Published online Sep 21, 2017. doi: 10.3748/wjg.v23.i35.6412
Peer-review started: February 24, 2017
First decision: June 5, 2017
Revised: June 21, 2017
Accepted: August 15, 2017
Article in press: August 15, 2017
Published online: September 21, 2017
Processing time: 210 Days and 8 Hours
To examine the effects of aspirin and enoxaparin on liver function, coagulation index and histopathology in a rat model of liver fibrosis.
Forty-five male Sprague-Dawley rats were randomly divided into the control group (n = 5) and model group (n = 40). Thioacetamide (TAA) was used to induce liver fibrosis in the model group. TAA-induced fibrotic rats received TAA continuously (n = 9), TAA + low-dose aspirin (n = 9), TAA + high-dose aspirin (n = 9) or TAA + enoxaparin (n = 9) for 4 wk. All rats were euthanized after 4 wk, and both hematoxylin-eosin and Masson staining were performed to observe pathological changes in liver tissue.
Liver fibrosis was assessed according to the METAVIR score. Compared with untreated cirrhotic controls, a significant improvement in fibrosis grade was observed in the low-dose aspirin, high-dose aspirin and enoxaparin treated groups, especially in the high-dose aspirin treated group. Alanine aminotransferase and total bilirubin were higher, albumin was lower and both prothrombin time and international normalized ratio were prolonged in the four treatment groups compared to controls. No significant differences among the four groups were observed.
Aspirin and enoxaparin can alleviate liver fibrosis in this rat model.
Core tip: Several lines of evidence show that intra-hepatic thrombosis is associated with liver fibrosis. Based on these observations, we hypothesized that routine use of antithrombotic drugs may prevent liver fibrosis. In our study, we successfully established a rat model of liver fibrosis after 8 wk and successfully established a rat model of liver cirrhosis after 12 wk through the thioacetamide-induced method. Our study clearly shows that aspirin and enoxaparin can reduce liver fibrosis in rats. However, reporting the results in humans is more complicated because other aspects, such as renal function, need to be considered.
- Citation: Li CJ, Yang ZH, Shi XL, Liu DL. Effects of aspirin and enoxaparin in a rat model of liver fibrosis. World J Gastroenterol 2017; 23(35): 6412-6419
- URL: https://www.wjgnet.com/1007-9327/full/v23/i35/6412.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i35.6412
Liver fibrosis is a common liver disease posing a heavy burden on human health, and is a common cause of death. The mortality rate due to end-stage liver disease caused by fibrosis and subsequent severe liver cirrhosis is 20%-50%. In recent years, liver fibrosis has become a research hotspot, but its occurrence and development have not been fully elucidated. Liver fibrosis is closely related to the chronic inflammatory response of the liver, and inflammation leading to liver fibrosis has been studied in detail. In some cases, other pathways may also contribute to liver fibrosis in addition to inflammation.
There is evidence showing that intra-hepatic thrombosis is associated with liver fibrosis. Wanless et al[1] found that microthrombosis in the intrahepatic portal vein and hepatic venous system was associated with hepatic atrophy and fibrosis in transplanted livers. Papatheodoridis et al[2] showed that, in patients with chronic viral hepatitis, risk factors for thrombosis were strongly associated with liver fibrosis and cirrhosis. Thrombin, a serine protease activated by proteolytic cleavage of prothrombin, can directly activate stellate cells through protease-activated receptors. Many antithrombotic therapies, such as standard heparin, low molecular weight heparin, vitamin K antagonists and antiplatelet agents, can reduce the proliferation and activation of stellate cells and reduce the fibrotic regions in various animal models with liver fibrosis.
Based on these observations, we hypothesized that routine use of antithrombotic drugs may prevent liver fibrosis in humans. To test this hypothesis, we used thioacetamide (TAA) to establish a rat model of liver fibrosis and examined the effect of antithrombotic therapy with aspirin and enoxaparin in this model.
Forty-five healthy and pure Sprague-Dawley (SD) male rats weighing 200-250 g were included in this study and were housed in a specific pathogen-free environment. The ambient temperature was maintained at 20-25 °C and humidity was maintained at 50%-70%. The animals were acclimated to their new environment for 2 wk prior to experimentation. The rats were treated in accordance with ethical requirements for laboratory animal care, and were weighed weekly to adjust the dose of TAA and drugs.
TAA was purchased from Sigma (St Louis, MO, United States). Aspirin enteric-coated tablets were produced by Bayer Healthcare Limited Corporation and enoxaparin was produced by Sanofi-Aventis (Beijing, China) Pharmaceutical Limited Corporation.
Preparation period: SD rats were randomly divided into two groups: the control group (Group A, n = 5) and the model group (n = 40). Hepatic fibrosis was induced by intraperitoneal injection of TAA at a dosage of 200 mg/kg body weight, given twice weekly for 8 wk. The control group received an intraperitoneal injection of saline. After 8 wk, four rats in the model group were sacrificed at random and liver tissue was obtained to verify the model.
Treatment period: The rats in the model group were subdivided into four groups: TAA group (Group B), TAA + low-dose aspirin group (Group C), TAA + high-dose aspirin group (Group D) and TAA + enoxaparin group (Group E), with 9 rats in each group. One milliliter of physiological saline was administered by gavage in Group A and B, 1 mL of aspirin (30 mg/kg) was administered by gavage in Group C, aspirin (300 mg/kg) was administered by gavage in Group D, and 400 L of enoxaparin (2 mg/kg) was injected subcutaneously in Group E. The frequency of administration in each group was once a day over a period of 4 wk.
During establishment of the model and the treatment period, the weight of the rats was measured twice a week and drug dosage was adjusted according to body weight. The dosage selected for each intervention was based on the results of previous studies using these agents in various fibrotic diseases[3,4]. To prevent spontaneous degeneration of liver cirrhosis, TAA (200 mg/kg) injections were continued twice a week during the treatment phase of the study in Groups B-E.
The main indices observed were activity, death and weight.
Serum and plasma were collected before the experi-ment, and at wk 8, 10 and 12 after treatment. Serum alanine aminotransferase (ALT), albumin (ALB), total bilirubin (TBIL) and plasma prothrombin time (PT) as well as international normalized ratio (INR) were measured.
Liver histopathology was determined by naked-eye observation and photography and by staining, microscopic observation and photography.
HE staining: Histopathological changes in the liver were observed, including necrosis of hepatocytes, the degree of fibrosis, the presence of regenerative nodules or pseudo-lobules, particularly the presence of thrombosis in sinusoidal or intrahepatic vessels, and the presence of luminal stenosis.
Masson staining: Liver collagen fibers were stained using Masson stain. The degree of fibrotic hyperplasia in sections was classified using the METAVIR liver fibrosis scoring system[5], which was blindly applied to each liver biopsy sample.
All data were statistically analyzed using SPSS 18.0 statistical software. The data were expressed as median, and the overall variance analysis was carried out using nonparametric Kruskal-Wallis H and Mann-Whitney U tests, as the data obtained were not consistent with normal distribution and variance was not homogeneous. P-values of < 0.05 were considered statistically significant.
At wk 12, healthy control rats had shiny hair and normal activities, with no mortality. The TAA group had disheveled hair and decreased activities; one rat died at wk 9 and another died at week 11, giving a mortality rate of 22.2%. Activity in the TAA + low-dose aspirin group, TAA + high-dose aspirin group and TAA + enoxaparin group decreased compared with the healthy control group, but was greater than that in the TAA group. The mortality rate in the TAA + low-dose aspirin group and TAA + high-dose aspirin group was 11.1%, with one death at wk 11 in each group. No deaths occurred in the TAA + enoxaparin group.
Compared with the healthy control group, body weight in rats in the model groups was lower at wk 8, 10 and 12 (P < 0.05; Table 1), but there was no statistically significant difference between them.
| Group | Body weighting | ||
| 8 wk | 10 wk | 12 wk | |
| Healthy control group, A | 510 | 536 | 527 |
| TAA group, B | 394 | 401 | 454 |
| TAA + low-dose aspirin group, C | 381 | 403 | 403 |
| TAA + high-dose aspirin group, D | 381 | 364 | 408 |
| TAA + enoxaparin group, E | 385 | 409 | 415 |
Biochemical changes in the rats are shown in Table 2. At wk 8, ALT and TBIL were higher in the model group than in the control group (P < 0.05). ALB decreased, and both PT and INR were prolonged, but were not statistically significant. Along with the formation of liver fibrosis, liver function became abnormal.
| Group | Wk | ALT | ALB | TBIL | PT | INR |
| Healthy control group, A | 8 | 35 | 29.1 | 2.95 | 9.2 | - |
| 10 | 41.0 | 30.1 | 2.3 | 8.8 | 0.83 | |
| 12 | 40.0 | 30 | 2.8 | 9.3 | 0.88 | |
| TAA group, B | 8 | 45.0 | 29 | 3.4 | 9.4 | 0.78 |
| 10 | 49.5 | 29 | 3.4 | 9.4 | 0.89 | |
| 12 | 60.0 | 28.5 | 4.2 | 10.3 | 0.97 | |
| TAA + low-dose aspirin group, C | 10 | 48.0 | 29.6 | 2.2 | 10.2 | 0.96 |
| 12 | 54.5 | 28.5 | 4.4 | 10.5 | 0.99 | |
| TAA + high-dose aspirin group, D | 10 | 60.5 | 29.7 | 3.5 | 9.45 | 0.895 |
| 12 | 47.0 | 29.25 | 4.75 | 10.1 | 0.950 | |
| TAA + enoxaparin group, E | 10 | 45.0 | 30 | 3.4 | 9.45 | 0.895 |
| 12 | 47.0 | 28.7 | 4.8 | 10.2 | 0.96 |
At wk 8, 10 and 12, ALT and TBIL increased, ALB decreased, and both PT and INR were prolonged in the four model groups (P < 0.05). However, there were no significant differences between the groups.
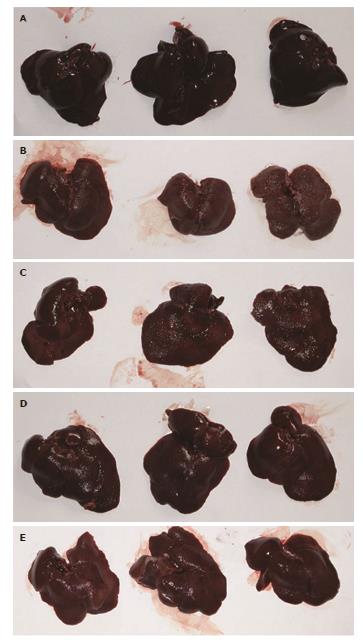
At wk 12, livers in the healthy control group were bright red, shiny and soft, with a sharp edge (Figure 1A). Livers in the TAA group were dark, dull and rough, and showed granular nodules, hard texture and a blunt edge (Figure 1B). Livers in the TAA + low-dose aspirin group (Figure 1C), TAA + high-dose aspirin group (Figure 1D) and TAA + enoxaparin group (Figure 1E) were slightly darker than in the healthy control group, but slightly softer than in the TAA group, and the granular nodules on the surface were not obvious compared to the TAA group. In particular, livers were improved in the TAA + high-dose aspirin group and TAA + enoxaparin group. TAA effectively induced liver fibrosis/cirrhosis in this rat model, while aspirin and enoxaparin reduced the degree of liver fibrosis/cirrhosis.
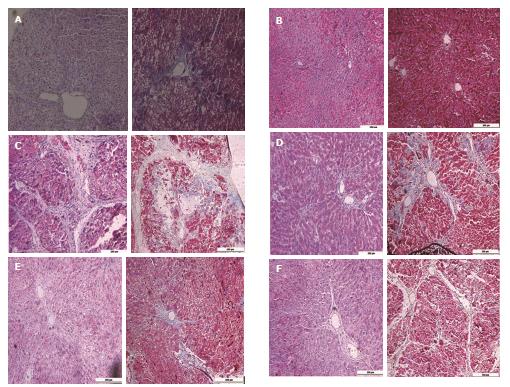
At wk 8, conventional HE staining of liver sections from the model group (Figure 2A) showed that hepatocytes around the central vein in lobules were disordered and both steatosis and fat vacuoles were visible in the cytoplasm. By Masson staining, collagen fibers around the central vein and portal area demonstrated significant proliferation, had a cord-like extension to the liver lobule and did not form pseudo-lobules. The rat model of liver fibrosis was successfully established.
At wk 12, HE staining of liver sections from the healthy control group (Figure 2B) showed normal hepatocyte morphology, complete structure of liver lobules and a neat radiation-like arrangement of liver tissue around the central vein. Masson staining showed no obvious collagen fibrosis. HE staining of liver sections from the TAA group (Figure 2C) showed damaged liver lobule structure and abnormal structure of pseudo-lobules due to regeneration of hepatocytes. Masson staining revealed significant proliferation of collagen tissue and the formation of pseudo-lobules. HE staining of liver sections in the remaining groups (Figure 2D-F) showed disordered hepatocyte arrangement, but no obvious formation of pseudo-lobules. Masson staining exhibited different degrees of collagen fiber proliferation.
The staging and scoring of liver fibrosis in each group at wk 12 are shown in Table 3. The difference in fibrosis grading between the healthy control group and the other model groups was statistically significant (P < 0.01), indicating that the model was successfully established. The difference between the TAA group and the other drug-intervention groups was statistically significant (P < 0.05), indicating that the drug intervention was effective.
Liver fibrosis/cirrhosis is a common outcome of chronic liver disease due to various etiologies, with high morbidity and mortality. Treatment of advanced liver fibrosis/cirrhosis is very unsatisfactory and is currently limited to the treatment of its underlying etiologies and remission of its complications. Therefore, there is an urgent need to develop new antifibrotic treatments that can prevent, halt or even reverse liver fibrosis. Recent, evidence has shown that intrahepatic thrombosis is associated with liver fibrosis. Mancuso[6] proposed that chronic microvascular ischemia of the liver may be a milestone in the development of liver cirrhosis. Chronic liver damage can eventually lead to endothelial injury and microvascular thrombosis, triggering inappropriate hepatocyte proliferation and fibrosis. In order to validate this theory, we established a rat model of liver fibrosis induced by TAA, determined the presence or absence of thrombosis in rat fibrotic livers, and examined whether antithrombotic therapy with aspirin and enoxaparin could reverse liver fibrosis.
The establishment of this animal model of liver fibrosis is of great significance in understanding the pathogenesis and treatment strategy of liver fibrosis and cirrhosis. To date, animal models have been developed using mice, rats, rabbits and pigs to mimic the complex processes of liver fibrosis and cirrhosis. Liver fibrosis induced by TAA is a widely used model and TAA can be administered by oral intake or peritoneal injection; however, peritoneal injection provides more consistent results. TAA-induced liver fibrosis is very similar to human liver fibrosis in terms of changes in hemodynamics, morphological and biochemical metabolism changes[7].
TAA damages DNA, RNA and protein synthetase in hepatocytes, leading to disorders of metabolism and necrosis of hepatocytes. An obvious feature of this model relative to the carbon tetrachloride model is that its fibrosis remains stable for several weeks after TAA withdrawal. However, TAA is a weak carcinogen and is both toxic and volatile. Large nodular cirrhosis and portal hypertension developed in the present model at 12 wk. A significantly highly powered cycle may be required to induce fibrosis for longer periods. Approximately 40% of rats develop ascites. After 18 wk of TAA administration, rats may develop cholangiocarcinoma.
In the present study, the rat liver fibrosis model was successfully induced by TAA in the 8th week. ALT and TBIL in the model group were significantly higher than those in the control group (P < 0.05). ALB decreased, and both PT and INR were prolonged, but prolongation was not statistically significant. These findings indicated that following the formation of liver fibrosis, liver function became abnormal. At the 12th week, the rat model of liver cirrhosis was successfully established.
In 1995, Wanless et al[8] showed that both congestion and non-congestion in liver cirrhosis were present in the hepatic circulation during microthrombosis. By studying liver biopsy specimens from patients with chronic viral hepatitis, Papatheodoridis et al[2] in 2003 found that thrombosis-related factors were closely related to hepatitis staging and the degree of liver fibrosis, and antithrombosis drugs seemed to reverse liver fibrosis. It was suggested that microthrombosis may be involved in the formation of liver fibrosis in chronic liver disease, rather than the result of cirrhosis. In the present study, both HE staining and Masson staining showed that liver fibrosis in model rats was significant, and progressed to liver cirrhosis at 12 wk. However, no apparent microthrombosis was found, probably because HE staining and Masson staining were not sensitive enough to detect microthrombi. It is necessary to identify a more sensitive experimental method to observe microthrombosis in the liver.
Aspirin is regarded as an agent for preventing arterial thrombosis, but can also reduce the risk of venous thromboembolism[9]. The mechanism underlying the antifibrotic properties of aspirin may be related to its antithrombotic properties. Sinusoidal endothelial cell injury can also lead to increased platelet adhesion and a coagulation state. Indeed, such mechanisms have been implicated in injury repair and hepatic sinus obstruction syndrome after liver transplantation, and aspirin has been shown to reduce the risk of chemotherapy-associated sinusoidal lesions.
Aspirin also exhibits anti-inflammatory activity and inhibits nuclear factor kappa B (NFκB) transcript adhesion molecules in endothelial cells and vascular smooth muscle cells in vitro, thereby affecting macrophage and T lymphocyte adherence to these cells. Aspirin also reduces the levels of interleukin-6, monocyte chemoattractant protein-1, transforming growth factor-beta (TGF-β), tumor necrosis factoral-pha and platelet-derived growth factor. Chavez et al[10] showed that in the liver fibrosis model induced by carbon tetrachloride, aspirin partially inhibited the expression of TGF-β and prevented translocation of NFκB into the nucleus.
The anti-inflammatory effects of aspirin are achieved by endogenous anti-inflammatory lipids known as “aspirin-triggering lipoxins” (ATLs). ATLs are derived from arachidonic acid (converted by COX-2 acetylation), and even low-dose aspirin can induce ATLs[11]. Interestingly, ATL analogues reduced the level of TGF-β and reversed the fibrosis process in a mouse model of pulmonary fibrosis induced by bleomycin[12]. Thus, the effect of aspirin in reducing liver fibrosis is mediated by its antithrombotic and/or anti-inflammatory properties.
Some studies have attempted to determine whether aspirin or other antiplatelet drugs play a role in the progression of liver fibrosis. In a rat model of nonalcoholic fatty liver disease, Fujita et al[13] reported that antiplatelet drugs, such as aspirin, ticlopidine and cilostazol, significantly reduced liver fibrosis. Wanless et al[14] also proved that in a rabbit model of liver sinus fibrosis induced by cholesterol and diethylstilbestrol, dipyridamole inhibited platelet aggregation and reduced fibrosis in vitro. Yoshida et al[15] recently reported that platelet activation directly promoted liver fibrosis, and aspirin prevented the progression of liver fibrosis in animal models.
In addition to animal studies, Poujol-Robert et al[16] reported that daily low-dose aspirin administration reduced the progression of liver fibrosis in patients with recurrent hepatitis C after liver transplantation. Our study found that aspirin reduced liver fibrosis in rats and that high-dose aspirin (300 mg/kg) was superior to low-dose aspirin (30 mg/kg) in terms of antifibrotic effects.
Current experimental evidence suggests that coagulation factors, particularly thrombin, are closely related to liver fibrosis. Thrombin, the major effector of the coagulation cascade, causes a series of cellular effects through a family of G protein-coupled receptors known as protease-activated receptors. Through these receptors, thrombin stimulates the proliferation of hepatic stellate cells, which are important mediators of liver fibrogenesis. Thrombin receptors are expressed in the liver, and during the process of acute and chronic liver injury, the expression of thrombin receptors is up-regulated. Heparin, through its interaction with antithrombin, induces a conformational change in the antithrombin molecule, which greatly facilitates the interaction between antithrombin and targets of serine proteases such as thrombin, as well as the factors Xa, IXa, XIa and XIIa[17]. Low molecular weight heparin induces inhibition of the coagulation cascade, and its potential beneficial effects in liver fibrosis have been evaluated[18]. In our study, enoxaparin was shown to reduce liver fibrosis in rats.
In conclusion, we successfully established a rat model of liver fibrosis after 8 wk and successfully established a rat model of liver cirrhosis after 12 wk induced by TAA. Our study clearly shows that aspirin and enoxaparin can reduce liver fibrosis in rats. However, it is unclear whether the antifibrotic mechanisms of the two drugs are directly related to their anticoagulant effects in preventing thrombosis, or to other mechanisms, Further investigations are needed using anticoagulants as potential treatment for liver fibrosis/cirrhosis.
The authors would like to thank the members of the East China Normal University for their technical support.
Liver fibrosis is a hotspot of research. It is closely related to the chronic inflammatory response of the liver, and inflammation leading to liver fibrosis has been studied in detail. At present, several lines of evidence show that intrahepatic thrombosis is associated with liver fibrosis. A variety of chronic liver damage can eventually lead to endothelial injury and microvascular thrombosis, triggering inappropriate hepatocyte proliferation and fibrosis. Based on these observations, they hypothesized that routine use of antithrombotic drugs may prevent liver fibrosis.
In the past, there was widespread belief that patients with liver cirrhosis experienced hypocoagulability. In recent years, however, this belief has been challenged. Studies found that patients with cirrhosis had hypercoagulability and even had a thrombus in some cases. There is evidence that coagulation is an important factor in the progression of cirrhosis. It is important to discuss the relationship among coagulation, anticoagulant therapy and liver fibrosis/cirrhosis.
Reports of studies on antithrombotic therapies and antiplatelet agents reducing liver fibrosis in animals are rare. In this study, the authors evaluated if aspirin and enoxaparin are beneficial in a thioacetamide-induced rat model of liver fibrosis.
Aspirin and enoxaparin can reduce liver fibrosis in a thioacetamide-induced rat model. They are beneficial in humans with liver fibrosis as well.
Liver fibrosis and subsequent liver cirrhosis are common outcomes of chronic liver disease caused by various etiologies, with high morbidity and mortality.
The manuscript is very interesting, describing a study using a rat model, but reporting the results in humans is more complicated because we should consider other aspects, such as renal function. The manuscript is well written.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lai S S-Editor S- Editor: Gong ZM
L- Editor: Filipodia E- Editor: Ma YJ
| 1. | Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238-1247. [PubMed] |
| 2. | Papatheodoridis GV, Papakonstantinou E, Andrioti E, Cholongitas E, Petraki K, Kontopoulou I, Hadziyannis SJ. Thrombotic risk factors and extent of liver fibrosis in chronic viral hepatitis. Gut. 2003;52:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Denda A, Tang Q, Endoh T, Tsujiuchi T, Horiguchi K, Noguchi O, Mizumoto Y, Nakae D, Konishi Y. Prevention by acetylsalicylic acid of liver cirrhosis and carcinogenesis as well as generations of 8-hydroxydeoxyguanosine and thiobarbituric acid-reactive substances caused by a choline-deficient, L-amino acid-defined diet in rats. Carcinogenesis. 1994;15:1279-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Frizelle S, Schwarz J, Huber SA, Leslie K. Evaluation of the effects of low molecular weight heparin on inflammation and collagen deposition in chronic coxsackievirus B3-induced myocarditis in A/J mice. Am J Pathol. 1992;141:203-209. [PubMed] |
| 5. | Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 644] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 6. | Mancuso A. Cirrhosis development probably arises from chronic micro-vascular ischemia. Med Hypotheses. 2014;82:244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Yang MC, Chang CP, Lei HY. Induction of liver fibrosis in a murine hepatoma model by thioacetamide is associated with enhanced tumor growth and suppressed antitumor immunity. Lab Invest. 2010;90:1782-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Wanless IR, Liu JJ, Butany J. Role of thrombosis in the pathogenesis of congestive hepatic fibrosis (cardiac cirrhosis). Hepatology. 1995;21:1232-1237. [PubMed] |
| 9. | Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, Bianchi M, Moia M, Ageno W, Vandelli MR. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 436] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 10. | Chávez E, Castro-Sánchez L, Shibayama M, Tsutsumi V, Pérez Salazar E, Moreno MG, Muriel P. Effects of acetyl salycilic acid and ibuprofen in chronic liver damage induced by CCl4. J Appl Toxicol. 2012;32:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178-15183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Martins V, Valença SS, Farias-Filho FA, Molinaro R, Simões RL, Ferreira TP, e Silva PM, Hogaboam CM, Kunkel SL, Fierro IM. ATLa, an aspirin-triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin-induced pulmonary fibrosis. J Immunol. 2009;182:5374-5381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Fujita K, Nozaki Y, Wada K, Yoneda M, Endo H, Takahashi H, Iwasaki T, Inamori M, Abe Y, Kobayashi N. Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut. 2008;57:1583-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Wanless IR, Belgiorno J, Huet PM. Hepatic sinusoidal fibrosis induced by cholesterol and stilbestrol in the rabbit: 1. Morphology and inhibition of fibrogenesis by dipyridamole. Hepatology. 1996;24:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Yoshida S, Ikenaga N, Liu SB, Peng ZW, Chung J, Sverdlov DY, Miyamoto M, Kim YO, Ogawa S, Arch RH. Extrahepatic platelet-derived growth factor-β, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology. 2014;147:1378-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Poujol-Robert A, Boëlle PY, Conti F, Durand F, Duvoux C, Wendum D, Paradis V, Mackiewicz V, Chazouillères O, Corpechot C. Aspirin may reduce liver fibrosis progression: Evidence from a multicenter retrospective study of recurrent hepatitis C after liver transplantation. Clin Res Hepatol Gastroenterol. 2014;38:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Laux V, Perzborn E, Heitmeier S, von Degenfeld G, Dittrich-Wengenroth E, Buchmüller A, Gerdes C, Misselwitz F. Direct inhibitors of coagulation proteins-the end of the heparin and low-molecular-weight heparin era for anticoagulant therapy? Thromb Haemost. 2009;102:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Abdel-Salam OM, Baiuomy AR, Ameen A, Hassan NS. A study of unfractionated and low molecular weight heparins in a model of cholestatic liver injury in the rat. Pharmacol Res. 2005;51:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |