient proliferation ability after large amount of PH, such as 70%.
Signaling pathways of liver regeneration are promoted by cytokines, i.e., interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha, and growth factors[20]. The expression of AKT, STAT3, and ERK1/2 protein play an important role in liver regeneration, and the IL-6/STAT3 signaling pathway accelerates liver proliferation[16,24,34-36]. STAT3 was expressed at high levels in a liver with steatosis; however, these phenomena did not induce liver regeneration[37]. The NASH liver received continuous damage and stress via inflammatory cytokines and cells; therefore, NASH liver was considered to exhibit limited proliferation, which was only induced by hepatectomy[38]. In this study, the expression of transcriptional factors induced by cytokines for liver regeneration was not recognized in the residual livers of the NASH PH groups. No significant difference was noted in the activation of these proteins in both NASH PH groups, but liver cell proliferation was significantly higher in the 30% PH NASH liver group than in the 70% PH NASH liver group. Although we need to confirm these findings in future studies, the consistent decrease in liver proliferation exists, especially in the 70% PH of NASH liver group, i.e., a large hepatectomy volume, which reduces the survival rate.
In general, the stress of liver resection promotes apoptosis in the residual liver[13,39], and liver damage after hepatectomy is considered to be the result of apoptosis to some degree[38]. The degree of liver damage also depends on the extent of the liver resection volume[37,39]. The STAT3 and AKT signaling pathways not only promote liver regeneration but also inhibit apoptosis[38]. In this study, STAT3 and AKT expression was significantly suppressed, and the number of TUNEL-positive cells was higher in the NASH PH groups than in the control. These results suggest that the difference in the expression of regenerative signaling proteins affected the degree of apoptosis. Resection of a large volume of the liver also enhanced necrosis[38]. In this study, microarray analysis revealed GADD45A upregulation in the NASH 70% PH group. GADD45A promotes apoptosis and cell cycle arrest[13]. The differences in the survival rate between 30% or 70% PH in the NASH groups are inversely proportional to the incidence of Ki-67-positive cells, apoptosis, and necrosis. GADD45A upregulation correlates with the differences between the NASH groups and the low survival rate in small residual NASH liver after 70% PH.
In conclusion, residual NASH liver dysfunction after hepatectomy is attributed to a reduction in liver regeneration and cell proliferation. These findings suggest that the resection volume is a more limiting factor in patients with NASH than in those with a normal liver. Regarding liver surgery, the risk of complications for patients diagnosed with NASH by liver biopsy should be determined before hepatectomy. Further studies are needed to clarify therapeutic agents for NASH using our novel NASH model.
The population of patients with nonalcoholic steatohepatitis (NASH) and NASH-related hepatocellular carcinoma (HCC) has been increasing. However, few animal models fully reflect both the histopathology and pathophysiology of NASH in humans, therefore, the metabolism of the residual liver after a hepatectomy with NASH has not been clarified. We succeeded to establish a novel experimental NASH model that had same characteristics of histopathology and pathophysiology of NASH in humans.
In NASH, continuous inflammation contributes to HCC. The cause of HCC is frequently infection with hepatitis B virus and hepatitis C virus (HCV). New antiviral medications for hepatitis are currently being used in clinics; therefore, the number of patients with virus-related HCC is expected to decrease in the future. By contrast, the number of patients with NASH-related HCC has been increasing recently, and this trend is expected to continue because no effective treatments are available
The aim of this study was to investigate whether a difference in liver resection volume in a novel NASH model influences surgical outcomes.
To establishment of a NASH model, mice were fed a high-fat diet for 4 wk, administered CCl4 for the last 2 wk and administered T0901317 for the last 5 d. These mice were divided into two groups: A 30% partial hepatectomy (PH) of NASH liver group and a 70% PH of NASH liver group (control). Evaluate the survival rate, regeneration, apoptosis, necrosis and DNA expression level after PH.
In the 70% PH of NASH group, the survival rate was significantly decreased compared with that in the control and 30% PH of NASH groups (P < 0.01). 10 of 32 mice in the NASH 70% PH group died within 48 h after PH. serum aspartate aminotransferase (AST) levels and total bilirubin (T-Bil) in the NASH 70% PH group were significantly higher than the levels in the other two groups (AST: P < 0.05, T-Bil: P < 0.01). In both PH of NASH groups, signaling proteins involved in regeneration were expressed at lower levels than those in the control group (P < 0.01). The 70% PH of NASH group also exhibited a lower number of Ki-67-positive cells and higher rates of apoptosis and necrosis than the NASH 30% PH group (P < 0.01). In addition, DNA microarray assays showed differences in gene expression associated with cell cycle arrest and apoptosis.
The residual NASH liver dysfunction after hepatectomy is attributed to a reduction in liver regeneration and cell proliferation. A larger residual volume is required to maintain liver functions in mice with NASH.
This study suggests that the resection volume is a more limiting factor in patients with NASH than in those with a normal liver. Regarding liver surgery, the risk of complications for patients diagnosed with NASH by liver biopsy should be determined before hepatectomy. Further studies are needed to clarify therapeutic agents for NASH using our novel NASH model.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Lin GM, Tarantino G, Ulasoglu C S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Chitturi S, Farrell GC, George J. Non-alcoholic steatohepatitis in the Asia-Pacific region: future shock? J Gastroenterol Hepatol. 2004;19:368-374. [PubMed] [Cited in This Article: ] |
| 2. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 2593] [Article Influence: 129.7] [Reference Citation Analysis (3)] |
| 3. | White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342-1359.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 527] [Article Influence: 43.9] [Reference Citation Analysis (2)] |
| 4. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 532] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 5. | Yokosuka O, Takaguchi K, Fujioka S, Shindo M, Chayama K, Kobashi H, Hayashi N, Sato C, Kiyosawa K, Tanikawa K. Long-term use of entecavir in nucleoside-naïve Japanese patients with chronic hepatitis B infection. J Hepatol. 2010;52:791-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1946] [Cited by in F6Publishing: 1900] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 7. | Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051-1055. [PubMed] [Cited in This Article: ] |
| 8. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [PubMed] [Cited in This Article: ] |
| 9. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1739] [Cited by in F6Publishing: 1648] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 10. | Tateishi R, Okanoue T, Fujiwara N, Okita K, Kiyosawa K, Omata M, Kumada H, Hayashi N, Koike K. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol. 2015;50:350-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Veteläinen R, van Vliet A, Gouma DJ, van Gulik TM. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Molla NW, Hassanain MM, Fadel Z, Boucher LM, Madkhali A, Altahan RM, Alrijraji EA, Simoneau EB, Alamri H, Salman A. Effect of non-alcoholic liver disease on recurrence rate and liver regeneration after liver resection for colorectal liver metastases. Curr Oncol. 2017;24:e233-e243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Inaba Y, Furutani T, Kimura K, Watanabe H, Haga S, Kido Y, Matsumoto M, Yamamoto Y, Harada K, Kaneko S. Growth arrest and DNA damage-inducible 34 regulates liver regeneration in hepatic steatosis in mice. Hepatology. 2015;61:1343-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Haga S, Ozawa T, Yamada Y, Morita N, Nagashima I, Inoue H, Inaba Y, Noda N, Abe R, Umezawa K. p62/SQSTM1 plays a protective role in oxidative injury of steatotic liver in a mouse hepatectomy model. Antioxid Redox Signal. 2014;21:2515-2530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Murata H, Yagi T, Iwagaki H, Ogino T, Sadamori H, Matsukawa H, Umeda Y, Haga S, Takaka N, Ozaki M. Mechanism of impaired regeneration of fatty liver in mouse partial hepatectomy model. Journal of gastroenterology and hepatology. 2007;2173-2180. [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Aoyama T, Ikejima K, Kon K, Okumura K, Arai K, Watanabe S. Pioglitazone promotes survival and prevents hepatic regeneration failure after partial hepatectomy in obese and diabetic KK-A(y) mice. Hepatology. 2009;49:1636-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Helling TS. Liver failure following partial hepatectomy. HPB (Oxford). 2006;8:165-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Bernal W, Lee WM, Wendon J, Larsen FS, Williams R. Acute liver failure: A curable disease by 2024? J Hepatol. 2015;62:S112-S120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 19. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [PubMed] [Cited in This Article: ] |
| 20. | Forbes SJ, Newsome PN. Liver regeneration [mdash] mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016;13:473-485. [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 21. | Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309. [PubMed] [Cited in This Article: ] |
| 22. | Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Millet G, Truant S, Leteurtre E, Hebbar M, Zerbib P, Huet G, Boleslawski E, Pruvot FR. Volumetric analysis of remnant liver regeneration after major hepatectomy in bevacizumab-treated patients: a case-matched study in 82 patients. Ann Surg. 2012;256:755-761; discussion 761-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Meier M, Andersen KJ, Knudsen AR, Nyengaard JR, Hamilton-Dutoit S, Mortensen FV. Liver regeneration is dependent on the extent of hepatectomy. J Surg Res. 2016;205:76-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How Much Remnant Is Enough in Liver Resection? Digestive surgery. 2012;29:6-17. [Cited in This Article: ] |
| 26. | Nakagawa H. Recent advances in mouse models of obesity- and nonalcoholic steatohepatitis-associated hepatocarcinogenesis. World J Hepatol. 2015;7:2110-2118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2300-2308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 379] [Cited by in F6Publishing: 378] [Article Influence: 31.5] [Reference Citation Analysis (2)] |
| 28. | Owada Y, Tamura T, Tanoi T, Ozawa Y, Shimizu Y, Hisakura K, Matsuzaka T, Shimano H, Nakano N, Sakashita S. Novel non-alcoholic steatohepatitis model with histopathological and insulin-resistant features. Pathol Int. 2018;68:12-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Rashidi B, An Z, Sun FX, Sasson A, Gamagammi R, Moossa AR, Hoffman RM. Minimal liver resection strongly stimulates the growth of human colon cancer in the liver of nude mice. Clin Exp Metastasis. 1999;17:497-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Wakai T, Shirai Y, Sakata J, Korita PV, Ajioka Y, Hatakeyama K. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011;15:1450-1458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Ninomiya M, Shirabe K, Terashi T, Ijichi H, Yonemura Y, Harada N, Soejima Y, Taketomi A, Shimada M, Maehara Y. Deceleration of regenerative response improves the outcome of rat with massive hepatectomy. Am J Transplant. 2010;10:1580-1587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Gerlach C, Sakkab DY, Scholzen T, Dassler R, Alison MR, Gerdes J. Ki-67 expression during rat liver regeneration after partial hepatectomy. Hepatology. 1997;26:573-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 34. | Sydor S, Gu Y, Schlattjan M, Bechmann LP, Rauen U, Best J, Paul A, Baba HA, Sowa JP, Gerken G. Steatosis does not impair liver regeneration after partial hepatectomy. Lab Invest. 2013;93:20-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Nakamura Y, Mizuguchi T, Tanimizu N, Ichinohe N, Ooe H, Kawamoto M, Meguro M, Hirata K, Mitaka T. Preoperative hepatocyte transplantation improves the survival of rats with nonalcoholic steatohepatitis-related cirrhosis after partial hepatectomy. Cell Transplant. 2014;23:1243-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, Hisakura K, Nakano Y, Kohno K, Kawasaki T. Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol. 2008;49:363-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Torbenson M, Yang SQ, Liu HZ, Huang J, Gage W, Diehl AM. STAT-3 overexpression and p21 up-regulation accompany impaired regeneration of fatty livers. Am J Pathol. 2002;161:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Fujiyoshi M, Ozaki M. Molecular mechanisms of liver regeneration and protection for treatment of liver dysfunction and diseases. J Hepatobiliary Pancreat Sci. 2011;18:13-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Kubota T, Takabe K, Yang M, Sekido H, Endo I, Ichikawa Y, Togo S, Shimada H. Minimum sizes for remnant and transplanted livers in rats. J Hep Bil Pancr Surg. 1997;4:398-404. [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |