Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.986
Peer-review started: August 31, 2016
First decision: September 28, 2016
Revised: October 11, 2016
Accepted: October 27, 2016
Article in press: October 27, 2016
Published online: February 14, 2017
Processing time: 165 Days and 7.8 Hours
AIM
To clarify the mechanisms involved in the critical endoplasmic reticulum (ER) stress initiating unfolded protein response pathway modified by melatonin.
METHODS
Hepatoma cells, HepG2, were cultured in vitro. Flow cytometry and TUNEL assay were used to measure HepG2 cell apoptosis. Western blotting and quantitative reverse transcription-polymerase chain reaction methods were used to determine the protein and messenger RNA levels of ER stress and apoptosis related genes’ expression, respectively. Tissue microarray construction from patients was verified by immunohistochemical analysis.
RESULTS
In the present study, we first identified that melatonin selectively blocked activating transcription factor 6 (ATF-6) and then inhibited cyclooxygenase-2 (COX-2) expression, leading to enhanced liver cancer cell apoptosis under ER stress condition. Dramatically increased CCAAT-enhancer-binding protein homologous protein level, suppressed COX-2 and decreased Bcl-2/Bax ratio by melatonin or ATF-6 siRNA contributed the enhanced HepG2 cell apoptosis under tunicamycin (an ER stress inducer) stimulation. In clinical hepatocellular carcinoma patients, the close relationship between ATF-6 and COX-2 was further confirmed.
CONCLUSION
These findings indicate that melatonin as a novel selective ATF-6 inhibitor can sensitize human hepatoma cells to ER stress inducing apoptosis.
Core tip: Endoplasmic reticulum (ER) stress plays an important role in tumor growth and resistance to treatment. Our previous studies have already shown that melatonin sensitizes the human hepatocellular carcinoma cell to ER stress-induced apoptosis and attenuates ER stress-induced doxorubicin resistance. In this study, we first identified that melatonin selectively blocked ER stress downstream activating transcription factor 6 (ATF-6) and then inhibited cyclooxygenase-2 expression, leading to enhanced liver cancer cell apoptosis under ER stress condition. Our findings indicate that melatonin as a novel selective ATF-6 inhibitor can sensitize human hepatoma cells to ER stress inducing apoptosis.
- Citation: Bu LJ, Yu HQ, Fan LL, Li XQ, Wang F, Liu JT, Zhong F, Zhang CJ, Wei W, Wang H, Sun GP. Melatonin, a novel selective ATF-6 inhibitor, induces human hepatoma cell apoptosis through COX-2 downregulation. World J Gastroenterol 2017; 23(6): 986-998
- URL: https://www.wjgnet.com/1007-9327/full/v23/i6/986.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i6.986
Hepatocellular carcinoma (HCC) was the fifth most frequently diagnosed cancer worldwide and the third cause of cancer-related death, responsible for approximately 700000 human deaths annually worldwide[1,2]. China accounts for the majority of total HCC incidence in the world[1]. Most patients with HCC are diagnosed at a late stage, when there is no chance of surgical therapy. Unfortunately, there are only a few effective chemotherapy agents for this type of malignant tumor. One of the major reasons for untreatable HCC is that the liver cancer cell has much greater tolerance towards a number of cellular stress conditions, such as endoplasmic reticulum (ER) stress, hypoxia, nutrient deprivation and so on[3,4]. Thus, approaches to overcoming this superior tolerance have been emerging as potential drug targets for the treatment of HCC[5].
All types of stress, including oncogenetic stress that interferes with ER function, cause accumulation of unfolded proteins in the ER lumen, referred to as ER stress, and activate a homeostatic signaling network known as the unfolded protein response (UPR)[6,7]. The three main ER transmembrane sensors that elicit the UPR are protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme-1 (IRE-1), and activating transcription factor 6 (ATF-6). In general, ER stress initiated by IRE-1a, PERK, and ATF-6 and activated in solid tumors is crucial for tumor growth and aggressiveness, as well as for microenvironment remodeling or drug resistance[3,4].
Melatonin (N-acetyl-5-methoxytryptamine), which is produced in the human pineal gland during the night phase of the light-dark cycle, plays important roles in physiological and pharmacological functions, such as circadian rhythms and antioxidants. More interestingly to us, however, is the fact that melatonin exerts anticancer effects through interplay with ER stress[8-10]. Our previous study demonstrated that melatonin could sensitize the human HCC cell line HepG2 to ER stress-induced apoptosis via the inhibition of cyclooxygenase-2 (COX-2)[11]. Furthermore, we found that melatonin attenuates ER stress-induced resistance to doxorubicin through reversing tunicamycin-induced ER stress[12]. However, the mechanisms involved in the critical UPR pathway modified by melatonin must still be clarified.
In the present study, we first identified that melatonin can selectively block ATF-6 and then inhibit COX-2 expression, leading to enhanced liver cancer cell apoptosis. In clinical HCC patients, the close relationship between ATF-6 and COX-2 was further confirmed. Our study explored the more detailed mechanisms of melatonin enhancing ER stress-induced apoptosis in human hepatoma cells via inhibition of COX-2 by selectively targeting ATF-6.
Melatonin (M5250) and tunicamycin (T7765) were obtained from Sigma Chemical (St. Louis, MO, United States). DMEM was purchased from Gibco-BRL Life Technologies (Grand Island, NY, United States). Anti-COX-2 (ab179800), anti-Bax (ab32503) and anti-CCAAT/enhancer-binding protein homologous protein (CHOP) (ab11419) were obtained from Abcam (Cambridge, MA, United States). Anti-ATF-6 (BS6476), anti-PERK (BS2156) and anti-Bcl-2 (BS3711) were obtained from Bioworld Technology Inc. (St Louis Park, MN, United States). The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) system was purchased from Roche (Indianapolis, IN, United States). The annexin V-FITC kit was obtained from Shanghai Bestbio (Shanghai, China). The reverse transcription kit (A3500) and TRIzol (15596-026) were obtained from Promega Inc. (Madison, WI, United States). The SYBR Green qPCR kit (11744-100) was obtained from Invitrogen Life Technologies (Grand Island, NY, United States).
Tissue samples were obtained from 100 patients with HCC. These 100 patients underwent surgery at the First Affiliated Hospital of Anhui Medical University between 2001 and 2007. Patients with HCC who had accepted chemotherapy or radiation therapy before surgery were excluded from the study. The pathohistological diagnosis of the specimens was consistent with HCC in accordance with the World Health Organization Guidelines. A total of 85 HCC patients were male, and 15 HCC patients were female. The median age of the HCC patient population was 50.7 years, ranging from 18 to 84 years. These 100 HCC patients were staged according to UICC as follows: 3 HCC patients were Stage I (3%), 73 HCC patients were Stage II (73%), 9 HCC patients were Stage III (9%), and 15 patients were Stage IV (15%). Tumors were pathologically graded according to WHO guidelines: 4 HCC patients were well-differentiated, 91 HCC patients were moderately differentiated, and 5 HCC patients were poorly differentiated. All the clinical specimens were collected from patients after obtaining written informed consent. The study was carried out in accordance with a protocol approved by the Ethics Committee of Anhui Medical University (Anhui, China).
Formalin-fixed paraffin-embedded specimens were obtained from the archives of the Department of Pathology at the First Affiliated Hospital of Anhui Medical University. Hematoxylin and eosin-stained tissue sections were reviewed for identification of the target area for tissue microarray construction. Three to five representative 1-mm cores were obtained from each sample and inserted in a grid pattern into a new recipient paraffin block using a manual tissue arrayer (Hengtai Instruments, Liaoning, China).
Tissue microarray sections were deparaffinized, and endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 min and heated in 0.01 mol/L sodium citrate buffer (pH 6.0) for 10 min for antigen retrieval. Subsequently, the sections were incubated with a primary antibody in a moist chamber for 1 h at ambient temperature. Then, the sections were washed in phosphate buffered saline (PBS) (pH 7.2), incubated with a biotinylated secondary antibody and then incubated with peroxidase-conjugated streptavidin. To observe positive binding of the antigen, the sections were incubated with diaminobenzidine solution and then counterstained with hematoxylin. Next, the sections were viewed under a microscope and scored on the basis of staining intensity and the percentage of stained cells relative to the background: > 10% of tumor cells stained was considered positive staining.
The human hepatoma cell line HepG2 was obtained from the Shanghai Cell Bank (Chinese Academy of Sciences, Shanghai, China). The cells were cultured in high glucose Dulbecco's modified Eagle's medium (DMEM) containing 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cell culture was carried out at 37 °C in a humidified 5% CO2 atmosphere.
After treatment, cells were detached from the 6-well plates by using 0.2% trypsin, harvested, washed twice with PBS, and centrifuged twice at 300 g for 5 min at 4 °C. Then, the supernatant was discarded, and the pellet was resuspended in 400 μL Annexin V binding buffer at 20 °C for at least 12 h. Cells were subsequently treated in PBS with RNase A for 30 min at room temperature and stained with propidium iodide (PI). Flow cytometric analysis was performed using an EPICS XL-MCL model counter (Beckman Coulter, Fullerton, CA, United States). A total of 1 × 106 cells/mL were analyzed for each sample, and the experiment was repeated at least three times.
Cells were cultured on coverslips in 6-well plates overnight. After treatment with various concentrations of the indicated compounds for each time period, the coverslips were washed twice with cold PBS and fixed in a 4% paraformaldehyde solution for 1 h at room temperature. Apoptotic cells were detected by the TUNEL assay (TUNEL System Kit; Roche, Basel, Switzerland), which was performed according to the manufacturer's instructions. The TUNEL assay results were quantitatively analyzed through the biological image analysis system from the Nikon ECLIPSE 80i biology microscope, Nikon Digital Camera DXM 1200F, ACT-1 version 2.63 software (Tokyo, Japan).
After drug treatment for the indicated time periods and concentrations, cells were lysed in RIPA lysis buffer [50 mmol/L Tris-HCl, (pH 7.4), 150 mmol/L NaCl, 10 mmol/L phenylmethylsulfonyl fluoride (PMSF), 1 mmol/L ethylene diamine tetraacetic acid (EDTA), 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-100 and 1% sodium deoxycholate] for 20-30 min on ice. Protein concentrations were determined by the Lowry protein assay. Lysates were incubated with 2 × Laemmli sample buffer (Bio-Rad, Hercules, CA, United States) and heated for 10 min at 95 °C. The proteins were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), then transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, United States) and incubated with blocking buffer [Tris-buffered saline/Tween 20/5% nonfat dry milk] overnight at 4 °C. Immunoblots were incubated with the indicated primary antibody followed by the appropriate horseradish peroxidase-conjugated secondary antibody and visualized with enhanced chemiluminescence (Pierce, Rockford, IL, United States) using hydrogen peroxide and luminol as substrate with Kodak X-AR film. Autoradiographs were scanned using a GS-700 Imaging Densitometer (Bio-Rad).
Total RNA was extracted from HepG2 cells using the Trizol reagent, and 1 mg RNA was reverse transcribed to cDNA using the Reverse Transcription System A3500 (Fermentas, Burlington, Canada). To determine the quantity of mRNA, the cDNA was amplified by real-time PCR with a SYBR Green PCR master mix kit (Invitrogen), and the housekeeping gene GAPDH was used as the internal control. The SYBR Green assays were performed in triplicate on a 7500 real-time instrument (Applied Biosystems Inc, Foster City, CA, United States). The primers to detect mRNA were 5'-CTGTATCCCGCCCTGCTGGTG-3' and 5'-ACTTGCGTTGATGGTGGCTGTCTT-3' for COX-2 and 5'-AGAAGGCTGGGGCTCATTTG-3' and 5'-AGGGGCCATCCACAGTCTTC-3' for GADPH. All samples were normalized to internal controls, and fold-changes were calculated by relative quantification. The conditions for quantitative reverse transcription polymerase chain reaction (qRT-PCR) were as follows: 5 min at 94 °C, and then 50 cycles of 94 °C for 30 s, 59 °C for 30 s, and 72 °C for 1 min.
Three or more separate experiments were performed for each experiment. Statistical analysis was performed by Student's t-test or ANOVA. Data are presented as the mean ± SD. Significance was noted at P < 0.05.
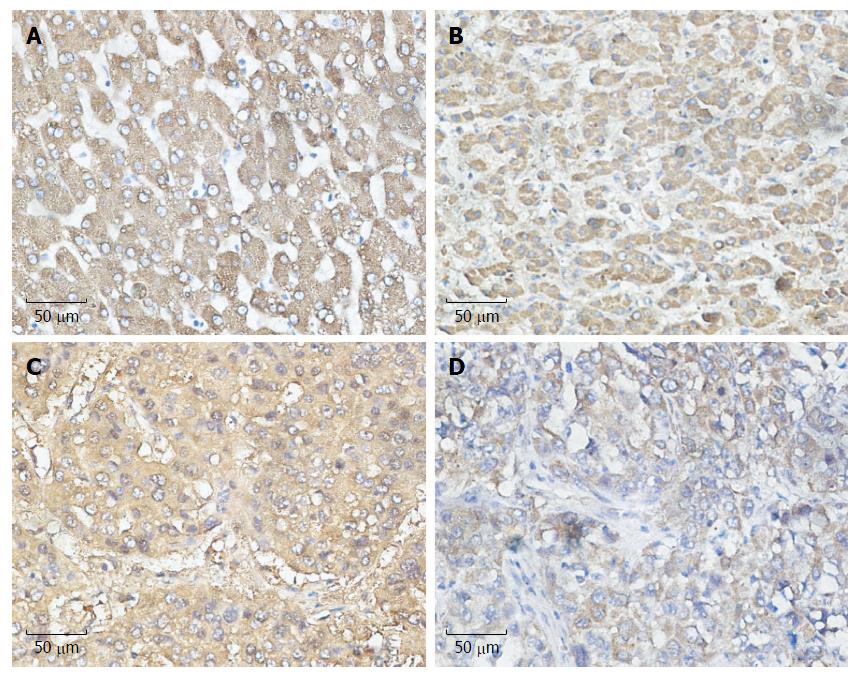
COX-2 is the inducible form of cyclooxygenase and is frequently highly expressed in tumor tissues, including liver cancer, playing an important role in tumor development. To determine which UPR pathway was associated with COX-2 expression under ER stress in HCC patients, we analyzed the paraffin-embedded, formalin-fixed HCC specimens by immunohistochemical staining. For all 100 specimens, COX-2, ATF-6, IRE-1 and PERK were stained in the nuclei and/or cytoplasm of tumor cells. We observed that the expression of COX-2 was more likely to be associated with the expression of ATF-6 than with IRE-1 and PERK, which reached statistical significance (P = 0.011) (Table 1). These data suggest the existence of a close relationship between ATF-6 and COX-2 (Figure 1).
| COX-2 | AFP-6 | IRE-1 | PERK | |||||||||
| - | + | r | P value | - | + | r | P value | - | + | r | P value | |
| Negative | 16 | 39 | 0.198 | 0.011 | 30 | 25 | 0.135 | 0.086 | 29 | 26 | 0.017 | 0.134 |
| Positive | 14 | 95 | 44 | 65 | 44 | 65 | ||||||
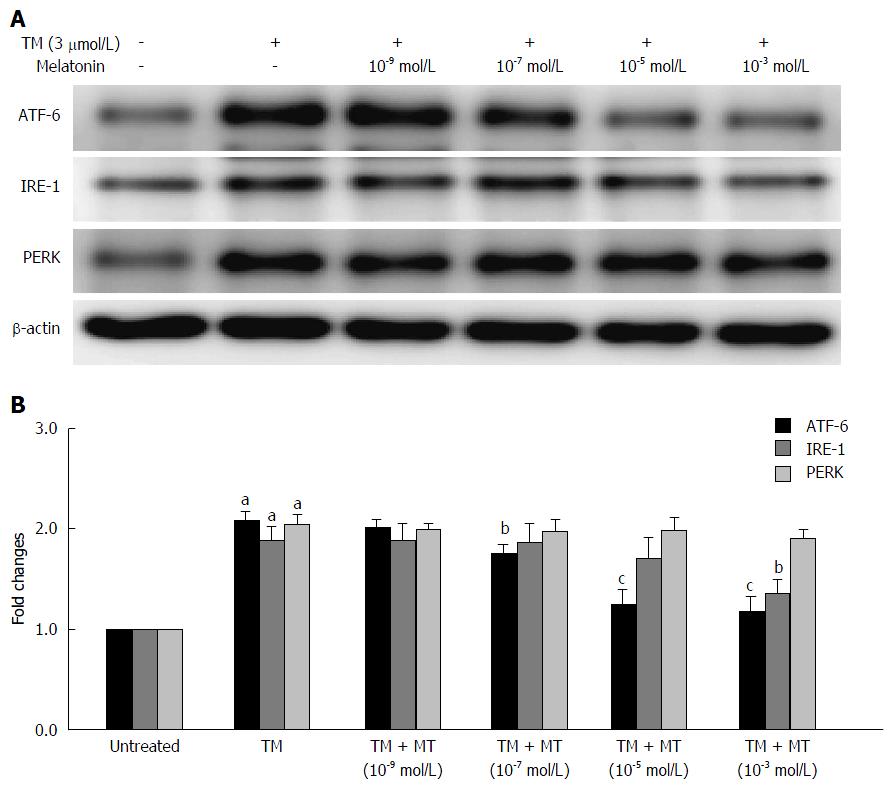
There is limited information about the effects of melatonin under ER stress conditions and the impact of melatonin on these three UPR pathways. To address this, we next evaluated the effects of melatonin on the UPR pathways in vitro. To mimic the ER stress condition, HepG2 cells were first pretreated with tunicamycin for 8 h; then, the cells were treated with melatonin at four different concentrations (10-3, 10-5, 10-7 and 10-9 mmol/L) for another 24 h. The expression of all three UPR pathways was evaluated by western blotting method. As illustrated in Figure 2A, and consistent with previous reports, the downstream signaling molecules ATF-6, IRE-1 and PERK were detected after ER stress activator tunicamycin treatment. Melatonin at concentrations between 10-7 to 10-3 mmol/L markedly inhibited ATF-6 expression. However, only high-concentration melatonin (10-3 mmol/L) slightly decreased the expression of IRE-1. Meanwhile, melatonin had no effect on the expression of PERK. These results indicate melatonin prominently affects the ATF-6 pathway under ER stress condition.
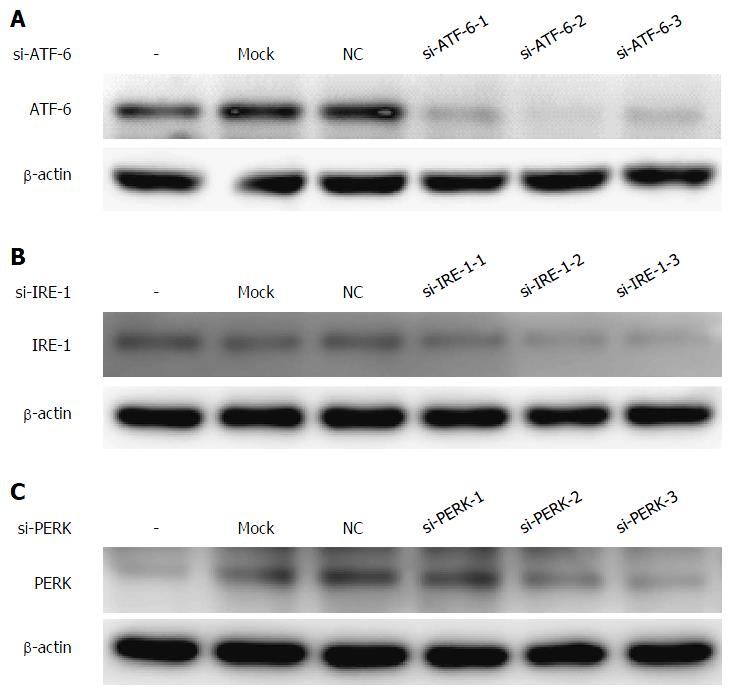
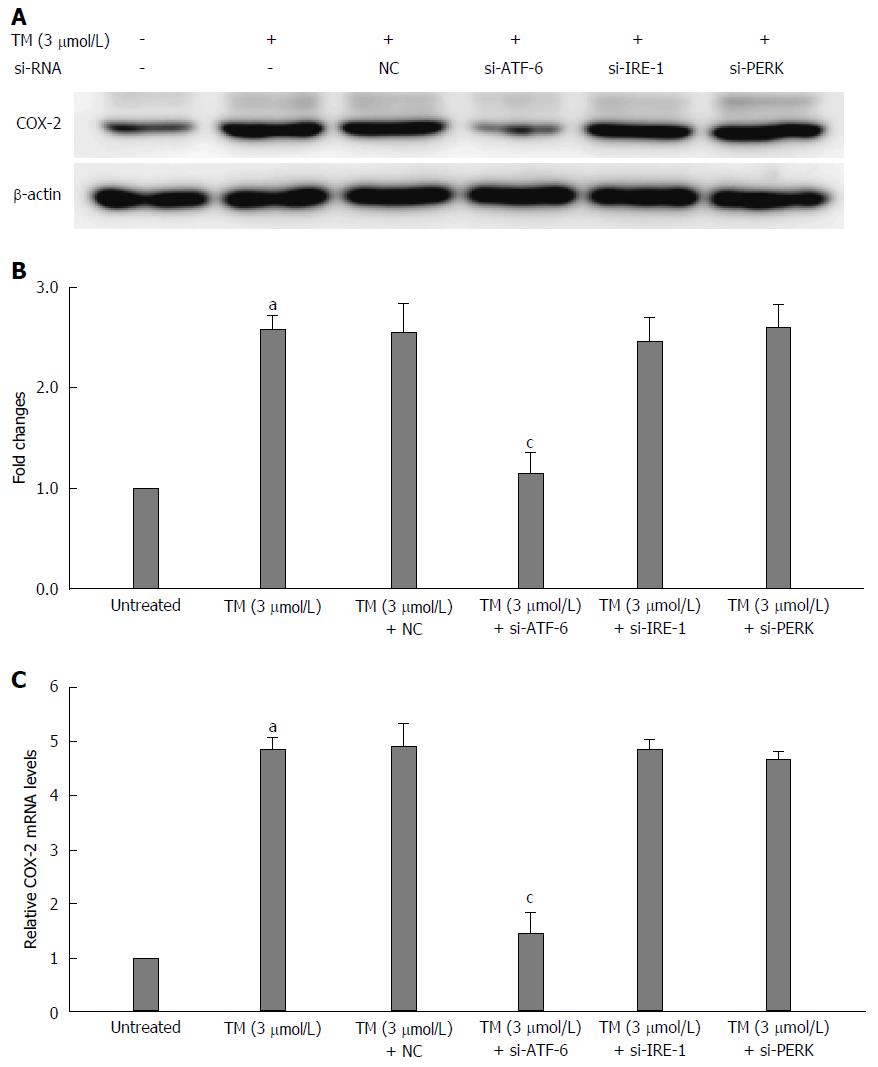
To investigate the underlying mechanisms of which pathway is associated with the expression of COX-2 under the condition of ER stress, we used RNA interference to knockdown the mRNA in all three UPR pathways and then observed the changes in COX-2 levels by western blotting. First, each UPR siRNA has three candidate sequences, and we chose the most effective ones by western blot staining (Figure 3A-C). As shown in Figure 4A and B, the COX-2 protein expression is downregulated after blocking the ATF-6 mRNA under ER stress condition. In addition, the mRNA level of COX-2 was also decreased by si-ATF-6 after ER stress activator tunicamycin pre-treatment. Thus, we concluded that COX-2 can be regulated by the ATF-6 pathway under ER stress.
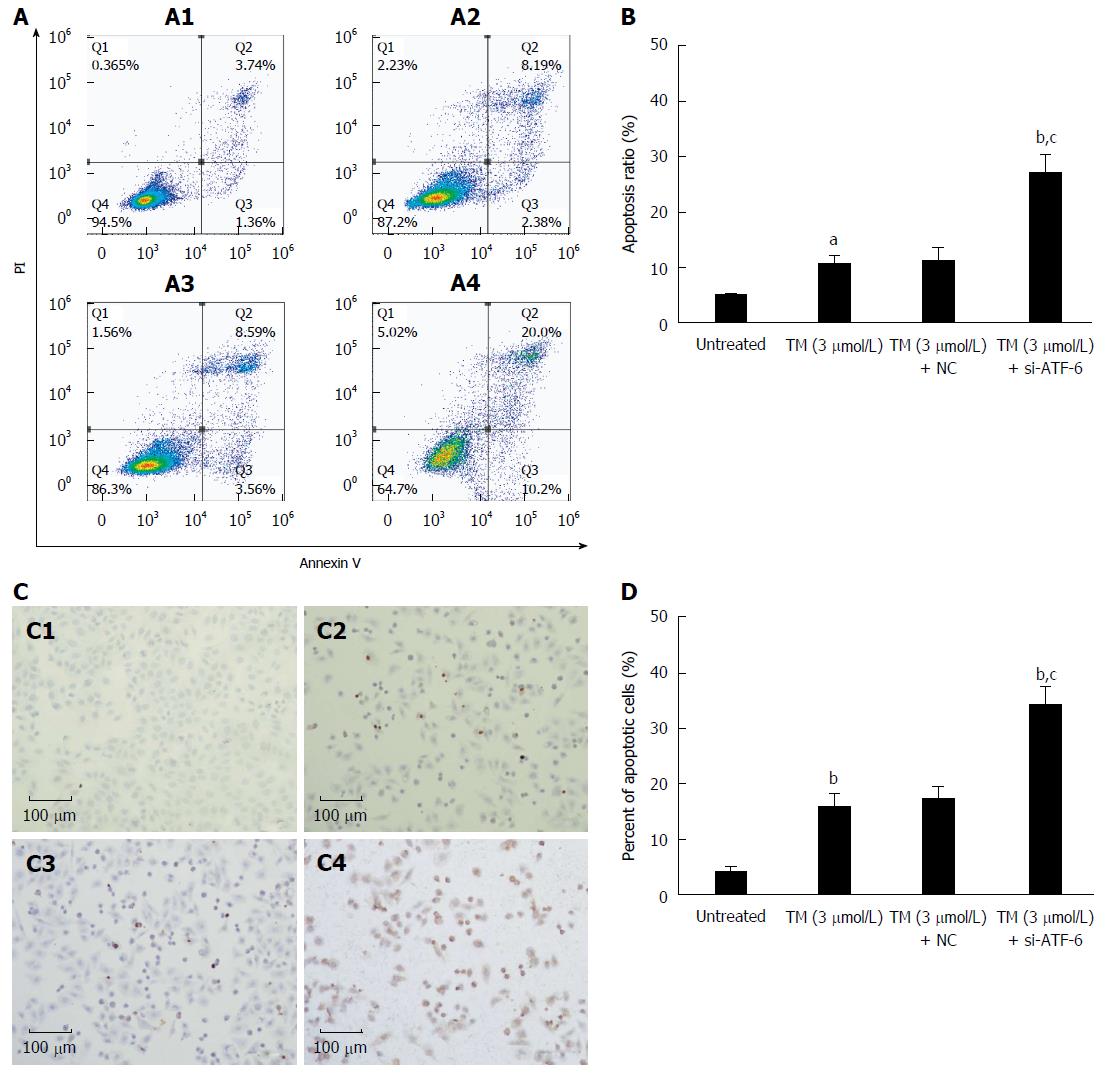
To further determine whether blocking the ATF-6 pathway influences ER stress-induced apoptosis, FACS analysis and TUNEL staining were performed using HepG2 cells. As shown in Figure 5, tunicamycin slightly but significantly induced tumor cell apoptosis to around 10%-20%. Interestingly, the percentage of apoptotic cells markedly increased to around 30% when combined with si-ATF-6. This result suggests that blocking ATF-6 pathway under ER stress can further aggravate liver tumor cell apoptosis.
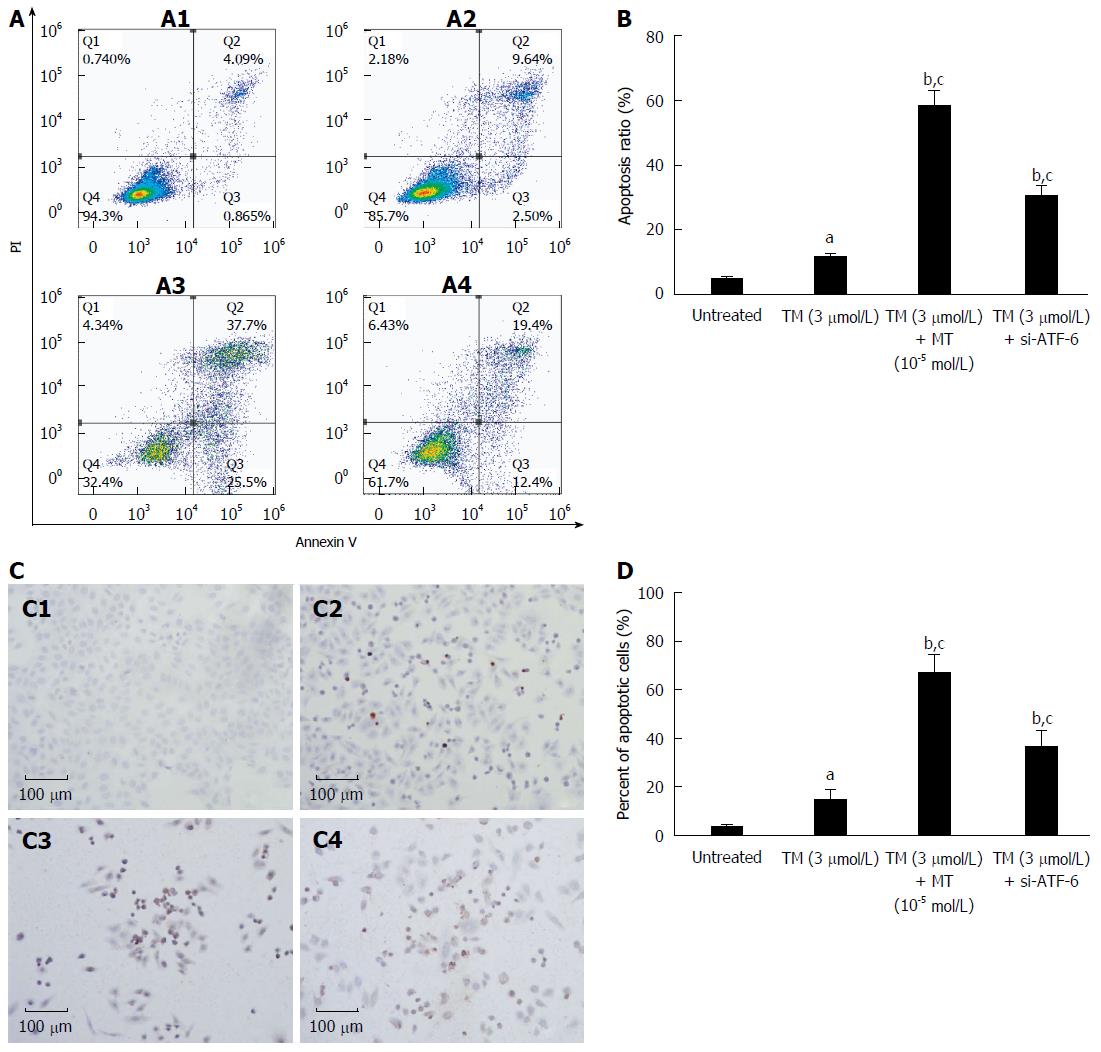
As has already been found, melatonin likely acts as an ER stress inhibitor by selectively blocking the ATF-6 pathway; thus, we next asked whether melatonin also can aggravate liver tumor cell apoptosis in a manner similar to si-ATF-6 after ER stress activation. To further determine whether melatonin influences ER stress-induced apoptosis, FACS analysis and TUNEL staining were performed in HepG2 cells in vitro. Similar to the results from si-ATF-6, and as shown in Figure 6, treatment with melatonin for 24 h after pretreatment with tunicamycin led to an obvious increase in apoptotic tumor cells. The morphological changes indicative of apoptosis were also assessed by TUNEL staining, as shown in Figures 5 and 6. Treatment with ATF-6-siRNA and melatonin resulted in a dramatic increase in the number of apoptotic HepG2 cells. This also suggested that melatonin can effectively downregulate ATF-6 and lead to an increased number of HepG2 cells, which supports the results of FACS.
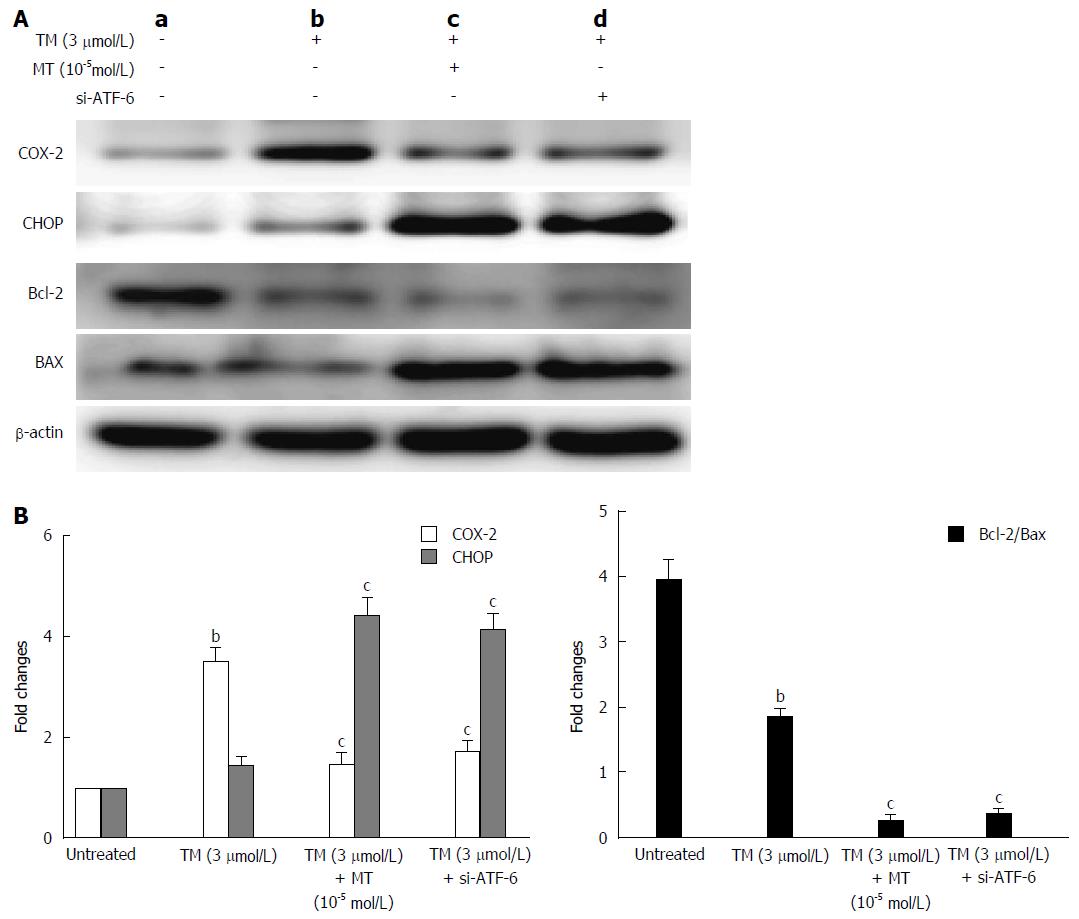
Further mechanism study, shown in Figure 7, showed that melatonin or si-ATF-6 inhibits COX-2 expression while increasing CHOP and the Bax/Bcl-2 ratio to induce cancer cell apoptosis. These data suggest that COX-2 expression may be directly involved in the adaptation of human hepatoma cells to ER stress-induced apoptosis. Based on the data of the relationship between COX-2 and UPR pathways, we concluded that melatonin can obviously knockdown ATF-6 and reduce apoptosis under ER stress by downregulating COX-2.
CHOP, also called GADD153, is one of the primary effectors of ER stress-mediated cell apoptosis. As shown in Figure 7A, the expression of CHOP was markedly increased in the presence of melatonin and ATF-6 siRNA. Similarly, the levels of the anti-apoptosis factor, Bcl-2, were decreased, and the levels of pro-apoptosis factor, Bax, were increased when cells were exposed to melatonin or ATF-6 siRNA. The Bcl-2/Bax ratio also decreased (Figure 7B). These data indicate that inhibition of COX-2 with melatonin by knocking down ATF-6 mRNA increases the number of apoptotic cells by upregulating the expression of CHOP.
In conclusion, the results of this study indicate that under tunicamycin-induced ER stress, melatonin can inhibit the expression COX-2 by downregulating one of the UPR pathways, ATF-6, which increases the apoptosis of HepG2 cells via CHOP and Bcl-2/Bax pathway.
ER stress produced by tumor cells exposed to intrinsic and external factors often causes tumor growth and resistance to treatment[4,5]. Targeting ER stress signaling in cancer is a potential therapeutic method. Our previous studies and other lab's data show that melatonin exerts its anticancer actions through modulation of the ER stress response[8,9,11,12]. However, there is limited information focused on the effect of melatonin under ER stress conditions and the impact of melatonin on the UPR pathways. In the present study, we obtained evidence suggesting that melatonin can selectively target ATF-6 signal, an important pathway of UPR response initiated by ER stress. We found that melatonin induced hepatoma cell apoptosis through inhibiting the ATF-6 pathway. Our studies demonstrated that downregulation of ATF-6 contributes to the increased susceptibility of liver cancer cells to melatonin treatment under ER stress condition.
HCC is one of the most common hepatobiliary malignant tumors, causing increased cancer mortalities worldwide. As the fifth most common cancer in the world, HCC is always associated with poor prognosis; the 5-year survival rate is less than 17%[2]. Hepatic resection and liver transplantation offers treatment to only 20% because most patients are diagnosed at a late stage[13]. To eliminate the early stages of HCC, local ablation, surgical resection, or liver transplantation was applied to the clinical treatment of HCC. Patients who suffer late stage HCC always present with distant metastasis and liver dysfunctions, and the tumor size no longer allows surgical management. Thus, there are few effective treatments for HCC patients to date.
A major obstacle that we have yet to overcome is chemotherapy resistance and we still need to clarify the underlying mechanisms. Among the complex mechanisms involved in HCC development and progress, ER stress induction associated with COX-2 is emerging as a very important contributor. COX-2 is a well-known inducible form of cyclooxygenase considered as a good drug target, which is frequently elevated in variety kinds of cancer tissues including HCC[14,15].
There is increasing evidence that shows COX-2 induction as closely associated with ER stress[16,17]. One research group observed that COX-2 and the eIF2α-ATF-4 pathway of ER stress were induced by heavy metal cadmium in both kidney tissues and cultured cells[16]. This finding indicates the ER stress eIF2α-ATF-4 pathway mediates COX-2 overexpression. In the present study, we found that the expression of COX-2 was more likely to be associated with the expression of ATF-6 but not IRE1 and PERK. An in vitro cell experiment in which the ATF-6 pathway was blocked using si-ATF-6 showed significant repression of COX-2 expression. These data suggest the existence of a close relationship between ATF-6 and COX-2. Thus, it is rational to search for specific inhibitors of ATF-6 as potential candidates for use as new therapeutic agents for HCC.
As HCC is resistant to systemic chemotherapy, identification of a new intervention or targeted therapy is urgently needed for patients[13]. One of the mechanisms by which liver cancer cells gain resistance to chemotherapy is ER stress[3,4,6]. The ER stress response is a process that can be activated by a number of cellular stress conditions, such as hypoxia, nutrient deprivation, alterations in glycosylation status, and disturbances of calcium flux[3,4,6]. Those conditions always cause imbalances in intracellular homeostasis.
ER stress plays an important role in post-translational modifications[3,4,6]. The ER responds to stress conditions by activating a range of stress response signaling pathways, which is referred to as the UPR. The UPR is fundamentally a cytoprotective response, but excessive or prolonged activation of the UPR can result in apoptosis. In the present study, single tunicamycin-induced ER stress only slightly induced HepG2 cell apoptosis, whereas combination with si-ATF-6 strongly increased the percentage of HepG2 cells undergoing apoptosis. This finding suggests that targeting the ATF-6 pathway in HepG2 cells enhances sensitivity to the apoptosis inducer.
Melatonin is mainly secreted by the human pineal gland, and it has been detected in many other tissues or as being secreted by other organs. Melatonin is a highly lipophilic molecule that can easily cross cell membranes to reach subcellular compartments, including mitochondria, where it exists in high concentrations[18]. Melatonin is able to prevent oxidative stress through both its free radical scavenging effect and by directly increasing antioxidant activity[19-25], and different studies have demonstrated its protective role against oxidative damage induced by drugs, toxins, and different diseases[26].
In addition, melatonin also acts upon complex functions through specific nuclear and plasma membrane receptors[27,28]. Melatonin MT1 and MT2 receptors are G protein coupled receptors expressed in various parts of the central nervous system and in peripheral organs, which mediate intracellular effects depending on the changes in intracellular cyclic nucleotides (cAMP, cGMP) and calcium levels, activation of certain protein kinase C subtypes, intracellular localization of steroid hormone receptors and regulation of G protein signaling proteins. Alterations in melatonin receptor expression and the following abnormal signaling pathway, as well as changes in endogenous melatonin production, contribute to the pathophysiology of various diseases, including sleep disorders, depression and Alzheimer's disease[27,28].
Interestingly, experimental and clinical studies recommend an increase in the awareness of melatonin as a therapeutic agent in cancers including gastrointestinal tract cancer[10,29,30]. Our previous studies and other labs' findings suggest that melatonin exerts its anticancer action through suppressing COX-2 and attenuating ER stress-induced drug resistance[11,12,31,32]. A recent study also showed that melatonin inhibits the expression of proangiogenic proteins HIF-1α and VEGF in conditions of normoxia and hypoxia using the HepG2 cell line[33].
ER stress induced by hepatitis B virus X (HBx) protein enhances COX-2 expression via activating transcription factor 4 (ATF-4). Further experiment showed that ATF-4 binding to the COX-2 promoter plays a critical role in HBx-mediated COX-2 induction[34]. In addition, melatonin enhances antitumor function through upregulation of the pro-apoptotic protein BimBim expression and downregulation of COX-2 expression in tunicamycin-treated breast carcinoma MDA-MB-231 cells[35].
The present study further explored the new mechanism of specific effect of melatonin on ATF-6, one of the UPR responses. We found that melatonin selectively inhibited the ATF-6 expression (Figure 1). As it is well-known that melatonin has an inhibitory effect on COX-2 activity[36], it is easy to speculate and understand that si-ATF-6 has a similar COX-2 suppression as melatonin (Figure 4). Furthermore, we confirmed that downregulation of ATF-6 by melatonin contributes to the increased susceptibility of liver cancer cells to melatonin treatment under ER stress condition (Figure 6). Dramatically increased CHOP level led to suppressed COX-2 and decreased Bcl-2/Bax ratio by melatonin, and ATF-6 siRNA contributed to the enhanced HepG2 cell apoptosis under ER stress stimulation (Figure 7). These findings indicate that melatonin, as a selective ATF-6 inhibitor, can sensitize human hepatoma cells to ER stress-induced apoptosis.
In summary, our study provides the new mechanism by which melatonin downregulates COX-2 expression and sensitizes apoptosis by selectively targeting ATF-6 in human HCC cells under ER stress. Our results raise the possibility that melatonin may be a promising approach in targeting ER stress-induced apoptosis as a therapeutic strategy for the treatment of HCC and other cancers. We also identified that of the three UPR pathways, ATF-6 was positively associated with COX-2 in HCC patient samples. Therefore, if there are any agents that can knockdown ATF-6, like melatonin, they can be used to treat HCC. However, we still need to investigate whether this effect of melatonin in ER stress-induced tumor apoptosis in vitro will also work well in vivo. Because of the low toxicity and well-documented oncostatic effects of melatonin, we believe melatonin has a promising future in the treatment of HCC.
Hepatocellular carcinoma (HCC) is a frequently diagnosed cancer in China and has a high lethality rate. Yet, there are few effective chemotherapy agents for this malignant tumor. One of the major reasons of untreatable HCC is that the liver cancer cell has greater tolerance towards a number of cellular stress conditions, especially endoplasmic reticulum (ER) stress. The published works by other authors have shown that melatonin can sensitize the human HCC cell to ER stress-induced apoptosis and that melatonin attenuates ER stress-induced resistance to doxorubicin. However, the precise mechanisms involved in the critical unfolded protein response (UPR) pathway modified by melatonin still need to be investigated.
To the best knowledge of the authors, this is the first study to identify melatonin as a selective ATF-6 blocker, thereby inhibiting COX-2 and leading to enhanced cell apoptosis in liver cancer.
This is the first study investigating the precise mechanisms of the critical UPR pathways modified by melatonin.
The promising findings presented in the current report suggest that melatonin, as a novel selective ATF-6 inhibitor, can sensitize human hepatoma cells to ER stress inducing apoptosis, which may be considered as a therapeutic strategy for the treatment of HCC and other cancers.
Melatonin plays important roles in human physiological and pharmacological functions, such as circadian rhythms and antioxidants. Interestingly, melatonin exerts anticancer effects through interplay with ER stress. The published studies show that melatonin sensitizes the human HCC cell to ER stress-induced apoptosis. Furthermore, melatonin also attenuates ER stress-induced resistance to doxorubicin through reversing tunicamycin-induced ER stress. In the current study, the authors first demonstrated that melatonin selectively blocks ATF-6 and then inhibits COX-2 expression, leading to enhanced liver cancer cell apoptosis. In clinical HCC patients, the close relationship between ATF-6 and COX-2 was further confirmed. The results presented raise the possibility that melatonin may be used in the treatment of HCC and other cancers.
In general, the enclosed set of data are very interesting. The article will be interesting for readers of this journal.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kleszczynski K, Zavodnik IB S- Editor: Yu J L- Editor: Filipodia E- Editor: Liu WX
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13196] [Article Influence: 1466.2] [Reference Citation Analysis (3)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12983] [Article Influence: 1442.6] [Reference Citation Analysis (2)] |
| 3. | Binet F, Sapieha P. ER Stress and Angiogenesis. Cell Metab. 2015;22:560-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 4. | Yadav RK, Chae SW, Kim HR, Chae HJ. Endoplasmic reticulum stress and cancer. J Cancer Prev. 2014;19:75-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 5. | Chevet E, Hetz C, Samali A. Endoplasmic reticulum stress-activated cell reprogramming in oncogenesis. Cancer Discov. 2015;5:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 6. | Hiramatsu N, Chiang WC, Kurt TD, Sigurdson CJ, Lin JH. Multiple Mechanisms of Unfolded Protein Response-Induced Cell Death. Am J Pathol. 2015;185:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Kato H, Nishitoh H. Stress responses from the endoplasmic reticulum in cancer. Front Oncol. 2015;5:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Fernández A, Ordóñez R, Reiter RJ, González-Gallego J, Mauriz JL. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res. 2015;59:292-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 397] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 9. | Wu SM, Lin WY, Shen CC, Pan HC, Keh-Bin W, Chen YC, Jan YJ, Lai DW, Tang SC, Tien HR. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPβ and NFκB cleavage. J Pineal Res. 2016;60:142-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Xin Z, Jiang S, Jiang P, Yan X, Fan C, Di S, Wu G, Yang Y, Reiter RJ, Ji G. Melatonin as a treatment for gastrointestinal cancer: a review. J Pineal Res. 2015;58:375-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Zha L, Fan L, Sun G, Wang H, Ma T, Zhong F, Wei W. Melatonin sensitizes human hepatoma cells to endoplasmic reticulum stress-induced apoptosis. J Pineal Res. 2012;52:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Fan L, Sun G, Ma T, Zhong F, Lei Y, Li X, Wei W. Melatonin reverses tunicamycin-induced endoplasmic reticulum stress in human hepatocellular carcinoma cells and improves cytotoxic response to doxorubicin by increasing CHOP and decreasing survivin. J Pineal Res. 2013;55:184-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1274] [Article Influence: 141.6] [Reference Citation Analysis (2)] |
| 14. | Breinig M, Schirmacher P, Kern MA. Cyclooxygenase-2 (COX-2)--a therapeutic target in liver cancer? Curr Pharm Des. 2007;13:3305-3315. [PubMed] |
| 15. | Misra S, Sharma K. COX-2 signaling and cancer: new players in old arena. Curr Drug Targets. 2014;15:347-359. [PubMed] |
| 16. | Luo B, Lin Y, Jiang S, Huang L, Yao H, Zhuang Q, Zhao R, Liu H, He C, Lin Z. Endoplasmic reticulum stress eIF2α-ATF4 pathway-mediated cyclooxygenase-2 induction regulates cadmium-induced autophagy in kidney. Cell Death Dis. 2016;7:e2251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J Biol Chem. 2004;279:46384-46392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res. 2010;48:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 298] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 19. | Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 841] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 20. | Tomás-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res. 2005;39:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 371] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 21. | Kleszczyński K, Zillikens D, Fischer TW. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J Pineal Res. 2016;61:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Kleszczyński K, Tukaj S, Kruse N, Zillikens D, Fischer TW. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J Pineal Res. 2013;54:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Fischer TW, Kleszczyński K, Hardkop LH, Kruse N, Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2’-deoxyguanosine) in ex vivo human skin. J Pineal Res. 2013;54:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 24. | Fischer TW, Scholz G, Knöll B, Hipler UC, Elsner P. Melatonin suppresses reactive oxygen species induced by UV irradiation in leukocytes. J Pineal Res. 2004;37:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol. 2003;196:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | García JJ, López-Pingarrón L, Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan DX, Reiter RJ, Ramírez JM, Bernal-Pérez M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J Pineal Res. 2014;56:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 339] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 27. | Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335-353. [PubMed] [DOI] [Full Text] |
| 28. | Emet M, Ozcan H, Ozel L, Yayla M, Halici Z, Hacimuftuoglu A. A Review of Melatonin, Its Receptors and Drugs. Eurasian J Med. 2016;48:135-141. [PubMed] [DOI] [Full Text] |
| 29. | Mills E, Wu P, Seely D, Guyatt G. Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J Pineal Res. 2005;39:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Reiter RJ, Rosales-Corral SA, Manchester LC, Liu X, Tan DX. Melatonin in the biliary tract and liver: health implications. Curr Pharm Des. 2014;20:4788-4801. [PubMed] |
| 31. | Carbajo-Pescador S, Steinmetz C, Kashyap A, Lorenz S, Mauriz JL, Heise M, Galle PR, González-Gallego J, Strand S. Melatonin induces transcriptional regulation of Bim by FoxO3a in HepG2 cells. Br J Cancer. 2013;108:442-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Carbajo-Pescador S, García-Palomo A, Martín-Renedo J, Piva M, González-Gallego J, Mauriz JL. Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HepG2 cell line: role of the MT1 receptor. J Pineal Res. 2011;51:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Colombo J, Maciel JM, Ferreira LC, DA Silva RF, Zuccari DA. Effects of melatonin on HIF-1α and VEGF expression and on the invasive properties of hepatocarcinoma cells. Oncol Lett. 2016;12:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Cho HK, Cheong KJ, Kim HY, Cheong J. Endoplasmic reticulum stress induced by hepatitis B virus X protein enhances cyclo-oxygenase 2 expression via activating transcription factor 4. Biochem J. 2011;435:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Woo SM, Min KJ, Kwon TK. Melatonin-mediated Bim up-regulation and cyclooxygenase-2 (COX-2) down-regulation enhances tunicamycin-induced apoptosis in MDA-MB-231 cells. J Pineal Res. 2015;58:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Aparicio-Soto M, Alarcón-de-la-Lastra C, Cárdeno A, Sánchez-Fidalgo S, Sanchez-Hidalgo M. Melatonin modulates microsomal PGE synthase 1 and NF-E2-related factor-2-regulated antioxidant enzyme expression in LPS-induced murine peritoneal macrophages. Br J Pharmacol. 2014;171:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |