Published online Jun 28, 2017. doi: 10.3748/wjg.v23.i24.4422
Peer-review started: March 2, 2017
First decision: April 10, 2017
Revised: April 19, 2017
Accepted: May 19, 2017
Article in press: May 19, 2017
Published online: June 28, 2017
Processing time: 116 Days and 23.4 Hours
To assess the accuracy of serum procalcitionin (PCT) as a diagnostic marker in verifying upper and lower gastrointestinal perforation (GIP).
This retrospective study included 46 patients from the surgical intensive care unit (ICU) of the Second Affiliated Hospital of Harbin Medical University who were confirmed to have GIP between June 2013 and December 2016. Demographic and clinical patient data were recorded on admission to ICU. Patients were divided into upper (n = 19) and lower (n = 27) GIP groups according to the perforation site (above or below Treitz ligament). PCT and WBC count was obtained before laparotomy and then compared between groups. Meanwhile, the diagnostic accuracy of PCT was analyzed.
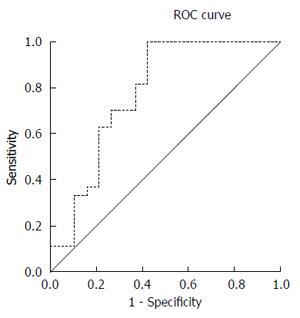
Patients with lower GIP exhibited significantly higher APACHE II score, SOFA score and serum PCT level than patients with upper GIP (P = 0.017, 0.004, and 0.001, respectively). There was a significant positive correlation between serum PCT level and APACHE II score or SOFA score (r = 0.715 and r = 0.611, respectively), while there was a significant negative correlation between serum PCT level and prognosis (r = -0.414). WBC count was not significantly different between the two groups, and WBC count showed no significant correlation with serum PCT level, APACHE II score, SOFA score or prognosis. The area under the receiver operating characteristic curve of PCT level to distinguish upper or lower GIP was 0.778. Patients with a serum PCT level above 17.94 ng/dL had a high likelihood of lower GIP, with a sensitivity of 100% and a specificity of 42.1%.
Serum PCT level is a reliable and accurate diagnostic marker in identifying upper or lower GIP before laparotomy.
Core tip: Procalcitionin (PCT) is a rapid, reliable and accurate predictive marker and contributes to assisting the clinicians in identifying upper or lower gastrointestinal perforation (GIP) before laparotomy, and it can be used as a useful supplementary tool for early clinical judgment of perforation site. The results showed that patients with lower GIP exhibited significantly higher APACHE II score, SOFA score and serum PCT level than patients with upper GIP, which might be related to the differences in bacterial load and the severity of sepsis between upper and lower GIP.
- Citation: Gao Y, Yu KJ, Kang K, Liu HT, Zhang X, Huang R, Qu JD, Wang SC, Liu RJ, Liu YS, Wang HL. Procalcitionin as a diagnostic marker to distinguish upper and lower gastrointestinal perforation. World J Gastroenterol 2017; 23(24): 4422-4427
- URL: https://www.wjgnet.com/1007-9327/full/v23/i24/4422.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i24.4422
Gastrointestinal perforation (GIP) is one of the most common acute abdominal diseases in department of general surgery. As a life-threatening disease with a high mortality rate, especially for elderly people, GIP could easily lead to shock and usually needs active rescue in the intensive care unit (ICU) and emergency laparotomy[1]. In general, GIP is related to many factors such as older age, diabetes, antecedent diverticulitis, glucocorticoid therapy, usage of non-steroidal anti-inflammatory drugs (NSAIDs) and so on[2-4]. It is still a challenge to accurately predict upper or lower GIP before laparotomy, despite increased available clinical and biological variables. Early diagnosis of perforation site has beneficial effects on operative procedure, selection of antibiotics and even judgement of the severity of infection and prognosis.
Serum procalcitionin (PCT) concentration was discovered to be significantly higher in patients with bacterial and fungal infections, and sepsis first in 1993[5], which has been proved to be a rapid, reliable and accurate diagnostic marker and is used to identify infectious and non-infectious diseases now[6-8]. Sepsis could promote the secretion of PCT through stimulating various types of cells in a variety of tissues[9], which is typically produced by C cells of the thyroid. More importantly, PCT concentration is related to bacterial load[10], the severity of sepsis[11], and even prognosis[12,13]. Owing to different perforation sites and contents, the severity of sepsis and bacterial load are obviously different, especially when comparing upper and lower GIP. We hypothesized that, due to fewer bacteria, the severity of sepsis caused by upper GIP was lower than that caused by lower GIP, which could be reflected by serum PCT level. Thus, serum PCT level might be a useful supplementary tool for clinical judgment of perforation site.
So far, the evidence regarding the diagnostic validity and accuracy of PCT in predicting upper or lower GIP is lacking. To the best of our knowledge, few studies have formally assessed its role in this area. In this retrospective study, serum PCT level was evaluated as a diagnostic marker in distinguishing upper or lower GIP before laparotomy. To our knowledge, this study is the first to evaluate the role of PCT in predicting upper or lower GIP before laparotomy as a useful supplementary tool.
The current study is a retrospective study performed in the surgical ICU of the Second Affiliated Hospital of Harbin Medical University (Harbin, China). Patients were enrolled from June 2013 to December 2016. The study protocol was approved by the ethics committee.
Patients who met the following criteria were included: (1) ICU admission; (2) patients were definitely diagnosed with GIP by laparotomy; (3) serum PCT level and WBC count were detected before laparotomy; and (4) patients aged > 18 years. Patients who met following criteria were excluded: (1) uncertain perforation site; (2) pregnant or breast-feeding women; (3) being on blood purification treatment; and (4) those receiving antibiotic therapy before ICU admission. In addition, patients with incomplete medical records were also excluded. All enrolled patients were treated by same experienced physicians.
GIP was defined as the destruction of integrity of the digestive tract, i.e., a complete non-traumatic penetration of the wall of the esophagus, stomach, small or large bowel[2]. Patients were divided into upper and lower GIP groups according to the perforation site (above or below Treitz ligament). Owing to the complexity of GIP diagnosis, a careful and thorough clinical examination was necessary.
Serum PCT level and WBC count were measured following ICU admission immediately before laparotomy. Mini VIDAS (Hain Lifescience GmbH; Nehren, Germany) was applied to measure serum PCT level.
Baseline data: Gender, age, height, weight, body mass index (BMI), prognosis, acute physiology and chronic health evaluation (APACHE) II score, sequential organ failure assessment (SOFA) score and perforation site were obtained from patient medical records.
Demographic and clinical data of selected patients were recorded on admission to SICU with blood samples taken for measurement of serum PCT level and WBC count immediately. APACHE II score and SOFA score were calculated using data collected from the first 24 h after admission.
Data are described as the mean ± SD and SPSS 13.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analyses. To compare baseline data between groups, independent sample t test and χ2 test were employed. Independent sample t test was used to compare APACHE II score, SOFA score and WBC count between groups. Owing to non-normality, serum PCT level between groups was analyzed by the Manny-Whitney rank sum test. Correlation between parameters was analyzed by Pearson or Spearman correlation. Area under the receiver operating characteristic (ROC) curve was calculated to evaluate the predictive value of PCT and to determine optimal cut-off value for distinguishing between upper and lower GIP before laparotomy. P-values < 0.05 were considered statistically significant.
A total of 46 patients were enrolled in this retrospective study who underwent serum PCT level and WBC count measurement following ICU admission immediately before laparotomy. In all patients, GIP was proved by laparotomy. Nineteen patients were included into the upper GIP group, while the rest patients in the lower GIP group. As shown in Table 1, no significant differences were observed in baseline data with the exception of age (P = 0.028).
| Upper | Lower | t value | P value | |
| n = 19 | n = 27 | |||
| Age | 58.84 | 67.59 | -2.280 | 0.028 |
| Gender | ||||
| Male | 7 | 14 | 1.013 | 0.314 |
| Female | 12 | 13 | ||
| Height | 168.47 | 165.37 | 1.316 | 0.195 |
| Weight | 63.42 | 58.37 | 1.589 | 0.119 |
| BMI | 22.27 | 21.21 | 1.176 | 0.246 |
| APACHEII | 12.47 | 18.15 | -2.477 | 0.017 |
| SOFA | 5.84 | 9.33 | -3.041 | 0.004 |
| PCT | 33.26 | 40.73 | -3.079 | 0.001 |
| WBC | 13.67 | 11.27 | 1.105 | 0.275 |
Patients with lower GIP exhibited significantly higher APACHE II score, SOFA score and serum PCT level than patients with upper GIP (P = 0.017, 0.004, and 0.001, respectively). However, WBC count showed no significant difference between the two groups, and WBC count showed no significant correlation with serum PCT level, APACHE II score, SOFA score or prognosis (Table 2). The area under the ROC curve of PCT level to distinguish upper and lower GIP was 0.778 (Figure 1). Patients with a serum PCT level above 17.94 ng/dL had a high likelihood of lower GIP, with a sensitivity of 100% and a specificity of 42.1% (Table 3).
| APACHEII | SOFA | PCT | WBC | Prognosis | |
| PCT | 0.715 | 0.611 | 1.000 | -0.143 | -0.414 |
| < 0.001 | < 0.001 | < 0.001 | 0.342 | 0.004 | |
| WBC | -0.242 | -0.033 | -0.143 | 1.000 | 0.112 |
| 0.105 | 0.829 | 0.342 | 0.457 |
| Area | Std. Error | Asymptotic Sig. | Asymptotic 95%CI | |
| Lower bound | Upper bound | |||
| 0.778 | 0.077 | 0.001 | 0.628 | 0.928 |
PCT is a prepropeptide precursor of calcitonin, which had been proved to be a sensitive and specific predictive marker for bacterial infection[14,15], and thus was used to guide antibacterial therapy and reduce its length[16-19], which is not affected by hepatic or renal dysfunction[20-22]. PCT has many advantages of an ideal marker for routine clinical application, including simplicity, accuracy, specificity, stability and availability[23]. However, little is known on the predictive value of PCT in judging perforation site before laparotomy. We speculated that, when upper or lower GIP happens, they are different in bacterial load and the severity of sepsis owing to the leakage of different digestive tract contents, which could be reflected by serum PCT level. Antibiotic therapy might have an impact on bacterial load and even serum PCT level, therefore, patients who had used antibiotics before ICU admission were excluded[24].
APACHE II score and SOFA score are usually applied to evaluate the severity of disease[25], especially suitable for critically ill adult patients in ICU, which are closely related to mortality[25-29]. Therefore, in our study, they were chosen to assess the degree of illness. In particular, SOFA score was an important part of sepsis diagnosis in The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)[30].
The results of the present study showed that patients with lower GIP exhibited significantly higher APACHE II score, SOFA score and serum PCT level than patients with upper GIP, which might be related to the differences in bacterial load and the severity of sepsis between upper and lower GIP. In contrast, our findings did not show a significant difference between the two groups regarding WBC count. In other words, WBC count did not have a predictive value for sepsis, which is in accordance with previous studies[31]. Further correlation analysis showed that there was a significant positive correlation between serum PCT level and APACHE II score or SOFA score, and a significant negative correlation between serum PCT level and prognosis. By analyzing the ROC curve, an optimal cut-off value was selected as a predictive value to distinguish between upper and lower GIP before laparotomy.
There were several limitations in the present study. First, patients were selected from a single center, which made the evidence level for this study relatively low. Second, the sample size was relatively small. Thus, experiments with larger sample size are needed to verify our findings in the future. At last, this study only concerned serum PCT level measured immediately after ICU admission and before laparotomy, but lacked of dynamic observation of changes in serum PCT level after laparotomy.
In conclusion, PCT is a rapid, reliable and accurate predictive marker and contributes to assisting clinicians toward identifying upper or lower GIP before laparotomy, which can be used as a useful supplementary tool for early clinical judgment of perforation site.
We thank the participants and colleagues from the SICU of the Second Affiliated Hospital of Harbin Medical University, and all the people who offered the advice and help to this study.
Gastrointestinal perforation (GIP) is one of the most common acute abdominal diseases in department of general surgery with high mortality rates, which could easily lead to shock and needs rescue therapy in the intensive care unit. It is still a challenge to accurately predict upper or lower GIP before laparotomy, despite increased available clinical and biological variables. Serum procalcitionin (PCT) concentration is related to bacterial load as well as the severity of sepsis, and even prognosis. Thus, the authors hypothesized that, due to fewer bacteria, the severity of sepsis caused by upper GIP is lower than that caused by lower GIP, which could be reflected by serum PCT level.
At present, the research on PCT has mainly focused on the following aspects: being used as a predictive marker for bacterial infection, distinguishing between bacterial and non-bacterial infections, guiding antibacterial therapy and reducing its length and so on. But so far the evidence regarding the diagnostic validity and accuracy of PCT in predicting upper or lower GIPs is lacking. To the best of our knowledge, few studies have formally assessed its role in this area.
This study is the first to evaluate the role of PCT in predicting upper or lower GIP before laparotomy as a useful supplementary tool. This study has confirmed that PCT is a rapid, reliable and accurate predictive marker and can be used for early clinical judgment of perforation site.
When upper or lower GIP happens, they are different in bacterial load and the severity of sepsis owing to leakage of different digestive tract contents, which could be reflected by serum PCT level. Therefore, PCT contributes to assisting clinicians toward identifying upper or lower GIP before laparotomy, which has beneficial effects on operative procedure, selection of antibiotics and even judgement of the severity of infection and prognosis.
PCT is a prepropeptide precursor of calcitonin, and its level was discovered to be significantly higher in patients with bacterial and fungal infections, and sepsis first in 1993. GIP was defined as the destruction of integrity of the digestive tract, i.e., a complete non-traumatic penetration of the wall of the esophagus, stomach, small or large bowel. Patients were divided into upper and lower GIP groups according to the perforation site (above or below Treitz ligament).
This is an interesting study about the procalcitionin as a diagnostic marker to distinguish upper and lower gastrointestinal perforation. In this retrospective study, the authors included 46 patients from the SICU of the Second Affiliated Hospital of Harbin Medical University who were confirmed to have GIP between June 2013 and December 2016. There was a significant positive correlation between serum PCT level and APACHE II score or SOFA score, while there was a significant negative correlation between serum PCT level and Prognosis. Patients with a serum PCT level above 17.94 ng/dL had a high likelihood of lower GIP, with a sensitivity of 100% and a specificity of 42.1%.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chadokufa S, Chamberlain MC S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Domínguez-Comesaña E, Ballinas-Miranda JR. Procalcitonin as a marker of intra-abdominal infection. Cir Cir. 2014;82:231-239. [PubMed] |
| 2. | Závada J, Lunt M, Davies R, Low AS, Mercer LK, Galloway JB, Watson KD, Symmons DP, Hyrich KL. The risk of gastrointestinal perforations in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the BSRBR-RA. Ann Rheum Dis. 2014;73:252-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Xie F, Yun H, Bernatsky S, Curtis JR. Brief Report: Risk of Gastrointestinal Perforation Among Rheumatoid Arthritis Patients Receiving Tofacitinib, Tocilizumab, or Other Biologic Treatments. Arthritis Rheumatol. 2016;68:2612-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Curtis JR, Xie F, Chen L, Spettell C, McMahan RM, Fernandes J, Delzell E. The incidence of gastrointestinal perforations among rheumatoid arthritis patients. Arthritis Rheum. 2011;63:346-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515-518. [PubMed] |
| 6. | Yang Y, Xie J, Guo F, Longhini F, Gao Z, Huang Y, Qiu H. Combination of C-reactive protein, procalcitonin and sepsis-related organ failure score for the diagnosis of sepsis in critical patients. Ann Intensive Care. 2016;6:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Arora R, Campbell JP, Simon G, Sahni N. Does serum procalcitonin aid in the diagnosis of bloodstream infection regardless of whether patients exhibit the systemic inflammatory response syndrome. Infection. 2016; Epub ahead of print. |
| 8. | Simon L, Saint-Louis P, Amre DK, Lacroix J, Gauvin F. Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr Crit Care Med. 2008;9:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Schneider HG, Lam QT. Procalcitonin for the clinical laboratory: a review. Pathology. 2007;39:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | van Nieuwkoop C, Bonten TN, van’t Wout JW, Kuijper EJ, Groeneveld GH, Becker MJ, Koster T, Wattel-Louis GH, Delfos NM, Ablij HC. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care. 2010;14:R206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Jiang L, Feng B, Gao D, Zhang Y. Plasma concentrations of copeptin, C-reactive protein and procalcitonin are positively correlated with APACHE II scores in patients with sepsis. J Int Med Res. 2015;43:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Liu D, Su LX, Guan W, Xiao K, Xie LX. Prognostic value of procalcitonin in pneumonia: A systematic review and meta-analysis. Respirology. 2016;21:280-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Jain S, Sinha S, Sharma SK, Samantaray JC, Aggrawal P, Vikram NK, Biswas A, Sood S, Goel M, Das M. Procalcitonin as a prognostic marker for sepsis: a prospective observational study. BMC Res Notes. 2014;7:458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Takakura Y, Hinoi T, Egi H, Shimomura M, Adachi T, Saito Y, Tanimine N, Miguchi M, Ohdan H. Procalcitonin as a predictive marker for surgical site infection in elective colorectal cancer surgery. Langenbecks Arch Surg. 2013;398:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Schlattmann P, Brunkhorst FM. Procalcitonin as a diagnostic marker for sepsis. Lancet Infect Dis. 2014;14:189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, Loef BG, Dormans T, van Melsen GC, Kluiters YC. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 567] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 17. | Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock--a systematic review and meta-analysis. Crit Care. 2013;17:R291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Oliveira CF, Botoni FA, Oliveira CR, Silva CB, Pereira HA, Serufo JC, Nobre V. Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Crit Care Med. 2013;41:2336-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Hohn A, Schroeder S, Gehrt A, Bernhardt K, Bein B, Wegscheider K, Hochreiter M. Procalcitonin-guided algorithm to reduce length of antibiotic therapy in patients with severe sepsis and septic shock. BMC Infect Dis. 2013;13:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Lin KH, Wang FL, Wu MS, Jiang BY, Kao WL, Chao HY, Wu JY, Lee CC. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: a systematic review and meta-analysis. Diagn Microbiol Infect Dis. 2014;80:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Rau BM, Frigerio I, Büchler MW, Wegscheider K, Bassi C, Puolakkainen PA, Beger HG, Schilling MK. Evaluation of procalcitonin for predicting septic multiorgan failure and overall prognosis in secondary peritonitis: a prospective, international multicenter study. Arch Surg. 2007;142:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Meisner M, Lohs T, Huettemann E, Schmidt J, Hueller M, Reinhart K. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur J Anaesthesiol. 2001;18:79-87. [PubMed] |
| 23. | Walker C. Procalcitonin-guided antibiotic therapy duration in critically ill adults. AACN Adv Crit Care. 2005;26:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect. 2015;21:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 25. | Naved SA, Siddiqui S, Khan FH. APACHE-II score correlation with mortality and length of stay in an intensive care unit. J Coll Physicians Surg Pak. 2011;21:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Stevens V, Lodise TP, Tsuji B, Stringham M, Butterfield J, Dodds Ashley E, Brown K, Forrest A, Brown J. The utility of acute physiology and chronic health evaluation II scores for prediction of mortality among intensive care unit (ICU) and non-ICU patients with methicillin-resistant Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2012;33:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | De Freitas ER. Profile and severity of the patients of intensive care units: prospective application of the APACHE II index. Rev Lat Am Enfermagem. 2010;18:317-323. [PubMed] |
| 28. | Al Tehewy M, El Houssinie M, El Ezz NA, Abdelkhalik M, El Damaty S. Developing severity adjusted quality measures for intensive care units. Int J Health Care Qual Assur. 2010;23:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Rocker G, Cook D, Sjokvist P, Weaver B, Finfer S, McDonald E, Marshall J, Kirby A, Levy M, Dodek P. Clinician predictions of intensive care unit mortality. Crit Care Med. 2004;32:1149-1154. [PubMed] |
| 30. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17122] [Article Influence: 1902.4] [Reference Citation Analysis (2)] |
| 31. | Seigel TA, Cocchi MN, Salciccioli J, Shapiro NI, Howell M, Tang A, Donnino MW. Inadequacy of temperature and white blood cell count in predicting bacteremia in patients with suspected infection. J Emerg Med. 2012;42:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |