Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.817
Peer-review started: September 14, 2016
First decision: October 20, 2016
Revised: November 4, 2016
Accepted: December 21, 2016
Article in press: December 21, 2016
Published online: February 7, 2017
Processing time: 130 Days and 23.3 Hours
To investigate the enhanced cytotoxic T lymphocyte responses against pancreatic cancer (PC) in vitro induced by dendritic cells (DCs) engineered to secrete anti-DcR3 monoclonal antibody (mAb).
DCs, T lymphocytes and primary PC cells were obtained from PC patients. DCs were transfected with a designed humanized anti-DcR3 monoclonal antibody heavy and light chain mRNA and/or total tumor RNA (DC-tumor-anti-DcR3 RNA or DC-total tumor RNA) by using electroporation technology. The identification, concentration and function of anti-DcR3 mAb secreted by DC-tumor-anti-DcR3 RNA were determined by western blotting and enzyme-linked immunosorbent assay. After co-culturing of autologous isolated PC cells with target DCs, the effects of secreting anti-DcR3 mAb on RNA-DCs’ viability and apoptosis were assessed by MTT assay and flow cytometry. Analysis of enhanced antigen-specific immune response against PC induced by anti-DcR3 mAb secreting DCs was performed using a 51Cr releasing test. T cell responses induced by RNA-loaded DCs were analyzed by measuring cytokine levels, including IFN-γ, IL-10, IL4, TNF-α and IL-12.
The anti-DcR3 mAb secreted by DCs reacted with recombinant human DcR3 protein and generated a band with 35 kDa molecular weight. The secreting mAb was transient, peaking at 24 h and becoming undetectable after 72 h. After co-incubation with DC-tumor-anti-DcR3 RNA for designated times, the DcR3 level in the supernatant of autologous PC cells was significantly down-regulated (P < 0.05). DCs secreting anti-DcR3 mAb could improve cell viability and slow down the apoptosis of RNA-loaded DCs, compared with DC-total tumor RNA (P < 0.01). The anti-DcR3 mAb secreted by DC-tumor-anti-DcR3 RNA could enhance the induction of cytotoxic T lymphocytes (CTLs) activity toward RNA-transfected DCs, primary tumor cells, and PC cell lines, compared with CTLs stimulated by DC-total tumor RNA or control group (P < 0.05). Meanwhile, the antigen-specific CTL responses were MHC class I-restricted. The CD4+ T cells and CD8+ T cells incubated with anti-DcR3 mAb secreting DCs could produce extremely higher level IFN-γ and lower level IL4 than those incubated with DC-total tumor RNA or controls (P < 0.01).
DCs engineered to secrete anti-DcR3 antibody can augment CTL responses against PC in vitro, and the immune-enhancing effects may be partly due to their capability of down-regulating DC apoptosis and adjusting the Th1/Th2 cytokine network.
Core tip: Dendritic cells co-transfection with tumor-associated antigens RNA and humanized anti-DcR3 monoclonal antibody mRNA may augment cytotoxic T lymphocyte responses against pancreatic cancer in vitro. This finding lays a good foundation for further investigation of tumor dendritic cells’ vaccine targeting DcR3 protein against pancreatic cancer.
- Citation: Chen J, Guo XZ, Li HY, Zhao JJ, Xu WD. Dendritic cells engineered to secrete anti-DcR3 antibody augment cytotoxic T lymphocyte response against pancreatic cancer in vitro. World J Gastroenterol 2017; 23(5): 817-829
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/817.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.817
Pancreatic cancer (PC) is the fourth leading cause of cancer-related deaths in the US, with 40560 deaths in 2015 alone[1,2]. It has an extremely poor prognosis with a total 5-year survival rate of < 5%[3]. The poor prognosis and high mortality rate in PC patients may be attributed in part to lack of effective treatments[2]. Existing therapies for PC are limited to systemic chemotherapy and surgical resection. However, neither of these two strategies can cure PC completely[4]. Thus, more effective therapeutic methods are urgently needed.
Cellular immunotherapy is a promising alternative that is currently considered the fourth line of cancer treatment[5]. In this approach, different kinds of immune cells, such as cytokine-induced killer cells, lymphokine-activated killer cells, natural killer (NK) cells and dendritic cells (DCs) are adopted for immunotherapy. Of these, DC is the most commonly used immune effector cell because of its potent antigen-presenting function in the initiation of antitumor immune responses and its pivotal function in cancer immunosurveillance. Cytotoxic T lymphocytes (CTLs) are capable of eliminating cancer cells directly in vivo, but their activities are primarily managed by DCs. The use of DC-based tumor vaccines has therefore become a promising alternative treatment method for cancer[2,6].
In clinical practice, antigen choice is essential in the design of an effective vaccine. Because of lacking the expression of MHC class II molecules and co-stimulatory molecules, PCs, with low level of expression of tumor-associated antigens (TAA), display weak antigenicity and high heterogeneity[7]. Therefore, loading whole antigens from PC cells may be an alternative method that can both generate a broad T cell immune response to TAA and reduce the possibility of PC escape from immune recognition. We have previously reported that DCs transfected with total tumor RNA can effectively induce anti-PC tumor-specific CTL responses[2]. However, although this method has been demonstrated to generate a vaccine-induced rise in tumor-specific cells, the immune and tumor reactions stay modest, suggesting the need for novel strategies to improve antitumor immunity[8]. Evidently, one cause of this insufficiency is that tumor cells can produce certain immunosuppressive molecules to induce an immunosuppressive microenvironment and inhibit the function of tumor-associated cells, such as T lymphocytes and DCs[9]. Decoy receptor (DcR) 3 is possibly one of these cells in the tumor microenvironment (TME)[10].
DcR3 is a decoy receptor for Fas ligand (FasL) and is a member of the tumor necrosis factor receptor (TNFR) superfamily[11]. DcR3 displays inducible expression, interacts with the herpes virus entry mediator (HVEM) and is presented by TNF-like molecule 1A (TL1A) and T lymphocytes (LIGHT)[12,13]. DcR3 lacks a transmembrane domain and works as a secreted protein instead of a membrane-bound one. DcR3 can bind to LIGHT and FasL, thereby blocking the interaction between LIGHT and LT-receptor (LTβR) or HVEM to inhibit the apoptosis induced by Fas-FasL interaction or LIGHT-mediated biological effects. DcR3 is frequently overexpressed in various tumors, including lung cancers[14], gastrointestinal tract tumors[15], virus-associated lymphomas[16], and PCs[17]. It has been reported that overexpression of DcR3 is correlated with shortened total survival time of cancer patients[18]. DcR3 has been postulated to promote tumor growth by escaping FasL- and LIGHT-mediated immunosurveillance. Specifically, DcR3 is able to suppress the activation and differentiation of DCs and macrophages[19], enhance osteoclast differentiation and angiogenesis[20], and sensitize T lymphocytes to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in cancer patients[21]. In addition, DcR3 is also regarded as an important immunosuppressive factor in defects associated with immune effector cell function. Therefore, we sought to determine whether neutralizing DcR3 expression in the TME can augment the CTL responses against PC in vitro induced by DCs loaded with total tumor RNA.
In the current study, we evaluated the novel approach of co-transfecting DCs with total tumor RNA and mRNA encoding humanized heavy (H) and light (L) chains of an anti-human DcR3 mAb together to achieve anti-DcR3 protein stimulation. Through co-culturing of autologous isolated PC cells with DCs, we found that DCs transfected with these RNAs secrete operational immune modulating proteins that can reduce DcR3 expression in TME of cultured PC cells. Then we demonstrated that CTLs induced by DCs co-transfected with total tumor RNA and anti-DcR3 monoclonal antibody (mAb) mRNA show more effective cytotoxic activities against PC cells in vitro compared with DCs loaded only with total tumor RNA alone. Furthermore, the immune-enhancing effect of DCs engineered to secrete anti-DcR3 mAb is partly due to their capability of down-regulating apoptosis of DCs and adjusting the T helper (Th)1/Th2 cytokine network. These findings are crucial for the development of tumor DC vaccines targeting DcR3 protein against PC.
Fifteen HLA-A2+ PC patients (9 males and 6 females; median age of 53.5 years, ranging from 35 years to 72 years) were included in this study. According to the TNM classification of AJCC[22], there were 10 stage II patients and 5 stage III patients. The location of tumor was divided into head (7 cases) and body/tail (8 cases). All patients underwent surgical resection and were pathologically diagnosed with invasive ducal adenocarcinoma.
Peripheral blood monocyte cells (PBMCs), isolated by Ficoll-Hypaque (Sigma, St Louis, MO, United States) density gradient separation, and was used as the nonmalignant control tissues. Pancreatic cancer specimens were obtained at the time of surgery and were stored in RNAlate (Ambion, Austin, TX, United States) at 4 °C until processing.
Autologous tumor cells were obtained as described by Wang et al[23]. Approximately 10 g of each tumor specimen was harvested in the operating room for primary cell culture. The tumor tissue was mechanically disrupted to generate approximately 1 mm3 sections. The tissue was digested in 10 mL of RPMI-1640 medium supplemented with 0.05% collagenase (Hyclone, South Logan, UT, United States) with gentle agitation at room temperature for 4-6 h. After culturing for 7 d, the immunohistochemistry technique was used to detect the expression of DcR3 protein (anti-DcR3 mAb obtained from Sigma).
The human PC cell lines Capan-2 (HLA-A2+) and AsPC-1 (HLA-A2-), as well as the leukemia cell line K562, were obtained from the American Type Culture Collection (Manassas, VA, United States). The cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine (Hyclone), 50 U/mL penicillin, and 50 mg/mL streptomycin (Hyclone). All cells were cultured for 7 d and maintained in the logarithmic phase growth at 37 °C in a humidified atmosphere supplemented with 5% CO2.
Total cellular RNA was extracted from autologous PC cells and PBMCs by using TRIzol Reagent (Sigma) according to the manufacturer’s instructions. Only RNA exhibiting a ratio of 28S:18S > 1 was subjected to further analysis.
Total RNA of anti-human DcR3 hybridoma clone 1B1 (a kindly gift from Dr. CF Wu of Jilin University, China)[24], was isolated with the RNeasy mini kit (Qiagen, Valencia, CA, United States). Five micrograms of RNA were used in a reverse transcription reaction with the RT primer 5’- ATT CTA GAG GCC GAG GCG GCC GAC ATG (T-30) VN-3’ and PowerScript RT (Clontech, Mountain View, CA, United States) and the primer 5’-AAG CAG TGG TAT CAA CGC AGA GTG GCC ATA TTG GCCr GrGrG 3AmMC7/-3’. The heavy (H) chain was amplified from 10% of the RT reaction by using Advantage 2 HF PCR mix (Clontech) and the primers 5’- AAA GAA TTC GGC CTT GTT GGC CTC ATT TAC CCA GAG ACC GGG AGA TG -3’ and 5’-GAA AAG CTT GGC CAT TGG GGC GGT ATC AAC GCA GAG TGG CCA TAT TG-3’. The L chain was amplified with the primers 5’-AAG AAT TCG GCC TTG TTG GCC TAA CAC TCA TTC CTG TTG AAG CTC TTG-3’ and 5’-GAA AAG CTT GGC CAT TGG GGC GGT ATC AAC GCA GAG TGG CCA TAT TG-3’. The resulting PCR fragments were digested with HindIII and EcoRI and cloned into the HindIII and EcoRI sites of the plasmid pSP73-Sph/A64, which possesses a T7 promoter and 64T nucleotides that allow for the production of in vitro transcribed RNA with a polyA tail of 64 residues. The gene encoding the full-length enhanced actin (as controls) was inserted into the pSP73-Sph/A64 plasmid, as well.
All plasmids were digested with SpeI for use as a template for in vitro transcription reactions using the mMESSAGE mMACHINE T7 kit (Ambion) according to the manufacturer’s protocol. mRNA was purified with the RNeasy mini kit.
DCs’ generation was performed as previously described by Zhu et al[25]. A concentrated leukocyte fraction was isolated from PBMCs that processed 200 mL of blood during each collection. Leukapheresis products were separated by density-gradient centrifugation over polysucrose sodium diatrizoate (Sigma), and cells were resuspended in serum-free AIM-V medium (Gibco, Burlington, Canada). Cells were incubated in a humidified incubator for 2 h at 37 °C to allow plastic adherence. The non-adherent fraction was removed, and the adherent cells were cultured for 7 d in serum-free AIM-V medium supplemented with human rIL-4 (500 U/mL) and recombinant human granulocyte macrophage colony-stimulating factor (GM-CSF) (800 U/mL) (R&D Systems, Minneapolis, MN, United States) at 37 °C under 5% CO2.
The DCs were transfected with RNA using a Gene Pulser II (Bio-Rad, Hercules, CA, United States). After 7 d, the immature DCs were transferred into a low-conductance medium (Cytofusion Medium Formula C; CytoPulse Sciences, Columbia, MO, United States) after centrifugation at 170 ×g at 4 °C for three times, each time for 7 min. Viable cells were resuspended to a final concentration of (10-40) × 106 cells/mL in the low-conductance medium. Subsequently, 0.5 mL cell suspension was mixed with 3 μg per 106 DCs and electroporated in a 0.4 cm cuvette at an optimum condition[26]. The cells were recovered for 5 min, and then the same protocols were repeated with 10 μg of H chain antibody RNA and 5 μg of L chain antibody RNA per 106 DCs. Cells were recovered for 15 min, and then the transfected DCs were matured by adding 10 ng/mL of TNF-α (Roche Molecular Biochemicals, Mannheim, Germany) for 24 h. The electroporation of actin mRNA and PBMC RNA was used as controls. Inverted phase contrast microscopy (I × 70; Olympus, Tokyo, Japan) and electron microscopy (scanning electron microscope JSM-7300EX; Hitachi, Tokyo, Japan) were used for the morphological characterization of DCs transfected with different RNA. The DcR3 protein expression in RNA-DCs was low to negative (mature DC ≤ 11%-14% and immature DC ≤ 9%-11%, data not shown).
The recombinant human DcR3 protein (Sigma) was dissolved in 0.02 mol/L phosphate-buffered saline (PBS, pH 7.4). The concentration was determined by the BCA Protein Assay Reagent method (Pierce Chemical Company, Rockford, IL, United States). Then, the proteins were resolved on sodium dodecyl sulfate (SDS)-polyacrylamide denaturing gels, and transferred onto nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany) overnight at 4 °C. The membranes were blocked in Tris-buffered saline containing 2% non-fat dry milk (Bio-Rad) and 0.05% Tween 20 (Sigma) for 1 h. Using 1:5000 dilution of the supernatant from DCs co-transfected with autologous PC cell total RNA and anti-DcR3 mAb-encoding mRNA for 24 h, the supernatant from DCs was transfected with actin mRNA for 24 h (negative control) and the commercial anti-DcR3 mAb (positive control; Qiagen) as primary antibodies, followed by incubation for 1 h in a horseradish peroxidase-conjugated secondary antibody. Protein bands were visualized using an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ, United States).
As described by our previous study[26], autologous tumor cells (1 × 106 cells) were co-incubated with DCs (1 × 106 cells) encoding anti-DcR3 mAb mRNA or actin mRNA (negative control) in 96-well plates in an overall volume of 200 μL at 37 °C for 0-72 h. A 1:5000 dilution of the commercial anti-DcR3 mAb was applied as positive control. Triplicate supernatant samples from these co-cultures were examined for specific DcR3 secretion using DcR3 enzyme-linked immunosorbent assay (ELISA) kit (Genzyme, Waltham, MA, United States). Each well was measured at 450 nm, and the optical density values were used to calculate the concentration of the samples.
As previously described by Pruitt[8], an indirect ELISA was simultaneously used to measure the DcR3 mAb concentration in the supernatants of DCs co-transfected with total tumor RNA and anti-DcR3 mAb-encoding mRNA for 0-72 h. DCs transfected with actin mRNA were used as positive control.
As described by Chen[27], the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) cell proliferation assay was used to defined the cell viability. In brief, RNA loaded DCs were inoculated into 96-well tissue culture plates (BD PharMingen, San Diego, CA, United States) with a density of 3000 cells per well and co-incubation with or without 3000 autologous PC cells. The cells were handled according to the instructions of MTT at the time points of 0-96 h. The formazan crystals that we acquired were dissolved in dimethylsulfoxide (BD Biosciences, Franklin Lakes, NJ, United States). Absorbance was monitored at 490 nm and the percentage of exposed cells to controls was used to show the cell viability. Cells that did not receive RNA transfections were regarded as the control cultures.
As described by our previous study[2], DCs were prepared using 2% paraformaldehyde after washing for three times with frigid PBS containing 0.5% of bovine serum albumin. Four fluorescein isothiocyanate (FITC)-conjugated mAbs, including anti-HLA-DR, anti-CD80, anti-CD86 and anti-CD83, were used and which came from BD PharMingen. Flow cytometry was used to analyze those stained cells.
Following the manner of Lin et al[28], apoptotic DCs were quantified by using annexin V-FITC and propidium iodide (PI) double staining. Briefly, autologous tumor cells were co-incubated with DC-total tumor RNA and DC-tumor-anti-DcR3 mAb mRNA for the specific times, and then 1 × 106 cells were resuspended in 100 μL binding buffer, after washing with frigid PBS. Two microliters of PI and FITC-annexin V were added into the cells that were resuspended and cultured for 15 min protecting from light. The cells were then added into 0.5 mL binding buffer and analyzed with flow cytometry.
Antigen-specific CTLs were produced utilizing a protocol described by Chen et al[2,26,27]. The PBMCs without adherence were cultured in serum-free medium containing 10 ng/mL IL-7 and 20 U/mL IL-2 (R&D Systems). The cells were encouraged weekly for a minimum of two times with RNA-DCs at a stimulator-to-effector ratio of 1:10. As determined by flow cytometric analysis, a minimum of 45% of purified effector cells were CD8+ T cells after 16 d of culture.
Target cells, including autologous DCs transfected with tumor antigen-encoding RNA and tumor cells, were resuspended in 1 mL RPMI-1640 medium at 37 °C in 5% CO2 for 1 h, and which contained 100 μCi NaCrO4 solution (Isotope Products, Beijing, China). The serial dilutions of effector CTLs at various E:T ratios and the 5 × 103 51Cr-labeled target cells were incubated in 200 μL RPMI-1640 in 96-well plates for 6 h. Fifty microliters of supernatant were then taken away, and 51Cr secretion was measured by a gamma counter (Beckmann, Heidelberg, Germany). In all the tests, the spontaneous discharge was less than 15% of the total release of the detergent. Specific lysis percentage was computed as [(experimental cpm-spontaneous cpm)/(maximum cpm-spontaneous cpm)] × 100.
As described by our previous study[2], after subjecting to different treatments, 5 × 103 DCs (DC-total tumor RNA and DC-tumor-anti-DcR3 RNA) were cultured in 96-well round bottom plates. T cells were isolated from proliferating peripheral blood lymphocytes (PBLCs) and 5 × 104 T cells were stimulated with RNA-DCs in a whole volume of 200 μL in 96-well plates for 24 h. The cytokines interleukin (IL)-12p70, interferon-γ (IFN-γ), IL-10, and TNF-α released by T cells were measured by ELISA kits (Endogen, Woburn, MA, United States). The results were obtained from triplicate wells and the examination of supernatant from cultured T cells alone for the four cytokines were used as control groups.
The measurement of multi-antigen specific CD4+ and CD8+ T cell responses were conducted by cytokine release assay as described by Chen et al[2] and Miyazawa et al[29]. Through using the instrument of autoMACSTM (Miltenyi Biotec, Bergisch Gladbach, Germany), CD4+ and CD8+ T cells were separated from proliferating PBLCs and that were civilized after three cycles of re-stimulation ex vivo. The cells were then incubated with CD4 or CD8 microbeads (Miltenyi Biotec) for 15 min at 4 °C and washed before separation. Separation was executed adopting an autoMACS column (Miltenyi Biotec). The pillar was set in the magnetic field, and magnetically-labeled cells were preserved in the pillar and then flushed out as positively chosen cells while the magnetic field was turned off. The sorted populations’ purity was determined by flow cytometry. The selected CD4+ and CD8+ T cells (5 × 104) were stimulated with RNA-DCs (DC-actin mRNA, DC-PBMC RNA, DC-total tumor RNA, and DC-tumor-anti-DcR3 mAb RNA, 5 × 103) in an overall volume of 200 μL of the entire medium in 96-well plates for 24 h. The supernatants were collected, and the IL-4 and IFN-γ levels were measured using IL-4 and human IFN-γ ELISA kits (Endogen). Each assay was carried out on duplicate samples.
Quantitative data are presented as mean ± SD. The ANOVA and post hoc test (S-N-K method) analyses were performed using Excel software (Microsoft, Redmond, WA, United States). P < 0.05 was considered for statistical significance.
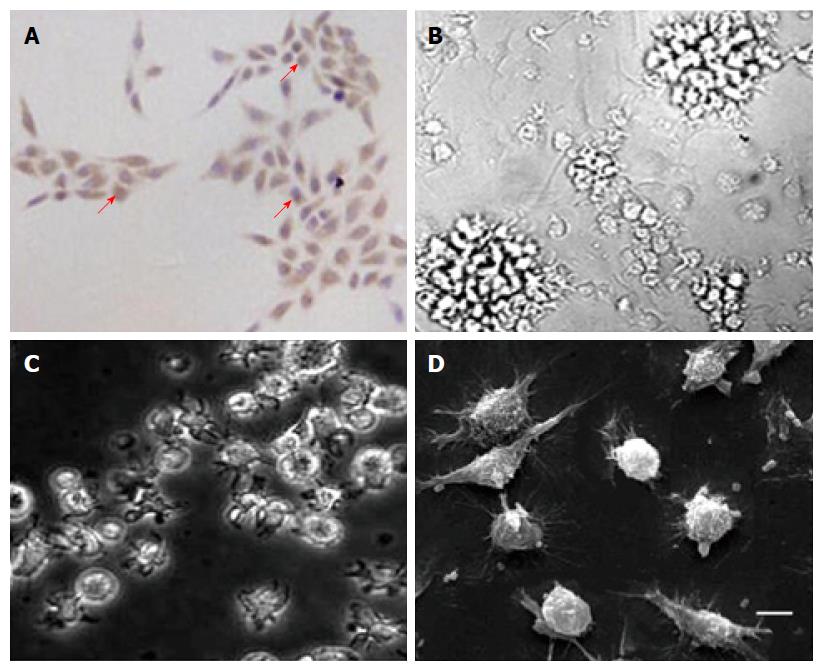
Primary tumor cells and DCs were cultured from HLA-A2+ PC patients. Most cultured primary tumor cells (> 90%) were found to be positive for DcR3 in the cytoplasm (Figure 1A). For DCs, cells cultured from PBMCs with stimulation of GM-CSF, TNF-α and IL-4 showed a series of typical morphologies of mature DCs. Most DCs without transfection with RNAs assembled non-cohesive colonies. Cells that were ablated from these colonies demonstrated typical villiform processes as shown by inverted phase contrast microscopy (Figure 1B). Both transfection with total tumor RNA (Figure 1C) and simultaneously loading with the total tumor RNA and anti-DcR3 mAb mRNA (Figure 1D) showed typical morphological characteristics of DCs.
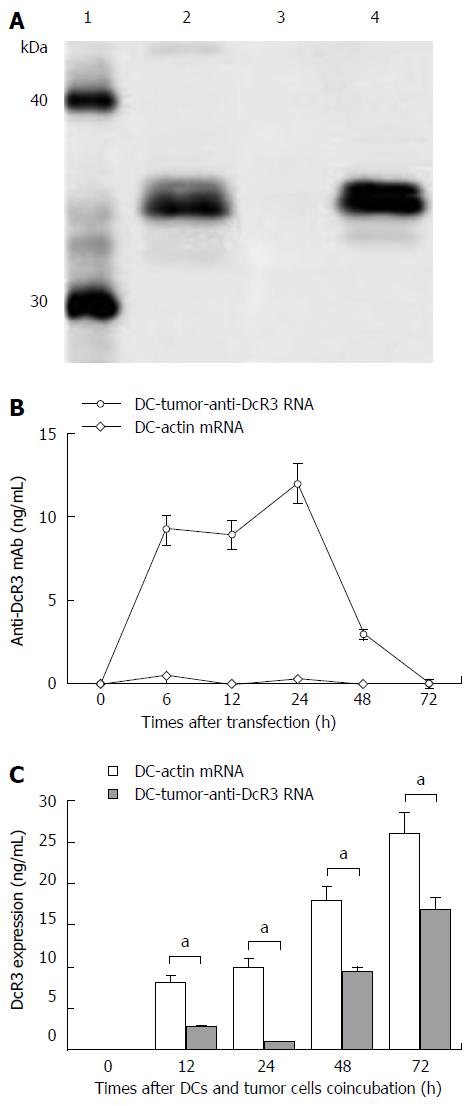
To determine if we could produce specific mAb by pulsing DCs with anti-DcR3 mAb-encoding RNA, we electroporated tumor-antigens-loaded DCs with IVT RNA encoding H and L mAb chains (anti-DcR3 H+L mRNA). The supernatants of DCs were obtained at the designated time points to identify the target mAb and measure their concentration. The supernatants of co-cultured autologous PC tumor cells and DC-tumor-anti-DcR3 RNA were harvested at specific time points to determine the effects of the mAb (Figure 2).
First, we used Western blotting to identify the anti-DcR3 mAb produced by DCs co-transfected with total tumor RNA and anti-DcR3 mAb mRNA. As shown in Figure 2A, the supernatant of DC-tumor-anti-DcR3 RNA could specifically neutralize the recombinant human DcR3 protein and generate a band with slightly higher molecular weight than 30 kDa, which was in line with the theoretical molecular weight of DcR3 protein. A commercially available anti-DcR3 mAb was used as positive control, while DC-actin was used as a negative control.
The amounts of anti-DcR3 mAb secreted by RNA-pulsed DCs were analyzed using an indirect ELISA assay. As shown in Figure 2B, mAb production by DC-tumor-anti-DcR3 RNA was transient and peaked at 24 h (containing 13.15 ± 1.9 ng of anti-DcR3 mAb per 1 × 106 cells) and then could not be detected after 72 h. However, no anti-DcR3 mAb was found in the supernatant of DC-actin RNA at any point.
The specific antigen-binding effect of anti-DcR3 mAb secreted by DCs co-transfected with total tumor RNA and humanized anti-DcR3 mAb mRNA was confirmed by ELISA. As shown in Figure 2C, the soluble DcR3 protein in the supernatant of autologous PC cells (1 × 106 cells) co-cultured with DC-tumor-anti-DcR3 RNA (1 × 106 cells) was significantly lower than that of tumor cells and the DC-actin RNA co-incubation group from 12 h to 72 h (aP < 0.05).
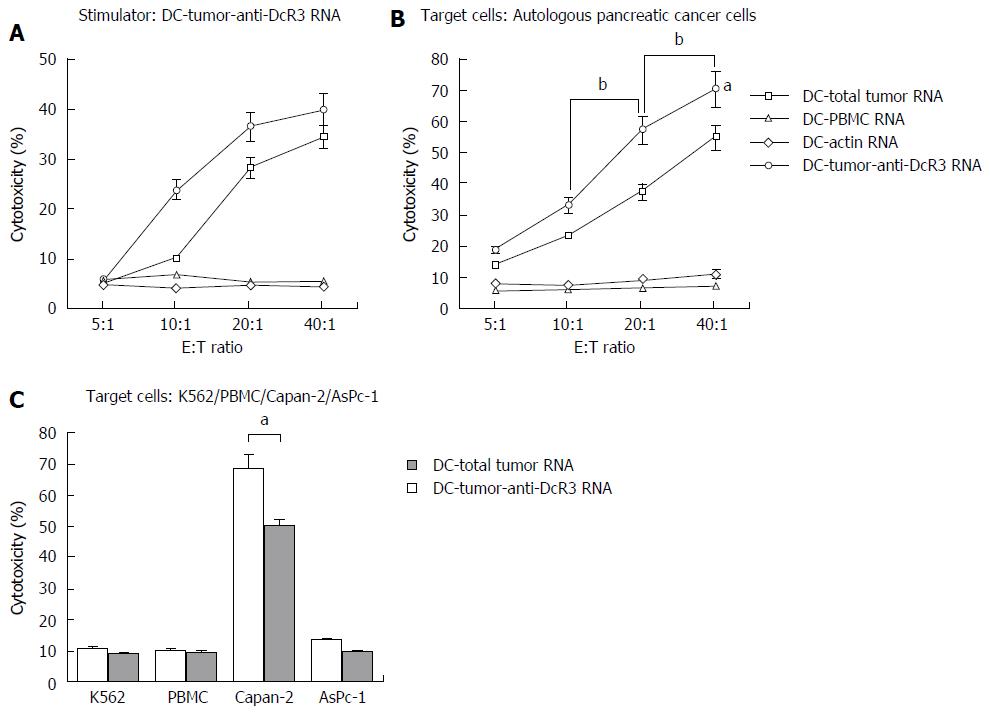
We next sought to determine whether anti-tumor responses could be enhanced by immunizing DCs co-transfected with total tumor RNA and anti-DcR3 mAb mRNA. Using cells from HLA-A2+ PC patients, we evaluated the ability of DC-tumor-anti-DcR3 RNA to augment the induction of anti-PC CTLs in response to DCs. As shown in the left panel of Figure 3A, DC-tumor-anti-DcR3 RNA was used as not only stimulator cells but also target cells, while DCs transfected with total tumor RNA alone or other autologous RNA-DCs were used as targets. DC-tumor-anti-DcR3 RNA demonstrated further enhancement of antigen-specific CTL induction compared with DCs only loaded with total tumor RNA without any increase in CTL activity above non-specific background.
CTL activity against PC cells was also assessed. Both CTLs induced by DC-tumor-anti-DcR3 RNA and DC-total tumor RNA were able to lyse their own cancer cells effectively, while CTLs induced by DC-PBMC RNA or DC-actin RNA were not, as shown in Figure 3A (right panel). Furthermore, DC-tumor-anti-DcR3 RNA showed greater effectiveness and superior ability to recognize and lyse HLA-A2+ autologous PC cells (P < 0.05). Meanwhile, with increase in the E:T ratio (from 10:1 to 40:1), the killing intensity increased concomitantly (P < 0.05).
No lysis of normal PBMCs or NK-sensitive K562 cells was observed. However, evident lysis against the cultured PC cell line occurred (Figure 3B). Effector T cells (HLA-A2+) stimulated by DCs transfected with total tumor RNA alone or together with anti-DcR3 mAb mRNA could lyse the Capan-2 cells, which expressed the HLA-A2+ antigen endogenously. On the other hand, HLA-A2- AsPC-1 cells were not identified and lysed. DC-tumor-anti-DcR3 RNA was more potent at inducing cytotoxicity in CTLs against Capan-2 cells with HLA-A2 adaptation in comparison with the CTLs induced by DC-total tumor RNA alone (P < 0.05). The expression of HLA alleles other than A2 was not evaluated in these experiments.
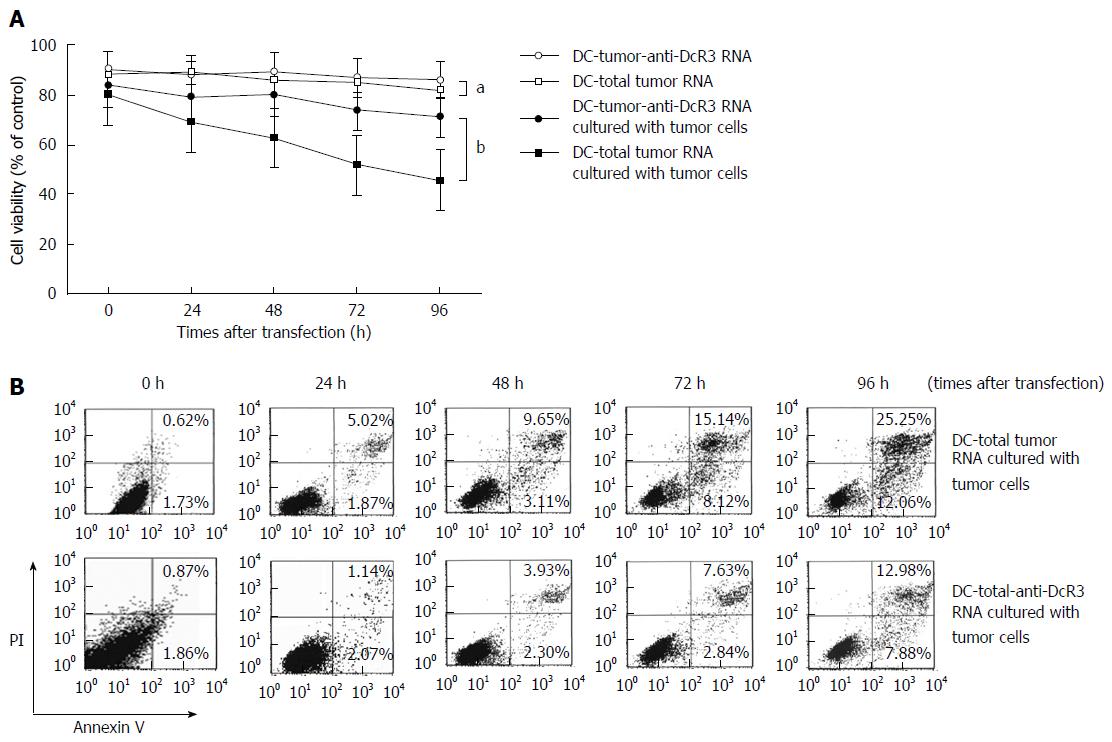
The effect of anti-DcR3 mAb secreting DCs on viability of RNA-loaded DCs was determined by MTT assay. As shown in Figure 4A, when RNA-DCs were co-incubated without autologous tumor cells, cell viability did not vary significantly at designed times, with around 85% survival throughout (P > 0.05). By contrast, the viability of DC-total tumor RNA cultured with tumor cells was evidently lower than that of DC-tumor-anti-DcR3 RNA (P < 0.01). The results of DC-PBMC RNA and DC-actin RNA were similar to those of DC-total tumor RNA. Meanwhile, both the DC-tumor-anti-DcR3 RNA and DC-tumor RNA demonstrated positive expression of CD80, CD83, CD86 (co-stimulatory molecules) and HLA-DR (MHC II molecules) after co-incubation with PC cells for 24 h, but there was no significant difference between the two methodologies (data not shown).
Annexin V and PI double staining was performed to demonstrate the inhibition of apoptosis of DCs co-transfected with total tumor RNA and anti-DcR3 mAb mRNA. After incubation with autologous PC cells for 0-96 h, annexin V-positive apoptotic cells were found to increase sharply in a time-dependent manner in DC-total tumor RNA, whereas apoptosis of DC-tumor-anti-DcR3 RNA were mitigated by anti-DcR3 mAb (Figure 4B).
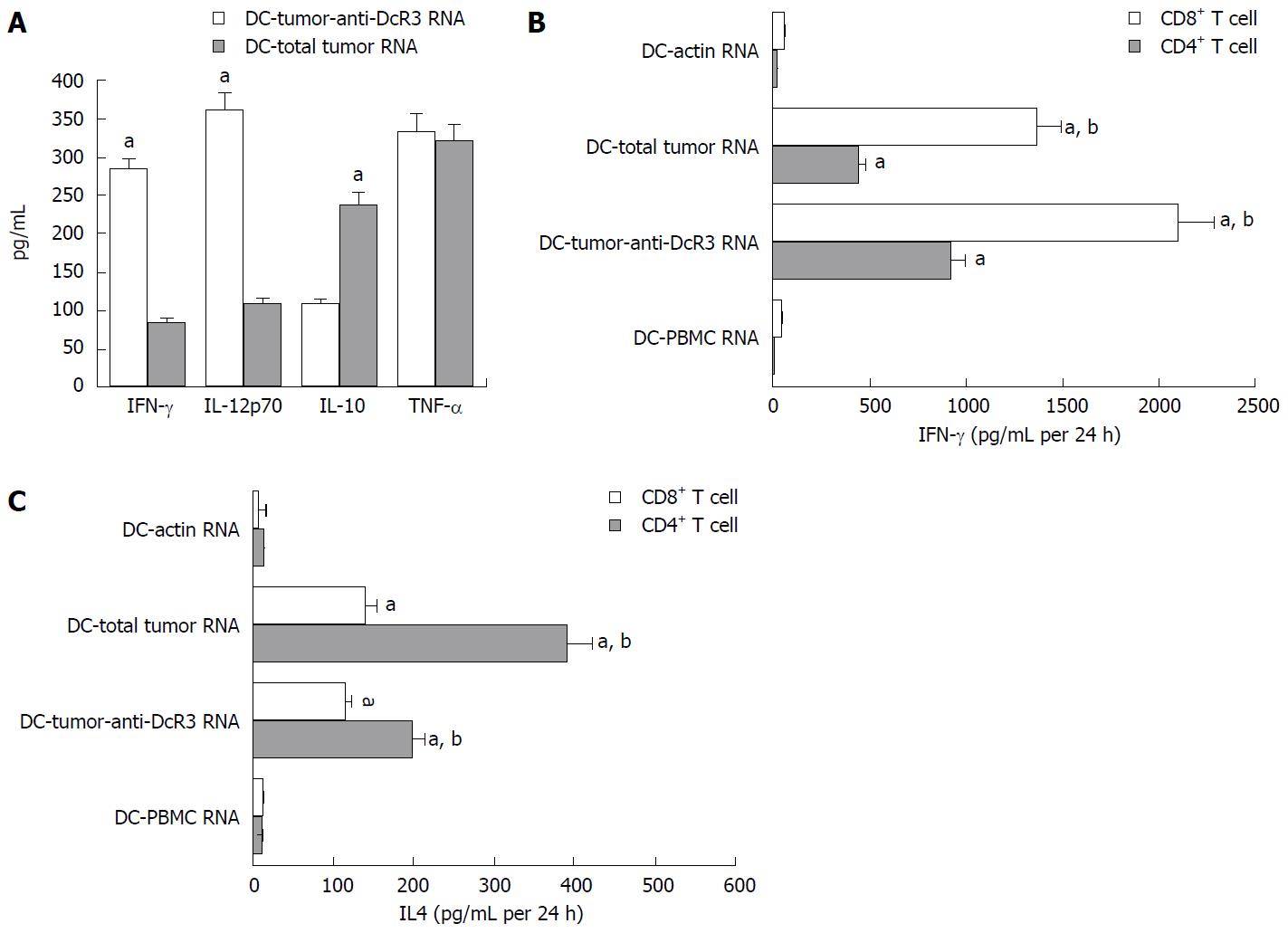
There were no significant differences in the amount of IL-10, TNF-α, IL-12p70 and IFN-γ cytokines released in the culture supernatants of T cells when measured by ELISA (data not shown). However, high levels of cytokines could be secreted by T cells after pulsing with RNA-DC (Figure 5A). The IFN-γ and IL-12p70 produced by DC-tumor-anti-DcR3 RNA pulsed T cells were higher than those secreted by T cell pulsed by DC-total tumor RNA (P < 0.01). At the same time, compared with the DC-total tumor RNA group, the TNF-α levels detected in the DC-tumor-anti-DcR3 RNA group was not changed significantly (P > 0.05). In addition, the IL-10 level was lower when it was detected in DCs co-transfected with both total tumor RNA and anti-DcR3 mAb RNA (P < 0.05).
As shown in Figure 5B, the CD4+ and CD8+ T cells incubated with DCs transfected with total tumor RNA alone or together with anti-DcR3 mAb mRNA produced significantly higher level of IFN-γ than those incubated with control DC (DC-actin RNA) or DCs exposed to normal tissues (DC-PBMC RNA) (P < 0.01). In addition, the CD4+ and CD8+ T cells that were cultured with DCs co-transfected with both total tumor RNA and anti-DcR3 mAb RNA produced a higher level of IFN-γ than those cultured with DC-total tumor RNA (P < 0.01). Meanwhile, CD4+ and CD8+ T cells incubated with DCs transfected with total tumor RNA alone or together with anti-DcR3 mAb mRNA were able to produce IL-4, but negative or weak secretion of IL-4 was observed in control groups (DC-actin RNA and DC-PBMC RNA) (P < 0.01). Furthermore, decreased levels of IL-4 production were detected in CD4+ T cells stimulated by DC-tumor-anti-DcR3 RNA compared with DC-total tumor RNA (P < 0.01). No significant difference in IL-4 secretion was detected between CD8+ T cells stimulated by DC-tumor RNA and those stimulated by DC-tumor-anti-DcR3 RNA.
Tumor cells can produce several immunomodulatory molecules to induce immunosuppressive TME and inhibit the function of tumor-associated DCs[9,30]. Therefore, new DC-based strategies for producing tumor vaccines are necessary to abrogate immunosuppressive molecules in tumor tissue-induced mechanisms for suppressing the activation of CTL responses that can treat established cancers[8].
DcR3 is one of the candidate target tumor-derived factors[31]. As we initially demonstrated, most cultured primary tumor cells showed a DcR3-positive expression in the cytoplasm of PC patients, which is consistent with the findings of Zhou et al[17]. Tumor cells engineered to release high amounts of DcR3 are able to protect themselves from apoptosis, consequently resulting in a decreased immune response and suggesting that DcR3 is involved in the immune evasion of malignant tumors[19,32]. As a powerful immunomodulatory factor, DcR3 can suppress actin polymerization in mitogen-stimulated T cells, prevent the formation of pseudopodia, down-regulate the activation of DCs and macrophages, induce abnormal aggregation of T cells after antigen stimulation, reduce the interaction between T cells and DCs, inhibit T cell chemotaxis, and induce T cell apoptosis[19,33]. Therefore, neutralizing the DcR3 protein secreted by tumor cells is particularly important in cancer immunotherapy.
Different methods have been developed for neutralizing immunosuppressive factors released by tumor cells. The effects of systemic administration of antibodies have been examined by numerous studies. Their findings showed that mAbs can improve special immune responses when administered systemically[34]. Human studies, however, have revealed that the side-effects of mAbs are unavoidable when delivered systemically, and one major concern is induction of autoimmunity[35-37]. Thus, our present study shows an alternative strategy of delivering mAb by transfecting DCs with the RNA that encodes both PC tumor-antigens and a defined immunosuppressive molecule, namely humanized anti-DcR3 mAb. Using this strategy, DCs were used both to produce anti-DcR3 mAb and as vehicles for delivery to the site of T cell activation considering their well-documented function as antigen-presenting cells.
Our results showed that the supernatant of cultured DCs co-transfected with total tumor RNA and anti-DcR3 H+L mRNA could specifically bind the recombinant human DcR3 protein and generate a band with molecular weight that was slightly greater than 30 kDa, which was in line with the theoretical molecular weight of DcR3 protein. In addition, the DcR3 level in the supernatant of autologous PC cells co-cultured with DC-tumor-anti-DcR3 RNA was significantly lower than that of the control group. These data suggest that this approach is feasible and can generate sufficient anti-DcR3 mAb to neutralize the DcR3 secreted by PC cells in vitro.
One advantage of the local release of anti-DcR3 mAb that is provided via DC mRNA transfection is its secretion over a relatively short time span at the precise site where T cell activation is needed[8]. We previously showed that mRNA subunits have a brief half-life of less than 24 h after DC transfection[2], implying that mRNA translating into protein is an instantaneous event in DC. Here, we confirmed that for anti-DcR3 mAb, most target proteins were freed in 12 h after mRNA transfection, but the extra secreting of target could last for 24-48 h. Considering that human PC cells showed unregulated production of DcR3 within 12 h, we found that the time course of anti-DcR3 mAb release by DCs is ideal for obstructing the DcR3 expressed by PC cells[8].
In the present study, we found that DCs transfected with total tumor RNA alone or together with anti-DcR3 mAb mRNA could induce tumor-specific cytotoxic T cells to recognize and lyse tumor RNA-loaded DCs and tumor cells effectively. In comparison, no damage in K562 cells were found, which implies that the two manners can not only be PC-specific but also eliminated the possibility of NK cell activity [2]. These data also indicate that CTLs induced by DC-tumor-anti-DcR3 RNA were more powerful at indicting lysis than the CTLs induced by DC-total tumor RNA. These results demonstrate that use of DCs co-transfected with RNA encoding humanized anti-DcR3 mAb and whole PC tumor-antigens may be a superior strategy for designing a DC-based tumor vaccine. In addition, it is known that, like Fas-Fc antibody, DcR3 can block apoptosis in Jurkat cells[21]. The more superior cytolytic function might be due to blockade of activation-induced cell death (AICD) in CTLs induced by DC-tumor-anti-DcR3 RNA rather than better CTL response generation due to superior priming of CTL precursors by engineered DCs. This should be addressed in future studies.
An HLA allele that matches the target cells and tumor-specific CTLs is necessary. All PC patients that were included in this study were HLA-A2+, and we are concerned with the function of HLA-A2 among different HLA alleles. We found that CTLs induced by DC-tumor-anti-DcR3 RNA and DC-total tumor RNA could deliver potent cytotoxicity towards Capan-2 cells. However, owing to HLA-A2 mismatching, AsPC-1 PC cells with HLA-A2- could not be lysed by HLAA2+ CTLs. This result indicates that HLA-A2 may be a key allele for presenting antigens, and PC-specific CTL immune response may be limited to MHC class I antigens.
The induction of autoimmunity is one potential problem that may limit the application of PC tumor and anti-DcR3 mAb RNA-transected DC vaccines, partly because RNAs loaded with both normal antigens and tumor antigens share the same antigen presentation pathway when they are delivered to DCs[26,38], and partly because RNA-encoding anti-DcR3 mAb in DCs may improve the risk of inducing autoimmunity just like systemic administration of neutralizing immunosuppressive factor antibody in TME[34]. Here, we adopted tissue cell enrichment by primary tumor cell culture, and found that CTLs stimulated by DC-total tumor RNA lysed tumor cells (autologous primary cultured tumor cells and PC cell lines) but not PBMCs (normal tissue cells). Meanwhile, using mRNA-transfected DCs to locally deliver anti-DcR3 mAb, no increase in non-specific background immune responses against control target cells or the normal tissue PBMCs in vitro was detected. On the basis of these results, we anticipate that local delivery of anti-DcR3 mAb by utilizing DCs transfected with RNA will bypass the adverse effects of autoimmune responses triggered by systemic delivery of mAb. At the same time, vaccine-induced anti-tumor immune response increased in patients. These findings indicate that harmful autoimmunity with pathological results may not be an issue with this method.
The mechanism by which DCs engineered to secrete anti-DcR3 mAb augments CTL response remains to be fully elucidated. In our study, we found that the cell viability of both DC-tumor-anti-DcR3 RNA and DC-total tumor RNA did not change significantly at the designated time points, with approximately 85% survival when cultured alone (without DcR3 influence). On the contrary, when co-cultured with autologous tumor cells (with DcR3 influence), the viability of DC-total tumor RNA was evidently lower than that of DC-tumor-anti-DcR3 RNA. Furthermore, when co-cultured with tumor cells for 0-96 h, apoptotic cells in DC-total tumor RNA evidently increased, whereas apoptotic cells in DC-tumor-anti-DcR3 RNA were found to increase slowly and mildly. Similar to the findings of You et al[39], results suggested that enhancement of CTL responses induced by anti-DcR3 mAb DCs may partly be due to its powerful DcR3 blocking capability, which down-regulated the apoptosis of DCs induced by DcR3 and increased DC viability at the site of T-cell activation in the process of whole tumor-antigen delivery.
The whole total tumor and anti-DcR3 mAb RNA electroporation likely acted as a powerful tumor vaccine which could effectively activate antigen-specific T cells against PCs, just like Th1 cells[2]. This interpretation is supported by our observations of a high percentage of killing of tumor cells and IFN-γ secretion of T cells. High expressions of cytokines, such as IL-12 and IFN-γ, and low expression of cytokine, such as IL-10, might be the reason why DC-tumor-anti-DcR3 RNA exhibited enhanced capability of inducing CTL responses. Th1 cells and Th2 cells are two important T regulatory (Treg) cells in the body. Transformation of Treg cells from Th1 to Th2 is a unique phenomenon in malignant tumors. Development of Th2 cells promotes long-term retention of cancer cells in the host body and protects them from immune surveillance and attack. Th1 cells and Th2 cells can both stimulate IFN-γ production, whereas Th2 cells preferentially induced production of IL-4 compared to Th1 cells[39,40]. In this study, we clearly demonstrated that CD4+ T cells induced by DCs co-transfected with total tumor RNA and anti-DcR3 mAb mRNA markedly decreased IL-4 secretion and increased IFN-γ production, indicating that DCs engineered to secrete anti-DcR3 mAb could increase the number of Th1 cells and decrease Th2 cells. Ojima et al[41] and Chen et al[2] reported that DCs that were loaded with TAA could induce antigen-specific CD4+Th1 cells and such CD4+ Th1 cells played a key role in the priming phase of CD8+ CTLs. In the present study, we showed that both DC-tumor-anti-DcR3 RNA and DC-total tumor RNA can activate not only tumor-specific CD4+ T cells but also CD8+ T cells assessed by IFN-γ release. Besides, CD4+ T cells and CD8+ T cells incubated with DC-tumor-anti-DcR3 RNA could produce more IFN-γ compared with those incubated with DC-total tumor RNA. These results indicate that adjusting the Th1/Th2 cytokine network and promoting the recovery of CD8+ anti-tumor cellular immunity may be the other two mechanisms for engineering DCs to secrete anti-DcR3 mAb to augment CTL response.
In summary, our results demonstrate that DCs engineered to secrete anti-DcR3 antibody can augment CTL responses against PC in vitro. The observed immune-enhancing effects may be partly due to their capability of down-regulating DC apoptosis and adjusting the Th1/Th2 cytokine network. Therefore, use of DCs engineered to secrete anti-DcR3 antibody vaccine may be an attractive and promising therapeutic strategy for a patient with PC.
Pancreatic cancer (PC) is considered as a highly destructive human malignant tumor without effective treatments. Dendritic cell (DC) tumor vaccines have emerged as an alternative treatment manner for advanced PC. But tumor cells can produce some immunosuppressive molecules to inhibit the function of tumor-associated cells, such as T lymphocytes and DCs.
DcR3, a soluble protein secreted by tumor cells, is overexpressed in carcinoma originating from the gastrointestinal tract system, including PC. DcR3 is regarded as an important immunosuppressive factor in immune effector cells’ defect and it is particularly important to neutralize DcR3 protein secreted by tumor cells in cancer immunotherapy.
A new strategy of delivering mAb by transfecting DCs with the RNA encoding both anti-DcR3 mAb and the whole tumor-antigens was shown by this study. Furthermore, the DCs engineered to secrete anti-DcR3 mAb could augment cytotoxic T lymphocyte responses against PC in vitro and the immune-enhancing effects may be partly due to their capability of down-regulating apoptosis of DCs and adjusting the Th1/Th2 cytokine network.
This study lays a good foundation for further investigation of tumor DC vaccine targeting DcR3 protein against PC.
As a tumor necrosis factor receptor superfamily’s member, DcR3 works as decoy receptor for Fas ligand, LIGHT and TNF-like molecule 1A to neutralize their cytotoxic and regulatory functions.
This manuscript has shown that DCs engineered to secrete anti-DcR3 antibody can stimulate cytotoxic T lymphocyte responses against pancreatic cancer cells in vitro. It provided important contribution to development of immunotherapy for pancreatic cancers. The proposed method is convincing.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chhabra A, Ferrante A, MatsudaY, Tarnawski AS S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 2. | Chen J, Guo XZ, Li HY, Wang D, Shao XD. Comparison of cytotoxic T lymphocyte responses against pancreatic cancer induced by dendritic cells transfected with total tumor RNA and fusion hybrided with tumor cell. Exp Biol Med (Maywood). 2015;240:1310-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2206] [Article Influence: 147.1] [Reference Citation Analysis (2)] |
| 4. | Li D, O’Reilly EM. Adjuvant and Neoadjuvant Therapy for Pancreatic Cancer. Surg Oncol Clin N Am. 2016;25:311-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Arslan C, Yalcin S. Current and future systemic treatment options in metastatic pancreatic cancer. J Gastrointest Oncol. 2014;5:280-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 6. | Darcy PK, Neeson P, Yong CS, Kershaw MH. Manipulating immune cells for adoptive immunotherapy of cancer. Curr Opin Immunol. 2014;27:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin Cancer Res. 2016;22:1897-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 456] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 8. | Pruitt SK, Boczkowski D, de Rosa N, Haley NR, Morse MA, Tyler DS, Dannull J, Nair S. Enhancement of anti-tumor immunity through local modulation of CTLA-4 and GITR by dendritic cells. Eur J Immunol. 2011;41:3553-3563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 749] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 10. | Lin WW, Hsieh SL. Decoy receptor 3: a pleiotropic immunomodulator and biomarker for inflammatory diseases, autoimmune diseases and cancer. Biochem Pharmacol. 2011;81:838-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699-703. [PubMed] |
| 12. | del Rio ML, Fernandez-Renedo C, Chaloin O, Scheu S, Pfeffer K, Shintani Y, Perez-Simon JA, Schneider P, Rodriguez-Barbosa JI. Immunotherapeutic targeting of LIGHT/LTβR/HVEM pathway fully recapitulates the reduced cytotoxic phenotype of LIGHT-deficient T cells. MAbs. 2016;8:478-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Zhan C, Patskovsky Y, Yan Q, Li Z, Ramagopal U, Cheng H, Brenowitz M, Hui X, Nathenson SG, Almo SC. Decoy strategies: the structure of TL1A: DcR3 complex. Structure. 2011;19:162-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Sung HY, Wu HG, Ahn JH, Park WY. Dcr3 inhibit p53-dependent apoptosis in gamma-irradiated lung cancer cells. Int J Radiat Biol. 2010;86:780-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Chen G, Luo D. Over-expression of decoy receptor 3 in gastric precancerous lesions and carcinoma. Ups J Med Sci. 2008;113:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Ho CH, Chen CL, Li WY, Chen CJ. Decoy receptor 3, upregulated by Epstein-Barr virus latent membrane protein 1, enhances nasopharyngeal carcinoma cell migration and invasion. Carcinogenesis. 2009;30:1443-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Zhou J, Song S, Li D, He S, Zhang B, Wang Z, Zhu X. Decoy receptor 3 (DcR3) overexpression predicts the prognosis and pN2 in pancreatic head carcinoma. World J Surg Oncol. 2014;12:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Jiang M, Lin X, He R, Lin X, Liang L, Tang R, Xiong D, Wei K, Dang Y, Feng Z. Decoy Receptor 3 (DcR3) as a Biomarker of Tumor Deterioration in Female Reproductive Cancers: A Meta-Analysis. Med Sci Monit. 2016;22:1850-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Wu SF, Liu TM, Lin YC, Sytwu HK, Juan HF, Chen ST, Shen KL, Hsi SC, Hsieh SL. Immunomodulatory effect of decoy receptor 3 on the differentiation and function of bone marrow-derived dendritic cells in nonobese diabetic mice: from regulatory mechanism to clinical implication. J Leukoc Biol. 2004;75:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Weissinger D, Tagscherer KE, Macher-Göppinger S, Haferkamp A, Wagener N, Roth W. The soluble Decoy Receptor 3 is regulated by a PI3K-dependent mechanism and promotes migration and invasion in renal cell carcinoma. Mol Cancer. 2013;12:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Wang W, Zhang M, Sun W, Yang S, Su Y, Zhang H, Liu C, Li X, Lin L, Kim S. Reduction of decoy receptor 3 enhances TRAIL-mediated apoptosis in pancreatic cancer. PLoS One. 2013;8:e74272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6461] [Article Influence: 430.7] [Reference Citation Analysis (0)] |
| 23. | Wang K, Zhou Q, Guo AL, Xu CR, An SJ, Wu YL. An autologous therapeutic dendritic cell vaccine transfected with total lung carcinoma RNA stimulates cytotoxic T lymphocyte responses against non-small cell lung cancer. Immunol Invest. 2009;38:665-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Wu CF, Ma ZS, Wang XL, Yang JL, Yang Y, Zhang Y, Tumen WL, Qiao JH. Preparation and identification of monoclonal antibodies against human DcR3. Xibao Yu Fenzi Mianyixue Zazhi. 2008;24:139-141. [PubMed] |
| 25. | Zhu M, Xu W, Su H, Huang Q, Wang B. Addition of CpG ODN and Poly (I: C) to a standard maturation cocktail generates monocyte-derived dendritic cells and induces a potent Th1 polarization with migratory capacity. Hum Vaccin Immunother. 2015;11:1596-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Chen J, Guo XZ, Li HY, Liu X, Ren LN, Wang D, Zhao JJ. Generation of CTL responses against pancreatic cancer in vitro using dendritic cells co-transfected with MUC4 and survivin RNA. Vaccine. 2013;31:4585-4590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Chen J, Li HY, Wang D, Zhao JJ, Guo XZ. Human dendritic cells transfected with amplified MUC1 mRNA stimulate cytotoxic T lymphocyte responses against pancreatic cancer in vitro. J Gastroenterol Hepatol. 2011;26:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Lin XY, He CD, Xiao T, Jin X, Chen J, Wang YK, Liu M, Wang KB, Jiang Y, Wei HC. Acitretin induces apoptosis through CD95 signalling pathway in human cutaneous squamous cell carcinoma cell line SCL-1. J Cell Mol Med. 2009;13:2888-2898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Miyazawa M, Iwahashi M, Ojima T, Katsuda M, Nakamura M, Nakamori M, Ueda K, Naka T, Hayata K, Iida T. Dendritic cells adenovirally-transduced with full-length mesothelin cDNA elicit mesothelin-specific cytotoxicity against pancreatic cancer cell lines in vitro. Cancer Lett. 2011;305:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Constantino J, Gomes C, Falcão A, Cruz MT, Neves BM. Antitumor dendritic cell-based vaccines: lessons from 20 years of clinical trials and future perspectives. Transl Res. 2016;168:74-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Bai C, Connolly B, Metzker ML, Hilliard CA, Liu X, Sandig V, Soderman A, Galloway SM, Liu Q, Austin CP. Overexpression of M68/DcR3 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc Natl Acad Sci USA. 2000;97:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Huang MT, Chen ST, Wu HY, Chen YJ, Chou TY, Hsieh SL. DcR3 suppresses influenza virus-induced macrophage activation and attenuates pulmonary inflammation and lethality. J Mol Med (Berl). 2015;93:1131-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Shi G, Wu Y, Zhang J, Wu J. Death decoy receptor TR6/DcR3 inhibits T cell chemotaxis in vitro and in vivo. J Immunol. 2003;171:3407-3414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Galluzzi L, Vacchelli E, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zucman-Rossi J, Zitvogel L, Kroemer G. Trial Watch: Monoclonal antibodies in cancer therapy. Oncoimmunology. 2012;1:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 35. | Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1589] [Cited by in RCA: 1469] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 36. | Postel-Vinay S, Aspeslagh S, Lanoy E, Robert C, Soria JC, Marabelle A. Challenges of phase 1 clinical trials evaluating immune checkpoint-targeted antibodies. Ann Oncol. 2016;27:214-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Weinstock M, McDermott D. Targeting PD-1/PD-L1 in the treatment of metastatic renal cell carcinoma. Ther Adv Urol. 2015;7:365-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 38. | Ganji A, Varasteh A, Sankian M. Aptamers: new arrows to target dendritic cells. J Drug Target. 2016;24:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | You RI, Chang YC, Chen PM, Wang WS, Hsu TL, Yang CY, Lee CT, Hsieh SL. Apoptosis of dendritic cells induced by decoy receptor 3 (DcR3). Blood. 2008;111:1480-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Hillyer P, Raviv N, Gold DM, Dougherty D, Liu J, Johnson TR, Graham BS, Rabin RL. Subtypes of type I IFN differentially enhance cytokine expression by suboptimally stimulated CD4(+) T cells. Eur J Immunol. 2013;43:3197-3208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Ojima T, Iwahashi M, Nakamura M, Matsuda K, Nakamori M, Ueda K, Naka T, Ishida K, Primus FJ, Yamaue H. Successful cancer vaccine therapy for carcinoembryonic antigen (CEA)-expressing colon cancer using genetically modified dendritic cells that express CEA and T helper-type 1 cytokines in CEA transgenic mice. Int J Cancer. 2007;120:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |