Published online Mar 7, 2017. doi: 10.3748/wjg.v23.i9.1618
Peer-review started: October 24, 2016
First decision: December 28, 2016
Revised: January 12, 2017
Accepted: February 6, 2017
Article in press: February 8, 2017
Published online: March 7, 2017
Processing time: 136 Days and 3.1 Hours
To quantify drug-drug-interactions (DDIs) encountered in patients prescribed hepatitis C virus (HCV) treatment, the interventions made, and the time spent in this process.
As standard of care, a clinical pharmacist screened for DDIs in patients prescribed direct acting antiviral (DAA) HCV treatment between November 2013 and July 2015 at the University of Colorado Hepatology Clinic. HCV regimens prescribed included ledipasvir/sofosbuvir (LDV/SOF), paritaprevir/ritonavir/ombitasvir/dasabuvir (OBV/PTV/r + DSV), simeprevir/sofosbuvir (SIM/SOF), and sofosbuvir/ribavirin (SOF/RBV). This retrospective analysis reviewed the work completed by the clinical pharmacist in order to measure the aims identified for the study. The number and type of DDIs identified were summarized with descriptive statistics.
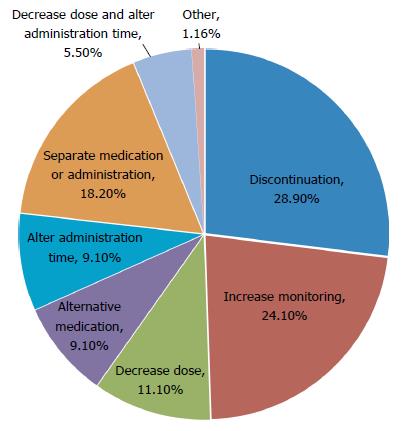
Six hundred and sixty four patients (83.4% Caucasian, 57% male, average 56.7 years old) were identified; 369 for LDV/SOF, 48 for OBV/PTV/r + DSV, 114 for SIM/SOF, and 133 for SOF/RBV. Fifty-one point five per cent of patients were cirrhotic. Overall, 5217 medications were reviewed (7.86 medications per patient) and 781 interactions identified (1.18 interactions per patient). The number of interactions were fewest for SOF/RBV (0.17 interactions per patient) and highest for OBV/PTV/r + DSV (2.48 interactions per patient). LDV/SOF and SIM/SOF had similar number of interactions (1.28 and 1.48 interactions per patient, respectively). Gastric acid modifiers and vitamin/herbal supplements commonly caused interactions with LDV/SOF. Hypertensive agents, analgesics, and psychiatric medications frequently caused interactions with OBV/PTV/r + DSV and SIM/SOF. To manage these interactions, the pharmacists most often recommended discontinuing the medication (28.9%), increasing monitoring for toxicities (24.1%), or separating administration times (18.2%). The pharmacist chart review for each patient usually took approximately 30 min, with additional time for more complex patients.
DDIs are common with HCV medications and management can require medication adjustments and increased monitoring. An interdisciplinary team including a clinical pharmacist can optimize patient care.
Core tip: Identification and management of potential drug-drug interactions (DDI) is a critical aspect of current hepatitis C virus (HCV) treatment. This retrospective analysis of patients prescribed common HCV treatments identifies DDIs and the interventions made by the clinical pharmacist, as well as the approximate time required to complete these activities. This novel review illustrates that DDIs are common in this population. Identification and management of DDIs is resource intensive and requires medication adjustments and increased monitoring. An interdisciplinary care team including a clinical pharmacist is critical to optimize patient care for new HCV therapies.
- Citation: Langness JA, Nguyen M, Wieland A, Everson GT, Kiser JJ. Optimizing hepatitis C virus treatment through pharmacist interventions: Identification and management of drug-drug interactions. World J Gastroenterol 2017; 23(9): 1618-1626
- URL: https://www.wjgnet.com/1007-9327/full/v23/i9/1618.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i9.1618
Approximately 4.6 million Americans are estimated to have been exposed to the hepatitis C virus (HCV), and 3.5 million of those have active chronic infection[1]. The "baby boomer" generation (persons born between 1945 and 1965) have the highest incidence of chronic HCV infection vs any other age group[2]. Fifteen to twenty percent of people infected with chronic HCV progress to liver cirrhosis within twenty years, which may lead to end-stage liver disease or hepatocellular carcinoma[3]. In the United States, at least 35% of patients on the liver transplant wait list have chronic HCV[4]. HCV-associated mortality is close to 500000 deaths per year world-wide[5]. The previous standard of care, treatment with peginterferon and ribavirin, had significant challenges to treatment including serious adverse events, non-oral administration, and low efficacy rates. Direct-acting antivirals (DAAs) have improved the treatment landscape through increased efficacy, improved safety and tolerability, and all-oral administration. However, drug-drug interactions (DDIs) are a significant challenge and managing the interactions can be complex and time-consuming[6].
Pharmacists are recognized as important members of the health care team. Pharmacists' involvement in anticoagulation services, human immunodeficiency virus (HIV) care, cystic fibrosis, and diabetes has been shown to increase adherence, reduce pill burden and dosing frequency, and decrease medication-related errors[7-19]. The role of pharmacists in improving medical care and managing adverse effects in HCV treatment is well-recognized, but the impact of pharmacy interventions on therapeutic outcomes has not been adequately assessed in patients with HCV[20-24]. Furthermore, there is a lack of evidence for managing real-world DDIs for HCV treatments, especially with oral DAAs.
The University of Colorado Hospital Outpatient Hepatology Clinic has a clinical pharmacist imbedded within the interdisciplinary care team. The hepatologist first assesses a patient with chronic HCV infection, determining the stage of liver fibrosis, diagnosing advanced liver disease or cirrhosis, investigating any disease complications, ordering all relevant baseline labs, and prescribing HCV treatment. The clinical pharmacist then reviews each patient prescribed HCV treatment to ensure correct dosing and dose adjustments as needed based on hepatic and renal function, to minimize therapeutic duplication, and to identify and manage DDIs. Each HCV medication has specific interactions with cytochrome P450 enzymes as well as transporters; and these medications can act as both victims and perpetrators in a number of DDIs. Potential DDIs are assessed using various resources including co-administration studies, medication package inserts, medication databases, and online tools such as http://www.hep-druginteractions.org. In situations where co-administration has not been studied, the pharmacology of each medication was reviewed to determine the potential for DDIs. Unfortunately, many herbal supplements lack data on pharmacokinetics and drug-drug interaction potential. If an herbal supplement did not have adequate data to ensure safe coadministration, the recommendation was often made to discontinue during HCV treatment. When managing DDIs, patient-specific factors such as vital signs, laboratory values, and concurrent use of other medications were accounted for. The clinical pharmacist coordinates with the internal medication access team to gain approval of the medication through the patient's prescription insurance plan or patient assistance programs. Once the patient is able to obtain medication, he or she meets with a physician assistant and the clinical pharmacist for a "medication start visit". During this visit, the specifics of treatment are reviewed, including the medication, dosing, administration, duration of treatment, potential side effects, and monitoring schedule. Clinically significant DDIs are reviewed with the patient and managed appropriately. The patient is then assessed through treatment by the physician assistant in conjunction with the clinical pharmacist.
Within the context of the role of the clinical pharmacist on the interdisciplinary team, the purpose of this study was to quantify (1) the type of DDI commonly encountered in patients prescribed HCV treatment in an academic outpatient hepatology clinic; (2) the interventions made; and (3) the time spent in identification and management of DDIs.
This retrospective review identified all patients with chronic HCV infection who were prescribed HCV treatment at the University of Colorado Outpatient Hepatology Clinic between November 2013 and July 2015. Patients were included regardless of their HCV genotype and stage of liver fibrosis. DAAs prescribed during the time period included ledipasvir/sofosbuvir (LDV/SOF), paritaprevir/ritonavir/ombitasvir/dasabuvir (OBV/PTV/r + DSV), simeprevir/sofosbuvir (SIM/SOF), and sofosbuvir/ribavirin (SOF/RBV). Ribavirin may or may not have been used with the first three regimens. Patients were excluded from the study if they were coinfected with HIV or were post-liver transplant.
Demographic data including age, body mass index (BMI), and self-identified gender, race, and ethnicity were collected using the electronic medical record. HCV genotype and stage of liver fibrosis were recorded. Patients were classified as minimal to moderate fibrosis (Stage 0-2), advanced fibrosis (Stage 3), and cirrhosis (Stage 4) including both compensated and decompensated[25]. Baseline medications for each patient were collected, including prescription medication, over-the-counter medication, and vitamin and herbal supplements. Combination products such as multivitamins, vitamin-mineral supplements, and vitamin-mineral-herbal supplements were classified as one product. The number, type, and recommended management of DDIs were recorded for each patient. Baseline medications that were involved with DDIs were classified into nine categories. These categories include analgesics, hypertension/heart failure agents, anticonvulsants, psychiatric agents, proton pump inhibitors (PPIs)/H2-receptor antagonists (H2RA), antacids, steroids, vitamin and herbal supplements, and others. The number and type of interactions were summarized with descriptive statistics, due to the heterogeneous nature of the retrospective study.
Management of interactions was classified as: (1) discontinue medication; (2) increase monitoring; (3) alter administration time; (4) separate administration; (5) decrease dose; and (6) continue. The approximate time required for the identification, assessment, and management for clinical pharmacist review was recorded.
664 patients fit the inclusion and exclusion criteria: 369 with LDV/SOF, 48 with OBV/PTV/r + DSV, 114 with SIM/SOF, and 133 with SOF/RBV. Patients were 57% male and averaged 56.7 years old. 83.4% of patients identified as Caucasian, 6.44% as Black or African American, 1.89% as Asian, 0.30% American Indian or Alaska Native, and 7.97% as other or unavailable. 87% identified as Non-Hispanic and 13% Hispanic. The majority (51.5%) of the patients in the study were cirrhotic. See Table 1 for full demographic information.
| Number of patients | 664 |
| Age (mean, yr) | 56.7% |
| Gender | |
| Female | 42.8% |
| Male | 57.2% |
| Race | |
| Caucasian | 83.4% |
| African American or Black | 6.44% |
| Asian American | 1.89% |
| American Indian or Alaska Native | 0.30% |
| Other and unavailable | 7.97% |
| Ethnicity | |
| Hispanic | 13.6% |
| Non-Hispanic | 86.4% |
| Number of patients on DAAs | |
| LDV/SOF | 369% |
| OBV/PTV/r + DSV | 48% |
| SIM/SOF | 114% |
| SOF/RBV | 133% |
| Fibrosis stage | |
| ≤ Stage 2 (minimal to moderate fibrosis) | 35.8% |
| Stage 3 (advanced fibrosis) | 10.8% |
| Stage 4 (cirrhosis) | 51.5% |
| Unknown or unavailable | 1.90% |
Overall, 5217 medications were reviewed (7.86 medications per patient) and 781 interactions identified (1.18 interactions per patient) (Table 2). The average number of medications for each regimen was similar and ranged from 6.50 (OBV/PTV/r + DSV) to 8.79 (SIM/SOF). The average number of DDIs was lowest for SOF/RBV with 0.17, then 1.28 and 1.48 for LDV/SOF and SIM/SOF, respectively. OBV/PTV/r + DSV had the most DDIs per patient with 2.48. When accounting for stage of liver disease, the number of medications per patient trended upward from 6.50 for patients with minimal fibrosis to 8.99 for patients with cirrhosis (Table 3). Despite the greater number of concomitant medications, there was a similar average number of DDIs in those with minimal vs more advanced disease. The most common interactions (identified as ≥ 10%) were vitamin and herbal supplements (284/781, 36.4%), PPI/H2RA agents (117/781, 15.0%), and other products (126/781, 16.1%). Table 4 summarizes the interactions amongst the different drug classes. Figure 1 shows the recommendations made for the management of DDIs.
| Regimen | n = 664 | Total number of meds | Total number of interactions, n (%) | Average number of meds per patient | Average number of interactions per patient | Contra-indications |
| LDV/SOF | 369 | 2996 | 472 (15.8) | 8.12 | 1.28 | 7 |
| OBV/PTV/r + DSV | 48 | 312 | 119 (38.1) | 6.50 | 2.48 | 4 |
| SIM/SOF | 114 | 1002 | 169 (16.8) | 8.79 | 1.48 | 19 |
| SOF/RBV | 133 | 964 | 21 (2.2) | 7.25 | 0.16 | 1 |
| Fibrosis stage | n = 664 | Total number of medications | Total number of interactions n (%) | Average number of medications per patient | Average number of interactions per patient |
| Minimal fibrosis (Stage 0-2) | 232 | 1508 | 249 (16.5) | 6.50 | 1.07 |
| Advanced fibrosis (Stage 3) | 72 | 575 | 91 (15.8) | 7.99 | 1.26 |
| Cirrhosis (Stage 4) | 341 | 3066 | 425 (13.8) | 8.99 | 1.25 |
| Drug class | Affected portion of the cohort n = 664 |
| PPI/H2RA agents | 117 (17.6) |
| Antacids | 72 (10.8) |
| Vitamin and herbal supplements | 284 (42.7) |
| Hypertensive agents | 53 (8.0) |
| Analgesics | 67 (10.1) |
| Psychiatric agents | 46 (6.9) |
| Anticonvulsants | 4 (0.6) |
| Steroids | 12 (1.8) |
| Others | 126 (19.0) |
In 369 patients prescribed LDV/SOF, 472 drug-drug interactions were identified. Common interactions (defined as ≥ 10%) with LDV/SOF included antacids (72/472, 15.3%), PPI/H2RA agents (107/472, 22.7%), and vitamin/herbal supplements (227/472, 48.1%). Ledipasvir, an NS5A inhibitor, is better absorbed in an acidic environment. When omeprazole 20 mg was administered once daily 2 h prior to LDV, area underneath the curve (AUC) decreases to 0.58[26]. Therefore, absorption is decreased with any medications that affect stomach acidity. Overall, interactions with antacids and PPI/H2RA agents occurred with (118/472, 25.0%) of our patients prescribed LDV/SOF. This interaction can be challenging for multiple reasons. These medications are available without prescription and often patients can forget to report them during medication reconciliation. Each patient prescribed LDV/SOF was explicitly asked if they were taking any prescription or non-prescription medications for heartburn or gastric esophagitis reflux diseas, or any other type of antacids and PPI/H2RA agents. Another challenging aspect is that PPIs are recommended for patients post banding ligation, a comorbidity common in patients with advanced liver disease. In order to manage the DDIs with PPI/H2RA agents, 54.2% were on the appropriate dose but were required to alter administration time; 40.2% were required to both decrease dose and alter administration time (40.2%). Supplements such as milk thistle, cod liver, krill oil, garlic cap, turmeric, and saw palmetto were recommended to be put on hold (70.4%) or separated from LDV/SOF (28.3%) administration time. Although there were few interactions with anticonvulsants (5/472, 0.85%), each occurrence was associated with a contraindication with LDV/SOF. For patients taking carbamazepine, oxcarbazepine, phenobarbital, and phenytoin, the recommendation was made to transition to an alternative anticonvulsant prior to initiation of HCV treatment.
Analgesics (22/119, 18.5%), vitamins and herbal supplements (21/119, 17.6%), and hypertensive agents (19/119, 16.0%) frequently interact with OBV/PTV/r + DSV. Managing interactions with analgesics such as morphine, oxycodone, tramadol, and hydrocodone, can be complicated due to the variability in dosing, patient response, and opioid tolerance. In order to manage the DDIs with analgesics, the dose was most often reduced and monitoring increased (81.8%). Supplements were recommended to be discontinued during HCV therapy (57.1%), separated by at least four hours (28.6%), or increased monitoring for adverse events (14.3%). Hypertensive agents including furosemide and amlodipine can have a greater affect due to the DDI with OBV/PTV/r + DSV. Depending on the dose of the hypertensive medications and the patient's blood pressure, the medications were continued at the same dose with increased monitoring for hypotension (7/19, 36.8%) or to decrease the dose (12/19, 63.2%) in anticipation for increased plasma concentration levels of the hypertensive agents. Other medication classes that interacted were erectile dysfunction agents (tadalafil), lipid lowering agents (pravastatin, rosuvastatin), allergy symptom medications (cetirizine), and insomnia agents (trazodone). Depending on the medication and indication, often the recommendation was to decrease the dose (23.5%) or discontinue the agent (11.8%).
Analgesics (21.3%), hypertensive agents (13.0%), psychiatrics (20.1%), and vitamins and herbal supplements (10.65%) frequently interact with SIM/SOF. Even though most of these medications have not been evaluated for DDIs with SIM/SOF, we anticipate the plasma concentration of these medications to increase due to mild inhibition of CYP3A4 and OAT1B1 from simeprevir[27]. As a result, increase monitoring for side effects and decrease dose were frequently recommended for analgesics (76.3%; 21.1%), hypertensive agents (70.0%; 25.0%), and psychiatrics (82.4%; 14.7%). Herbal supplements such as St. John's wort and milk thistle are contraindicated with SIM/SOF. When administered with inducers or inhibitors of CYP3A4 enzyme, the plasma concentration of SIM is expected to change[28,29]. Therefore, both St. John's wort and milk thistle were recommended to be discontinued during the course of HCV therapy (100%). Other medications that had potential interaction with SIM/SOF were lipid lowering agents, anti-nausea medications, bladder dysfunction medications, anti-bacterial agents, and insomnia medications. Based on the metabolism of these medications, the most common recommendations were to increase monitoring for side effects (56.0%) or to reduce the dose (28.0%) of these medications (Figure 1).
SOF/RBV had the fewest identified DDIs of any regimen. Ribavirin has the fewest direct interactions for any of the HCV medications, and sofosbuvir has relatively few as well. Vitamins and herbal supplements that have not been studied with SOF/RBV represented the largest group of potential DDIs (81.8%). Since DDIs have not been evaluated with supplements such as milk thistle, turmeric, mushroom extract, and horny goat weed, it was recommended to discontinue these while on therapy for treating HCV infection (100%). Due to the DDI between sofosbuvir and carbamazepine, the contraindicated medication was discontinued prior to initiating HCV treatment in one patient.
The clinical pharmacist was able to record his time spent reviewing medications in 105 consults. These consults included LDV/SOF, OBV/PTV/r + DSV, and SOF/RBV, but did not include SIM/SOF. The time requirement increased with the complexity of the patient and the number of baseline medications and drug-drug interactions identified. Consults for patients prescribed OBV/PTV/r + DSV took the longest for the pharmacist to complete, averaging 30 min per consult. SOF/RBV and LDV/SOF were slightly shorter averaging 20 min per consult. This is consistent in relation to our data that shows more DDIs were identified in patients prescribed OBV/PRV/r + DSV.
Drug-drug interactions continue to be a considerable challenge for managing patients with HCV treatment. This study assessed the frequency and pharmacological category of identified DDIs in real-world patients with HCV in addition to describing the management of the most common DDIs encountered with the DAAs. Published data on this topic are lacking. In 2010, a US insurance cohort showed the top four medication classes in patients with chronic HCV were analgesics and/or antipyretics, antidepressants, antivirals, and gastrointestinal agents including proton pump inhibitors[29]. Additionally, the study identified the average number of baseline medications as 9.0 and 11.4 in HCV treated and untreated patients, respectively. Höner Zu Siederdissen et al[30] published an account of potential drug-drug interactions in a cohort of patients in Hanover, Germany. Our result for the number of medications per patient at baseline study (7.86 medications per patient) was comparable with the US cohort. A significant number of DDIs predicted in our patient cohort were with analgesics (9%), anti-hypertensives (7%), and psychiatrics (6%). With the exception of psychiatrics and analgesics, this is comparable with both the US and German cohorts.
Use of over-the-counter medications and herbal supplements presents a challenge for assessing and managing DDIs in patients. Herbal products are gaining popularity in the US and there is a perception that herbal medications are safer than conventional medications[31]. These supplements can be recommended by people other than the patient's primary healthcare provider. Other times, patients will forget or not realize to inform the provider about non-prescription medications. In the HALT-C study, 44% of 1145 participants in the study with HCV infection admitted past or current use of herbal supplements[29]. Because the supplements are not prescribed, often they are not documented in the electronic medical records. Additionally, non-prescription medications and herbal supplements are rarely studied for interactions. When data were available or the potential interaction was minimal, the clinical pharmacist usually recommended continuing the product. However, if there were no data to support the safe use of the supplement with HCV treatment, the clinical pharmacist generally recommended it to be discontinued. This was especially true with supplements that had no clear benefit to the patient. Supplements such as multivitamins, fish oil, and probiotics that have shown benefits in certain populations, were most often recommended to separate from the DAAs in order to avoid any potential absorption interaction.
As with most hepatology clinics, the University of Colorado Hospital Hepatology Clinic largely acts as a consult service for liver disease management. As a result, providers through other clinics prescribe most concomitant medications. Adjusting or switching a medication due to DDIs can be relatively straightforward with certain medication classes such as lipid lowering agents and antihypertensive medications. However, management can be complex with other DDIs. All currently available HCV therapies have DDIs with certain anticonvulsants. These anticonvulsants are normally prescribed and managed through a specialty neurology clinic, sometimes outside of the health-system. The clinical pharmacist may not have access to the neurology clinic notes, and vice versa. Depending on the disease state being treated and specific patient factors, an alternative anticonvulsant that does not interact may not be appropriate. If an appropriate alternative is found, the switch can involve specific titration schedules and overlap, requiring management and specific monitoring. DDIs involving antipsychotic medication or other mental health medications are also complex. Patient responses are varied and sometimes unpredictable to certain agents, and exacerbation of mental health disease is a significant health concern. In these situations, it may be pertinent to choose a different HCV medication regimen with fewer interactions as opposed to switching the concomitant medications. Prescription insurance companies often have a specific formulary agent, and obtaining approval for an alternative HCV regimen can be challenging and time consuming.
Serious adverse events have been reported due to DDIs. This includes a case of rhabdomyolysis associated with telaprevir and simvastatin, renal failure related to the increased levels of tacrolimus after starting protease inhibitor therapy, new-onset diabetes due to the interactions between LDV/SOF and tenofovir, and severe bradyarrhythmias due to the interaction between amiodarone and sofosbuvir[32-35]. Although relatively rare, these interactions can lead to very serious patient harm or potentially death. These cases illustrate the importance of having knowledge of possible drug-drug interactions to prevent severe side effects. In our study, there were no documented significant adverse events due to drug-drug interactions. By understanding the pharmacodynamics and pharmacokinetic profiles of the drugs involved with interactions, therapy can be safely and effectively managed in patients with HCV infection.
This study is limited by the retrospective nature of the review. Additionally, it is a single-center study at an academic medical center and regional transplant center, thereby limiting the relatability of the results to smaller clinics with less complexity. Additionally, the region lacks significant diversity and the result may differ with patients of different racial and ethnic backgrounds. Potential selection bias can also limit the results, as the patients were not randomized to each medication regimen but selected based on patient specific characteristics, medication regimen characteristics, provider experience and judgment, and insurance formulary. For patients with cirrhosis, depending on compensation and Child-Pugh Score, he or she may not be eligible to receive certain medication regimens. Due to all of the reasons, only descriptive statistics were used to analyze the results. Additionally, measuring the time requirement for the pharmacists to complete a consult was challenging. Anecdotally, as the pharmacist was more familiar with the medications and common DDIs, the time spent per patient was shortened. Additionally, due to the nature of a consult position, interruptions were common making it hard to capture the true amount of time spent per consult. For these reasons, caution should be warranted when applying these data to other clinics.
In conclusion, DDIs are common in patients prescribed HCV medications and the involvement of a clinical pharmacist can be beneficial to the interdisciplinary hepatology team. Identification and management of DDIs is resource intensive and requires medication adjustments and increased monitoring. Clinical pharmacists can encourage preventive measures on reducing HCV transmission, increase education adherence, assist in initiating HCV treatment, assist in monitoring clinical and adverse effects, and facilitate medication acquisition.
Identification and management of potential drug-drug interactions (DDIs) is a critical aspect of current hepatitis C virus (HCV) treatment. Direct-acting antivirals (DAAs) have improved the treatment landscape through increased efficacy, improved safety and tolerability, and all-oral administration. However, DDIs are a significant challenge and managing the interactions can be complex and time-consuming.
Drug-drug interactions continue to be a considerable challenge for managing patients with HCV treatment. This study assessed the frequency and pharmacological category of identified DDIs in real-world patients with HCV in addition to describing the management of the most common DDIs encountered with the DAAs.
This retrospective analysis of patients prescribed common HCV treatments identifies DDIs and the interventions made by the clinical pharmacist, as well as the approximate time required to complete these activities. This novel review illustrates that DDIs are common in this population. Identification and management of DDIs is resource intensive and requires medication adjustments and increased monitoring. An interdisciplinary care team including a clinical pharmacist is critical to optimize patient care for new HCV therapies.
Drug-drug interactions continue to be a considerable challenge for managing patients with HCV treatment. In this study the authors assessed the frequency and pharmacological category of identified drug-drug-interactions in real-world patients with HCV. This study suggests an interdisciplinary approach for managing DDIs. In my opinion, publication will be valuable.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B,B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Akyar E, Altintas E, Ho SB S- Editor: Yu J L- Editor: A E- Editor: Liu WX
| 1. | Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 2. | Smith BD, Patel N, Beckett GA, Jewett A, Ward JW. Hepatitis C virus antibody prevalence, correlates and predictors among persons born from 1945 through 1965, United States, 1999-2008 [Abstract]. November 6, 2011. San Francisco, CA: American Association for the Study of Liver Disease 2011; abstract 241. |
| 3. | Hiller KM, Haukoos JS, Heard K, Tashkin JS, Paradis NA. Impact of the Final Rule on the rate of clinical cardiac arrest research in the United States. Acad Emerg Med. 2005;12:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1382] [Article Influence: 138.2] [Reference Citation Analysis (1)] |
| 5. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9582] [Article Influence: 737.1] [Reference Citation Analysis (0)] |
| 6. | Maasoumy B, Port K, Calle Serrano B, Markova AA, Sollik L, Manns MP, Cornberg M, Wedemeyer H. The clinical significance of drug-drug interactions in the era of direct-acting anti-viral agents against chronic hepatitis C. Aliment Pharmacol Ther. 2013;38:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Bunting BA, Cranor CW. The Asheville Project: long-term clinical, humanistic, and economic outcomes of a community-based medication therapy management program for asthma. J Am Pharm Assoc (2003). 2003;46:133-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Bungard TJ, Gardner L, Archer SL, Hamilton P, Ritchie B, Tymchak W, Tsuyuki RT. Evaluation of a pharmacist-managed anticoagulation clinic: Improving patient care. Open Med. 2009;3:e16-e21. [PubMed] |
| 9. | Garabedian-Ruffalo SM, Gray DR, Sax MJ, Ruffalo RL. Retrospective evaluation of a pharmacist-managed warfarin anticoagulation clinic. Am J Hosp Pharm. 1985;42:304-308. [PubMed] |
| 10. | Young S, Bishop L, Twells L, Dillon C, Hawboldt J, O'Shea P. Comparison of pharmacist managed anticoagulation with usual medical care in a family medicine clinic. BMC Fam Pract. 2011;12:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Mooney K, Ryan C, Downey DG. Pharmacists' perspectives on monitoring adherence to treatment in Cystic Fibrosis. Int J Clin Pharm. 2016;38:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 591] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 13. | Viktil KK, Blix HS. The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin Pharmacol Toxicol. 2008;102:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Tseng A, Foisy M, Hughes CA, Kelly D, Chan S, Dayneka N, Giguère P, Higgins N, Hills-Nieminen C, Kapler J. Role of the Pharmacist in Caring for Patients with HIV/AIDS: Clinical Practice Guidelines. Can J Hosp Pharm. 2012;65:125-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Henderson KC, Hindman J, Johnson SC, Valuck RJ, Kiser JJ. Assessing the effectiveness of pharmacy-based adherence interventions on antiretroviral adherence in persons with HIV. AIDS Patient Care STDS. 2011;25:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Ma A, Chen DM, Chau FM, Saberi P. Improving adherence and clinical outcomes through an HIV pharmacist's interventions. AIDS Care. 2010;22:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | March K, Mak M, Louie SG. Effects of pharmacists' interventions on patient outcomes in an HIV primary care clinic. Am J Health Syst Pharm. 2007;64:2574-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Merchen BA, Gerzenshtein L, Scarsi KK, Achenbach C, Postelnick M, Zavod RM. HIV-specialized pharmacists' impact on prescribing errors in hospitalized patients on antiretroviral therapy [abstract H2-794]. 51st Interscience Conference on Antimicrobial Agents and Chemotherapy;. 2011;Sep 17-20; Chicago (IL). |
| 19. | Heelon M, Skiest D, Tereso G, Meade L, Weeks J, Pekow P, Rothberg MB. Effect of a clinical pharmacist's interventions on duration of antiretroviral-related errors in hospitalized patients. Am J Health Syst Pharm. 2007;64:2064-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Rodis J. Chronic hepatitis C virus infection: a review for pharmacists. J Am Pharm Assoc (2003). 2007;47:508-520; quiz 508-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Smith JP. Treatment options for patients with hepatitis C: role of pharmacists in optimizing treatment response and managing adverse events. Pharmacotherapy. 2008;28:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Shatin D, Schech SD, Patel K, McHutchison JG. Population-based hepatitis C surveillance and treatment in a national managed care organization. Am J Manag Care. 2004;10:250-256. [PubMed] |
| 23. | Smith JP, Dong MH, Kaunitz JD. Evaluation of a pharmacist-managed hepatitis C care clinic. Am J Health Syst Pharm. 2007;64:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Kolor B. Patient education and treatment strategies implemented at a pharmacist-managed hepatitis C virus clinic. Pharmacotherapy. 2005;25:1230-1241. [PubMed] [DOI] [Full Text] |
| 25. | Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 644] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 26. | Harvoni(R) [package insert]. Foster City, CA: Gilead Sciences; 2016. |
| 27. | Olysio(R) [package insert]. Latina, Italy: Janssen; 2015. |
| 28. | Solvadi(R) [package insert]. Foster City, CA: Gilead Sciences; 2015. |
| 29. | Lauffenburger JC, Mayer CL, Hawke RL, Brouwer KL, Fried MW, Farley JF. Medication use and medical comorbidity in patients with chronic hepatitis C from a US commercial claims database: high utilization of drugs with interaction potential. Eur J Gastroenterol Hepatol. 2014;26:1073-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Höner Zu Siederdissen C, Maasoumy B, Marra F, Deterding K, Port K, Manns MP, Cornberg M, Back D, Wedemeyer H. Drug-Drug Interactions With Novel All Oral Interferon-Free Antiviral Agents in a Large Real-World Cohort. Clin Infect Dis. 2016;62:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Siddiqui U, Weinshel EH, Bini EJ. Prevalence and predictors of herbal medication use in veterans with chronic hepatitis C. J Clin Gastroenterol. 2004;38:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Premji R, Roopnarinesingh N, Qazi N, Nylen ES. New-Onset Diabetes Mellitus With Exposure to Ledipasvir and Sofosbuvir. J Investig Med High Impact Case Rep. 2015;3:2324709615623300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Fontaine H, Lazarus A, Pol S, Pecriaux C, Bagate F, Sultanik P, Boueyre E, Corouge M, Mallet V, Vallet-Pichard A. Bradyarrhythmias Associated with Sofosbuvir Treatment. N Engl J Med. 2015;373:1886-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Kanter CT, Luin Mv, Solas C, Burger DM, Vrolijk JM. Rhabdomyolysis in a hepatitis C virus infected patient treated with telaprevir and simvastatin. Ann Hepatol. 2014;13:452-455. [PubMed] |
| 35. | Werner CR, Egetemeyr DP, Lauer UM, Nadalin S, Königsrainer A, Malek NP, Berg CP. Telaprevir-based triple therapy in liver transplant patients with hepatitis C virus: a 12-week pilot study providing safety and efficacy data. Liver Transpl. 2012;18:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |